نقش درمان بیماری پریودنتال در پیشگیری از وقوع پیامدهای نامطلوب زایمان در زنان باردار
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Location: UK Setting: Guy's and St Thomas Hospitals, UK Recruitment period: unclear | |
| Participants | Inclusion criteria: 12 weeks gestation, severe periodontal disease (6 or more sites with 5 mm or more probing depth and 3 or more sites with 3 mm or more loss of periodontal attachment) Exclusion criteria: not stated Age: not stated Gestational age: 12 weeks History of preterm delivery: not stated Number randomised: n = 198 Number evaluated: n = 140 (attrition n = 58 lost to follow‐up) | |
| Interventions | 1) Antenatal periodontal treatment (n = 102): 5 visits (baseline assessment, oral hygiene instruction, scaling, hand and ultrasonic instrumentation, follow‐up at 30 weeks and maintenance every month until birth) 2) Control (n = 96): could choose to attend own dentist after birth No information on the expertise of the dental professional who administered intervention | |
| Outcomes | Gestational age; birth weight; miscarriage/stillbirth | |
| Funding | Unclear as full study was not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Administrative staff allocated subjects via a random allocation table to one of the two groups, following stratification for age, ethnicity, and smoking status" |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation. The outcome in question was assumed to be the obstetric since the study did not report any periodontal outcome |
| Incomplete outcome data (attrition bias) | High risk | 40% (41/102) of the treatment group did not receive any periodontal treatment and 57% (58/102) did not attend the follow‐up visit ‐ high attrition rate |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | Unclear risk | Imbalance in numbers in the 2 groups (102 in the treatment in pregnancy group and 96 in the control group) ‐ about 6% difference |
| Methods | Study design: RCT Location: Colombia Setting: Hospital Universitario del Valle, Cali, Colombia Recruitment period: March 2006 and December 2007 | |
| Participants | Periodontal characteristics: 62% of women had chronic periodontitis (American Academy of Periodontology criteria) Inclusion criteria: pregnant women with mild pre‐eclampsia (blood pressure < 160/11 and proteinuria ≥ 300 mg/L in 24 hours urine) with gestational age between 26 and 34 weeks (no restriction on parity or mother's age); women who had not received antibiotics in the previous 3 months, or periodontal treatment in the previous 6 months before inclusion in study Exclusion criteria: history of chronic hypertension, kidney or cardiovascular disease, diabetes or past history of infections (apart from periodontal or HIV) Mean age (± standard deviation (years)): Group A = 24.7 ± 6.4, Group B = 27 ± 7.6 (P = 0.01) Mean gestational age at trial entry (weeks): Group A = 31.2, Group B = 32.4 History of preterm delivery: not reported Number randomised: n = 60 Number evaluated: n= 60 | |
| Interventions | A) Antenatal periodontal treatment (n = 28): between 26 and 34 weeks supragingival and subgingival cleaning with ultrasonic and manual devices (oral health education, hygiene, dental plaque removal, scaling and root planing (if necessary), subgingival irrigation without antibiotic administration in 1 single session of 1 to 2 hours) B) Postnatal periodontal treatment (n = 32): at 48 hours postpartum Periodontal treatment was performed by periodontists | |
| Outcomes | Progression from mild to severe pre‐eclampsia; eclampsia or HELLP syndrome; number of days of clinical stability; percentile of birth weight adjusted for gestational age; preterm birth; probing depth; clinical attachment level; gingival bleeding (at probing) | |
| Funding | No funding source reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised by blocks" Comment: no further details |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment intention was determined at random, in closed envelopes prepared by professionals external to the research group" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Periodontists did not know the objectives of the research" Comment: this was not considered as adequate blinding |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Periodontal health outcomes on the same population were reported in a linked article (Contreras A, Botero J, Jaramillo A, Soto J, Velez S, Herrera JA. Effects of periodontal treatment on the preterm delivery and low weight newborn in women with preeclampsia ‐ clinical controlled trial. Revista Odontológica Mexicana 2010;14(4):226‐30) |
| Other bias | High risk | More (57% (16/28)) of women in the treatment group had chronic mild periodontitis compared with 37% (12/32) in the control group. There were also differences in age and gestational age at entry |
| Methods | Study design: RCT Location: USA Setting: Periodontal Clinic, University of Alabama School of Dentistry, Alabama, USA Recruitment period: not stated | |
| Participants | Inclusion criteria: pregnant women between 21 and 25 weeks gestational age; screened for ≥ 3 sites clinical attachment loss ≥ 3 mm; ambulatory; willingness to participate and give consent Exclusion criteria: women participating in any other treatment study; undergoing periodontal therapy; taking antibiotics during pregnancy; or using antibiotic mouthrinse; requiring treatment for bacterial vaginosis Mean age (± standard deviation (years)): Group A = 22.2 ± 4 .3, Group B = 22.8 ± 4.6, Group C = 22.4 ± 5 (P = 0.62) Gestational age at trial entry: 21 to 25 weeks (P = not reported) History of spontaneous preterm birth < 35 weeks, n (%): Group A = 6 (4.9%), Group B = 5 (4.1%), Group C = 4 (3.3%) (P = 0.83) Number randomised: n = 368 Number analysed: n = 366 (attrition n = 2 participants delivered elsewhere) | |
| Interventions | A) Antenatal periodontal treatment ‐ SRP + placebo capsule (n = 123): scaling and root planing was performed according to usual clinical procedures and clinicians were instructed to spend as much time and as many visits as needed B) Antenatal periodontal treatment ‐ SRP + metronidazole capsule (n = 120): metronidazole was taken 250 mg 3 times a day for 1 week. Scaling and root planing was performed according to usual clinical procedures and clinicians were instructed to spend as much time and as many visits as needed C) Antenatal periodontal treatment ‐ Dental prophylaxis + placebo capsule (n = 123): tooth cleaning and polish (supragingival scaling and rubber cup polish) + placebo capsule 3 times daily All women: received oral hygiene instructions from a dental hygienist and supplies of toothbrushes, dental floss and fluoride toothpaste Dental hygienists carried out examinations at baseline supervised by periodontists. SRP was administered by "clinicians" | |
| Outcomes | Preterm birth rate (< 35 weeks); preterm birth rate (< 37 weeks) Intention‐to‐treat analysis was applied and the prevalence of preterm birth calculated for each of the 3 randomised treatment groups | |
| Funding | Not stated | |
| Notes | Stratification by BMI (< 19.8 versus ≥ 19.8); presence of bacterial vaginosis as assessed by Gram stain; previous spontaneous birth prior to 35 weeks gestation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "University research pharmacist generated the randomisation code" |
| Allocation concealment (selection bias) | Low risk | Pharmacist provided a double packet with coding information for each participant ‐ the code did not need to be broken during the study |
| Blinding of participants and personnel (performance bias) | Low risk | Study was placebo‐blinded and code breaking seems to have occurred only at the end of the study |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "The clinicians delivering periodontal care had no role in determining the outcome of the study... research obstetric nurses abstracted maternal records to determine the predefined age at delivery. These abstractors were completely blinded as to the periodontal status or the patients' periodontal treatment" Comment: outcome seems to have been assessed by different personnel from caregivers |
| Incomplete outcome data (attrition bias) | Low risk | There were only 2 dropouts and intention‐to‐treat analysis was applied |
| Selective reporting (reporting bias) | High risk | Periodontal health outcome was not reported |
| Other bias | Low risk | No other apparent biases |
| Methods | Study design: RCT Location: Chile Setting: Consultorio Carol Urzua of Penalolen, a district of Santiago, Chile Recruitment period: recruited over a 20‐month period | |
