여성의 분만 입원시 간헐적 모니터링과 분만 감시 장치 (CTG)에 의한 태아 심장의 전자 모니터링 비교
Referencias
References to studies included in this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: RCT. Duration of study: 1999. | |
| Participants | Setting: Glasgow Royal Maternity Hospital, Scotland. Inclusion criteria: healthy women who had experienced a normal pregnancy, presented at term in spontaneous labour and were eligible for admission to the Midwives Birth Unit. Randomisation on admission in labour. | |
| Interventions | Admission CTG: a routine 20‐minute period of EFM at the time of admission. Intermittent auscultation: the fetal heart was auscultated during and immediately following a contraction for a minimum of 60 seconds. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
| |
| Notes | Unpublished data to permit re‐inclusion of women to groups as randomised kindly provided by author. This study was funded by North Glasgow University Hospitals NHS Trust. Declaration of interest were not mentioned in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated in order to allocate participants equally between the two groups..." |
| Allocation concealment (selection bias) | Low risk | "...sequentially numbered, sealed opaque envelopes, which contained allocation to the appropriate group." |
| Blinding of participants and personnel (performance bias) | High risk | Although not documented, we judged, given nature of intervention, that women and clinicians were not blind to the interventions used. |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: in the trial report 22 women (7%) are excluded from the analysis (21 women entered into the study and found not to be in labour and 1 randomisation card missing). However, data for these 21 of 22 women were identified and extracted subsequently by the trial author and kindly provided to the review team. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were reported adequately in results. |
| Other bias | Low risk | None identified. |
| Methods | Study design: RCT. Duration of study: 1997 to 2001. | |
| Participants | Setting: National Maternity Hospital in Dublin, Ireland. Inclusion criteria: women were eligible for inclusion if they were admitted in labour, a singleton pregnancy, fewer than 42 completed weeks of gestation, no suspicion or evidence of antenatal fetal compromise, no adverse obstetric history, clear amniotic fluid, and maternal temperature of 37.5°C or less at admission. Randomisation on admission in labour. A relatively small number (fewer than 5%) of women who had a previous caesarean section and who went into labour prior to 37 completed weeks' gestation were included in this study and were randomised. The trial author kindly provided data separately for the outcomes for women (i) between 37 and 42 completed weeks with (ii) an absence of previous caesarean section and these data were used in the main analyses for this review. Sensitivity analyses were conducted in which the outcomes for all randomised women were used. | |
| Interventions | Admission CTG: a 20‐minute admission CTG immediately after early amniotomy done on diagnosis of labour in women presenting to the delivery ward. Intermittent auscultation: intermittent auscultation was used for 1 minute after a contraction every 15 minutes in the first stage and every 5 minutes in the second stage of labour. This was done after early amniotomy on diagnosis of labour in women presenting to the delivery ward. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
| |
| Notes | See Participants (above) The study was funded by the Research Committee of the National Maternity Hospital, Holles St, Dublin, Ireland. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the randomisation sequence was from a commercial package 10 and used a fixed block size of 100. It was changed after 2621 patients had been recruited, and was generated by the National Perinatal Epidemiology Unit with random block sizes of 100–250." |
