Compresión neumática intermitente de la pierna y profilaxis farmacológica combinadas para la profilaxis de la tromboembolia venosa en pacientes de alto riesgo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: controlled clinical trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: unfractionated heparin (5000 iu BID, subcutaneously) and sequential compression devices with elastic stockings | |
| Outcomes | Symptomatic PE, confirmed with ventilation‐perfusion scan | |
| Notes | The study was planned to be randomized but due to administrative errors the randomisation protocol was violated Sequential compression devices were started in the operating room and discontinued when the patients were ambulatory, usually 18 hours postoperatively Heparin was started two hours before the operation and was continued for 7 days or the time of discharge Study was discontinued because of bleeding complications associated with heparin use. No specific bleeding definitions were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study was planned to be randomized but due to administrative errors the randomization protocol was violated. Method of planned randomizations was stated as patient order |
| Allocation concealment (selection bias) | High risk | Alternating patients received the study medication and in most cases the surgeon was aware of which patients received heparin |
| Blinding of participants and personnel (performance bias) | High risk | In most cases the surgeon was aware of which patients received heparin, and the same perhaps applies to the anesthesia personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing the pulmonary ventilation‐perfusion scans or angiograms were aware of which patients received heparin |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | PE was the only VTE event stated in methodology and was reported |
| Other bias | Unclear risk | No baseline characteristics, apart from age, were provided |
| Methods | Study design: controlled clinical trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: sequential compression devices and pharmacological prophylaxis (unfractionated heparin or coumadin) | |
| Outcomes | DVT diagnosed with I‐125 fibrinogen scanning, IPG, Doppler ultrasound and venography | |
| Notes | Patients who received aspirin or dextran as an exclusive pharmacological modality or elastic stockings as an exclusive mechanical modality were not included in our review All modalities were started with the preoperative medication and continued until the patients were well ambulatory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were placed into each category in rotation by the vascular technicians |
| Allocation concealment (selection bias) | High risk | No details on the allocation procedure were provided |
| Blinding of participants and personnel (performance bias) | High risk | Placebo medications or devices were not used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing DVT testing were aware of which patients received heparin |
| Incomplete outcome data (attrition bias) | Low risk | All patients had an event reported |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE event stated in methodology and was reported |
| Other bias | Unclear risk | No baseline characteristics were provided |
| Methods | Study design: controlled clinical trial | |
| Participants | Country: UK | |
| Interventions | Intervention group: unfractionated heparin (5000 iu BID, subcutaneously), graduated compression stockings (TEDs), and pneumatic foot compression on the side to be operated on | |
| Outcomes | DVT on bilateral lower extremity venography performed postoperative day 12 | |
| Notes | The foot pump started at the beginning of surgery and continued until discharge from the hospital. No details were provided for heparin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients with an even date of birth were randomized to receive IPC |
| Allocation concealment (selection bias) | High risk | Patients with an even date of birth were allocated to receive IPC ‐ allocation therefore predictable |
| Blinding of participants and personnel (performance bias) | High risk | No placebo device was used |
| Blinding of outcome assessment (detection bias) | Low risk | The radiologist who read the venograms was blinded to patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE outcome event stated in methodology and was reported |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: subcutaneous heparin injections (5000 iu BID) combined with the use of a thigh‐length sequential pneumatic compression device (Kendall Health Care, Manchester, Mass, USA) Control groups: | |
| Outcomes | DVT on duplex ultrasound and also clinically evident DVT and PE | |
| Notes | Investigation on the effect of study interventions on fibrinolytic activity, but also reported VTE outcomes DVT prophylaxis was initiated in the operating room after induction of anesthesia and continued until postoperative day 5 (or discharge, if this occurred sooner). If the patient remained hospitalized after postoperative day 5, DVT prophylaxis was left to the discretion of the primary surgeon, and the patient was no longer participating in the research study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | Not provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo anticoagulants or IPC devices were used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel who performed the DVT screening were blinded to the treatment regimens |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE outcome event stated in methodology and was reported |
| Other bias | Low risk | No significant baseline imbalances |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: Enoxaparin (Lovenox; Rhône‐Poulenc Rorer Pharmaceuticals) subcutaneously at a dose of 30 mg in the anesthesia holding room, and continued at a dose of 30 mg BID combined with thigh‐high SCDs (Kendall), a type of IPC, functioning on the patient before induction of anesthesia | |
| Outcomes | DVT on duplex ultrasonography between days 1 and 3, between days 5 and 7, at the wound check appointment between days 10 and 14, and at the 1‐month follow‐up appointment Incidence of adverse events (including bleeding) was assessed by principal investigator by thorough review of medical records. No specific bleeding definitions were provided | |
| Notes | The IPC devices functioned throughout the surgical procedure and remained on the patient during the postoperative period, until the patient was walking without assistance. If the patient remained nonambulatory, the devices were discontinued at the time of discharge from the Neurosurgery Service Enoxaparin was started in the anesthesia holding room and was discontinued at the time of discharge from the Neurosurgery Service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | Not provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo anticoagulants or IPC devices were used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel who performed the DVT screening were blinded to the treatment regimens |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE outcome event stated in methodology and was reported |
