鼻内类固醇治疗急性鼻窦炎
Abstract
研究背景
急性鼻窦炎是初诊的常见原因。它导致显著的症状,并经常导致休班或休学。
研究目的
我们检查了鼻内皮质类固醇(INCS)是否有效缓解成人和儿童急性鼻窦炎的症状。
检索策略
我们检索了Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)(2013 年第 4 期),MEDLINE(1966 年 1 月到 2013 年 5 月第二周)、EMBASE(1990 年到 2013 年 5 月)和所纳入研究的参考书目。
标准/纳入排除标准
纳入INCS对比安慰剂或无干预治疗成人和儿童急性鼻窦炎的随机对照试验(Randomised controlled trials,RCTs)。急性鼻窦炎由临床诊断和影像学或鼻内窥镜进行确诊。主要结局是症状缓解或改善的受试者比例。次要结果是任何需要停止治疗、在研究结束前辍学、复发率、并发症和重返学校或工作的任何不良事件。
数据收集与分析
两名综述作者独立提取资料,并评价试验质量和偏倚风险。
主要结果
这次的更新并没有发现新的试验。涉及1943名急性鼻窦炎受试者的四项研究符合我们的纳入标准。这些试验设计良好并采用双盲法,将INCS对照安慰剂或不干预,周期为15天或21天。失访率分别为7%、11%、41%和10%。当我们综合了系统综述中所纳入的三项试验结果时,接受INCS的受试者比接受安慰剂的受试者更有可能经历症状的解决或改善(73%比66.4%;风险比(RR)=1.11;95%置信区间(CI)[1.04, 1.18]。高剂量的INCS对改善症状或完全缓解有更强烈的影响:糠酸莫米松400μg比200μg(RR=1.10,95%CI [1.02, 1.18])比(RR=1.04,95%CI [0.98,1.11])。未报告重大不良事件,两个治疗组和接受较高剂量的INCS组的辍学率和复发率没有显著差异。
作者结论
目前的证据仅限于经放射科或鼻内窥镜证实的急性鼻窦炎,但支持使用INCS作为单一疗法或作为抗生素的辅助疗法。临床医生在开处方治疗时,应进行临床上重要疗效获益相比可能的轻微不良事件之间的利弊权衡。
PICO
Plain language summary
类固醇用于成人和儿童急性鼻窦炎
急性鼻窦炎是初级保健就诊的常见原因;它是门诊的10种最常见的诊断之一,表现出各种症状和体征,包括化脓性鼻腔分泌物、充血和咳嗽,持续时间超过典型病毒性上呼吸道感染的7至10天。根据对过敏性鼻炎和慢性鼻炎的研究,有人建议鼻内皮质类固醇 (intranasal corticosteroids, INCS)由于其抗炎特性,可以缓解急性鼻窦炎的症状并加速其恢复。
一项重要的系统性文献综述发现,有四项进行良好、随机、安慰剂对照的干预研究,涉及1943名参与者,治疗时间为15天或21天。这项综述结果表明,INCS在症状的解决或改善方面可能具有一定作用。且只有轻微的不良事件被报告,如鼻出血、头痛和鼻痒。鉴于本综述所纳入研究数量少,建议进行进一步的随机对照试验。证据检索日期截止到2013年5月。
Authors' conclusions
Background
Description of the condition
Acute sinusitis is a common reason for primary care visits. It causes significant symptoms and often results in time off work and school. It is one of the 10 most common diagnoses in ambulatory practice and is the fifth most common diagnosis for which an antibiotic is prescribed. Primary care physicians tend to think of sinusitis as an acute bacterial infection and consequently prescribe antibiotics in 85% to 98% of cases. However, sinusitis is frequently caused by a viral infection. According to epidemiological estimates, only 0.2% to 2% of viral upper respiratory tract infections in adults are complicated by bacterial rhinosinusitis. It will often resolve in most patients without antibiotic treatment, even if it is bacterial in origin. Since no simple and accurate practice‐based test exists for acute bacterial sinusitis, clinicians rely on clinical findings to make the diagnosis. Signs and symptoms of acute bacterial sinusitis and those of prolonged viral upper respiratory tract infection are very similar, resulting in frequent misclassification of viral cases (Snow 2001).
The common cold is associated with frequent and variable anatomical involvement of the upper airways, including occlusion and abnormalities in the sinus cavities (Gwaltney 1994). Rhinorrhoea, sinus tenderness, purulent secretions and a history of sinusitis were significant predictors for the diagnosis of sinusitis in a retrospective analysis (Little 2000). Acute sinusitis is defined as an inflammation of the sinuses with the symptom complex lasting less than eight weeks in adults and less than 12 weeks in children (Kaliner 1997).
Clinical diagnosis is made through the appearance of a characteristic constellation of symptoms and signs, including purulent nasal discharge and congestion and cough lasting beyond the typical seven to 10 days for a viral upper respiratory infection. Fever and facial pain may also occur. Diagnosis is often confirmed by sinus imaging; in this area, the use of computerised tomography (CT) scanning is gaining favour (Gwaltney 1995).
