Pentasaccharides for the prevention of venous thromboembolism
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multi‐centre, randomised, open‐label, dose‐finding study | |
| Participants | Total number of participants: 243 Number of participants allocated to each group: fondaparinux (FX) 4 mg group: 86, FX 2 mg group: 78, enoxaparin (EN) 40 mg group: 79 Number of participants excluded and/or lost to follow‐up: FX 4 mg group: 4 (1 adverse event, 1 lack of efficacy, 2 other reasons), FX 2 mg group: 3 (1 adverse event, 0 lack of efficacy, 2 other reasons), EN 40 mg group: 3 (1 adverse event, 1 lack of efficacy, 1 other reasons) Inclusion: Men and postmenopausal women aged > 40 years with body weight 50 to 100 kg inclusive who were undergoing first single non‐revision total hip replacement (subsequently amended to non‐revision total hip replacement), with no contraindication to undergo phlebography on day 8 ± 1 Exclusion: Patients were excluded from study participation on the basis of their bleeding risk at the time of randomisation (e.g. known bleeding tendency, thrombocytes < 150 × 109/L, prothrombin time < 65%, APTT/control > 1.2 or other medical conditions associated with a bleeding risk), other significant conditions (e.g. history of PE or DVT, serum creatinine > 2.3 mg% (200 µmol/L), severe hepatic disease or uncontrolled severe high blood pressure (systolic blood pressure/diastolic blood pressure > 200/120 mmHg) or use of anticoagulant or fibrinolytic therapy within 1 week before randomisation. | |
| Interventions | FX: Phase I: 4 mg FX once daily. Phase II: 2 mg FX once daily; FX was administered for 7 days from day 2 (first injection planned 6 hours after surgery) to day 8. EN: Phase I: 40 mg EN once daily (first control group (CG). Phase II: 40 mg EN once daily (second CG). EN was administered for 8 days, from day 1 to day 8 (first injection planned 12 hours before surgery and first postoperative injection planned 6 hours after surgery). | |
| Outcomes | Primary efficacy outcome: incidence of any DVT; DVT was assessed on day 8 ± 1 by phlebography Primary safety outcome: major bleeding; major bleeding was defined as a clinically overt haemorrhage (except drain < 500 mL/d) in addition to 1 of the following criteria: haemoglobin (Hb) reduction to < 8 g/dL or Hb decrease > 2 g/dL over any 48‐hour period between day 3 and day 9 inclusive, or reoperation or intracranial bleeding or retroperitoneal or withdrawal | |
| Notes | Use of adjunctive prophylaxis methods: No adjunctive method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label, dose finding study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label, dose finding study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mention that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A central evaluation was performed blindly by two independent experts" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 95.3%, 96.2%, 96.2% of participants in the 3 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, double‐blind, parallel‐group trial | |
| Participants | Total number of participants: 1309 Number of participants allocated to each group: fondaparinux (FX) group: 650, placebo group: 659 Number of participants excluded and/or lost to follow‐up: FX group: 57 (23 adverse events, 1 lack of efficacy, 33 other reasons), placebo group: 58 (17 adverse events, 2 lack of efficacy, 39 other reasons) Inclusion: Patients were eligible if they were undergoing abdominal surgery (defined as surgery between the diaphragm and the pelvic floor), lasting longer than 45 minutes (from anaesthesia induction to incision closure); over 40 years old | |
| Interventions | FX: 2.5 mg FX sodium given sc starting 6 to 8 hours postoperatively, then once daily for 7 ± 2 days (day 1 was the day of surgery) or until the mandatory venography was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 10, but not more than 1 calendar day after the last study treatment administration. All participants were to receive IPC therapy concomitantly. Placebo: Placebo was given sc starting 6 to 8 hours postoperatively, then once daily for 7 ± 2 days (day 1 was the day of surgery) or until mandatory venography was performed, whichever came first. Mandatory venography had to be performed between day 5 and day 10, but not more than 1 calendar day after the last study treatment administration. All participants were to receive IPC therapy concomitantly. | |
| Outcomes | Primary efficacy outcome: cluster of 1 or more of the following VTE outcomes, evaluated (by an independent adjudicating committee) up to the first venography or up to day 10, whichever came first: venogram positive for DVT between day 5 and day 10, symptomatic DVT and/or non‐fatal PE, fatal PE Primary safety outcome: incidence of major bleeding (any investigator‐reported unusual bleeding) recorded during treatment period (between first injection of study drug and 2 calendar days after last injection) and adjudicated as a major bleeding event by the Central Adjudication Committee (CAC). Major bleeding was defined as: fatal bleeding, surgical bleeding leading to intervention; non‐surgical site bleeding: retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, adrenal gland, pericardium, spine) or leading to intervention, and/or a bleeding index ≥ 2. | |
| Notes | Use of adjunctive prophylaxis methods: Both groups received background mechanical prophylaxis with IPC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study……2.5 mg FX sodium (or FX placebo) given subcutaneously (s.c.)" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "evaluated (by an independent adjudicating committee)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Less than 10% (9% and 8.9% of the 2 study groups) of participants withdrawn Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, open‐label study | |
| Participants | Total number of participants: 127 Number of participants allocated to each group: fondaparinux (FX) group: 83, intermittent pneumatic compression (IPC) group: 44 Number of participants excluded and/or lost to follow‐up: FX group: 7 (2 adverse events, 0 lack of efficacy, 5 other reasons), IPC group: 1 (0 adverse events, 0 lack of efficacy, 1 other reasons) Inclusion: patients aged 40 years undergoing the following abdominal (area between diaphragm and pelvic floor) surgery under general anaesthesia lasting longer than 45 minutes: general or urological surgery, cancer surgery, gynaecological surgery, radical surgery for pelvic malignancy Exlcusion: Patients were excluded if any of the exclusion criteria based on contraindications and precautions for use of anticoagulants currently approved in Japan (e.g. active, clinically significant bleeding, bleeding tendency) or exclusion criteria related to venography (e.g. severe renal disorder, hypersensitivity to contrast media) were applied, or if any of the prohibited medications were used within 1 week before first study drug administration, or use of IPC was contraindicated or inappropriate. | |
| Interventions | FX: 2.5 mg FX was administered once daily by sc injection for 4 to 8 days. First injection of study drug was given 24 ± 2 hours after surgical closure. Second and subsequent injections of study drug were given at approximately the same time every day as far as possible (but longer than 12 hours after the first dose). IPC was prohibited during surgical and treatment periods. IPC: IPC was initiated before or after surgery and was continued until an appropriate time point. Procedures and methods usually employed at each individual study centre were followed as a rule. | |
| Outcomes | Primary efficacy outcome: rate of VTE (symptomatic PE and any DVT) during main efficacy period Primary safety outcomes: major bleeding Major bleeding defined as:
BI calculated as "number of units* transfused" within 48 hours of the bleed + prebleed Hb (g/dL) – postbleed Hb within 48 hours of the bleed (g/dL) * 450 mL of whole blood or red blood cells derived from 450 mL of whole blood is considered as 1 unit. | |
| Notes | Use of adjunctive prophylaxis methods: No adjunctive prophylaxis method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A multicenter, randomised, open‐label study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "A multicenter, randomised, open‐label study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mention that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Rate of VTE (symptomatic PE and any DVT) during main efficacy period, adjudicated by Central Independent Adjudication Committee of Efficacy (CIACE)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 85.5% and 93.2% of randomised participants in the 2 study groups respectively finished their treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, multi‐national, randomised, double‐blind, placebo‐controlled study | |
| Participants | Total number of participants: 849 Number of participants allocated to each group: fondaparinux group: 429; placebo group: 420 Number of participants excluded and/or lost to follow up: fondaparinux group: 4; placebo group: 6 Inclusion: Participants were acutely ill medical patients, aged ≥ 60 years and expected to require bed rest for at least 4 days at the moment of inclusion; hospitalised for congestive heart failure New York Heart Association (NYHA) class III/IV, and/or acute respiratory illness in the presence of chronic lung disease, and/or acute infectious or inflammatory disease Exclusion: Patients were excluded from study participation on the basis of their bleeding risk at the time of randomisation (e.g. active clinically significant bleeding or medical conditions associated with a bleeding risk) criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 48 hours before randomisation. | |
| Interventions | Fondaparinux (FX): Administration of 2.5 mg FX (2.5 mg once daily as sc injection) started 2 hours after randomisation. Study treatment was to be given at least up to and including day 6 but not after day 14. Venography had to be performed within 1 day after cessation of treatment on days 6 to 15, or earlier in case of symptomatic VTE. Placebo: Placebo was started 2 hours after randomisation. Study treatment was to be given at least up to and including day 6 but not after day 14. Venography had to be performed within 1 day after cessation of treatment on days 6 to 15, or earlier in case of symptomatic VTE. | |
| Outcomes | Primary efficacy outcome: composite of the following VTE events recorded up to day 15 or up to the first venography, whichever came first: venogram positive for DVT, symptomatic DVT, non‐fatal PE or fatal PE. Venography and all other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc) were blindly adjudicated by experts of the Central Independent Adjudication Committee (CIAC). Primary safety outcome: major bleeding during treatment and 2 days thereafter, defined as fatal bleeding, bleeding in a critical location, bleeding leading to surgical intervention or overt bleeding associated with a drop in haemoglobin (Hb) concentration ≥ 20 g/L or leading to transfusion of 2 or more units of red blood cells Efficacy and safety outcomes were adjudicated by a central independent committee (CIAC), whose members were unaware of the treatment assignment. Accumulated safety data were regularly reviewed by an independent committee. | |
| Notes | Use of adjunctive prophylaxis methods: Use of aspirin or non‐steroidal anti‐inflammatory drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out using a predefined central randomisation list, balanced in blocks of four" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Multi‐centre, multi‐national, randomised, double‐blind, placebo‐controlled study Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Study drugs were provided in identical boxes" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All venograms ……were blindly adjudicated by experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 89.4% and 88.7% of randomised participants in the 2 study groups, respectively, finished their treatment Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Prospective, randomised, 3‐arm study | |
| Participants | Total number of participants: 355 Number of participants allocated to each group: variable‐dose warfarin group: 118, fondaparinux group: 118, 1 mg daily warfarin group: 119 Number of participants excluded and/or lost to follow‐up: 2 in variable‐dose warfarin group did not receive allocated intervention, 1 was not analysed; 10 in fondaparinux group did not receive allocated intervention, 6 were not analysed; 7 in 1 mg daily warfarin group did not receive allocated intervention, 6 were not analysed Inclusion criteria: Participants were recruited from among patients over 20 years of age planning elective primary unilateral total hip or knee replacement surgery at an orthopaedic specialty hospital. Exclusion criteria
| |
| Interventions | ARM A Fixed low‐dose warfarin 1.0 mg daily, beginning 7 days preoperatively, and continued at 1.0 mg daily until day 28 ± 2 of follow‐up | |
| Outcomes | Primary endpoint was composite DVT, PE or death due to VTE. Secondary endpoints included frequency of proximal vs distal DVT, estimated blood loss (EBL) at surgery and haemorrhagic complications. | |
| Notes | Use of adjunctive anticoagulative methods: Patients had early postoperative ambulation. All patients wore pneumatic compression stockings while in‐patients. Elastic compression stockings were prescribed to be used after discharge until follow‐up ultrasonography. Hydroxyethyl starch (HES) 6% was allowed intraoperatively for case‐specific reasons. Use of platelet function suppressive drugs, such as non‐steroidal anti‐inflammatory drugs (NSAIDs), was discouraged but was not prohibited by the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A member of the pharmacy department pulled randomized cards as prepared by the statisticians" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "A member of the pharmacy department pulled randomized cards as prepared by the statisticians" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Radiology technicians and the radiologists were blinded to patient randomization" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 99.1%, 94.9% and 94.9% of participants in 3 groups completed the study. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All endpoints listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias identified |
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, 2‐parallel‐group study | |
| Participants | Total number of participants: 3002 Number of participants allocated to each group: fondaparinux (FX) group: 1502, placebo group: 1500 Number of participants excluded and/or lost to follow‐up: FX group: 21 (2 adverse events, 0 lack of efficacy, 19 other reasons), placebo group: 33 (1 adverse event, 0 lack of efficacy, 32 other reasons) Inclusion: Hospitalised and non‐hospitalised male and female patients 18 years of age or older with acute symptomatic isolated superficial venous thrombosis (SVT) of the lower limbs at least 5 cm long, documented by standard compression ultrasonography (CUS) were eligible to enter the study. Exclusion: Patients at high risk of VTE were excluded (e.g. those with DVT on the qualifying ultrasound exam and/or documented PE at inclusion, with SVT within 3 cm from the sapheno‐femoral junction (SFJ) requiring ligation of the SFJ or thrombectomy, with active cancer, or with documented DVT or PE within the previous 6 months). | |
| Interventions | FX: 2.5 mg FX sc once daily (self‐administered or not self‐administered). Treatment was presented as prefilled (0.5 mL) syringes. Duration of treatment was 45 days with 30‐day follow‐up. Placebo: matching placebo administered sc once daily (self‐administered or not self‐administered). Treatment was presented as prefilled (0.5 mL) syringes. Duration of treatment was 45 days with 30‐day follow‐up. | |
| Outcomes | Primary efficacy outcome: incidence of VTE and/or death from any cause recorded up to day 47. VTE was defined as a composite of symptomatic DVT, symptomatic PE, symptomatic extension of SVT or symptomatic recurrence of SVT. All VTEs were confirmed by objective tests and were then adjudicated by an independent central adjudication committee (CAC), whose members were blinded to treatment assignment. Primary safety outcome: major bleeding | |
| Notes | Use of adjunctive methods: Participants were encouraged to use graduated compression stockings and were allowed to take acetaminophen or topical non‐steroidal anti‐inflammatory drugs as needed. Use of oral antiplatelet agents or aspirin at a low dose (≤ 325 mg per day) was discouraged. In total, 1131 participants in FX group used graduated stockings, and 347 participants used antiplatelet agents of all 1502 participants; 1147 participants in placebo group used graduated stockings, and 364 used antiplatelet agents of all 1500 participants, as adjunctive anticoagulative methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "With the use of a central telephone system and a computer‐generated randomisation list" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "With the use of a central telephone system and a computer‐generated randomisation list" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "to fondaparinux at a dose of 2.5 mg or matching placebo" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "adjudicated by an independent central adjudication committee (CAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 1481 (98.6%) and 1467 (97.8%) participants in the 2 study groups finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Double‐blind, prospective, randomised, controlled trial | |
| Participants | Total number of participants: 148 Number of participants allocated to each group: fondaparinux group: 74, placebo group: 74 Number of participants excluded and/or lost to follow‐up: FX group: 0, placebo group: 0 Inclusion: All adult patients with a diagnosis of primary osteoarthritis of the knee and undergoing elective unilateral primary TKA were considered for inclusion in the study. Exclusion: patients undergoing bilateral knee replacements; patients with diagnosed chronic or acute DVT preoperatively; patients with active bleeding or documented congenital or acquired bleeding disorders such as haemophilia, current ulcerative or angiodysplastic gastrointestinal disease; hemorrhagic stroke or brain, spinal or ophthalmologic surgery within the previous 3 months. Additional exclusion criteria were contraindication to anticoagulant therapy, serum creatinine concentration > 2 mg/dL in a well‐hydrated patient and platelet count < 100,000 per cubic millimetre. | |
| Interventions | FX: subcutaneous doses of 2.5 mg of fondaparinux (Arixtra; GlaxoSmith‐Kline, UK) once daily Placebo: 0.25 mL of isotonic saline once daily The first postoperative injection was administered 6 to 8 hours after surgery, and the second injection was given 24 hours after the first. The day of surgery was defined as day 1. Treatment was scheduled to continue with a daily single dose until day 5. | |
| Outcomes | Primary efficacy outcome: prevalence of DVT ‐ total, proximal and distal ‐ and symptomatic PE up to day 7 Primary safety outcome: incidence of major bleeding. Major bleeding included clinically overt bleeding requiring transfusion of ≥ 2 units of blood products (considering 450 mL of reinfused shed blood as 1 U), bleeding with a serious or life‐threatening clinical event or requiring surgical intervention, bleeding in retroperitoneal, intracranial or intraocular locations or bleeding resulting in death. | |
| Notes | Use of adjunctive anticoagulative methods: Graduated compression stockings were applied in all participants. All were managed by the same rehabilitation protocol, which included range of motion, quadriceps, hamstring and | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "a double‐blind, prospective randomised controlled trial" and "Eligible patients were randomly assigned prior to the surgery through a computer‐derived randomisation table with block sizes of four to receive……" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule was known to the research pharmacist who prepared the study medication but was not involved in any way with the care of the patients. The patients, surgeon, health care providers, and outcome assessors were blinded to the randomisation till the end of the study" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, surgeon, health care providers, and outcome assessors were blinded to the randomisation till the end of the study" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The radiologist was blinded to the treatment assignment" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | All participants in the 2 groups finished treatment, and primary efficacy and safety results were assessed. |
| Selective reporting (reporting bias) | Low risk | All results planned in the study design section were assessed. |
| Other bias | Low risk | No risk of other bias was identified. |
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group, dose‐response study | |
| Participants | Total number of participants: 411 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 82, FX 1.5 mg group: 82, FX 2.5 mg group: 82, FX 3.0 mg group: 83, placebo group: 82 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 8 (6 adverse events, 0 lack of efficacy, 2 other reasons), FX 1.5 mg group: 6 (5 adverse events, 0 lack of efficacy, 1 other reasons), FX 2.5 mg group: 3 (3 adverse events, 0 lack of efficacy, 0 other reasons), FX 3.0 mg group: 5 (5 adverse events, 0 lack of efficacy, 0 other reasons), placebo group: 3 (2 adverse event, 0 lack of efficacy, 1 other reasons) Inclusion: Patients undergoing primary elective THR surgery or revision of a THR; ≥ 20 years of age Exclusion: Exclusion criteria were based on the Japanese labelling for anticoagulants in force at the time study was conducted (e.g. active, clinically significant bleeding; documented congenital or acquired bleeding tendency/disorders, other medical condition associated with a bleeding risk), or criteria related to use of contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 1 week before first dose of study medication. | |
| Interventions | FX: once daily sc dosing of FX 0.75, 1.5, 2.5 or 3.0 mg for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. The first dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration Placebo: once daily sc placebo for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 17 or to first venography, whichever occurred first: adjudicated mandatory venogram positive for DVT between day 11 and day 17; adjudicated symptomatic DVT; adjudicated positive fatal or non‐fatal PE. All venography sessions, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE). Primary safety outcome: incidence of major bleeding (any investigator‐reported bleeding adjudicated as a major bleeding event by the Central Independent Adjudication Committee of Safety (CIACS)) recorded during \treatment period (i.e. from first injection of study drug to 2 days after last dose). Major bleeding was defined as fatal bleeding, or clinically overt bleeding including retroperitoneal or intracranial bleeding or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/hematoma at the operative site; clinically overt bleeding leading to Hb fall > 2 g/dL (1.6 mmol/L) within 48 hours of the bleed; clinically overt bleeding that required a transfusion of red blood cell or whole blood derived from > 900 mL of whole blood within 48 hours of the bleed (excluding autologous transfusion except for treatment of bleeding adverse event (AE)); clinically overt bleeding leading to bleeding index > 2 (within 48 hours of the bleed, calculated as "number of units transfused" + prebleed Hb (g/dL) – postbleed Hb (g/dL) | |
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, placebo controlled, parallel group, dose response study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, placebo controlled, parallel group, dose response study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 56 of 71 randomised participants, 62 of 82 randomised participants, 63 of 82 randomised participants, 67 of 82 randomised participants, 68 of 83 randomised participants in the 5 study groups, respectively, finished their treatment and were involved in the efficacy analysis. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group, dose‐response study | |
| Participants | Total number of participants: 432 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 86, FX 1.5 mg group: 87, FX 2.5 mg group: 86, FX 3.0 mg group: 86, placebo group: 87 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 4 (4 adverse events, 0 lack of efficacy, 0 withdrawn consent, 0 other reasons), FX 1.5 mg group: 7 (3 adverse events, 0 lack of efficacy, 4 withdrawn consents, 0 other reasons), FX 2.5 mg group: 6 (2 adverse events, 0 lack of efficacy, 4 withdrawn consents, 0 other reasons), FX 3.0 mg group: 5 (4 adverse events, 0 lack of efficacy, 0 withdrawn consent, 1 other reasons), placebo group: 7 (4 adverse events, 0 lack of efficacy, 1 withdrawn consent, 2 other reasons) Inclusion: patients who were undergoing elective primary TKR surgery or revision surgery of a TKR; ≥ 20 years of age Exclusion: Exclusion criteria were based on the Japanese labelling for anticoagulants in force at the time study was conducted (e.g. active, clinically significant bleeding; documented congenital or acquired bleeding tendency/disorders or other medical conditions associated with a bleeding risk), criteria related to use of contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 1 week before first dose of study medication. | |
| Interventions | FX: once daily sc dosing of FX 0.75, 1.5, 2.5 or 3.0 mg for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration. Placebo: once daily sc placebo for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but no later than 2 calendar days after last study drug administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 17 or to first venography, whichever occurred first: adjudicated mandatory venogram positive for DVT between day 11 and day 17; adjudicated symptomatic DVT; adjudicated positive fatal or non‐fatal PE. All venography procedures, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE). Primary safety outcome: incidence of major bleeding (any investigator‐reported bleeding adjudicated as a major bleeding event by the Central Independent Adjudication Committee of Safety (CIACS)). This was recorded during treatment period (i.e. from first injection of study drug to 2 days after the last dose). Major bleeding was defined as fatal bleeding, clinically overt bleeding including retroperitoneal or intracranial bleeding or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/haematoma at the operative site; clinically overt bleeding leading to Hb fall > 2 g/dL (1.6 mmol/L) within 48 hours of the bleed; clinically overt bleeding that required a transfusion of red blood cell or whole blood derived from > 900 mL of whole blood within 48 hours of the bleed (excluding autologous transfusion, except for treatment of bleeding adverse event (AE)); clinically overt bleeding leading to bleeding index > 2 (within 48 hours of the bleed, calculated as "number of units* transfused" + prebleed Hb (g/dL) – postbleed Hb (g/dL)). Other safety variables were minor bleeding (defined as clinically overt bleeding not meeting the criteria for major bleeding and considered more than expected in the clinical context), transfusion requirements, AEs/serious AEs (SAEs) and deaths. | |
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, PBO controlled, parallel group, dose response study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, PBO controlled, parallel group, dose response study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A central, independent adjudication committee reviewed both safety and efficacy outcomes" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 74 of 85 randomised participants, 74 of 85 randomised participants, 74 of 85 randomised participants, 74 of 85 randomised participants and 74 of 85 randomised participants in the 5 study groups, respectively, finished treatment and were involved in the efficacy analysis. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Randomised, double‐blind, pilot trial | |
| Participants | Total number of participants: 198 Number of participants allocated to each group: fondaparinux group: 100, enoxaparin group: 98 Number of participants excluded and/or lost to follow‐up: Of the 198 randomised participants, 7 in the fondaparinux group and 7 in the enoxaparin group did not receive treatment according to protocol, but all randomised participants were analysed. Inclusion: Patients were eligible for the study if they were 18 years of age or older with a body mass index (BMI) of 35 to 59 kg/m2, and were undergoing laparoscopic vertical sleeve gastrectomy (VSG) or laparoscopic Roux‐en Y gastric bypass (LRYGB) Exclusion: Patients with BMI > 60 were excluded, as they may have required extended DVT prophylaxis. Patients with contraindications to low molecular weight heparin or selective antithrombin III agonists, previous history of DVT or PE, documented clotting/coagulation disorders, history of treatment for cancer within the past year, history of venous stasis or superficial thrombophlebitis, vein stripping or ligation, obesity hypoventilation syndrome or recent history of smoking (within the past year) were excluded. Patients with severe hepatic impairment, creatinine clearance < 30 mL per minute or platelet count < 100,000 per cubic millimetre were also excluded, as were women of childbearing age if they were pregnant or were taking oestrogen‐based birth control medication within 1 month of surgery | |
| Interventions | FX: The fondaparinux group received a placebo on call to the operating room. Six hours after surgery stop time, participants were given 5 mg fondaparinux subcutaneously. Beginning on postoperative day 1, participants received 5 mg of fondaparinux subcutaneously once daily in the morning and placebo (saline) injections subcutaneously once daily in the evening for the duration of their hospital stay. Enoxaparin: In accordance with current practice, the enoxaparin group received a dose of enoxaparin 40 mg subcutaneously on call to the operating room. To maintain blinding, participants randomised to enoxaparin received placebo (saline) injection 6 hours after surgery stop time. Beginning on postoperative day 1, 40 mg of enoxaparin was administered subcutaneously twice daily for the duration of the participant's hospital stay. | |
| Outcomes | Primary outcome was the effect of preoperative enoxaparin vs postoperative fondaparinux prophylaxis on antifactor Xa concentrations in participants undergoing bariatric surgery. Attainment of a target antifactor Xa level was determined on the basis of blood samples drawn 3 hours after the drug was received on postoperative day 1. This cutoff was the standard for adequate prophylaxis used by our in‐patient haematology lab (Z 0.20 IU/mL for enoxaparin and Z 0.39 mg/L for fondaparinux). Secondary outcomes were asymptomatic DVT, defined as a positive MRV within 2 weeks after surgery, and symptomatic DVT. Safety outcomes included perioperative bleeding, perioperative complications and death. | |
| Notes | Use of adjunctive anticoagulative methods: All participants had sequential compression devices and antiembolic stockings placed before induction of anaesthesia; 4 to 6 hours after surgery stop time, participants were ambulated in the hallways. Sequential compression devices were removed during ambulation. Use of aspirin, non‐steroidal anti‐inflammatory drugs and other antiplatelet agents was prohibited during participants' hospital stay. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated randomization scheme (Microsoft Excel 2007 data analysis tool pack)" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was performed by the pharmacy and was concealed from patients and study personnel" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "placebo doses were prepared to maintain the blind. Active and placebo syringes were prepared by our inpatient pharmacy and were not identifiable by external appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "asymptomatic DVT, defined as a positive MRV within 2 weeks following surgery, and symptomatic DVT" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 83 of 98 in the enoxaparin group, 94 of 100 in the fondaparinux group had MRV results. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company provided study material and additional financial support but was not involved in the design and procedure of the study. |
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study | |
| Participants | Total number of participants: 2309 Number of participants allocated to each group: fondaparinux (FX) group: 1155, enoxaparin (EN) group: 1154 Number of participants excluded and/or lost to follow‐up: FX group: 70 (18 adverse events, 7 lack of efficacy, 45 other reasons), EN group: 58 (15 adverse events, 5 lack of efficacy, 38 other reasons) Inclusion: Patients were eligible if they were undergoing an elective, primary THR surgery or a revision of at least 1 component of a THR; ≥ 18 years of age; men and women of non‐childbearing potential or of childbearing potential and having a negative pregnancy test within 48 hours before surgery or first study drug administration, whichever came first; written informed consent Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time of study conduct (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk), criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 2 days before first dose of study medication. | |
| Interventions | FX: administration of FX (2.5 mg once daily sc injection) started postoperatively at 6 ± 2 hours after surgery closure EN: administration of EN (40 mg once daily as sc injection) started preoperatively at 12 ± 2 hours before the start of surgery, then postoperatively at least 12 hours after the preoperative dose but not longer than 24 hours after surgery Placebo: Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method). Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until mandatory venography was performed, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after last study treatment administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; adjudicated PE. All venography procedures, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC). Primary safety endpoint: incidence of major bleeding (any investigator‐reported unusual bleeding adjudicated as a major bleeding event by the CIAC) recorded between first injection of study drug (active drug or placebo) and day 11. Major bleeding was defined as fatal bleeding; clinically overt bleeding including retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, spine, pericardium, adrenal gland); reoperation due to bleeding/haematoma at the operative site; clinically overt bleeding leading to a fall in Hb ≥ 2 g/dL (1.6 mmol/L) and/or transfusion of ≥ 2 units of packed red blood cells or whole blood AND for which the combined calculated index was ≥ 2. Other safety variables were minor bleeding (defined as clinically overt bleeding not meeting the criteria for major bleeding and considered more than expected in the clinical context), transfusion requirements, adverse events (AEs)/serious AEs (SAEs), deaths and changes in laboratory parameters recorded between first injection of study drug and day 11. In addition, all safety parameters were recorded between first injection and day 49. | |
| Notes | Use of adjunctive anticoagulative methods: In FX group, 29 participants received prohibited treatment (intermittent pneumatic compression, dextran, thrombolytic treatment and any other anticoagulant agents), 483 received discouraged treatment (aspirin or non‐steroidal anti‐inflammatory drugs) and 649 wore graduated compression stockings; in EN group, 30 participants received prohibited treatment (intermittent pneumatic compression, dextran, thrombolytic treatment and any other anticoagulant agents), 493 received discouraged treatment (aspirin or non‐steroidal anti‐inflammatory drugs) and 654 wore graduated compression stockings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, double‐dummy, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly described use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, double‐dummy, parallel‐group study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method)" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 93.9% and 94.9% of participants in the 2 study groups, respectively, finished treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, open‐label, controlled, 2‐parallel‐group, Phase III study | |
