Usporedba adenozina i intravenskih antagonista kalcijevih kanala u liječenju supraventrikularne tahikardije
Abstract
Background
People with supraventricular tachycardia (SVT) frequently are symptomatic and present to the emergency department for treatment. Although vagal manoeuvres may terminate SVT, they often fail, and subsequently adenosine or calcium channel antagonists (CCAs) are administered. Both are known to be effective, but both have a significant side effect profile. This is an update of a Cochrane review previously published in 2006.
Objectives
To review all randomised controlled trials (RCTs) that compare effects of adenosine versus CCAs in terminating SVT.
Search methods
We identified studies by searching CENTRAL, MEDLINE, Embase, and two trial registers in July 2017. We checked bibliographies of identified studies and applied no language restrictions.
Selection criteria
We planned to include all RCTs that compare adenosine versus a CCA for patients of any age presenting with SVT.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane. Two review authors independently checked results of searches to identify relevant studies and resolved differences by discussion with a third review author. At least two review authors independently assessed each included study and extracted study data. We entered extracted data into Review Manager 5. Primary outcomes were rate of reversion to sinus rhythm and major adverse effects of adenosine and CCAs. Secondary outcomes were rate of recurrence, time to reversion, and minor adverse outcomes. We measured outcomes by calculating odds ratios (ORs) and assessed the quality of primary outcomes using the GRADE approach through the GRADEproGDT website.
Main results
We identified two new studies for inclusion in the review update; the review now includes seven trials with 622 participants who presented to an emergency department with SVT. All included studies were RCTs, but only three described the randomisation process, and none had blinded participants, personnel, or outcome assessors to the intervention given. Moderate‐quality evidence shows no differences in the number of people reverting to sinus rhythm who were treated with adenosine or CCA (89.7% vs 92.9%; OR 1.51, 95% confidence interval (CI) 0.85 to 2.68; participants = 622; studies = 7; I2 = 36%). Low‐quality evidence suggests no appreciable differences in major adverse event rates between CCAs and adenosine. Researchers reported only one case of hypotension in the CCA group and none in the adenosine group (0.66% vs 0%; OR 3.09, 95% CI 0.12 to 76.71; participants = 306; studies = 3; I2 = 0%). Included trials did not report length of stay in hospital nor patient satisfaction.
Authors' conclusions
Moderate‐quality evidence shows no differences in effects of adenosine and calcium channel antagonists for treatment of SVT on reverting to sinus rhythm, and low‐quality evidence suggests no appreciable differences in the incidence of hypotension. A study comparing patient experiences and prospectively studied adverse events would provide evidence on which treatment is preferable for management of SVT.
PICO
Laički sažetak
Usporedba adenozina i intravenskih antagonista kalcijevih kanala u liječenju tahikardije odraslih
Dosadašnje spoznaje
Supraventrikularna tahikardija (SVT) je čest poremećaj srčanog ritma. Uzrokuje veoma ubrzane otkucaje srca. Taj poremećaj srčanog ritma obično se javlja u inače zdravih ljudi, a uobičajeni simptomi uključuju lupanje srca, omaglice i bol u prsima. Povremeno, SVT može uzrokovati i zbunjenost ili gubitak svijesti. SVT se ponekad može liječiti jednostavnom fizičkom radnjom, kao što je prisilno zadržavanje daha. Kada takva jednostavna radnja ne djeluje, SVT se može liječiti na hitnom prijemu različitim lijekovima. Dvije vrste lijekova koje se najčešće koriste su adenozin i antagonisti kalcijevih kanala (CCAs) (verapamil se najviše koristi među lijekovima iz te skupine).
Obilježja uključenih istraživanja
Ovaj Cochrane sustavni pregled literature uspoređuje učinkovitost i nuspojave adenozina i CCAs u prekidanju napadaja SVT. U pregled smo uključili sedam istraživanja u kojima su sudjelovala 622 pacijenta. Dokazi se temelje na literaturi objavljenoj do srpnja 2017.
Ključni rezultati
Kombinirana analiza tih ispitivanja pokazala je da nema razlike između adenozina i CCAs u uspješnosti liječenja SVT. Ti rezultati temelje se na dokazima srednje kvalitete. Privremeni pad krvnog tlaka koji ne zahtijeva liječenje prijavljen je samo kod jednog od 152 sudionika liječenih pomoću CCA, a dokazi niske kvalitete pokazuju da nijedan pacijent liječen adenozinom nije doživio nizak krvni tlak. Nemamo podataka o duljini boravka u bolnici, niti o zadovoljstvu pacijenta.
Zaključak
Dokazi srednje kvalitete pokazuju da nema razlike u učincima između adenozina i antagonista kalcijevih kanala u liječenju SVT ako gledamo sinusni ritam, dok dokazi niske kvalitete pokazuju da nema razlike u slučajevima hipotenzije. Niti jedno od ovih ispitivanja nije ispitalo želje pacijenata, što je važan čimbenik u odlučivanju koji je lijek "najbolja" terapija.