Inflammation of nasal mucosa plays an essential role in the development of sinusitis (Tutkun 1996). Sinusitis is invariably accompanied by inflammation of the contiguous nasal mucosa, therefore rhinosinusitis has become the preferred term (Snow 2001). The precipitating factor in acute sinusitis appears to be blockage of the sinus ostium. The obstruction, as well as mucus retention and infection, produce the characteristic signs and symptoms of rhinosinusitis. Although many conditions may lead to ostial closure, viral upper respiratory infections and allergic inflammation are by far the most frequent and important (Shapiro 1992).
Description of the intervention
Treatment of sinusitis is aimed at eliminating causative factors and controlling the inflammatory and infectious components (Becker 2003). It has been theorised that by decreasing the inflammatory response and reducing the mucosal swelling, a topical intranasal steroid would promote drainage and increase aeration of the sinuses, thus hastening the elimination of infectious organisms and decreasing the frequency and severity of recurrences (Mygind 1976). There is evidence that asthma, otitis media with effusion and acute sinusitis may all benefit from such therapy as well (Scadding 2000). A recent Cochrane review found that systemic corticosteroids as adjunctive to antibiotic treatment were effective for the short‐term relief of symptoms in acute sinusitis; the authors mention that the data for this review are limited and there is a significant risk of bias (Venekamp 2011).
How the intervention might work
In addition to treating seasonal and perennial rhinitis (possible predisposing factors to the development of acute rhinosinusitis), intranasal corticosteroids (INCS) might be beneficial in reducing inflammation in the treatment of sinusitis and may help decrease secondary rhinovirus infections (Gawchik 2000). The mode of action of INCS is complex. It is not known whether INCS penetrate the nasal mucosa or act on target cells. However, their low systemic activity supports the concept of local action on nasal mucosa. This local effect can influence a variety of inflammatory cells and their mediators such as epithelial cells, lymphocytes, basophiles, mast cells and Langerhans cells. Corticosteroid‐induced inhibition of the immunoglobulin E dependent release of histamine is a possible but unproven mode of action (Mygind 2001).
Why it is important to do this review
The management of rhinosinusitis depends on a number of variables related to the duration and severity of symptoms in the individual patient. Since there are a variety of conservative and pharmacological interventions available, the physician can find it difficult to develop a cohesive and logical approach to treatment (Benninger 1997). A small benefit for clinical outcomes was observed in patients treated with antibiotics for uncomplicated acute sinusitis; 80% of participants treated without antibiotics improved within two weeks (Ahovuo‐Saloranta 2011). No clear evidence of efficacy of decongestants, antihistamines and nasal irrigations for acute sinusitis in children was found in a recent Cochrane Review (Shaikh 2012). Recent practice guidelines for the diagnosis and management of rhinosinusitis suggest considering the use of INCS as adjunctive therapy (Slavin 2005; Spector 1998). Although the guidelines reflect the belief of many clinicians that INCS are a valuable component of rhinosinusitis management, limited clinical data are available on their use in this disease. A recent experimental prospective study on rabbits with surgically introduced sinusitis demonstrated no clear advantage of steroids in the treatment of sinus infections using this model (Cable 2000). The use of adjunctive medications for acute sinusitis such as antihistamines, decongestants and nasal steroids also remains controversial (Shrum 2001). Several recent studies tested the effectiveness of inhaled steroids for relieving symptoms in acute sinusitis in humans, concluding that this treatment is effective. A systematic review that addresses the effectiveness of this therapy will provide useful information to all primary care practitioners and could assist in formulating the best treatment plan for the individual patient.
Objectives
We examined whether intranasal corticosteroids (INCS) are effective in relieving symptoms of acute sinusitis in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing topical intranasal steroids with placebo or no intervention.
Types of participants
-
Children and adults, irrespective of age, with acute sinusitis.
-
Acute sinusitis is defined by clinical diagnosis and nasal endoscopy or radiological evidence or nasal endoscopy.
-
We included trials including a mixed population of acute and non‐acute sinusitis if outcomes were reported separately for these subgroups.
Types of interventions
Studies which used intranasal corticosteroids (INCS) ‐ any preparation, dose or route of administration (for example, inhaled or drops) versus placebo or no intervention in the control group. We included trials reporting combined interventions only if the control arm received the same co‐treatments as the intervention arm, except for topical steroids.
Types of outcome measures
Primary outcomes
-
Proportion of participants with resolution or improvement of symptoms.
Secondary outcomes
-
Any adverse event that necessitated discontinuation of treatment.
-
Proportion of participants that developed complications.
-
Drop‐outs before the end of the study.
-
Rates of relapse in symptoms.
-
Proportion of participants that returned to school or work within a specific time frame.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 4, part of The Cochrane Library, www.thecochranelibrary.com (accessed 22 May 2013), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (April 2011 to May week 2, 2013) and EMBASE (April 2011 to May 2013). See Appendix 1 for details of previous searches.