| Participants | Total number of participants: 1243 Number of participants allocated to each group: fondaparinux (FX) group: 621, nadroparin (NA) group: 622 Number of participants excluded and/or lost to follow‐up: FX group: 14 (3 adverse events, 4 withdrawals by participant, 4 lost to follow‐up, 0 immobilisation, 1 investigator/orthopaedic surgeon decision, 2 orthopaedic surgery, 0 visits not performed, 0 deep vein thrombosis), NA group: 8 (0 due to adverse events, 0 due to lack of efficacy, 8 due to other reasons) Inclusion: requiring rigid or semirigid immobilisation (e.g. with a plaster cast or brace) for at least 21 days and up to 45 days because of isolated non‐surgical below‐knee injury, with a no weight‐bearing recommendation at the time of inclusion (partial weight bearing is permitted, e.g. crutches, walking cast, relief shoes); presenting at least 1 of the following risk factors for venous thromboembolism: below‐knee fracture or Achilles tendon rupture, age ≥ 40 years, body mass index > 30 kg/m2, oestrogen‐containing hormonal replacement therapy or oral contraception, active cancer (treatment ongoing or stopped for less than 1 year), history of VTE, congenital or acquired hypercoagulable stat; requiring thromboprophylaxis according to the investigator's judgement up to complete mobilisation (corresponding to cast or brace removal; able and willing to provide written informed consent) Exclusion: delay between injury and randomisation greater than 2 days; treatment with antithrombotic or anticoagulant therapy, including low‐dose anticoagulation, for longer than 2 days before randomisation; anticoagulant therapy required or likely to be required during the study period for another reason (e.g. planned surgery justifying pharmacological thromboprophylaxis, curative dose for treatment of VTE); shown hypersensitivity to fondaparinux or nadroparin or their excipient; known history of heparin‐induced thrombocytopenia; women of childbearing potential not using a reliable contraceptive method throughout the study period; women pregnant or breast‐feeding during the study period; active, clinically significant bleeding; clinically significant bleeding within past 6 months; major surgery within previous 3 months; intraocular (other than cataract), spinal and/or brain surgery within previous 12 months; haemorrhagic stroke within previous 12 months; severe head injury within previous 3 months; documented congenital or acquired bleeding tendency/disorder(s); previous (within 12 months) or active or currently treated peptic ulcer disease; uncontrolled arterial hypertension (systolic blood pressure over 180 mmHg or diastolic blood pressure over 110 mm Hg); treatment with more than 1 antiplatelet agent (e.g. clopidogrel and aspirin) at any dose; need for long‐term aspirin at doses ≥ 325 mg or long‐term NSAIDs; bacterial endocarditis; severe hepatic impairment; calculated creatinine clearance < 30 mL/min; thrombocytopenia (< 100 x 109/L); body weight < 50 kg; any condition that could prevent the patient from providing written informed consent or from adhering to study treatment; life expectancy < 6 months; participation in any study using an investigational drug during previous 3 months; patient in whom V3 is unlikely to be feasible (e.g. patient moving house); In France, a patient was not be eligible for inclusion in this study if not affiliated with or a beneficiary of a social security system. This is an additional exclusion criterion that applies only to individuals enrolled in France. | |
| Interventions | FX: 2.5 mg in 0.5 mL or 1.5 mg in 0.3 mL for at least 21 days and up to 45 days NA: 2850 anti‐Xa IU in 0.3 mL administered sc once daily. Treatments were presented as prefilled syringes for at least 21 days and up to 45 days | |
| Outcomes | Primary efficacy outcome: composite of VTE and death up to complete mobilisation, corresponding to cast or brace removal (plus 2 days). VTE was defined in this study as asymptomatic DVT detected by systematic compression ultrasonography, symptomatic DVT or symptomatic fatal or non‐fatal PE. Primary safety outcome: major bleeding, non‐major clinically relevant bleeding and minor bleeding up to complete mobilisation (V3) plus 4 days, and up to the final visit or contact | |
| Notes | Use of adjunctive anticoagulative methods: No adjunctive anticoagulative method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "multicenter, randomised, open‐label, controlled, two‐parallel‐group, phase III study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "multicenter, randomised, open‐label, controlled, two‐parallel‐group, phase III study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Multi‐centre, randomised, open‐label, controlled, 2‐parallel‐group, phase III study Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All events were blindly adjudicated by an independent committee" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 97.7% and 98.7% of participants in the 2 study groups, respectively, finished treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Randomised, open‐label, controlled study | |
| Participants | Total number of participants: 43 Number of participants allocated to each group: fondaparinux (FX) group: 21, edoxaban group: 22 Number of participants excluded and/or lost to follow‐up: FX group: 3 participants discontinued, edoxaban group: 2 participants discontinued Inclusion: patients ≥ 20 years of age, with serious renal injury (creatinine clearance ≥ 20 mL/min to < 30 mL/min) who were undergoing unilateral TKA or THA (excluding revision surgeries) or hip fracture surgery for medial or lateral femoral neck fracture (trochanteric or subtrochanteric section of the femur) within 10 days of presurgical examination. Informed consent was obtained from all participants. Exclusion: Presurgical exclusion criteria included, but were not limited to, patients undergoing or possibly undergoing haemodialysis; risk of bleeding; risk of thromboembolism; and hepatic dysfunction. Postsurgical exclusion criteria included, but were not limited to, creatinine clearance < 15 mL/min; abnormal bleeding at the site of spinal anaesthesia; abnormal or excessive bleeding during or immediately after surgery; and inability to take oral medication. | |
| Interventions | Fondaparinux: subcutaneous fondaparinux 1.5 mg sc once daily Edoxaban: oral edoxaban 15 mg once daily | |
| Outcomes | Primary efficacy outcome: incidence of symptomatic VTE (composite of symptomatic DVT or PE) during treatment period Primary safety outcome: major bleeding defined as fatal bleeding; clinically overt bleeding accompanied by a decrease in haemoglobin > 2 g/dL or requiring a transfusion of > 4 units of blood (1 unit = ˜200 mL); retroperitoneal, intracranial, intraocular or intrathecal bleeding; or bleeding requiring repeat surgery | |
| Notes | Use of adjunctive anticoagulative methods: Concomitant physiotherapy (intermittent pneumatic compression devices or elastic stockings) was permitted throughout the treatment period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, controlled study applied permuted block method with SAS software to generate random sequence. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Randomised, controlled study applied permuted block method with SAS software to generate random sequence. Comment: probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label study Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | All thromboembolic events were assessed by a thromboembolic event assessor, who was blinded to treatment group, on the basis of imaging results. Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 18/21 and 20/22 participants in the fondaparinux and edoxaban groups, respectively finished their treatment. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | This study was supported by a pharmaceutical company that was involved in the study design and analysis of the data and provided writing and editorial support. |
| Methods | Randomised, placebo‐controlled, double‐blind study | |
| Participants | Total number of participants: 78 Number of participants allocated to each group: fondaparinux group: 41, placebo group: 37 Number of participants excluded and/or lost to follow‐up: 2 participants in the fondaparinux group and no participants in the placebo group withdrew; all randomised participants were analysed. Inclusion criteria: All patients scheduled to undergo a first or a repeat isolated CABG operation were considered for enrolment in the study. Exclusion criteria
| |
| Interventions | Fondaparinux (FX): Intervention group received 2.5 mg subcutaneous injections of fondaparinux sodium daily, starting at a mean of 12 ± 2 hours after wound closure or on the morning of the first postoperative day. Second dose was administered at a mean of 24 ± 2 hours after the first dose, and subsequent injections were administered once daily for 9 days or until the patient was discharged from the hospital, whichever happened first. Placebo: Control group received similar amounts of subcutaneous isotonic saline on the same schedule as the intervention group. | |
| Outcomes | Primary study endpoint: composite, up to day 11, of cumulative incidence of all VTE events, defined as symptomatic and asymptomatic DVT, and fatal and non‐fatal pulmonary embolisms Primary safety endpoint: cumulative incidence of major haemorrhages | |
| Notes | Use of adjunctive anticoagulative methods: Both groups routinely received graduated compression stockings and/or intermittent pneumatic compression (mechanical antithrombotic prophylaxis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study was conducted in compliance with the Good Clinical Practice guidelines" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "This study was conducted in compliance with the Good Clinical Practice guidelines" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The control group received similar amounts of subcutaneous isotonic saline on the same schedule as the interventional group" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients who developed symptomatic DVT or VTE underwent DUS scan of the lower extremities. An independent Data and Safety Monitoring Board monitored the safety of the study." Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Two patients in the fondaparinux group, who withdrew their consent at 3 and 8 days Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No other bias noted |
| Methods | Multi‐centre, randomised, single‐blind, parallel‐group control study | |
| Participants | Total number of participants: 237 Number of participants allocated to each group: fondaparinux (FX) group: 119, enoxaparin (EN) group: 118 Number of participants excluded and/or lost to follow‐up: FX group: 6 (5 adverse events, 0 lack of efficacy, 1 other reasons), EN group: 3 (2 adverse events, 0 lack of efficacy, 1 other reasons) Inclusion: Male/female patients (aged 18 to 75 years) who were to undergo an elective hip or knee replacement or revision, and who gave written informed consent, were included in the study. | |
| Interventions | FX: 2.5 mg for 7 ± 2 days (once daily sc injection) EN: 40 mg for 7 ± 2 days First treatment injection (placebo or enoxaparin) was administered at 12 ± 2 hours before surgery, then was continued for 7 ± 2 days post surgery, via daily sc injection. | |
| Outcomes | Primary efficacy outcome: overall DVT events as confirmed by colour ultrasound imaging conducted within 2 days after the last dose following orthopaedic surgery Primary safety outcome: major bleeding recorded between day 1 and day 9 post surgery | |
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method Used for sensitivity analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multi‐centre, randomised, single‐blind, parallel control study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multi‐centre, randomised, single‐ blind, parallel control study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Multi‐centre, randomised, single‐blind, parallel control study" Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clearly described Comment: unclear |
| Incomplete outcome data (attrition bias) | Low risk | 94.96% and 97.46% of participants in both groups completed the study. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Randomised, open label, evaluator‐blinded | |
| Participants | Total number of participants: 51 Number of participants allocated to each group: fondaparinux (FX) group: 28, enoxaparin (EN) group: 23 Number of participants excluded and/or lost to follow‐up: FX group: 4 (0 adverse events, 0 lack of efficacy, 4 other reasons), EN group: 1 (1 adverse event, 0 lack of efficacy, 0 other reasons) Inclusion: Patients ≥ 20 years of age scheduled for primary elective total knee replacement surgery were included in the study. Exclusion: Patients were excluded if they had leg oedema, peripheral vascular disease, diabetes with peripheral neuropathy or any condition likely to increase the risk of bleeding. | |
| Interventions | FX: 2.5 mg FX 2.5 sc once daily for 7 days. First postoperative dose was given ≥ 6 hours after closure of the surgical wound, and the second dose 18 to 24 hours after the first dose. Thereafter, daily at 8 PM ± 2 hours for 5 days. | |
| Outcomes | Primary efficacy outcome: incidence of occurrence of VTE events (DVT, as determined by clinical assessment and compression Doppler) up to day 10 Primary safety outcome: Major bleeding, minor bleeding, no bleeding, adverse events (AEs) and serious adverse events (SAEs) were monitored from day 0 up to day 37. | |
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized, open label" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "open‐label" study Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "evaluator‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 85.7% and 95.7% of participants in the 2 study groups completed medication. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Randomised controlled study | |
| Participants | Total number of participants: 36 Number of participants allocated to each group: fondaparinux group: 18, low molecular weight heparin (LMWH) group: 18 Number of participants excluded and/or lost to follow‐up: 0 Inclusion criteria: confirmed infection in trauma patients; hypercoagulopathy: prothrombin time (PT) < 3 seconds or longer than normal, abnormal international normalised ratio (INR) or activated partial prothrombin time (APTT) < 10 seconds or longer than normal; > 18 and < 70 years old; without haematological disorders; an informed consent Exclusion criteria: anticoagulation therapy before enrolment; haematological or bleeding disorders; active or recent‐stroked peptic ulcer; malignant disease; diluting coagulopathy and low platelets counts; hepatic and renal failure | |
| Interventions | Fondaparinux (FX): Participants in group F were given fondaparinux sodium (2.5 mg, 1/d for 11 d). Low molecular weight heparin (LMWH): Participants in group L were given the standard LMWH (4100 U, 1/12 hours for 11 days) recipe and served as controls. | |
| Outcomes | Endpoints of clinical observation were discharge and death. All participants were followed up for 3 months. Clinical parameters included deep vein thrombosis (DVT), bleeding events, occurrence of multiple organ dysfunction syndrome (MODS) and mortality. Laboratory parameters included serum fibrinogen, D‐dimer and antithrombin III. Observations were made on days 1, 3, 5, 7 and 11 after admission. | |
| Notes | Use of adjunctive prophylaxis methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used the randomisation sequence list to generate the random sequence. This information was obtained by contacting the study authors. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Investigators used the randomisation sequence list to generate the random sequence. This information was obtained by contacting the study authors. Comment: probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinded to participants but not to healthcare staff This information was obtained by contacting the study authors. Comment: may affect participants' treatment and outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to outcome evaluator This information was obtained by contacting the study authors. Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 100% of enrolled participants received responsive treatment, and data were analysed. |
| Selective reporting (reporting bias) | Low risk | All endpoints listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias was identified. |
| Methods | Multi‐centre, multi‐national, randomised, double‐blind study | |
| Participants | Total number of participants: 2927 Number of participants allocated to each group: fondaparinux (FX) group: 1465, dalteparin (DA) group: 1462 Number of participants excluded and/or lost to follow‐up: FX group: 127 (62 adverse events, 9 lack of efficacy, 56 other reasons), DA group: 119 (57 adverse events, 2 lack of efficacy, 60 other reasons) Inclusion: Patients undergoing abdominal surgery under general anaesthesia, planned to last longer than 45 minutes (from incision to incision closure), and > 60 years of age with or without any other risk factor for VTE, or > 40 years of age and at risk for thromboembolic complications, were eligible. Patients at risk included those who were obese (body mass index (BMI) > 30 kg/m2 for men and 28.6 kg/m2 for women), undergoing cancer surgery, with a history of DVT or PE, with congestive heart failure (CHF) (grade III or IV of the New York Heart Association (NYHA) classification), with chronic obstructive pulmonary disease or with inflammatory bowel disease Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk, hypersensitivity to heparin or LMWH), or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or criteria related to trial methods (e.g. use of anticoagulant or fibrinolytic therapy within 2 days before first drug administration). | |
| Interventions | FX: 2.5 mg once daily given by sc injection up to day 7 ± 2, with the first injection between 6 and 7 hours after incision closure, provided haemostasis had been established | |
| Outcomes | Primary efficacy outcome: VTE (asymptomatic and/or symptomatic DVT or PE or both) recorded until the time of screening venography or day 10, whichever occurred first. Secondary efficacy outcomes included individual events of total DVT, proximal DVT, distal DVT, symptomatic VTE up to day 10 and symptomatic VTE up to day 30 ± 2. Venography was considered positive if an intraluminal filling defect was seen on 2 different views, or after repeated injection of contrast medium; thrombi in the popliteal vein or above were considered proximal. A venogram was considered adequate if the entire deep venous system was visualised | |
| Notes | Use of adjunctive anticoagulative methods: The use of graded‐pressure elastic stockings was permitted. Eleven (0.4 %) of 2858 participants were given a diagnosis of CHF at baseline. Results were not stratified by baseline illness, but owing to the small numbers, we decided to include this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A multicenter, multinational, randomised, double‐blind study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "A multicenter, multinational, randomised, double‐blind study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients given fondaparinux received a placebo injection 2 h before surgery ……received a placebo injection 6 h after surgery to correspond with the fondaparinux injection schedule" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote:"VTE outcomes evaluated (by an independent adjudicating committee)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 91.1% and 91.6% of patients in 2 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, parallel‐group study | |
| Participants | Total number of participants: 1049 Number of participants allocated to each group: fondaparinux (FX) group: 526, enoxaparin (EN) group: 523 Number of participants excluded and/or lost to follow‐up: FX group: 36 (20 adverse events, 2 lack of efficacy, 7 withdrawn consent, 7 other reasons ), EN group: 36 (13 adverse events, 6 lack of efficacy, 11 withdrawn consent, 6 other reasons). Inclusion: Study population had to conform to the following criteria: men or women (of non‐childbearing potential, i.e. postmenopausal or with hysterectomy or bilateral tubal ligation), or women of childbearing potential using highly effective birth control and having a negative pregnancy test within 48 hours before randomisation; aged ≥ 18 years; undergoing elective major knee surgery or revision of at least 1 component (enrolment of participants with surgery limited to an osteotomy was not permitted); and haemostasis established on the calendar day of surgery, no later than 8 hours after closure of the incision. Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk), or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media). | |
| Interventions | FX: Administration of FX (2.5 mg once daily as sc injection) started 6 ± 2 hours after surgical closure on day 1 (day of surgery). EN: EN (30 mg twice daily as sc injection) at least 12 hours but less than 24 hours after surgical closure To protect blinding (double‐dummy method), all participants received placebo to the active treatment they were not receiving. Study treatment was given up to 7 ± 2 days after surgical closure, or until the final venogram (positive unscheduled or mandatory) was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after the last study treatment administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic or asymptomatic DVT; adjudicated non‐fatal PE or fatal PE. All venography procedures, scheduled and unscheduled, were adjudicated by a blinded Central Independent Adjudication Committee (CIAC). Primary safety outcome: incidence of major bleeding, which included fatal bleeding; bleeding that was retroperitoneal, intracranial or intraspinal or that involved any other critical organ; bleeding leading to reoperation; and overt bleeding with a bleeding index ≥ 2. The bleeding index was calculated as the number of units of packed red cells or whole blood transfused plus Hb values before the bleeding episode minus Hb values after the episode (in grams per decilitre). Secondary safety outcomes were death, other bleeding, need for transfusion, thrombocytopenia and any other adverse event. Efficacy and safety outcomes were adjudicated by a central independent committee, whose members were unaware of treatment assignments, and included reviews of all venograms and reports of bleeding and death. | |
| Notes | Use of adjunctive anticoagulative methods: In the FX group, 4 participants received prohibited treatment (anticoagulant or antiplatelet agents other than aspirin or thrombolytic therapy), 44 received discouraged treatment (non‐steroidal anti‐inflammatory agents or aspirin) and 298 wore graduated compression stockings; in the EN group, 11 participants received prohibited treatment (anticoagulant or antiplatelet agents other than aspirin or thrombolytic therapy), 60 received discouraged treatment (non‐steroidal anti‐inflammatory agents or aspirin) and 294 wore graduated compression stockings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Immediately after surgery, patients were randomly assigned (in a ratio of 1:1 in blocks of four, stratified according to centre), through a central computer‐derived randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately after surgery, patients were randomly assigned (in a ratio of 1:1 in blocks of four, stratified according to centre), through a central computer‐derived randomisation scheme" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "To protect the blind (double‐dummy method) all subjects received placebo (PBO) to the active treatment they were not receiving" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "were adjudicated by a blinded Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 93% participants completed the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, parallel, dose‐ranging study of FX with an assessor‐blind, comparative control group of EN | |
| Participants | Total number of participants: 950 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 185, FX 1.5 mg group: 190, FX 3.0 mg group: 181, FX 6.0 mg group: 73, FX 8.0 mg group: 52; enoxaparin (EN) 60 mg group: 269 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 14 (8 adverse events, 0 lack of efficacy, 6 other reasons), FX 1.5 mg group: 15 (8 adverse events, 0 lack of efficacy, 7 other reasons), FX 3.0 mg group: 13 (7 adverse events, 0 lack of efficacy, 6 other reasons), FX 6.0 mg group: 12 (10 adverse events, 0 lack of efficacy, 2 other reasons), FX 8.0 mg group: 13 (9 adverse events, 0 lack of efficacy, 4 for other reasons); EN group: 27 (18 adverse events, 1 lack of efficacy, 8 other reasons). Inclusion: males and females of non‐childbearing potential ≥ 18 years of age undergoing elective primary hip replacement or revision of a primary procedure Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (such as active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L) or known bleeding disorder), criteria related to venogram (such as creatinine clearance > 1.6 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy 1 week before the start of the study. | |
| Interventions | FX: Participants received a once daily sc injection of FX 0.75, 1.5, 3.0, 6.0 or 8.0 mg, starting 6 ± 2 hours postoperatively on day 1 (day of surgery). EN: twice daily sc injection of EN 30 mg that started within 12 to 24 hours postoperatively (day 1 or day 2) Participants were treated for a minimum of 5 days from day 1 until the final venogram was obtained, up to a maximum of 10 days. | |
| Outcomes | Primary efficacy outcome: incidence of participants with adjudicated mandatory venogram positive for DVT and/or symptomatic adjudicated PE. Independent experts of the Central Independent Adjudication Committee (CIAC) evaluated blindly all venograms and lung scans performed during the study. Primary safety outcome: major bleeding; bleeding was defined as major if it was clinically overt and fatal, intracranial or retroperitoneal; involved a critical organ; or led to reoperation for bleeding or hematoma at the operative site. Overt bleeding was also defined as major if Hb levels declined by more than 2 grams per decilitre, if more than 2 units of packed red cells or whole blood was transfused or if the number of units transfused plus the decline in Hb level in grams per decilitre was greater than 2. | |
| Notes | Use of adjunctive anticoagulative methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, parallel, dose ranging study of FX with an assessor‐blind, comparative control group of EN" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, parallel, dose ranging study of FX with an assessor‐blind, comparative control group of EN" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Independent experts of the Central Independent Adjudication Committee (CIAC) evaluated blindly all venograms and lung scans performed during the study" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 92.4%, 92%, 92.7%, 83.3%, 75% and 89.6% of participants in the 6 different treatment groups finished their treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐centre, randomised, double‐blind study | |
| Participants | Total number of participants: 2275 Number of participants allocated to each group: fondaparinux (FX) group: 1138, enoxaparin (EN) group: 1137 Number of participants excluded and/or lost to follow‐up: FX group: 61 (33 adverse events, 4 lack of efficacy, 19 withdrawn consent, 5 other reasons ), EN group: 66 (35 adverse events, 2 lack of efficacy, 15 withdrawn consent, 14 other reasons) Inclusion: Patients were eligible if they were undergoing an elective, primary, THR surgery or a revision of at least 1 component of a THR; > 18 years of age; men and women of non‐childbearing potential or women of childbearing potential using effective birth control with a negative pregnancy test within 48 hours before randomisation; haemostasis established on the calendar day of surgery, no later than 8 hours after incision closure; written informed consent Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x109/L), medical condition associated with a bleeding risk) or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media). | |
| Interventions | FX: administration of FX (2.5 mg once daily as sc injection) started postoperatively at 6 ± 2 hours after surgical closure EN: administration of EN (30 mg twice daily as sc injection) started postoperatively at least 12 hours but no more than 24 hours post surgical closure Respective placebo to each drug was administered to protect the double‐blind (double‐dummy) method. Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until the final venogram (positive unscheduled or mandatory) was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after the last study treatment administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; and adjudicated PE. All venograms, scheduled and unscheduled, and other diagnostic tests (ultrasonography, ventilation/perfusion, lung scan, pulmonary angiography, spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC). Primary safety outcome: incidence of major bleeding (any Investigator‐reported unusual bleeding adjudicated as major or minor bleeding by the CIAC) recorded between first injection of study drug (active drug or placebo) and day 11. Major bleeding was defined as fatal bleeding; clinically overt bleeding including retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/haematoma at the operative site; and clinically overt bleeding leading to a fall in Hb > 2 g/dL (1.6 mmol/L) and/or transfusion ≥ 2 units of packed red blood cells or whole blood AND for which the combined calculated index was > 2. | |
| Notes | Use of adjunctive anticoagulative methods: use of graduated compression stockings and physiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a central computer‐derived randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "a central computer‐derived randomisation scheme" Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method)" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 94.6% and 94.2% of participants in the 2 study groups finished their relative treatments. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study | |
| Participants | Total number of participants: 1711 Number of participants allocated to each group: fondaparinux (FX) group: 849, enoxaparin (EN) group: 862 Number of participants excluded and/or lost to follow‐up: FX group: 55 (29 adverse events, 1 lack of efficacy, 25 other reasons), EN group: 67 (32 adverse events, 2 lack of efficacy, 33 other reasons) Inclusion: patients undergoing standard surgery for fracture of the upper third of the femur, including femoral head and neck, not more than 48 hours after admission; ≥ 18 years of age; men and women of non‐childbearing potential or women with a negative pregnancy test Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (such as active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L) or known bleeding disorder), criteria related to venogram (such as creatinine clearance > 2.0 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy during the screening period. | |
| Interventions | FX: administration of FX (2.5 mg once daily as sc injection) started postoperatively (6 ± 2 hours after end of surgery) EN: EN (40 mg once daily as sc injection) preoperatively (12 ± 2 hours before surgery) when surgery was planned within 24 hours after hospital admission If surgery was delayed to 24 to 48 hours after admission, both study treatments were administered 12 ± 2 hours before the start of surgery. Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until the mandatory venogram was obtained, whichever came first. Mandatory venography had to be performed between days 5 and 11, but not more than 2 calendar days after the last study treatment administration. | |
| Outcomes | Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; adjudicated non‐fatal and fatal PE. All venograms, scheduled and unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography, spiral computed tomography scan, autopsy report, etc) were adjudicated blindly by independent experts of the CIAC. Primary safety outcome: incidence of major bleeding, which included fatal bleeding; bleeding that was retroperitoneal, intracranial or intraspinal or that involved any other critical organ; bleeding leading to reoperation; and overt bleeding with a bleeding index ≥ 2. The bleeding index was calculated as the number of units of packed red cells or whole blood transfused plus Hb values before the bleeding episode minus Hb values after the episode (in g/dL). | |
| Notes | Use of adjunctive anticoagulative methods: Use of graduated compression stockings and physiotherapy was recommended. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to treatment groups in blocks of four, with stratification according to centre, with the use of a computer‐generated randomisation list" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study Comment: probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Study medications were packaged in boxes of identical appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "were adjudicated blindly by independent experts of the CIAC" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 93.4% and 92% of participants in the 2 study groups finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Total number of participants: 656 Number of participants allocated to each group: fondaparinux (FX) group: 326, placebo group: 330 Number of participants excluded and/or lost to follow‐up: FX group: 40 (20 adverse events, 2 lack of efficacy, 18 other reasons), placebo group: 46 (14 adverse events, 12 lack of efficacy, 20 for other reasons) Inclusion: patients undergoing standard surgery for fracture of the upper third of the femur, including femoral head and neck, not more than 48 hours after admission; ≥ 18 years of age; men and women of non‐childbearing potential or women with a negative pregnancy test Exclusion: Exclusion criteria included active clinically significant bleeding, or known bleeding disorder, criteria related to venography (such as creatinine clearance > 2.0 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy during the screening period. | |
| Interventions | Before randomisation, participants received open‐label fondaparinux 2.5 mg once daily, sc postoperatively for 7 ± 1 days (day 1 = day of surgery). They then were randomised to receive double‐blind fondaparinux 2.5 mg once daily sc for 21 ± 2 days or placebo. | |
| Outcomes | Primary efficacy outcome: cluster of the following adjudicated VTE outcomes, evaluated from day 1 (first day of double‐blind treatment) to day 24 of postrandomisation period: mandatory venogram positive for DVT; symptomatic DVTs and/or adjudicated non‐fatal and fatal PE. During the study, the investigator had to perform appropriate evaluations in case of clinical suspicion of VTE. In the absence of previous confirmation of VTE, a mandatory venogram had to be performed between day 19 and day 24, but not more than 1 calendar day after the last study treatment administration. A Central Independent Adjudication Committee (CIAC) adjudicated any diagnostic tests performed during the double‐blind period, and all reported bleedings and deaths. Primary safety outcome: incidence of major bleeding rate | |
| Notes | Use of adjunctive anticoagulative methods: The use of graduated elastic stockings was permitted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Double‐blind study with effective method to confirm blindness Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "For the double blind period, study medications were packaged in the boxes" Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A Central Independent Adjudication Committee (CIAC) adjudicated any diagnostic tests performed during the double‐blind period, and all reported bleedings and deaths" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 87.7% and 86.1% of participants in 2 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
| Methods | Randomised controlled study | |
| Participants | Total number of participants enrolled: 121 Number of participants allocated to each group: fondaparinux (FX) group: 59, nadroparin calcium (NA) group: 57 Number of participants excluded and/or lost to follow‐up: not mentioned Inclusion: clinical diagnosis of oesophageal carcinoma and planned for oesophagectomy; clinical diagnosis of lung carcinoma and planned for lung resection; general anaesthesia combined with epidural anaesthesia Exclusion: blood clotting dysfunction before surgery; anticoagulating or antiplatelet history before surgery; low blood platelet count; haemorrhagic disease; cerebral haemorrhage; cerebral, spinal or ophthalmologic operation history; peptic ulcer; bleeding > 400 mL during operation; bleeding > 100 mL/h after operation; blood transfusion during or after operation; severe renal or liver dysfunction; severe hypertension | |
| Interventions | FX: 2.5 mg IH once daily after operation NA: nadroparin calcium 4100 AxaIU IH once daily after operation | |
| Outcomes | Primary efficacy outcomes: VTE events and drainage volume | |
| Notes | Use of adjunctive anticoagulative methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors confirmed that they used the computer‐derived randomisation table. |
| Allocation concealment (selection bias) | Low risk | Study authors confirmed that they used the computer‐derived randomisation table. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study publication did not clearly describe methods of blinding, and communication with study authors did not provide further information. Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Study authors confirmed that outcome assessors did not know the group information. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 121 patients were enrolled in this study, and 116 eligible patients were randomly assigned (Group H: 57 patients; Group F: 59 patients)" Comment: low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Results of VTE rates were reported, but the number of events was not clearly reported. Drainage volumes of study participants were reported. |