| Other bias | High risk | The trial stopped early (enrolment was planned for 120 subjects) |
| Methods | Study design: randomized controlled trial Losses to follow up: none | |
| Participants | Country: USA | |
| Interventions | Intervention group: Enoxaparin (30 mg BID, starting the morning after surgery for 7 ‐ 8 days) combined with IPC (CECT device, ActiveCare DVT; Medical Compression Systems, Or Akiva, Israel) with calf sleeves Control group: Enoxaparin (30 mg BID, starting the morning after surgery for 7 ‐ 8 days) | |
| Outcomes | DVT on duplex ultrasonography before discharge and also clinically evident DVT and PE at three months | |
| Notes | IPC was placed on the calves of the patient in the operating room and continued during hospitalisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | Not provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo devices were used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel who performed the DVT screening were blinded to the treatment regimens |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT and PE were VTE events stated in methodology and were reported |
| Other bias | High risk | A large number of post‐randomization exclusions |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Germany | |
| Interventions | Intervention group: LMWH, certoparin (3000 iu 12 hours pre‐op, 12 post‐op then daily, subcutaneously), compression stockings (18 to 20 mmHg), and rapid‐inflation intermittent pneumatic compression | |
| Outcomes | Symptomatic DVT and DVT on duplex‐color coded ultrasound performed on the day of discharge | |
| Notes | "The DVT prophylaxis regimen was randomly assigned in the operating theatre at the time of completion of surgery and the randomisation was stratified by age." No information on PE was provided Patients in the intermittent pneumatic compression group had the intermittent pneumatic compression system applied to both calves in the recovery room shortly after the completion of surgery. Intermittent pneumatic compression therapy was applied daily during the time that the patient was confined to bed postoperatively, and it was terminated at the time that the patient was able to walk LMWH was started 12 hours preoperatively and continued throughout hospitalization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided, apart from the information that it was stratified by patient age, so that an assumption that a computer generated sequence or the sealed envelope method was used may be made |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo device was used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel who performed the DVT screening were blinded to the treatment regimens |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE event stated in methodology and was reported |
| Other bias | Low risk | Baseline number of risk factors for deep venous thrombosis per patient were comparable |
| Methods | Study design: quasi‐randomized controlled trial | |
| Participants | Country: Turkey | |
| Interventions | Intervention group: LMWH (40 mg/day) combined with IPC | |
| Outcomes | DVT on duplex ultrasonography and clinically evident DVT and PE | |
| Notes | Information on randomization and blinding was obtained from the study authors. No information on start and discontinuation of IPC or LMWH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized trial, randomized by the last digit of year of birth |
| Allocation concealment (selection bias) | High risk | Quasi‐randomized trial so predictable |
| Blinding of participants and personnel (performance bias) | High risk | No placebo anticoagulants were used |
| Blinding of outcome assessment (detection bias) | Low risk | The radiologist who performed the ultrasound tests was not aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | All study participants were reported in the results section |
| Selective reporting (reporting bias) | Low risk | DVT and PE were the VTE events stated in methodology and results were provided |
| Other bias | Unclear risk | Insufficient details were provided to allow a conclusion to be made |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: unfractionated heparin (5000 iu BID, subcutaneously) and sequential compression devices | |
| Outcomes | Symptomatic PE, confirmed by ventilation perfusion scan and/or pulmonary angiography | |
| Notes | Both prophylactic methods were started immediately after surgery and continued for 4 to 5 days or until patients were fully ambulatory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo device was used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing the pulmonary ventilation‐perfusion scans or angiograms were aware of which patients used a compression device |
| Incomplete outcome data (attrition bias) | High risk | A large number of patients were excluded after randomization |
| Selective reporting (reporting bias) | Low risk | PE was the only VTE event stated in methodology and was reported |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Japan | |
| Interventions | Intervention group: Edoxaban (15 mg or 30 mg OD) and a foot pump (A‐V Impulse System foot pump) Control group: Edoxaban (15 mg or 30 mg OD) | |
| Outcomes | Symptomatic VTE by postoperative day 28 and asymptomatic DVT on compression ultrasonography on the postoperative day 10 Bleeding: major bleeding was defined as wound hematoma or hemorrhage occurring at a critical site and bleeding required for > 2 units of red blood cell concentrates. Minor bleeding was defined as bleeding that did not fulfil the criteria for major bleeding | |
| Notes | Both groups also used bilateral knee‐high antithromboembolic stockings The foot pump was activated in the recovery room and used for four days Edoxaban started 12 hours postoperatively and was used for a mean of 11.5 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | High risk | Sealed enveloped contained the randomization slip, but no statement that these were opaque |
| Blinding of participants and personnel (performance bias) | High risk | No placebo device was used |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was provided on who performed the ultrasound and if that person was blinded to patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | Minimal losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | DVT and PE were VTE events stated in methodology and were reported |
| Other bias | High risk | Trial stopped prematurely |
| Methods | Study design: controlled clinical trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: unfractionated heparin (5000 iu BID, subcutaneously) and sequential compression devices | |
| Outcomes | Symptomatic DVT or PE | |
