무릎 관절경검사를 받는 성인의 정맥 혈전색전증 예방을 위한 중재
초록
배경
무릎 관절경검사(KA)는 십자인대 및 반월상연골 손상을 복구하고 적절한 경우 지속적인 무릎 통증 진단을 돕기 위해 권장되는 일상적인 정형외과 시술이다. KA와 관련된 혈전 색전증의 위험이 적다. 이 체계적인 검토는 약리학적 또는 비약리학적 중재가 이러한 위험을 줄일 수 있는지 평가하는 것을 목표로 한다. 이것은 이전 문헌고찰의 업데이트이다.
목적
KA를 겪고 있는 성인의 혈전 예방을 위한 중재(기계적, 약리학적 또는 둘 다의 조합)의 효능과 안전성을 평가한다.
검색 전략
표준적이고 광범위한 코크란 검색 방법을 사용했다. 최근 검색 날짜는 2021년 6월 1일이었다.
선정 기준
KA를 겪고 있는 18세 이상의 남성과 여성의 심부 정맥 혈전증(DVT)을 예방하기 위해 사용된 모든 유형의 중재에 대해 맹검 또는 비맹검 무작위 대조 시험(RCT) 및 대조 임상 시험(CCT)을 포함했다.
자료 수집 및 분석
표준 코크란 방법을 사용했다. 주요 결과는 폐색전증(PE), 증상이 있는 DVT, 무증상 DVT 및 모든 원인으로 인한 사망이었다. 이차 결과는 부작용, 주요 출혈 및 경미한 출혈이었다. 근거의 확실성을 평가하기 위해 GRADE 기준을 사용했다.
주요 결과
이 업데이트에 대한 새로운 연구를 확인하지 않았다. 이 리뷰에는 혈전색전증 병력이 없는 성인 3,818명을 대상으로 한 8건의 연구가 포함되어 있다. 5건의 연구는 매일 피하 저분자량 헤파린(LMWH)을 예방하지 않는 것과 비교했다. 한 연구는 경구용 리바록사반 10mg과 위약을 비교했다. 한 연구는 일일 피하 LMWH와 눈금 압박 스타킹을 비교했다. 그리고 한 연구는 아스피린과 예방 요법을 비교하지 않았다.
모든 연구에서 PE 발생률은 3818명의 참여자 중 7건으로 낮았다. 중재 또는 대조군에서 사망은 없었다.
저분자량 헤파린 대 예방 없음
예방 조치가 없는 경우와 비교할 때 LMWH는 KA를 겪고 있는 사람들의 PE 발병률에 거의 또는 전혀 차이가 없을 것이다(위험비[RR] 1.81, 95% 신뢰 구간[CI] 0.49 ~ 6.65; 3건의 연구, 1820명의 참가자; 중간‐ 확실한 근거). LMWH는 증상이 있는 DVT 발생률에 거의 또는 전혀 영향을 미치지 않을 수 있다(RR 0.61, 95% CI 0.18 ~ 2.03, 4개 연구, 1848명의 참가자, 근거 확실성 낮음). LMWH가 무증상 DVT의 위험을 줄이는지 여부는 불확실하다(RR 0.14, 95% CI 0.03 ~ 0.61, 2건의 연구, 369명의 참가자, 근거 확실성 매우 낮음). LMWH는 아마도 모든 부작용(RR 1.85, 95% CI 0.95~3.59, 5개 연구, 1978명의 참가자, 근거 확실성 중간), 주요 출혈(RR 0.98, 95% CI 0.06~15.72 ;1451명의 참가자, 중간 정도의 근거 확실성) 또는 경미한 출혈(RR 1.79, 95% CI 0.84 ~ 3.84, 5개의 연구, 1978명의 참가자, 중간 정도의 근거 확실성)에 영향을 주지 않는다.
리바록사반 대 위약
234명의 참가자를 대상으로 한 한 연구에서 경구용 리바록사반 10mg과 위약을 비교했다. 보고된 PE 사례는 없었다. 리바록사반은 아마도 증상이 있는 DVT에서 거의 또는 전혀 차이가 없었을 것이다(RR 0.16, 95% CI 0.02 ~ 1.29; 근거 확실성 중간). 근거의 확실성이 매우 낮기 때문에(RR 0.95, 95% CI 0.06~15.01) 리바록사반이 무증상 DVT의 위험을 감소시키는지는 불확실하다. 이 연구에서는 출혈 부작용만 보고했다. 두 그룹 모두에서 주요 출혈이 발생하지 않았으며 리바록사반은 경미한 출혈과 거의 또는 전혀 차이가 없었을 것이다(RR 0.63, 95% CI 0.18 ~ 2.19; 근거 확실성 중간).
아스피린 대 예방 없음
한 연구는 아스피린을 예방하지 않은 것과 비교했다. 두 그룹 모두에서 PE, DVT 또는 무증상 사례가 발견되지 않았다. 연구 저자는 통증과 부종을 포함한 부작용을 보고했지만 어떤 그룹에서 이러한 부작용이 발생했는지는 밝히지 않았다. 보고된 출혈은 없었다.
저분자량 헤파린 대 압박 스타킹
1317명의 참가자를 대상으로 한 한 연구에서 LMWH와 압박 스타킹을 비교했다. LMWH는 압박 스타킹과 비교하여 PE 위험의 차이가 거의 또는 전혀 없을 수 있지만(RR 1.00, 95% CI 0.14 ~ 7.05; 근거 확실성 낮음) 증상이 있는 DVT의 위험을 줄일 수 있다(RR 0.17, 95% CI 0.04 ~ 0.75, 근거 확실성 낮음). LMWH가 무증상 DVT에 어떤 영향을 미치는지 불확실하다(RR 0.47, 95% CI 0.21 ~ 1.09, 근거 확실성 매우 낮음). 결과는 LMWH가 주요 출혈(RR 3.01, 95% CI 0.61 ~ 14.88; 근거 확실성 중간) 또는 경미한 출혈(RR 1.16, 95% CI 0.64 ~ 2.08; 근거 확실성 중간)에서 차이가 거의 또는 전혀 없음을 시사한다.
전반적인 작은 사례 수로 인한 부정확성, 눈가림 부족에 대한 우려로 인한 비뚤림 위험, 무증상 DVT 발견의 직접적인 임상적 관련성에 대한 불확실성으로 인한 간접성에 대한 근거의 확실성을 하향 조정했다.