We searched MEDLINE and CENTRAL using the following search strategy. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the strategy to search EMBASE (Appendix 2).
MEDLINE (OVID)
1 exp Sinusitis/
2 sinusit*.tw.
3 (rhinosinusit* or nasosinusit*).tw.
4 or/1‐3
5 exp Steroids/
6 steroid*.tw.
7 exp Adrenal Cortex Hormones/
8 adrenal cortex hormone*.tw.
9 exp Anti‐Inflammatory Agents/
10 anti‐inflammat*.tw.
11 corticosteroid*.tw.
12 or/5‐11
13 exp Administration, Intranasal/
14 exp Administration, Topical/
15 (nasal* or intranasal* or topical*).tw.
16 or/13‐15
17 12 and 16
18 4 and 17
Searching other resources
We inspected the reference lists in all identified studies for further relevant studies. We also scrutinised the existing review literature (for example, Mucha 2003). We contacted trial authors for information about possible unpublished studies. There were no language or publication restrictions. We also searched the WHO ICTRP and ClinicalTrials.gov trials registries (14 May 2013) for completed and ongoing trials.
Data collection and analysis
Selection of studies
The two review authors independently reviewed the abstracts of potential studies to be included in the review. We obtained the full article and independently inspected it for relevance.
Data extraction and management
The two review authors independently extracted data from included trials. We documented disagreements and resolved them by discussion. We contacted the trial authors for clarification when necessary. We also documented justification for excluding studies from the review in the Characteristics of excluded studies table. We reported on the following domains.
-
Characteristics of trials: publication status, year, country of study, setting, design, inclusion and exclusion criteria, recruitment, methods, analysis, results.
-
Characteristics of participants: study population, number of participants in each group, age, gender, nationality, diagnostic criteria.
-
Characteristics of interventions: preparation used, dose, length of treatment and follow‐up, compliance, co‐interventions.
-
Outcomes: resolution of symptoms, improvement of symptoms, relapse, complications, return to school/work, adverse events related to the intervention, drop‐outs before the end of the study and reasons for dropping out.
Assessment of risk of bias in included studies
The two review authors independently assessed the methodological quality of each study in the 'Risk of bias' tables, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete out come data;
-
selective reporting; and
-
other bias.
We included trials if they met the following criteria: randomisation method described that would not allow the investigator/participant to know or influence intervention group before the eligible participant entered in the study (low risk of bias) and randomisation stated but no information on method used is available (moderate risk of bias). There were no disagreements and we observed no selective reporting or other potential bias. We obtained additional information from the trial authors when the publications presented insufficient detail.
Measures of treatment effect
We analysed dichotomous data by calculating the risk ratio (RR) and risk difference (RD) for each trial with the uncertainty in each result being expressed as 95% confidence interval (CI). We expressed the results using the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed all analyses on the basis of intention‐to‐treat (ITT). We divided study data as far as possible from published and unpublished information into subgroups for children less than 18 years, adults and co‐interventions. We planned subgroup analyses to assess the impact of these possible sources of heterogeneity. We used the fixed‐effect model for combining studies in the absence of heterogeneity.
Unit of analysis issues
We included RCTs with standard designs and parallel groups in the review.
Dealing with missing data
We tried to contact study authors for missing data.
Assessment of heterogeneity
We assessed heterogeneity by inspection of the graphical presentations and I2 statistic for heterogeneity.
Assessment of reporting biases
We did not have sufficient studies for performing funnel plot analysis to assess possible publication bias. We did not observe other reporting bias.
Data synthesis
We did not find any evidence of heterogeneity between studies as assessed by inspection of the graphical presentations; therefore we used the fixed‐effect model for combining the studies.
Subgroup analysis and investigation of heterogeneity
We did not perform the planned subgroup analyses as the included studies did not report data for these subgroups.
Sensitivity analysis
We planned no sensitivity analyses in the absence of heterogeneity.
Results
Description of studies
Results of the search
We did not identify any trials to include or exclude in this 2013 update from the 82 new references identified. In the previous review (Zalmanovici Trestioreanu 2011) 495 references were identified and the abstracts were inspected by the two review authors.
Included studies
Four studies with 1943 participants assigned to intranasal corticosteroids (INCS) or placebo met the inclusion criteria for this review. Three studies were multicentre trials; one was conducted at 22 sites ‐ 12 primary care and 10 otolaryngology clinics (Dolor 2001), one study involved outpatients from 61 treatment centres in the USA (Nayak 2002), one study was conducted at 71 medical centres in 14 countries (Meltzer 2005) and one study involved participants from the Marmara University Hospital Pediatric Outpatient Clinic (Barlan 1997).
One trial had three treatment arms; two arms for different doses of INCS and one arm for placebo (Nayak 2002). One trial had four treatment arms; two arms for different doses of INCS, one arm for antibiotic and one arm for placebo (Meltzer 2005). We performed meta‐analyses for treatment arms using different doses of INCS combined and separately.
