La cardiotocographie comparée à l'auscultation intermittente du cœur fœtal lors de l'admission en salle d'accouchement pour l'évaluation du bien‐être du fœtus
Résumé scientifique
Contexte
La cardiotocographie (CTG) à l'admission est un test de dépistage couramment utilisé qui consiste en un bref enregistrement (durant généralement 20 minutes) de la fréquence cardiaque fœtale (FCF) et de l'activité utérine, qui est réalisé lors de l'admission de la mère en salle de travail. Cet article est une mise à jour d'une revue publiée en 2012.
Objectifs
Comparer les effets de la cardiotocographie à l'admission à une auscultation intermittente de la FCF sur les résultats pour la mère et le nourrisson chez les femmes enceintes sans facteurs de risque lors de leur admission en salle de travail.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre d'essais cliniques du groupe Cochrane sur la grossesse et la naissance le 30 novembre 2016 et nous avons prévu d'examiner les références bibliographiques des articles trouvés.
Critères de sélection
Tous les essais randomisés et quasi‐randomisés comparant la CTG à l'admission à une auscultation intermittente de la FCF chez les femmes enceintes entre 37 et 42 semaines révolues de grossesse et présentant de faibles risques d'hypoxie fœtale intrapartum et de développement de complications pendant l'accouchement.
Recueil et analyse des données
Deux auteurs ont indépendamment évalué l'éligibilité et la qualité des essais et extrait les données. L'exactitude des données a été vérifiée.
Résultats principaux
Nous n'avons pas inclus de nouvel essai dans cette mise à jour. Nous avons inclus quatre essais portant sur plus de 13 000 femmes, réalisés au Royaume‐Uni et en Irlande et qui incluaient des femmes qui accouchaient. Trois essais étaient financés par les hôpitaux où les essais ont été réalisés et un essai a été financé par le gouvernement écossais. Aucun conflit d'intérêt n'a été déclaré dans deux essais ; et les deux derniers essais n'ont pas offert de déclaration d'intérêt. Dans l'ensemble, les études ont été évaluées comme présentant un faible risque de biais. Les résultats rapportés dans la revue de 2012 restent inchangés.
Bien que les données ne soient pas statistiquement significatives à l'aide d'un critère P < 0,05 strict, celles‐ci indiquaient de manière cohérente que les femmes assignées à une CTG à l'admission avaient, en moyenne, une augmentation de l'incidence des césariennes par rapport aux femmes assignées à une auscultation intermittente (risque relatif (RR) 1,20, intervalle de confiance à 95 % (IC) 1,00 à 1,44, 4 essais, 11 338 femmes, I² = 0 %, preuves de qualité modérée). Il n'y avait aucune différence claire dans l'effet moyen du traitement parmi les essais inclus entre les femmes assignées à une CTG à l'admission et celles assignées à une auscultation intermittente au niveau des accouchements instrumentaux par voie basse (RR 1,10, IC à 95 % 0,95 à 1,27, 4 essais, 11 338 femmes, I² = 38 %, preuves de faible qualité) et des taux de mortalité périnatale (RR 1,01, IC à 95 % 0,30 à 3,47, 4 essais, 11 339 nourrissons, I² = 0 %, preuves de qualité modérée).
Les femmes assignées à une CTG à l'admission présentaient, en moyenne, des taux plus élevés de surveillance fœtale électronique continue pendant le travail (RR 1,30, IC à 95 % 1,14 à 1,48, 3 essais, 10 753 femmes, I² = 79 %, preuves de faible qualité) et de prélèvements de sang fœtal (RR 1,28, IC à 95 % 1,13 à 1,45, 3 essais, 10 757 femmes, I² = 0 %) par rapport aux femmes assignées à une auscultation intermittente. Il n'y avait aucune différence entre les groupes pour les autres mesures des critères de jugement secondaires, notamment l'incidence et la gravité de l'encéphalopathie hypoxique ischémique (seule l'incidence était rapportée) (RR 1,19, IC à 95 % 0,37 à 3,90 ; 2367 nourrissons ; 1 essai ; preuves de très faible qualité) et l'incidence des convulsions dans la période néonatale (RR 0,72, IC à 95 % 0,32 à 1,61 ; 8056 nourrissons ; 1 essai ; preuves de faible qualité). Aucune donnée n'a été rapportée quant à l'incapacité neurodéveloppementale sévère évaluée à l'âge de 12 mois ou plus.