| Other bias | Low risk | No risk of other bias was identified. |
| Methods | Randomised controlled trial | |
| Participants | Total number of participants: 250 Number of participants allocated to each group: fondaparinux (FX) group: 85, enoxaparin (EN): 86, placebo group: 85 Number of participants excluded and/or lost to follow‐up: FX group: 1, EN group: 2, placebo group: 2 Inclusion: patients older than 20 years of age undergoing elective primary unilateral THR Exclusion: patients who had undergone bilateral and revision THR. Other exclusion criteria included long‐term anticoagulation treatment such as unfractionated heparin, LMWH, vitamin K antagonists and antiplatelet agents for pre‐existing cardiac or cerebrovascular disease; history of VTE; coagulation disorder including antiphospholipid syndrome; presence of a solid malignant tumour or a peptic ulcer; and major surgery in the preceding 3 months. The study also excluded Caucasian patients. | |
| Interventions | FX: postoperative sc injections of fondaparinux (2.5 mg once daily) for 10 consecutive days EN: postoperative sc injections of enoxaparin (40 mg or 20 mg twice daily) for 10 consecutive days Placebo: placebo (0.5 mL of isotonic saline) for 10 consecutive days The first postoperative injections of fondaparinux, enoxaparin and saline took place at an average of 18 hours (SD 2), 17 hours (SD 2) and 18 hours (SD 2), respectively, after the operation. | |
| Outcomes | Primary efficacy outcome: incidence of DVT or VTE assessed by bilateral ultrasonographic studies from the external iliac vein to proximal portions of the calf veins at postoperative day 11. All scans were performed by experienced vascular technicians and were read by experienced radiologists who were blinded to participants' randomisation. Those with a negative scan were followed clinically for 12 weeks (until postoperative day 84) for signs or symptoms of DVT, pulmonary emboli or readmission to hospital because of a complication related to the chemical prophylaxis, a bleeding complication, a wound problem or any other clinical event. Participants who were found to have a distal (calf) DVT did not receive any chemical treatment. Those with proximal DVT received anticoagulant therapy, with initial administration of unfractionated heparin along with initiation of warfarin therapy. Primary safety outcome: incidence of any bleeding, major or minor. This was assessed daily during the treatment period of 10 days and within 24 hours after completion or discontinuation of treatment. An episode of bleeding was classified as major if it was retroperitoneal, intracranial or intraocular, or if it was associated with death, transfusion of more than 2 units of packed red blood cells or whole blood (except autologous), a reduction in level of Hb > 2 g/dL or a serious or life‐threatening clinical event requiring medical intervention. Suspected intra‐abdominal or intracranial bleeding was confirmed by ultrasonography, CT or MRI. Minor episodes of bleeding were defined as those with at least 1 of the following features: epistaxis lasting longer than 5 minutes or requiring intervention, ecchymosis or haematoma with maximum size of > 5 cm, haematuria not associated with trauma from the urinary catheter, gastrointestinal haemorrhage not related to intubation or to passage of a nasogastric tube, wound haematoma or haemorrhagic wound complications not associated with major haemorrhage or subconjunctival haemorrhage, requiring cessation of medication. | |
| Notes | Use of adjunctive anticoagulative methods: All participants received the same routine mechanical prophylaxis (intermittent pneumatic compression device and a thigh‐high elastic compression bandage) during and after operation. Used for sensitivity analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not clearly describe what randomisation method was used. Comment: unclear |
| Allocation concealment (selection bias) | Unclear risk | The study did not clearly describe what randomisation method was used and whether a strategy was used to confirm allocation concealment. Comment: unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study did not clearly describe blinding Comment: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All the scans were performed by experienced vascular technicians and were read by experienced radiologists who were blinded to the patient’s randomisation" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 250 of 267 participants finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias was identified. |
AE: adverse event.
APTT: activated partial thromboplastin time.
BI: bleeding index.
BMI: body mass index.
CG: control group.
CUS: compression ultrasonography.
DVT: deep vein thrombosis.
EBL: estimated blood loss.
IPC: intermittent pneumatic compression.
EN: enoxaparin.
FX: fondaparinux.
HES: hydroxy ethyl starch.
INR: international normalised ratio.
LMWH: low molecular weight heparin.
MRV: magnetic resonance venography.
NYHA: New York Heart Association.
PE: pulmonary embolism.
PT: prothrombin time.
PTT: partial thromboplastin time.
SAE: serious adverse event.
sc: subcutaneous.
SFJ: sapheno‐femoral junction.
SVT: superficial venous thrombosis.
THR: total hip replacement.
TKA: total knee arthroplasty.
TKR: total knee replacement.
VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Dose studied in the trial markedly extended the normal dose. | |
| Study investigated idraparinux for prevention of embolism in patients with atrial fibrillation. Most thrombosis events in the study were arterial embolism, not venous thrombosis, and study results did not distinguish arterial embolism and venous thrombosis events. | |
| This study focused on initial treatment of VTE, not on the prophylaxis of VTE. | |
| Both groups in the study received fondaparinux therapy. | |
| Both groups in the study received fondaparinux therapy. | |
| No reliable primary efficacy and safety results were reported. | |
| The study did not report on VTE evaluation methods. In addition, it did not report the outcome major bleeding and did not indicate whether the evaluator was blinded to group allocation. | |
| The study investigated idrabiotaparinux for prevention of thromboembolism in patients with atrial fibrillation. Study results did not distinguish between systemic embolism and venous thromboembolism. | |
| This study focused on initial treatment of VTE. | |
| Both groups in the study received fondaparinux therapy. | |
| Both groups in the study received pentasaccharide therapy; 1 group received idraparinux, and the other received idrabiotaparinux. | |
| This study focused on treatment of VTE. | |
| Both groups in the study received fondaparinux therapy. | |
| This was not a randomised controlled study. | |
| This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. | |
| This study examined initial treatment of VTE, not prophylaxis of VTE. | |
| This study examined initial treatment of VTE, not prophylaxis of VTE. | |
| This study was stopped owing to lack of accrual without outcomes reported. | |
| This study was terminated because of problems with accrual. | |
| Participants in all 5 study groups received fondaparinux therapy. | |
| This study focused on initial treatment of VTE. | |
| This study examined initial treatment of VTE, not prophylaxis of VTE. | |
| This study focused on fondaparinux for anticoagulation treatment of electric cardioversion of atrial fibrillation that was mainly done to prevent arterial embolism. | |
| This was a quasi‐randomised study. | |
| This was a quasi‐randomised study. | |
| The outcome of the study was different from the outcomes that we studied. | |
| This was not a randomised controlled study. | |
| This study focused on initial treatment of VTE. | |
| This study focused on initial treatment of VTE. | |
| This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. | |
| This was not a randomised controlled study. | |
| This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. | |
| This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. |
VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Prospective randomised open study on the comparison of fondaparinux with the low‐molecular‐weight heparin enoxaparin in patients undergoing femoro‐distal venous bypass operation |
| Methods | Randomised controlled trial |
| Participants | Periperal arterial occlusive disease Fontaine IIb to IV Possibility of venous bypass reconstruction with intended oral anticoagulation Women with childbearing potential were included only when they were using correctly and consistently a highly effective method of birth control (i.e. Pearl‐Index < 1) during the study. Regarded as highly effective were sterilised women, vasectomised partner, combined oral contraceptives and hormone‐eluting IUDs. |
| Interventions | Trade name: Arixtra Product name: Arixtra Pharmaceutical form: anticoagulant and preservative solution for blood INN or proposed INN: fondaparinux‐sodium Concentration unit: mg/mL Concentration number: 2.5/0.5 Trade name: Clexane Product name: Clexane Pharmaceutical form: anticoagulant and preservative solution for blood INN or proposed INN: enoxaparin‐sodium Concentration unit: mg/mL Concentration number: 100 |
| Outcomes | Primary endpoint(s):
|
| Starting date | 27 March 2008 |
| Contact information | |
| Notes |
| Trial name or title | Markers of hypercoagulability and risk of death and rehospitalization in heart failure patients: a pilot study on the effects of fondaparinux ‐ fondaparinux and heart failure |
| Methods | Randomised placebo‐controlled trial |
| Participants | Diagnosis of heart failure according to the Framingham criteria (III to IV NYHA) for patients aged ≥ 60 years. Patients had a planned hospital stay ≥ 4 days. |
| Interventions | Trade name: ARIXTRA Pharmaceutical form: solution for injection INN or proposed INN: fondaparinux Concentration unit: mg/mL milligram(s)/millilitre Concentration type: equal Concentration number: 5 |
| Outcomes | Main objective: to evaluate the effects of fondaparinux on biochemical parameters and clinical events according to different duration of treatment in heart failure patients (III to IV NYHA). Primary endpoint: circulating D‐dimer plasma levels 2 months after enrolment Primary endpoint(s): circulating D‐dimer plasma levels 2 months after enrolment Secondary objective: secondary endpoints: circulating thrombin‐antithrombin complexes (TAT), prothrombin fragment 1+2 (F1+2), interleukin‐6 (IL‐6), C‐reactive protein (CRP), tumour necrosis factor‐alpha (TNF‐alpha) at discharge and 1, 2 and 12 months after enrolment. Circulating D‐dimer plasma levels at discharge and 1 and 12 months after enrolment. Total mortality, cardiovascular mortality and need for rehospitalisation 12 months after enrolment. Pulmonary emboly and/or deep vein thrombosis. Minor and major bleeding |
| Starting date | 9 May 2008 |
| Contact information | |
| Notes |
| Trial name or title | Randomised study of anticoagulant therapy to prevent postoperative DVT/PE |
| Methods | Randomised double‐blind parallel trial |
| Participants | Patients older than 20 years of age after digestive surgery classified as at high risk of DVT/PE |
| Interventions | Fondaparinux 2.5 mg 1/d for 7 days Enoxaparin 2000 IU 2/d for 7 days Heparin sodium 5000 IU 2/d for 7 days |
| Outcomes | Primary efficacy outcome: frequency of VTE Primary safety outcome: frequency of adverse events, including haemorrhage and abnormal serological findings |
| Starting date | 1 September 2009 |
| Contact information | [email protected]‐u.ac.jp |
| Notes |
| Trial name or title | The efficacy and safety of anticoagulant therapy Arixtra injection for the prevention of the vein thromboembolism in laparoscopic colorectal surgery |
| Methods | Randomised parallel open‐label study |
| Participants | Patients underwent laparoscopic surgery for colorectal cancer. |
| Interventions | Fondaparinux and no intervention |
| Outcomes | Efficacy of the incidence of venous thromboembolism. Safety of the incidence of major bleeding |
| Starting date | 10 January 2012 |
| Contact information | Osaka Medical College |
| Notes |
| Trial name or title | Phase III study of efficacy of fondaparinux on the prevention of post‐operative venous thromboembolism in patients undergoing with laparoscopic colorectal cancer surgery |
| Methods | Randomised controlled trial |
| Participants | Patients undergoing laparoscopic colorectal surgery with additional risk factor for VTE |
| Interventions | Once daily fondaparinux (2.5 mg or 1.5 mg) for 4 to 8 days after 24 hours of surgery Intermittent pneumatic compression according to institutional guidelines |
| Outcomes | Incidence of venous thromboembolism (VTE) Incidence of major bleeding |
| Starting date | 1 September 2012 |
| Contact information | Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group, 2‐2‐E2 Yamadaoka, Suita, Osaka 565‐0871, Japan Telephone number: 06‐6879‐3251 |
| Notes |
| Trial name or title | Prophylaxis of thromboembolic complications trial: thromboprophylaxis needed in below knee plaster cast immobilisation for ankle and foot fractures (PROTECT) |
| Methods | Prospective, randomised, controlled, single‐blinded, multi‐centre trial |
| Participants | Patients 18 years of age or older with a non‐surgical fracture of the lower extremity requiring immobilisation in a below‐knee plaster cast for a minimum of 4 weeks |
| Interventions | One group receiving nadroparin (2850 IU anti‐Xa = 0.3 mL once daily), 1 group receiving fondaparinux (2.5 mg = 0.5 mL once daily) and 1 group receiving no prophylaxis |
| Outcomes | Primary outcome measure: deep vein trombosis as detected by venous duplex. Secondary outcome measure: bleeding complications |
| Starting date | 13 April 2009 |
| Contact information | |
| Notes |
CABG: coronary artery bypass graft.
DVT: deep vein thrombosis.
INN: international non‐proprietary name.
IU: international unit.
IUD: intrauterine device.
PE: pulmonary embolism.
VTE: venous thromboembolism.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| Analysis 1.1  Comparison 1 Fondaparinux versus placebo, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| Analysis 1.2  Comparison 1 Fondaparinux versus placebo, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| Analysis 1.3 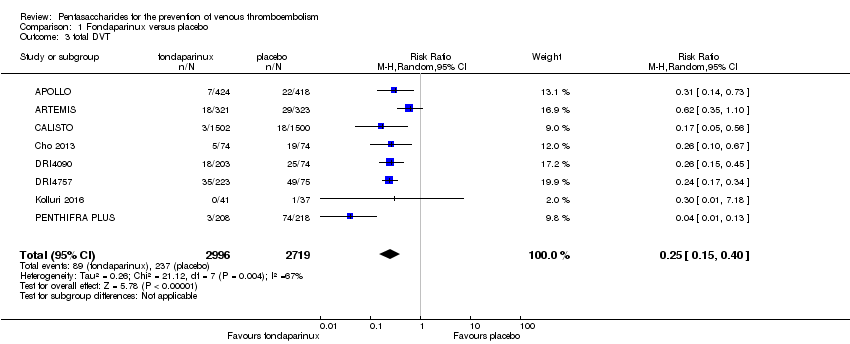 Comparison 1 Fondaparinux versus placebo, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| Analysis 1.4 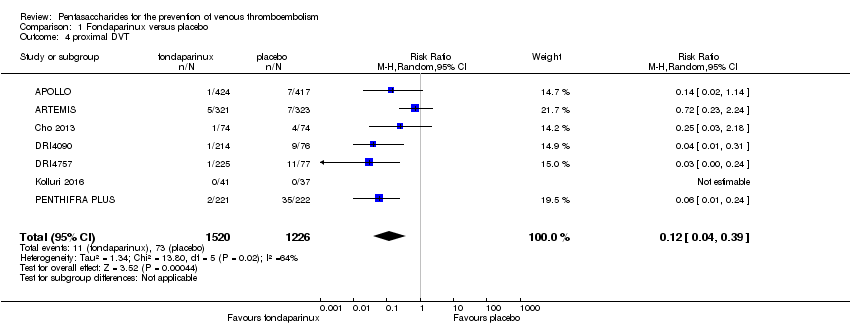 Comparison 1 Fondaparinux versus placebo, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| Analysis 1.5  Comparison 1 Fondaparinux versus placebo, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| Analysis 1.6 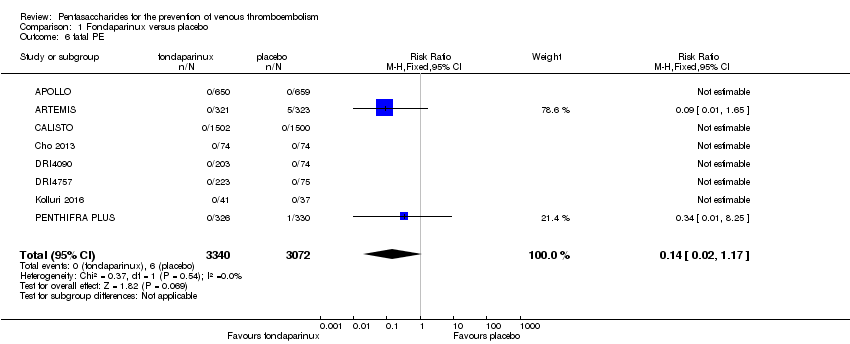 Comparison 1 Fondaparinux versus placebo, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| Analysis 1.7  Comparison 1 Fondaparinux versus placebo, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| Analysis 1.8  Comparison 1 Fondaparinux versus placebo, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| Analysis 1.9 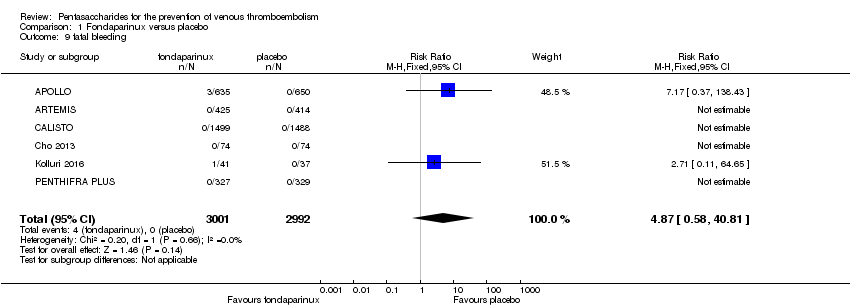 Comparison 1 Fondaparinux versus placebo, Outcome 9 fatal bleeding. | ||||
| 10 MI Show forest plot | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| Analysis 1.10 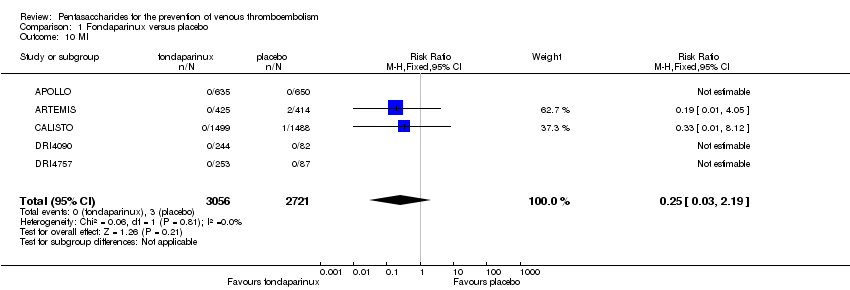 Comparison 1 Fondaparinux versus placebo, Outcome 10 MI. | ||||
| 11 all causes of death Show forest plot | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| Analysis 1.11 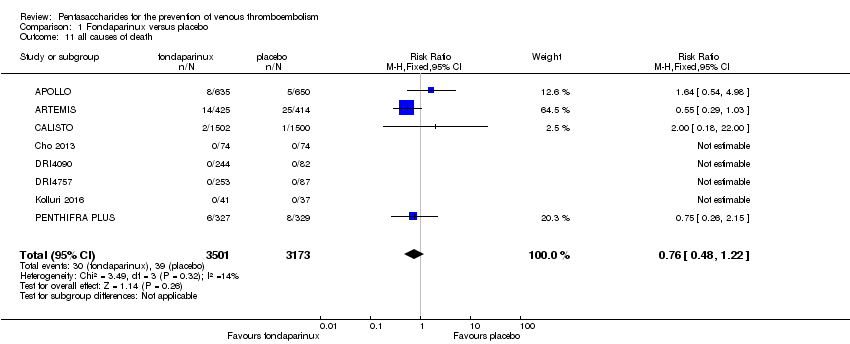 Comparison 1 Fondaparinux versus placebo, Outcome 11 all causes of death. | ||||
| 12 other serious adverse effects Show forest plot | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Analysis 1.12  Comparison 1 Fondaparinux versus placebo, Outcome 12 other serious adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 9 | 5884 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.43] |
| Analysis 2.1 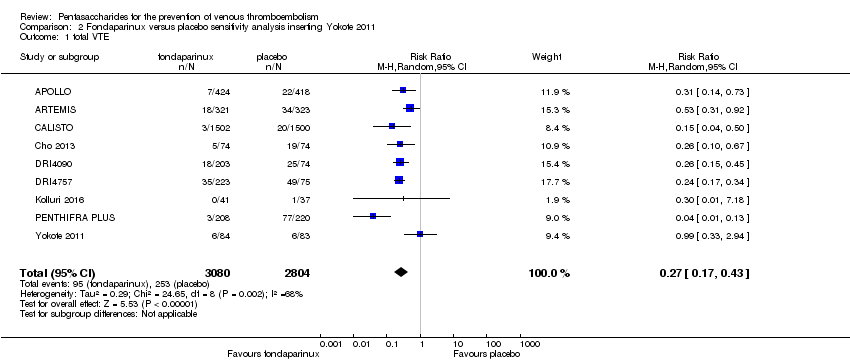 Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 9 | 6670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.42] |
| Analysis 2.2  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 9 | 5882 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.45] |
| Analysis 2.3  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 8 | 2913 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.05, 0.51] |
| Analysis 2.4  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| Analysis 2.5  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| Analysis 2.6 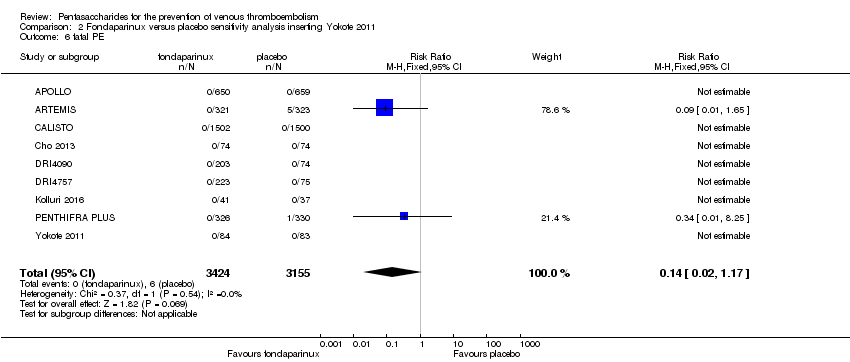 Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| Analysis 2.7 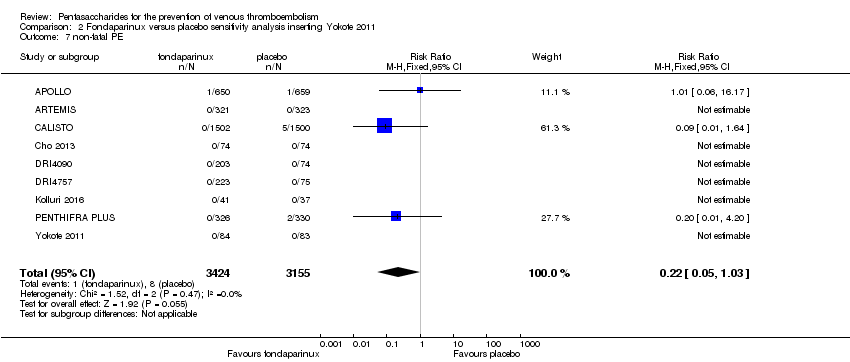 Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 9 | 6829 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| Analysis 2.8  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 7 | 6163 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| Analysis 2.9  Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 9 fatal bleeding. | ||||
| 10 all causes of death Show forest plot | 8 | 6766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| Analysis 2.10 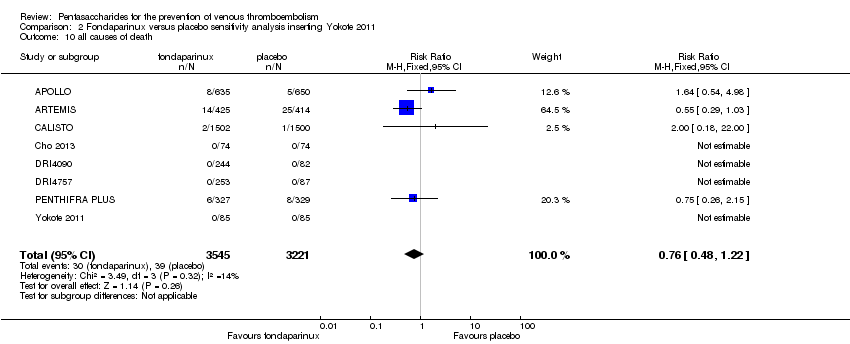 Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 10 all causes of death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 7 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| Analysis 3.1 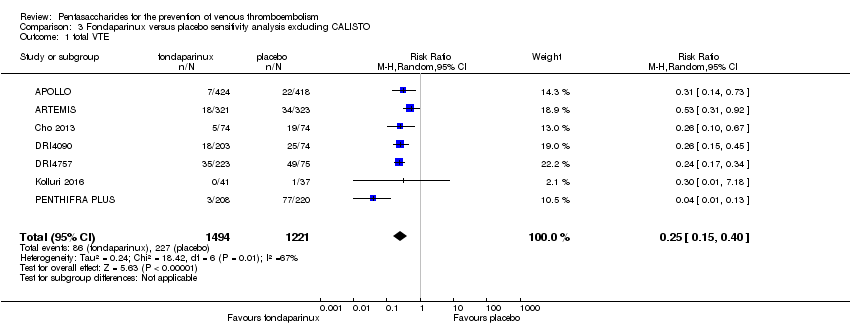 Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 7 | 3501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.54] |
| Analysis 3.2  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 7 | 2713 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.43] |
| Analysis 3.3  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| Analysis 3.4 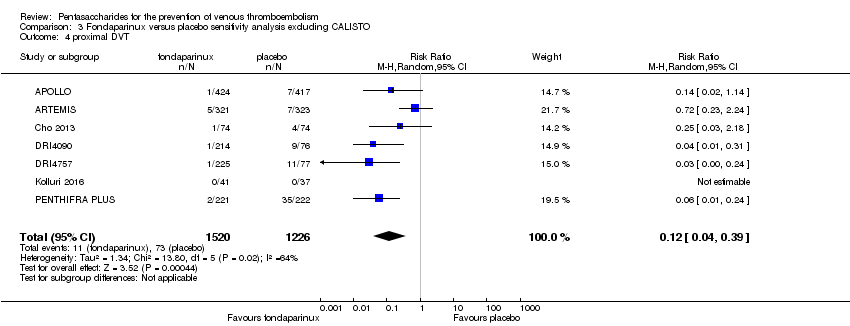 Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 7 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.92] |
| Analysis 3.5 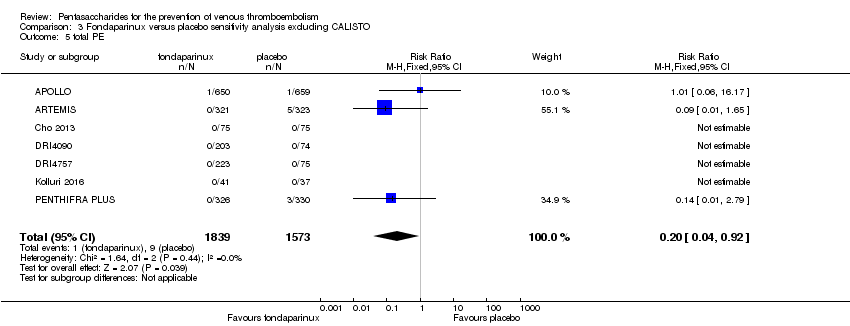 Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| Analysis 3.6  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| Analysis 3.7  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.52, 4.67] |
| Analysis 3.8 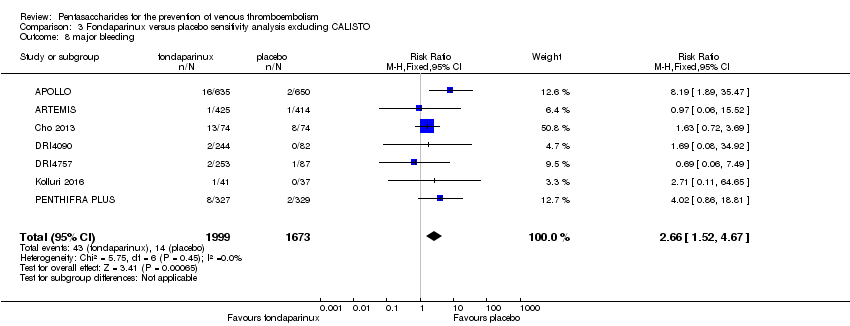 Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 5 | 3006 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| Analysis 3.9  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 9 fatal bleeding. | ||||
| 10 MI Show forest plot | 4 | 2790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| Analysis 3.10  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 10 MI. | ||||
| 11 all causes of death Show forest plot | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| Analysis 3.11 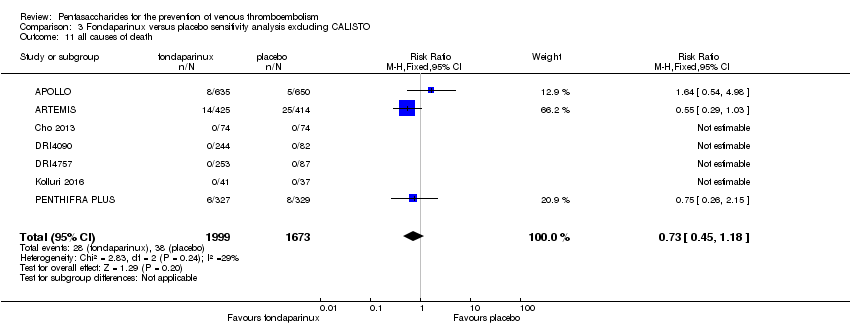 Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 11 all causes of death. | ||||
| 12 other serious adverse effects Show forest plot | 6 | 3594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.32] |
| Analysis 3.12  Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 12 other serious adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| Analysis 4.1  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 1 total VTE. | ||||
| 1.1 surgery patients | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 1.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.50] |
| 1.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.92] |
| 2 symptomatic VTE Show forest plot | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| Analysis 4.2 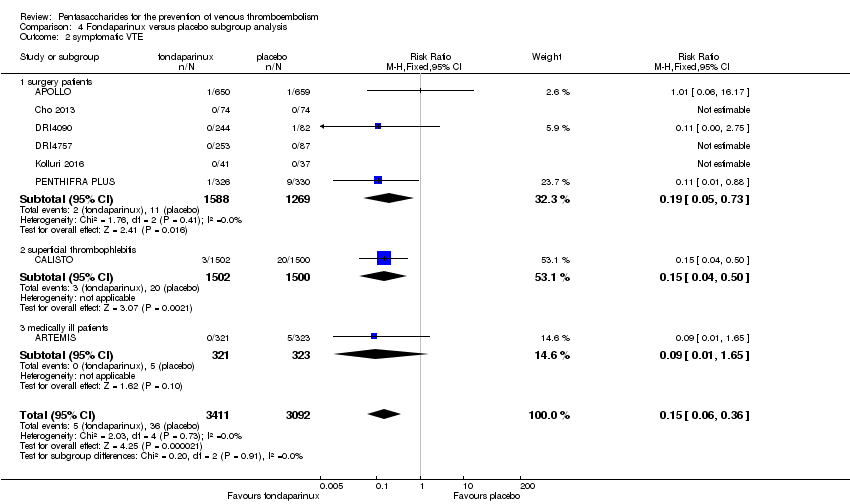 Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 2 symptomatic VTE. | ||||
| 2.1 surgery patients | 6 | 2857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.73] |
| 2.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.50] |
| 2.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 3 total DVT Show forest plot | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| Analysis 4.3  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 3 total DVT. | ||||
| 3.1 surgery patients | 6 | 2069 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 3.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.56] |
| 3.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.10] |
| 4 proximal DVT Show forest plot | 7 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.07, 0.22] |
| Analysis 4.4  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 4 proximal DVT. | ||||
| 4.1 surgery patients | 6 | 2101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.15] |
| 4.2 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.24] |
| 5 total PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| Analysis 4.5  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 5 total PE. | ||||
| 5.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.05, 2.14] |
| 5.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 5.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 6 fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| Analysis 4.6  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 6 fatal PE. | ||||
| 6.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.25] |
| 6.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 7 non‐fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| Analysis 4.7  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 7 non‐fatal PE. | ||||
| 7.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| 7.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 7.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding Show forest plot | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| Analysis 4.8 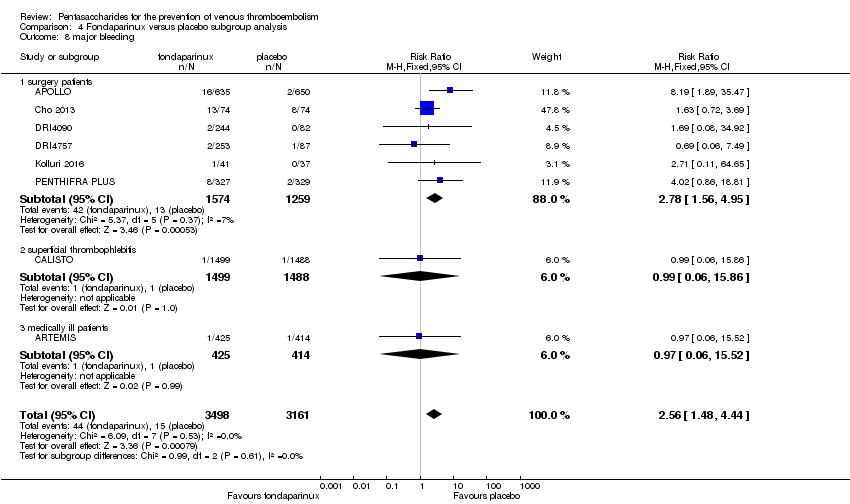 Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 8 major bleeding. | ||||
| 8.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.56, 4.95] |
| 8.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.86] |
| 8.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.52] |
| 9 fatal bleeding Show forest plot | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| Analysis 4.9  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 9 fatal bleeding. | ||||
| 9.1 surgery patients | 4 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 9.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI Show forest plot | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| Analysis 4.10  Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 10 MI. | ||||
| 10.1 surgery patients | 3 | 1951 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 10.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| 11 all causes of death Show forest plot | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| Analysis 4.11 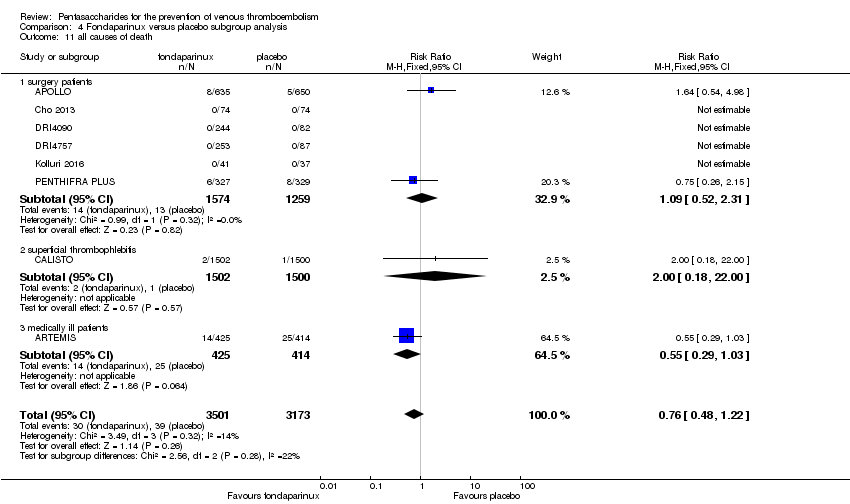 Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 11 all causes of death. | ||||
| 11.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.31] |
| 11.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.00] |
| 11.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.29, 1.03] |
| 12 other serious adverse effects Show forest plot | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Analysis 4.12 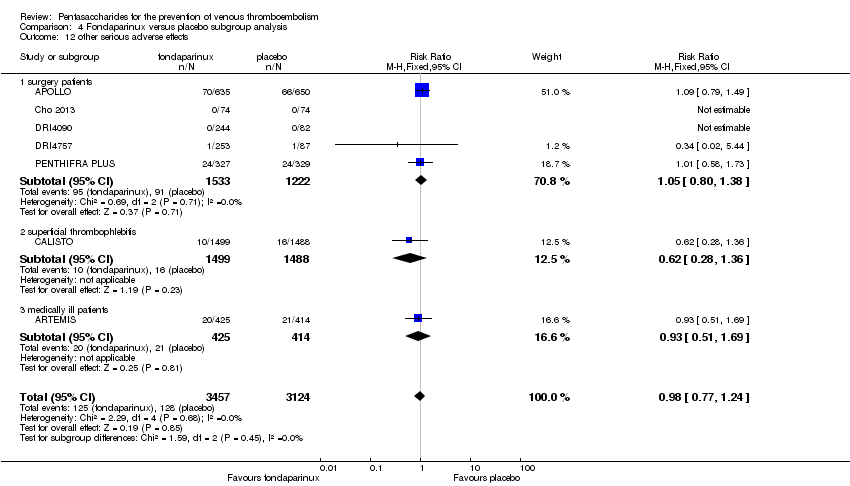 Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 12 other serious adverse effects. | ||||
| 12.1 surgery patients | 5 | 2755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 12.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.28, 1.36] |
| 12.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| Analysis 5.1  Comparison 5 Fondaparinux versus LMWH, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| Analysis 5.2  Comparison 5 Fondaparinux versus LMWH, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| Analysis 5.3 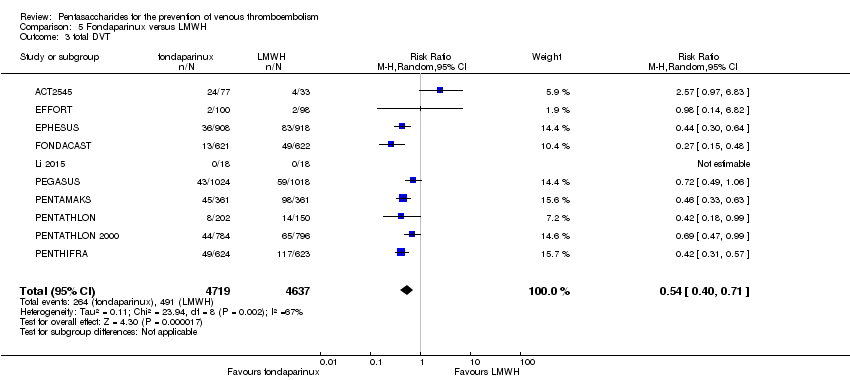 Comparison 5 Fondaparinux versus LMWH, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| Analysis 5.4 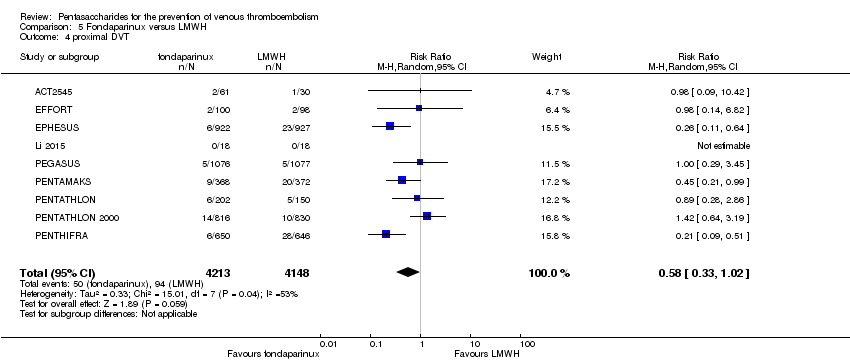 Comparison 5 Fondaparinux versus LMWH, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| Analysis 5.5  Comparison 5 Fondaparinux versus LMWH, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| Analysis 5.6  Comparison 5 Fondaparinux versus LMWH, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| Analysis 5.7 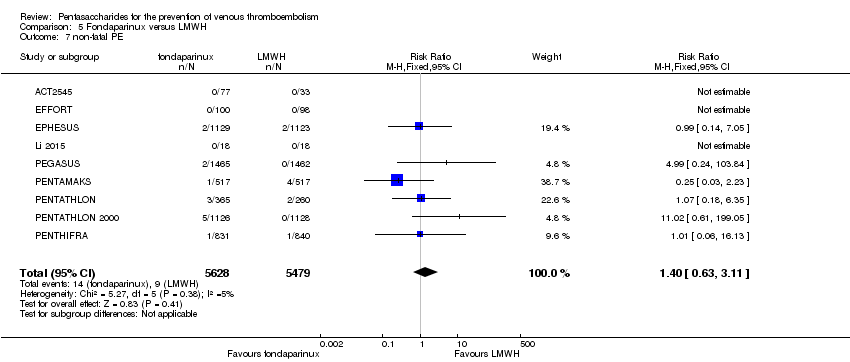 Comparison 5 Fondaparinux versus LMWH, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| Analysis 5.8  Comparison 5 Fondaparinux versus LMWH, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 6 | 10293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| Analysis 5.9  Comparison 5 Fondaparinux versus LMWH, Outcome 9 fatal bleeding. | ||||
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| Analysis 5.10  Comparison 5 Fondaparinux versus LMWH, Outcome 10 MI. | ||||
| 11 all causes of death Show forest plot | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| Analysis 5.11  Comparison 5 Fondaparinux versus LMWH, Outcome 11 all causes of death. | ||||
| 12 death associated with VTE or bleeding Show forest plot | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| Analysis 5.12  Comparison 5 Fondaparinux versus LMWH, Outcome 12 death associated with VTE or bleeding. | ||||
| 13 other serious adverse effects Show forest plot | 10 | 12465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
| Analysis 5.13  Comparison 5 Fondaparinux versus LMWH, Outcome 13 other serious adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 13 | 9709 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.74] |
| Analysis 6.1 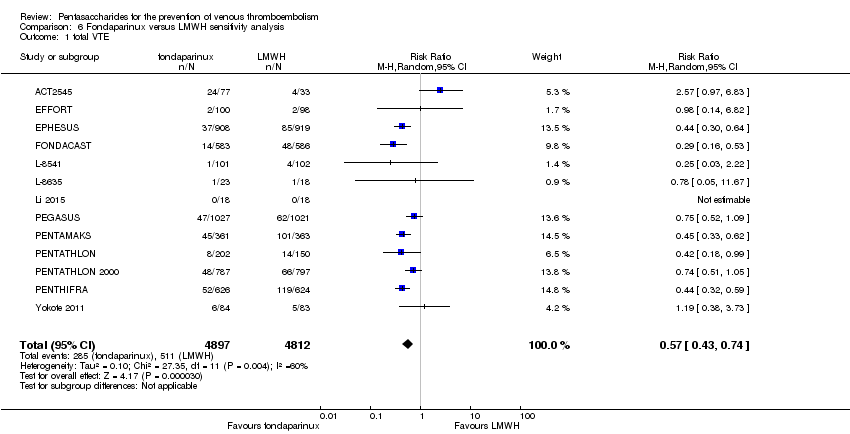 Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 11 | 12569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.70] |
| Analysis 6.2  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 12 | 9726 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.72] |
| Analysis 6.3  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 10 | 8528 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.06] |
| Analysis 6.4 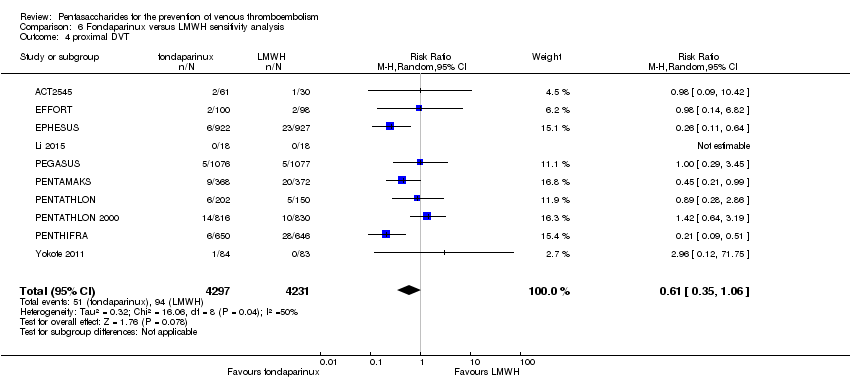 Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 12 | 12720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| Analysis 6.5 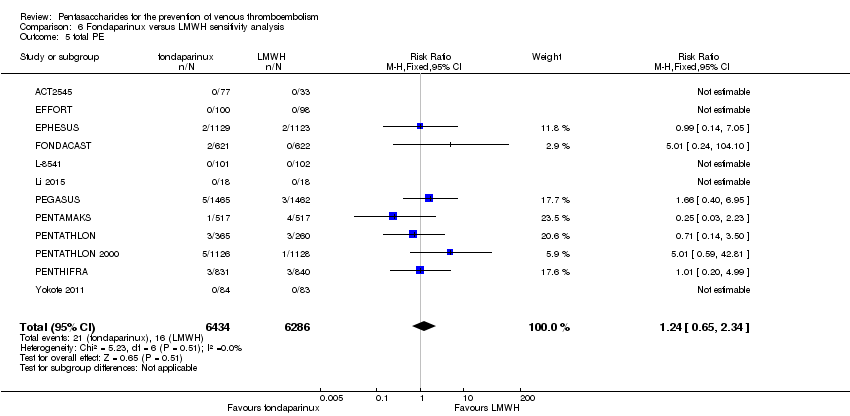 Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 11 | 11477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| Analysis 6.6  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 11 | 11486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| Analysis 6.7  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 13 | 12874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.06, 1.68] |
| Analysis 6.8  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 8 | 10499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| Analysis 6.9  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 9 fatal bleeding. | ||||
| 10 all causes of death Show forest plot | 12 | 12603 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| Analysis 6.10  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 10 all causes of death. | ||||
| 11 other serious adverse effects Show forest plot | 11 | 12707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
| Analysis 6.11  Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 11 other serious adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 10 | 9141 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| Analysis 7.1  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 8 | 12042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| Analysis 7.2  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 9 | 9158 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| Analysis 7.3 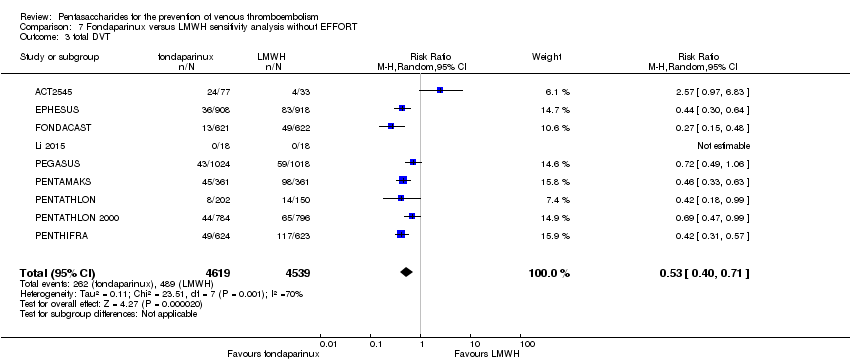 Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 8 | 8163 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.03] |
| Analysis 7.4 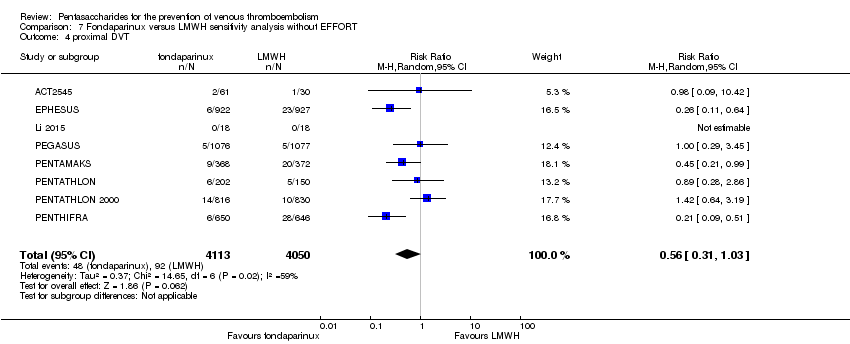 Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 9 | 12152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| Analysis 7.5  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 5 total PE. | ||||
| 6 fatal PE Show forest plot | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| Analysis 7.6  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 6 fatal PE. | ||||
| 7 non‐fatal PE Show forest plot | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| Analysis 7.7  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 7 non‐fatal PE. | ||||
| 8 major bleeding Show forest plot | 10 | 12303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| Analysis 7.8  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 8 major bleeding. | ||||
| 9 fatal bleeding Show forest plot | 5 | 10095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| Analysis 7.9  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 9 fatal bleeding. | ||||
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| Analysis 7.10  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 10 MI. | ||||
| 11 all causes of death Show forest plot | 10 | 12202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| Analysis 7.11  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 11 all causes of death. | ||||
| 12 death associated with VTE or bleeding Show forest plot | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| Analysis 7.12 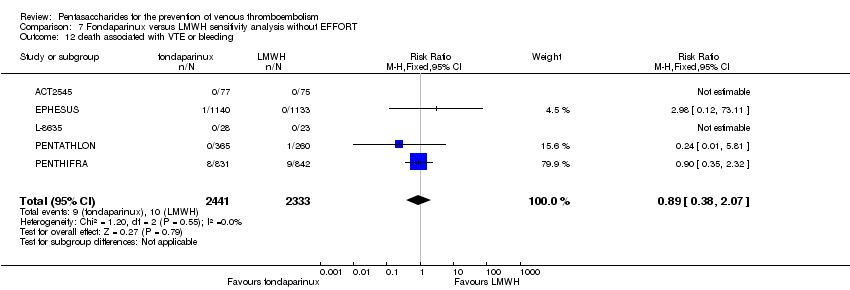 Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 12 death associated with VTE or bleeding. | ||||
| 13 other serious adverse effects Show forest plot | 9 | 12267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
| Analysis 7.13  Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 13 other serious adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| Analysis 8.1  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 1 total VTE. | ||||
| 1.1 orthopaedic patients | 8 | 7057 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.38, 0.70] |
| 1.2 abdominal surgery | 1 | 2048 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.09] |
| 1.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 2 symptomatic VTE Show forest plot | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| Analysis 8.2  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 2 symptomatic VTE. | ||||
| 2.1 orthopaedic patients | 6 | 9079 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.65] |
| 2.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.37, 3.92] |
| 2.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 total DVT Show forest plot | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| Analysis 8.3 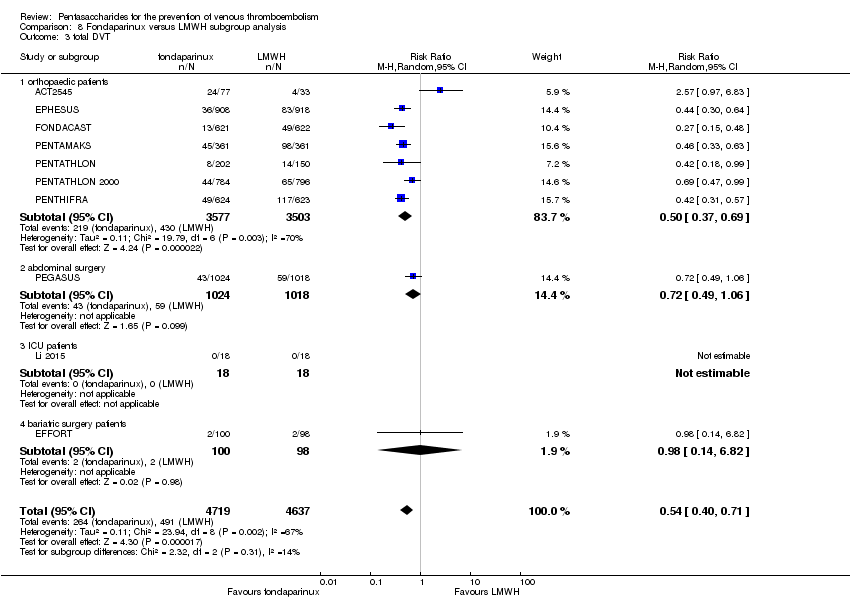 Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 3 total DVT. | ||||
| 3.1 orthopaedic patients | 7 | 7080 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.37, 0.69] |
| 3.2 abdominal surgery | 1 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.06] |
| 3.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 4 proximal DVT Show forest plot | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| Analysis 8.4  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 4 proximal DVT. | ||||
| 4.1 orthopaedic patients | 6 | 5974 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.02] |
| 4.2 abdominal surgery | 1 | 2153 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.29, 3.45] |
| 4.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 5 total PE Show forest plot | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| Analysis 8.5  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 5 total PE. | ||||
| 5.1 orthopaedic patients | 7 | 9189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.34] |
| 5.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.95] |
| 5.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| Analysis 8.6  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 6 fatal PE. | ||||
| 6.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.29] |
| 6.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.94] |
| 6.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 non‐fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| Analysis 8.7  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 7 non‐fatal PE. | ||||
| 7.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.83] |
| 7.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.99 [0.24, 103.84] |
| 7.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 bariatric surgery | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding Show forest plot | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| Analysis 8.8 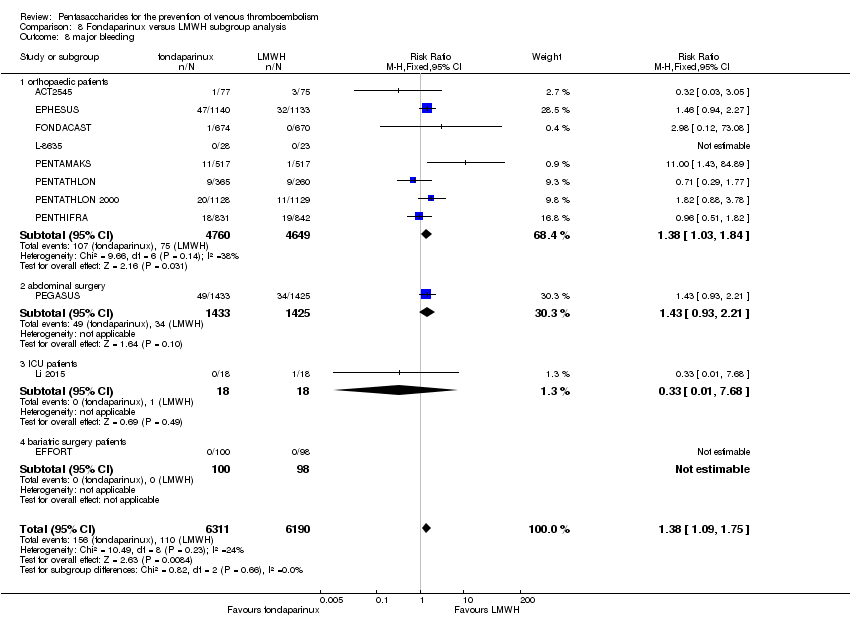 Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 8 major bleeding. | ||||
| 8.1 orthopaedic patients | 8 | 9409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.03, 1.84] |
| 8.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.93, 2.21] |
| 8.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.68] |
| 8.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 fatal bleeding Show forest plot | 7 | 10329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| Analysis 8.9 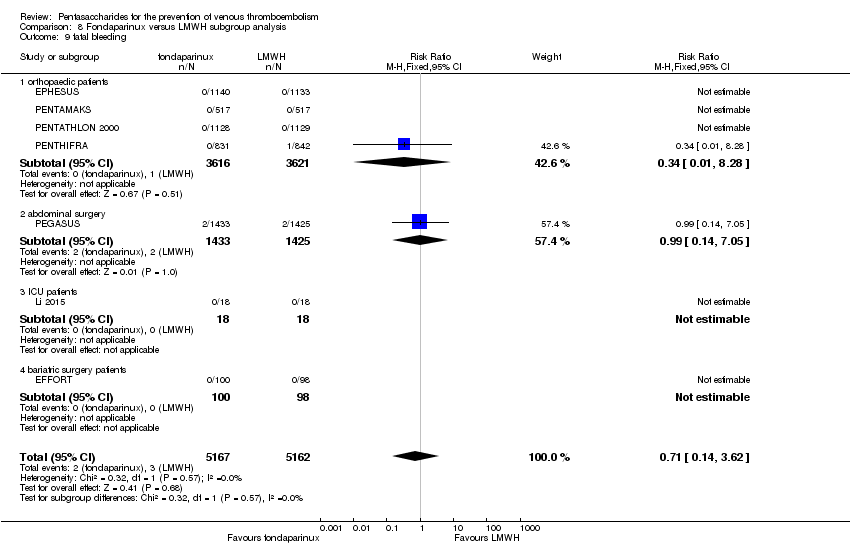 Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 9 fatal bleeding. | ||||
| 9.1 orthopaedic patients | 4 | 7237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.28] |
| 9.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.05] |
| 9.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| Analysis 8.10  Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 10 MI. | ||||
| 10.1 orthopaedic patients | 5 | 7862 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.61, 2.39] |
| 10.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.92] |
| 11 all causes of death Show forest plot | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| Analysis 8.11 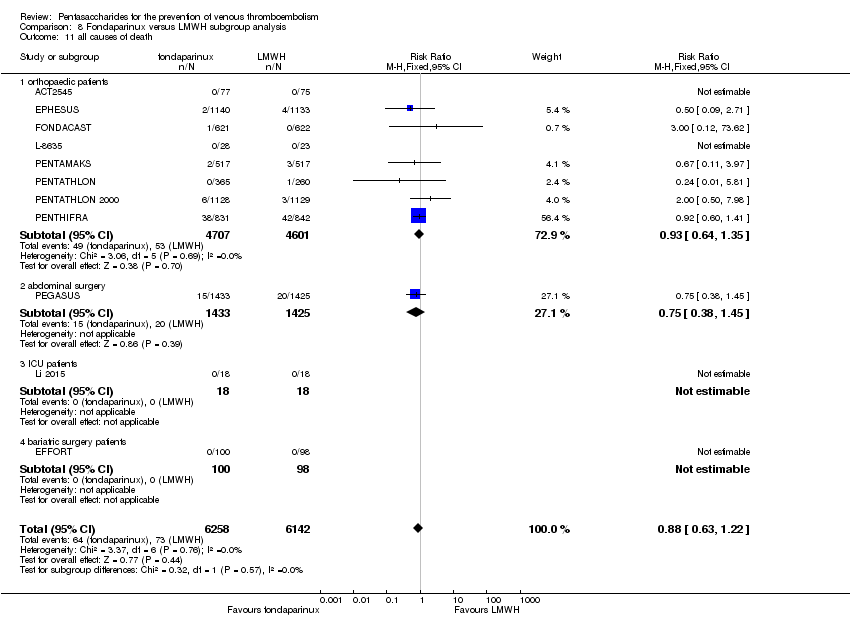 Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 11 all causes of death. | ||||
| 11.1 orthopaedic patients | 8 | 9308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.35] |
| 11.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.45] |
| 11.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 other serious adverse effects Show forest plot | 10 | 12470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| Analysis 8.12 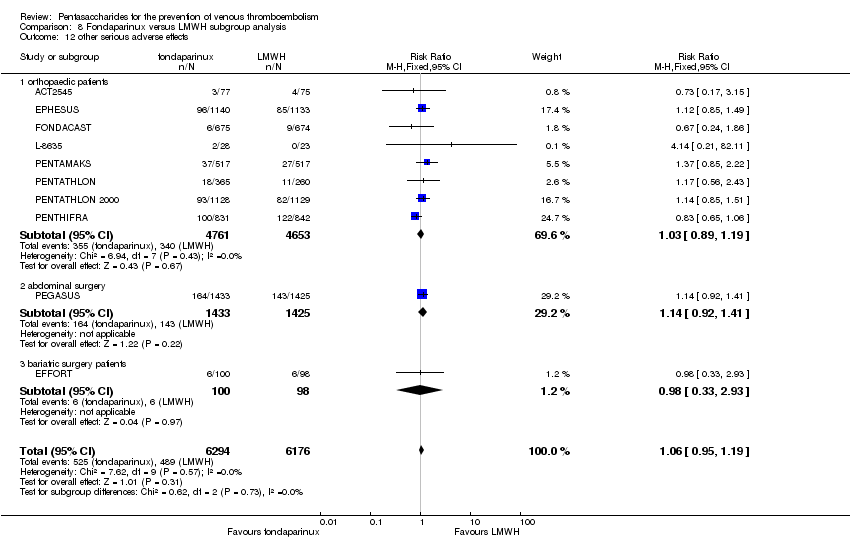 Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 12 other serious adverse effects. | ||||
| 12.1 orthopaedic patients | 8 | 9414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.19] |
| 12.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.41] |
| 12.3 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.33, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.4  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.5  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 5 total PE. | ||||
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.6  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 6 major bleeding. | ||||
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.7  Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 7 all causes of death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.2 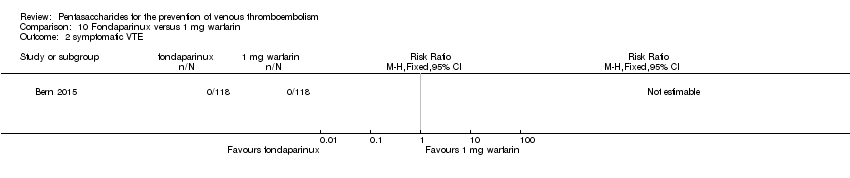 Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.4  Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.5 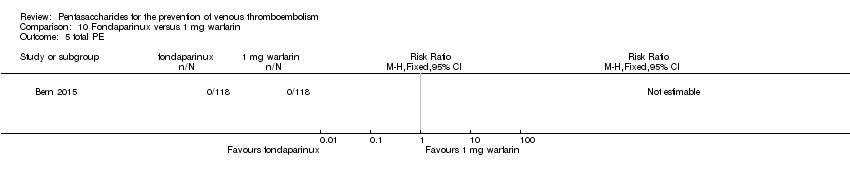 Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 5 total PE. | ||||
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.6 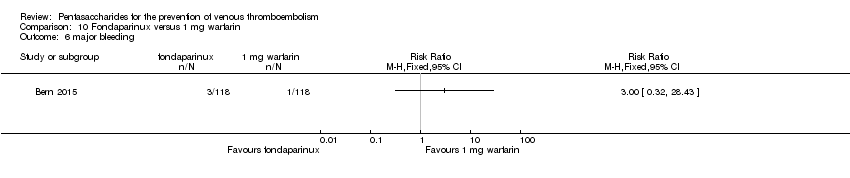 Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 6 major bleeding. | ||||
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.7 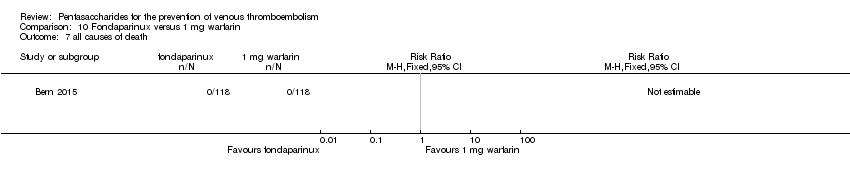 Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 7 all causes of death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Fondaparinux versus edoxaban, Outcome 1 total VTE. | ||||
| 2 major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.2 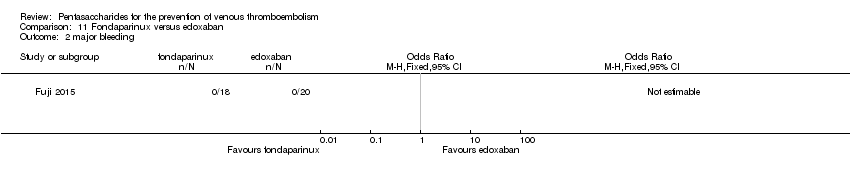 Comparison 11 Fondaparinux versus edoxaban, Outcome 2 major bleeding. | ||||
| 3 all causes of death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.3  Comparison 11 Fondaparinux versus edoxaban, Outcome 3 all causes of death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 1 total VTE. | ||||
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.2  Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 2 symptomatic VTE. | ||||
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.3 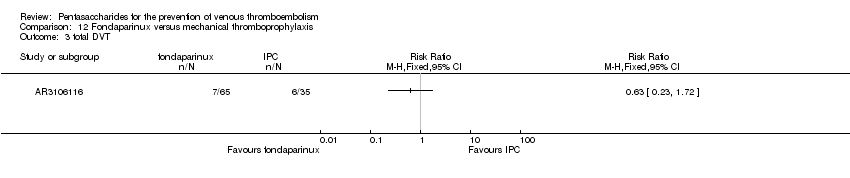 Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 3 total DVT. | ||||
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.4 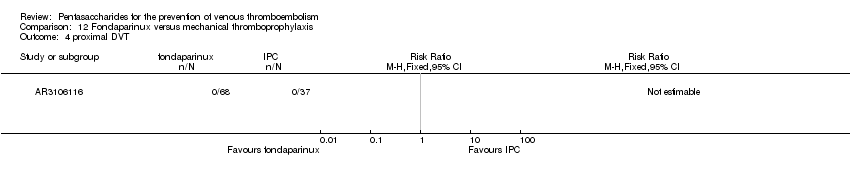 Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 4 proximal DVT. | ||||
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.5 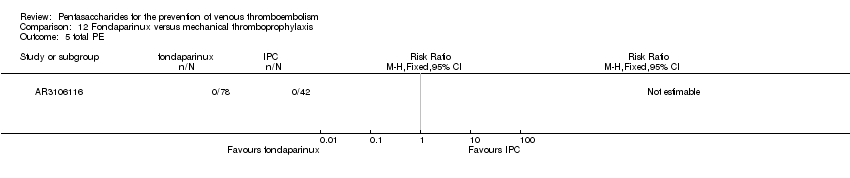 Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 5 total PE. | ||||
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.6  Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 6 major bleeding. | ||||
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.7  Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 7 all causes of death. | ||||
| 8 other serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.8  Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 8 other serious adverse effects. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Funnel plot of comparison: 1 fondaparinux versus placebo, outcome: 1.1 total VTE.
Funnel plot of comparison: 5 fondaparinux versus LMWH, outcome: 5.1 total VTE.
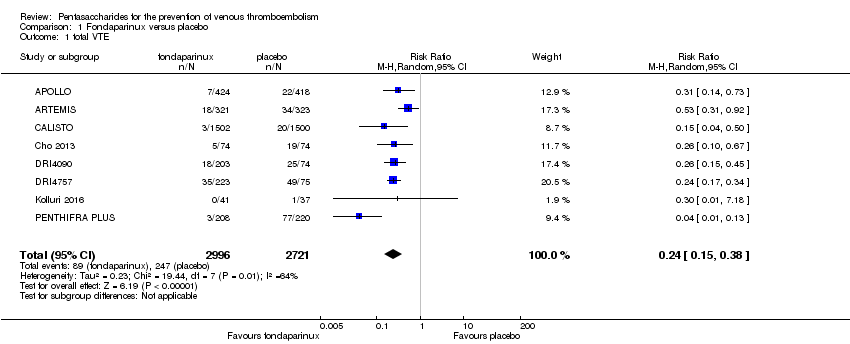
Comparison 1 Fondaparinux versus placebo, Outcome 1 total VTE.