Authors' conclusions
Summary of findings
| Adenosine compared with calcium channel antagonists for supraventricular tachycardia | |||||||||
| Patient or population: patients with supraventricular tachycardia | |||||||||
| Outcomes | Number of participants | Number of studies | Odds ratio | Absolute effects (95% CI) | Follow‐up | Quality of the evidence | What happens | ||
| With adenosine | With CCA | Difference | |||||||
| Odds of reversion | 622 | 7 RCTs | OR 1.51 (0.85 to 2.68) | 89.7% | 92.9% | 3.2% lower odds of reversion with adenosine | Until reversion occurred | ⊕⊕⊕⊝ | Higher odds of reversion indicate better effect. |
| Major adverse event: | 306 | 3 RCTs | OR 3.09 | 0.0% | 0.0% (0.0 to 0.0) | 0.0% fewer (0 fewer to 0 fewer) | Up to 2 hours after infusion | ⊕⊕⊝⊝ | Lower hypotension rate indicates fewer adverse events. |
| Length of stay in hospital | Not reported | 0 | |||||||
| Patient satisfaction | Not reported | 0 | |||||||
| CI: confidence interval; OR: odds ratio. | |||||||||
| GRADE Working Group grades of evidence | |||||||||
| aQuality of the evidence downgraded by one level for imprecision. Moderate to wide confidence intervals. bQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies, as none of the studies were blinded. | |||||||||
Background
Description of the condition
Definitions
Supraventricular tachycardia (SVT) includes all tachyarrhythmias that originate in supraventricular tissue or incorporate supraventricular tissue in the re‐entrant circuit and have sudden onset and termination. Atrioventricular nodal re‐entrant tachycardia (AVNRT) and atrioventricular re‐entrant tachycardia (AVRT) (such as Wolff‐Parkinson‐White syndrome) are two major types of SVT; other types include atrial tachycardia, paroxysmal atrial flutter, and paroxysmal atrial fibrillation (Jayam 2004). Most patients with SVT due to AVNRT or AVRT do not have associated structural heart disease (Ferguson 2003).
Epidemiology
SVT is a common arrhythmia with a prevalence of 2 per 1000 adults. The incidence of SVT is 36 per 100,000 people per year, and women have twice the risk of developing SVT compared with men (Orejarena 1998).
Clinical presentation and diagnosis
SVTs are often recurrent and occasionally persistent, and are a frequent cause of visits to emergency departments and primary care physicians' offices. Common symptoms of SVT include palpitations, anxiety, light‐headedness, chest pain, neck pounding, and dyspnoea (Delacrétaz 2006; Medi 2009). For patients presenting with SVT, a 12‐lead electrocardiogram (ECG) that shows a narrow complex tachycardia is essential for making the diagnosis and may reveal the mechanism of the arrhythmia.
Treatment
Treatment in stable, symptomatic patients is aimed at terminating the rhythm by decreasing conduction through the atrioventricular (AV) node. Increasing vagal tone by the Valsalva manoeuvre or by carotid sinus massage will effectively revert up to 53% of patients to sinus rhythm (Wen 1998). A modified Valsalva manoeuvre with leg elevation and supine positioning can further improve success (Appelboam 2015). A recent Cochrane review assessed effectiveness of the Valsalva manoeuvre in terminating SVT and showed a reversion success rate between 19.4% and 54.3% in two studies. However, evidence was insufficient overall to support its effectiveness in terminating SVT (Smith 2015). For patients in whom vagal manoeuvres are not effective, calcium channel antagonists (CCAs), adenosine, sotalol, beta‐blockers, and magnesium sulphate have been shown to be more effective than placebo (Dougherty 1992; Gupta 1999; Jordaens 1991; Joshi 1995). However, for acute management, adenosine and non‐dihydropyridine CCAs ‐ verapamil and diltiazem ‐ are the intravenous drugs of choice for termination of SVT (Mangrum 2002). The 2015 American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation and the 2015 European Resuscitation Council (ERC) Guidelines for Resuscitation for regular narrow complex SVT recommend use of adenosine if vagal manoeuvres have failed to terminate the SVT. CCAs are recommended as a second‐line drug if adenosine is contraindicated or fails to terminate the SVT (Page 2016; Soar 2015). The decision as to which agent should be used is generally determined by clinician preference, personal experience, and institutional culture.
Description of the intervention
Adenosine and CCAs have been widely used in SVT with similar efficacy (Bolton 2000; Delaney 2011). Moreover, the previous version of this Cochrane review, which compared these agents, showed no significant differences in reversion rate between the two drugs (Holdgate 2006). However, adenosine is significantly more costly than most intravenous (IV) CCAs.
Adenosine has a half‐life of less than a minute, and reversion to sinus rhythm may be short‐lived, as a subsequent ectopic beat may reinitiate SVT. Many patients experience short‐lived but unpleasant side effects following administration of adenosine, including dyspnoea, flushing, and, perhaps most dreadfully, a sense of impending death or doom that can be very frightening (Bolton 2000; Katzung 1995). The recommended adult dosage of adenosine for peripheral infusion is 6 mg, followed by a 12‐mg dose if needed. Because of the ultrashort duration of action, cumulative effects of sequential doses are not seen (Ferguson 2003).