연구진 결론
KA를 겪고 있는 건강한 성인이 정맥 혈전색전증(PE 또는 DVT)이 발생할 위험이 적다. PE 또는 증상이 있는 DVT의 작은 위험을 줄이는 데 LMWH 또는 리바록사반의 이점이 거의 또는 전혀 없다는 중간에서 낮은 확실성 근거를 발견했다. 이 연구는 LMWH가 예방 조치가 없는 경우에 비해 무증상 DVT의 위험을 감소시킬 수 있다는 매우 낮은 확실성의 근거를 제공했지만, 이것이 KA를 겪고 있는 건강한 사람의 DVT 또는 PE 발병률과 직접적으로 어떤 관련이 있는지는 불확실하다. 부작용(대출혈 및 경미한 출혈 포함)에는 차이가 거의 또는 전혀 없을 수 있지만, 이러한 결과와 관련된 데이터는 이러한 결과를 보고하는 연구에서 적은 수의 사례로 인해 제한되었다.
PICOs
쉬운 말 요약
무릎의 키홀 수술을 받은 성인의 정맥 혈전색전증 예방을 위한 치료
주요 메시지
• 최소 침습 무릎 수술을 받는 건강한 성인에게 혈전 예방 약물(저분자량 헤파린(LMWH), 아스피린 또는 리바록사반)을 사용하면 깊은 정맥에서 혈전이 발생하는 작은 위험이나 이러한 혈전이 폐의 혈관으로 이동할 작은 위험을 줄일 수 있다는 명확한 근거를 찾지 못했다.
• LMWH, 아스피린 또는 리바록사반이 이러한 사람들에게 출혈과 같은 해로운 영향을 미친다는 명확한 근거를 찾지 못했다.
이 질문이 왜 중요한가?
무릎 관절경검사에서 의료 서비스 제공자는 사람의 무릎에 작은 상처를 통해 작은 카메라를 삽입한다. 무릎 관절경검사는 다양한 유형의 무릎 부상을 진단하고 복구하는 데 사용된다. 이 시술을 받는 사람은 다리 심부정맥에 혈전이 생길 위험이 적다(심부정맥 혈전증). 심부정맥혈전증에 걸리면 종아리 통증이나 부종(증상성 심부정맥혈전증) 등의 증상이 나타날 수 있다. 혈전이 폐로 이동할 위험도 있다(폐색전증). 수술을 받은 사람들은 종종 혈전 발생 위험을 낮추기 위해 약물을 투여받는다. 이러한 약물은 혈액을 묽게 하여 혈전을 예방하는 데 도움이 되며 출혈과 같은 부작용을 일으킬 수 있다. 무릎 관절경검사 후 혈전이 생길 위험이 적기 때문에 혈전 예방을 위한 약물 복용이 필요한지 아는 것이 중요하다.
무엇을 했는가?
이 체계적 검토에는 무릎 관절경검사를 받았고 혈전 예방 약물을 받았거나 받지 않은 사람들에 대한 무작위 통제 시험이 포함되었다. 무작위 연구는 사람들이 받는 치료가 무작위로 결정되기 때문에 치료 효과에 대한 가장 신뢰할 수 있는 근거를 제공한다.
무엇을 찾았는가?
검토에 포함된 연구에서 대조군 참가자(약물을 투여받지 않은 사람들)는 6건의 연구에서 치료를 받지 않았고, 1건의 연구에서는 더미 알약(위약)을 받았으며, 1건의 연구에서는 압박 스타킹을 착용했다.
5건의 연구에서 얻은 정보를 종합한 결과 사람들이 매일 복부에 주사하는 약인 LMWH가 치료를 받지 않은 경우에 비해 폐색전증 또는 증후성 심부 정맥 혈전증의 위험을 명확하게 감소시키지 못하는 것으로 나타났다. LMWH는 무증상 심부 정맥 혈전증(증상이 없는 다리의 혈전, 일반적으로 검사로 감지됨)의 위험을 줄일 수 있지만 결과에 대해서는 매우 불확실하다. LMWH는 출혈에 명확한 영향을 미치지 않았다.
한 연구에서 경구용 리바록사반은 심부 정맥 혈전증의 위험을 감소시키지 않았고 위약과 비교할 때 경미한 출혈도 증가시키지 않았다. 이 연구에서는 폐색전증이나 주요 출혈 사례가 보고되지 않았다.
아스피린을 무치료와 비교한 한 연구에서 폐색전증, 심부 정맥 혈전증 또는 출혈이 있는 참가자는 없었다.
압박 스타킹에 비해 LMWH는 폐색전증이나 출혈을 감소시키지는 않았지만 증상이 있는 심부 정맥 혈전증을 감소시켰다.
근거에 대해 얼마나 확신하는가?
근거에 대한 확신은 치료와 치료 결과에 따라 다르다. 폐색전증과 증후성 심부정맥 혈전증의 근거에 대한 총 사례(혈전) 수가 적었기 때문에 확신할 수 없다. 참가자들이 자신이 받은 치료에 대해 알고 있었고 이 정보를 실수로든 고의로든 혈전을 찾고 있던 의료진에게 공개했을 수 있기 때문에 무증상 혈전에 대한 근거를 확신할 수 없다. 또한, 무증상 혈전은 혈전 위험이 낮거나 무릎 관절경검사 후 신속하게 이동성을 회복하는 사람들에게 임상적으로 덜 중요할 수 있다.
이 근거는 얼마나 최신인가?
이 코크란리뷰는 이전 근거의 업데이트이다. 근거는 2021년 6월 1일까지 검색했다.