| Allocation concealment (selection bias) | Low risk | "...sealed, opaque, sequentially numbered envelope." |
| Blinding of participants and personnel (performance bias) | High risk | Although not documented, we judged, given nature of intervention, that women and clinicians were not blind to the interventions used. |
| Blinding of outcome assessment (detection bias) | Low risk | "...Data were entered and neonatal assessment was made without knowledge of the randomised assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up = 22 (0.5%); admission CTG 26 (0.6%). Intermittent auscultation. For outcome 'pH less than 7 or BD/E > than 12 mmol/L' 7.5% and 7.8% data missing for admission CTG and intermittent auscultation respectively. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were reported adequately in results. |
| Other bias | Low risk | None identified. |
| Methods | Study design: RCT. Duration of study: not stated. | |
| Participants | Setting: Dundee, Scotland. Inclusion criteria: "Women were eligible to join the study if they were booked for hospital delivery, attended a hospital or community based consultant led clinic in the third trimester of pregnancy, and had no obstetric complications at that visit that would warrant continuous intrapartum monitoring of FHR (pre eclampsia or hypertension in previous or index pregnancy; essential hypertension; diabetes (insulin dependent or gestational); suspected intrauterine growth restriction; placental abruption or praevia or vaginal bleeding of unknown origin; multiple pregnancy; fetal malformation; previous caesarean section; breech presentation; or rhesus isoimmunisation)." A total of 3752 women were recruited to the study and randomised during the third trimester. However, some women developed an obstetric complication between randomisation and admission in labour that warranted continuous FHR monitoring in labour, such that only 2367 women were judged to be low‐risk when in labour (1186 admission CTG, 1181 intermittent auscultation). The trial author kindly provided data separately for the outcomes in this subgroup of women and these data are used in the main analyses in this review. Sensitivity analyses were done in which the outcomes for all randomised women were used. | |
| Interventions | Admission CTG: a 20‐minute CTG on admission in spontaneous uncomplicated labour. Intermittent auscultation: auscultation of the fetal heart with a hand‐held Doppler device during and immediately after at least 1 contraction. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
| |
| Notes | See Participants (above) This study was funded by Chief Scientists Office of the Scottish Executive, Edinburgh. Declarations of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...commercially available computer randomisation program." |
| Allocation concealment (selection bias) | Low risk | "The allocation was placed in a sealed envelope..." |
| Blinding of participants and personnel (performance bias) | High risk | Although not documented, we judged, given nature of intervention, that women and clinicians were not blind to the interventions used. |
| Blinding of outcome assessment (detection bias) | Low risk | "The data analysts were blind to the randomisation code." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up for the primary outcome of metabolic acidosis was high (admission CTG N = 310, 26% and intermittent auscultation N = 321, 27%). However, metabolic acidosis was defined as "pH less than 7.20 or BD (Base Deficit) > than 8 mmol/L". Data were unavailable for the outcome metabolic acidosis as defined in this review, i.e. 'pH less than 7 or BD/E > than 12 mmol/L', therefore this study does not provide data for this outcome in this review. All other outcomes had low rates of missing data, hence rating as low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were reported adequately in results. |