Participants
Participants included in the trials were children and adults with a documented episode of acute sinusitis, confirmed by radiology or nasal endoscopy. The entry criteria in the trials were similar.
Intervention
All the studies used a placebo in the control group. Participants in the treatment groups in three studies received INCS for 21 days as fluticasone propionate two puffs daily in each nostril, giving a total dose of 200 µg (Dolor 2001), MFNS (mometasone furoate) twice daily giving a total dose of 400 µg or 800 µg (Nayak 2002) and budesonide 50 µg twice daily to each nostril as a nasal spray (Barlan 1997) as adjuvant therapy to antibiotics. One study used MFNS 200 µg and 400 µg total daily dose in the treatment arms for 15 days as monotherapy (Meltzer 2005). Other concomitant therapies were similar in all groups, in every study.
Outcomes
The included studies reported the proportion of participants with clinical success; the length of time until clinical success; difference over time in sinusitis symptoms; quality of life scores; relapse (Dolor 2001); improvement in total and individual symptoms scores; onset of relief and evaluation of changes in computerised tomography (CT) sinus scans (Nayak 2002); difference in weekly symptom scores as difference between groups or change from baseline (Barlan 1997); global response to treatment; time to onset of action; mean major symptom scores; mean total symptom scores; individual symptom scores; treatment failure and disease recurrence (Meltzer 2005). Information on adverse events that occurred during the trials is presented in Table 1. Drop‐outs before the end of the study and the reasons for leaving were described in all the studies. One study did not report separate data for the groups for this outcome and the number of participants initially randomised in each group had a high drop‐out rate. It reported results as medians of scores using non‐parametric tests because a wide range of scores were without normal distribution; it was not included in the meta‐analyses (Barlan 1997).
| Study | Intervention | Side effects | Comments |
| Fluticasone propionate 2 puffs ‐ total dose 200 µg or placebo nasal spray once daily in addition to 250 mg cefuroxime axetil orally twice daily and 2 puffs of xylometazoline hydrochloride twice daily | Headache, bloody nose, vaginal itching, yeast infection, nausea, stomach irritation, diarrhoea, increased congestion, hay fever, light‐headed, sore throat, thirsty, itching, rash, cough, fatigue, metallic taste, felt dried out, nasal tissue felt inflamed | No serious unexpected adverse events reported | |
| Amoxicillin‐clavulanate potassium 875 mg | Epistaxis was the most frequently reported adverse event | Treatment well‐tolerated, adverse events similar for all 3 arms of mild/moderate intensity: 12%, 15%, 15% in the MFNS 400, 800 µg and placebo arms | |
| Budesonide 50 µg or placebo nasal spray to each nostril bid in addition to amoxicillin clavulanate potassium 40 mg/kg/day tid | Rash after 1 week attributed to the antibiotic in 1 subject that was switched to cefaclor | No specific adverse events related to the INCS use were reported | |
| MFNS 200 µg once daily or twice daily nasal spray | Headache and epistaxis were most common reported | Most adverse events were mild or moderate with a similar incidence among treatment groups: 36.2%, |
bid: twice daily
INCS: intranasal corticosteroid
MFNS: mometasone furoate
tid: three times daily
Excluded studies
We excluded 491 studies for one or more of the following reasons: not acute sinusitis; not randomised; observational studies; intervention of interest not used; no relevant outcomes reported; repeated reports of the same study; and review articles. Thirteen reports were considered potentially eligible for inclusion but after inspection of the full papers, we excluded nine (Bachert 2007; Gehanno 2000; Jurkiewicz 2004; Meltzer 1993; Meltzer 2000; Quarnberg 1992; Tutkun 1996; Williamson 2007; Yilmaz 2000) (see Characteristics of excluded studies table). In the first publication of this review (Zalmanovici 2007) two studies were awaiting further assessment for missing data (Meltzer 2000; Tutkun 1996). We excluded these studies in the first update (Zalmanovici 2009) as data were not made available from the trial authors, whom we contacted. The reasons for exclusion are added to the Characteristics of excluded studies table. In addition, for one study (Jurkiewicz 2004), no abstract or full paper was available.
Risk of bias in included studies
The studies were well‐designed, randomised, double‐blind, placebo‐controlled trials. The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
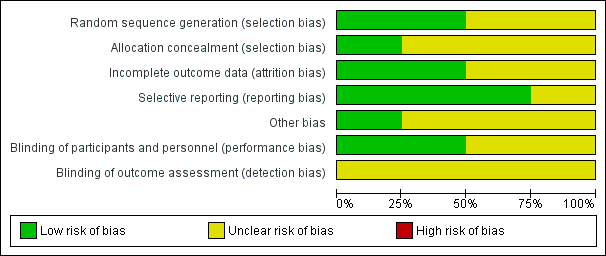
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
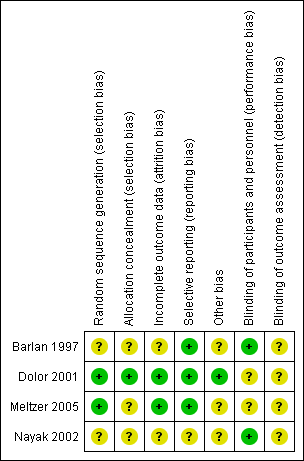
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four studies were RCTs. However, only two contained an adequate report of the generation of allocation sequence (Dolor 2001; Meltzer 2005) and one study reported concealment of allocation (Dolor 2001). The assessment for trial inclusion was based on allocation concealment.