Comparison 1 Fondaparinux versus placebo, Outcome 2 symptomatic VTE.
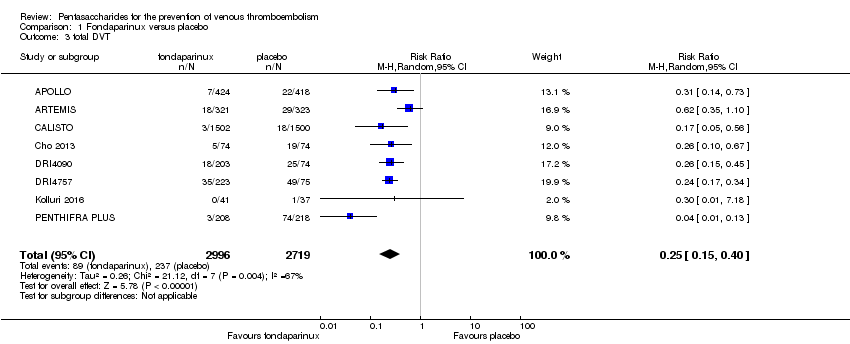
Comparison 1 Fondaparinux versus placebo, Outcome 3 total DVT.
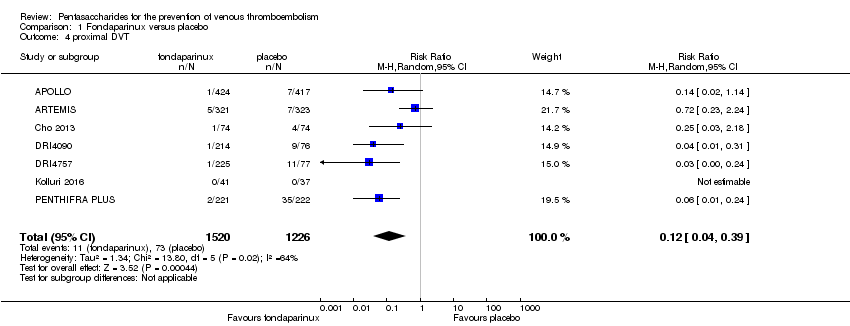
Comparison 1 Fondaparinux versus placebo, Outcome 4 proximal DVT.
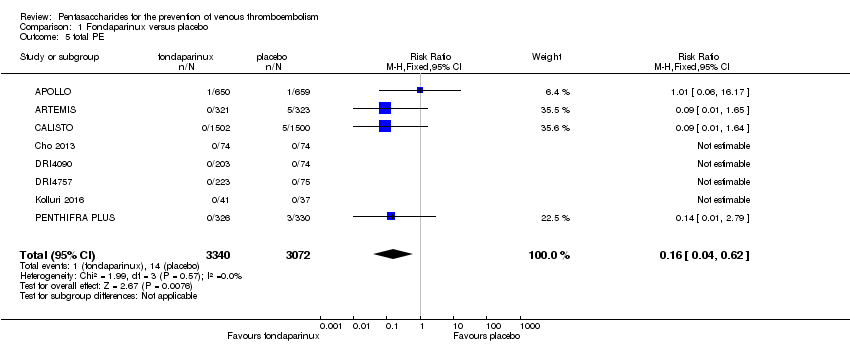
Comparison 1 Fondaparinux versus placebo, Outcome 5 total PE.
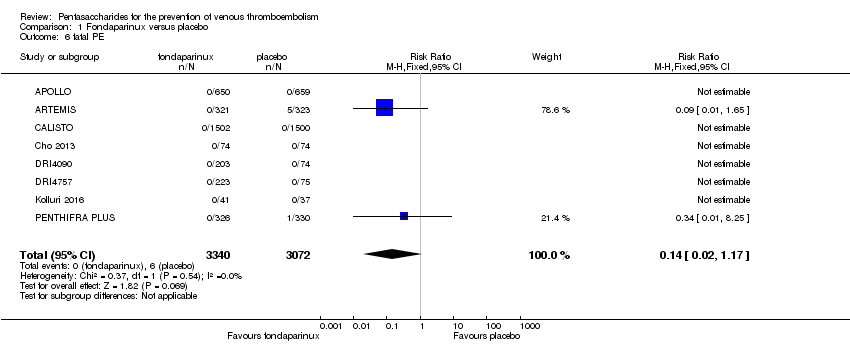
Comparison 1 Fondaparinux versus placebo, Outcome 6 fatal PE.