| Other bias | Low risk | "Between randomisation during the third trimester of pregnancy and admission in labour, 1384 women (37%) developed an obstetric complication that warranted continuous fetal heart rate monitoring in labour". A total of 3752 women were recruited to the study and randomised during the third trimester. However, some women developed complications between randomisation and admission in labour, such that only 2367 women were judged to be low risk when in labour (1186 admission CTG, 1181 intermittent auscultation). There are similar levels of attrition in both groups due to development of complications suggesting that allocation concealment remained intact. The trial author kindly provided data separately for the outcomes in this low‐risk subgroup of women and these data are used in the main analyses in this review. |
| Methods | Study design: RCT. Duration of study: 2002 to 2006. | |
| Participants | Setting: Buckinghamshire, England. Inclusion criteria: labouring women considered to be "low risk" of fetal or maternal complications on admission. Exclusion criteria: any minor maternal medical complication, e.g. diabetes or essential hypertension; previous caesarean section; preterm labour (< 37 completed weeks); multiple pregnancy; prolonged pregnancy (> 42 completed weeks); prolonged membrane rupture (more than 24 hours); induction of labour; meconium‐stained liquor; maternal pyrexia; rhesus sensitisation; polyhydramnios; oligohydramnios; pre‐eclampsia or blood pressure over 140/90 mmHg; abnormal presentation or lie (e.g. breech, transverse); high head (5/5ths palpable per abdomen); antepartum or intrapartum haemorrhage; known or suspected intrauterine growth retardation; any known or suspected fetal medical complication; abnormal Doppler artery velocimetry; known fetal malformation; poor obstetric history (e.g. history of stillbirth); un‐booked. Randomisation on admission in labour. | |
| Interventions | Admission CTG: a 15‐minute CTG on admission in spontaneous uncomplicated labour. Intermittent auscultation: auscultation of the fetal heart for one continuous minute using a Pinard stethoscope or Doppler ultrasound device, after a contraction, at least every 15 minutes in the first stage of labour, and every 5 minutes in the second stage of labour. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
| |
| Notes | See Participants. The study was funded by Buckinghamshire Hospitals NHS Trust's Research Department and through the establishment of a research midwife role within the maternity unit. Declaration of interest not mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...via a random number table." |
| Allocation concealment (selection bias) | Low risk | "Allocation to control and experimental arms was via opening of the next envelope in a series of sequentially numbered envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | Although not documented, we judged, given nature of intervention, that women and clinicians were not blind to the interventions used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data reported with exception of "augmentation with oxytocin" where missing data were low (admission CTG N = 2, 0.7% and intermittent auscultation N = 4, 1.4%). |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were reported adequately in results. |
| Other bias | Low risk | None identified. |
BD: base deficit
BD/E: base deficit/excess
CTG: cardiotocograph
EFM: electronic fetal monitoring
FHR: fetal heart rate
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Trial name or title | Foetal cardiotocography versus intermittent auscultation during labour ward admission: a randomised controlled trial (the ADCAR trial) |
| Methods | RCT |
| Participants |
|
| Interventions |
|
| Outcomes | Primary: incidence of caesarean section |
| Starting date | 2008 |
| Contact information | Declan Devane |
| Notes |
CTG: cardiotocograph
EFM: electronic fetal monitoring
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.00, 1.44] |
| Analysis 1.1  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 1 Caesarean section. | ||||