Blinding
The trials were double‐blinded and the method of blinding was adequate. One study did not describe the method of blinding (Barlan 1997).
Incomplete outcome data
Drop‐outs before the end of the study and the reasons for leaving were described in the studies. The total loss to follow‐up was 7% (Dolor 2001), 11% (Nayak 2002), 10% (Meltzer 2005) and 41% (Barlan 1997), respectively.
Selective reporting
The studies reported what was pre‐stated in their protocol.
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
Four studies that included 1943 participants met our inclusion criteria (Barlan 1997; Dolor 2001; Meltzer 2005; Nayak 2002). Two studies had more than two arms, two treatment arms for different doses of intranasal corticosteroids (INCS), and we performed separate and combined dose meta‐analyses (Meltzer 2005; Nayak 2002). One study was included in the review but not in the meta‐analysis as it was not possible to extract data, non‐parametric tests were used and it had a high drop‐out rate (Barlan 1997).
Primary outcome
Proportion of participants with resolution or improvement of symptoms
Information on our primary outcome was found in three trials, assessed at 15 days in one study (Meltzer 2005) and at 21 days in two other studies (Dolor 2001; Nayak 2002). When combined using intention‐to‐treat (ITT) analysis, 73% of INCS‐treated participants and 66.4% of controls had resolution or marked improvement of symptoms (for every 100 patients treated with INCS seven additional patients had complete or marked symptom relief). Individuals treated with INCS (combined results for the 200 µg, 400 µg and 800 µg doses) were more likely to have complete relief or improvement than the placebo group (risk ratio (RR) 1.11; 95% confidence interval (CI) 1.04 to 1.18) (Analysis 1.3); (risk difference (RD) 0.07; 95% CI 0.03 to 0.11) and this result was statistically significant. When we performed separate meta‐analyses for different doses of INCS, a stronger and statistically significant effect was obtained when patients were treated with 400 µg than 200 µg mometasone furoate (MFNS) total daily dose (RR 1.10; 95% CI 1.02 to 1.18 versus RR 1.04; 95% CI 0.98 to 1.11) (Analysis 1.1; Analysis 1.2); (RD 0.06; 95% CI 0.02 to 0.11 versus RD 0.04; CI 95% ‐0.02 to 0.09). The attributable risk percentage (AR%) calculated for the results that were statistically significant means that 8% (one in 12) of all patients who, having received the 400 µg dose of INCS, had resolution or improvement in symptoms could attribute that relief to the treatment. When calculated from results combined across all doses, the number is 9% (one in 11). One study that used in one of the treatment arms an 800 µg MFNS daily dose found a statistically significant effect for this dose (RR 1.21; 95% CI 1.05 to 1.39) (Nayak 2002).
Secondary outcomes
Any adverse event that necessitated discontinuation of treatment
This outcome was reported in two studies (Meltzer 2005; Nayak 2002). No separate data for each treatment arm were available in one study (Nayak 2002) and the participants were equally distributed among the three arms. One study reported a drop‐out rate from treatment of 1%, 3%, 2% and 2% because of adverse events in the INCS 200 µg, 400 µg, antibiotic and placebo arms (Meltzer 2005) (Table 1).
Proportion of participants that developed complications
No studies reported this outcome.
Drop‐outs before the end of the study
This outcome is reported in three studies (Dolor 2001; Meltzer 2005; Nayak 2002). No statistically significant difference could be found for participants that were lost to follow‐up in the two groups (RR 0.85; 95% CI 0.64 to 1.12) (Analysis 1.6). Using a higher dose of INCS did not change the results (RR 0.86; 95% CI 0.61 to 1.20) (Analysis 1.4) for MFNS 400 µg versus MFNS 200 µg (RR 0.75; 95% CI 0.46 to 1.21) (Analysis 1.5).
Rates of relapse in symptoms
Two studies reported data for this outcome (Dolor 2001; Meltzer 2005). No statistically significant differences could be found between groups; 6.3% and 10% had relapse in the INCS and placebo groups (RR 0.71; 95% CI 0.44 to 1.15) (Analysis 1.7). The median time to first recurrence was three days earlier in the placebo group (22 versus 25 days) in one study (Dolor 2001). One study did not find significant differences between groups for different doses of INCS (Meltzer 2005).