| Notes | Participants were assigned to heparin and control groups by the primary surgeon Sequential compressive stockings were placed at the time of surgery and left in place for 48 hours after surgery for all patients Heparin was started preoperatively and continued for three days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Allocation concealment (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Blinding of participants and personnel (performance bias) | High risk | No placebo injection for heparin was given |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | DVT and PE were VTE events stated in methodology and were reported |
| Other bias | Unclear risk | Insufficient details were provided to allow a conclusion to be made |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Germany | |
| Interventions | Intervention group: LMWH, enoxaparin (40 mg daily, subcutaneously) and pneumatic sequential compression | |
| Outcomes | Symptomatic and asymptomatic DVT (on ultrasound) | |
| Notes | The calf cuffs were applied to both lower limbs directly after the operation in the recovery room and the system was activated. The use of the IPC was continued until the tenth postoperative day whenever the patient was in bed Enoxaparin was started the evening before surgery and continued for 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | A placebo device was not used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Color duplex ultrasonography was performed by an independent angiologist who was unaware of the patients' participation in the study or of the method of prophylaxis, but only to confirm the findings of compression ultrasonography, which was not reported to be performed by a blinded or not observer, hence unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Eight patients who were randomized were subsequently excluded (two from the LMWH/IPC and six from the LMWH/GCS group) for various reasons, but they represent a small percentage of the total patient number, unlikely to change they results and conclusions whatever their outcome might have been |
| Selective reporting (reporting bias) | Low risk | Thromboembolic (VTE) events were stated in methodology to be the outcome measures of the study and they were reported as such |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Italy | |
| Interventions | Intervention group: IPC + UFH | |
| Outcomes | DVT on venography | |
| Notes | No information on start and discontinuation of IPC or UFH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | A placebo device was not used |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | DVT was the only VTE event stated in methodology and was reported |
| Other bias | Unclear risk | Insufficient details were provided to allow a conclusion to be made |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Korea | |
| Interventions | Intervention group: IPC combined with enoxaparin 40 mg OD | |
| Outcomes | DVT on duplex ultrasound but also clinically evident DVT and PE Bleeding: major and minor, no specific bleeding definitions provided | |
| Notes | Interim analysis The IPC was initiated preoperatively and continued until postoperative discharge Enoxaparin started postoperatively but the exact time of start and discontinuation was not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐randomized treatment assignments |
| Allocation concealment (selection bias) | High risk | Sequential sealed envelopes, but no statement that these were opaque |
| Blinding of participants and personnel (performance bias) | High risk | No placebo injection was given |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | High risk | A relatively large number of patients in the combined group did not have duplex ultrasonography |
| Selective reporting (reporting bias) | Low risk | DVT and PE were stated in methodology to be the outcome measures of the study and results were reported |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Heparin/aspirin versus intermittent foot compression versus combined heparin/aspirin and intermittent foot compression | |
| Outcomes | Asymptomatic DVT, symptomatic DVT, any DVT, PE | |
| Notes | The pumps were started in the recovery room immediately after surgery and used until the end of the study, with the exact time not specified Heparin was started 8 hours before the operation and after 3 days of use it was replaced with 325 mg aspirin twice daily for an undefined duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo for compression, heparin and aspirin |
| Blinding of outcome assessment (detection bias) | Low risk | All duplex results and venograms were read by one of the authors who was blinded to the prophylactic modality used |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | DVT was stated in methodology to be the outcome measure of the study and results were reported |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: controlled clinical trial | |
| Participants | Country: Japan | |
| Interventions | Intervention group: IPC (stopped 24 hours after surgery) combined with fondaparinux (subcutaneous injections of fondaparinux at 2.5 mg OD) | |
| Outcomes | Clinically evident DVT and PE Bleeding: major bleeding was defined as bleeding that was fatal, retroperitoneal, intracranial, involving any other critical organ, led to intervention being discontinued, or was associated with a need for transfusion of more than 3 units of packed red blood cell. Other types of bleeding was included and defined as bleeding that did not fulfil the criteria for major bleeding | |
| Notes | IPC was used for 24 hours after surgery, but no information on when it was started Fondaparinux was started 24 hours after surgery and was continued until days 5‐7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Allocation concealment (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Blinding of participants and personnel (performance bias) | High risk | No placebo for fondaparinux |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled patients had results reported |
| Selective reporting (reporting bias) | Low risk | Thromboembolic events (DVT and PE) were stated in methodology to be the outcome measures of the study and they were reported as such |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized, double‐blind, placebo‐controlled, superiority trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: fondaparinux and intermittent pneumatic compression | |
| Outcomes | Venous thromboembolism (defined as DVT detected by mandatory screening and/or documented symptomatic DVT or PE, or both) and individual components up to day 10. Symptomatic venous thromboembolism up to day 10 and day 32 Major bleeding (defined as bleeding that was fatal, retroperitoneal, intracranial, or involved any other critical organ, led to intervention being discontinued, or was associated with a bleeding index of 2.0 or more) detected during the treatment period Death during the treatment period and up to day 32 | |