Conclusions des auteurs
Bien que la CTG soit couramment utilisée dans certains domaines cliniques, nous n'avons trouvé aucune preuve d'un bénéfice de l'utilisation de la CTG chez les femmes présentant des grossesses à faible risque à leur admission en salle d'accouchement.
Par ailleurs, il est possible que la CTG à l'admission augmente les taux de césariennes d'environ 20 %. Les données n'étaient pas suffisamment puissantes pour détecter d'éventuelles différences importantes concernant la mortalité périnatale. Cependant, il est peu probable qu'un essai, ou une méta‐analyse, ait une puissance statistique adéquate pour détecter ces différences. Les résultats de cette revue soutiennent les recommandations indiquant que la CTG à l'admission ne doit pas être utilisée chez les femmes présentant de faibles risques lors de leur admission en salle de travail. Les femmes doivent être informées qu'une CTG à l'admission est probablement liée à une augmentation de l'incidence des césariennes et n'apporte pas de bénéfice établi.
La qualité des preuves était modérée à très faible. Nous l'avons rabaissée en raison de l'imprécision, des incohérences et du manque de mise en aveugle des participants et du personnel. Les quatre essais inclus ont été réalisés dans des pays développés d'Europe occidentale. Une étude supplémentaire est en cours.
L'utilité des résultats de cette revue pour les pays en développement dépendra des pratiques de surveillance de la FCF. Cependant, l'absence de bénéfice et les probables effets délétères associés à une CTG à l'admission sont pertinents à considérer pour les pays s'interrogeant sur le rôle de la CTG à l'admission.
Les futures études évaluant les effets de la CTG à l'admission devraient considérer l'inclusion de femmes présentant des signes d'accouchement avant un diagnostic formel d'accouchement. Cela signifie inclure une cohorte de femmes recevant actuellement une CTG à leur admission qui ne sont pas encore incluses dans les essais actuels.
PICO
Résumé simplifié
Comparer la surveillance électronique de la fréquence cardiaque du bébé lors de l'admission en salle d'accouchement de la femme à l'aide de la cardiotocographie (CTG) avec la surveillance intermittente
Quelle est la problématique ?
La cardiotocographie (CTG) ou l'auscultation de la fréquence cardiaque fœtale (FCF) pendant une minute après une contraction permettent‐elles de conduire à de meilleurs résultats pour les mères et leurs bébés, chez les femmes enceintes en bonne santé présentant une grossesse à faible risque et admises en salle d'accouchement ?
Pourquoi est‐ce important ?
La surveillance de la FCF est l'une des méthodes les plus courantes pour vérifier le bien‐être d'un bébé. Les méthodes les plus courantes de surveillance de la FCF consistent à écouter les battements cardiaques à l'aide d'un stéthoscope fœtal, le Pinard (un dispositif spécial en forme de trompette), d'un dispositif d'échographie par Doppler portatif (connu sous le nom d'auscultation intermittente) ou d'une machine de surveillance fœtale électronique (SFE) qui génère une version imprimée de la fréquence cardiaque du bébé et des contractions de la mère (un CTG).
Le CTG à l'admission est un test couramment utilisé qui consiste en un bref enregistrement, de 20 minutes en général, de la FCF et de l'activité utérine et qui est réalisé lorsque la femme est admise en salle d'accouchement et présente des signes de travail. Le CTG à l'admission est pratiqué pour essayer d'identifier les bébés les plus exposés à des risques de complications liées à un manque d'oxygène pendant l'accouchement. Ces bébés pourraient être surveillés de façon plus soutenue par une SFE continue, ou ils pourraient bénéficier d'une intervention immédiate, telle qu'un accouchement par césarienne.
Les preuves observées :
Nous avons comparé la CTG à l'admission à une auscultation intermittente de la FCF réalisée lors de l'admission en salle d'accouchement de la femme. Nous avons recherché des preuves jusqu'au 30 novembre 2016, mais nous n'avons pas trouvé de nouvelles études pour cette mise à jour de la revue (publiée précédemment en 2012). Cette revue comprend quatre études et a identifié une étude encore en cours. Les études incluses (réalisées au Royaume‐Uni et en Irlande) portaient sur plus de 13 000 femmes ayant une grossesse à faible risque. Trois essais étaient financés par les hôpitaux où les essais ont été réalisés et un essai a été financé par le gouvernement écossais.