Proportion of participants that returned to school or work within a specific time frame
No studies reported this outcome. One study (Dolor 2001) reported a higher subjective level of work performance that was significantly different on day 21 (P value = 0.009) in the INCS treatment group versus placebo. The difference between groups with respect to the total number of hours missed from work was not significant (P value = 0.40).
Discussion
Acute sinusitis is typically first seen as an upper respiratory tract infection that has persisted beyond five to seven days. The diagnosis of sinusitis is based on a combination of clinical history with physical examination, nasal cytology or imaging studies (or both). Factors that may predispose to sinusitis include allergic or occupational rhinitis, vasomotor rhinitis, nasal polyps, rhinitis medicamentosa and immunodeficiency (Spector 1998). Although acute sinusitis is an infectious disease in which several bacterial species play a major aetiological role, there is an important interaction between respiratory viruses (for example, common cold viruses) and bacteria in the pathogenesis of acute community‐acquired sinusitis (Winther 1990). Upper respiratory tract infections and allergic inflammation are recognised as the important risk factors for acute sinusitis, with upper respiratory tract infection being the most common (Wald 1988).
Summary of main results
Four studies met the inclusion criteria in our review. They were well‐conducted and produced results that suggest a clinically relevant, earlier resolution of symptoms in participants treated with intranasal corticosteroids (INCS), without the risk of severe adverse events, even when higher doses in the therapeutic range were used. All four of the trials reached statistical significance for this outcome. One in 12 of all patients who having received the 400 µg dose had resolution or improvement in symptoms could attribute that relief to the treatment. Across all doses, the number is one in 11. No statistically significant difference in the relapse rate between groups was found. One study (Barlan 1997) found that INCS may be a useful ancillary treatment to antibiotics in childhood sinusitis and effective in reducing the cough and nasal discharge earlier in the course of acute sinusitis. Clinical signs and symptoms decreased significantly in both groups in comparison to baseline (P < 0.01) and in the intervention group when compared to placebo in the scores for cough and nasal discharge at the end of the second week (P < 0.05). This study was not included in the meta‐analyses as it had a high drop‐out rate (41%), drop‐outs were not described separately for both groups, outcomes were reported as weekly scores using non‐parametric tests and it was not possible to extract data for our outcomes. One of the included studies (Nayak 2002) found a significant improvement in the total symptom score and in individual symptom scores during the treatment period.
The mean change in the score from computerised tomography (CT) scans of the sinuses from baseline to day 21 was not statistically significant between the treatment and control groups. One other included study (Dolor 2001) found the median number of days to clinical success in those treated with INCS was six days compared to nine and a half days in those treated with a placebo. The subjective level of work performance at 21 days was significantly better in the treatment group. Improvement in sinusitis symptoms scores, sinusitis‐related quality of life and the total number of hours of work missed were not significantly different in the two groups. Mometasone furoate (MFNS) 400 µg daily demonstrated significant superiority over MFNS 200 µg daily in nasal congestion/stuffiness score (P = 0.013) and global response to treatment (P = 0.002) was more consistently superior across the endpoints and over amoxicillin in one study (Meltzer 2005), suggesting that higher doses are needed. Also, this study found significant improvement in the major symptom score (P < 0.001), total symptom score (P < 0.001), global response to treatment (P = 0.001) and individual symptom scores (rhinorrhoea, nasal congestion/stuffiness, sinus headache, facial pain) for MFNS 400 µg over placebo.
The results of these studies and reviews support the current clinical rationale of adding an INCS to antibiotic therapy for acute episodes of rhinosinusitis and suggest that higher doses are needed; effectiveness as monotherapy remains to be demonstrated by further studies. The included studies enrolled adults and children and the samples were representative of participants that physicians would recognise as common in their practice. Clinical improvement was assessed by patient‐derived (subjective) symptom reports and this outcome met one of our study goals: evaluating alleviation of symptoms together with possible adverse events.
Overall completeness and applicability of evidence
It is important that the mucous membranes and ciliary function are restored to normal as soon as possible, to avoid recurrence or development of chronic sinusitis (Quarnberg 1992). Two surveys of primary care and specialty physicians suggested considerable variability in approaches to treatment (Piccirillo 2001; Williams 1993). Recommendations for appropriate treatment for acute sinusitis range from symptomatic treatment alone (Snow 2001) to a prolonged course of antibiotic therapy (Winther 1990). A variety of ancillary treatments aimed at improving nasal and sinus ostial patency (antihistamines, decongestants, INCS and nasal irrigation) might be helpful in the treatment of sinusitis but there are few controlled studies to support or deny their effectiveness (Zeiger 1992). Numerous clinical trials attest to the efficacy of topical corticosteroids in controlling symptoms of allergic rhinitis (Juniper 1990; Seigel 1988). The similarity of the respiratory epithelium in the nose and paranasal sinuses, as well as the contiguity of these areas, would lead one to expect that sinusitis might also be treatable with inhaled corticosteroids.
