Mengelakkan penggunaan botol semasa memulakan penyusuan susu ibu pada bayi pramatang
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, stratified by gestational age < 28 weeks and 28 to < 34 weeks and by study centre. Study duration ‐ 3 years, 1996 to 1999 | |
| Participants | Two Australian Neonatal Intensive Care Units Inclusion criteria: gestational age < 34 weeks (experimental: mean 29.4 weeks, SD 2.6, range 23 to 33; control: mean 30.0 weeks, SD 2.5, range 24 to 33), mother wishes to breast feed, infant had not been fed by cup or bottle, no congenital abnormality precluding sucking feeds, dummy use ≤ 48 hours Sample size: 319 randomised (161 experimental/cup, 158 control/bottle). 303 included in analysis (151 experimental/cup, 152 control/bottle) | |
| Interventions | Randomised to cup/no dummy, cup/dummy, bottle/no dummy, bottle/dummy Experimental: supplementary feeds given by cup according to Lang 1994b recommendations; 60 mL medicine cup used Control: supplementary feeds given by bottle Both groups: Infants breast fed when mother was present; cup/bottle was used in addition to nasogastric tube. | |
| Outcomes | Breast feeding prevalence any and full at discharge, and 'all' and any at 3 and 6 months; days to all sucking feeds; length of hospitalisation; weight gain from birth to discharge home; necrotising enterocolitis | |
| Notes | Initial analyses showed no clinically important nor significant interaction between use of cups and dummies; therefore, additional comparisons were performed on marginal groups with cup vs bottle. High proportion of non‐compliance: experimental group: 85/151 (46%) had a bottle introduced; control group: 1/152 (0.7%) had a cup introduced. Participants were analysed in the groups to which they were randomised regardless of the intervention they actually received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent researcher developed a separate randomisation schedule for each recruiting hospital by using a random number table to select balanced blocks of varying size with stratification for gestation (< 28 weeks, 28 ‐ < 34 weeks)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignments were sealed in sequentially numbered, opaque envelopes. Researchers determined allocation by telephoning an independent ward, available 24 hours a day, within the recruiting hospitals". |
| Blinding (performance bias and detection bias) | High risk | Quote: "Particpants, care providers, and researchers were not blinded to treatment allocation; data entry and analysis were undertaken unblinded". Comment: blinding of intervention not possible |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 16, 5%) due to attrition (experimental 10, control 6):
Comment: low risk of bias due to incomplete outcome data |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 36, 11%) due to attrition (experimental 17, control 19):
Comment: low risk of bias due to incomplete outcome data |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 38, 12%) due to attrition (experimental 19, control 19):
Comment: low risk of bias due to incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Before clinical trial registration requirements; however, outcomes reported as per PhD thesis |
| Other bias | Low risk | |
| Methods | Randomised controlled trial, stratified by gestational age < 31 weeks and 31 to < 35 weeks. Study duration ‐ 2 years, 2002 to 2004 | |
| Participants | Single centre, Neonatal Intensive Care Unit, UK Inclusion criteria: < 35 weeks' gestation (experimental: median 31 weeks, range 25 to 34; control: median 32 weeks, range 26 to 34 weeks), > 30 weeks' postmenstrual age at trial entry, ability to tolerate full strength, full volume of nasogastric feeds for 48 hours or longer, anticipated stay ≥ 1 week, mother's intention to breast feed Sample size: 54 randomised, 54 included in analysis (additional information from study author). Number randomised to each group: 27 (experimental/cup), 27 (control/bottle) | |
| Interventions | Experimental: supplementary feeds given by cup when mother not present to breast feed Control: supplementary feeds given by bottle when mother not present to breast feed Both groups: Infants breast fed when mother was present; cup/bottle was used in addition to nasogastric tube. | |
| Outcomes | Breast feeding prevalence any and full on discharge home, at term and at 6 weeks post term; postmenstrual age at nasogastric tube withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomized, non‐blinded stratified controlled trial" Comment: unable to determine whether sequence generation was adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was by selection of concealed cards in envelopes, stratified by gestation" |
| Blinding (performance bias and detection bias) | High risk | Quote: “randomized, non‐blinded stratified controlled trial" Quote (from correspondence): "No one was blinded in the study once the envelope was opened". Comment: blinding of intervention not possible |
| Incomplete outcome data (attrition bias) | Low risk | 3 infants not accounted for in paper, additional information provided by study author 14 women counted as withdrawals in the paper, as they no longer wanted to breast feed. With additional information from study author, reanalysed in this review Comment: outcome data complete |
| Selective reporting (reporting bias) | Low risk | Before clinical trial registration requirements, all expected outcomes were reported. |
| Other bias | Low risk | Nil noted |
| Methods | Randomised controlled trial. Study duration ‐ 22 months | |
| Participants | Single centre, Neonatal Intensive Care Unit, USA Inclusion criteria: birth weight 1000 g to 2500 g, < 1 week of age, no congenital or neurological abnormalities that interfered with cardiopulmonary status Gestational age at birth ‐ experimental: 32 weeks, SD not reported, range 26 to 35 weeks; control: 32 weeks, SD not reported, range 28 to 35 weeks; birth weight ‐ experimental: 1.73 kg, range 1.05 kg to 2.43 kg; control: 1.64 kg, range 1.0 kg to 2.35 kg; twins ‐ experimental: 8 (21%); control: 16 (35%) Sample size: 99 randomised (47 experimental/tube alone, 52 control/bottle); 84 included in analysis (38 experimental/tube alone, 46 control/bottle) | |