On the other hand, CCAs have been used in SVT for many years and are effective in up to 90% of patients (Bolton 2000; Delaney 2011). Calcium channel blockade causes negative inotropy and peripheral vasodilation, which may result in hypotension, particularly among patients with impaired left ventricular function. CCAs have a relatively long half‐life of three to six hours, thus adverse effects may be prolonged. They are relatively contraindicated in patients who are already taking beta‐blockers, as the combined effect may lead to significant bradycardia (Katzung 1995). The recommended dosage of verapamil is 5 mg IV over 2 minutes, followed in 5 to 10 minutes by a second dose of 5 to 7.5 mg. The recommended dosage of diltiazem is 20 mg, followed, if necessary, by a second dose of 25 to 35 mg; SVT termination should occur within 5 minutes of infusion completion (Ferguson 2003).
How the intervention might work
Both adenosine and CCAs inhibit conduction through the AV node, which facilitates termination of SVT. Adenosine is an endogenous nucleoside that acts by inhibiting cyclic adenosine monophosphate (cAMP)‐mediated calcium influx and enhancing potassium conduction. This leads to inhibition of AV nodal conduction and expansion of the AV nodal refractory period. In contrast, CCAs act by blocking voltage‐dependent calcium channels, thus reducing intracellular calcium and leading to blockade of calcium‐dependent conduction through the AV node (Katzung 1995).
Why it is important to do this review
The previous version of this review showed that adenosine and CCAs are reasonably effective but have a significant side effect profile (Holdgate 2006). This review update looks at new studies conducted over the past 10 years and aims to further explore uncertainty while helping clinicians and decision makers to regulate the choice between adenosine and CCAs. Recent American and British guidelines recommend adenosine as first pharmacological treatment for stable patients with SVT after vagal manoeuvres are attempted (Blomstrom‐Lundqvist 2003; Page 2016; Resuscitation Council (UK) 2015).
Objectives
To review all randomised controlled trials (RCTs) that compare effects of adenosine versus CCAs in terminating SVT.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include RCTs. We excluded studies reported to be randomised but exhibiting major violations in randomisation methods or treatment allocation, or major differences in baseline characteristics unlikely to have occurred by chance (Athar 2013; Riaz 2012). We contacted authors of studies with protocol violations or for whom we had questions regarding the randomisation process or approval. We excluded these studies from the analysis until we receive further information.
Types of participants
We included patients of any age with SVT diagnosed on 12‐lead ECG within 24 hours of onset.
We excluded RCTs of patients with an SVT induced in the electrophysiology lab, as they do not meet the aim of this review, which focuses on SVT (see Differences between protocol and review section).
Types of interventions
We included all interventions that directly compare any intravenous CCA (e.g. verapamil, diltiazem) versus IV adenosine, at any dosage or infusion rate of either drug.
Types of outcome measures
Primary outcomes
-
Reversion to sinus rhythm
-
Major adverse events (defined as cardiac arrest, prolonged hypotension, symptomatic bradycardia requiring treatment, and acute cardiac failure)
Secondary outcomes
-
Time to immediate reversion to sinus rhythm
-
Rate of relapse to SVT within two hours following reversion
-
Length of stay in hospital
-
Minor adverse events (defined as any reported adverse events other than those defined above)
-
Patient satisfaction as measured on any validated scale
Search methods for identification of studies
Electronic searches
We updated searches conducted in 2006 for the original review (Appendix 1) by searching the following databases on 5 July 2017 for relevant RCTs (Appendix 2).
-
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (2017; Issue 6 of 12).
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and MEDLINE (Ovid, 1946 to 5 July 2017).
-
Embase (Ovid, 1980 to 2017 Week 27).
We applied the sensitivity‐maximising version of the Cochrane RCT filter to our MEDLINE search, and we applied terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions to our Embase search (Lefebvre 2011). We imposed no restrictions on date or language of publication.
Searching other resources
We searched the following sources.
-
Reference lists of relevant identified publications.
-
Two databases of ongoing trials‐ ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) ‐ on 7 July 2017 (Appendix 2).
Data collection and analysis
Selection of studies
Two review authors (MQ and AS) independently screened titles and abstracts for inclusion of all eligible studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. For disagreements, we asked a third review author (SA) to arbitrate. We retrieved full‐text study reports/publications; two review authors (MQ and AS) independently screened the full texts and identified studies for inclusion, or recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion or through consultation with a third review author (SA). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail and completed a PRISMA flow diagram and Characteristics of excluded studies tables.
We excluded all publications that were reviews, retrospective studies, or studies of observational design, as well as those that were not randomised, or did not focus on adenosine or CCAs or SVT.
Data extraction and management
Four review authors (MQ, AS, EA, and TJAC) extracted data for the eligible studies so that each eligible study was independently extracted by two authors. We extracted and collated data using a standardised, agreed upon data extraction form. Data collected include:
-
general information: publication type; title, authors, source, country, year of publication, trial dates, additional publications;
-
trial characteristics: design, setting, duration, types of interventions, types of outcome measures, aim of study, randomisation (and method), allocation concealment (and method), blinding (outcome assessors), check of blinding, funding/conflict of interest;
-
participants: unit of allocation, method of recruitment, inclusion criteria, exclusion criteria, total number and numbers in comparison groups, sex/age, ethnicity, severity of illness, subgroups reported, similarity of groups at baseline, withdrawals/losses to follow‐up;
-
intervention: dosage, delivery, timing, administration rate, type of CCA, length of intervention, co‐interventions, costs, compliance;
-
outcomes: outcomes as specified above, the main outcome assessed in the study, other events, length of follow‐up; and
-
results: for outcomes assessed.
