Adenosin berbanding penghalang saluran kalsium intravena untuk takikardia supraventricular
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT | |
| Participants | Age not stated, presumed adult | |
| Interventions | Gp 1: ATP 20 mg bolus | |
| Outcomes | Reversion rate | |
| Notes | Country: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to determine whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding intervention was made. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, withdrawals, dropouts, or protocol deviations were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
| Methods | RCT | |
| Participants | Adults 18 to 75 years | |
| Interventions | Gp 1: Adenosine 3 mg, then 6 mg, then 9 mg every 1 to 2 minutes if no response to previous dose. Mean dose 9.63 mg | |
| Outcomes | Reversion rate | |
| Notes | Country: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to determine whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding intervention was made. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, withdrawals, dropouts, or protocol deviations were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
| Methods | RCT with cross‐over design | |
| Participants | Adults | |
| Interventions | Gp 1: ATP 10 mg, then 20 mg bolus if needed. Mean dose 10.8 mg | |
| Outcomes | Reversion rate | |
| Notes | Country: Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to determine whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding intervention was made. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, withdrawals, dropouts, or protocol deviations were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
| Methods | RCT with cross‐over design | |
| Participants | Adults (25 M,25 F) | |
| Interventions | Gp 1: ATP 5 mg, then 10 mg, then 20 mg every 1 minute if previous dose not effective | |
| Outcomes | Reversion rate | |
| Notes | Country: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to determine whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding intervention was made. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, withdrawals, dropouts, or protocol deviations were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
| Methods | RCT with cross‐over design | |
| Participants | Children < 13 years | |
| Interventions | Gp 1: ATP titrated to effect, mean dose 7.46 mg | |
| Outcomes | Reversion rate | |
| Notes | Two‐part study; only participants in second part included, as no randomisation in first part Country: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to determine whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding intervention was made. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, withdrawals, dropouts, or protocol deviations were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
| Methods | RCT with cross‐over design | |
| Participants | 233 participants with spontaneous regular narrow complex tachycardia and failed Valsalva manoeuvres Gp 1: 104 participants on adenosine, mean age 50.6 ± 17.0, 42% males Gp 1: 102 participants on verapamil (57 people) and diltiazem (59 people). Mean age 48.9 ± 18.3, 40% males 27 excluded from analysis after enrolment, as they had an arrhythmia other than SVT Inclusion criteria: at least 10 years of age with regular narrow complex tachycardia and an electrocardiographic (ECG) diagnosis of SVT, not converted by vagal manoeuvres (Valsalva manoeuvre or carotid sinus massage or both) Exclusion criteria: signs of impaired cerebral perfusion (e.g. altered mental state) or acute pulmonary oedema | |
| Interventions | Gp 1: adenosine, initially a 6‐mg bolus, then a 12‐mg bolus after 2 minutes, if needed Gp 2: verapamil and diltiazem Diltiazem: slow intravenous infusion at a rate of 2.5 mg per minute, up to a maximum dose of 50 mg Refractory cases were crossed‐over if initial intervention was not successful after repeated admissions. These cases were counted as failures of the intervention and were not included in the final analysis. | |
| Outcomes | Reversion rate Relapse rate: recurrences during 2‐hour observation period Major adverse event: hypotension | |
| Notes | ED of the Singapore General Hospital Country: Singapore | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a nurse who drew a serialised sealed envelope. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised with the use of sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Interventions were given by different methods, and no attempt at blinding intervention was made. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Twenty‐seven participants were excluded from analysis, as they were found not to have SVT after enrolment. Therefore, 15% of participants were not analysed in the groups to which they were randomised. However, as participants were randomised, excluded patients were closely distributed across intervention groups and had similar reasons for exclusion. |
| Selective reporting (reporting bias) | Low risk | The main outcomes reported are the same as those planned at a prospective trial registration. |
| Other bias | Low risk | Study authors declared no conflicts of interest. The Department of Clinical Research, Singapore General Hospital, funded adenosine and diltiazem. |
| Methods | RCT | |
| Participants | Adults with spontaneous SVT or WPW 64 consecutive patients with diagnosis of acute SVT or WPW syndrome Males 48.4% Mean age of men was 47 ± 12 years, and women 48 ± 12 years Inclusion criteria: older than 18 years of age with abrupt onset of SVT lasting 20 to 30 minutes Exclusion criteria: presence of atrial flutter, asthma or chronic obstructive pulmonary disease, long‐term use of dipyridamole or theophylline derivatives, pregnant or breastfeeding women, any heart disease apart from coronary artery disease (different forms of stenotic lesions of major arteries or veins), heart failure or pulmonary heart disease, history of bleeding diathesis, stroke, hypertension over 200/110 mmHg, severe diseases of liver or renal function (anamnestic data), confirmed malignancies, severe genetic diseases, severe anaemia, alcohol or narcotic addiction, psychiatric disorders, AV block of second or third degree, sick sinus syndrome | |
| Interventions | Gp 1: adenosine IV bolus of 6 mg, then 12 mg if needed Gp 2: verapamil or IV 5 mg up to maximum dose of 10 mg if needed | |
| Outcomes | Cardioversion into sinus rhythm Duration to sinus rhythm conversion Relapse Biomarkers outcomes | |
| Notes | Intensive care unit and emergency centre at Clinical Center of Serbia Country: Serbia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation and randomisation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Interventions given by different methods and no attempt at blinding intervention made |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Interventions were applied and outcomes were assessed within the department. No losses to follow‐up, withdrawals, or dropouts were reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available for comparison of intended study outcomes vs reported outcomes. |
| Other bias | Unclear risk | No mention of funding and no mention of possible conflicts of interest |
A/E: adverse events.
