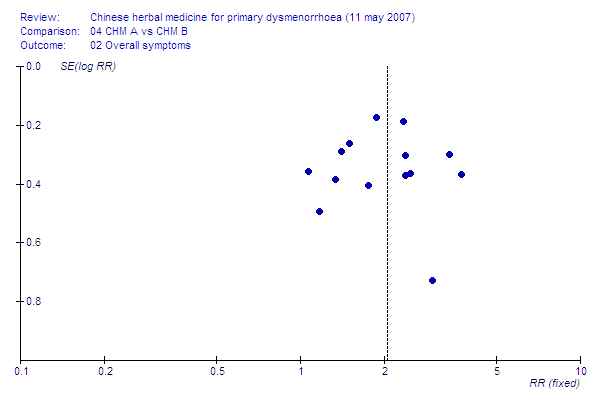
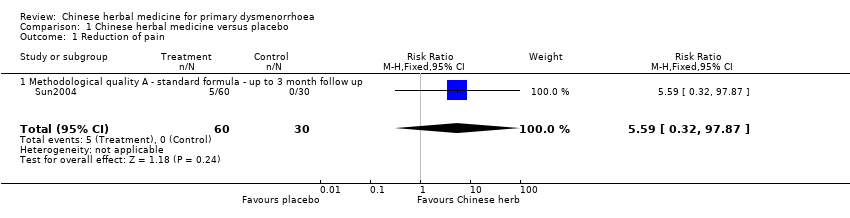
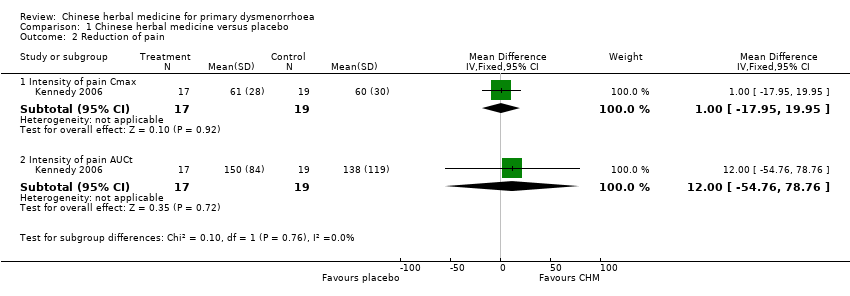
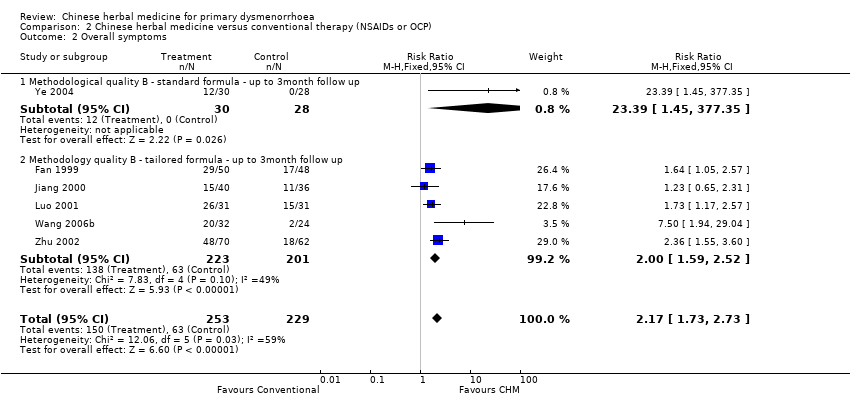
Contenido relacionado
Revisiones y protocolos relacionados
Zheng Jing, Xunzhe Yang, Khaled MK Ismail, Xiao Y Chen, Taixiang Wu | 21 enero 2009
Andrew Flower, Jian Ping Liu, George Lewith, Paul Little, Qing Li | 16 mayo 2012
Hai Bo Qu, Wang Dengfeng, Taixiang Wu, Jane Marjoribanks, Sun Ying, Jia Haijun, Jing Zhang, Lina Hu | 6 julio 2011
Kunyan Zhou, Jing Zhang, Liangzhi Xu, Chi Eung Danforn Lim | 4 junio 2021
Xiaoshu Zhu, Yuklan Liew, Zhao Lan Liu | 15 marzo 2016
Jian Ping Liu, Hong Yang, Yun Xia, Francesco Cardini | 30 abril 2013
Porjai Pattanittum, Naowarat Kunyanone, Julie Brown, Ussanee S Sangkomkamhang, Joanne Barnes, Vahid Seyfoddin, Jane Marjoribanks | 22 marzo 2016
Rachel A Earl, Rosalie M Grivell | 18 diciembre 2021
Caroline A Smith, Mike Armour, Xiaoshu Zhu, Xun Li, Zhi Yong Lu, Jing Song | 18 abril 2016
Jane Marjoribanks, Reuben Olugbenga Ayeleke, Cindy Farquhar, Michelle Proctor | 30 julio 2015