| Participants | Inclusion criteria: healthy pregnant women with periodontal disease (≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm at the same site) randomised, aged 18 to 35 years, singleton pregnancy, between 9 and 21 weeks gestation; with fewer than 18 natural teeth Exclusion criteria: history of congenital heart disease requiring prophylactic antibiotics for invasive procedures, existing diabetes before pregnancy, current use of corticosteroids, chronic renal disease, and the intention to give birth at another hospital Mean age (± standard deviation (years)): Group A = 28 ± 4.5, Group B = 27 ± 4.3 (P = 0.04) Mean gestational age (± standard deviation (weeks)): Group A = 39.6 ± 1.2; Group B = 39 ± 2 (P = 0.002) History of preterm low birth weight (%): Group A = 4.3, Group B = 7.4 (P = 0.21) Number randomised: n = 400 Number evaluated: n = 351 (attrition n = 49: loss to follow‐up n = 10, discontinuation of treatment n = 18, spontaneous abortion n = 14, indicated preterm delivery n = 7) | |
| Interventions | 1) Antenatal periodontal treatment (n = 200): plaque control instructions, scaling and root planing performed under local anaesthesia, each woman was instructed to rinse once a day with 0.12% chlorhexidine; periodontal therapy was completed before 28 weeks gestation and maintenance therapy was provided every 2 to 3 weeks until birth 2) Postnatal periodontal treatment (n = 200): monitoring every 4 to 6 weeks during pregnancy and treatment after birth All women: at study entry, all women received a full‐mouth periodontal examination and the following were determined: oral hygiene status, gingival inflammation, probing depth, clinical attachment level. Periodontal examination was given after 28 weeks of gestation. Carious lesions were treated and all teeth indicated for extraction were extracted from both groups No information on the expertise of the dental professional who administered intervention | |
| Outcomes | Preterm birth < 37 weeks; low birth weight < 2500 g; preterm low birth weight; number of teeth after 28 weeks gestational age; % of sites with plaque; bleeding on probing; redness; probing depth, clinical attachment loss; after 28 weeks gestational age | |
| Funding | Supported by project grant 1981094 Fondo de Investigación Científica y Tecnológica. Dental instruments partially provided by Hu‐Friedy Co. of Chicago Illinois | |
| Notes | It was believed that 280 women in each group might detect a significant difference of preterm low birth weight between groups with a power of 80%. Data to determine the odds ratios for preterm birth, low birth weight and preterm low birth weight were analysed on an intention‐to‐treat basis. 29 women in the treatment group had severe aggressive periodontitis and were given metronidazole and amoxicillin (3 times daily) for 7 days in addition to mechanical treatment Antibiotics were always prescribed in women with severe periodontitis after they had completed at least 16 weeks of gestation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done equalizing periodontal disease as the relevant variable and probing depth was selected as the variable describing periodontal disease. Patients were assigned to 1 of 2 categories: those with a mean probing depth < 2.5 mm and those with a mean probing depth ≥ 2.5 mm. Patients were matched on the basis of the mean probing depth. Each patient of the matched pair was allocated to the treatment or the control group by a coin toss" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Labour and delivery management decisions were made by personnel who had no knowledge that the patients were participating a research study. The obstetrician who reviewed records of patients with preterm or low birth weight was masked from the mother's periodontal data |
| Incomplete outcome data (attrition bias) | High risk | 24/200 (12%) of women in the intervention group were lost to follow‐up (n = 6) or withdrew (n = 18); 4/200 (2%) in the control group were lost to follow‐up There was a difference in attrition rates between groups |
| Selective reporting (reporting bias) | Low risk | No apparent evidence of selective reporting |
| Other bias | High risk | The trial was stopped early due to benefit (preterm low birth weight) ‐ 400 women recruited from target sample size of 580, statistically significant difference in maternal and gestational age between groups |
| Methods | Study design: RCT Location: Chile Setting: Public Health Clinic, Santiago, Chile Recruitment period: not stated | |
| Participants | Inclusion criteria: healthy pregnant women with gingivitis aged 18 to 42; single gestation; ≤ 22 weeks of gestation; gingival inflammation with ≥ 25% of sites with bleeding on proving, and no sites with clinical attachment loss > 2 mm Exclusion criteria: < 18 natural teeth; indication of prophylactic antibiotics for invasive procedures; diabetes previous to pregnancy and the intention to deliver at a hospital other than that of the study Mean age (± standard deviation (years)): Group A = 25.54 ± 5.41, Group B = 24.98 ± 4.55 (P = 0.31) Gestational age: ≤ 22 weeks Previous preterm low birth weight (%): Group A = 3.44, Group B = 7.47 (P = 0.009) Number randomised: n = 870 Number evaluated: n = 834 (attrition n = 36: loss to follow‐up n = 5, withdrawal from treatment and study n = 9, spontaneous abortion n = 10, preterm delivery n = 11, stillbirth n = 1) | |
| Interventions | A) Antenatal periodontal treatment (n = 580): plaque control instructions (toothbrushes and mouthrinse daily), supra and subgingival scaling, and crown polishing before 28 weeks of gestation + maintenance therapy (oral hygiene instruction and supragingival plaque removal by instrumentation) every 2 to 3 weeks until delivery B) Postnatal periodontal treatment (n = 290): monitoring 2 to 3 times during pregnancy All women: repeated periodontal examinations after 30 weeks of gestation No information on the expertise of the dental professional who administered intervention | |
| Outcomes | Preterm birth (< 37 weeks gestational age with birth weight < 2500 g following spontaneous labour and/or rupture of the membranes, regardless of route of delivery); low birth weight; gestational age; infant birth weight; plaque; bleeding on probing; probing depth; clinical attachment loss | |
| Funding | Not stated | |
| Notes | 290 women were required to detect a significant difference of preterm/low birth weight between groups with 80%. To increase statistical power a 2:1 allocation of participants to the treatment and control groups was adopted. Intention‐to‐treat principle was applied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done equalizing gingivitis as the relevant variable, and the percentage of bleeding on probing sites was selected as the variable describing gingivitis... One woman of each group of the three was selected by rolling a dice" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Obstetrician researcher who obtained pregnancy outcome data from hospital records was masked to the periodontal characteristics of the patients. Staff involved in labour and delivery management decisions had no knowledge that the patients were participating in a research study. However, it is not clear whether periodontal outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were similarly low and balanced across groups (1.7% versus 1.3%) |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | High risk | More participants in the control group had a history of previous preterm/low birth weight compared to the treatment group (P = 0.009) |
| Methods | Study design: RCT Location: USA Setting: Periodontal Infections and Prematurity Study (PIPS), a multicentre trial, was conducted in 3 antenatal clinics in metropolitan Philadelphia, USA Recruitment period: not stated | |
| Participants | Inclusion criteria: women between 6 and 20 weeks gestation with periodontal disease who returned for the scheduled treatment visit Exclusion criteria: periodontal treatment during pregnancy, antibiotic use within 2 weeks; use of antimicrobial mouthwash within 2 weeks, multiple gestation, and known mitral valve prolapse Mean age (± standard deviation (years)): Group A = 24.1 ± 5.2, Group B = 24.4 ± 5.7 (P = 0.41) Gestational age: 6 to 20 weeks History of preterm delivery (%): Group A = 11.7, Group B = 12.9 (P = 0.62) Periodontal characteristics: periodontal disease was defined as attachment loss ≥ 3 mm on ≥ 3 teeth. Moderate/severe ‐ Group A = 54.8, Group B = 55.3 (P = 0.9) Number randomised: n = 756 Number evaluated: n = 713 (attrition n = 43; lost to follow‐up n = 43) | |
| Interventions | A) Antenatal periodontal treatment ‐ Scaling and root planing (n = 376) B) Antenatal periodontal treatment ‐ Superficial tooth cleaning procedure (n = 380): superficial tooth cleaning procedure involved using the rotating cup to remove stains and plaque from the supragingival portion of the tooth using minimally abrasive polishing past. No sharp instruments were used for the subgingival removal of calculus Interventions were delivered by hygienists | |
| Outcomes | Spontaneous preterm birth (occurring < 35 weeks of gestation because of either idiopathic preterm labour or from preterm premature rupture of the amniotic membranes); < 37 weeks of gestational age, < 35 weeks gestational age; gestational age at delivery; birth weight; neonatal adverse outcomes (respiratory distress syndrome, chronic lung disease, necrotizing enterocolitis, grade III/IV intraventricular haemorrhage (IVH), sepsis, death), stillbirth, miscarriage | |