| Notes | Study medications were packaged in boxes of identical appearance During the on‐study‐drug period of 5–9 days, all patients were to receive venous thromboembolism prophylaxis with intermittent pneumatic compression using any type of device, except a foot pump, for a duration left to the investigator's discretion. The first injection of fondaparinux or placebo was scheduled 6–8 h after surgical closure, provided that hemostasis was achieved. The duration of the on‐study‐drug period was 5–9 days. If the patient was discharged from hospital before completing the on‐study‐drug period, a visiting nurse administered the remaining trial injections | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized computer‐generated schedule randomization (1:1 randomization in blocks of four and stratified by centre) |
| Allocation concealment (selection bias) | Low risk | Centralized computer‐generated schedule randomization |
| Blinding of participants and personnel (performance bias) | Low risk | Use of placebo injections |
| Blinding of outcome assessment (detection bias) | Low risk | Reports double‐blind (use of placebo injections) but it is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | High risk | A large number of exclusions in both trial arms, around 35% of the total number of participants, mainly because of lack of mandatory or interpretable venography |
| Selective reporting (reporting bias) | Low risk | DVT and PE were the primary efficacy outcomes and they were reported in the results |
| Other bias | Low risk | Demographic variables and risk factors at baseline, type of anesthesia, and type and duration of surgery were similar in the two groups among both randomized and treated patients (Tables 1 and 2) and among patients analysed for primary efficacy |
| Methods | Study design: controlled clinical trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: pneumatic sequential compression and warfarin | |
| Outcomes | DVT on ultrasound of the ipsilateral lower external iliac, common femoral, superficial femoral, deep femoral, and popliteal veins | |
| Notes | No symptomatic VTE was observed Three patients on warfarin developed bleeding complications The IPC device was applied over the duration of the patient's preoperative and postoperative stay until the time of discharge. Patients sent to a rehabilitation center were told to continue using the IPC until their final discharge home. Warfarin or aspirin started on the night before surgery but no duration of use was provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Allocation concealment (selection bias) | High risk | The type of the study (CCT) makes it high risk for selection bias |
| Blinding of participants and personnel (performance bias) | High risk | Not a double‐blind study |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions/participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | DVT and PE were the main study outcomes and they were reported in the results |
| Other bias | Unclear risk | Insufficient details were provided to allow a conclusion to be made |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: pneumatic sequential compression and enoxaparin | |
| Outcomes | DVT on ultrasound before discharge on postoperative days 3 to 5, and 4 to 6 weeks after surgery | |
| Notes | Bleeding complications were documented, no specific bleeding definitions provided Upon their arrival in the recovery room, the patients received a VenaFlow calf compression device that was placed on both of their lower extremities. The compression device was used during each patient's entire hospital stay Enoxaparin was initiated 2 hours after epidural catheter removal (approx. 48 hours postoperatively). Patients received 30 mg of enoxaparin twice daily until their hospital discharge; upon discharge, their dosage was changed to 40 mg once daily for 3 weeks. Aspirin started on the night of their surgery in the recovery room and was continued for 4 weeks postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | Not a blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | High risk | A large number of patients were lost to follow‐up, likely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | DVT was the main study outcome and was reported in the results |
| Other bias | Low risk | Baseline characteristics were comparable |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Germany | |
| Interventions | Intervention group: IPC (foot pump) combined with enoxaparin (40 mg OD, beginning 24 hours prior to the operation) | |
| Outcomes | DVT on duplex ultrasonography, but also clinically evident DVT and PE | |
| Notes | Reports none of the participants needed to be operated upon for hemarthrosis, no other details regarding bleeding were provided The AVI system was attached in the recovery room to both feet of the participants only shortly after completion of the operation; patients were free to discontinue its use at will Enoxaparin was started 24 hours before surgery, duration was not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | A placebo device was not used |
| Blinding of outcome assessment (detection bias) | Low risk | Sonographers were unaware of treatment allocations |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions/patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | DVT and PE were VTE events stated in methodology and were reported |
| Other bias | Low risk | There were no baseline imbalances |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: USA | |
| Interventions | Intervention group: pneumatic sequential compression, thigh‐high graduated elastic compression stockings, and warfarin (one group); or pneumatic sequential compression, thigh‐high graduated elastic compression stockings, and aspirin (second group) | |
| Outcomes | Proximal DVT on venography, B‐mode ultrasonography, or both, on discharge | |
| Notes | Warfarin dose was 7.5 or 10 mg orally on the evening before the operation, then titrated to maintain the prothrombin time at 1.2 to 1.3 times the control value. Aspirin started the evening before surgery and continued at a dose of 650 mg twice daily. For both agents duration of use was not reported IPC was started in the operating theater, as soon as the patient was draped and used discharge Bleeding: one patient in each of the three groups had a wound hematoma, that required evacuation in the two intervention group patients but not in the control group. No specific definition of bleeding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of sealed envelope method |
| Allocation concealment (selection bias) | Unclear risk | Does not mention if the sealed envelopes were sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) | High risk | Not a blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear if the personnel performing diagnostic testing were aware of patient allocation |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | DVT was the main study outcome and was reported in the results |
| Other bias | Low risk | There were no baseline imbalances |
| Methods | Study design: randomized controlled trial | |
| Participants | Country: Japan | |
| Interventions | Intervention group: 1. Enoxaparin (20 mg BID) + IPC 2. Fondaparinux (2.5 mg OD) + IPC | |