'Summary of findings' table
We used the GRADE approach, adopted by Cochrane, to interpret findings (Schünemann 2011). We used the GRADE profiler website (www.gradepro.org) to create a 'Summary of findings' table. Two review authors (SA, AS) independently assessed the quality of included studies.
With GRADEproGDT (GRADEproGDT 2015), evidence relative to each specific outcome is rated as having high, moderate, low, or very low quality. We started rating outcomes of all randomised trials as high quality and downgraded them depending on limitations in study design or execution, indirectness of evidence, unexplained heterogeneity, imprecision of results, and high probability of publication bias. By using GRADEproGDT, we produced a 'Summary of findings' table to show outcome‐specific ratings and to present information about the overall quality of evidence.
We selected all primary outcomes for inclusion in the 'Summary of findings' table. In addition, we had planned to include length of stay in hospital and patient experience as patient‐relevant outcomes, but included studies did not report this information.
Assessment of risk of bias in included studies
For this updated review, two review authors (MQ, AS, EA, and TJAC) independently carried out risk of bias assessment.
We assessed risk of bias of included trials, using the methods detailed in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We rated the risk of selection bias by assessing randomisation and allocation concealment. We rated performance, detection, and attrition bias by assessing blinding to treatment, blinding to outcome assessment, and losses to follow‐up. We planned to assess selective reporting bias by cross‐checking study outcomes against published protocols or trial registrations.
We coded each risk of bias criterion as having high risk, low risk, or unclear risk of bias, and we resolved disagreements by discussion. When necessary, we contacted study authors to try to clarify trial methods.
Measures of treatment effect
We followed the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions, Sections 9.2 and 9.4, for measuring effects of different data types (Higgins 2011). For continuous outcomes (e.g. time to reversion), we calculated mean differences (MDs) and 95% confidence intervals (CIs), and for dichotomous outcomes (e.g. odds of reversion, adverse events), we calculated odds ratios (ORs) and 95% CIs.
Unit of analysis issues
Our unit of analysis was the participant. For cross‐over trials, we included only data from the pre‐cross‐over phase, as time between drugs was short and did not allow drug washout. We did not encounter any cluster‐randomised trials.
Dealing with missing data
When possible, we extracted data relevant to intention‐to‐treat analyses.
Assessment of heterogeneity
We analysed statistical heterogeneity by visually inspecting the forest plot and carrying out both Chi2 and I2 tests, as recommended in Chapter 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For the Chi2 test on N‐1 degrees of freedom, we defined P < 0.1 as showing substantial heterogeneity. We used the I2 statistic to quantify statistical inconsistency and to assess the impact of heterogeneity on the meta‐analysis. We determined that I2 > 50% demonstrated high heterogeneity.
If no heterogeneity was present, we performed analysis using a fixed‐effect model. When we detected substantial heterogeneity, we investigated possible sources of heterogeneity (e.g. study quality, outcome measures, participants, interventions). When the source of heterogeneity could not be explained, we did not combine study results.
Assessment of reporting biases
To assess the risk of publication bias, we had planned to construct funnel plots for each outcome with at least 10 trials; however, this was not possible owing to the limited number of included studies (Sterne 2011).
Data synthesis
We used Review Manager 5 software to perform data analysis (RevMan 5.3).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis based on participant age, gender, duration of symptoms, intercurrent drug therapy, presence of underlying heart disease, prior treatments, and drug dosage to explore different effects amongst different groups. However, we found insufficient data to carry out these subgroup analyses.
Sensitivity analysis
We had planned to conduct sensitivity analyses on the primary outcomes to re‐analyse exclusion of studies that we judged to be at high risk of bias across one or more domains of the Cochrane 'Risk of bias' tool. This was not possible, as all included studies had at least one domain with high risk of bias.
Results
Description of studies
Results of the search
We identified 237 new references in our updated literature review. We screened 192 records on the basis of title and abstract after removing duplicates. We excluded most studies on abstract review because they were not RCTs or did not compare adenosine versus a CCA. We assessed eight full‐text records and included two studies (Lim 2009; Vranic 2006). Figure 1 shows a flow chart of the updated search.

Study flow diagram.
We found no trials from reference checking and no ongoing trials upon searching ClinicalTrials.gov and the International Clinical Trials Registry Platform.
The original review included eight trials (Cabrera‐Sole 1989; Cheng 2003; DiMarco 1990; Ferreira 1996; Gil Madre 1995; Greco 1982; Hood 1992; Kulakowski 1998). In this updated review, we excluded three of these, as they included patients with induced SVT (DiMarco 1990; Hood 1992; Kulakowski 1998). See Differences between protocol and review for clarification.
Included studies
Study designs
Four studies included a cross‐over component in which the alternate study drug was administered if the first drug was unsuccessful (Lim 2009; Ferreira 1996; Gil Madre 1995; Greco 1982). The authors of one study did not report results after cross‐over but counted them as showing failure of the initial treatment intervention (Lim 2009). Otherwise, we included only data from the pre‐cross‐over phase, as time between drugs was short and did not allow drug washout (particularly for verapamil). Another study included a third treatment arm given digitalis and did not provide data from this component of the trial (Greco 1982). The Lim trial,divided the CCA arm into verapamil and diltiazem (Lim 2009). We combined these arms in a single CCA group for the purposes of our meta‐analysis.