Authors' conclusions
Summary of findings
| LMWH compared to no prophylaxis for preventing VTE in adults undergoing KA | ||||||
| Patient or population: adults undergoing KA | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Interpretation | |
|---|---|---|---|---|---|---|
| Without LMWH | With LMWH | |||||
| PE: assessed by CT arteriography (follow‐up: 30 days to 3 months) | 1820 | RR 1.81 | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of PE. | |
| 3 per 1000 | 6 per 1000 (2 to 22) | |||||
| Symptomatic DVT: assessed by presence of symptoms and confirmed by a positive compression ultrasound (follow‐up: 30 days to 3 months) | 1848 | RR 0.61 | Study population | ⊕⊕⊝⊝
| LMWH may lead to little or no difference in symptomatic DVT. | |
| 11 per 1000 | 7 per 1000 (2 to 22) | |||||
| Asymptomatic DVT: assessed with compression ultrasound (follow‐up: 30 days to 8 weeks) | 369 | RR 0.14 | Study population | ⊕⊝⊝⊝ | It is uncertain if LMWH reduces the risk asymptomatic DVT. | |
| 81 per 1000 | 11 per 1000 (2 to 49) | |||||
| All‐cause mortality (follow‐up: 30 days to 3 months) | 1978 | See comment | Not estimable | No deaths occurred in any of the included studies. | ||
| All adverse effects, including allergies at injection site, minor and major bleeding, others (follow‐up: 30 days to 3 months) | 1978 | RR 1.85 | Study population | ⊕⊕⊕⊝ Moderatea | LMWH probably leads to little or no difference in adverse effects. | |
| 13 per 1000 | 24 per 1000 | |||||
| Major bleeding (follow‐up: 30 days to 3 months) | 1451 | RR 0.98 (0.06 to 15.72). | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of major bleeding. | |
| 1 per 1000 | 1 per 1000 (0 to 22) | |||||
| Minor bleeding (follow‐up: 30 days to 3 months) | 1978 | RR 1.79 | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of minor bleeding. | |
| 11 per 1000 | 20 per 1000 (9 to 43) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision. | ||||||
Background
Description of the condition
Knee arthroscopy (KA) is indicated to repair the anterior cruciate ligament (ACL) and meniscal tears, and, in suitable people, to assist the diagnosis of persistent knee pain (AAOS 2014). Complications of KA are uncommon, with rates of less than 1%. The most frequent complications are local hematoma, pyogenic arthritis and thromboembolic events. In a large cohort study of 20,770 KA procedures (Maletis 2012), the risk of deep vein thrombosis (DVT) was 0.25% (95% confidence interval (CI) 0.18% to 0.31%). For pulmonary embolism (PE), the risk was 0.017% (95% CI 0.11% to 0.22%). Two prospective studies reported incidence rates of asymptomatic DVT of 7.84% to 18% (Delis 2001; Demers 1998). The risk of thromboembolic events after KA was higher in participants who smoked, in those taking oral contraceptives, in those with a previous history of thromboembolic diseases, and in those with a body mass index over 30 kg/m2.
Proximal DVT (DVT above the popliteal vein) increases the risk of PE. Half of people presenting with proximal symptomatic DVT will have evidence of PE in lung scans (Kearon 2003). Immobilization and limb surgery also increase the risk of thromboembolic disease because of decreased blood flow and an increase in inflammatory markers that activate the coagulation cascade (Delis 2001; Maletis 2012). The clinical relevance of asymptomatic distal DVT is unclear; the consensus is that isolated asymptomatic calf DVT rarely progresses to symptomatic DVT (Gaskill 2015; Kearon 2003).
The accuracy of asymptomatic DVT diagnosis by sonography (ultrasound) varies depending on the technique used: according to Goodacre 2005, duplex ultrasound achieves higher sensitivity (71%), while compression ultrasound alone achieves higher specificity (98%). Thromboprophylaxis is recommended in hip replacement and knee replacement surgeries, as thromboembolic events are relatively frequent in those clinical circumstances. Two probable reasons for this are the longer duration of the surgery and the fact that some people are unable to regain early mobility.
Description of the intervention
For pharmacological prophylaxis in orthopedic surgery, clinical guidelines recommend apixaban, aspirin, dabigatran etexilate, fondaparinux sodium, low‐molecular‐weight heparin (LMWH), and rivaroxaban. Where there is a contraindication for any pharmacological strategy, the guidelines recommend using graduated elastic compression stockings and intermittent pneumatic compression (Falck‐Ytter 2012; NICE 2019). The aim of all the above interventions is to prevent venous thromboembolism.
How the intervention might work
Pharmacological interventions include parenteral anticoagulants (LMWH, fondaparinux), oral direct thrombin inhibitors (dabigatran), oral factor Xa inhibitors (rivaroxaban and apixaban), and oral antiplatelets (aspirin). They all work by inhibiting stages of the coagulation process (coagulation cascade). LMWH selectively binds to antithrombin (AT; a serine protease inhibitor) and creates a conformational change of the molecule. This change promotes inhibition of activated factor X. Fondaparinux is a synthetic pentasaccharide factor Xa inhibitor; it also works by binding to AT, but it is selective for factor Xa (Ritter 2019). Dabigatran reversibly binds to the active site on the thrombin molecule, preventing thrombin‐mediated activation of coagulation factors (Ritter 2019). Rivaroxaban inhibits both free factor Xa and factor Xa bound in the prothrombinase complex. It is a highly selective direct factor Xa inhibitor with a rapid onset of action. Aspirin inhibits platelet generation of thromboxane A2, resulting in an antithrombotic effect (Ritter 2019). Mechanical interventions facilitate the circulation of the venous flow in lower limbs, preventing thrombus formation (Benko 2001).
Why it is important to do this review
It is relevant to clinical practice to determine whether thromboprophylaxis reduces the already low risk of thromboembolic disease in people undergoing common procedures such as KA. Whether prophylaxis used routinely in orthopedic surgery should also be used in KA remains unclear, owing to the very low risk of severe thromboembolic events (proximal DVT and PE), the uncertain significance of asymptomatic distal DVT (which is also of low prevalence), and the fact that people quickly regain mobility after the procedure. To assist decision‐making, it is important to present up‐to‐date evidence on thromboprophylaxis in people undergoing KA who have no previous history of thromboembolic events.
Objectives
To evaluate the efficacy and safety of interventions – whether mechanical, pharmacological, or a combination of both – for thromboprophylaxis in adults undergoing KA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and controlled clinical trials (CCTs), regardless of blinding (i.e. double‐blind, single‐blind and unblinded trials were eligible for inclusion).
Types of participants
Eligible studies included adults (aged 18 years and older) undergoing KA.
Types of interventions
We included any type of pharmacological or mechanical intervention, whether used singly or in combination, to prevent DVT in adults undergoing KA. We excluded interventions involving arthroscopically assisted osteosynthesis of tibial plateau fractures.