ATP: adenosine triphosphate.
AV: atrioventricular.
CCF: congestive cardiac failure.
ECG: electrocardiogram.
ED: emergency department.
MI: myocardial infarction.
RCT: randomised controlled trial.
SBP: systolic blood pressure.
SVT: supraventricular tachycardia.
UAP: unstable angina pectoris.
WPW: Wolff‐Parkinson‐White.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT, as allocation to treatment was changed according to previous patient experience with adenosine/verapamil. In addition, significant differences in baseline characteristics suggest that no appropriate randomisation method was used. Study authors have not yet replied to our request for further data/information. | |
| Retrospective chart review and no relevant outcomes measured | |
| Review article, not a trial | |
| Editorial only | |
| Included participants with induced SVT | |
| Not a randomised trial. Participants with induced SVT were given adenosine, then were re‐induced and given verapamil. | |
| Not a randomised trial | |
| Included participants with induced SVT | |
| Included participants with induced SVT | |
| Review article, not a trial | |
| Significant differences in baseline characteristics suggest that no appropriate randomisation method was used. Study authors have not yet replied to our request for further data/information. | |
| Retrospective chart review | |
| Not a randomised trial. Participants with induced SVT were given adenosine, then were re‐induced and given verapamil. | |
| Comparison of intravenous adenosine vs intravenous adenosine with oral verapamil | |
| Comparison of adenosine vs ajmaline (class 1A antiarrhythmic). No calcium antagonist arm included | |
| Not a randomised trial | |
| Only participants with induced SVT were included. |
RCT: randomised controlled trial.
SVT: supraventricular tachycardia.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Odds of reversion Show forest plot | 7 | 622 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.85, 2.68] |
| Analysis 1.1  Comparison 1 Adenosine vs CCA, Outcome 1 Odds of reversion. | ||||
| 2 Time to reversion (seconds) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Adenosine vs CCA, Outcome 2 Time to reversion (seconds). | ||||
| 3 Relapse to SVT post reversion Show forest plot | 4 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.69] |
| Analysis 1.3  Comparison 1 Adenosine vs CCA, Outcome 3 Relapse to SVT post reversion. | ||||
| 4 Minor adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 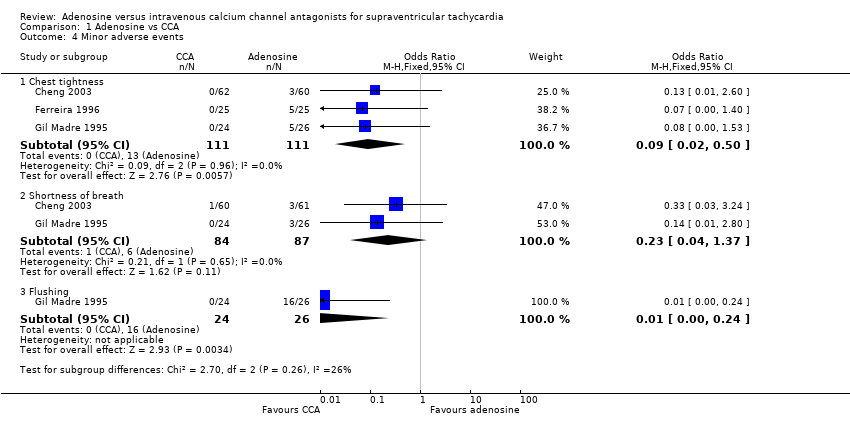 Comparison 1 Adenosine vs CCA, Outcome 4 Minor adverse events. | ||||
| 4.1 Chest tightness | 3 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.50] |
| 4.2 Shortness of breath | 2 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.37] |