Les femmes assignées à une CTG à l'admission étaient probablement plus susceptibles d'avoir une césarienne par rapport aux femmes assignées à une auscultation intermittente (preuves de qualité modérée). Il n'y avait aucune différence dans le nombre d'accouchements par voie basse avec assistance instrumentale (preuves de faible qualité) ou quant au nombre de bébés décédés pendant ou peu après l'accouchement (preuves de qualité modérée) entre les femmes dans les deux groupes. La CTG à l'admission était associée à une augmentation de l'utilisation des SFE continues (avec une électrode placée sur le cuir chevelu du bébé) (preuves de faible qualité) et des prélèvements de sang fœtal (un petit échantillon sanguin prélevé sur le cuir chevelu du bébé) pendant le travail. Il n'y avait pas de différences dans les autres critères de jugement mesurés, comme la rupture artificielle des membranes, l'accélération du travail, l'utilisation d'une anesthésie péridurale, les lésions du cerveau du bébé en raison du manque d'oxygène (preuves de très faible qualité), ou les spasmes voire crises épileptiques du bébé juste après la naissance (preuves de faible qualité). Aucune étude n'a indiqué si les bébés ont développé de quelconques problèmes graves au niveau du cerveau, du système nerveux central, de la croissance ou du développement après un an d'âge.
Qu'est‐ce que cela signifie ?
Bien que de nombreux hôpitaux réalisent des CTG sur les femmes lorsqu'elles sont admises à l'hôpital pendant l'accouchement, nous n'avons trouvé aucune preuve indiquant que cette pratique apporte un bénéfice chez les femmes ayant une grossesse à faible risque. Nous avons constaté que les CTG à l'admission peuvent augmenter le nombre de femmes subissant une césarienne d'environ 20 %.
Les études incluses n'incluaient pas suffisamment de femmes pour montrer si les CTG à l'admission ou l'auscultation intermittente étaient plus efficaces pour maintenir les bébés en sécurité. Pour que des études permettent de déterminer laquelle des interventions est la plus efficace pour préserver la sécurité des bébés, il faudrait qu'elles soient à très large échelle. Sur la base de cette revue, les femmes présentant une grossesse à faible risque et recevant une CTG à l'admission pourraient être plus susceptibles d'avoir une césarienne. Le bénéfice de la CTG à l'admission reste incertain pour ces femmes.
Toutes les études incluses ont été réalisées dans des pays développés d'Europe occidentale. Les résultats de cette revue pourraient ne pas être utiles pour les personnes vivant dans des pays très différents ou lorsque d'autres méthodes de surveillance de la FCF sont utilisées. Cependant, les pays qui utilisent les CTG à l'admission devraient questionner cette pratique, parce qu'elle n'amène pas de bénéfices clairs et parce que la CTG peut causer du tort aux femmes en les rendant plus susceptibles d'avoir une césarienne.
Authors' conclusions
Summary of findings
| Admission cardiotocography compared to Intermittent auscultation (low‐risk women) for assessment of fetal wellbeing | ||||||
| Patient or population: Low risk pregnant women. All of the women were in labour. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Intermittent auscultation (low‐risk women) | Risk with admission cardiotocography | |||||
| Incidence of caesarean section | Study population | RR 1.20 | 11338 | ⊕⊕⊕⊝ | ||
| 36 per 1000 | 44 per 1000 | |||||
| Incidence of operative vaginal birth | Study population | RR 1.10 | 11338 | ⊕⊕⊝⊝ | ||
| 126 per 1000 | 139 per 1000 | |||||
| Perinatal mortality rate (fetal and neonatal deaths excluding lethal congenital anomalies) | Study population | RR 1.01 | 11339 | ⊕⊕⊕⊝ | ||
| 1 per 1000 | 1 per 1000 | |||||
| Severe neurodevelopmental disability assessed ≥ 12 months of age | Study population | ‐ | (0 RCTs) | ‐ | None of the included studies reported data for the outcome | |
| see comment | see comment | |||||
| Incidence of continuous electronic fetal monitoring during labour | Study population | RR 1.30 | 10753 | ⊕⊕⊝⊝ | ||
| 417 per 1000 | 542 per 1000 | |||||
| Incidence and severity of hypoxic ischaemic encephalopathy (incidence only reported) | Study population | RR 1.19 | 2367 | ⊕⊝⊝⊝ | ||
| 4 per 1000 | 5 per 1000 | |||||
| Incidence of seizures in the neonatal period | Study population | RR 0.72 | 8056 | ⊕⊕⊝⊝ | ||