| Outcomes | DVT on duplex ultrasonography and also clinically evident DVT and PE Any bleeding, both major or minor. Major bleeding: retro‐peritoneal, intracranial or intraocular, or if associated with either death, transfusion of more than two units of packed red blood cells or whole blood (except autologous), a reduction in the level of hemoglobin of > 2 g/dL, or a serious or life‐threatening clinical event requiring medical intervention. Suspected intra‐abdominal or intracranial bleeding was confirmed by ultrasonography, CT or MRI Minor bleeding: epistaxis lasting for more than five minutes or requiring intervention, ecchymosis or hematoma with a maximum size of > 5 cm, hematuria not associated with trauma from the urinary catheter, gastrointestinal hemorrhage not related to intubation or the passage of a nasogastric tube, a wound hematoma or hemorrhagic wound complications not associated with major hemorrhage or subconjunctival hemorrhage, requiring cessation of medication | |
| Notes | The pneumatic devices were initiated in the operating theater (before surgery for the contralateral leg and just after surgery for the operated leg) and removed on the "second post‐operative day when the day of surgery was defined as post‐operative day 1" Pharmacological prophylaxis was started postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Scans were read by experiences radiologist blinded to randomisation |
| Incomplete outcome data (attrition bias) | Low risk | A small percentage of exclusions (5/255, 2%) |
| Selective reporting (reporting bias) | Low risk | DVT and PE were the main study outcomes and they were reported in the results |
| Other bias | Low risk | Baseline characteristics were comparable |
BID: twice daily
DVT: deep vein thrombosis
IPC: intermittent pneumatic compression
IPG: impedence plethysmography
iu: international units
LMWH: low molecular weight heparin
mg: milligrams
NYHA: New York Hospital Association
OD: once daily
PE: pulmonary embolism
THA: total hip arthroplasty
THR: total hip replacement
TKR: total knee replacement
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Retrospective case‐control study | |
| Use of combined modalities was not concurrent in the intervention group | |
| Controlled before and after study | |
| Registry study, non‐randomized | |
| Pharmacological prophylaxis was not the same in the two study groups | |
| Controlled before and after study | |
| Investigation restricted to intraoperative period up to 10 min after extubation | |
| The control (single modality) group included patients who were allocated to heparin or pneumatic compression | |
| Pharmacological prophylaxis consisted of aspirin, which has limited thromboprophylactic properties | |
| Pharmacological prophylaxis was not the same in the two study groups | |
| Only aggregated VTE rates and not separate DVT and PE rates were provided and the authors did not reply when individual data were requested | |
| Prospective case‐control study | |
| Retrospective study | |
| Pneumatic compression was used only intraoperatively | |
| Pharmacological prophylaxis was not the same in the two study groups | |
| Use of enoxaparin was not concurrent in the two study groups | |
| Controlled before and after study | |
| Retrospective study | |
| Pharmacological prophylaxis consisted of aspirin, which has limited thromboprophylactic properties | |
| Retrospective case‐control study investigating preoperative anticoagulation in patients on postoperative low molecular‐weight heparin and SCDs | |
| Retrospective case‐control study |
DVT: deep vein thrombosis
PE: pulmonary embolism
SCD: sequential compression device
VTE: venous thromboembolism
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The mechanical and medical prevention of lower extremity deep venous thrombosis formation post gynecologic pelvic surgery, a multiple center randomized case control study |
| Methods | Randomized parallel controlled trial |
| Participants | Women undergoing gynecologic pelvic surgery |
| Interventions | GCS: graduated compression stockings; GCS + LMWH: graduated compression stockings + low molecular weight heparin; GCS + IPC: graduated compression stockings + intermittent pneumatic compression; GCS + LMWH + IPC: graduated compression stockings + low molecular weight heparin + intermittent pneumatic compression |
| Outcomes | DVT on ultrasound of the leg veins (primary); hemoglobin; white blood cell count; hematocrit; platelets; PT; APTT; Fbg; TT; D‐Dimer; AT‐III; t‐PA; PAI; VIII factor; X factor; Protein c; Protein s; CTPA (all secondary) |
| Starting date | November 2015 |
| Contact information | Cuiqin Sang, 22 South Sanlitun Road, Beijing, China |
| Notes | Target sample size: GCS: 250; GCS + LMWH: 250; GCS + IPC: 250; GCS + LMWH + IPC: 250 |
| Trial name or title | The PREVENT Trial: pneumatic compression for PREventing VENous Thromboembolism |
| Methods | RCT in ICU patients already receiving anticoagulants |
| Participants | No further information is provided |
| Interventions | Patients were randomized to use IPC or not |
| Outcomes | Incidence of proximal leg DVT up to 30 days (primary), PE up to 30 days (secondary), ICU and hospital mortality (secondary) |
| Starting date | December 2013 |
| Contact information | Dr Yaseen Arabi |
| Notes | Trial completed, no longer recruiting |
| Trial name or title | Efficacy of the association mechanical prophylaxis plus anticoagulant prophylaxis on venous thromboembolism incidence in intensive care unit (ICU) (CIREA2) |
| Methods | RCT in ICU patients without high risk of bleeding |
| Participants | 621 ICU patients |
| Interventions | Patients were randomized to use IPC or not |
| Outcomes | Primary outcome measures: combined criterion evaluated at day 6 ± 2 days after randomization: symptomatic venous thromboembolic event, non‐fatal, objectively confirmed; death related to PE; asymptomatic DVT of the lower limbs detected by CUS on day 6 (time frame: 6 ± 2 days) |
| Starting date | October 2007 |
| Contact information | Karine Lacut, MD. CHU Brest France, Univ Brest, EA 3878 |
| Notes | Study completed in January 2015, with no results being presented or published at the time of writing this review |
APTT: activated partial thromboplastin time
CTPA: computed tomography pulmonary angiogram
CUS: colour ultrasound
DVT: deep vein thrombosis
Fbg: fibrinogen
GCS: graduated compression stockings
ICU: intensive care unit
IPC: intermittent pneumatic compression
LMWH: low molecular weight heparin
PAI: plasminogen activator inhibitor
PE: pulmonary embolism
PT: prothrombin time
RCT: randomized controlled trial
TT: thrombin time
t‐PA: tissue plasminogen activator
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 12 | 3017 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| Analysis 1.1  Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| Analysis 1.2 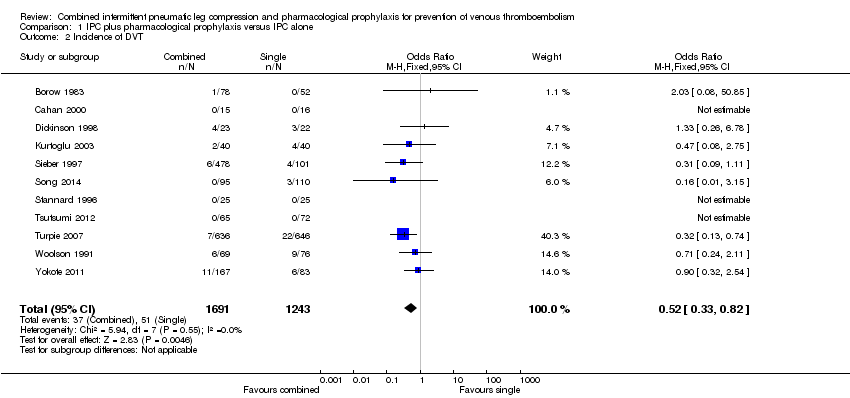 Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 2 Incidence of DVT. | ||||
| 3 Incidence of symptomatic DVT Show forest plot | 6 | 2526 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.47] |