Whether nasal steroid therapy can sufficiently decrease nasal inflammation and improve mucociliary transport to the point where the ostiomeatal complex becomes competent is unknown. Topical corticosteroids offer the theoretical advantage of a localised therapeutic action in nasal tissues, without the occurrence of undesirable systemic effects (Sahay 1980). Inhaled corticosteroids have been used safely in patients with allergic rhinitis or asthma. There exists a theoretical concern regarding the potential spread of infection in acute sinusitis. However, this does not occur when topical corticosteroids are administered concurrently with antibiotics (Druce 1990; Druce 1991). Investigations of whether INCS promotes resolution of symptoms and prevents recurrences of sinusitis have yielded conflicting results (Meltzer 1993; Quarnberg 1992).
Acute sinusitis is a very common infection in childhood but its management remains a controversial issue. A considerable proportion of children, especially those with mild or improving symptoms, may not have to be treated at all (Contopoulos 2003). Management of acute sinusitis usually includes an oral antibiotic. However, it has been estimated that about 45% of cases will resolve without antibiotics (Spector 1998).
Considering the host of symptoms associated with acute rhinosinusitis, recovery can take time and be of substantial discomfort to the affected patient. The burden of affected individuals in terms of decreased productivity, absenteeism from the workplace and diminished quality of life, when added to the cost of care and the growing public health menace of antibiotic‐resistant bacteria, makes rhinosinusitis a serious disease that warrants a precise diagnosis and effective therapy. Recognised pitfalls in acute rhinosinusitis management are the injudicious use of antibiotics and antihistamines (Winstead 2003). The decision on the best treatment for the specific patient should be based on the severity of symptoms, adapted individually, taking in consideration the existing evidence and the patient's preferences.
Most clinicians diagnose acute sinusitis using only clinical symptoms, without additional diagnostic tests. Over‐diagnosis of acute bacterial rhinosinusitis is not surprising, considering the lack of specific clinical features that distinguish it from non‐bacterial upper respiratory tract infections. Often, patients and physicians believe that an upper respiratory tract infection has gone on too long and that antibiotic treatment is therefore needed. Symptomatic treatment and reassurance are the preferred initial management strategy for patients with mild symptoms. Antibiotic therapy should be reserved for patients with severe symptoms who meet the criteria for the clinical diagnosis of acute bacterial rhinosinusitis, regardless of the duration of the illness. The greatest barrier to efficient antibiotic treatment of acute bacterial rhinosinusitis is the lack of a simple and accurate diagnostic test. Until a better test is widely available in clinical practice, the primary diagnosis of acute bacterial rhinosinusitis will remain imprecise (Snow 2001).
Quality of the evidence
Currently, nasal steroid therapy has become an acceptable adjunct in treating both acute and chronic sinusitis. Several intranasal steroids are now available: flunisolide, beclomethasone, triamcinolone, fluticasone, budesonide and mometasone. Each of these has proven to be effective in the treatment of allergic rhinitis and may be a useful addition in sinus disease (Spector 1998). The International Consensus Conference Proceedings on Rhinitis recommends the use of INCS as a first‐line therapy, since they have been found to be well‐tolerated and effective with minimal adverse events (Gawchik 2000).
The evidence available suggests that some intranasal steroids, such as beclomethasone dipropionate, may slow growth when used regularly for prolonged periods (Allen 2000). Studies of MFNS in adults and children with allergic rhinitis showed a lack of hypothalamic‐pituitary axis suppression, no childhood growth suppression and were consistent with extremely low bioavailability of MFNS after intranasal administration (Brannan 1997; Davies 1997; Schenkel 2000). Reducing the systemic activity of nasal corticosteroids to the lowest possible level is desirable. Pharmacologically, newer drugs such as MFNS and fluticasone propionate appear to have substantially higher topical potencies, higher lipid solubilities and lower systemic bioavailabilities than older compounds. With respect to adverse events, emerging data suggest that MFNS and fluticasone may have less potential for systemic effects during prolonged use, particularly in children (Corren 1999). For short‐term therapy of one to two months, the first‐generation INCS (beclomethasone, triamcinolone, budesonide and flunisolide) could be used and MFNS and fluticasone (second‐generation drugs) could be considered for long‐term therapy. With the exception of fluticasone for children aged four years and older and MFNS for those aged three years and older, the other INCS including beclomethasone, triamcinolone, budesonide and flunisolide are approved for children six years and older. All are effective, so the major considerations are cost and safety (Galant 2001).
The decision on the best treatment for the specific patient should be based on the severity of symptoms, adapted individually, taking in consideration the existing evidence and the patient's preferences.
Potential biases in the review process
A small number of studies were included in this review and not all reported an adequate concealment of allocation to treatment.
Agreements and disagreements with other studies or reviews
The minor effects of inhaled corticosteroids for acute sinusitis observed in this review are supported by other existing evidence, including the evidence mentioned here.