| Funding | Not stated | |
| Notes | For a prevalence of preterm birth at < 35 weeks of gestation of 7%, it was estimated that 636 participants would be needed per treatment group and the goal was to recruit 700 subjects per treatment group. However, because of temporal restraints that were mandated by the mechanism of funding, enrolment stopped after 3 years of recruitment, which was well before the target sample size was reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished centrally at the University of Pennsylvania, although each clinical site had its own randomisation scheme. A permuted block randomisation procedure was used to formulate assignment lists to assure close to equal numbers of subjects in each treatment group. A uniform block size of 4 was used" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was accomplished centrally at the University of Pennsylvania..." Comment: this suggests central allocation |
| Blinding of participants and personnel (performance bias) | High risk | Caregivers were unblinded |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "Members of the investigative team (including the obstetricians) who assessed our primary and secondary end points were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were similarly low and balanced across groups for all outcomes |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | High risk | There were more participants of high socioeconomic status in the control group. Enrollment stopped after 3 years of recruitment due to restraints mandated by the funding mechanism |
| Methods | Study design: RCT Location: USA Setting: the Obstetrics and Periodontal Therapy (OPT) Study, a multicentre trial, was conducted in Hennepin County Medical Centre (Minnesota), the University of Kentucky, the University of Mississippi Medical Center and Harlem Hospital (New York) Recruitment period: March 2003 to June 2005 | |
| Participants | Inclusion criteria: at least 16 years of age, less than 16 weeks 6 days gestation, at least 20 natural teeth, and the presence of periodontal disease (4 or more teeth with a probing depth of at least 4 mm and a clinical attachment loss of at least 2 mm, as well as bleeding on probing at 35% or more of tooth sites) Exclusion criteria: multiple pregnancy, required antibiotic prophylaxis for periodontal procedures, medical condition that precluded elective dental treatment, had extensive tooth decay, or likely to have fewer than 20 teeth after treatment of moderate to severe caries, abscesses or other non‐periodontal pathoses. Baseline assessments were conducted between 13 weeks 0 days and 16 weeks 6 days gestation Mean age (± standard deviation (years)): Group A = 26.1 ± 5.6, Group B = 25.9 ± 5.5 (P = 0.56) Mean gestational age (± standard deviation (weeks)): Group A = 15 ± 1.3, Group B = 15 ± 1.3 (P = 0.85) History of preterm delivery (%): Group A = 12.5, Group B = 16.5 (P = 0.18) Periodontal characteristics: tooth sites with probing depth ≥ 4 mm ‐ Group A = 26.5 ± 16.6, Group B = 24.8 ± 15.9 (P = 0.13). Most women were judged to have generalised early‐moderate periodontitis Number randomised: n = 823 Number evaluated: n = 823 (for gestational age); (attrition n = 11: lost to follow‐up n = 7, withdrawal n = 2, elective abortion n = 2) | |
| Interventions | A) Antenatal periodontal treatment; before 21 weeks gestation (n = 413): scaling and root planing until birth; removal of dental plaque and calculus from the tooth enamel and root (up to 4 treatment visits were allowed); instruction in oral hygiene, monthly tooth polishing and reinstruction in oral hygiene (actual treatment time = mean 127.7 minutes and 2.0 visits) B) Postnatal periodontal therapy (n = 410): brief oral examination at monthly follow‐ups; attended the same number of visits as the treatment in pregnancy group; periodontal therapy after birth All women: topical or systemic antimicrobials were not used; at study entry, all women were screened for periodontal disease in the obstetric clinic (assessed attachment loss, probing depth, bleeding on probing on 6 sites on each tooth, evaluation of dental plaque and calculus on selected teeth). Women were referred to a dentist for treatment of teeth that were abscessed, fractured or likely to become symptomatic during the study. Full‐mouth assessments were repeated at 21 to 24 weeks gestation and again at 29 to 32 weeks gestation Over half the women (59%) were judged to need essential dental care (239 (61%) in the treatment group and 244 (57%) in the control group) and 73% of these women (74% in the treatment group and 71% in the control group) completed the recommended treatment. Control women with progressive periodontitis at 6 or more sites were offered full‐mouth scaling and root planing. Treatment group participants with progressive disease at 6 or more tooth sites were referred to a consulting periodontist and could receive a second course of full‐mouth scaling and root planing and/or systemic antibiotics, or subgingival irrigation with antimicrobial solutions No information on the expertise of the dental professional who administered intervention | |
| Outcomes | Periodontitis progression (increase in clinical attachment loss from baseline of at least 3 mm); birth weight; gestational age at birth; labour induced before 37 weeks (due to hypertension, diabetes or pre‐eclampsia); spontaneous abortion (loss before 20 weeks); stillbirth (loss from 20 weeks to 36 weeks and 6 days); maternal death; bacteria from subgingival plaque sampled at 29 to 32 weeks gestation; child neurodevelopment | |
| Funding | Funding from the National Institute of Dental and Craniofacial Research | |
| Notes | Calculations showed that 405 patients per group would be required to show statistical significance with a power of 90% for gestational age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization, stratified by center with the use of permuted randomized blocks of 2 and 4, was made by a telephone call to the coordinating center" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization, stratified by center with the use of permuted randomized blocks of 2 and 4, was made by a telephone call to the coordinating center" |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible to blind intervention for participants and some personnel |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "(obstetrical) examiners and nurses were not aware of the study group assignments" |
| Incomplete outcome data (attrition bias) | Low risk | The numbers evaluated varied between outcomes, however, attrition rates were similarly low and balanced across groups. 395/413 women in the treatment in pregnancy group received treatment (18 women failed to attend treatment visits or withdrew); 413 women in the treatment group and 410 women in the control group received monthly follow‐ups and 407 and 405 women respectively, were included in the gestational age analysis (99% of women overall). During pregnancy, 6 women in the treatment group withdrew (4 were lost to follow‐up, 1 withdrew consent and 1 had an elective abortion). In the control group 5 women withdrew (3 were lost to follow‐up, 1 withdrew consent and 1 had an elective abortion) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No indication of other sources of bias |
| Methods | Study design: RCT Location: Australia Setting: Smile Study was 'single‐centre' study conducted at 7 sites in public and private antenatal clinics and offices across Perth, Western Australia Recruitment period: February 2005 and December 2007 | |
| Participants | Inclusion criteria: > 16 years of age; absence of maternal cardiac disease that would warrant the need for antibiotics for periodontal examination or treatment; not already received periodontal treatment during the current pregnancy; ≥ 20 natural teeth; single pregnancy of > 12 and < 20 weeks gestational age; did not have any known fetal anomalies or other risk factors such as hydramnios that would place the pregnancy at imminent risk of complications; able to attend regularly for periodontal treatment if required Exclusion criteria: not stated Mean age (± standard deviation (years)): Group A = 30.5 ± 5.5, Group B = 30.5 ± 5.5 (P = 0.842) Mean gestational age (± standard deviation (weeks)): Group A = 18.1 ± 2.3, Group B = 18.2 ± 2.2 (P = 0.451) History of preterm delivery (%): Group A = 13.2, Group B = 11.1 (P = 0.412) Periodontal disease: defined as periodontal probing depth ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth (excluding wisdom teeth) Number randomised: n = 1087 Number evaluated: n = 1078 (attrition n = 9: loss to follow‐up n = 1, miscarriage before treatment n = 2, multiple pregnancy n = 1, withdrew consent n = 5) | |