| Analysis 1.3 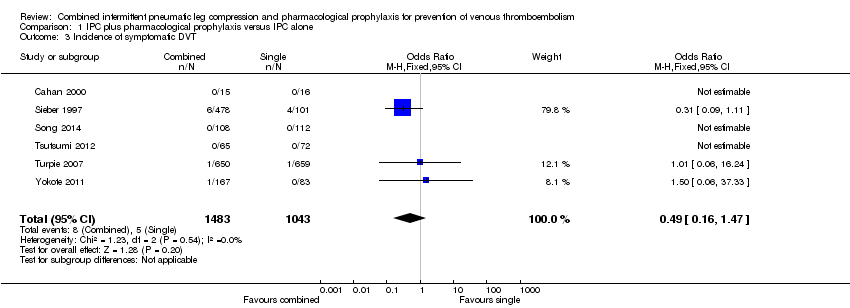 Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 3 Incidence of symptomatic DVT. | ||||
| 4 Incidence of DVT by foot IPC or other IPC Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| Analysis 1.4 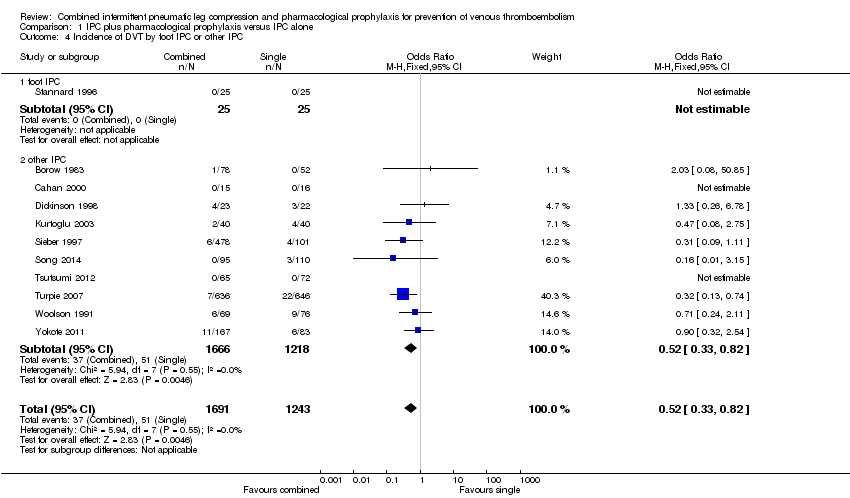 Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 4 Incidence of DVT by foot IPC or other IPC. | ||||
| 4.1 foot IPC | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 other IPC | 10 | 2884 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 5 Incidence of bleeding Show forest plot | 7 | 2155 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.04 [2.36, 10.77] |
| Analysis 1.5  Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 5 Incidence of bleeding. | ||||
| 6 Incidence of major bleeding Show forest plot | 7 | 2155 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.81 [1.99, 23.28] |
| Analysis 1.6  Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 6 Incidence of major bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 10 | 3544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.64] |
| Analysis 2.1  Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| Analysis 2.2 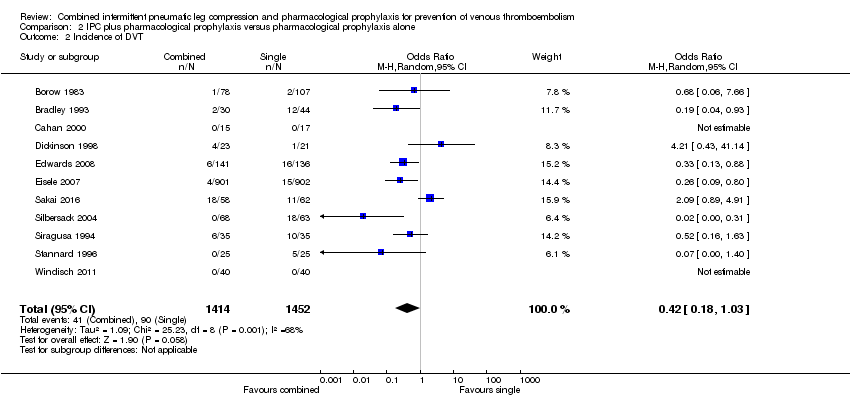 Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 2 Incidence of DVT. | ||||
| 3 Incidence of symptomatic DVT Show forest plot | 5 | 2312 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.29, 3.54] |
| Analysis 2.3  Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 3 Incidence of symptomatic DVT. | ||||
| 4 Incidence of DVT by foot IPC or other IPC Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| Analysis 2.4  Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 4 Incidence of DVT by foot IPC or other IPC. | ||||
| 4.1 foot IPC | 4 | 324 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.47] |
| 4.2 other IPC | 7 | 2542 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.16, 0.96] |
| 5 Incidence of bleeding Show forest plot | 3 | 244 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.30, 2.14] |
| Analysis 2.5  Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 5 Incidence of bleeding. | ||||
| 6 Incidence of major bleeding Show forest plot | 3 | 244 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.35, 4.18] |
| Analysis 2.6  Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 6 Incidence of major bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 3 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| Analysis 3.1  Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 3 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.42] |
| Analysis 3.2 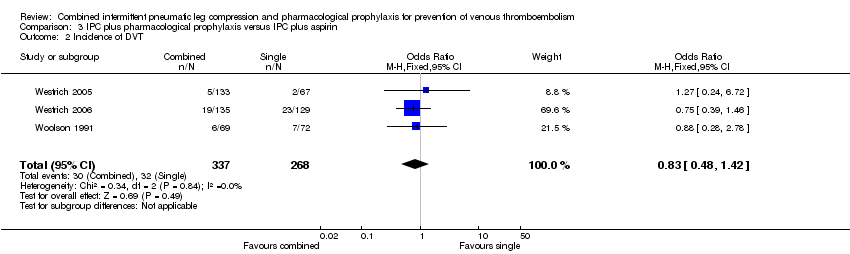 Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 2 Incidence of DVT. | ||||
| 3 Incidence of symptomatic DVT Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 3 Incidence of symptomatic DVT. | ||||
| 4 Incidence of bleeding Show forest plot | 3 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.27, 5.53] |
| Analysis 3.4  Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 4 Incidence of bleeding. | ||||
| 5 Incidence of major bleeding Show forest plot | 3 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.15, 4.17] |
| Analysis 3.5 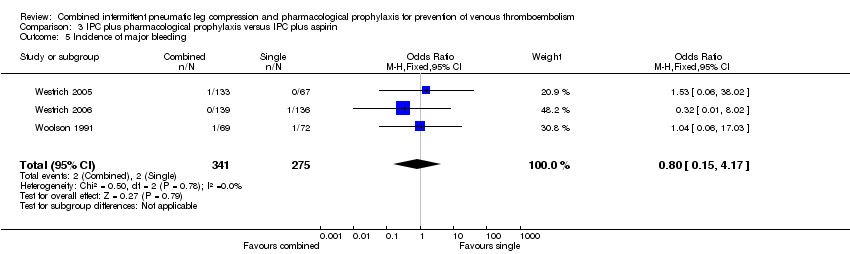 Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 5 Incidence of major bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 12 | 3017 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| Analysis 4.1  Comparison 4 IPC plus pharmacological prophylaxis versus IPC alone ‐ subgroups, Outcome 1 Incidence of PE. | ||||
| 1.1 Orthopedic patients | 3 | 445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Non‐orthopedic patients | 9 | 2572 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| 2 Incidence of DVT Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| Analysis 4.2  Comparison 4 IPC plus pharmacological prophylaxis versus IPC alone ‐ subgroups, Outcome 2 Incidence of DVT. | ||||
| 2.1 Orthopedic patients | 3 | 445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.69] |
| 2.2 Non‐orthopedic patients | 8 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 10 | 3544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.64] |
| Analysis 5.1 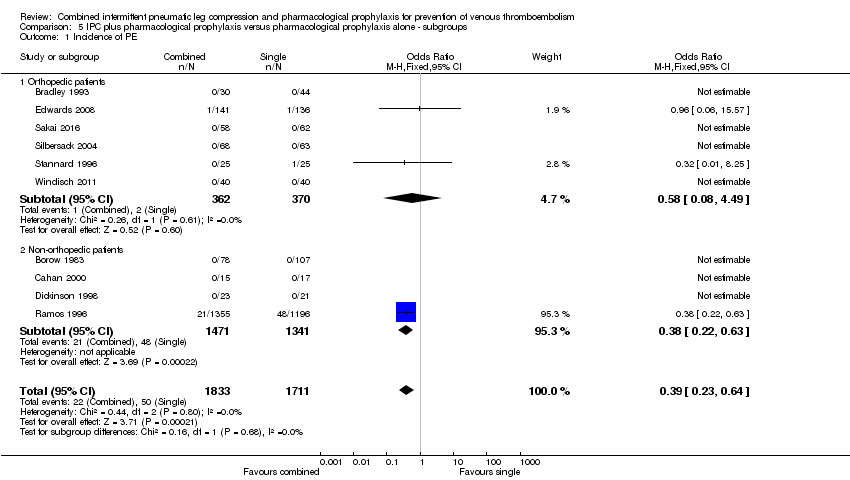 Comparison 5 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ subgroups, Outcome 1 Incidence of PE. | ||||
| 1.1 Orthopedic patients | 6 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.08, 4.49] |
| 1.2 Non‐orthopedic patients | 4 | 2812 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.63] |
| 2 Incidence of DVT Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| Analysis 5.2  Comparison 5 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ subgroups, Outcome 2 Incidence of DVT. | ||||
| 2.1 Orthopedic patients | 8 | 2605 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.86] |
| 2.2 Non‐orthopedic patients | 3 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.30, 10.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 7 | 2023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.76] |
| Analysis 6.1  Comparison 6 IPC plus pharmacological prophylaxis versus IPC alone ‐ RCTs only, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 7 | 2008 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.90] |
| Analysis 6.2 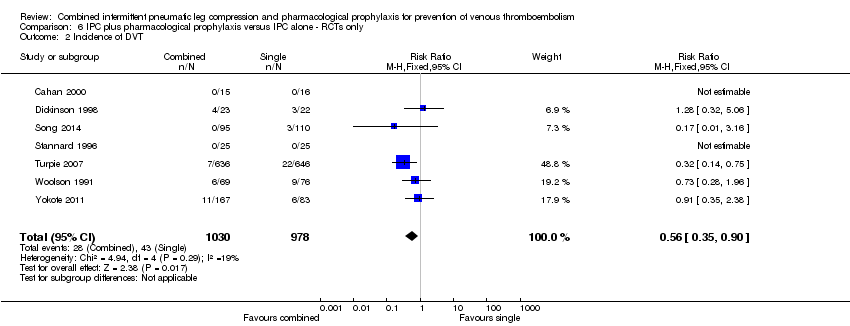 Comparison 6 IPC plus pharmacological prophylaxis versus IPC alone ‐ RCTs only, Outcome 2 Incidence of DVT. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 8 | 3285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.65] |
| Analysis 7.1 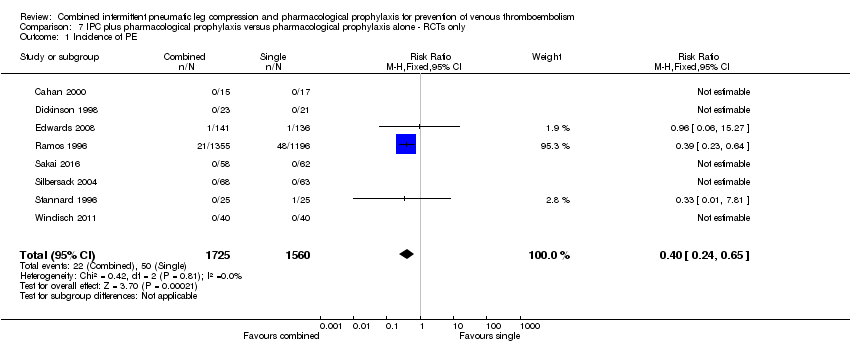 Comparison 7 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ RCTs only, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 9 | 2607 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.19, 1.26] |
| Analysis 7.2  Comparison 7 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ RCTs only, Outcome 2 Incidence of DVT. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.17] |
| Analysis 8.1  Comparison 8 IPC plus pharmacological prophylaxis versus IPC plus aspirin ‐ RCTs only, Outcome 1 Incidence of PE. | ||||
| 2 Incidence of DVT Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.33] |
| Analysis 8.2 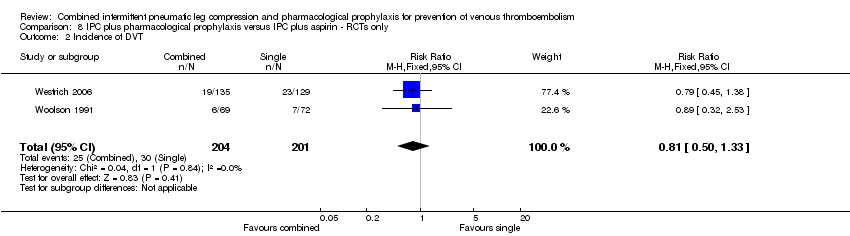 Comparison 8 IPC plus pharmacological prophylaxis versus IPC plus aspirin ‐ RCTs only, Outcome 2 Incidence of DVT. | ||||
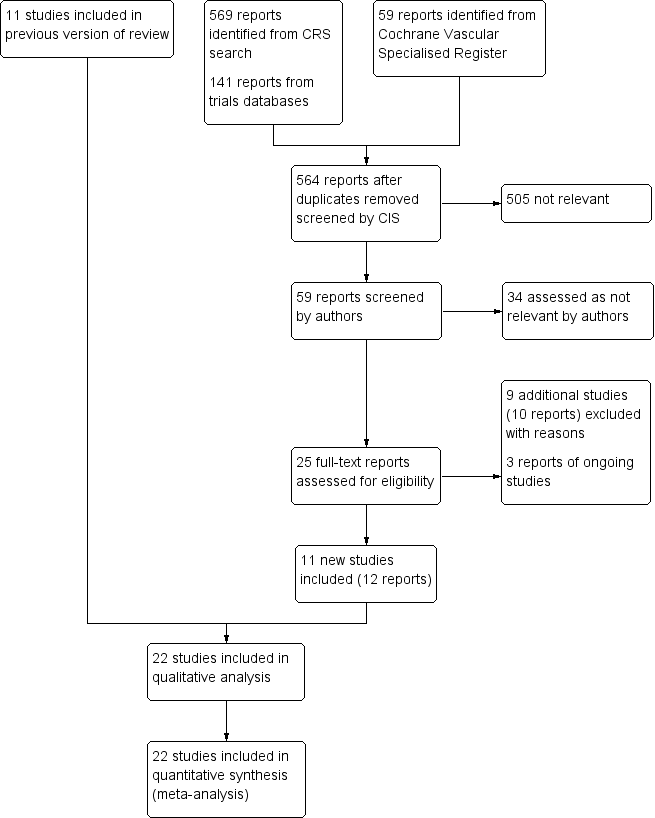
Study flow diagram.
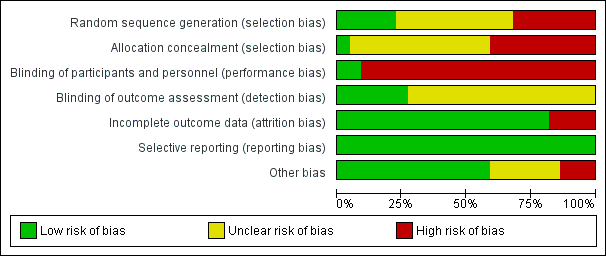
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
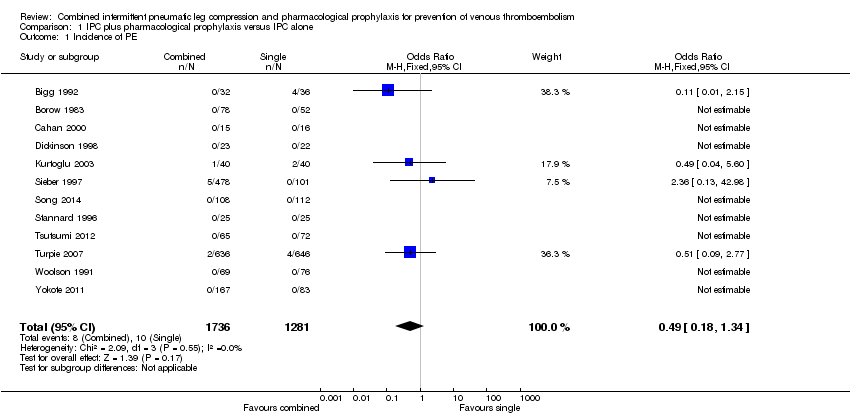
Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 1 Incidence of PE.
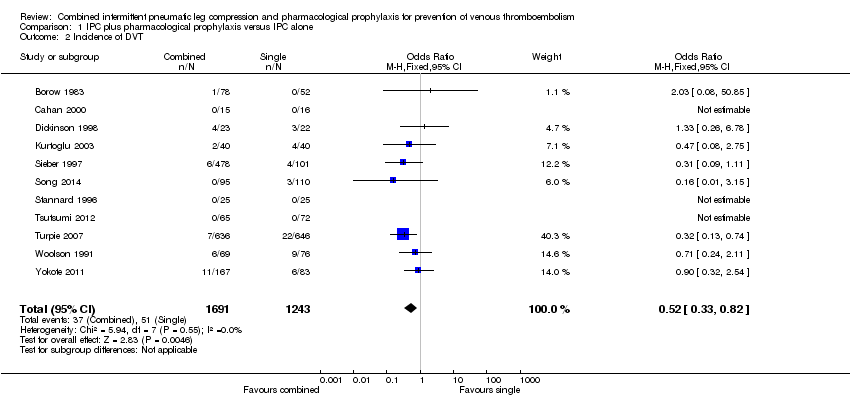
Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 2 Incidence of DVT.
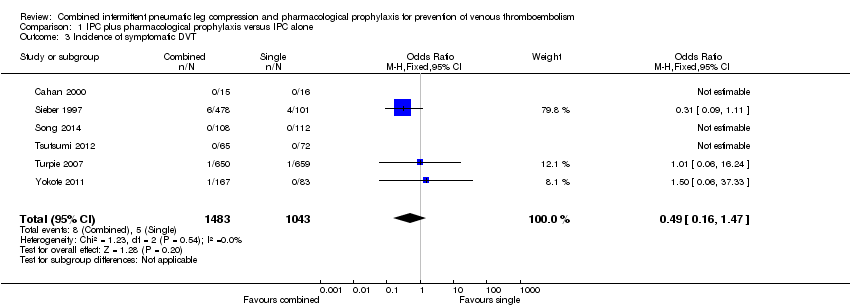
Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 3 Incidence of symptomatic DVT.
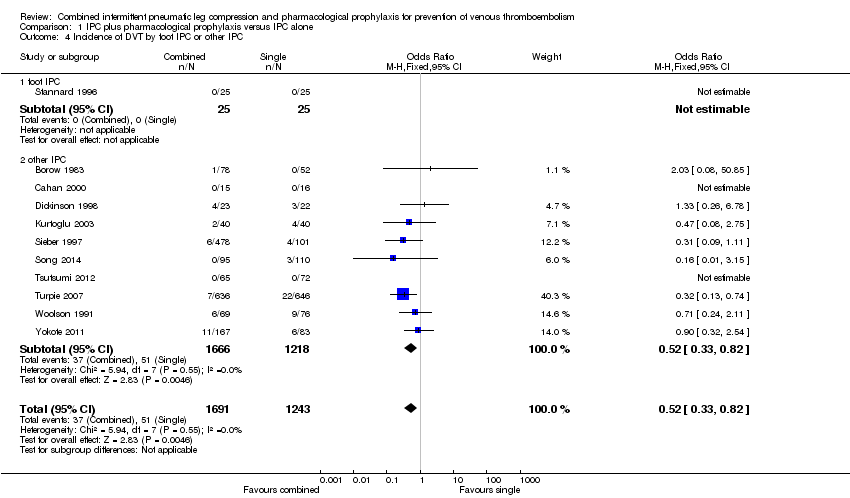
Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 4 Incidence of DVT by foot IPC or other IPC.