Types of outcome measures
Primary outcomes
-
PE confirmed by ventilation/perfusion (V/Q) lung scan, spiral computed tomography (CT), or pulmonary angiography
-
Symptomatic DVT (distal and proximal combined), diagnosed clinically, venographically or sonographically, and defined as symptomatic when participants spontaneously experienced DVT symptoms (calf pain, swelling, edema) or when participants referred to symptoms of DVT while undergoing echo‐Doppler or compression ultrasound in the context of the study
-
Asymptomatic DVT, defined as asymptomatic when participants were actively screened for DVT using echo‐Doppler or compression ultrasound and did not indicate any symptoms during ultrasound
-
All‐cause mortality
Secondary outcomes
-
All reported adverse effects (pain, allergies, minor and major bleeding)
-
Major bleeding (overt and requiring transfusion of red blood cells or surgical intervention, and which may lead to permanent disability)
-
Minor bleeding (not meeting the criteria for intervention), including hematoma, hemarthrosis and thrombocytopenia (reduction of circulating platelets) with a platelet count below 80,000/mm3 or less than half of the initial count
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and CCTs, with no restrictions on language, publication year or publication status.
-
The Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web, searched 1 June 2021).
-
The Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (2021, Issue 5).
-
MEDLINE: Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE (searched 14 August 2019 to 1 June 2021).
-
Embase Ovid (searched 14 August 2019 to 1 June 2021).
-
CINAHL Ebsco (searched 14 August 2019 to 1 June 2021).
-
AMED Ovid (searched 14 August 2019 to 1 June 2021).
The Information Specialist modeled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, strategies were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and CCTs, as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 1 June 2021.
-
The World Health Organization International Clinical Trials Registry Platform (who.int/clinical-trials-registry-platform).
-
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We searched the reference lists of identified studies for additional citations.
Data collection and analysis
Selection of studies
Two authors (CP, JR) independently screened the initial reference list, identified relevant citations, and used a standardized form to determine whether they met the inclusion criteria.
Data extraction and management
Three authors (JR, JC, GB) extracted the data into a custom spreadsheet before entering them into Review Manager 5 (Review Manager 2014). If necessary, we planned to contact study authors for additional information or data.
Assessment of risk of bias in included studies
Using RoB 1 (Higgins 2011), three review authors (JC, JR, GB) independently assessed the risk of bias for each of the included studies according to the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessors (ascertainment bias).
-
Incomplete outcome data (attrition bias).
-
Selective outcome reporting bias.
-
Other sources of bias.
The review authors judged whether each study was at low, high or unclear risk of bias in each domain. An unclear risk of bias judgement could be due to lack of information or uncertainty over the potential for bias. Disagreements among review authors were resolved by consensus.
Measures of treatment effect
We investigated the treatment effect on our outcomes of interest by calculating risk ratios (RRs) with 95% CIs. We planned to include clinical trials that had zero events. None of our outcomes were continuous.
Unit of analysis issues
We considered individual participants to be the units of randomization in the included studies. In the original protocol, we stated that the unit of analysis would be the knee (Ramos 2005), but no included studies enrolled people undergoing bilateral KA.
Dealing with missing data
Had we identified any studies with missing data for the relevant outcomes (thromboembolic events and adverse effects), we would have contacted the authors to request the missing data.
Assessment of heterogeneity
We assessed the heterogeneity of estimated effects in the included studies by visually inspecting the forest plot generated from the meta‐analysis of studies. We assessed statistical heterogeneity using the I² statistic (Higgins 2003). Very divergent results or I² greater than 50% were considered to represent substantial heterogeneity. We also used the Chi² statistic, assuming statistically significant heterogeneity where Chi² exceeded the degrees of freedom (df) and the P value was less than 0.05. If heterogeneity was present, we aimed to explore sources of heterogeneity that could be relevant to the clinical question.
Assessment of reporting biases
If we had included sufficient studies (10 or more) in the meta‐analysis, we would have assessed the presence of publication bias and other reporting bias using funnel plots (Higgins 2011).
Data synthesis
Where studies compared similar interventions and reported comparable outcomes, we pooled effect estimates using the inverse variance method through a fixed‐effect model, and calculated 95% CIs.
If heterogeneity was present, we used a random‐effects model and, where possible, carried out subgroup analyses to explain the source of heterogeneity. We reported the results narratively when meta‐analysis was not possible.
We used Review Manager 5 to perform the analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We did not have any preplanned subgroup analyses. To investigate heterogeneity, we attempted to explain differences as per study design by looking at subgroups of studies, specifically how the outcomes were defined and assessed.
Sensitivity analysis
We carried out a sensitivity analysis to determine any impact of using either intention‐to‐treat (ITT) data or per‐protocol (PP) data in van Adrichem 2017.
Summary of findings and assessment of the certainty of the evidence
Using GRADEpro GDT, we prepared a summary of findings table to present the main findings from the meta‐analysis for the outcomes of PE, symptomatic DVT, asymptomatic DVT, all‐cause mortality, all adverse effects, major bleeding and minor bleeding.
We created a summary of findings table for the comparison of LMWH versus no prophylaxis but not for the remaining comparisons, which were based on data from single studies.
Using the GRADE criteria, we ranked the certainty of the evidence for each outcome based on risk of bias, inconsistency, indirectness, imprecision and publication bias (Guyatt 2008), justifying all decisions to downgrade the certainty of the evidence.
Results
Description of studies
Results of the search
See Figure 1. The searches retrieved a total of 755 records. After removing duplicates, we screened 631 records, considering all of them to be clearly irrelevant. We did not include any new studies in this update; nor did we assess any new excluded or ongoing studies.
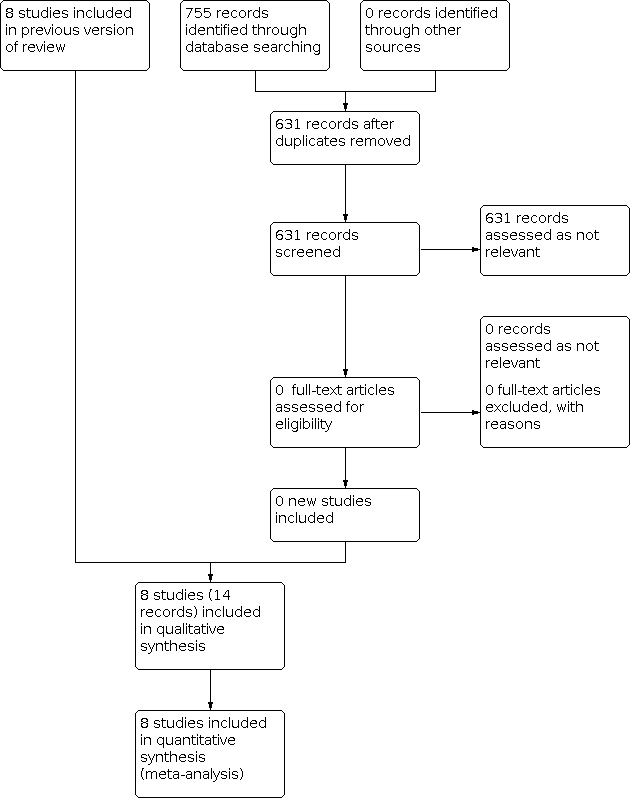
Flow diagram
Included studies
See the Characteristics of included studies table for further details.