The other three included studies reported that they were randomised and provided no further explanation (Cabrera‐Sole 1989; Cheng 2003; Vranic 2006).
None of the included studies attempted blinding of participants or personnel.
Study design characteristics of the included studies can be found in the Characteristics of included studies tables.
Participants
The seven included trials were conducted in six different countries, were published between 1982 and 2009, and included 622 participants (Cabrera‐Sole 1989; Cheng 2003; Ferreira 1996; Gil Madre 1995; Greco 1982; Lim 2009; Vranic 2006). All studies but one were conducted in adults (Greco 1982). Inclusion criteria for one trial included people above the age of 10. However, it was not possible to determine how many children younger than 18 were included in this trial (Lim 2009).
All included studies enrolled patients with SVT only.
Interventions
Four trials used adenosine in the form of ATP (adenosine triphosphate) (Cabrera‐Sole 1989; Ferreira 1996; Gil Madre 1995; Greco 1982); the remaining five used adenosine. ATP is rapidly converted to adenosine (the free base form) following exogenous administration; 10 mg ATP is equipotent to 6 mg adenosine, and a linear dosage relationship has been noted between these two forms of the drug (Belhassen 1984; Faulds 1991). Verapamil was the CCA used in all trials. One trial included an arm of diltiazem that was analysed with verapamil in a combined CCA group (Lim 2009). One trial administered adenosine in doubling doses (3 mg‐6 mg‐12 mg), and another used dosing starting at 10 mg ATP (equivalent to 6 mg adenosine) followed by 20 mg ATP (Cheng 2003; Ferreira 1996). Two trials gave adenosine 6 mg IV bolus followed by 12 mg IV bolus if SVT was not reverted with the first bolus (Lim 2009; Vranic 2006).
Cheng 2003, Ferreira 1996, and Gil Madre 1995 gave verapamil in 5‐mg boluses. Cheng 2003 gave a 5‐mg verapamil infusion over five minutes, Gil Madre 1995 in three minutes, Riaz 2012a in two minutes, and Ferreira 1996 in one minute. One trial did not specify whether the 5‐mg verapamil bolus was infused over time or was given as an injection (Vranic 2006).
Verapamil was given at a fixed dose of 10 mg in Cabrera‐Sole 1989. Another trial administered adenosine by slow intravenous infusion at a rate of 1 mg per minute up to a maximum dose of 20 mg while assessing the rhythm every two minutes (Lim 2009).
One trial administered diltiazem by slow intravenous infusion at a rate of 2.5 mg per minute up to a maximum dose of 50 mg, while assessing the rhythm every two minutes (Lim 2009).
Outcomes
All trials reported reversion to sinus rhythm as the main outcome. Researchers reported continuous ECG monitoring or ECG recording in Cheng 2003, Gil Madre 1995, Greco 1982, and Vranic 2006. Infusions were given until successful conversion to sinus rhythm occurred without further details on how this was assessed in Cabrera‐Sole 1989, Ferreira 1996, and Lim 2009. Investigtors in all included studies monitored heart rate and blood pressure throughout infusion.
No studies reported length of stay in hospital nor outcomes derived from patient satisfaction surveys.
Excluded studies
We excluded five studies after acquiring full texts (Athar 2013; Gill 2014; Riaz 2012; Shaker 2015; Turkoglu 2009).
Riaz 2012 mentions randomisation only in the title and provides no further explanation in the Methods section. We contacted study authors for further clarification. This study mentions that a lottery method was used as the allocation method without providing further explanation about what this involved. When contacted, study authors described potentially significant differences in baseline characteristics (four‐year difference in age and no P for comparison) and explained that no other baseline comparisons were available). We deemed that this trial did not use an appropriate randomisation method and therefore excluded it from this review.
Athar 2013 reports a quasi‐experimental trial, with participants "randomly" allocated to two groups. However, study authors did not conceal allocation, as randomised participants received the alternate study drug (rather than the allocated drug) if they had a personal preference for the other drug owing to previous exposure. This article makes no further mention and provides no details of randomisation; multiple attempts to contact study authors were met with no response for clarification.
Gill 2014 makes no mention of randomisation; our attempts to contact study authors for clarification resulted in no response. Shaker 2015 was an RCT that compared IV adenosine versus IV adenosine and oral verapamil. Turkoglu 2009 enrolled only participants with induced SVT.
We excluded three studies that were initially included in the original review, as they enrolled patients with induced SVT (DiMarco 1990; Hood 1992; Kulakowski 1998). See Differences between protocol and review for clarification.
We have provided study design characteristics of all excluded studies in the Characteristics of excluded studies tables.