| Interventions | A) Antenatal periodontal treatment (n = 542): 3‐week protocol which included non‐surgical debridement of the subgingival and supragingival plaque, removal of local predisposing factors such as calculus, root planing, and adjustment of overhanging restorations. Oral hygiene instruction and motivation were provided at each visit. The advice included toothbrushing and flossing after mean and rinsing with chlorhexidine mouthwash. Local anaesthesia was used as required. Sessions were provided on 3 occasions at weekly intervals commencing around 20 weeks of gestation.Those women in whom the treatment had not been successful (19.6%) were offered a further 3‐week treatment regimen. In addition to the baseline and 28‐week examinations, examinations were also carried out at 32 and 36 weeks gestation B) Postnatal periodontal treatment (n = 540): periodontal care after birth commencing 6 weeks after delivery All women: examinations were carried out at baseline and 4 weeks after treatment (28 weeks gestational age) in both groups Treatments were conducted either by the hygienists or periodontists | |
| Outcomes | Preterm birth; stillbirth; neonatal death; gestational age; onset of labour; birth weight; sepsis necessitating antibiotics; birth weight less than 10th percentile; sites with probing depth > 4 mm | |
| Funding | Not stated | |
| Notes | A sample size of 1082 women was required to detect a reduction in the preterm birth rate with 80% power. However the independent data safety monitoring committee recommended proceeding without an interim analysis after data on treatment safety and pregnancy outcomes from the trial conducted by Michalowicz 2006 were published. Primary data analysis was performed on the intention‐to‐treat principle, however, the per‐protocol analysis showed similar results as the intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted using computer randomisation software specifically designed to allocate each case at random with stratification for nulliparity, history of preterm birth, and current smoking" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and caregivers not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | All medical, nursing, perinatal pathology staff members as well as research midwives who extracted details of all medical, obstetric and neonatal outcomes from the medical records were also unaware of the treatment allocation of each woman |
| Incomplete outcome data (attrition bias) | Low risk | Primary data analysis was performed on intention‐to‐treat principle, similarly low attrition rates (1.4% versus 0.2%) |
| Selective reporting (reporting bias) | Low risk | Birth weight and gestational age were reported as median and interquartile range |
| Other bias | Low risk | No apparent source of other biases |
| Methods | Study design: RCT (pilot) Location: USA Setting: 2 antenatal clinics (1 high risk) in Raleigh, NC, USA Recruitment period: January 2001 to November 2003 | |
| Participants | Inclusion criteria: initially pregnant women with a history of a previous preterm/low birth weight birth, but this was subsequently dropped due to very low eligibility rates. Pregnant women < 22 weeks gestation ≥ 18 years of age at time of scaling and root planing or supragingival polish, 2 or more sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1 to 2 mm at 1 or more sites with probing depths ≥ 5 mm; ≥ 20 teeth Exclusion criteria: multiple births, a positive history of HIV, AIDS, diabetes (gestational diabetes was acceptable), any medical contraindication to periodontal probing (e.g. congenital heart disease), and use of phentermine and fenfluramine (phen‐fen) for weight loss; currently undergoing periodontal treatment, chronic regimen of aspirin or non‐steroidal anti‐inflammatory drugs, chronic use of medications that cause gingival enlargement such as phenytoin, cyclosporin‐A, or calcium channel antagonists, 5 or more teeth requiring extraction, rampant decay or any other oral condition that, in the clinician's judgement, would place the woman at unacceptable risk if treatment was delayed, prescribed or using chlorhexidine or other mouthrinses with known antiplaque or anti‐inflammatory effects Mean age (± standard deviation (years)): Group A = 26.8 ± 5.5, Group B = 25.7 ± 5.4 (P = not significant) Gestational age: < 22 weeks History of preterm delivery: Group A = 75, Group B = 88.2 (P = not stated) Number randomised: n = 109 Number evaluated: n = 67 (74 completed baseline examinations) | |
| Interventions | A) Antenatal periodontal treatment ‐ Scaling and root planing and polishing + oral health instructions and a sonic power toothbrush and instructions for use (n = 56 (40)) B) Antenatal periodontal treatment ‐ Supragingival debridement + manual toothbrush with no instruction (n = 53 (34)): postnatal scaling and root planing therapy was provided ˜ 6 weeks postpartum with sonic toothbrushes and instruction in their use All participants were interviewed by the dental hygienist, however, it is not clear whether they also administered intervention | |
| Outcomes | Preterm birth; gingival index (0 = normal gingiva; 1 = mild inflammation; 2 = moderate inflammation; 3 = severe inflammation); plaque index (0 = absence of plaque on clinical crown; 3 = soft deposits covering more than 2‐thirds of the crown); probing depth (6 sites per tooth on all teeth present in the mouth); recession (6 sites per tooth on all teeth present in the mouth or isolated teeth); bleeding on probing (for each quadrant ‐ 0 = absence of bleeding; 1 = bleeding present) | |
| Funding | Study was principally supported by Philips Oral Healthcare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "109 subjects were randomised" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Study was referred to as "examiner‐blinded". No further details stated |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate: 16 of the 56 women (29%) assigned to the intervention group and 19 of the 53 women assigned to the control group (36%) did not complete baseline periodontal examinations (due to moving or dropping out of the study). This includes 2 fetal deaths (not reported which group(s) these were from). A further 5 women in the intervention group and 2 in the control group did not have birth outcome data, leaving 35 women in the intervention group and 32 in the control group. Postpartum periodontal examinations were collected from 25 intervention and 28 control mothers |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | High risk | "Randomization did not balance the primary exposure" of periodontal status |
| Methods | Study design: RCT Location: USA Setting: Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) Study was a multicentre trial conducted at the Duke University Medical Center (and affiliated clinic at Lincoln Health Center), The University of Alabama at Birmingham Medical Center and 2 obstetric sites of the University of Texas Health Science Center at San Antonio, USA Recruitment period: December 2003 and October 2007 | |
| Participants | Inclusion criteria: pregnant women presenting for obstetric care of legal age (16 years) to consent and able to complete periodontal treatment before 23 6/7 weeks gestation; with at least 20 weeks and at least 3 periodontal sites with at least 3 mm of clinical attachment: before randomisation women could receive limited dental care to reduce the likelihood of an acute infectious event during pregnancy (including extraction of hopeless teeth and restoration of pulp‐threatening caries) Exclusion criteria: women with multiple gestation; history of human immunodeficiency virus infection, acquired immunodeficiency syndrome; autoimmune disease; or diabetes (women with gestational diabetes were eligible); need for antibiotic prophylaxis for periodontal probing or periodontal treatment; any obstetric finding that precluded enrolment in the study; women with advanced caries or advanced periodontal disease requiring multiple immediate extractions Mean age (± standard deviation (years)): Group A = 25.3 ± 5.5, Group B = 25.4 ± 5.5 Mean gestational age (± standard deviation (weeks)): Group A = 19.6 ± 2.2, Group B = 19.7 ± 2.1 History of preterm delivery: Group A = 9, Group B = 10.6 (P = 0.244) Number randomised: n = 1806 Number evaluated: n = 1806 (for preterm pregnancy < 37 weeks only). Attrition ranged from 21 to 119 depending on the outcome | |
| Interventions | A) Antenatal periodontal treatment (n = 903 women randomised): received ≤ 4 sessions of supragingival and subgingival scaling and root planing (non‐surgical) using hand and ultrasonic instruments. Local anaesthesia was used as needed early in second trimester; plus full‐mouth polishing and oral hygiene home instructions B) Postnatal periodontal treatment (n = 903 women randomised): received periodontal care after delivery Treatment was administered by dental therapists | |
| Outcomes | Gestational age < 37 weeks (including induced or spontaneous births, fetal demise, and miscarriage but not therapeutic abortions) [this primary outcome was originally specified as < 35 weeks]; gestational age < 35 weeks; birth weight; composite of neonatal morbidity before discharge; fetal demise after randomisation; neonatal death before discharge; respiratory distress syndrome; proven sepsis, intraventricular haemorrhage (IVH) III or IV; necrotizing enterocolitis (NEC); probing depth | |