| 2 Instrumental vaginal birth Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| Analysis 1.2  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 2 Instrumental vaginal birth. | ||||
| 3 Continuous EFM during labour Show forest plot | 3 | 10753 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| Analysis 1.3  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 3 Continuous EFM during labour. | ||||
| 4 Amniotomy Show forest plot | 2 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| Analysis 1.4  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 4 Amniotomy. | ||||
| 5 Oxytocin for augmentation of labour Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.17] |
| Analysis 1.5  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 5 Oxytocin for augmentation of labour. | ||||
| 6 Epidural Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.87, 1.41] |
| Analysis 1.6 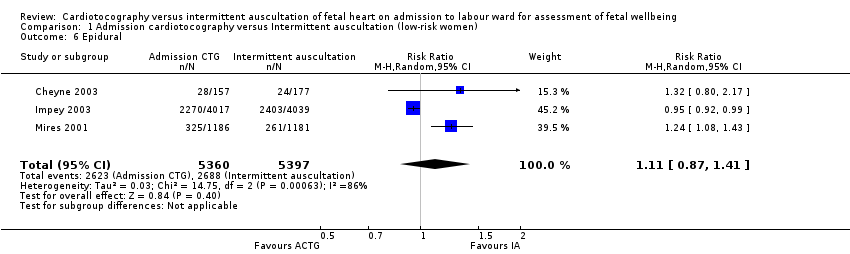 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 6 Epidural. | ||||
| 7 Fetal blood sampling Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.13, 1.45] |
| Analysis 1.7  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 7 Fetal blood sampling. | ||||
| 8 Fetal and neonatal deaths Show forest plot | 4 | 11339 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.30, 3.47] |
| Analysis 1.8 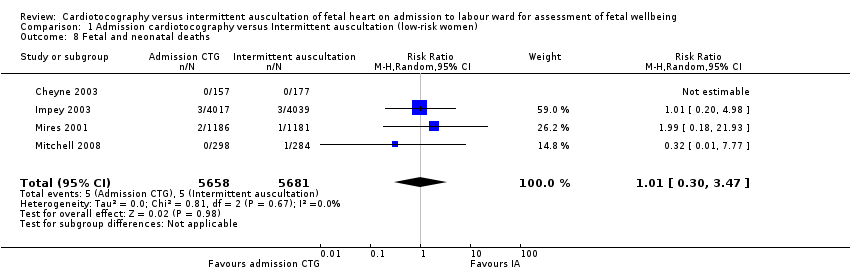 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 8 Fetal and neonatal deaths. | ||||
| 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.67] |
| Analysis 1.9  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth. | ||||
| 10 Admission to neonatal intensive care Show forest plot | 4 | 11331 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.24] |
| Analysis 1.10 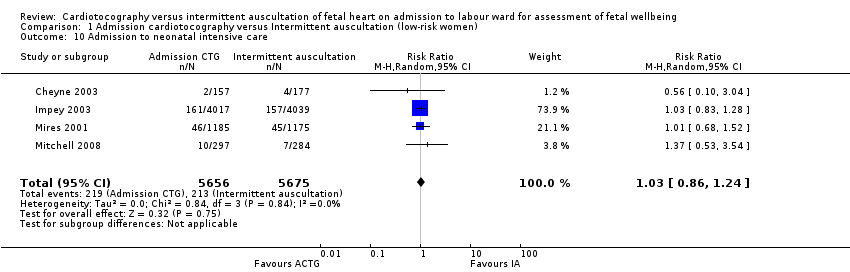 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 10 Admission to neonatal intensive care. | ||||
| 11 Apgar score < 7 at or after 5 minutes Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.85] |
| Analysis 1.11  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 11 Apgar score < 7 at or after 5 minutes. | ||||
| 12 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.90] |
| Analysis 1.12 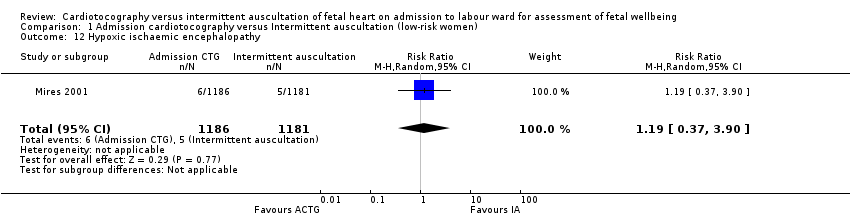 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 12 Hypoxic ischaemic encephalopathy. | ||||
| 13 Neonatal seizures Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.61] |
| Analysis 1.13 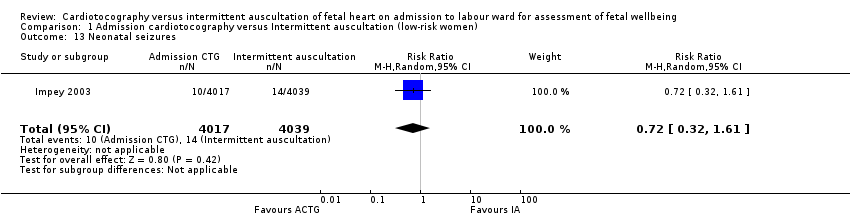 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 13 Neonatal seizures. | ||||
| 14 Length of stay in neonatal intensive care (days) Show forest plot | 1 | 91 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.59, 4.19] |
| Analysis 1.14  Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 14 Length of stay in neonatal intensive care (days). | ||||
| 15 Length of stay in neonatal intensive care (hours) Show forest plot | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 6.20 [‐8.70, 21.10] |
| Analysis 1.15 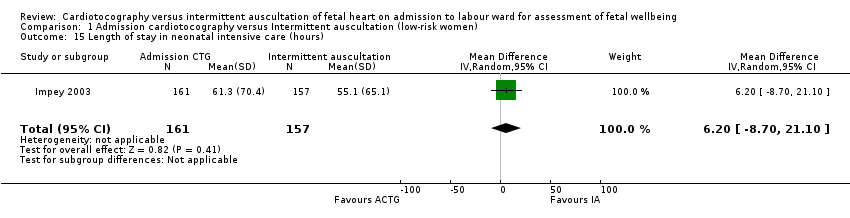 Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 15 Length of stay in neonatal intensive care (hours). | ||||
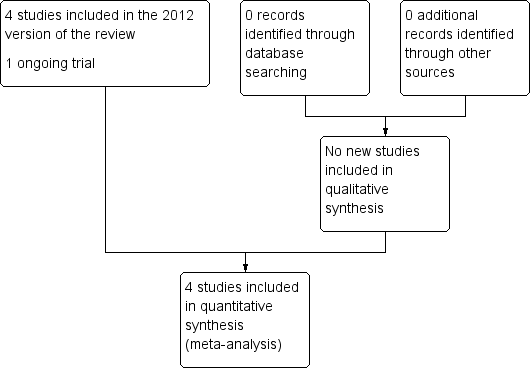
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 1 Caesarean section.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 2 Instrumental vaginal birth.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 3 Continuous EFM during labour.
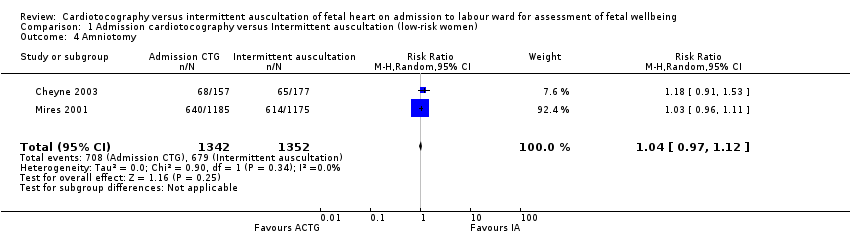
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 4 Amniotomy.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 5 Oxytocin for augmentation of labour.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 6 Epidural.
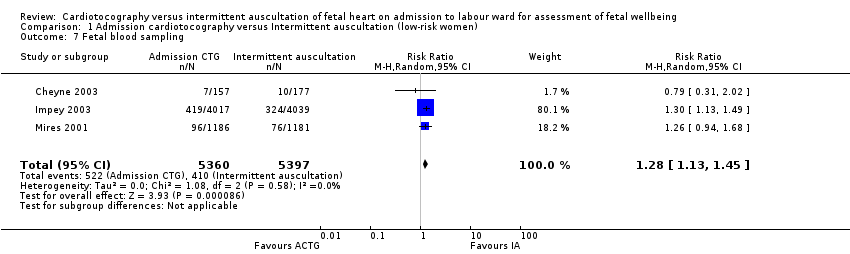
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 7 Fetal blood sampling.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 8 Fetal and neonatal deaths.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 10 Admission to neonatal intensive care.