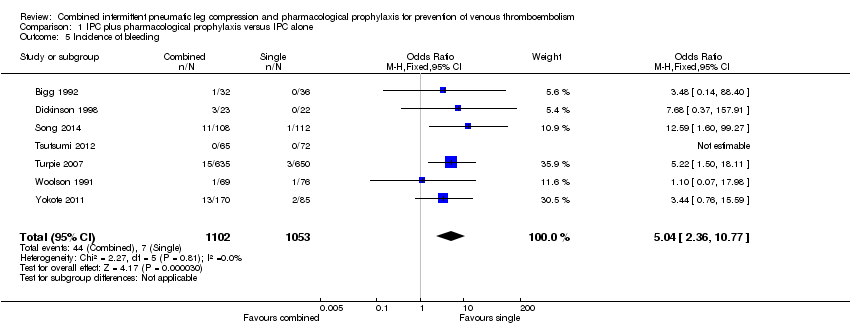
Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 5 Incidence of bleeding.

Comparison 1 IPC plus pharmacological prophylaxis versus IPC alone, Outcome 6 Incidence of major bleeding.

Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 1 Incidence of PE.

Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 2 Incidence of DVT.
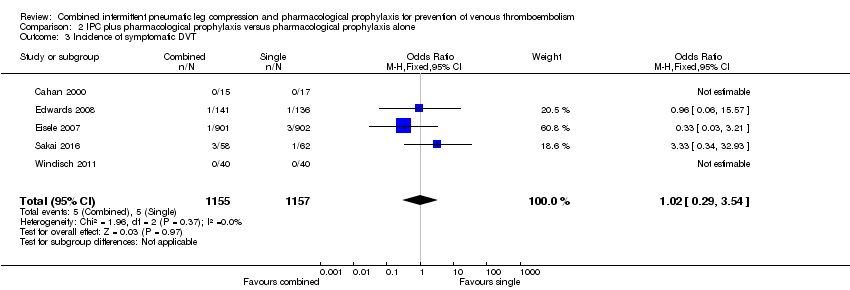
Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 3 Incidence of symptomatic DVT.
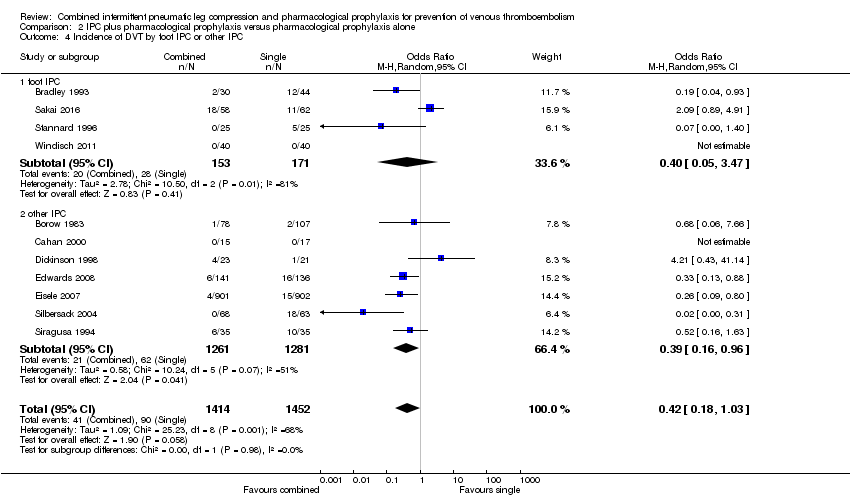
Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 4 Incidence of DVT by foot IPC or other IPC.

Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 5 Incidence of bleeding.

Comparison 2 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone, Outcome 6 Incidence of major bleeding.

Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 1 Incidence of PE.

Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 2 Incidence of DVT.

Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 3 Incidence of symptomatic DVT.

Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 4 Incidence of bleeding.
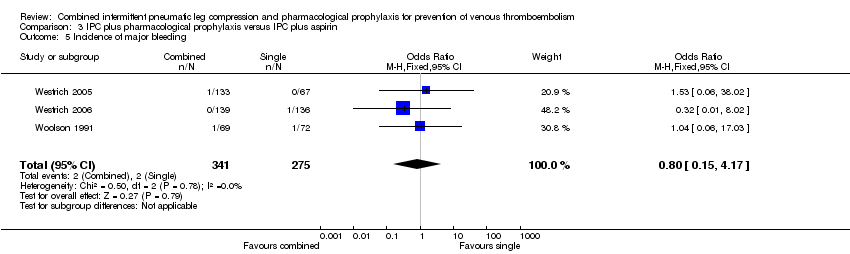
Comparison 3 IPC plus pharmacological prophylaxis versus IPC plus aspirin, Outcome 5 Incidence of major bleeding.
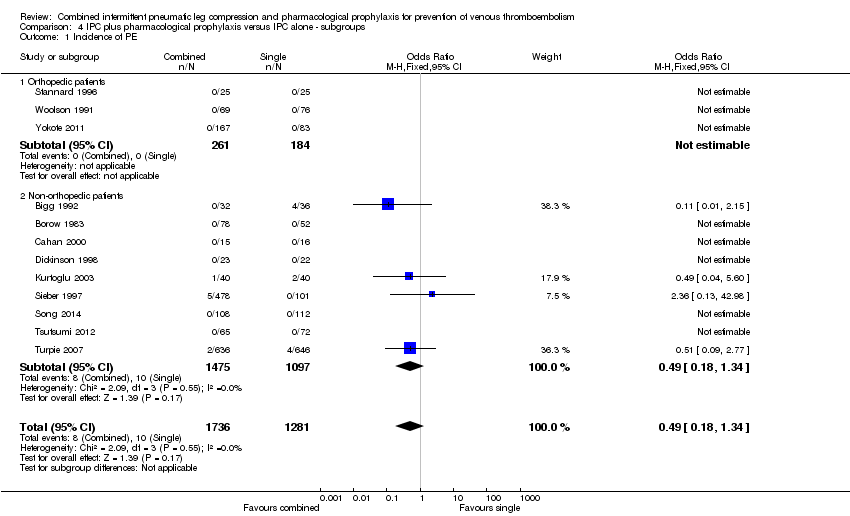
Comparison 4 IPC plus pharmacological prophylaxis versus IPC alone ‐ subgroups, Outcome 1 Incidence of PE.

Comparison 4 IPC plus pharmacological prophylaxis versus IPC alone ‐ subgroups, Outcome 2 Incidence of DVT.

Comparison 5 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ subgroups, Outcome 1 Incidence of PE.

Comparison 5 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ subgroups, Outcome 2 Incidence of DVT.
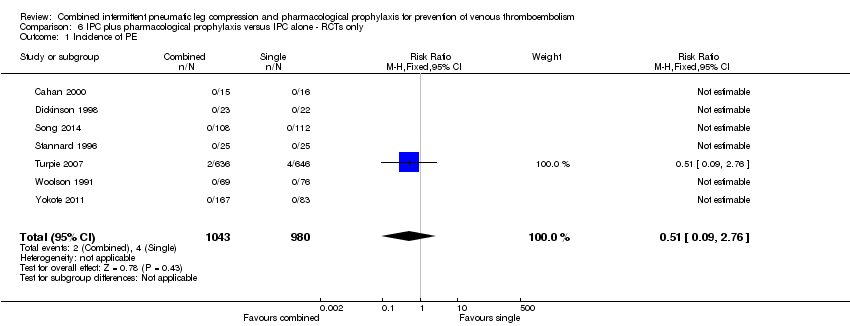
Comparison 6 IPC plus pharmacological prophylaxis versus IPC alone ‐ RCTs only, Outcome 1 Incidence of PE.
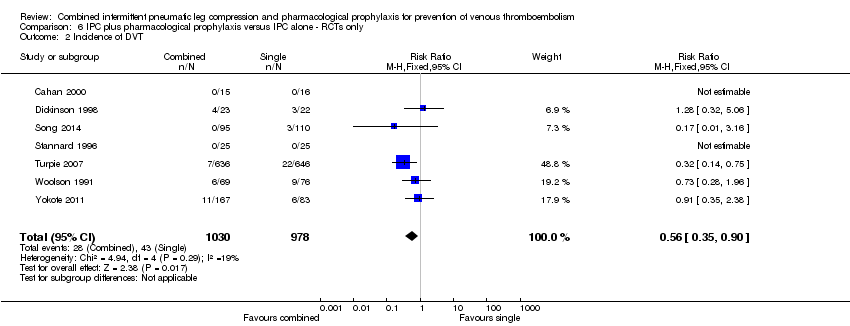
Comparison 6 IPC plus pharmacological prophylaxis versus IPC alone ‐ RCTs only, Outcome 2 Incidence of DVT.