| Funding | Supported by National Institute of Dental and Craniofacial Research (NIDCR) grant U01‐DE014577 and National Center for Research Resources (NCRR) grants RR00046 and UL1RR025747 | |
| Notes | Before randomisation, women could receive limited dental care to reduce the likelihood of an acute infectious event during pregnancy including the extraction of hopeless teeth and restoration of pulp‐threatening caries. Sample size determination used data from the University of Alabama pilot trial and estimated a preterm (gestational age < 35 weeks) birth rate of 6% in the delayed periodontal therapy group compared with 2% in the periodontal therapy group. A sample size of 900 per treatment group would provide power of 91%, however, the primary outcome was changed due to advice from the monitoring board without change of sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A permuted block randomisation scheme with a random mixture of block sizes was used, stratifying participants by clinical center" Comment: although computer generation was not mentioned, we have judged the method of sequence generation to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "Dental examiners were masked to treatment assignment of participants until after the postpartum examination, after the primary obstetric outcome was collected. Dental therapists were instructed not to divulge treatment status to study staff assigned to postnatal data collection. Participants and staff were instructed to not inform the postpartum examiner of the pregnancy outcome. The managing physicians were totally unaware of oral treatment assignments" |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates varied across outcome, ranged from 2.3% to 23% and was imbalanced for periodontal outcome, yet intention‐to‐treat analysis was only applied to the preterm pregnancy (< 37 weeks) outcome |
| Selective reporting (reporting bias) | Low risk | The authors changed the primary outcome from < 35 weeks to < 37 weeks gestational age, as recommended by the data and safety monitoring board. However, we considered this not to be a source of bias |
| Other bias | Unclear risk | Number of nulliparous pregnancies and alcohol use were higher in the treatment group compared to the control group, however, history of previous adverse pregnancy outcome was balanced between groups. Unclear whether this could be a source of bias |
| Methods | Study design: RCT Location: Brazil Setting: prenatal care programmes at 2 public hospitals in Belo Horizonte, Brazil Recruitment period: not stated | |
| Participants | Inclusion criteria: healthy pregnant women from low socioeconomic status aged 18‐35 years, between 12‐20 weeks gestational age, current single gestation, ≥ 20 natural teeth and the presence of periodontitis Exclusion criteria: current genitourinary infection, chronic hypertension, diabetes, human immunodeficiency virus infection and/or acquired immunodeficiency syndrome, current use of tobacco (smoking), alcohol and/or illicit drug use, and any medical condition requiring antibiotic prophylaxis for dental treatment, use of any antibiotic or nonsteroidal ant‐inflammatory agents, antiseptic mouthwashes and drugs able to induce gingival overgrowth, women undergoing current periodontal treatment Mean age (± standard deviation (years)): Group A = 29.96 ± 4.38, Group B = 26.58 ± 3.96 (P = 0.5) History of preterm delivery: not stated Gestational age: 12 to 20 weeks Periodontitis was defined as: presence of 4 or more teeth with 1 or more sites with probing depth ≥ 4 mm and clinical attachment level as ≥ 3 mm Number randomised: n = 246 Number evaluated: n = 225 (attrition n = 21: spontaneous abortion n = 5, eligible preterm birth n = 8, stillbirth n = 1, abandonment n = 7) | |
| Interventions | A) Antenatal periodontal treatment (n = 122): informed of periodontal status and received a kit containing toothbrushes, dental floss and toothpastes, oral hygiene instructions, plaque index evaluations, dental prophylaxis, and mechanical debridement, when necessary, under local anaesthesia on all affected sites each month during the second trimester; final examination 30‐40 days later; periodontal maintenance every 3 weeks until birth. "The personnel who performed the periodontal therapy were trained", however, there was no information on the expertise of the dental professional who administered the intervention B) Postnatal periodontal treatment (n = 124): informed of their periodontal status and received a kit containing toothbrushes, dental floss and toothpastes. Examination at baseline and final periodontal examination between 30 to 32 weeks gestation; postpartum periodontal treatment offered All women: received a complete periodontal examination (probing depth, clinical attachment level, bleeding on probing at 6 sites per tooth) and were informed of their periodontal status | |
| Outcomes | Preterm birth; low birth weight; probing depth; clinical attachment loss; and bleeding on probing | |
| Funding | Funded by Research Fund of Pontifical Catholic University of Minas Gerais, Brazil | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly divided" |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Albeit assessed independently of the caregiver, it is not clear whether obstetric outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 9/122 (7%) women from the intervention group withdrew (2 spontaneous abortion, 3 'eligible preterm birth', 4 abandonment'); 12/124 (10%) women from the control group withdrew (3 spontaneous abortion, 1 stillbirth, 5 eligible preterm birth, 3 'abandonment') Attrition was similarly low and balanced across groups |
| Selective reporting (reporting bias) | Low risk | No apparent reporting bias |
| Other bias | High risk | Intervention group had worse periodontal outcomes at baseline |
| Methods | Study design: RCT Location: Northern Ireland Setting: Royal Jubilee Maternity Service, Belfast, Northern Ireland Recruitment period: Februrary 2005 and December 2007 | |
| Participants | Inclusion criteria: females >18 years old with singleton pregnancy and ≥ 20 natural teeth Exclusion criteria: multiple pregnancy, diabetes/pregnancy complications, requiring antibiotic prophylaxis before periodontal scaling, had been provided with specialist periodontal treatment in the previous 12 months or aggressive periodontitis requiring urgent intervention Mean age (± standard deviation (years)): Group A = 30.5 ± 4.5, Group B = 30.5 ± 5.5 Mean gestational age (± standard deviation (days)): Group A = 97.6 ± 10.2, Group B = 98.8 ± 10.8 Previous preterm: Group A = 0, Group B = 1 Periodontitis: it was defined as ≥ 4 mm probing depth at ≥ 4 sites and clinical attachment level ≥ 2 mm at ≥ 4 sites Number randomised: n = 99 Number evaluated: n = 99 | |
| Interventions | A) Antenatal periodontal treatment ‐ SRP (n = 49): oral hygiene instruction, followed by supragingival and subgingival scaling and root planing of sites with probing depths ≥ 4 mm and polishing of all the teeth. Therapy was performed over 2 1‐hour sessions under local anaesthetic (9 patients refused anaesthetic due to anxiety). Treatment was completed by 24 weeks gestational age. No information on the expertise of the dental professional who administered intervention B) Antenatal periodontal treatment ‐ Alternative mechanical treatment (n = 50): oral hygiene instruction and supragingival cleaning of all teeth at their baseline visit and the option of postpartum periodontal treatment All women: periodontal examination by calibrated examiner. Post‐treatment clinical periodontal related data were collected at 8 weeks after treatment (32 weeks gestational age) | |
| Outcomes | Pregnancy complications such as pre‐eclampsia, type of delivery, birth weight, gestational age, probing depth, clinical attachment loss, plaque, bleeding on probing | |
| Funding | Supported by Research and Development Office, Department of Health, Northern Ireland Grant EAT/2560/03 | |
| Notes | For sample size, 50 participants in each group were to achieve a power of 80% to detect a difference of 0.6 in mean birth weight standard deviation score equating to a difference of 300 g in birth weight | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocations were computer generated by a third person who was not otherwise involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly allocated to either the control or test arm of the study using sealed opaque envelopes labelled with a study number and containing the group allocation" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Birth outcomes were completed at delivery by delivery‐suite staff. These staff members were masked to the group assignments of the participants |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat principle applied |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | Both groups were balanced for age, weight, height, BMI, alcohol consumption, smoking, periodontal condition, obstetric history except for social class with high socioeconomic status in the test group compared to the control (P = 0.02). However, the review authors did not consider this to be sufficient to bias the results |