| 3 per 1000 | 2 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations: outcome may have been affected by lack of blinding as all studies judged to be at high risk of performance bias (‐1) 2 Good sample size (> 3000), no measurable heterogeneity (I² = 0%), however 95% confidence interval touches the line of no effect (not downgraded) 3 Good sample size (> 3000), though wide confidence intervals cross the line of no effect (‐1) 4 Studies contributing data had design limitations: unlikely this outcome was affected by lack of blinding (not downgraded) 5 Few events but good sample size (not downgraded) 6 Very wide confidence intervals crossing the line of no effect (‐1) 7 Statistical heterogeneity (I² = 79%) (‐1) 8 Wide confidence interval crossing the line of no effect, few events & small sample size (based on one study) (‐2) 9 Wide confidence intervals crossing the line of no effect, large sample size with data from one study (‐1) | ||||||
Background
Assessment of fetal wellbeing throughout pregnancy, labour and birth is widely regarded as a fundamental component of maternity care and essential for optimising fetal outcomes. Although a variety of methods are used to assess fetal wellbeing, including fetal movement counting and biophysical tests such as Doppler ultrasound, monitoring of the fetal heart rate (FHR) remains the most common method for the assessment of fetal wellbeing (Alfirevic 2013; NCCWCH 2007).
The FHR undergoes constant changes in response to changes in the intrauterine environment and to other stimuli such as uterine contractions. These changes in the FHR can be monitored to assess the wellbeing of the fetus during pregnancy and labour.
Description of the condition
Two common methods of monitoring the FHR are by intermittent auscultation and by an electronic fetal monitoring (EFM) machine that produces a printout called a cardiotocograph (CTG) (Ayres‐de‐Campos 2015). Intermittent auscultation involves listening to the fetal heart at predetermined intervals using either a Pinard stethoscope or a hand‐held Doppler ultrasound device. The CTG is a graphical printout of the FHR and uterine contractions. The FHR recorded on a CTG may be captured externally via an ultrasound transducer attached to the mother's abdomen, or internally via a fetal scalp electrode placed directly on the baby's head. Uterine contractions are recorded via a pressure transducer attached to the mother's abdomen or, less commonly, by an intrauterine pressure device placed in the uterine cavity (Ayres‐de‐Campos 2015).
Description of the intervention
The admission CTG is a commonly‐used screening test consisting of a short, usually 20 minute, recording of the FHR and uterine activity performed on the mother's admission to the labour ward with signs of labour (Cheyne 2003; Impey 2003; Mires 2001). Anecdotally, some women will have an admission CTG performed before assessments aimed at diagnosing the onset of labour, while others will not have the admission CTG until a diagnosis of labour has been established. The implications of this are that some women will have an admission CTG performed on admission to the labour ward or labour assessment room where, on subsequent assessment, a diagnosis of not being in labour is made. Differences in timing of the admission CTG with respect to the onset of labour may result in differences in outcomes assessed. We planned to explore this through subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
How the intervention might work
Pioneered in the 1950s and 1960s as an alternative to intermittent auscultation of the FHR by stethoscope or Pinard (Caldeyro‐Barcia 1966; Hammacher 1968; Hon 1958), EFM was introduced into widespread clinical practice in the 1970s to 1980s on the premise that it would facilitate early detection of abnormal FHR patterns thought to be associated with hypoxia (lack of oxygen), to enable earlier intervention to prevent fetal neurological damage and death or both (Nelson 1996).
However, because antenatal risk factors do not identify all fetuses who will subsequently experience morbidity, mortality, or both, the admission CTG was introduced as a means of attempting to identify those fetuses of low‐risk mothers at greatest risk of intrapartum hypoxia (Arulkumaran 2000; RCOG 2001) who might benefit from more intensive monitoring by continuous EFM and fetal scalp blood gas analysis or both, or from immediate intervention (e.g. expedited birth).