| 4.3 Flushing | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.24] |
| 5 Hypotension Show forest plot | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.71] |
| Analysis 1.5  Comparison 1 Adenosine vs CCA, Outcome 5 Hypotension. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
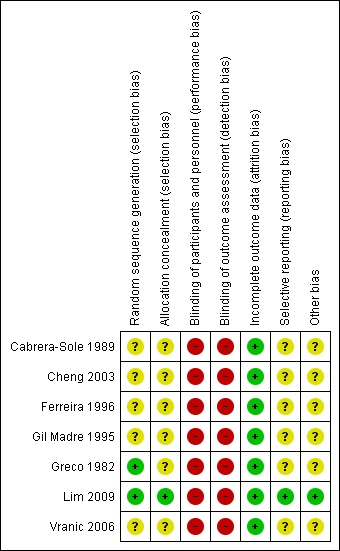
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
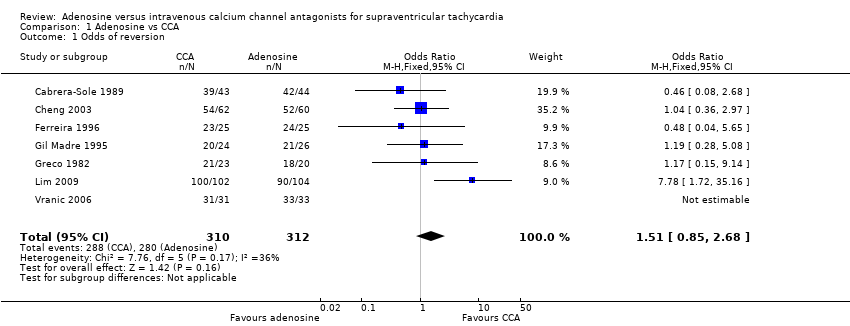
Comparison 1 Adenosine vs CCA, Outcome 1 Odds of reversion.
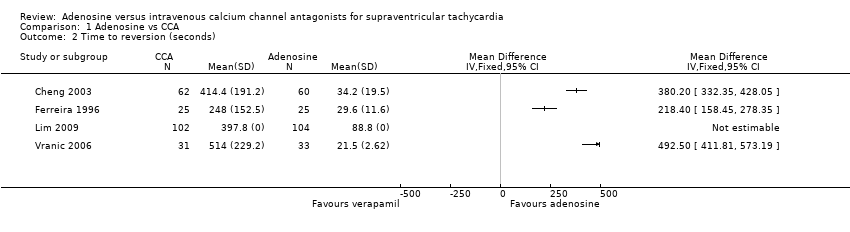
Comparison 1 Adenosine vs CCA, Outcome 2 Time to reversion (seconds).

Comparison 1 Adenosine vs CCA, Outcome 3 Relapse to SVT post reversion.

Comparison 1 Adenosine vs CCA, Outcome 4 Minor adverse events.

Comparison 1 Adenosine vs CCA, Outcome 5 Hypotension.
| Adenosine compared with calcium channel antagonists for supraventricular tachycardia | |||||||||
| Patient or population: patients with supraventricular tachycardia | |||||||||
| Outcomes | Number of participants | Number of studies | Odds ratio | Absolute effects (95% CI) | Follow‐up | Quality of the evidence | What happens | ||
| With adenosine | With CCA | Difference | |||||||
| Odds of reversion | 622 | 7 RCTs | OR 1.51 (0.85 to 2.68) | 89.7% | 92.9% | 3.2% lower odds of reversion with adenosine | Until reversion occurred | ⊕⊕⊕⊝ | Higher odds of reversion indicate better effect. |
| Major adverse event: | 306 | 3 RCTs | OR 3.09 | 0.0% | 0.0% (0.0 to 0.0) | 0.0% fewer (0 fewer to 0 fewer) | Up to 2 hours after infusion | ⊕⊕⊝⊝ | Lower hypotension rate indicates fewer adverse events. |
| Length of stay in hospital | Not reported | 0 | |||||||
| Patient satisfaction | Not reported | 0 | |||||||
| CI: confidence interval; OR: odds ratio. | |||||||||
| GRADE Working Group grades of evidence | |||||||||
| aQuality of the evidence downgraded by one level for imprecision. Moderate to wide confidence intervals. bQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies, as none of the studies were blinded. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Odds of reversion Show forest plot | 7 | 622 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.85, 2.68] |
| 2 Time to reversion (seconds) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Relapse to SVT post reversion Show forest plot | 4 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.69] |
| 4 Minor adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chest tightness | 3 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.50] |
| 4.2 Shortness of breath | 2 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.37] |
| 4.3 Flushing | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.24] |
| 5 Hypotension Show forest plot | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.71] |