To date, we have included a total of eight studies with 3818 participants in our review (Camporese 2008; Camporese 2016; Canata 2003; Kaye 2015; Michot 2002; Roth 1995; van Adrichem 2017; Wirth 2001).
Overall, the included studies investigated four different treatments for the prevention of thromboembolic disease in adults undergoing KA.
Pregnancy, cancer and previous thromboembolic disease were exclusion criteria in most studies. Kaye 2015 excluded smokers, and Wirth 2001 excluded people with three or more of a predefined set of risk factors (obesity, nicotine abuse, oral contraceptives, family history of thrombosis).
Five studies, with a total of 1942 participants, compared LMWH to no prophylaxis (Canata 2003; Michot 2002; Roth 1995; van Adrichem 2017; Wirth 2001). One study with 241 participants compared rivaroxaban to placebo (Camporese 2016). One study with 170 participants compared aspirin to no prophylaxis (Kaye 2015). One study with 1317 participants compared LMWH for seven or 14 days to use of compression stockings (Camporese 2008).
All studies evaluated thromboembolic disease (PE, symptomatic DVT, asymptomatic DVT, or a combination of these), mortality, minor and major bleeding or other adverse effects.
The types of KA performed were meniscectomy, ACL reconstruction and removal of loose fragments and, in a small proportion of participants, diagnostic procedures. Most of the studies did not tabulate events in relation to the type of surgery performed or to participant characteristics such as body mass index or smoking status.
Excluded studies
No studies reached the full‐text review stage in this update, so we did not assess any new excluded studies. We reassessed one study that we had considered irrelevant in the previous version of this review (Muñoa 2014), to confirm our decision. This was a non‐randomized study that compared rivaroxaban with bemiparin after KA. On reassessment, we maintained that it should not be included. Marlovits 2004 was excluded previously as it compared LMWH treatment for three to eight days with LMWH for 20 days, without a control arm. See the Characteristics of excluded studies table for further details.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the overall risk of bias.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Overall, the included studies were of acceptable methodological quality, given the type of intervention. We judged no studies at high risk of bias in relation to randomization, allocation, selection bias or reporting bias. We flagged a few issues, such as a lack of participant or personnel blinding, and the lack of a placebo in the studies comparing LMWH to no prophylaxis. We believe that the lack of placebo would not have impacted more objective outcomes such as death, PE or symptomatic DVT (participants seeking medical advice due to DVT symptoms with confirmation by a positive echo‐Doppler or compression ultrasound). There is a risk of bias in outcomes that could be influenced by study personnel or participants being aware of, or disclosing, participant status in treatment arms.
All studies except van Adrichem 2017 included asymptomatic venous thrombosis in their combined outcome. Camporese 2008 and Michot 2002 identified symptomatic DVT by actively asking participants, during study visits or through telephone interviews, if they had any symptoms of DVT (e.g. calf pain, edema, redness), followed by color‐coded Doppler (Camporese 2008), or compression ultrasonography (Michot 2002). We considered that there is an unclear risk of bias, due to indirectness of the outcome in the case of Camporese 2008 (a Doppler sonograph is not a usual procedure after KA), and because actively asking participants about their symptoms before performing a Doppler ultrasound could introduce recall bias. There are also differences in the accuracy of the methods of determining DVT (symptomatic and asymptomatic) in both studies (Goodacre 2005). We addressed the impact of any potential bias on our confidence in the effect estimates of individual outcomes while assessing the certainty of evidence with GRADE.
Allocation
We considered allocation to be adequate in five studies, where randomization strategies included using sealed opaque envelopes; using sequentially numbered, unlabeled boxes; and blinding research staff to block sizes (Camporese 2008; Camporese 2016; Kaye 2015; van Adrichem 2017; Wirth 2001). We judged these studies at low risk of selection bias. The remaining three studies did not provide sufficient information to prove adequate allocation concealment, and were therefore judged at unclear risk of selection bias (Canata 2003; Michot 2002; Roth 1995).
Blinding
Camporese 2016 used oral medications as the intervention and a placebo in the control group. This study was at low risk of both performance and detection bias, as participants, personnel and outcome assessors were blinded to the treatment group. In van Adrichem 2017, participants were aware of their treatment arm, but outcomes were assessed by checking hospital registries. In this case, therefore, lack of blinding should not have influenced outcome measurement. In five studies, the participants or personnel were aware of the treatment group, though the outcome assessors were not (Camporese 2008; Canata 2003; Kaye 2015; Michot 2002; Wirth 2001). We considered that lack of blinding would not have affected the outcomes of PE, symptomatic DVT or adverse effects, but may have influenced the assessment of asymptomatic DVT. We judged these studies at unclear risk of performance and detection bias. We also considered Roth 1995 at unclear risk of performance and detection bias because it provided insufficient information.
Incomplete outcome data
We considered five studies at low risk of attrition bias because they reported complete outcome data, or because any losses to follow‐up were accounted for and balanced between groups (Camporese 2008; Camporese 2016; Kaye 2015; Michot 2002; Wirth 2001). van Adrichem 2017 described losses and reported results from PP and ITT analyses. As there were no differences in the estimates, we judged the risk of attrition bias to be low. The authors of some studies reported combined outcomes and individual outcomes (all thromboembolic events, including DVT, PE and asymptomatic DVT), so we were able to report the outcomes of interest individually. We judged Roth 1995 at unclear risk of attrition bias because it provided incomplete information on dropouts or losses to follow‐up. We judged Canata 2003 at unclear risk of attrition bias because there were no details on missing data or losses to follow‐up.
Selective reporting
We judged seven studies at low risk of reporting bias as there were no concerns in relation to selective reporting (Camporese 2008; Camporese 2016; Canata 2003; Kaye 2015; Michot 2002; van Adrichem 2017; Wirth 2001). Roth 1995 did not have complete reporting for all the outcomes of interest, and so was judged at unclear risk of reporting bias.