Current prevalence rates of perinatal mortality, neonatal encephalopathy and cerebral palsy are relatively low and, of those, only a small proportion are thought to be attributable directly to intrapartum causes (RCOG 2001). Changes in FHR patterns are neither sensitive (the ability of a test to identify those who have the disease or condition) nor specific (the ability of the test to correctly identify those without the disease or condition) to any particular cause (MacLennan 1999). Multiple late decelerations and decreased FHR variability have been shown to be associated with an increased risk of cerebral palsy (Nelson 1996). However, the associated false positive rate is reported as high as 99.8% in the presence of tracings displaying these abnormalities in the FHR pattern (Nelson 1996). This poor positive predictive value implies that to identify the fetus who may be compromised, EFM identifies abnormal FHR patterns in many healthy fetuses who are not truly compromised.
Why it is important to do this review
There is a lack of evidence of benefit supporting the use of the admission CTG in low‐risk pregnancy. Despite recommendations that it should not be recommended for this group of women (Liston 2007; NCCWCH 2007; RCOG 2001), the admission CTG was used by approximately 79% of maternity units in the UK in 2000 (CESDI 2001), by 96% of units in Ireland in 2004 (Devane 2007) and by approximately 76% of Canadian hospitals (Kaczorowski 1998). More recently, the admission CTG was used in all (100%, n = 42) labour units in Sweden in 2008 (Holzmann 2010).
Although the admission CTG remains in widespread use, several issues remain controversial. These include whether the admission CTG (a) should be offered routinely to all women without risk factors for intrapartum hypoxia; (b) whether the admission CTG is effective at predicting those fetuses who will subsequently develop intrapartum hypoxia; and (c) the effect of the admission CTG on neonatal mortality and on maternal and neonatal morbidity.
It was important to undertake this systematic review to explore these issues and to evaluate the efficacy of admission CTG compared to intermittent auscultation as a method of assessing fetal wellbeing in women on admission to the labour ward, or labour assessment room, with signs of possible labour. This review complements other Cochrane systematic reviews evaluating the effectiveness of other interventions for the assessment of fetal wellbeing including the following.
-
Amniotic fluid index versus single deepest vertical pocket as a screening test for predicting adverse pregnancy outcomes (Nabhan 2008).
-
Antenatal cardiotocography for fetal assessment (Grivell 2015).
-
Biochemical tests for placental function for assessment in pregnancy (Neilson 2012).
-
Biophysical profile for fetal assessment in high‐risk pregnancies (Lalor 2008).
-
Fetal and umbilical Doppler ultrasound in high‐risk pregnancies (Alfirevic 2013).
-
Fetal manipulation for facilitating tests of fetal wellbeing (Tan 2013a).
-
Fetal movement counting for assessment of fetal wellbeing (Mangesi 2015).
-
Fetal vibroacoustic stimulation for facilitation of tests of fetal wellbeing (Tan 2013b).
-
Maternal glucose administration for facilitating tests of fetal wellbeing (Tan 2012).
-
Regimens of fetal surveillance for impaired fetal growth (Grivell 2012).
-
Utero‐placental Doppler ultrasound for improving pregnancy outcome (Stampalija 2010).
-
Vibroacoustic stimulation for fetal assessment in labour in the presence of a non‐reassuring FHR trace (East 2013).
Objectives
To compare the effects of admission cardiotocograph with intermittent auscultation of the fetal heart rate on maternal and infant outcomes for pregnant women without risk factors for intrapartum hypoxia on their admission to the labour ward.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi randomised trials comparing admission cardiotocograph (CTG) with intermittent auscultation of the fetal heart rate (FHR).
Types of participants
Pregnant women between 37 and 42 completed weeks of pregnancy and considered to be at low risk of intrapartum fetal hypoxia and of developing complications during labour were included. It is recognised that there is much debate surrounding the definition of what constitutes 'normality' and concerns have been expressed at what some regard as the disempowering concept of risk classification (Gail‐Thomas 2003). In addition, the predictive value of risk scoring during pregnancy is poor (WHO 1999). However, given the consensus of opinion that continuous electronic fetal monitoring (EFM) should be reserved for women whose fetuses are at high or increased risk of cerebral palsy, neonatal encephalopathy or perinatal death (Liston 2007; NCCWCH 2007; RANZCOG 2002; RCOG 2001), where sufficient detail was provided by trial authors, we determined eligibility of participants based on absence of risk factors identified in international guidelines for EFM (Characteristics of included studies).
Types of interventions
Admission CTG compared with intermittent auscultation of the FHR on admission to the labour ward.
For the purpose of this review we used the following operational definitions.
-
Admission CTG is defined as a commonly‐used screening test consisting of a short, usually 20 minute, recording of the FHR and uterine activity performed on the mother's admission to the labour ward.