| Methods | Study design: RCT Location: Hungary Setting: Department of Obstetrics and Gynaecology Szeged, Hungary Recruitment period: 2005 and 2006 | |
| Participants | Inclusion criteria: women with initial localised chronic periodontitis, and hospitalised due to threatening preterm birth, otherwise healthy with a singleton pregnancy Exclusion criteria: women with any systemic medical problem, multiple pregnancy, history of previous preterm birth or miscarriage, smokers, high consumption of alcohol, drug use, malnourished or women requiring antibiotics for invasive procedures Mean age (± standard deviation (years)): Group A = 29.1 ± 6.4, Group B = 28.9 ± 5.4 (P = 0.888) Mean gestational age (± standard deviation (weeks)): Group A = 31.63 ± 2.6, Group B = 31.45 ± 2.8 (P = 0.822) Previous preterm: not stated Periodontitis: it was defined as ≥ 4 mm probing depth, at least 1 site, and bleeding on probing for ≥ 50% of teeth Number randomised: n = 89 Number evaluated: n = 83 (attrition n = 6 lost to follow‐up) | |
| Interventions | A) Antenatal periodontal treatment (n = 43 (41)): treatment around 32 weeks gestational age (oral hygiene instruction), supra and subgingival scaling with hand instruments and/or ultrasonic scaler, teeth polishing with a fluoride paste. The women were examined and treated by a periodontist B) Postnatal periodontal treatment (n = 46 (42)): treatment was suggested postbirth | |
| Outcomes | Preterm birth (< 37 weeks); low birth weight (< 2500 g); gestational age at birth | |
| Funding | Funded through 'institutional support' | |
| Notes | Power calculation was performed which related birth weight assessment and time of gestation. Assuming a 500 g birth weight difference at a 95% test power for a 2‐sample t‐test, n = 39 was the necessary minimum case number. To show a 2‐week difference in delivery time, at 2.5 standard deviation and 95% power, the desired minimum sample size was n = 42 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "generated a random sequence of 1's and 2's, and the treatment was allocated accordingly to the 1st or 2nd person in the blocks, leaving the other for the control group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were similarly low and balanced across groups ‐ 2/43 (4.7%) women were lost to follow‐up in the intervention group and 4/46 (8.7%) were lost to follow‐up in the control group |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | Low risk | No indication of other sources of bias |
| Methods | Study design: RCT Location: Iran Setting: not stated Recruitment period: not stated | |
| Participants | Inclusion criteria: pregnant women 18‐35 years of age, with moderate or advanced periodontitis, in 13th to 20th week of pregnancy Exclusion criteria: women with a history of congenital heart disease requiring prophylactic antibiotics, diabetes, current use of corticosteroids, chronic renal disease, or with fetal congenital abnormality (evaluated by ultrasound until 20th week), obstetric disorders such as gestational diabetes, placenta previa, pre‐eclampsia eclampsia and polyhydramnios Periodontal disease: it was defined as women with at least 4 teeth, with at least 1 site of pocket depth of at least 4 mm, and clinical attachment loss of at least 3 mm Mean age (± standard deviation (years)): Group A = 29.1 ± 4.3, Group B = 28.4 ± 4.1 Gestational age: 13‐20 weeks History of preterm delivery: not stated Number randomised: n = 30 Number evaluated: n = 30 | |
| Interventions | A) Antenatal periodontal treatment (n = 15): first phase ‐ ultrasonic scaling and hand instrument root planing under local anaesthesia using lidocaine or mepivastesin, if needed; maintenance phase ‐ oral hygiene instructions, use of 0.2% chlorhexidine mouthrinse once a night for 1 week, and periodontal evaluation every fortnight before birth. No information on the expertise of the dental professional who administered intervention B) Postnatal periodontal treatment (n = 15) All women: repeated periodontal examination: 28th week of pregnancy for the control group and 2nd week after treatment in the intervention group (during the 30th week) | |
| Outcomes | Probing depth; clinical attachment; bleeding on probing; preterm birth < 37 weeks; birth weight; gestational age at birth | |
| Funding | No funding source stated | |
| Notes | None of the subjects were excluded due to abortion, eclampsia, pre‐eclampsia, pregnancy diabetes, placenta previa and polyhydramnios | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomly divided into two groups" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No postrandomisation exclusions or losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | No apparent evidence of selective reporting bias (although perinatal mortality was not reported) |
| Other bias | Low risk | No indication of other sources of bias |
| Methods | Study design: RCT Location: India Setting: The Department of Obstetrics and Gynaecology, Dr BR Ambedkar Medical College and Hospital, Bangalore, Karnataka India Recruitment period: August 2004 to August 2005 | |
| Participants | Inclusion criteria: healthy pregnant women aged 18 to 35 years; single gestation between 9 and 21 weeks; with ≥ 20 completely erupted teeth, excluding the third molars, and women with ≥ 2 mm attachment loss at ≥ 50% of examined sites Exclusion criteria: current use of tobacco (smoking/smokeless) or alcohol; history of congenital heart disease, current use of corticosteroids, diabetes, asthma, glomerulonephritis, or hyperthyroidism; mothers with twin pregnancy and Rh factor isoimmunity, and clinically evident systemic infection, inadequate antenatal care (< 6 visits) Mean age (± standard deviation (years)): Group A = 23 ± 3.3, Group B = 22.9 ± 3.6 (P = 0.935) Gestational age: 9 to 21 weeks History of preterm delivery: not reported Number randomised: n = 220 Number evaluated: n = 188 (attrition n = 32: loss to follow‐up n = 16, spontaneous abortions n = 4, did not receive allocated intervention n = 12) | |
| Interventions | A) Antenatal periodontal treatment (n = 120): plaque control instructions (rinsing twice daily with 0.2% chlorhexidine until periodontal therapy was completed) + scaling and root planing performed under local anaesthesia. Full‐mouth scaling and root planing was performed over 4 to 5 appointments, with a 1 week interval between appointments. Periondontal therapy was completed before 28 weeks gestation and maintenance therapy was provided (oral prophylaxis and reinforcement of oral hygiene instructions every 3 to 4 weeks until birth). Treatment was provided by a periodontist B) Control ‐ Plaque control (brushing) instructions only + checkups at 4 to 5‐week intervals (n = 100) All women: full‐mouth periodontal examination, including oral hygiene index (simplified); bleeding index, and clinical attachment level | |
| Outcomes | Preterm birth (< 37 weeks); low birth weight (< 2500 g); gestational age at birth | |
| Funding | Not stated | |
| Notes | The authors claim to have undertaken intention‐to‐treat analysis involving all of the subjects regardless of whether they underwent the prescribed treatment, however, this is not reflected in the 'numbers evaluated' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Based on the intention‐to‐treat analysis applied to the treatment group, there was no difference in attrition between groups (16% versus 9%) |
| Selective reporting (reporting bias) | High risk | Periodontal data at follow‐up were not reported clearly |
| Other bias | Low risk | Some imbalance in numbers of women randomised to each group |
BMI = body mass index; CAL = clinical attachment loss; PD = probing depth; RCT = randomised controlled trial; SRP = scaling and root planing.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The control group consisted of women who refused to participate and were classed as the 'non‐treated' group. There was no randomisation | |
| Not an RCT. The study was used in generating preliminary data for another RCT | |
| Overlap with an included study (Jeffcoat 2003) and does not contain useful additional information to supplement the primary publication | |
| The study compared antimicrobial mouthrinse with toothbrushing. Antimicrobial mouthrinse alone is not considered a periodontal treatment, however, it can be used as an adjunct to a mechanical periodontal treatment | |
| Population consisted of a subsample of participants included in a study (Weidlich 2013) which was excluded for the wrong study population. There is lack of clarity on whether the concerns we had about the primary study were addressed in this study and obstetric outcomes were not reported | |
| Obstetric outcome not reported | |
| Participants were a subset of study population in an included study (Newnham 2009) | |
| Obstetric outcome not reported | |
| Included pregnant women regardless of their periodontal status |
RCT = randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | 34 pregnant women aged between 15 and 43 years who had at least 4 teeth with probing depth ≥ 4 mm or clinical attachment loss ≥ 3 mm, with bleeding on probing in the same place |