Other potential sources of bias
In Camporese 2008, the data and safety monitoring board stopped the 14‐day treatment group during a planned interim analysis. The study continued with two groups (7‐day treatment versus no prophylaxis). We were unsure if this could have biased the results, so we judged there to be an unclear risk of other bias. In Michot 2002, the trialists stopped recruiting before reaching the desired sample size because they considered it unethical to withhold LMWH from the control participants. We judged this study at unclear risk of other bias. All remaining studies were at low risk of other bias.
Effects of interventions
Low‐molecular‐weight heparin versus no prophylaxis
Pulmonary embolism
Of the five studies comparing LMWH to no prophylaxis (Canata 2003; Michot 2002; Roth 1995; van Adrichem 2017; Wirth 2001), three reported on PE (Michot 2002; van Adrichem 2017; Wirth 2001). Overall, there were few events, and pooling the data from these showed no clear difference in the rates of PE in the LMWH group compared to the control group (6/914 with LMWH versus 3/906 with control; RR 1.81, 95% CI 0.49 to 6.65; 3 studies, 1820 participants; moderate‐certainty evidence; Analysis 1.1). We downgraded the certainty of the evidence by one level for imprecision around the estimate of effect.
Symptomatic deep vein thrombosis
Four studies reported symptomatic DVT as a separate outcome (Canata 2003; Roth 1995; van Adrichem 2017; Wirth 2001). There was no clear difference in the rates of symptomatic DVT when comparing LMWH to no prophylaxis (RR 0.61, 95% CI 0.18 to 2.03; 4 studies, 1848 participants; low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence by one level for imprecision due to the small absolute number of events and small sample size, and by one level for risk of bias due to lack of a valid placebo, uncertainty about blinding and reporting of symptoms with potential recall bias.
Asymptomatic deep vein thrombosis
Two studies reported on asymptomatic DVT (Michot 2002; Wirth 2001). Compared to no prophylaxis, LMWH may reduce the prevalence of DVT diagnosed by compression ultrasonography, but the evidence is very uncertain (RR 0.14, 95% CI 0.03 to 0.61; 2 studies, 369 participants; very low‐certainty evidence; Analysis 1.3).
We downgraded the evidence by three levels for imprecision, risk of bias and indirectness. The use of compression ultrasonography to actively look for asymptomatic DVT is not routinely recommended in KA. Participants were not blinded to the intervention, and there is a possibility that they discussed their allocation intervention with assessors. Additionally, this is not an outcome of interest in participants who are at low risk of thromboembolic disease or who will regain full mobility within a week of the procedure.
All‐cause mortality
No deaths occurred in any study.
All reported adverse effects (pain, allergies, minor and major bleeding)
The five studies reported 39 adverse effects in 1978 participants (Canata 2003; Michot 2002; Roth 1995; van Adrichem 2017; Wirth 2001). Adverse effects included minor bleeding at the injection site, pain at the injection site, and allergies manifesting as inflammation at the injection site. No studies assessed thrombocytopenia. LMWH had no clear effect on risk of all adverse effects combined (RR 1.85, 95% CI 0.95 to 3.59; 5 studies, 1978 participants; moderate‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence by one level for imprecision due to the low numbers of events.
Major bleeding
Only one study reported any cases of major bleeding (van Adrichem 2017), with one case in each treatment arm (RR 0.98, 95% CI 0.06 to 15.72; 1451 participants; moderate‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence by one level for imprecision.
Minor bleeding
All five studies assessed minor bleeding (Canata 2003; Michot 2002; Roth 1995; van Adrichem 2017; Wirth 2001). There was no clear difference in minor bleeding events with LMWH versus no prophylaxis (RR 1.79, 95% CI 0.84 to 3.84; 5 studies, 1978 participants; moderate‐certainty evidence; Analysis 1.6). We downgraded the certainty of the evidence by one level for imprecision.
As van Adrichem 2017 presented both PP and ITT data, we carried out a sensitivity analysis to investigate both datasets. There were no differences in the results, and we included the ITT data in our analysis.
Rivaroxaban versus placebo
Pulmonary embolism
In the single study (234 participants) comparing rivaroxaban to placebo, there were no PE events during the study period (Camporese 2016).
Symptomatic deep vein thrombosis
There was no clear difference in the risk of symptomatic DVT with oral rivaroxaban 10 mg compared to placebo (RR 0.16, 95% CI 0.02 to 1.29; 234 participants; moderate‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence by one level for imprecision of the estimate.
Asymptomatic deep vein thrombosis
There was no clear difference in the risk of asymptomatic DVT, as diagnosed by compression ultrasonography, with oral rivaroxaban 10 mg compared to placebo (RR 0.95, 95% CI 0.06 to 15.01; 234 participants; very low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence by three levels for imprecision, risk of bias and indirectness of the outcome.
All‐cause mortality
No deaths occurred in either group.
All reported adverse effects (pain, allergies, minor and major bleeding)
Camporese 2016 did not assess any adverse effects other than major and minor bleeding, which are reported below.
Major bleeding
No major bleeding events occurred.
Minor bleeding
There was no clear difference in the risk of minor bleeding with rivaroxaban compared to placebo (RR 0.63, 95% CI 0.18 to 2.19; 234 participants; moderate‐certainty evidence; Analysis 2.3). We downgraded the certainty of the evidence by one level for the imprecision of the estimate.
Aspirin versus no prophylaxis
Pulmonary embolism
In the single study comparing aspirin to no prophylaxis, no PE events occurred during the study period (Kaye 2015).
Symptomatic deep vein thrombosis
No symptomatic DVT events occurred.
Asymptomatic deep vein thrombosis
No asymptomatic DVT events occurred. Asymptomatic events were investigated using compression ultrasonography.
All‐cause mortality
No deaths occurred.
All reported adverse effects (pain, allergies, minor and major bleeding)
Kaye 2015 reported complications including pain and swelling (15 cases, 9%) and residual joint line tenderness (5 cases, 3%), but did not specify which groups they occurred in. Three participants developed knee swelling. Two of these participants were randomized to the aspirin group.
Major bleeding
No major bleeding events occurred.
Minor bleeding
No minor bleeding events occurred.
Low‐molecular‐weight heparin versus compression stockings
Pulmonary embolism
In the single study comparing LMWH to compression stockings, there was no clear difference in the risk of PE between the groups (RR 1.00, 95% CI 0.14 to 7.05; 1317 participants; low‐certainty evidence; Analysis 3.1; Camporese 2008). We downgraded the certainty of the evidence by two levels for imprecision due to the low number of events and for risk of bias concerns.
Symptomatic deep vein thrombosis
LMWH resulted in a lower risk of symptomatic DVT compared to compression stockings (RR 0.17, 95% CI 0.04 to 0.75; 1317 participants; low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence by two levels for imprecision of the absolute effects and for risk of bias.
Asymptomatic deep vein thrombosis
There was no clear difference in the risk of asymptomatic DVT, as diagnosed by compression ultrasonography, with LMWH compared to compression stockings (RR 0.47, 95% CI 0.21 to 1.09; 1317 participants; very low‐certainty evidence; Analysis 3.3). We downgraded the certainty of the evidence by three levels for imprecision of the estimates due to the low number of events, for indirectness and for risk of bias concerns.
Mortality
No deaths occurred in either group.
All reported adverse effects (pain, allergies, minor bleeding and major bleeding)
In addition to major bleeding and minor bleeding, Camporese 2008 reported pain, tenderness, and edema. The study showed no clear difference between the groups (RR 1.17, 95% CI 0.95 to 1.43; 1317 participants; moderate‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence by one level for imprecision due to the small number of events and small sample size. In the compression stockings group, 152 participants reported symptoms (pain, 92; tenderness, 23; edema, 24; discoloration, 10; and nonvaricose collateral symptoms, 3), compared with 131 participants in the LMWH group (pain, 85; tenderness, 18; edema, 18; discoloration, 8; and nonvaricose collateral symptoms, 2). Thrombocytopenia was not assessed.
Major bleeding
There was no clear difference between the groups for major bleeding (RR 3.01, 95% CI 0.61 to 14.88; 1317 participants; moderate‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence by one level for imprecision due to the small sample size and small number of events.
Minor bleeding
There was no clear difference between the groups for minor bleeding (RR 1.16, 95% CI 0.64 to 2.08; 1317 participants; moderate‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence by one level for imprecision due to the small sample size and small number of events.
Discussion
Summary of main results
LMWH probably leads to little or no difference in PE when compared to no prophylaxis (moderate‐certainty evidence) and may lead to little or no difference when compared with stockings (low‐certainty evidence). There were no PE events reported for aspirin and rivaroxaban. The overall risk of PE was low, with 7 cases in 3499 participants (less than 0.2%), evenly distributed between intervention and control groups. There were no deaths due to PE or any other cause in any of the included studies.
LMWH may lead to little or no difference in the risk of symptomatic DVT (people presenting with symptoms of DVT at the emergency department, or symptoms with a positive screening) compared to no prophylaxis (low‐certainty evidence). Rivaroxaban probably leads to little or no difference in symptomatic DVT (moderate‐certainty evidence). Compared with compression stockings, LMWH may lead to a reduced rate of DVT (low‐certainty evidence).
It is unclear whether LMWH, compression, or rivaroxaban reduce the risk of asymptomatic DVT because the certainty of the evidence is very low. No asymptomatic DVT events were detected in the study investigating aspirin versus no prophylaxis.
There is probably little or no difference in adverse effects for any comparison (moderate‐certainty evidence), but results were limited by low numbers of studies reporting clearly on this outcome. When reported, there was probably little or no difference in the rates of major or minor bleeding (moderate‐certainty evidence).
In conclusion, there is moderate to low‐certainty evidence of little or no difference with the use of LMWH, aspirin or rivaroxaban compared to placebo or no prophylaxis for the risk of PE or symptomatic DVT in low‐risk adults undergoing KA.
Overall completeness and applicability of evidence
This evidence is applicable to otherwise healthy adults, at low risk of DVT, undergoing KA for minor conditions. Study participants had no history of any thromboembolic events, were not pregnant, had no history of any coagulation disorder that would put them at risk of thromboembolic events, and had accessible health care systems. People taking birth control pills were excluded in only one study.
The population included participants who smoked or were obese, with similar numbers of each in the intervention and control groups. We were unable to perform subgroup analyses according to these variables owing to the low rate of events, but study authors did not report more events in participants with these characteristics.
A major limitation in all the studies is the low event rate for severe thromboembolic events (symptomatic DVT or PE). The event rate was similar to that reported in observational studies. This confirms that KA in healthy individuals carries a small risk of symptomatic thrombosis, but it also makes it difficult to detect any benefit from the proposed interventions. Interestingly, the incidence of symptomatic DVT was higher in the compression stocking group of the Camporese 2008 study than in any other arm across all included studies in this review. This group had a 2% incidence of symptomatic DVT while the literature reports rates below 0.5% (Maletis 2012).
Only one study reflected a 'real life' scenario (van Adrichem 2017): rather than actively looking for thromboembolic events, the study authors identified participants who had attended an emergency department or had been admitted for PE or DVT. PE and symptomatic DVT data were obtained from hospital registries up to three months after the procedure. It is possible but unlikely that the study authors missed significant events with this approach. In any case, it reflects what occurs in daily practice within a healthcare system that has good accessibility.
It is possible that in healthcare systems with poor accessibility or that are highly fragmented, thromboembolic events may be missed. As there is still a risk – however small – for thromboembolic events following KA, the healthcare team looking after these people (general practitioners, orthopedic surgeons, physiotherapists, emergency department team) need to advise them to seek medical care if any thromboembolic symptoms develop.
The evidence presented in this review may not be applicable to people who are at higher risk of PE or DVT, who are unable to reach their surgeons or do not have access to healthcare, or who are unable to achieve early mobility after the procedure.
Quality of the evidence
The certainty of the evidence is moderate to low for the outcomes PE, symptomatic DVT and adverse effects (including major and minor bleeding). The certainty of the evidence for asymptomatic DVT is very low. Outcomes were more prone to bias in cases where interventions were not blinded. Those who assessed outcomes were blinded to the allocated treatment arm, provided the participants did not break this blinding (e.g. by revealing the injection site). We downgraded for risk of bias concerns when we felt this could have impacted outcome assessment.
There was significant imprecision in all the clinical outcomes, mostly due to the low rate of symptomatic thromboembolic events after KA in low‐risk participants. It is possible that larger sample sizes would provide different results.
There is very low‐certainty evidence that LMWH reduces the occurrence of asymptomatic DVT compared to no prophylaxis. The clinical significance of DVT diagnosed through an echo‐Doppler or compression ultrasonography scan is uncertain in the context of KA in a healthy person, so we downgraded the certainty of the evidence for indirectness, as well as for imprecision and for risk of bias concerns.