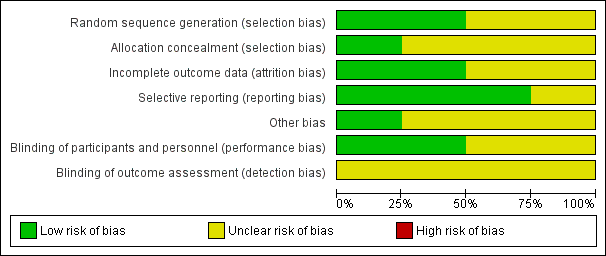
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily).
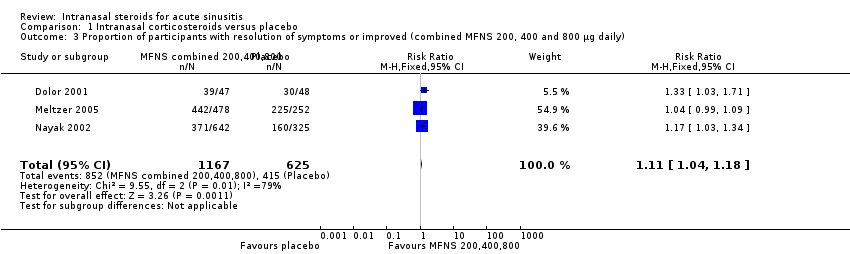
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily).
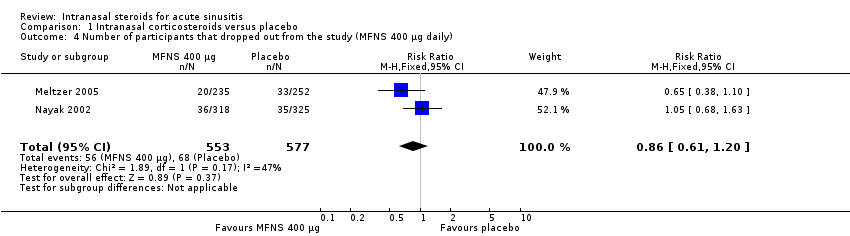
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 4 Number of participants that dropped out from the study (MFNS 400 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 5 Number of participants that dropped out from the study (MFNS 200 µg daily).
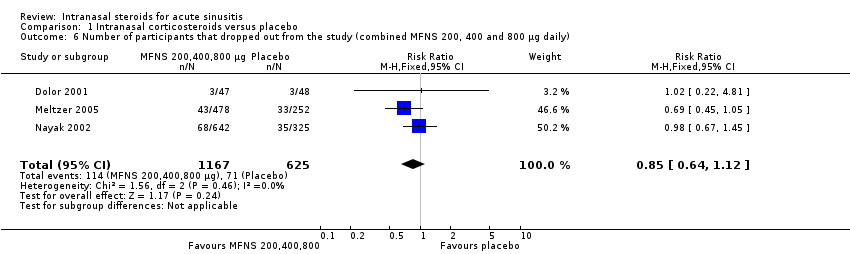
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily).

Comparison 1 Intranasal corticosteroids versus placebo, Outcome 7 Relapse (combined 200 and 400 µg daily).
| Study | Intervention | Side effects | Comments |
| Fluticasone propionate 2 puffs ‐ total dose 200 µg or placebo nasal spray once daily in addition to 250 mg cefuroxime axetil orally twice daily and 2 puffs of xylometazoline hydrochloride twice daily | Headache, bloody nose, vaginal itching, yeast infection, nausea, stomach irritation, diarrhoea, increased congestion, hay fever, light‐headed, sore throat, thirsty, itching, rash, cough, fatigue, metallic taste, felt dried out, nasal tissue felt inflamed | No serious unexpected adverse events reported | |
| Amoxicillin‐clavulanate potassium 875 mg | Epistaxis was the most frequently reported adverse event | Treatment well‐tolerated, adverse events similar for all 3 arms of mild/moderate intensity: 12%, 15%, 15% in the MFNS 400, 800 µg and placebo arms | |
| Budesonide 50 µg or placebo nasal spray to each nostril bid in addition to amoxicillin clavulanate potassium 40 mg/kg/day tid | Rash after 1 week attributed to the antibiotic in 1 subject that was switched to cefaclor | No specific adverse events related to the INCS use were reported | |
| MFNS 200 µg once daily or twice daily nasal spray | Headache and epistaxis were most common reported | Most adverse events were mild or moderate with a similar incidence among treatment groups: 36.2%, | |
| bid: twice daily | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with resolution of symptoms or improved (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| 2 Proportion of participants with resolution of symptoms or improved (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| 3 Proportion of participants with resolution of symptoms or improved (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 4 Number of participants that dropped out from the study (MFNS 400 µg daily) Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 5 Number of participants that dropped out from the study (MFNS 200 µg daily) Show forest plot | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.21] |
| 6 Number of participants that dropped out from the study (combined MFNS 200, 400 and 800 µg daily) Show forest plot | 3 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| 7 Relapse (combined 200 and 400 µg daily) Show forest plot | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |