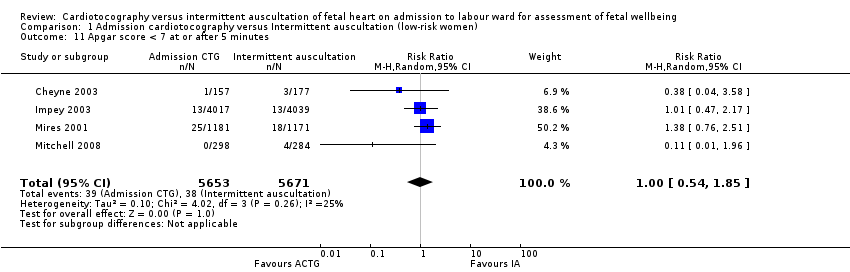
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 11 Apgar score < 7 at or after 5 minutes.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 12 Hypoxic ischaemic encephalopathy.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 13 Neonatal seizures.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 14 Length of stay in neonatal intensive care (days).

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 15 Length of stay in neonatal intensive care (hours).
| Admission cardiotocography compared to Intermittent auscultation (low‐risk women) for assessment of fetal wellbeing | ||||||
| Patient or population: Low risk pregnant women. All of the women were in labour. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Intermittent auscultation (low‐risk women) | Risk with admission cardiotocography | |||||
| Incidence of caesarean section | Study population | RR 1.20 | 11338 | ⊕⊕⊕⊝ | ||
| 36 per 1000 | 44 per 1000 | |||||
| Incidence of operative vaginal birth | Study population | RR 1.10 | 11338 | ⊕⊕⊝⊝ | ||
| 126 per 1000 | 139 per 1000 | |||||
| Perinatal mortality rate (fetal and neonatal deaths excluding lethal congenital anomalies) | Study population | RR 1.01 | 11339 | ⊕⊕⊕⊝ | ||
| 1 per 1000 | 1 per 1000 | |||||
| Severe neurodevelopmental disability assessed ≥ 12 months of age | Study population | ‐ | (0 RCTs) | ‐ | None of the included studies reported data for the outcome | |
| see comment | see comment | |||||
| Incidence of continuous electronic fetal monitoring during labour | Study population | RR 1.30 | 10753 | ⊕⊕⊝⊝ | ||
| 417 per 1000 | 542 per 1000 | |||||
| Incidence and severity of hypoxic ischaemic encephalopathy (incidence only reported) | Study population | RR 1.19 | 2367 | ⊕⊝⊝⊝ | ||
| 4 per 1000 | 5 per 1000 | |||||
| Incidence of seizures in the neonatal period | Study population | RR 0.72 | 8056 | ⊕⊕⊝⊝ | ||
| 3 per 1000 | 2 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations: outcome may have been affected by lack of blinding as all studies judged to be at high risk of performance bias (‐1) 2 Good sample size (> 3000), no measurable heterogeneity (I² = 0%), however 95% confidence interval touches the line of no effect (not downgraded) 3 Good sample size (> 3000), though wide confidence intervals cross the line of no effect (‐1) 4 Studies contributing data had design limitations: unlikely this outcome was affected by lack of blinding (not downgraded) 5 Few events but good sample size (not downgraded) 6 Very wide confidence intervals crossing the line of no effect (‐1) 7 Statistical heterogeneity (I² = 79%) (‐1) 8 Wide confidence interval crossing the line of no effect, few events & small sample size (based on one study) (‐2) 9 Wide confidence intervals crossing the line of no effect, large sample size with data from one study (‐1) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.00, 1.44] |
| 2 Instrumental vaginal birth Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| 3 Continuous EFM during labour Show forest plot | 3 | 10753 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| 4 Amniotomy Show forest plot | 2 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 5 Oxytocin for augmentation of labour Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.17] |
| 6 Epidural Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.87, 1.41] |
| 7 Fetal blood sampling Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.13, 1.45] |
| 8 Fetal and neonatal deaths Show forest plot | 4 | 11339 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.30, 3.47] |
| 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.67] |
| 10 Admission to neonatal intensive care Show forest plot | 4 | 11331 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.24] |
| 11 Apgar score < 7 at or after 5 minutes Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.85] |
| 12 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.90] |
| 13 Neonatal seizures Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.61] |
| 14 Length of stay in neonatal intensive care (days) Show forest plot | 1 | 91 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.59, 4.19] |
| 15 Length of stay in neonatal intensive care (hours) Show forest plot | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 6.20 [‐8.70, 21.10] |