Risk of bias in included studies
For details on risk of bias in included studies, see 'Risk of bias' tables (Characteristics of included studies). We have presented information on overall risk of bias in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
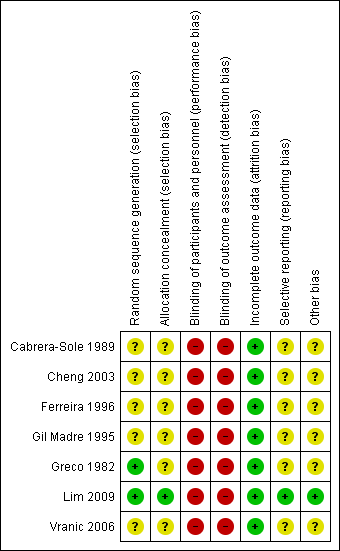
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Two trials described the randomisation process (Greco 1982; Lim 2009). Investigators used a random numbers table to allocate participants to treatment in Greco 1982. A nurse drawing a serialised sealed envelope performed randomisation In Lim 2009. All included studies provided data showing that participants in both drug groups were of similar age and had similar physiological parameters at the time of enrolment.
Allocation concealment
Only one trial reported adequate allocation concealment using an envelope method (Lim 2009). The remaining six trials did not provide sufficient information to reveal whether allocation concealment was adequate.
Blinding
We rated blinding as introducing high risk of bias in all included studies, as none reported blinding of participants, caregivers, outcome assessors, or investigators. As these two drugs are usually given by different methods (adenosine as a rapid bolus, and CCAs as a slower IV infusion), it would be possible to achieve blinding only by using a double‐dummy method, which would require substantial resources. However, no investigators discussed this issue.
Incomplete outcome data
Trialists applied all interventions and assessed all outcomes in the emergency department during admission of patients. All studies reported outcomes for all included patients and reported no withdrawals or dropouts; therefore we rated risk of attrition bias as low for all studies.
Selective reporting
No protocols for included studies were available for cross‐checking of reported study outcomes versus published protocols. All included trials described outcomes in the Methods sections.
We found a prospective trial registration in clinicaltrials.gov for Lim 2009. Planned outcomes included conversion to sinus rhythm as a primary outcome, and recurrence of SVT and vital signs as secondary outcomes. Trial authors reported these outcomes in the published article.
Other potential sources of bias
Inclusion and exclusion criteria
All trials described inclusion criteria, although most studies stated that a diagnosis of SVT was the main inclusion criterion without defining SVT by rate or QRS width, and provided no time limit on duration of symptoms. All but one study described exclusion criteria well (Cabrera‐Sole 1989).
Publication bias
A funnel plot was not appropriate for assessment of publication bias, as this review includes fewer than 10 studies (Sterne 2011).
Conflicts of interest and funding
Only one study included a declaration of interests and reported its source of financial support (Lim 2009). Trial authors reported the absence of any conflicts of interests and receipt of funding from the Department of Clinical Research of Singapore General Hospital for costs of adenosine and diltiazem.
Effects of interventions
Odds of reversion
All seven studies reported odds of reversion or 'efficacy' of adenosine versus CCA as an outcome measure, noting no difference in the odds of reversion to sinus rhythm among participants treated with adenosine or CCA (Analysis 1.1: 89.7% vs 92.9%; odds ratio (OR) 1.51, 95% confidence interval (CI) 0.85 to 2.68; participants = 622; studies = 7; I2 = 36%). This result is based on evidence of moderate quality (summary of findings Table for the main comparison).
Low heterogeneity between trials can be explained by differences in doses of adenosine and verapamil given. All but one study used sequentially increasing doses of each trial drug until reversion occurred or the predetermined maximum dose was reached, whichever occurred first (Cabrera‐Sole 1989). Six trials reported odds of reversion as overall cumulative reversion for participants who received one or more doses of each drug. Trialists in one study used a fixed dose of each drug with no escalation of drug dosage in the absence of reversion (Cabrera‐Sole 1989).
Major adverse events
Three trials reported outcomes of hypotension, noting only one episode of hypotension with CCA and none with adenosine (Analysis 1.5: 0.66% vs 0%; OR 3.09, 95% CI 0.12 to 76.71; participants = 306; studies = 3; I2 = 0%) (Cabrera‐Sole 1989; Ferreira 1996; Lim 2009). This result is not precise, and the confidence interval is wide. These results are based on evidence of low quality (summary of findings Table for the main comparison).
Two of the three trials reporting hypotension specifically excluded patients with systolic blood pressure (BP) < 90 mmHg at enrolment. A hypotensive episode in one trial occurred at infusion of 7.5 mg of verapamil and did not require specific treatment (Lim 2009).
Only one study specifically reported absence of major bradycardia in either group (Ferreira 1996). No studies reported acute heart failure.
The only paediatric study reported that two participants experienced cardiac arrest after receiving treatment with verapamil (Greco 1982). One was an infant with cyanotic heart disease and electrolyte disturbances, the other was an infant already receiving treatment with a beta‐blocker for Wolff‐Parkinson‐White syndrome. Both children were successfully resuscitated.
Time to reversion
Average time to reversion was reported in four studies (Cheng 2003; Ferreira 1996; Lim 2009; Vranic 2006). Each study showed a statistically significantly shorter time to reversion with adenosine than with verapamil. Average time to reversion in all studies combined was 44 seconds for adenosine and 394 seconds for CCAs. Very high heterogeneity between studies made pooling of results inappropriate. This heterogeneity may be due to differences in timing and dosing protocols between trials. Cheng 2003 reported 'average time after dose'; trialists did not report how time to reversion was estimated in two trials (Lim 2009; Vranic 2006).