Comparison 1 Fondaparinux versus placebo, Outcome 7 non‐fatal PE.

Comparison 1 Fondaparinux versus placebo, Outcome 8 major bleeding.

Comparison 1 Fondaparinux versus placebo, Outcome 9 fatal bleeding.
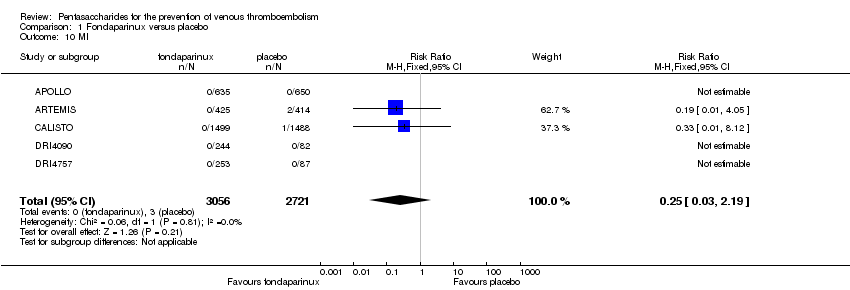
Comparison 1 Fondaparinux versus placebo, Outcome 10 MI.
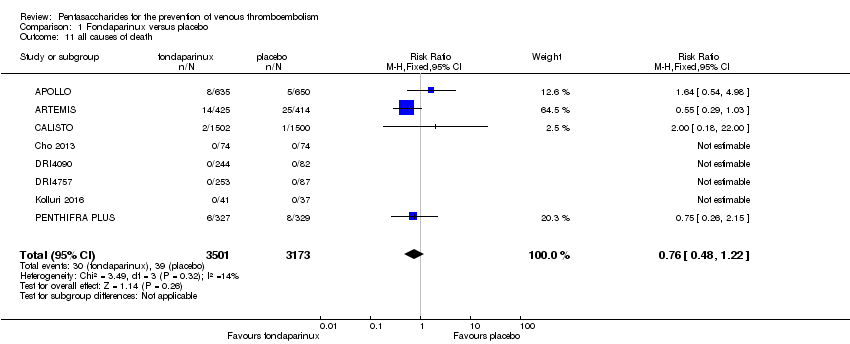
Comparison 1 Fondaparinux versus placebo, Outcome 11 all causes of death.