| Interventions | Both groups of infants breast fed when mother was present. Experimental group: feeds given by indwelling size 3.5 FG nasogastric tube when mother not available, or top‐up after breast feed required. Tube was removed during last 24 to 48 hours of parent 'rooming‐in' period; a cup or syringe was used during this time if needed. Control group: fed by bottle when mother not available, or top‐up after breast feed required. Indwelling nasogastric tube was removed as directed by clinicians. | |
| Outcomes | Breast feeding, exclusive and partial, at discharge home, and at 3 days, 3 months and 6 months post discharge. Length of hospital stay, apnoea/bradycardia, weight gain to discharge home, infection rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by using sealed envelopes, which were physically mixed and drawn in random sequence after enrolment of the dyad into the study". |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed envelopes" |
| Blinding (performance bias and detection bias) | High risk | Comment: Blinding of intervention not possible. Blinding of outcome assessment not reported |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data (n = 15, 15%) (experimental 9, control 6):
Comment: high risk of bias due to incomplete outcome data. Difference in proportion of missing data across groups (19% experimental, 12% control). For 4 infants, valid reasons were given for missing outcome data (1 died, 2 were transferred to another hospital). |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data (n = 15, 15%) (experimental 9, control 6):
Comment: high risk of bias due to incomplete outcome data |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data (n = 15, 15%) (experimental 9, control 6):
Comment: high risk of bias due to incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Before clinical trial registration requirements, all expected outcomes reported |
| Other bias | Low risk | Nil noted |
| Methods | Randomised controlled trial, pilot study. Study duration ‐ 3 months | |
| Participants | Single centre, Special Care Baby Unit, District General Hospital, England Inclusion criteria: gestational age 32 to 37 weeks, mother wishes to breast feed, no congenital abnormality, no maternal preference for cup or bottle, infant had not been fed by cup or bottle Experimental group: mean gestational age 35.5 weeks, SD not reported; control group: 35.2 weeks, SD not reported Sample size: 16 randomised (8 experimental/cup, 8 control/bottle); 14 included in analysis (6 experimental/cup, 8 control/bottle) | |
| Interventions | Experimental: supplementary feeds given by cup Control: supplementary feeds given by bottle | |
| Outcomes | Prevalence exclusive breast feeding on discharge home | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "There were 10 instructions to cup feed and ten to bottle feed. These details were then put in the envelopes, shuffled thoroughly and then the envelopes were numbered sequentially". |
| Allocation concealment (selection bias) | Low risk | Quote: "Midwife/nurse responsible was asked to select a sealed, numbered, opaque envelope, which contained information on the feeding method to be adopted". |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind intervention. No information provided on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 2, 13%) (experimental 2, control 0):
Comment: Although difference in proportion of incomplete outcome data was noted across groups (25% experimental, 0% control), the sample size was so small that we are unable to sensibly assess the impact of missing data. Low risk of bias due to incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Before clinical trial registration requirements, all expected outcomes reported |
| Other bias | Low risk | Nil noted |
| Methods | Randomised controlled trial, stratified by weight (500 to 999 g, 1000 to 1499 g, 1500 to 1699 g). Study duration ‐ 18 months, August 1998 to February 2000 | |
| Participants | Single centre, Neonatal Intensive Care Unit, University Hospital, Brazil Inclusion criteria: gestational age at birth 32 to 34 weeks (experimental: mean 32.7 weeks, SD 1.8, range not reported; control: mean 32.5 weeks, SD 2, range not reported) and birth weight < 1700g (experimental: mean 1276 g, SD 283 g; control: mean 1262 g, SD 270 g), mothers wished to breast feed, clinically stable, not initially on parenteral nutrition Sample size: 83 randomised (46 experimental/cup, 37 control/bottle); 78 included in analysis (44 experimental/cup, 34 control/bottle) | |
| Interventions | Infants in both groups fed by orogastric tube until 1600 g. Experimental: supplements or complements given by cup according to the recommendations of Kuehl 1997 and Lang 1994a. Dummy not offered. Control: supplements or complements given by bottle | |
| Outcomes | Breast feeding prevalence on discharge, at first follow‐up visit and at 3 months post discharge. Weight gain (calculated as the difference between weight at the beginning of the intervention and weight at the end of 1 week during feeding observation, reported in g/kg/d). Length of feeding time (1 week after beginning oral feeds). Oxygen saturation Breast feeding was defined as an infant exclusively or partially breast fed directly at the breast. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ". controlled experimental study with stratified randomisation"; "Within each stratum, the infants were randomly assigned to 1 of 2 feeding groups by drawing lots". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Infants were randomly assigned to 1 of 2 feeding groups by drawing lots". Comment: Mechanism for drawing of lots not reported, therefore unclear whether allocation was concealed |
| Blinding (performance bias and detection bias) | High risk | Blinding of intervention not possible. Blinding of outcome assessment not reported |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 5, 6%) (experimental 2, control 3):
Comment: low risk of bias due to incomplete outcome data. Small difference in proportions of missing data across groups, although protocol violations only in experimental group (4% experimental, 8% control). Overall small proportion of missing data (6%) |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 5, 6%) (experimental 2, control 3):
Comment: low risk of bias due to incomplete outcome data. Small difference in proportions of missing data across groups, although protocol violations only in experimental group (4% experimental, 8% control). Overall small proportion of missing data (6%) |