Relapse rate
Four studies reported rate of relapse to SVT following reversion to sinus rhythm (Ferreira 1996; Gil Madre 1995; Lim 2009; Vranic 2006). Results show no differences in relapse rates between adenosine and CCAs (Analysis 1.3: 3.3% vs 1.14%; OR 0.38, 95% CI 0.09 to 1.69; participants = 358; studies = 4; I2 = 0%). Two studies reported the period of observation following drug administration as 2 hours and 24 hours, respectively (Lim 2009; Vranic 2006). Ferreira 1996 reported relapse at 10 minutes for one participant given adenosine but did not mention time to relapse for the other participant given verapamil.
Length of stay in hospital
None of the included studies reported this outcome.
Minor adverse events
Studies reported numbers of specific adverse events rather than numbers of participants experiencing minor adverse events. Reported minor adverse events included chest tightness, nausea, shortness of breath, headache, and flushing. As patients might experience several different minor adverse events, double counting and exaggeration of estimated effects may occur. Therefore, we have not provided a total pooled estimate of minor adverse event subgroups.
Three trials reported that chest tightness occurred more frequently among participants treated with adenosine compared with verapamil (Analysis 1.4.1: 11.7% vs 0%; OR 0.09, 95% CI 0.02 to 0.50; participants = 222; studies = 3; I2 = 0%) (Cheng 2003; Ferreira 1996; Gil Madre 1995).
Two trials reported shortness of breath, noting no differences between adenosine and CCAs (Analysis 1.4.2: 6.9% vs 1.2%; OR 0.23, 95% CI 0.04 to 1.37; participants = 171; studies = 2; I2 = 0%) (Cheng 2003; Gil Madre 1995). These trials also reported nausea and headache, but high heterogeneity for these outcomes made pooling of results inappropriate.
Flushing as reported in trial was higher in the adenosine group (Analysis 1.4.3: 61.5% vs 0%; OR 0.01, 95% CI 0.00 to 0.24; participants = 50; studies = 1; I2 = 0%) (Gil Madre 1995).
Greco 1982 also reported nausea, chest tightness, shortness of breath, and headache at higher rates among participants treated with adenosine. However, data included results from a non‐randomised component of the study; therefore, we did not include these outcomes in the pooled analysis.
Two trials did not report any minor adverse events (Lim 2009; Vranic 2006).
Patient satisfaction
None of the included studies reported this outcome.
Subgroup analysis
We found insufficient data to carry out intended subgroup analyses.
Sensitivity analysis
All included studies had one or more component at high risk of bias; therefore, a sensitivity analysis for studies with low risk of bias was not possible.
Discussion
Summary of main results
Adenosine and CCA efficiency
Our review aimed to examine the relative efficacy and safety of adenosine and calcium channel antagonists (CCAs) for patients presenting with supraventricular tachycardia (SVT). We included seven trials with 622 participants.
We used three outcomes to compare efficiency of these agents: odds of reversion, time to reversion, and relapse rates. Reversion and relapse rates were similar with adenosine and CCAs. We could not reliably examine time to reversion in a pooled analysis owing to severe heterogeneity. Time to reversion was on average less than a minute with adenosine and longer than six minutes with CCAs. The difference between these two treatments is probably of little clinical significance for patients who are haemodynamically stable.
Adverse effects
Investigators reported only one episode of hypotension among patients treated with verapamil and none in those treated with adenosine. Two cardiac arrests occurred in a paediatric study published in the 1980s, in clinical circumstances for which current practice guidelines would not recommend verapamil without expert consultation (ACLS 2015).
Minor adverse events occurred more frequently with adenosine and affected approximately one in ten patients. No studies specifically defined minor adverse events. Study authors relied on post hoc reporting; therefore, it is possible that the actual rate was higher than was reflected in the data. From a medical perspective, short‐lived symptoms such as chest pain may be perceived as minor; however, no studies explored patients' perception of the relative severity of these events. No study commented on the sense of impending death or doom associated with adenosine treatment.
Patient‐centred outcomes
The two outcomes for which we could find no data (i.e. patient satisfaction and length of hospital stay) may be helpful in the clinical decision as to which treatment should be used. From the patient's perspective, the risk of brief but unpleasant side effects, such as feeling close to death, may be unacceptable.
Overall completeness and applicability of evidence
The main gap in current knowledge involves patient preference. None of the included studies reported results on patient experiences.
Quality of the evidence
The GRADE approach shows that the quality of the evidence is moderate for the odds of reversion outcome (i.e. the result is likely to be close to the true effect but can be substantially different). The quality of evidence is low for the outcome of rate of major adverse events, but this result should be viewed with caution (summary of findings Table for the main comparison). Reasons for downgrading the quality of evidence for adverse events were the presence of high risk of bias in the blinding domain for all included studies and imprecision of results with wide confidence intervals. As studies objectively assessed reversion to sinus rhythm using electrocardiograms (ECGs), lack of blinding of participants or outcome assessors is not expected to have an impact on this endpoint.
Authors of all seven included studies stated that these were randomised trials; however, randomisation was poorly or incompletely reported. Only two studies specified how randomisation was undertaken (Greco 1982; Lim 2009). One study described allocation concealment (Lim 2009). None of the included studies were blinded. Most included trials used a cross‐over design; however we have provided only pre‐cross‐over data in this review.