| Interventions | Group 1 received supra and subgingival scaling associated with oral hygiene orientation (OHO) and Group 2 received only supragingival scaling with OHO |
| Outcomes | Probing depth, clinical attachment level, hyperplasia, recession, bleeding, presence of plaque and tooth mobility on a standardized form. Quantitative parameters were evaluated at 6 sites per tooth: mesio/medium/distobuccal and mesio/medium/distolingual through millimetre periodontal probe‐type Williams. The bleeding and the presence of plaque in dichotomous variables were measured: present and absent. All patients received oral hygiene orientation |
| Notes | Reported on clinical registry as completed ‐ study not available |
| Methods | Single‐blind RCT |
| Participants | Inclusion criteria: provide written informed consent prior to participation and be given a signed copy of the informed consent form; be at least the age of legal consent; be between 8 and 24 weeks of pregnancy; have at least 20 natural teeth; have moderate‐to‐severe gingivitis during pregnancy, including at least 30 intraoral sites with evidence of marginal gingival bleeding |
| Interventions | Active comparator: regular oral hygiene. Toothpaste, toothbrush and dental floss. Interventions: drug: 0.243% sodium fluoride; device: toothbrush; device: dental floss Experimental: advanced oral hygiene + counselling. Toothpaste, toothbrush, mouthrinse and dental floss + specialized education. Interventions: drug: 0.454% stannous fluoride; device: toothbrush; drug: 0.07% cetylpyridinium chloride; device: dental floss |
| Outcomes | Gestational age (weeks); neonate birth weight (grams); preterm birth (gestational age < 37 weeks) |
| Notes | Reported on clinical registry as completed in April 2014 ‐ study not available |
RCT = randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A clinical, biochemical and interventional evaluation of possible relationship between periodontal disease and adverse pregnancy outcomes ‐ A randomized controlled trial (CTRI/2015/02/005581) |
| Methods | RCT |
| Participants | Pregnant women with periodontal disease |
| Interventions | Periodontal treatment |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Vaibhavi Joshipura; [email protected] |
| Notes | Author was contacted in January 2015 and confirmed that the study was not yet published |
RCT = randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
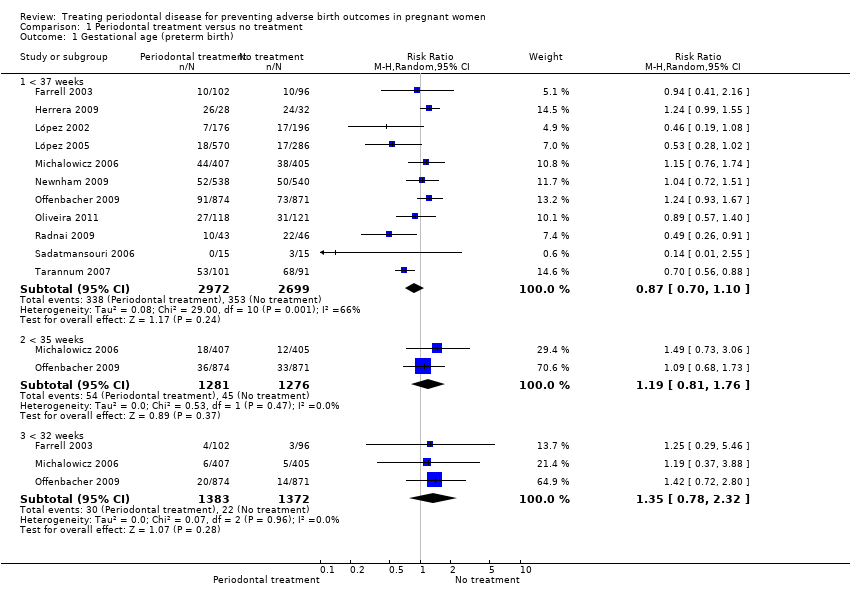
| 1 Gestational age (preterm birth) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 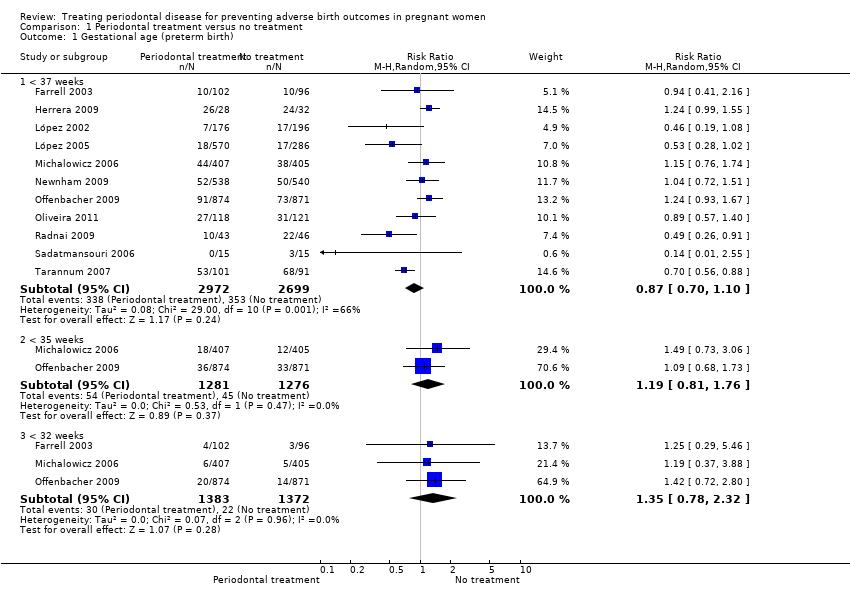 Comparison 1 Periodontal treatment versus no treatment, Outcome 1 Gestational age (preterm birth). | ||||
| 1.1 < 37 weeks | 11 | 5671 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.10] |
| 1.2 < 35 weeks | 2 | 2557 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 1.3 < 32 weeks | 3 | 2755 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.78, 2.32] |
| 2 Birth weight (low birth weight) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Periodontal treatment versus no treatment, Outcome 2 Birth weight (low birth weight). | ||||
| 2.1 < 2500 g | 7 | 3470 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 2.2 < 1500 g | 2 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.70] |
| 3 Small for gestational age Show forest plot | 3 | 3610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| Analysis 1.3  Comparison 1 Periodontal treatment versus no treatment, Outcome 3 Small for gestational age. | ||||
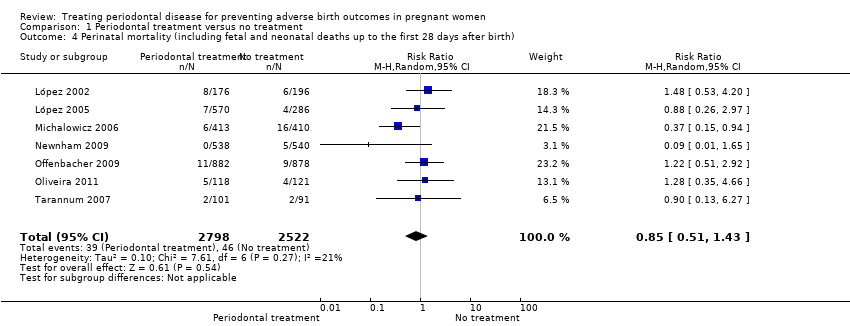
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 7 | 5320 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.43] |
| Analysis 1.4  Comparison 1 Periodontal treatment versus no treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth). | ||||
| 5 Pre‐eclampsia Show forest plot | 3 | 2946 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.62] |
| Analysis 1.5  Comparison 1 Periodontal treatment versus no treatment, Outcome 5 Pre‐eclampsia. | ||||
| 6 Probing depth Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 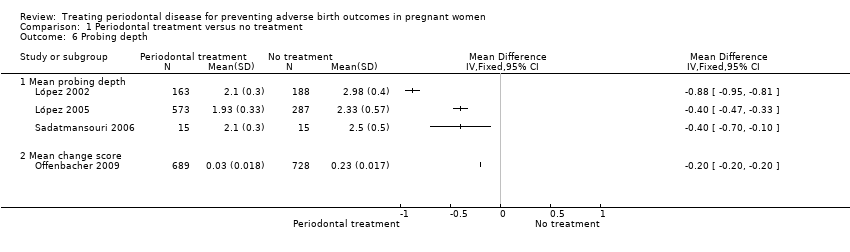 Comparison 1 Periodontal treatment versus no treatment, Outcome 6 Probing depth. | ||||
| 6.1 Mean probing depth | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Bleeding on probing Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Periodontal treatment versus no treatment, Outcome 7 Bleeding on probing. | ||||
| 7.1 Mean bleeding on probing | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Plaque index Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8 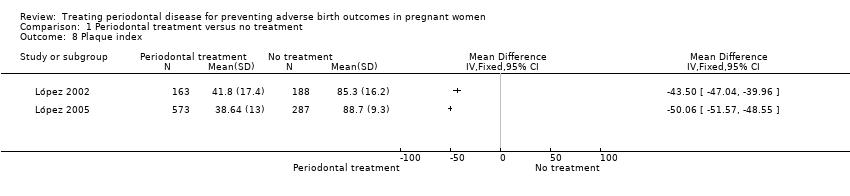 Comparison 1 Periodontal treatment versus no treatment, Outcome 8 Plaque index. | ||||
| 9 Clinical attachment level Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9 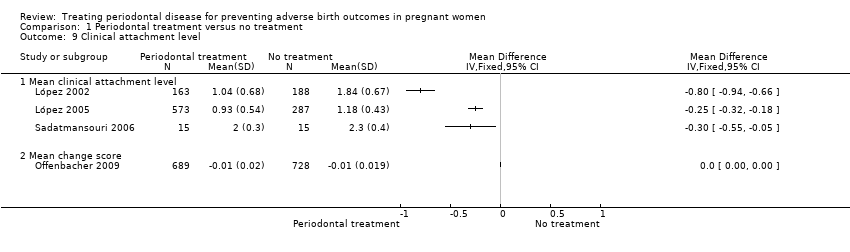 Comparison 1 Periodontal treatment versus no treatment, Outcome 9 Clinical attachment level. | ||||
| 9.1 Mean clinical attachment level | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational age (preterm birth < 37 weeks) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1 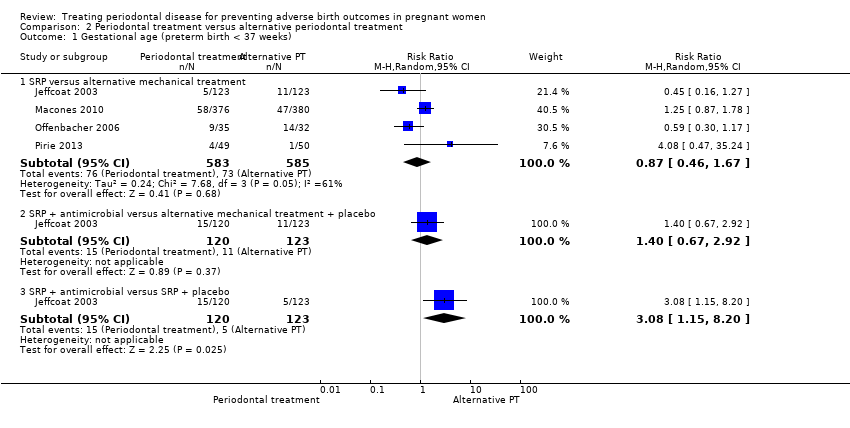 Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 1 Gestational age (preterm birth < 37 weeks). | ||||
| 1.1 SRP versus alternative mechanical treatment | 4 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 1.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.67, 2.92] |
| 1.3 SRP + antimicrobial versus SRP + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.15, 8.20] |
| 2 Gestational age (preterm birth < 35 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 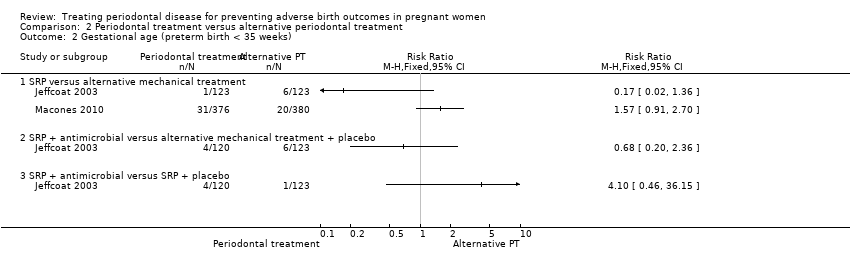 Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 2 Gestational age (preterm birth < 35 weeks). | ||||
| 2.1 SRP versus alternative mechanical treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 SRP + antimicrobial versus SRP + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Birth weight (low birth weight) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 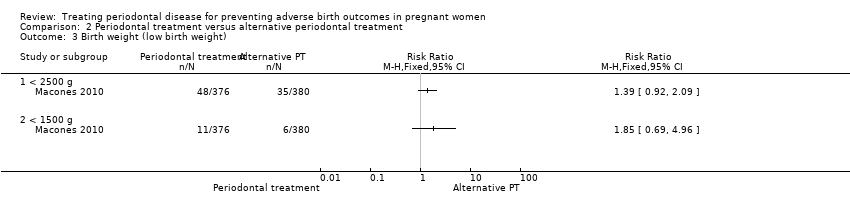 Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 3 Birth weight (low birth weight). | ||||
| 3.1 < 2500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 < 1500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.60, 1.85] |
| Analysis 2.4  Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth). | ||||
| 5 Probing depth Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5 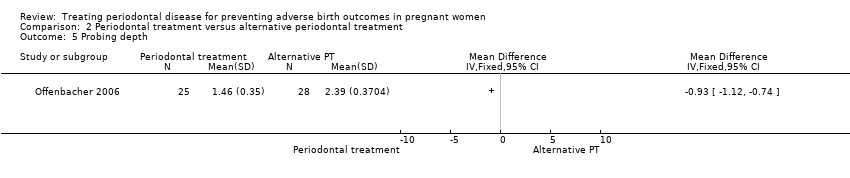 Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 5 Probing depth. | ||||
| 6 Clinical attachment level Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 6 Clinical attachment level. | ||||
| 7 Bleeding on probing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 7 Bleeding on probing. | ||||
| 8 Gingival index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.8 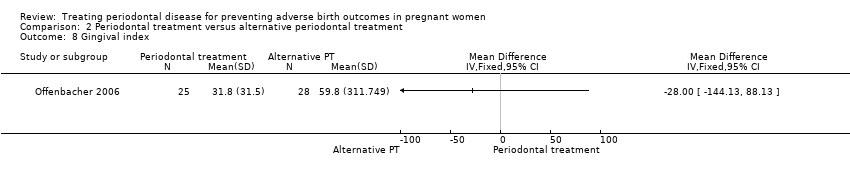 Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 8 Gingival index. | ||||
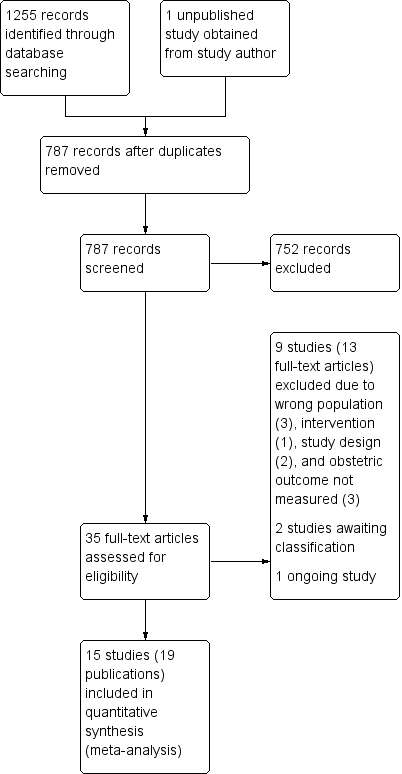
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
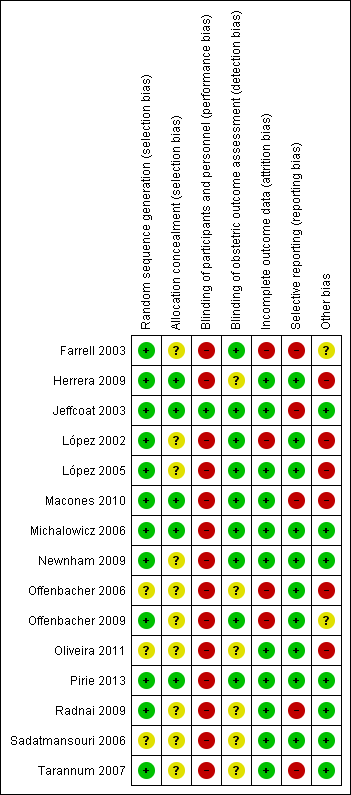
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
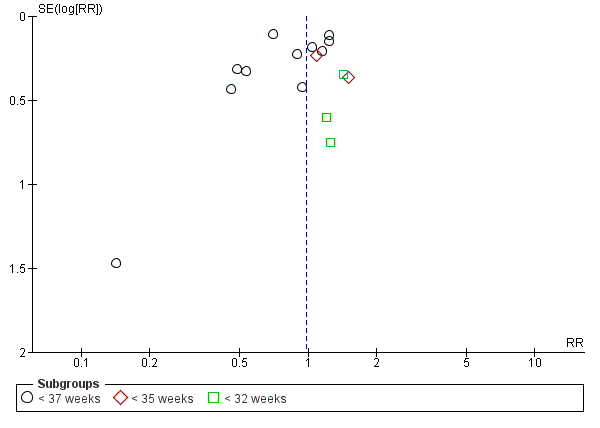
Funnel plot of Comparison 1 Periodontal treatment versus no treatment, Outcome 1.1 Gestational age (preterm birth).
Comparison 1 Periodontal treatment versus no treatment, Outcome 1 Gestational age (preterm birth).

Comparison 1 Periodontal treatment versus no treatment, Outcome 2 Birth weight (low birth weight).

Comparison 1 Periodontal treatment versus no treatment, Outcome 3 Small for gestational age.
Comparison 1 Periodontal treatment versus no treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).

Comparison 1 Periodontal treatment versus no treatment, Outcome 5 Pre‐eclampsia.

Comparison 1 Periodontal treatment versus no treatment, Outcome 6 Probing depth.
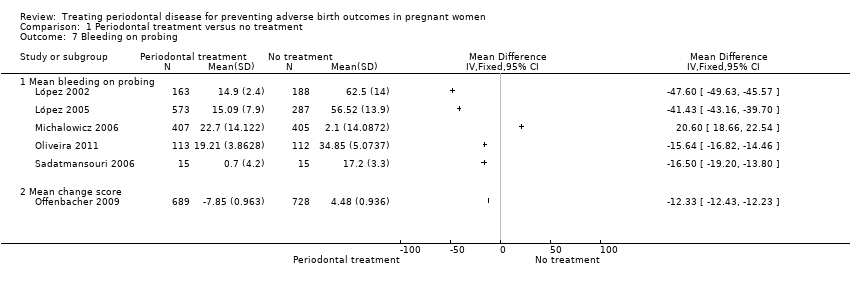
Comparison 1 Periodontal treatment versus no treatment, Outcome 7 Bleeding on probing.

Comparison 1 Periodontal treatment versus no treatment, Outcome 8 Plaque index.

Comparison 1 Periodontal treatment versus no treatment, Outcome 9 Clinical attachment level.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 1 Gestational age (preterm birth < 37 weeks).
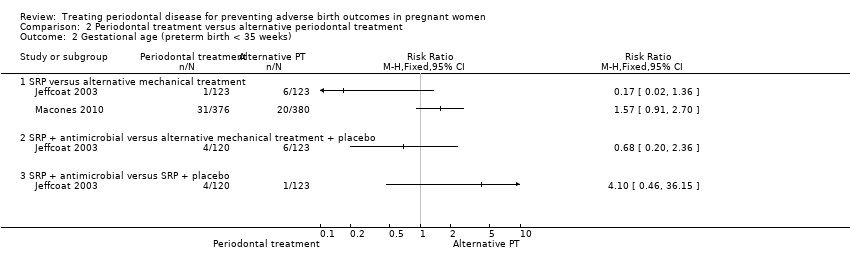
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 2 Gestational age (preterm birth < 35 weeks).

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 3 Birth weight (low birth weight).
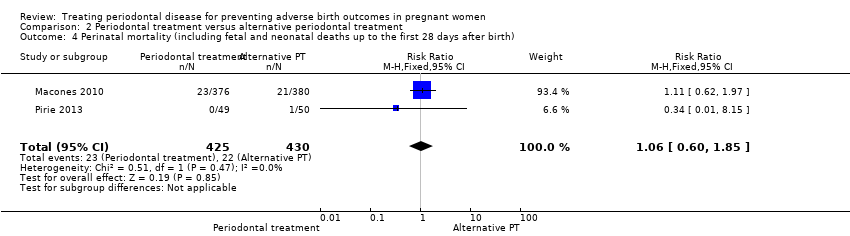
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).
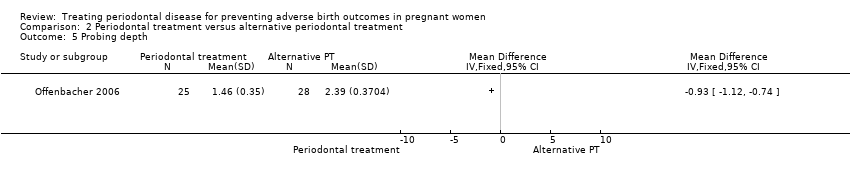
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 5 Probing depth.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 6 Clinical attachment level.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 7 Bleeding on probing.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 8 Gingival index.