| Selective reporting (reporting bias) | Unclear risk | Before clinical trial registration requirements, all expected outcomes reported |
| Other bias | Low risk | |
| Methods | Randomised controlled trial, stratified by 25 to 29 weeks' and 30 to 33 weeks' gestational age. Twins randomised to same group. Conducted from 1 August 2011 to 30 June 2012 | |
| Participants | Single centre, Neonatal Intensive Care Unit, Australia Inclusion criteria: gestational age 25 to 34 weeks (experimental: 30.1, SD 2.7 weeks, birth weight 1310, SD 422 g; control: 30.1, SD 2.6 weeks, birth weight 1430, SD 507 g); mother intended to breast feed; required 75% enteral feeds by intragastric tube with remainder provided by parental nutrition Exclusion criteria: congenital anomalies, grade 4 intracerebral haemorrhage, periventricular leukomalacia, oral anomalies (e.g. ankyloglossia, cleft palate) Sample size: 100 randomised (54 experimental/novel teat, 46 control/bottle with conventional teat), 97 included in analysis (51 experimental/novel teat, 46 control/bottle with conventional teat) | |
| Interventions | Bottles were offered only if a bottle feed was scheduled, and duration of feed was limited to 30 minutes. Non‐nutritive sucking encouraged up to 33 weeks before suck feeds, after which increasing suck feeds replaced non‐nutritive sucking Experimental: a feeding system (Medela AG, Baar, Switzerland) that combined strategies known to improve oral feeding skills: development of vacuum and self paced feeding. A shut‐off valve incorporated in the system to ensure that milk flowed only when infant created a vacuum; venting prevented collapse of the teat. Two different threshold levels for the valve of ‐10, SD 5 mmHg and ‐30, SD 15 mmHg Control: conventional teat that allowed milk flow with gravity and compression of the teat (Grow, Growbaby, Icon Health, Victoria, Australia, or Peristaltic Narrow Neck Slow Flow, Pigeon, Seoul, South Korea) | |
| Outcomes | Primary outcomes: time to first and full suck feeds, length of hospital stay, breast feeding (full and any) at discharge Secondary outcomes: breast feeding rates (full and any) at 3, 6 and 12 weeks post discharge, late‐onset sepsis | |
| Notes | The manufacturer of the feeding system (Medela AG, Baar, Switzerland) provides an unrestricted research grant from which the salaries of 2 authors were paid; the research nurse was partially funded by the manufacturer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " ....computer generated treatment allocation..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed opaque coded envelopes containing the computer generated treatment allocation |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind families and staff. Analysis done by biostatistician who was not involved in data collection and was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data (n = 3, 3%) (experimental 3, control 0):
Comment: low risk of bias due to incomplete outcome data |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Selective reporting (reporting bias) | Low risk | Prospectivley registered on clinical trial register, all outcomes reported |
| Other bias | Low risk | Nil noted |
| Methods | Randomised controlled trial with stratification by gestation (gestational age stratification category not stated) Study conducted April 2006 to February 2008 | |
| Participants | Three Neonatal Intensive Care Units, Turkey Inclusion criteria: singleton birth, 32 to 35 weeks’ gestation (experimental: gestation 32.8, SD 0.9 weeks, birth weight 1539, SD 332; control: 32.8, SD 0.9, birth weight 1547, SD 330), maternal intention to breast feed, no supplemental oxygen required, fed intermittently by gastric tube only at the time of recruitment Exclusion criteria: no prerandomisation exclusion criteria stated. Infants excluded post randomisation have been listed in the exclusion criteria (development of a disease that prevented oral feeding for more than 2 consecutive days and non‐compliance with assigned feed method). Sample size: 607 randomised (299 experimental/cup, 308 control/bottle); 522 included in analysis (254 experimental/cup, 268 control/bottle | |
| Interventions | Infants in both randomised groups were breast fed whenever the mother was available; mothers were welcome to stay in the NICU 24 hours a day and had access to a comfortable chair/recliner, bed, or mattress while nursing. If supplementation required once home, the same assigned method was used (cup or bottle). Experimental: supplementary feeds (formula or breast milk) given by cup (small plastic medicine cup) by NICU nurses or parents who had been trained in the cup feeding technique described by Lang 1994a Control: supplementary feeds (formula or breast milk) given by bottle by nursing staff or parents | |
| Outcomes | Primary: weight gain (g/d) at day 7 of study; proportion of exclusively or any breast fed infants on discharge home Secondary: length of hospital stay and proportion of exclusive or any breast feeding at 3 and 6 months of age. Also reported feeding time (min/feeding during first week of study for cup or bottle) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Separate randomisation schedule for each recruiting hospital by using a random number table to select balanced blocks of varying size with stratification for gestation” |
| Allocation concealment (selection bias) | Low risk | Quote: “Assignments were sealed in sequentially numbered, opaque envelopes”. |
| Blinding (performance bias and detection bias) | High risk | Unable to blind assigned treatment groups Primary outcome data collected by researcher from data recorded in medical records Secondary outcome assessment data collection at 3 and 6 months post discharge collected at home visit, not blinded |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data: 85/607 (14%) (45/299, 15%, experimental (cup); and 40/308, 13%, control (bottle)):
Missing outcome data: 14%; reasons missing similar between groups |
| Incomplete outcome data (attrition bias) | Low risk | No further missing data ‐ as for discharge home |
| Incomplete outcome data (attrition bias) | Low risk | No further missing data ‐ as for discharge home |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not reported in manuscript. All expected outcomes reported |
| Other bias | Low risk | Nil noted |
FG: French gauge.