We created a summary of findings table for the comparison of LMWH versus no prophylaxis but not for the remaining comparisons, which were based on data from single studies. See summary of findings Table 1.
Potential biases in the review process
We carried out a thorough search and are confident that we have assessed all relevant studies. However, the available evidence is limited by the small number of studies eligible for inclusion. We followed the procedures outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with review authors independently assessing studies of inclusion, extracting data and assessing risk of bias.
Agreements and disagreements with other studies or reviews
One systematic review investigating only LMWH reached similar conclusions to our review (Huang 2018). We aimed to include all types of interventions to prevent DVT, including pharmacological and mechanical interventions. Compared with Huang 2018, our review also included studies using aspirin and rivaroxaban as pharmacological interventions (Camporese 2016; Kaye 2015). Of note, Huang 2018 included Marlovits 2004, which we excluded because it compared short‐duration LMWH with extended‐duration LMWH. Huang 2018 also excluded distal DVT from their analysis. One network meta‐analysis agreed with our findings of no difference between interventions to reduce thrombosis after KA (Lameire 2022).

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 1: Pulmonary embolism

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 2: Symptomatic deep vein thrombosis

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 3: Asymptomatic deep vein thrombosis

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 4: Adverse effects

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 5: Major bleeding

Comparison 1: Low‐molecular‐weight heparin (LMWH) versus no prophylaxis, Outcome 6: Minor bleeding

Comparison 2: Rivaroxaban versus placebo, Outcome 1: Symptomatic deep vein thrombosis

Comparison 2: Rivaroxaban versus placebo, Outcome 2: Asymptomatic deep vein thrombosis

Comparison 2: Rivaroxaban versus placebo, Outcome 3: Minor bleeding

Comparison 3: Low‐molecular‐weight heparin (LMWH) versus compression stockings, Outcome 1: Pulmonary embolism

Comparison 3: Low‐molecular‐weight heparin (LMWH) versus compression stockings, Outcome 2: Symptomatic deep vein thrombosis

Comparison 3: Low‐molecular‐weight heparin (LMWH) versus compression stockings, Outcome 3: Asymptomatic deep vein thrombosis

Comparison 3: Low‐molecular‐weight heparin (LMWH) versus compression stockings, Outcome 4: Adverse effects
| LMWH compared to no prophylaxis for preventing VTE in adults undergoing KA | ||||||
| Patient or population: adults undergoing KA | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Interpretation | |
|---|---|---|---|---|---|---|
| Without LMWH | With LMWH | |||||
| PE: assessed by CT arteriography (follow‐up: 30 days to 3 months) | 1820 | RR 1.81 | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of PE. | |
| 3 per 1000 | 6 per 1000 (2 to 22) | |||||
| Symptomatic DVT: assessed by presence of symptoms and confirmed by a positive compression ultrasound (follow‐up: 30 days to 3 months) | 1848 | RR 0.61 | Study population | ⊕⊕⊝⊝
| LMWH may lead to little or no difference in symptomatic DVT. | |
| 11 per 1000 | 7 per 1000 (2 to 22) | |||||
| Asymptomatic DVT: assessed with compression ultrasound (follow‐up: 30 days to 8 weeks) | 369 | RR 0.14 | Study population | ⊕⊝⊝⊝ | It is uncertain if LMWH reduces the risk asymptomatic DVT. | |
| 81 per 1000 | 11 per 1000 (2 to 49) | |||||
| All‐cause mortality (follow‐up: 30 days to 3 months) | 1978 | See comment | Not estimable | No deaths occurred in any of the included studies. | ||
| All adverse effects, including allergies at injection site, minor and major bleeding, others (follow‐up: 30 days to 3 months) | 1978 | RR 1.85 | Study population | ⊕⊕⊕⊝ Moderatea | LMWH probably leads to little or no difference in adverse effects. | |
| 13 per 1000 | 24 per 1000 | |||||
| Major bleeding (follow‐up: 30 days to 3 months) | 1451 | RR 0.98 (0.06 to 15.72). | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of major bleeding. | |
| 1 per 1000 | 1 per 1000 (0 to 22) | |||||
| Minor bleeding (follow‐up: 30 days to 3 months) | 1978 | RR 1.79 | Study population | ⊕⊕⊕⊝ | LMWH probably leads to little or no difference in rates of minor bleeding. | |
| 11 per 1000 | 20 per 1000 (9 to 43) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pulmonary embolism Show forest plot | 3 | 1820 | Risk Ratio (IV, Fixed, 95% CI) | 1.81 [0.49, 6.65] |
| 1.2 Symptomatic deep vein thrombosis Show forest plot | 4 | 1848 | Risk Ratio (IV, Fixed, 95% CI) | 0.61 [0.18, 2.03] |
| 1.3 Asymptomatic deep vein thrombosis Show forest plot | 2 | 369 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.03, 0.61] |
| 1.4 Adverse effects Show forest plot | 5 | 1978 | Risk Ratio (IV, Fixed, 95% CI) | 1.85 [0.95, 3.59] |
| 1.5 Major bleeding Show forest plot | 1 | 1451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.72] |
| 1.6 Minor bleeding Show forest plot | 5 | 1978 | Risk Ratio (IV, Fixed, 95% CI) | 1.79 [0.84, 3.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Symptomatic deep vein thrombosis Show forest plot | 1 | 234 | Risk Ratio (IV, Fixed, 95% CI) | 0.16 [0.02, 1.29] |
| 2.2 Asymptomatic deep vein thrombosis Show forest plot | 1 | 234 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.06, 15.01] |
| 2.3 Minor bleeding Show forest plot | 1 | 234 | Risk Ratio (IV, Fixed, 95% CI) | 0.63 [0.18, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Pulmonary embolism Show forest plot | 1 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.05] |
| 3.2 Symptomatic deep vein thrombosis Show forest plot | 1 | 1317 | Risk Ratio (IV, Fixed, 95% CI) | 0.17 [0.04, 0.75] |
| 3.3 Asymptomatic deep vein thrombosis Show forest plot | 1 | 1317 | Risk Ratio (IV, Fixed, 95% CI) | 0.47 [0.21, 1.09] |
| 3.4 Adverse effects Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Pain, tenderness and edema | 1 | 1317 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.95, 1.43] |
| 3.4.2 Major bleeding | 1 | 1317 | Risk Ratio (IV, Fixed, 95% CI) | 3.01 [0.61, 14.88] |
| 3.4.3 Minor bleeding | 1 | 1317 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [0.64, 2.08] |