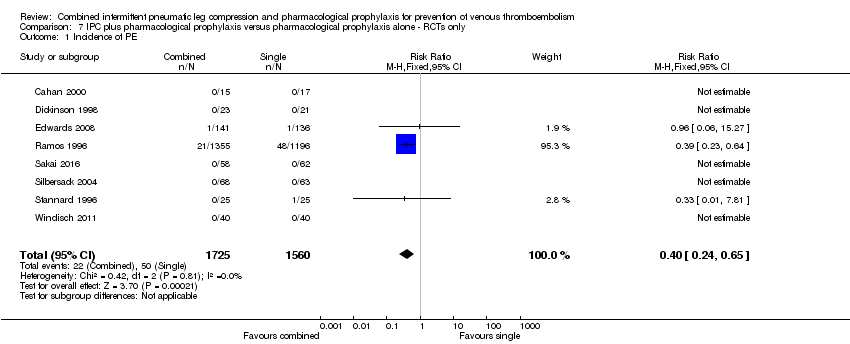
Comparison 7 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ RCTs only, Outcome 1 Incidence of PE.

Comparison 7 IPC plus pharmacological prophylaxis versus pharmacological prophylaxis alone ‐ RCTs only, Outcome 2 Incidence of DVT.

Comparison 8 IPC plus pharmacological prophylaxis versus IPC plus aspirin ‐ RCTs only, Outcome 1 Incidence of PE.
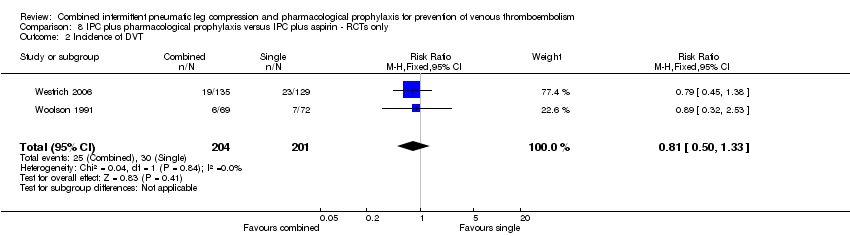
Comparison 8 IPC plus pharmacological prophylaxis versus IPC plus aspirin ‐ RCTs only, Outcome 2 Incidence of DVT.
| Does combined intermittent pneumatic compression (IPC) plus pharmacological prophylaxis increase prevention of venous thromboembolism compared with IPC alone? | ||||||
| Patient or population: patients undergoing surgery or at risk of developing VTE because of other reasons (e.g. trauma) Settings: hospital (surgery, trauma or ICU stay) Intervention: combined IPC plus pharmacological prophylaxis Comparison: IPC alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single modalities | Combined modalities | |||||
| Incidence of PEa | 8 per 1000 | 4 per 1000 (1 to 10) | OR 0.49 (0.18 to 1.34) | 3017 (12) | ⊕⊕⊕⊝ | |
| Incidence of DVTb | 41 per 1000 | 22 per 1000 (14 to 34) | OR 0.52 (0.33 to 0.82) | 2934 (11) | ⊕⊕⊕⊝ | |
| Incidence of bleedingc | 7 per 1000 | 33 per 1000 (16 to 67) | OR 5.04 (2.36 to 10.77) | 2155 (7) | ⊕⊕⊕⊝ moderate3 | |
| Incidence of major bleedingd | 1 per 1000 | 6 per 1000 (2 to 22) | OR 6.81 (1.99 to 23.28) | 2155 (7) | ⊕⊕⊕⊝ moderate3 | |
| * The basis for the assumed risk was the average risk in the single modalities group (i.e. the number of participants with events divided by total number of participants of the single modalities group included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| a PE assessed by pulmonary angiography or scintigraphy, computed tomography (CT), angiography, or autopsy | ||||||
| Does combined intermittent pneumatic compression (IPC) plus pharmacological prophylaxis increase prevention of venous thromboembolism compared with pharmacological prophylaxis alone? | ||||||
| Patient or population: patients undergoing surgery or at risk of developing VTE because of other reasons (e.g. trauma) Settings: hospital (surgery, trauma or ICU stay) Intervention: combined IPC plus pharmacological prophylaxis Comparison: pharmacological prophylaxis alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single modalities | Combined modalities | |||||
| Incidence of PEa | 29 per 1000 | 12 per 1000 (7 to 19) | OR 0.39 (0.23 to 0.64) | 3544 (10) | ⊕⊕⊕⊝ | |
| Incidence of DVTb | 62 per 1000 | 27 per 1000 (12 to 64) | OR 0.42 (0.18 to 1.03) | 2866 (11) | ⊕⊕⊕⊝ | |
| Incidence of bleedingc | 81 per 1000 | 66 per 1000 (26 to 159) | OR 0.8 (0.3 to 2.14 | 244 (3) | ⊕⊝⊝⊝ very low3 | |
| Incidence of major bleedingd | 41 per 1000 | 49 per 1000 (15 to 150) | OR 1.21 (0.35 to 4.18) | 244 (3) | ⊕⊝⊝⊝ very low3 | |
| *The basis for the assumed risk was the average risk in the single modalities group (i.e. the number of participants with events divided by total number of participants of the single modalities group included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| a PE assessed by pulmonary angiography or scintigraphy, computed tomography (CT), angiography, or autopsy 1 Downgraded by one level due to risk of detection and attrition bias affecting effect estimate as shown by sensitivity analysis | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 12 | 3017 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| 2 Incidence of DVT Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 3 Incidence of symptomatic DVT Show forest plot | 6 | 2526 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.47] |
| 4 Incidence of DVT by foot IPC or other IPC Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 4.1 foot IPC | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 other IPC | 10 | 2884 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 5 Incidence of bleeding Show forest plot | 7 | 2155 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.04 [2.36, 10.77] |
| 6 Incidence of major bleeding Show forest plot | 7 | 2155 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.81 [1.99, 23.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 10 | 3544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.64] |
| 2 Incidence of DVT Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| 3 Incidence of symptomatic DVT Show forest plot | 5 | 2312 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.29, 3.54] |
| 4 Incidence of DVT by foot IPC or other IPC Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| 4.1 foot IPC | 4 | 324 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.47] |
| 4.2 other IPC | 7 | 2542 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.16, 0.96] |
| 5 Incidence of bleeding Show forest plot | 3 | 244 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.30, 2.14] |
| 6 Incidence of major bleeding Show forest plot | 3 | 244 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.35, 4.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 3 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 2 Incidence of DVT Show forest plot | 3 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.42] |
| 3 Incidence of symptomatic DVT Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Incidence of bleeding Show forest plot | 3 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.27, 5.53] |
| 5 Incidence of major bleeding Show forest plot | 3 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.15, 4.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 12 | 3017 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| 1.1 Orthopedic patients | 3 | 445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Non‐orthopedic patients | 9 | 2572 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.34] |
| 2 Incidence of DVT Show forest plot | 11 | 2934 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 2.1 Orthopedic patients | 3 | 445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.69] |
| 2.2 Non‐orthopedic patients | 8 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 10 | 3544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.64] |
| 1.1 Orthopedic patients | 6 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.08, 4.49] |
| 1.2 Non‐orthopedic patients | 4 | 2812 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.63] |
| 2 Incidence of DVT Show forest plot | 11 | 2866 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.03] |
| 2.1 Orthopedic patients | 8 | 2605 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.86] |
| 2.2 Non‐orthopedic patients | 3 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.30, 10.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 7 | 2023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.76] |
| 2 Incidence of DVT Show forest plot | 7 | 2008 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 8 | 3285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.65] |
| 2 Incidence of DVT Show forest plot | 9 | 2607 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.19, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of PE Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.17] |
| 2 Incidence of DVT Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.33] |