NEC: necrotising enterocolitis.
NICU: neonatal intensive care unit.
SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomised. Thirty infants received usual practice (bottle supplementation), and the next 30 the intervention (supplementation using cup feeds). | |
| Randomised cross‐over study. Infants fed by paladai or bottle on consecutive feeds. The aim of the study was to assess amount of spillage, volume consumed, time taken and physiological stability during both a cup feed and a bottle feed. | |
| Not randomised. Aim was to determine effects of spoon feeding compared with bottle feeding on breast feeding success. Conducted in 2 neonatal intensive care units ‐ 1 that used bottle feeds and 1 that used spoon feeds | |
| Not randomised, a retrospective study | |
| Involves non‐nutritive sucking only, not related to mode of sucking feeds nor to breast feeding outcomes | |
| Randomised groups do not include a bottle group; nasogastric tube alone compared with spoon feeding | |
| Involves sucking and swallowing exercises, not related to mode of sucking feeds nor to breast feeding outcomes | |
| Randomised cross‐over trial. Assessed swallowing and spilling when fed by cup and by bottle during first sucking feed only; did not include breast feeding outcomes | |
| Randomised groups do not include a bottle group, have compared feeding by cup with feeding by paladay (paladai) | |
| Qualitative study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Four‐group, parallel, randomised controlled trial |
| Participants | 132 infants born at 26 to 36 weeks' gestation |
| Interventions | Randomised to 1 of 4 groups: (1) nasogastric tube with pacifier, (2) bottle with preterm teat, (3) cup feeding with 30 mL medicine cup, (4) Haberman infant feeder (Medela) |
| Outcomes | Primary outcome: breast feeding ability at discharge and tolerance to supplementary method of feeding Secondary outcomes: breast feeding rate at discharge, at 2 and 4 weeks post discharge; weight gain; hospital length of stay; frequency of skin‐to‐skin contact; maternal satisfaction with the feeding method |