| Periodontal treatment compared to no treatment for preventing adverse birth outcomes in pregnant women | ||||||
| Patient or population: pregnant women considered to have periodontal disease after dental examination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Periodontal treatment | |||||
| Gestational age (preterm birth < 37 weeks) | Study population | RR 0.87 | 5671 | ⊕⊕⊝⊝ | Preterm birth < 35 weeks and < 32 weeks were also reported. There was no evidence of a difference in preterm birth < 35 weeks (RR 1.19 (0.81 to 1.76), 2 studies; 2557 participants) and < 32 weeks (RR 1.35 (0.78 to 2.32), 3 studies; 2755 participants) (VERY LOW2 quality evidence) | |
| 131 per 1000 | 114 per 1000 | |||||
| Birth weight (low birth weight < 2500 g) | Study population | RR 0.67 | 3470 | ⊕⊕⊝⊝ | Low birth weight < 1500 g was reported in 2 studies. There was no evidence of a difference in low birth weight < 1500 g (RR 0.80 (0.38 to 1.70); 2550 participants) (VERY LOW2 quality evidence) | |
| 126 per 1000 | 84 per 1000 | |||||
| Small for gestational age | Study population | RR 0.97 (0.81 to 1.16) | 3610 | ⊕⊕⊝⊝ | ||
| 115 per 1000 | 111 per 1000 | |||||
| Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | Study population | RR 0.85 (0.51 to 1.43) | 5320 | ⊕⊝⊝⊝ | ||
| 18 per 1000 | 16 per 1000 (9 to 26) | |||||
| Maternal mortality | 0% in both groups | Not estimated | 2134 (4 RCTs) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.10 | 2946 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 70 per 1000 | |||||
| Adverse effects of therapy | 0% in both groups | Not estimated | 2389 (4 RCTs) | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 2 levels: serious limitation ‐ high risk of bias due to other bias (imbalance in baseline characteristics); serious inconsistency ‐ substantial heterogeneity (I2 = 66%). | ||||||
| Outcome | Study ID |
| Birth weight ≥ 2500 g | |
| Small for gestational age (10th percentile) | |
| Preterm/low birth weight | López 2002; López 2005; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Pirie 2013 |
| Birth length | Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013 |
| Head circumference | |
| Amniotic fluid index (< 5 cm, > 25 cm) | |
| Umbilical artery S/D ratios | |
| Umbilical cord artery/vein blood (number, pH, PCO2, PO2, base excess) | |
| Meconium in amniotic fluid | |
| Decision on delivery based on electronic fetal heart rate monitoring | |
| Scalp pH measured in labour | |
| Nonreassuring fetal heart rate pattern | |
| Caesarean delivery for nonreassuring fetal heart rate | |
| Electronic fetal heart rate monitoring in labour | |
| Ventilation | |
| Continuous Positive Airway Pressure (CPAP) | |
| Oxygen | |
| Special care nursery admission | |
| 1‐min Apgar score | |
| 5‐min Apgar score (0‐3, 4‐7, 8‐10) | |
| Apgar score (< 7 at 1 min, < 7 at 5 min) | |
| Admission to neonatal intensive care unit (number admitted, length of stay > 2 days, discharged alive) | |
| Sepsis necessitating antibiotics | |
| Composite neonatal morbidity/mortality | |
| HELLP syndrome, severe pre‐eclampsia | |
| Prenatal visits | |
| Onset of labour (spontaneous, induced, augmented, no labour) | |
| Mode of delivery (spontaneous vaginal, assisted vaginal, elective caesarean, emergency caesarean) | |
| Fever > 37o C in labour | |
| Postpartum haemorrhage (> 1000 mL) | |
| Retained placenta | |
| Fraction of expected birth weight | |
| Urinary tract infection | |
| Vaginosis, underweight, onset prenatal care after 20 weeks of gestation | |
| S/D = systolic/diastolic ratio. | |
| Study ID | Case definition |
| ≥ 3 sites with CAL ≥ 3 mm | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm | |
| ≥ 6 sites with ≥ 5 mm probing depth and ≥ 3 sites with ≥ 3 mm loss of periodontal attachment | |
| AAP criteria ‐ PPD up to 6 mm with CAL up to 4 mm | |
| Gingival inflammation with over ≥ 25% of sites with BOP and no sites with CAL > 2 mm | |
| Attachment loss ≥ 3 mm on ≥ 3 teeth | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 2 mm | |
| PPD ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth | |
| ≥ 2 sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1‐2 mm at ≥ 1 sites with probing depths ≥ 5 mm | |
| ≥ 4 sites with PD ≥ 4 mm and ≥ 4 sites with CAL ≥ 4 mm | |
| Chronic: ≥ 4 mm probing depth, at least 1 site, and BOP for ≥ 50% of teeth | |
| ≥ 2 mm attachment loss at ≥ 50% of examined sites | |
| AAP = American Academy of Periodontology; BOP = bleeding on probing; CAL = clinical attachment level; PD = pocket depth; PPD = periodontal pocket depth. | |
| Study | Number of visits | When | Intervention | Comparator | |
| Periodontal treatment versus no treatment | |||||
| 5 visits | 12 weeks | Plaque assessment Oral hygiene instruction | None | ||
| 1 session lasting 1‐2 hours | Supragingival and subgingival cleaning | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Up to 4 visits | SRP | None | |||
| 3 treatments over 3 weeks | 20 weeks | Nonsurgical debridement of subgingival and supragingival plaque | None | ||
| Up to 4 sessions (mean 1.3 ± 0.4) | Supragingival and subgingival SRP | None | |||
| Maintenance therapy every 3 weeks till delivery | Dental prophylaxis | Tooth cleaning kit | |||
| Not stated | 32 weeks | Supragingival and subgingival scaling and polishing | None | ||
| Not reported | 28 weeks | SRP | None | ||
| 4‐5 sessions with a 1‐week interval between each appointment | Unclear | SRP | Plaque control instruction (toothbrushing) | ||
| Periodontal treatment versus alternative periodontal treatment | |||||
| Antibiotics 3 times daily for 1 week | SRP | SRP | Dental prophylaxis | ||
| Not stated | SRP | Superficial tooth cleaning | |||
| 4‐6 weeks follow‐up visit | SRP | Supragingival debridement | |||
| Performed over 2 1‐hour sessions | Completed by end of 24 weeks | Supragingival and subgingival SRP | Supragingival cleaning | ||
| CHX = chlorhexidine; SRP = scaling and root planing. | |||||
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 39.6 | 1.2 | 163 | 39 | 2 | 188 | |
| 39.26 | 1.5 | 560 | 38.9 | 1.7 | 283 | |
| 39.1 | 2.1 | 538 | 39.2 | 2.1 | 540 | |
| 37.5 | 1.7 | 41 | 36.1 | 2.8 | 42 | |
| 38.5 | 0.8 | 15 | 37.9 | 1.3 | 15 | |
| 33.8 | 2.8 | 99 | 32.7 | 2.8 | 89 | |
| Mean birth weight (grams) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 3501 | 429 | 163 | 3344 | 598 | 188 | |
| 3426 | 477 | 560 | 3325 | 535 | 283 | |
| 3239 | 586 | 406 | 3258 | 575 | 403 | |
| 3370.6 | 613.4 | 538 | 3423.4 | 597.3 | 540 | |
| 3227 | 612 | 872 | 3241 | 590 | 866 | |
| 3079 | 592.3 | 41 | 2602.4 | 668.3 | 42 | |
| 3371 | 394.2 | 15 | 3059 | 389 | 15 | |
| 2565.3 | 331.2 | 99 | 2459.6 | 380.7 | 89 | |
| SD = standard deviation. | ||||||
| Study ID | Outcome | Periodontal treatment | Number of participants | Alternative periodontal/no treatment | Number of participants | P value |
| % sites with PD 4‐6 mm (mean ± SD) | 2.9 ± 3.9 | 163 | 27 ± 14 | 188 | 0.001 | |
| % sites with CAL ≥ 3 mm (mean ± SD) | 6.1 ± 7.8 | 163 | 25.4 ± 17.2 | 188 | 0.001 | |
| % sites with PD > 4 mm (mean ± SD) | 1.8 ± 2.9 | 573 | 14.5 ± 2.8 | 287 | 0.0001 | |
| Change PD at sites initially 4‐6 mm (mean ± SE) | 0.38 ± 0.02 | 405 | 0.88 ± 0.02 | 407 | < 0.001 | |
| Change PD at sites initially ≥ 7 mm (mean ± SE) | 1.07 ± 0.14 | 405 | 1.84 ± 0.14 | 407 | < 0.001 | |
| Change % sites with CAL ≥ 2 mm (mean ± SD) | 0.84 ± 0.85 | 405 | 9.72 ± 0.87 | 407 | < 0.001 | |
| % sites with PD > 4 mm (median (IQR)) | 3.3 (1.2‐7) | 354 | Not reported | Not reported | < 0.001 | |
| % sites BOP (median (IQR)) | 28.7 (17.9‐42.5) | 354 | Not reported | Not reported | < 0.001 | |
| Extent of PD ≥ 4 mm (mean ± SE) | 13.7 ± 1.5 | 25 | 10.5 ± 1.2 | 28 | < 0.0001 | |
| PI ≥ 1 (mean ± SE) | 67.8 ± 5.6 | 25 | 87 ± 5.3 | 28 | 0.02 | |
| Change PD at sites initially ≥ 4 mm (mean ± SD) | 1.47 ± 0.574 | 689 | 7.81 ± 0.559 | 728 | Not reported | |
| Sites with PD ≥ 4 mm (% (95% CI)) | 1.19 (1‐1.39) | 113 | 6.36 (5.92‐6.81) | 112 | < 0.0001 | |
| Sites with CAL ≥ 3 mm (% (95% CI)) | 5.72 (5.3‐6.14) | 113 | 6.58 (6.13‐7.03) | 112 | 0.0069 | |
| Number of sites PD ≥ 4 mm (median (IQR)) | 10 (6‐22) | 45 | Not reported | 45 | Not reported | |
| Number of sites PD ≥ 5 mm (median (IQR)) | 1 (0‐4) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 4 mm (median (IQR)) | 10 (5‐19) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 5 mm (median (IQR)) | 0 (0‐2) | 45 | Not reported | 45 | Not reported | |
| Number of sites plaque present (median (IQR)) | 57 (40‐82.5) | 45 | Not reported | 45 | Not reported | |
| Number of sites BOP present (median (IQR)) | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % of sites plaque present | 37 (28‐54.8) | 45 | Not reported | 45 | Not reported | |
| % of sites BOP present | 50 (42.9‐54.1) | 45 | Not reported | 45 | Not reported | |
| % of sites PD ≥ 4 mm | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % sites with PD 4 mm (mean ± SD) | 53.31 ± 18.5 | 15 | 68.6 ± 20.2 | 15 | 0.04 | |
| % sites with CAL 3 mm (mean ± SD) | 41.4 ± 18.4 | 15 | 67.1 ± 15.6 | 15 | 0.000 | |
| AL = attachment loss; BOP = bleeding on probing; CAL = clinical attachment level; IQR = interquartile range; PD = probing depth; PPD = periodontal pocket depth; SD = standard deviation; SE = standard error. | ||||||
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | Alternative periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 38.6 | 2.8 | 376 | 38.8 | 2.3 | 380 | |
| 39.4 | 2.3 | 49 | 40 | 2.5 | 50 | |
| Mean birth weight (grams) | ||||||
| 3076.1 | Not reported | 376 | 3143.8 | Not reported | 380 | |
| 3510 | 650 | 49 | 3580 | 630 | 50 | |
| SD = standard deviation. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational age (preterm birth) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 37 weeks | 11 | 5671 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.10] |
| 1.2 < 35 weeks | 2 | 2557 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 1.3 < 32 weeks | 3 | 2755 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.78, 2.32] |
| 2 Birth weight (low birth weight) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2500 g | 7 | 3470 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 2.2 < 1500 g | 2 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.70] |
| 3 Small for gestational age Show forest plot | 3 | 3610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 7 | 5320 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.43] |
| 5 Pre‐eclampsia Show forest plot | 3 | 2946 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.62] |
| 6 Probing depth Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Mean probing depth | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Bleeding on probing Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Mean bleeding on probing | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Plaque index Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Clinical attachment level Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Mean clinical attachment level | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational age (preterm birth < 37 weeks) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 SRP versus alternative mechanical treatment | 4 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 1.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.67, 2.92] |
| 1.3 SRP + antimicrobial versus SRP + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.15, 8.20] |
| 2 Gestational age (preterm birth < 35 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 SRP versus alternative mechanical treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 SRP + antimicrobial versus SRP + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Birth weight (low birth weight) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 < 2500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 < 1500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.60, 1.85] |
| 5 Probing depth Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Clinical attachment level Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Bleeding on probing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Gingival index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