Comparison 1 Fondaparinux versus placebo, Outcome 12 other serious adverse effects.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 1 total VTE.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 2 symptomatic VTE.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 3 total DVT.
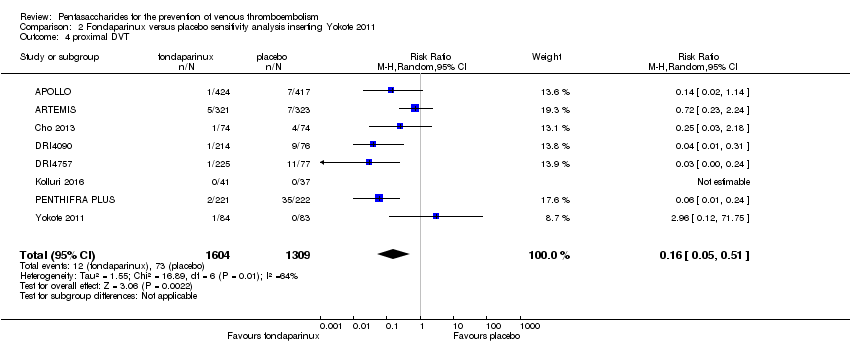
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 4 proximal DVT.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 5 total PE.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 6 fatal PE.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 7 non‐fatal PE.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 8 major bleeding.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 9 fatal bleeding.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 10 all causes of death.
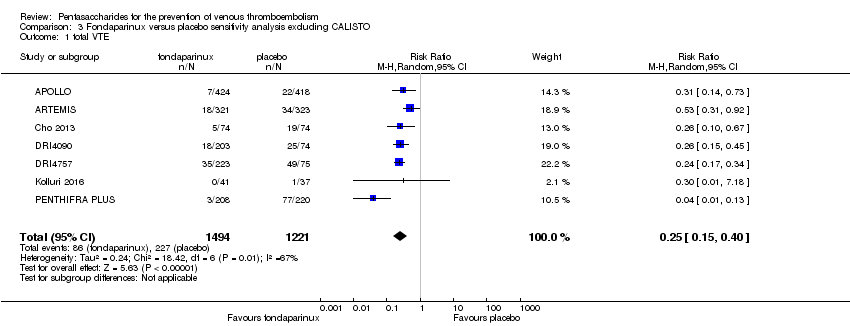
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 1 total VTE.
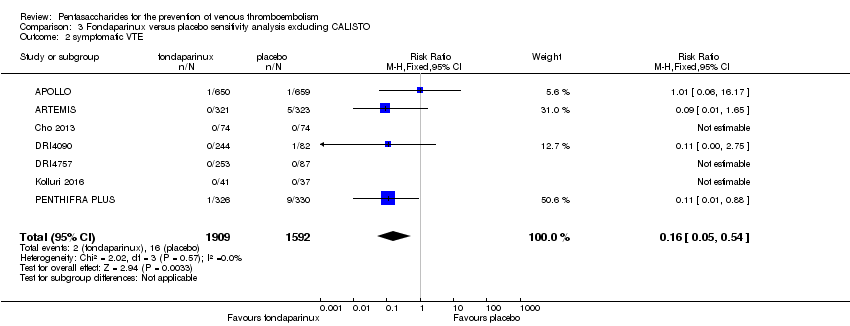
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 2 symptomatic VTE.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 3 total DVT.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 4 proximal DVT.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 5 total PE.
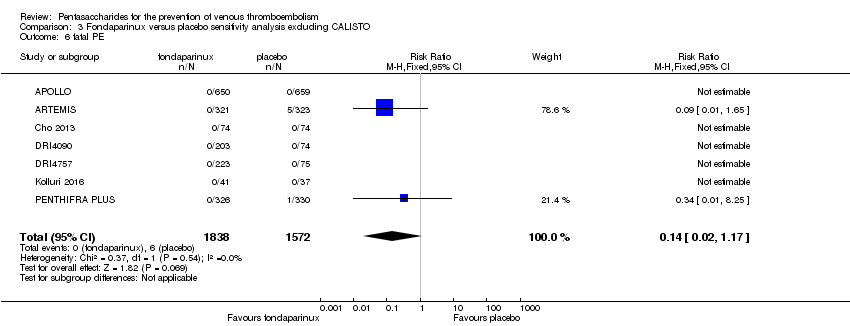
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 6 fatal PE.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 7 non‐fatal PE.
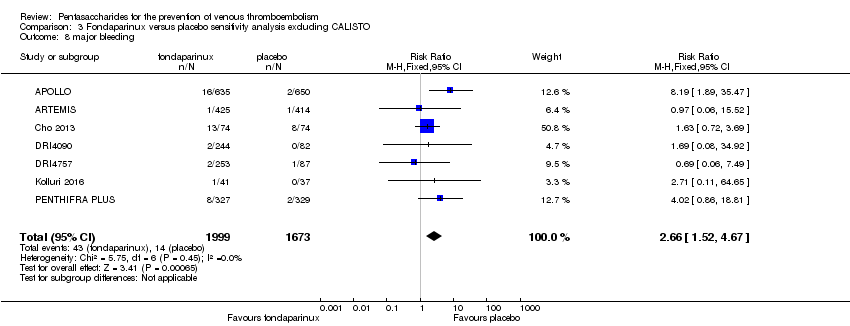
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 8 major bleeding.
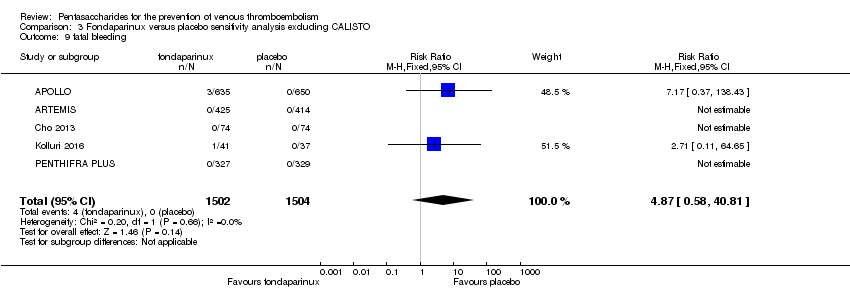
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 9 fatal bleeding.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 10 MI.

Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 11 all causes of death.
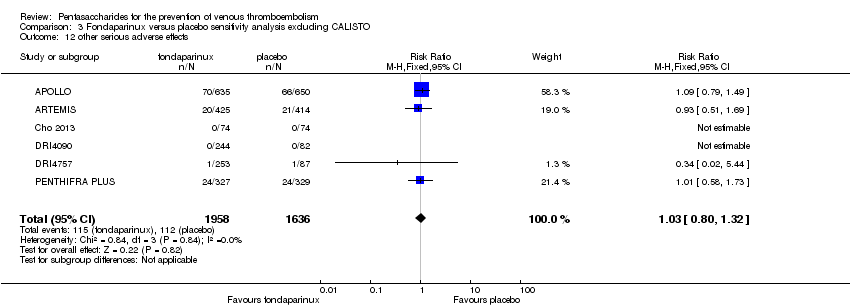
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 12 other serious adverse effects.
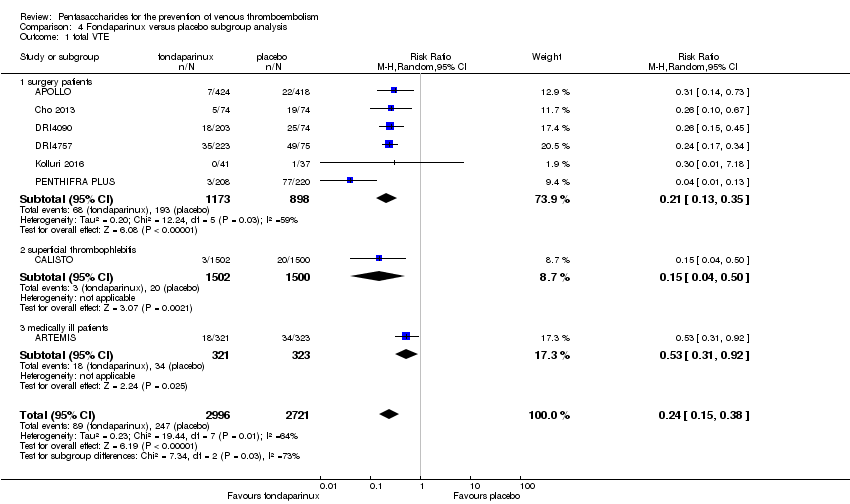
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 1 total VTE.
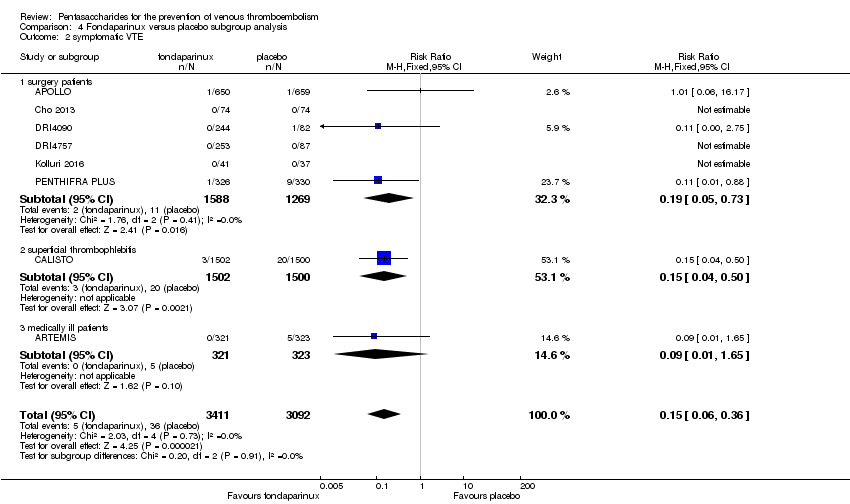
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 2 symptomatic VTE.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 3 total DVT.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 4 proximal DVT.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 5 total PE.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 6 fatal PE.
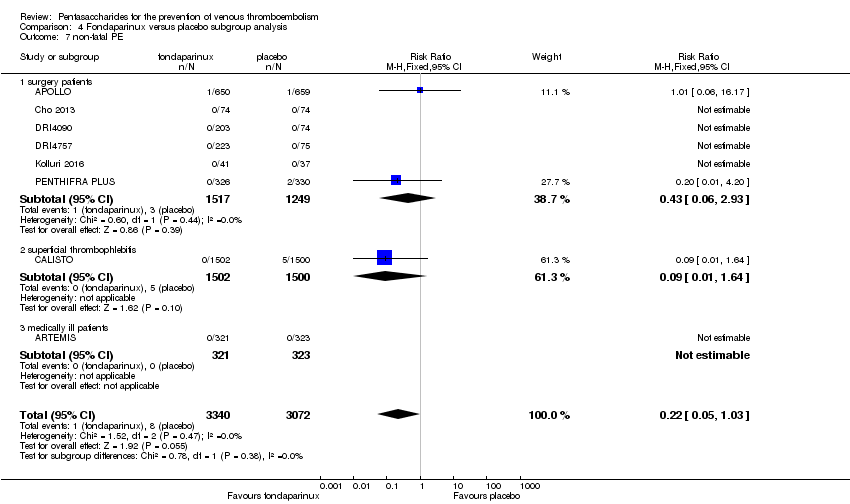
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 7 non‐fatal PE.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 8 major bleeding.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 9 fatal bleeding.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 10 MI.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 11 all causes of death.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 12 other serious adverse effects.