| Notes | Abstract only; review authors have attempted to contact the study author |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
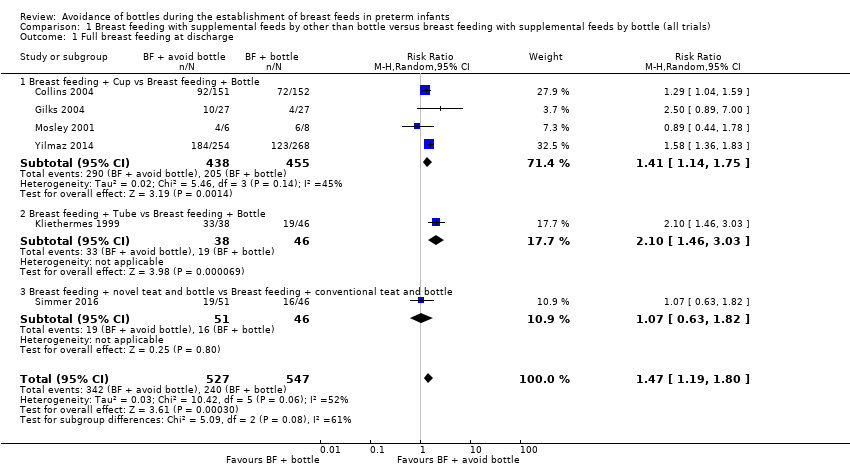
| 1 Full breast feeding at discharge Show forest plot | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.80] |
| Analysis 1.1 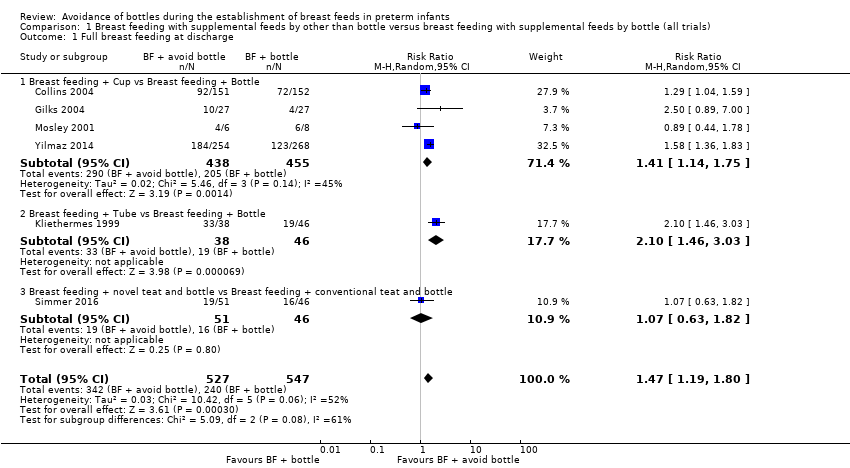 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 1 Full breast feeding at discharge. | ||||
| 1.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 893 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.14, 1.75] |
| 1.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.46, 3.03] |
| 1.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.82] |
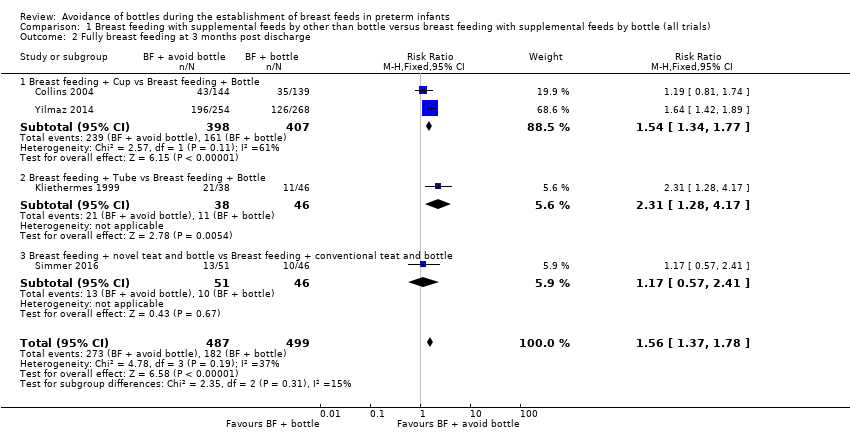
| 2 Fully breast feeding at 3 months post discharge Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| Analysis 1.2 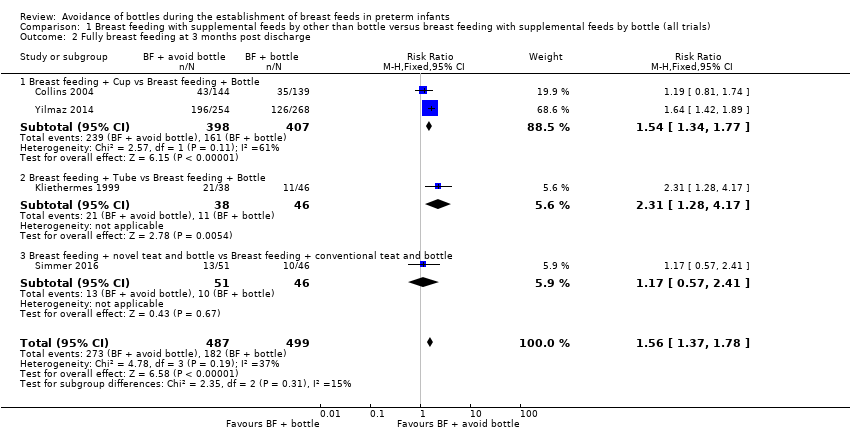 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 2 Fully breast feeding at 3 months post discharge. | ||||
| 2.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.34, 1.77] |
| 2.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.28, 4.17] |
| 2.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
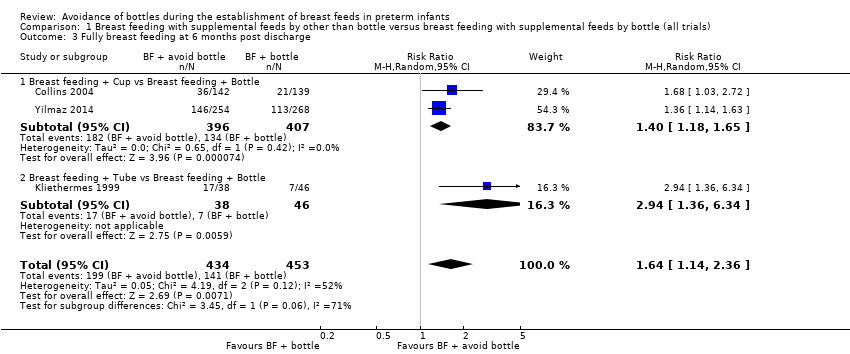
| 3 Fully breast feeding at 6 months post discharge Show forest plot | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.14, 2.36] |
| Analysis 1.3 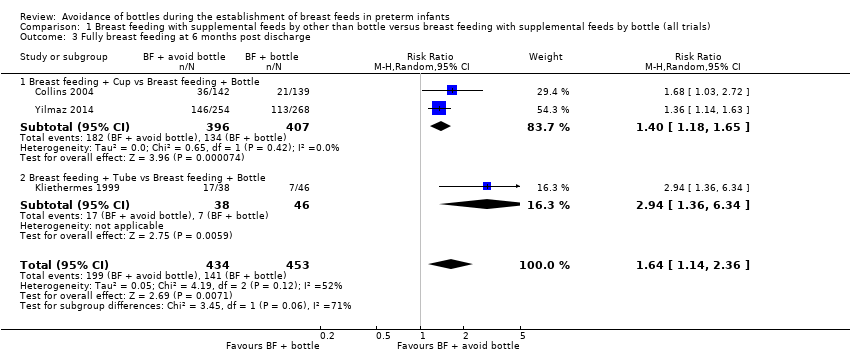 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 3 Fully breast feeding at 6 months post discharge. | ||||
| 3.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.18, 1.65] |
| 3.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.36, 6.34] |
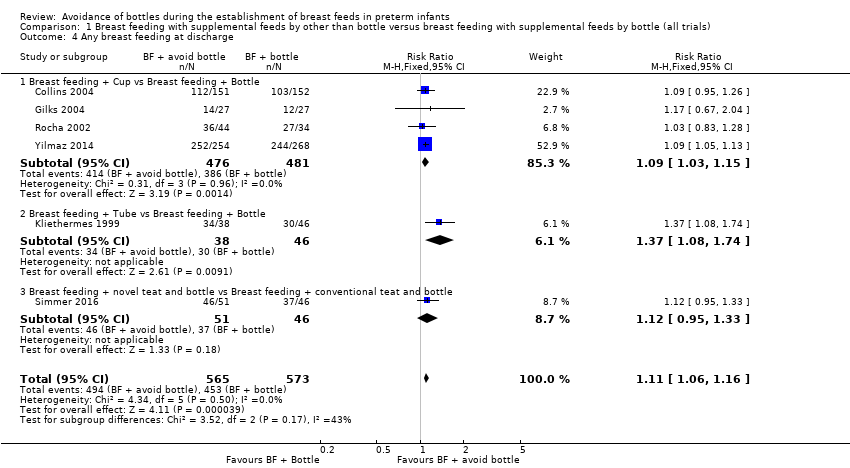
| 4 Any breast feeding at discharge Show forest plot | 6 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.16] |
| Analysis 1.4 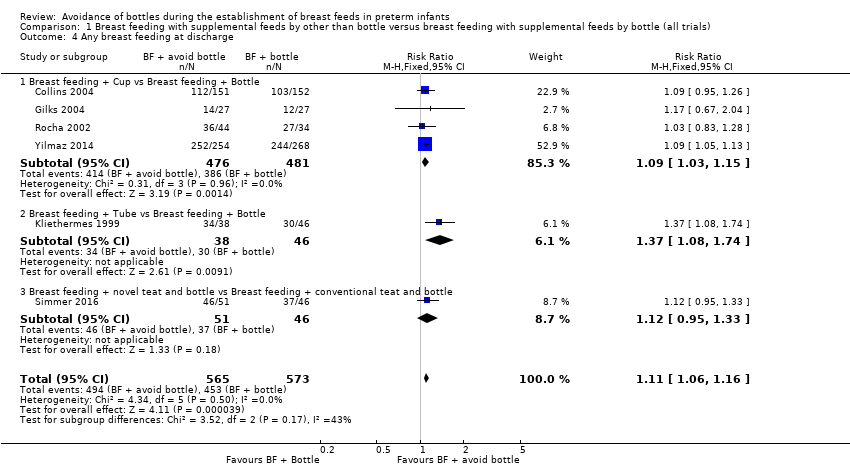 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 4 Any breast feeding at discharge. | ||||