Potential biases in the review process
We performed a comprehensive literature search to find all relevant trials for inclusion in this review. Two review authors independently performed the literature search, selected studies, extracted data, and assessed risk of bias to minimise review bias. We contacted study authors to request further information when needed.
We conducted the review according to the previously published protocol. However, in some ways, we deviated from the protocol during the review process. We have documented deviations under Differences between protocol and review.
Agreements and disagreements with other studies or reviews
Delaney 2011 is a systematic review and meta‐analysis of adenosine versus verapamil for treatment of stable SVT. This review included eight studies (Cabrera‐Sole 1989; Cheng 2003; DiMarco 1990; Ferreira 1996; Gil Madre 1995; Hood 1992; Kulakowski 1998; Lim 2009). Review authors concluded that both adenosine and verapamil are effective and safe and included studies with induced SVT that we excluded from our review.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
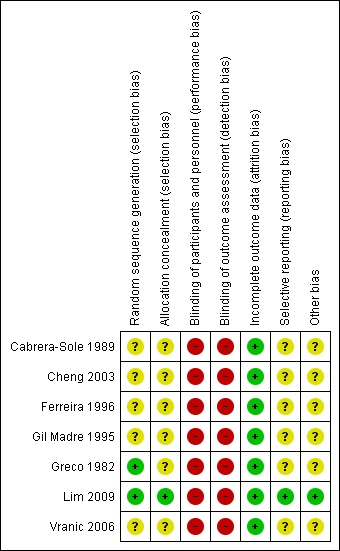
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
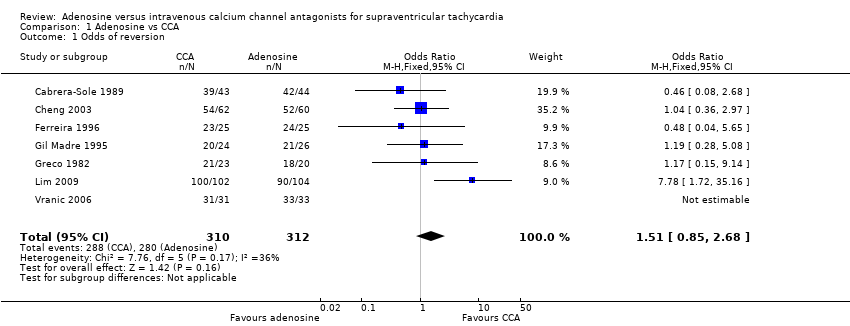
Comparison 1 Adenosine vs CCA, Outcome 1 Odds of reversion.
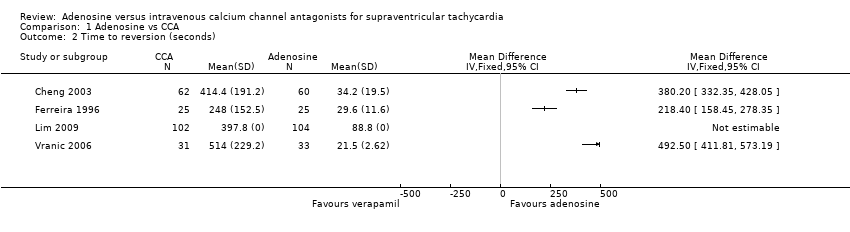
Comparison 1 Adenosine vs CCA, Outcome 2 Time to reversion (seconds).

Comparison 1 Adenosine vs CCA, Outcome 3 Relapse to SVT post reversion.

Comparison 1 Adenosine vs CCA, Outcome 4 Minor adverse events.

Comparison 1 Adenosine vs CCA, Outcome 5 Hypotension.
| Adenosine compared with calcium channel antagonists for supraventricular tachycardia | |||||||||
| Patient or population: patients with supraventricular tachycardia | |||||||||
| Outcomes | Number of participants | Number of studies | Odds ratio | Absolute effects (95% CI) | Follow‐up | Quality of the evidence | What happens | ||
| With adenosine | With CCA | Difference | |||||||
| Odds of reversion | 622 | 7 RCTs | OR 1.51 (0.85 to 2.68) | 89.7% | 92.9% | 3.2% lower odds of reversion with adenosine | Until reversion occurred | ⊕⊕⊕⊝ | Higher odds of reversion indicate better effect. |
| Major adverse event: | 306 | 3 RCTs | OR 3.09 | 0.0% | 0.0% (0.0 to 0.0) | 0.0% fewer (0 fewer to 0 fewer) | Up to 2 hours after infusion | ⊕⊕⊝⊝ | Lower hypotension rate indicates fewer adverse events. |
| Length of stay in hospital | Not reported | 0 | |||||||
| Patient satisfaction | Not reported | 0 | |||||||
| CI: confidence interval; OR: odds ratio. | |||||||||
| GRADE Working Group grades of evidence | |||||||||
| aQuality of the evidence downgraded by one level for imprecision. Moderate to wide confidence intervals. bQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies, as none of the studies were blinded. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Odds of reversion Show forest plot | 7 | 622 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.85, 2.68] |
| 2 Time to reversion (seconds) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Relapse to SVT post reversion Show forest plot | 4 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.69] |
| 4 Minor adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chest tightness | 3 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.50] |
| 4.2 Shortness of breath | 2 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.37] |
| 4.3 Flushing | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.24] |
| 5 Hypotension Show forest plot | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.71] |