Comparison 5 Fondaparinux versus LMWH, Outcome 1 total VTE.
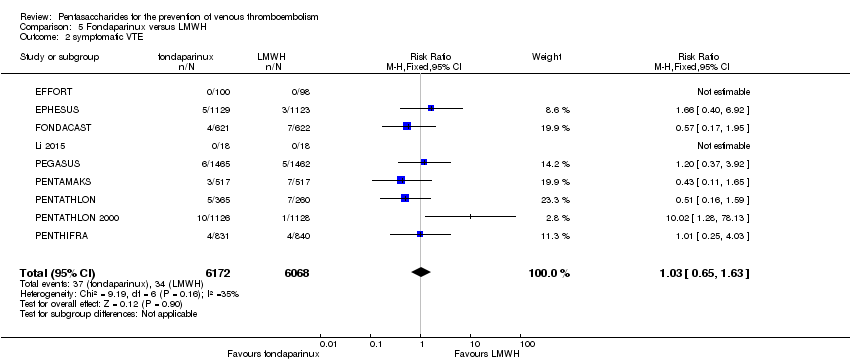
Comparison 5 Fondaparinux versus LMWH, Outcome 2 symptomatic VTE.

Comparison 5 Fondaparinux versus LMWH, Outcome 3 total DVT.

Comparison 5 Fondaparinux versus LMWH, Outcome 4 proximal DVT.

Comparison 5 Fondaparinux versus LMWH, Outcome 5 total PE.

Comparison 5 Fondaparinux versus LMWH, Outcome 6 fatal PE.

Comparison 5 Fondaparinux versus LMWH, Outcome 7 non‐fatal PE.

Comparison 5 Fondaparinux versus LMWH, Outcome 8 major bleeding.
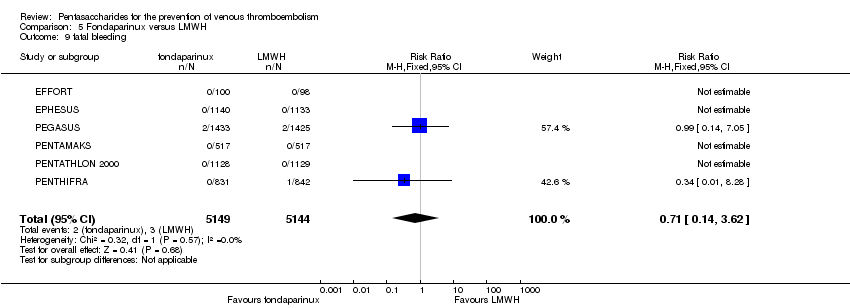
Comparison 5 Fondaparinux versus LMWH, Outcome 9 fatal bleeding.
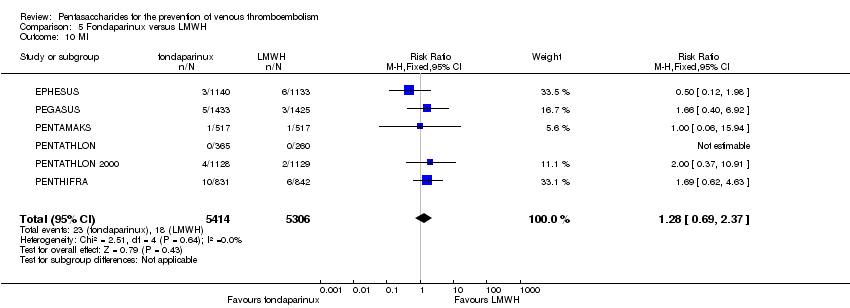
Comparison 5 Fondaparinux versus LMWH, Outcome 10 MI.

Comparison 5 Fondaparinux versus LMWH, Outcome 11 all causes of death.
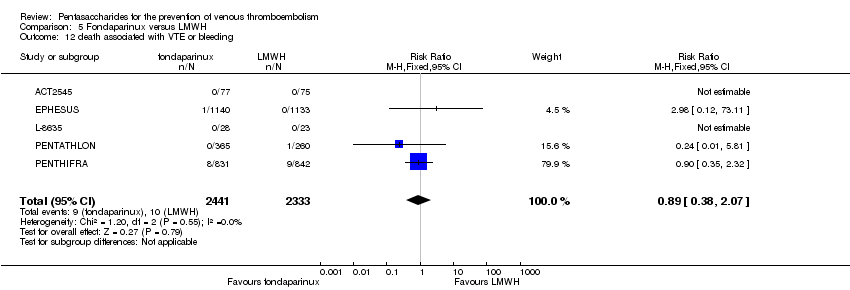
Comparison 5 Fondaparinux versus LMWH, Outcome 12 death associated with VTE or bleeding.
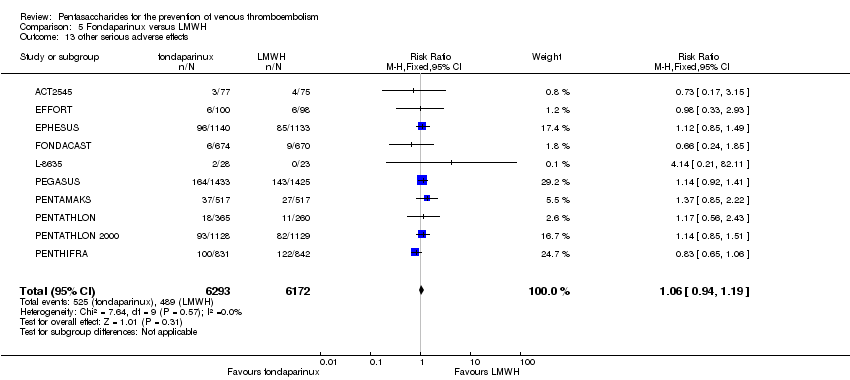
Comparison 5 Fondaparinux versus LMWH, Outcome 13 other serious adverse effects.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 1 total VTE.
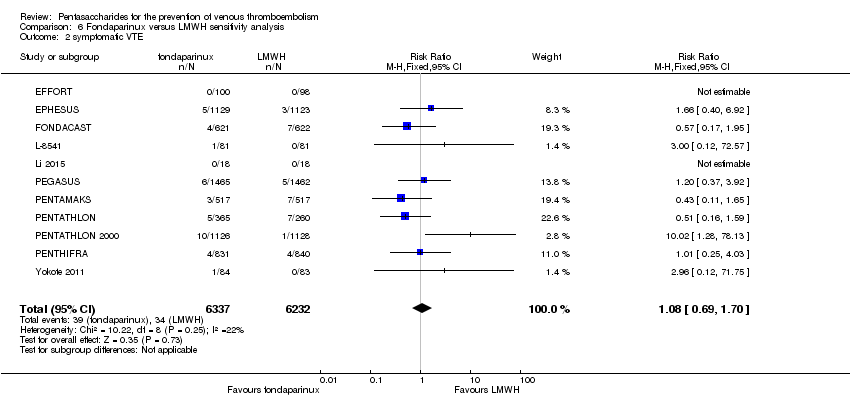
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 2 symptomatic VTE.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 3 total DVT.
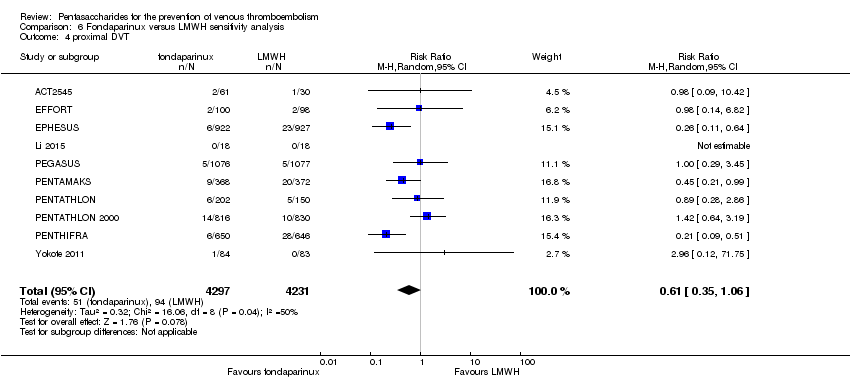
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 4 proximal DVT.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 5 total PE.
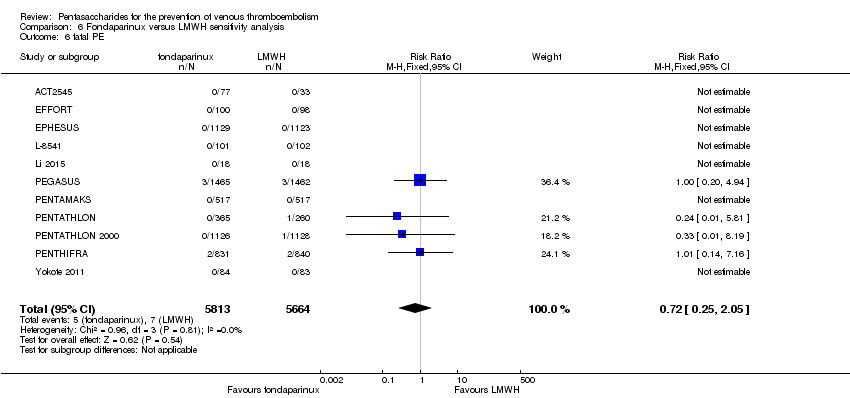
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 6 fatal PE.
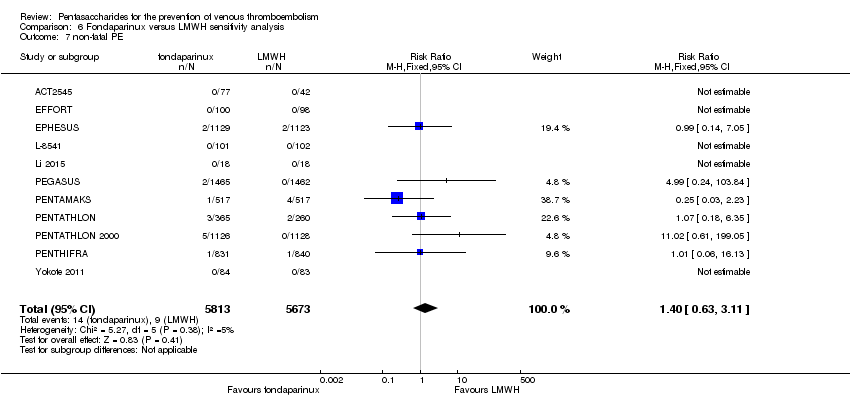
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 7 non‐fatal PE.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 8 major bleeding.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 9 fatal bleeding.
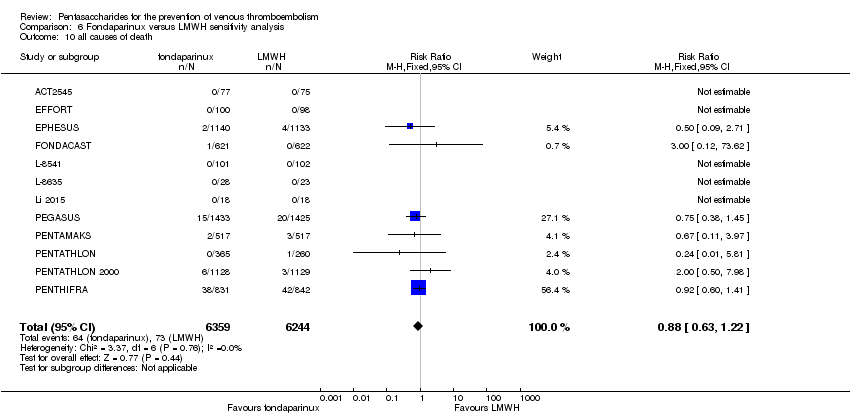
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 10 all causes of death.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 11 other serious adverse effects.
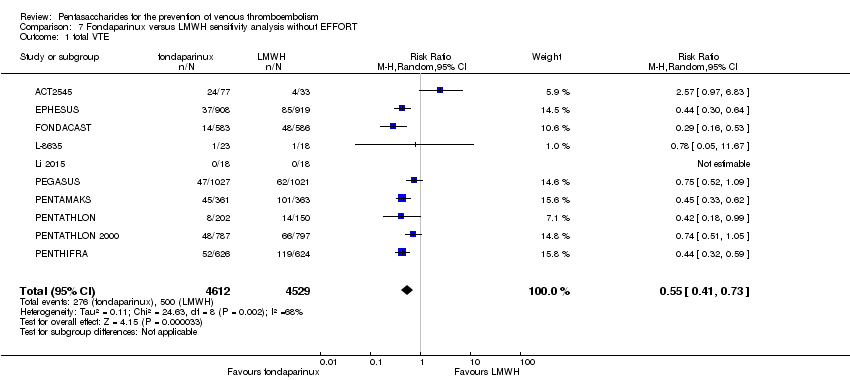
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 1 total VTE.
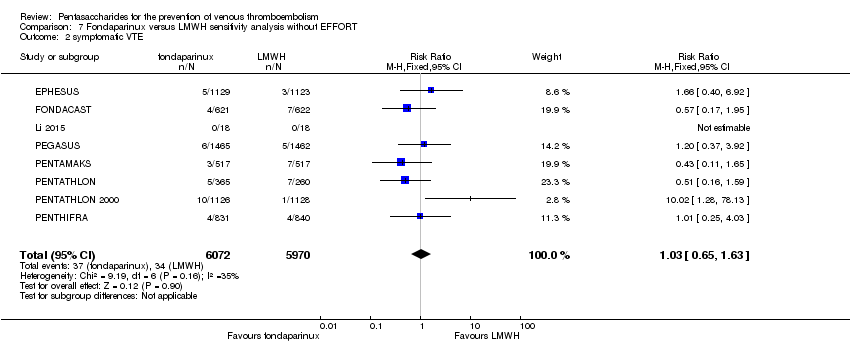
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 2 symptomatic VTE.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 3 total DVT.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 4 proximal DVT.
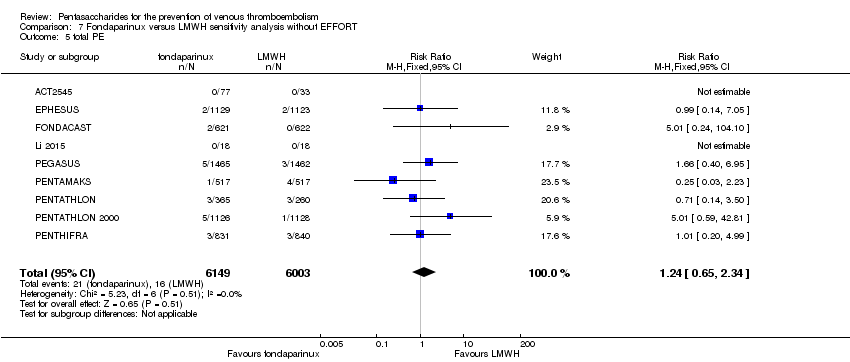
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 5 total PE.
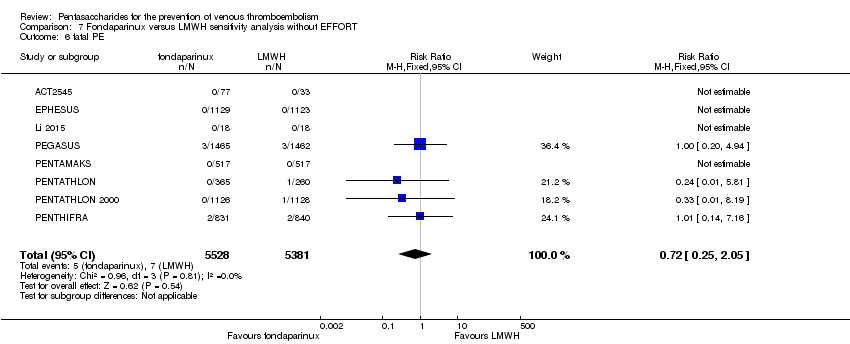
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 6 fatal PE.
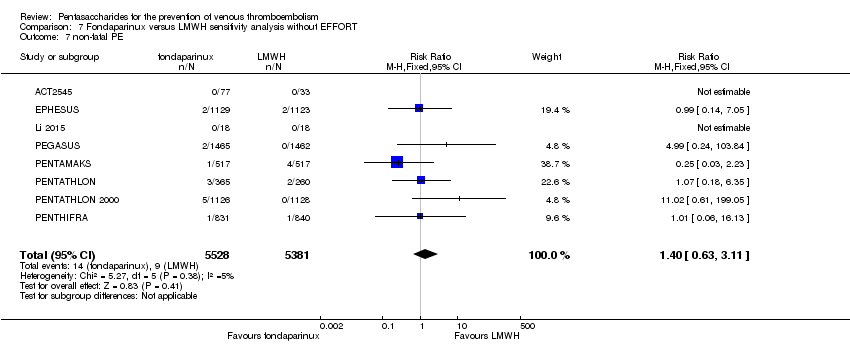
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 7 non‐fatal PE.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 8 major bleeding.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 9 fatal bleeding.
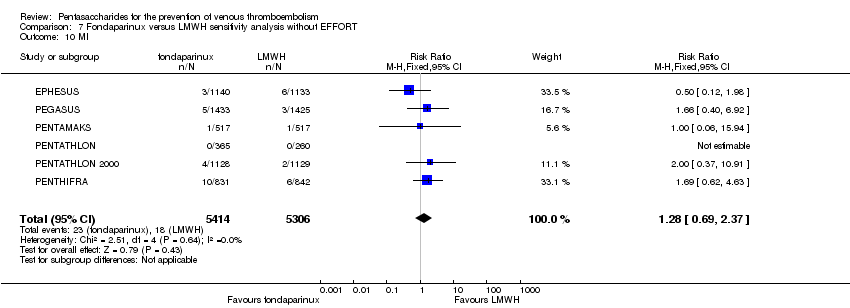
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 10 MI.
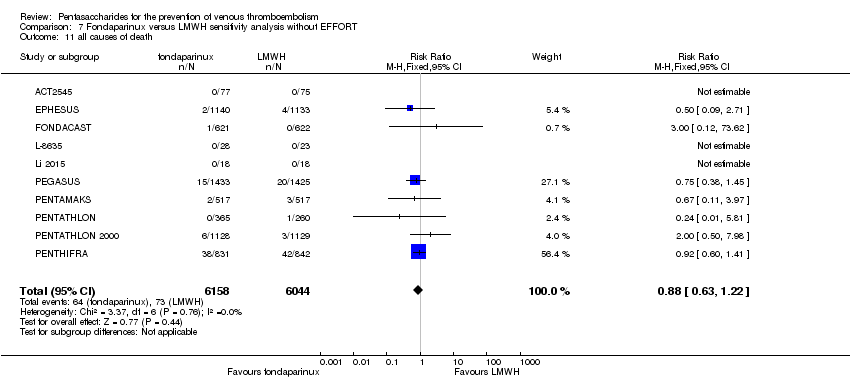
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 11 all causes of death.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 12 death associated with VTE or bleeding.

Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 13 other serious adverse effects.
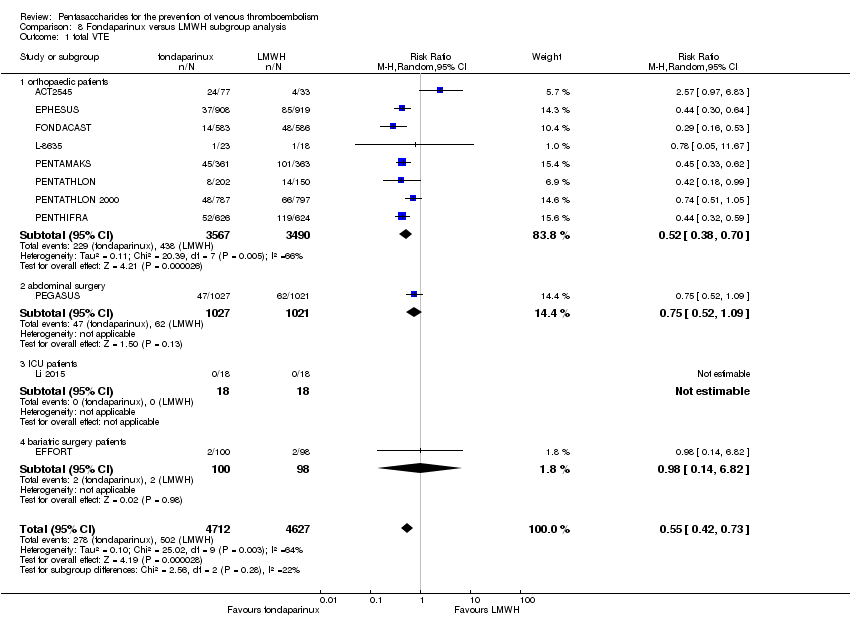
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 1 total VTE.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 2 symptomatic VTE.
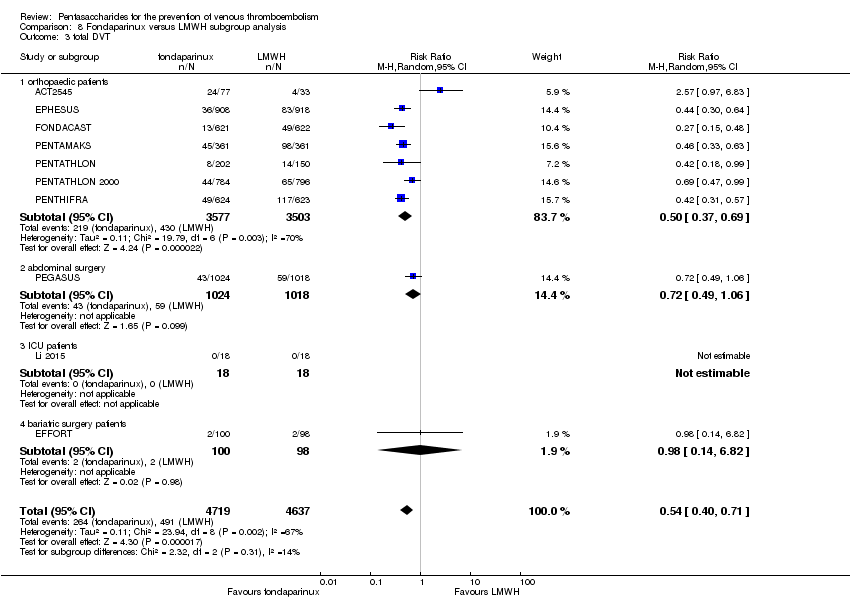
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 3 total DVT.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 4 proximal DVT.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 5 total PE.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 6 fatal PE.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 7 non‐fatal PE.
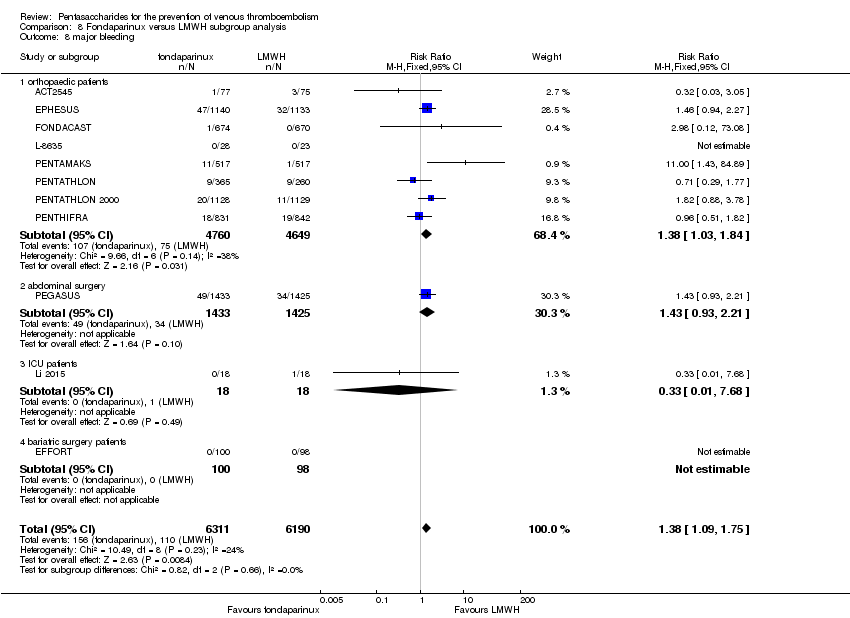
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 8 major bleeding.
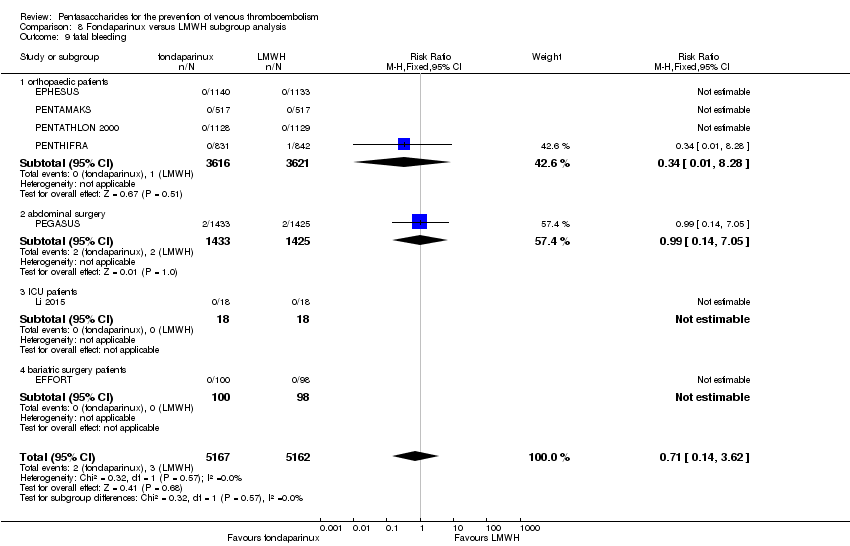
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 9 fatal bleeding.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 10 MI.
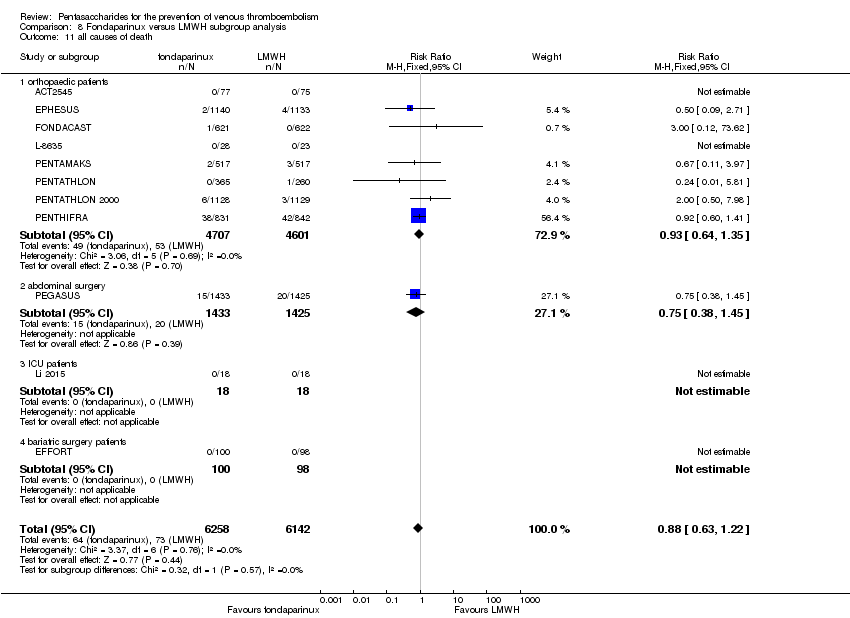
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 11 all causes of death.
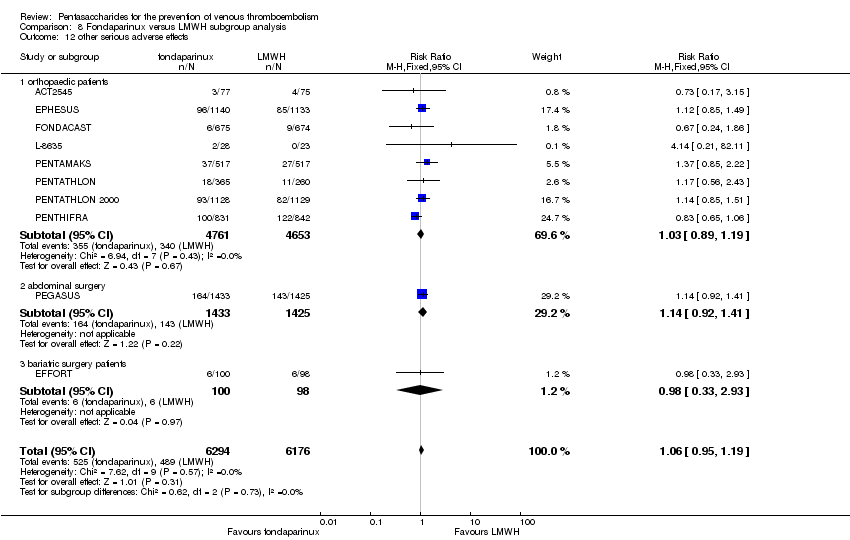
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 12 other serious adverse effects.

Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 1 total VTE.

Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 2 symptomatic VTE.

Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 3 total DVT.
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 4 proximal DVT.

Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 5 total PE.
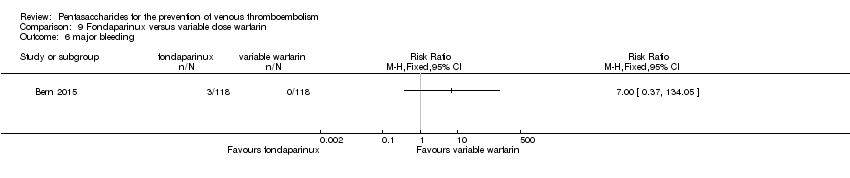
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 6 major bleeding.

Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 7 all causes of death.

Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 1 total VTE.
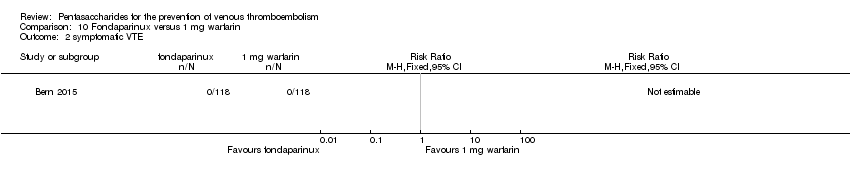
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 2 symptomatic VTE.
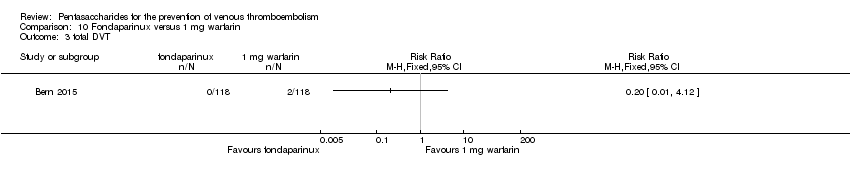
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 3 total DVT.

Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 4 proximal DVT.

Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 5 total PE.
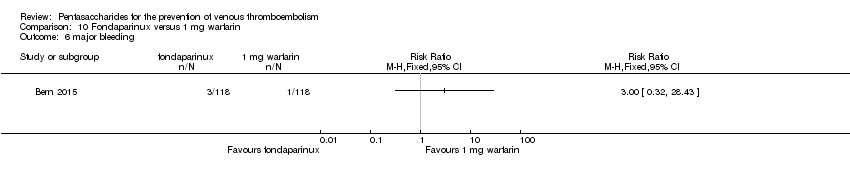
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 6 major bleeding.

Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 7 all causes of death.
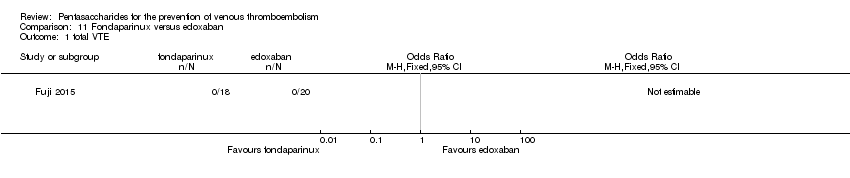
Comparison 11 Fondaparinux versus edoxaban, Outcome 1 total VTE.

Comparison 11 Fondaparinux versus edoxaban, Outcome 2 major bleeding.

Comparison 11 Fondaparinux versus edoxaban, Outcome 3 all causes of death.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 1 total VTE.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 2 symptomatic VTE.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 3 total DVT.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 4 proximal DVT.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 5 total PE.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 6 major bleeding.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 7 all causes of death.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 8 other serious adverse effects.
| Fondaparinux versus placebo for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Fondaparinux | |||||
| Total VTE | Study population | RR 0.24 | 5717 | ⊕⊕⊕⊝ | ||
| 91 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 181 per 1000 | 43 per 1000 | |||||
| Symptomatic VTE | Study population | RR 0.15 | 6503 | ⊕⊕⊕⊕ | ||
| 12 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 1 per 1000 | |||||
| Total DVT | Study population | RR 0.25 | 5715 | ⊕⊕⊕⊝ | ||
| 87 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 173 per 1000 | 43 per 1000 | |||||
| Proximal DVT | Study population | RR 0.12 | 2746 | ⊕⊕⊕⊝ | ||
| 60 per 1000 | 7 per 1000 | |||||
| Moderate | ||||||
| 54 per 1000 | 6 per 1000 | |||||
| Total PE | Study population | RR 0.16 | 6412 | ⊕⊕⊕⊕ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 0 per 1000 | |||||
| Major bleeding | Study population | RR 2.56 | 6659 | ⊕⊕⊕⊝ | ||
| 5 per 1000 | 12 per 1000 | |||||
| Moderate | ||||||
| 3 per 1000 | 8 per 1000 | |||||
| All causes of death | Study population | RR 0.76 | 6674 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Data were pooled with a random‐effects model owing to heterogeneity ‐ downgraded by one level. | ||||||
| Fondaparinux versus LMWH for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| LMWH | Fondaparinux | |||||
| Total VTE | Study population | RR 0.55 | 9339 | ⊕⊕⊕⊝ | ||
| 108 per 1000 | 60 per 1000 | |||||
| Moderate | ||||||
| 83 per 1000 | 46 per 1000 | |||||
| Symptomatic VTE | Study population | RR 1.03 | 12240 | ⊕⊕⊕⊝ | ||
| 6 per 1000 | 6 per 1000 | |||||
| Moderate | ||||||
| 3 per 1000 | 3 per 1000 | |||||
| Total DVT | Study population | RR 0.54 | 9356 | ⊕⊕⊕⊝ | ||
| 106 per 1000 | 57 per 1000 | |||||
| Moderate | ||||||
| 86 per 1000 | 46 per 1000 | |||||
| Proximal DVT | Study population | RR 0.58 | 8361 | ⊕⊕⊝⊝ | ||
| 23 per 1000 | 13 per 1000 | |||||
| Moderate | ||||||
| 25 per 1000 | 14 per 1000 | |||||
| Total PE | Study population | RR 1.24 | 12350 | ⊕⊕⊕⊝ | ||
| 3 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| Major bleeding | Study population | RR 1.38 | 12501 | ⊕⊕⊕⊕ | ||
| 18 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 23 per 1000 | 32 per 1000 | |||||
| All causes of death | Study population | RR 0.88 | 12400 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 10 per 1000 | |||||
| Moderate | ||||||
| 3 per 1000 | 3 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Data were pooled with the random‐effects model because of heterogeneity ‐ downgraded by one level. | ||||||
| Fondaparinux versus variable dose warfarin for the prevention of venous thromboembolism | ||||||
| Patient or population: patients requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Variable dose warfarin | Fondaparinux | |||||
| Total VTE | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No VTE events recorded |
| Symptomatic VTE | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No VTE events recorded |
| Total DVT | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No DVT events recorded |
| Proximal DVT | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No proximal DVT events recorded |
| Total PE | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No PE events recorded |
| Major bleeding | Study population | RR 7 | 236 | ⊕⊝⊝⊝ | ||
| No cases of bleeding (0/118) were reported in the variable warfarin group. Three cases (3/118) of bleeding were reported in the fondaparinux group. | ||||||
| All causes of death | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study was included in this comparison; the study sample was small, and the events were rare ‐ downgraded by three levels. | ||||||
| Fondaparinux versus 1 mg warfarin for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 1 mg warfarin | Fondaparinux | |||||
| Total VTE | Study population | RR 0.2 | 236 | ⊕⊝⊝⊝ | ||
| 17 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 3 per 1000 | |||||
| Symptomatic VTE | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No systematic VTE events recorded |
| Total DVT | Study population | RR 0.2 | 236 | ⊕⊝⊝⊝ | ||
| 17 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 3 per 1000 | |||||
| Proximal DVT | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No proximal DVT events recorded |
| Total PE | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No PE events recorded |
| Major bleeding | Study population | RR 3 | 236 | ⊕⊝⊝⊝ | ||
| 8 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 27 per 1000 | |||||
| All causes of death | See comment | See comment | Not estimable | 236 | ⊕⊝⊝⊝ | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study was included in this comparison; the study sample was small, and the events were rare ‐ downgraded by three levels. | ||||||
| Fondaparinux versus edoxaban for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Edoxaban | Fondaparinux | |||||
| Total VTE | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No VTE events recorded |
| Symptomatic VTE | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No VTE events recorded |
| Total DVT | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No DVT events recorded |
| Proximal DVT | See comment | See comment | 38 | Proximal DVT not an outcome of this study | ||
| Total PE | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No PE events recorded |
| Major bleeding | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No major bleeding events recorded |
| All causes of death | See comment | See comment | Not estimable | 38 | ⊕⊝⊝⊝ | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study was included in this comparison; the study sample was small, and no events were recorded ‐ downgraded by three levels. | ||||||
| Fondaparinux versus mechanical thromboprophylaxis for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mechanical thromboprophylaxis | Fondaparinux | |||||
| Total VTE | Study population | RR 0.61 | 99 | ⊕⊕⊝⊝ | ||
| 176 per 1000 | 108 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 108 per 1000 | |||||
| Symptomatic VTE | See comment | See comment | Not estimable | 120 | ⊕⊕⊝⊝ | No cases of symptomatic VTE recorded |
| Total DVT | Study population | RR 0.63 | 100 | ⊕⊕⊝⊝ | ||
| 171 per 1000 | 108 per 1000 | |||||
| Moderate | ||||||
| 171 per 1000 | 108 per 1000 | |||||
| Proximal DVT | See comment | See comment | Not estimable | 105 | ⊕⊕⊝⊝ | No cases of proximal DVT recorded |
| Total PE | See comment | See comment | Not estimable | 120 | ⊕⊕⊝⊝ | No cases of PE recorded |
| Major bleeding | See comment | See comment | Not estimable | 120 | ⊕⊕⊝⊝ | No cases of major bleeding recorded |
| All causes of death | See comment | See comment | Not estimable | 120 | ⊕⊕⊝⊝ | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study included in this comparison, small study sample, funded by pharmaceutical company, very short follow‐up ‐ downgraded by two levels. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| 2 symptomatic VTE Show forest plot | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| 3 total DVT Show forest plot | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 4 proximal DVT Show forest plot | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| 5 total PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 6 fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 8 major bleeding Show forest plot | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 9 fatal bleeding Show forest plot | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 MI Show forest plot | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 11 all causes of death Show forest plot | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| 12 other serious adverse effects Show forest plot | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 9 | 5884 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.43] |
| 2 symptomatic VTE Show forest plot | 9 | 6670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.42] |
| 3 total DVT Show forest plot | 9 | 5882 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.45] |
| 4 proximal DVT Show forest plot | 8 | 2913 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.05, 0.51] |
| 5 total PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 6 fatal PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE Show forest plot | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 8 major bleeding Show forest plot | 9 | 6829 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 9 fatal bleeding Show forest plot | 7 | 6163 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 all causes of death Show forest plot | 8 | 6766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 7 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 2 symptomatic VTE Show forest plot | 7 | 3501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.54] |
| 3 total DVT Show forest plot | 7 | 2713 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.43] |
| 4 proximal DVT Show forest plot | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| 5 total PE Show forest plot | 7 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.92] |
| 6 fatal PE Show forest plot | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE Show forest plot | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| 8 major bleeding Show forest plot | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.52, 4.67] |
| 9 fatal bleeding Show forest plot | 5 | 3006 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 MI Show forest plot | 4 | 2790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| 11 all causes of death Show forest plot | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| 12 other serious adverse effects Show forest plot | 6 | 3594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| 1.1 surgery patients | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 1.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.50] |
| 1.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.92] |
| 2 symptomatic VTE Show forest plot | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| 2.1 surgery patients | 6 | 2857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.73] |
| 2.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.50] |
| 2.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 3 total DVT Show forest plot | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 3.1 surgery patients | 6 | 2069 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 3.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.56] |
| 3.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.10] |
| 4 proximal DVT Show forest plot | 7 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.07, 0.22] |
| 4.1 surgery patients | 6 | 2101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.15] |
| 4.2 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.24] |
| 5 total PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 5.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.05, 2.14] |
| 5.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 5.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 6 fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 6.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.25] |
| 6.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 7 non‐fatal PE Show forest plot | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 7.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| 7.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 7.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding Show forest plot | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 8.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.56, 4.95] |
| 8.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.86] |
| 8.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.52] |
| 9 fatal bleeding Show forest plot | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 9.1 surgery patients | 4 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 9.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI Show forest plot | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 10.1 surgery patients | 3 | 1951 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 10.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| 11 all causes of death Show forest plot | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| 11.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.31] |
| 11.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.00] |
| 11.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.29, 1.03] |
| 12 other serious adverse effects Show forest plot | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| 12.1 surgery patients | 5 | 2755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 12.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.28, 1.36] |
| 12.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 2 symptomatic VTE Show forest plot | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 3 total DVT Show forest plot | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| 4 proximal DVT Show forest plot | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| 5 total PE Show forest plot | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding Show forest plot | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 9 fatal bleeding Show forest plot | 6 | 10293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 11 all causes of death Show forest plot | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 12 death associated with VTE or bleeding Show forest plot | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| 13 other serious adverse effects Show forest plot | 10 | 12465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 13 | 9709 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.74] |
| 2 symptomatic VTE Show forest plot | 11 | 12569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.70] |
| 3 total DVT Show forest plot | 12 | 9726 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.72] |
| 4 proximal DVT Show forest plot | 10 | 8528 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.06] |
| 5 total PE Show forest plot | 12 | 12720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE Show forest plot | 11 | 11477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE Show forest plot | 11 | 11486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding Show forest plot | 13 | 12874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.06, 1.68] |
| 9 fatal bleeding Show forest plot | 8 | 10499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 all causes of death Show forest plot | 12 | 12603 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 11 other serious adverse effects Show forest plot | 11 | 12707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 10 | 9141 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 2 symptomatic VTE Show forest plot | 8 | 12042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 3 total DVT Show forest plot | 9 | 9158 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| 4 proximal DVT Show forest plot | 8 | 8163 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.03] |
| 5 total PE Show forest plot | 9 | 12152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE Show forest plot | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE Show forest plot | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding Show forest plot | 10 | 12303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 9 fatal bleeding Show forest plot | 5 | 10095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 11 all causes of death Show forest plot | 10 | 12202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 12 death associated with VTE or bleeding Show forest plot | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| 13 other serious adverse effects Show forest plot | 9 | 12267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 1.1 orthopaedic patients | 8 | 7057 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.38, 0.70] |
| 1.2 abdominal surgery | 1 | 2048 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.09] |
| 1.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 2 symptomatic VTE Show forest plot | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 2.1 orthopaedic patients | 6 | 9079 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.65] |
| 2.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.37, 3.92] |
| 2.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 total DVT Show forest plot | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| 3.1 orthopaedic patients | 7 | 7080 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.37, 0.69] |
| 3.2 abdominal surgery | 1 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.06] |
| 3.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 4 proximal DVT Show forest plot | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| 4.1 orthopaedic patients | 6 | 5974 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.02] |
| 4.2 abdominal surgery | 1 | 2153 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.29, 3.45] |
| 4.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 5 total PE Show forest plot | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 5.1 orthopaedic patients | 7 | 9189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.34] |
| 5.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.95] |
| 5.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 6.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.29] |
| 6.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.94] |
| 6.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 non‐fatal PE Show forest plot | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 7.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.83] |
| 7.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.99 [0.24, 103.84] |
| 7.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 bariatric surgery | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding Show forest plot | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 8.1 orthopaedic patients | 8 | 9409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.03, 1.84] |
| 8.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.93, 2.21] |
| 8.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.68] |
| 8.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 fatal bleeding Show forest plot | 7 | 10329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 9.1 orthopaedic patients | 4 | 7237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.28] |
| 9.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.05] |
| 9.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI Show forest plot | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 10.1 orthopaedic patients | 5 | 7862 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.61, 2.39] |
| 10.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.92] |
| 11 all causes of death Show forest plot | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 11.1 orthopaedic patients | 8 | 9308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.35] |
| 11.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.45] |
| 11.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 other serious adverse effects Show forest plot | 10 | 12470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| 12.1 orthopaedic patients | 8 | 9414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.19] |
| 12.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.41] |
| 12.3 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.33, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 all causes of death Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 total VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 other serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