| 4.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.15] |
| 4.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.08, 1.74] |
| 4.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.33] |
| 5 Any breast feeding at 3 months post discharge Show forest plot | 5 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.01, 1.71] |
| Analysis 1.5  Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 5 Any breast feeding at 3 months post discharge. | ||||
| 5.1 Breast feeding + Cup vs Breast feeding + Bottle | 3 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.89, 1.71] |
| 5.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.41] |
| 5.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 6 Any breast feeding at 6 months post discharge Show forest plot | 3 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.10, 1.41] |
| Analysis 1.6 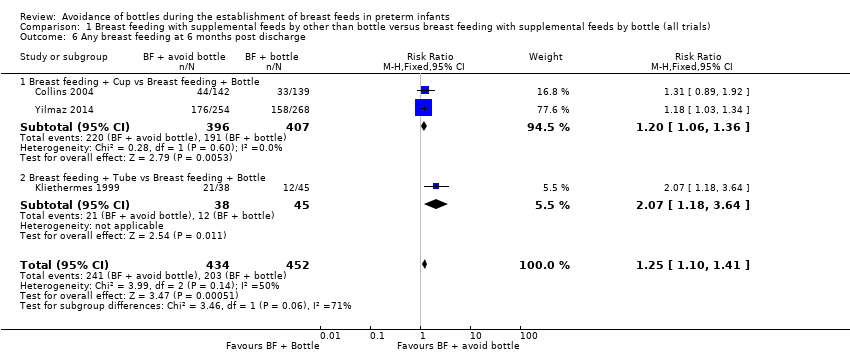 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 6 Any breast feeding at 6 months post discharge. | ||||
| 6.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
| 6.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.18, 3.64] |
| 7 Days to reach full sucking feeds Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐7.17, 12.28] |
| Analysis 1.7 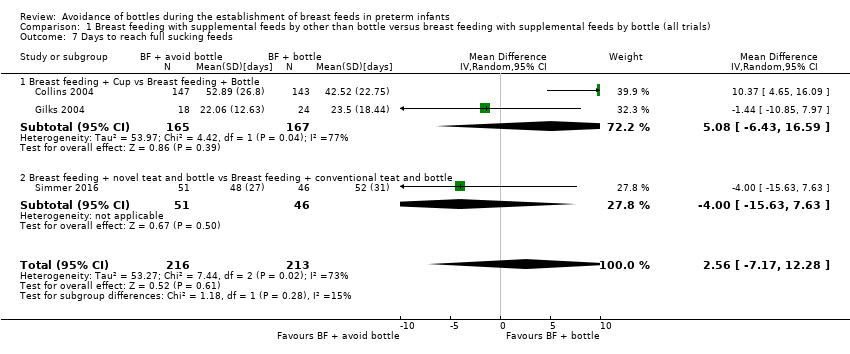 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 7 Days to reach full sucking feeds. | ||||
| 7.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 5.08 [‐6.43, 16.59] |
| 7.2 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐15.63, 7.63] |
| 8 Weight gain Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 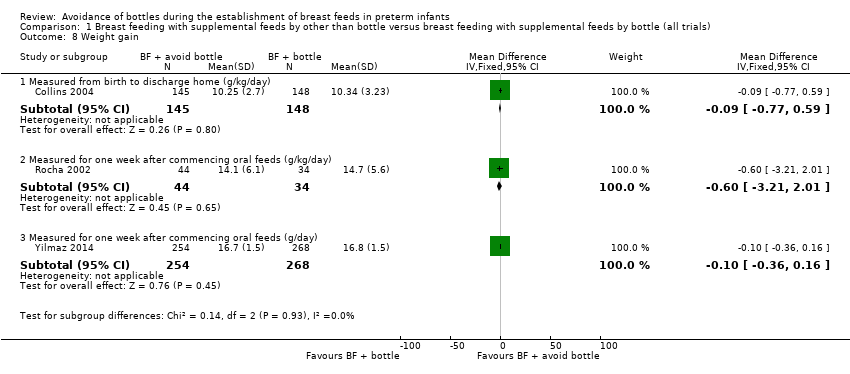 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 8 Weight gain. | ||||
| 8.1 Measured from birth to discharge home (g/kg/day) | 1 | 293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.77, 0.59] |
| 8.2 Measured for one week after commencing oral feeds (g/kg/day) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.21, 2.01] |
| 8.3 Measured for one week after commencing oral feeds (g/day) | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 9 Length of hospital stay Show forest plot | 4 | 1004 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐3.36, 7.86] |
| Analysis 1.9  Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 9 Length of hospital stay. | ||||
| 9.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 823 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐5.57, 14.48] |
| 9.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.89, 9.09] |
| 9.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐17.25, 7.45] |
| 10 Duration of supplementary feed Show forest plot | 2 | 600 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.96, 1.12] |
| Analysis 1.10  Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 10 Duration of supplementary feed. | ||||
| 11 Episodes of infection Show forest plot | 3 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.42] |
| Analysis 1.11 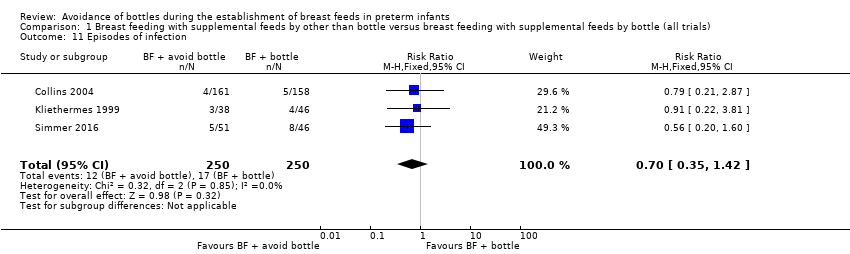 Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 11 Episodes of infection. | ||||

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 1 Full breast feeding at discharge.
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 2 Fully breast feeding at 3 months post discharge.
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 3 Fully breast feeding at 6 months post discharge.
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 4 Any breast feeding at discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 5 Any breast feeding at 3 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 6 Any breast feeding at 6 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 7 Days to reach full sucking feeds.
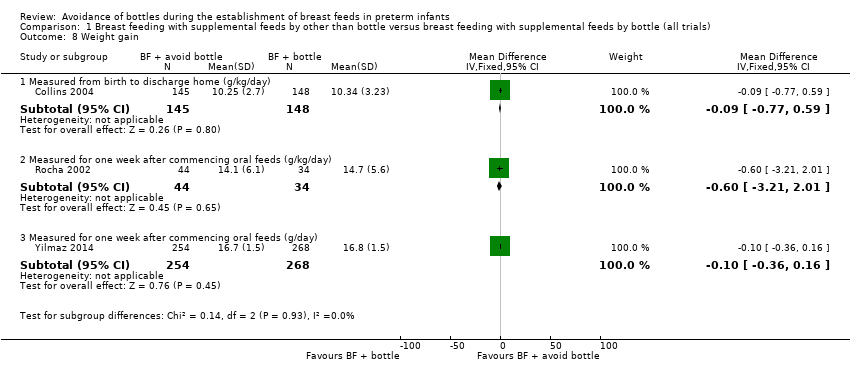
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 8 Weight gain.
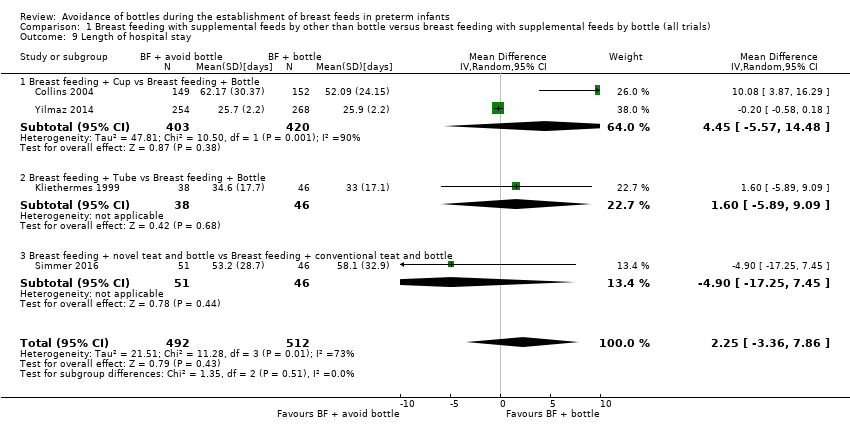
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 9 Length of hospital stay.
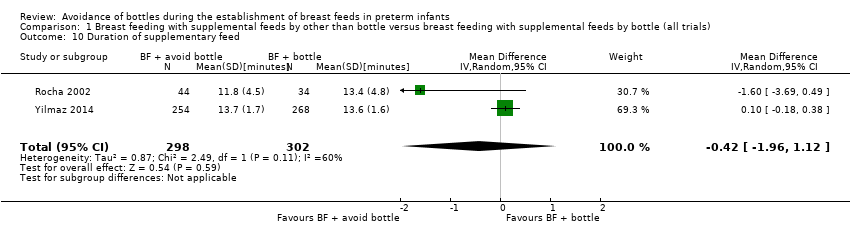
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 10 Duration of supplementary feed.
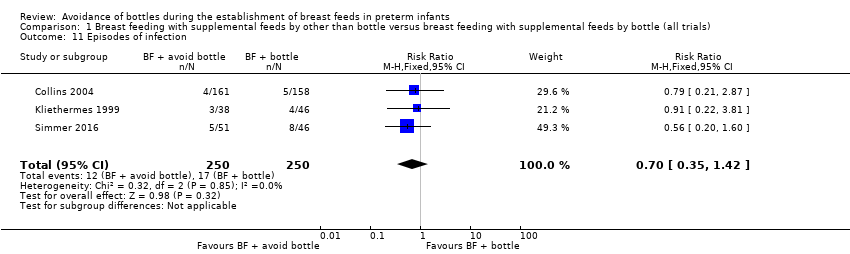
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 11 Episodes of infection.
| Breast feeding with supplemental feeds by other than bottle compared with breast feeding with supplemental feeds by bottle (all trials) in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with breast feeding with supplemental feeds by bottle (all trials) | Risk with breast feeding with supplemental feeds by other than bottle | |||||
| Full breast feeding at discharge | Study population | RR 1.47 | 1074 | ⊕⊕⊝⊝ | ||
| 44 per 100 | 64 per 100 | |||||
| Full breast feeding at 3 months post discharge | Study population | RR 1.56 | 986 | ⊕⊕⊕⊝ | ||
| 36 per 100 | 57 per 100 | |||||
| Full breast feeding at 6 months post discharge | Study population | RR 1.64 | 887 | ⊕⊕⊝⊝ | ||
| 31 per 100 | 51 per 100 | |||||
| Any breast feeding at discharge | Study population | RR 1.11 | 1138 | ⊕⊕⊕⊝ | ||
| 79 per 100 | 88 per 100 | |||||
| Any breast feeding at 3 months post discharge | Study population | RR 1.31 | 1063 | ⊕⊝⊝⊝ | ||
| 60 per 100 | 78 per 100 | |||||
| Any breast feeding at 6 months post discharge | Study population | RR 1.25 | 886 | ⊕⊝⊝⊝ | ||
| 45 per 100 | 56 per 100 | |||||
| Length of hospital stay | MD 2.25 higher | ‐ | 1004 | ⊕⊝⊝⊝ | ||
| Episodes of infection | Study population | RR 0.70 | 500 | ⊕⊕⊕⊝ | ||
| 7 per 100 | 5 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAttrition bias (14% and 15% attrition in two included studies). bModerate heterogeneity (I2 = 52%). cModerate heterogeneity (I2 = 73%). dModerate heterogeneity (I2 = 71%). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full breast feeding at discharge Show forest plot | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.80] |
| 1.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 893 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.14, 1.75] |
| 1.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.46, 3.03] |
| 1.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.82] |
| 2 Fully breast feeding at 3 months post discharge Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| 2.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.34, 1.77] |
| 2.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.28, 4.17] |
| 2.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
| 3 Fully breast feeding at 6 months post discharge Show forest plot | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.14, 2.36] |
| 3.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.18, 1.65] |
| 3.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.36, 6.34] |
| 4 Any breast feeding at discharge Show forest plot | 6 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.16] |
| 4.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.15] |
| 4.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.08, 1.74] |
| 4.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.33] |
| 5 Any breast feeding at 3 months post discharge Show forest plot | 5 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.01, 1.71] |
| 5.1 Breast feeding + Cup vs Breast feeding + Bottle | 3 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.89, 1.71] |
| 5.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.41] |
| 5.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 6 Any breast feeding at 6 months post discharge Show forest plot | 3 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.10, 1.41] |
| 6.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
| 6.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.18, 3.64] |
| 7 Days to reach full sucking feeds Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐7.17, 12.28] |
| 7.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 5.08 [‐6.43, 16.59] |
| 7.2 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐15.63, 7.63] |
| 8 Weight gain Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Measured from birth to discharge home (g/kg/day) | 1 | 293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.77, 0.59] |
| 8.2 Measured for one week after commencing oral feeds (g/kg/day) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.21, 2.01] |
| 8.3 Measured for one week after commencing oral feeds (g/day) | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 9 Length of hospital stay Show forest plot | 4 | 1004 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐3.36, 7.86] |
| 9.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 823 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐5.57, 14.48] |
| 9.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.89, 9.09] |
| 9.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐17.25, 7.45] |
| 10 Duration of supplementary feed Show forest plot | 2 | 600 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.96, 1.12] |
| 11 Episodes of infection Show forest plot | 3 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.42] |