-
Intermittent auscultation is defined as intermittent surveillance of the FHR at predetermined intervals, using either a Pinard stethoscope or a hand‐held Doppler, performed on the mother's admission to the labour ward.
Types of outcome measures
Main outcomes
Maternal
-
Incidence of caesarean section.
-
Incidence of operative vaginal delivery.
Infant
-
Perinatal mortality rate (fetal and neonatal deaths excluding lethal congenital anomalies).
-
Severe neurodevelopmental disability assessed at 12 months of age or more. We defined severe neurodevelopmental disability as any one or a combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70), auditory and visual impairment. Development should have been assessed by means of a previously validated tool, such as Bayley Scales of Infant Development (Psychomotor Developmental Index and Mental Developmental Index (Bayley 1993)).
Other important outcomes
Maternal
-
Incidence of serious maternal complications (e.g. admission to intensive care unit, septicaemia (a form of blood infection), organ failure).
-
Incidence of continuous EFM during labour.
-
Incidence of artificial rupture of membranes during labour.
-
Incidence of oxytocin augmentation of labour.
-
Mobility during labour.
-
Perceived control and self‐confidence or both during labour.
-
Incidence of use of pharmacological analgesia including regional analgesia.
-
Incidence of use of non‐pharmacological methods of coping with labour and birth, e.g. transcutaneous electrical nerve stimulation (TENS), hydrotherapy.
-
Satisfaction with labour experience.
-
Incidence of fetal blood sampling.
-
Length of hospital stay.
Infant
-
Cardiorespiratory and neurological depression or both at birth as demonstrated by an Apgar score less than seven for longer than five minutes, or evidence of acidaemia indicated by a pH less than 7.0 or base deficit greater than 12 mmol/L in umbilical arterial cord blood, or neonatal blood sample within the first hour of life, or both.
-
Incidence and severity of hypoxic ischaemic encephalopathy. Severity of hypoxic ischaemic encephalopathy assessed using Sarnat staging (Sarnat 1976):
-
stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures;
-
stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro reflexes;
-
stage 3 (severe): stupor, flaccidity, small to midposition pupils which react poorly to light, decreased stretch reflexes, hypothermia and absent Moro reflex.
-
-
Incidence of seizures in the neonatal period, either apparent clinically or detected by electro‐encephalographic recordings.
-
Evidence of multi‐organ compromise within the first 24 hours after birth: for example, renal failure, hepatic injury, cardiac damage, respiratory complications, or haematological insult.
-
Incidence of admission to neonatal special care and intensive care unit or both.
-
Length of stay to neonatal special care and neonatal intensive care unit or both.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 November 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the "Specialized Register" section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by the group's Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Ongoing studies).
For details of additional author searching carried out in the previous version of the review, please see Devane 2012.
Searching other resources
We planned to search the reference list of papers identified using the search strategy described to assess their suitability for inclusion in the review. However, we did not find any new studies for inclusion in this update.
We did not apply any language or date restrictions.
Data collection and analysis
No new reports were identified from the updated search for this update. For methods used in the previous version of this review, see Devane 2012.
The Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (DD, JGL) assessed independently for inclusion all the potential studies identified as a result of the search strategy. We did not encounter any disagreement and therefore did not need to consult a third review author (SD, WM or VS).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (DD, JGL) extracted data using the data extraction form. We resolved any discrepancies through discussion and did not need to consult a third review author. Two review authors (DD, JGL) entered all data into the Review Manager (RevMan) software (RevMan 2014) and checked for accuracy. When information regarding any of the steps was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
Given the nature of the intervention, we did not expect blinding or participants or personnel to have been likely.
(4) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(5) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(6) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(7) Other bias (checking for bias due to problems not covered by criteria (1) to (6))
We described for each included study any important concerns we had about other possible sources of bias.
Assessment of the quality of the evidence using the GRADE approach
We assessed evidence quality using the GRADE approach as outlined in the GRADE handbook to assess the quality of the body of evidence relating to the following outcomes for the main comparison: Admission cardiotocography versus Intermittent auscultation (low‐risk women).
-
Incidence of caesarean section.
-
Incidence of operative vaginal delivery.
-
Perinatal mortality rate (fetal and neonatal deaths excluding lethal congenital anomalies).
-
Severe neurodevelopmental disability assessed at or after 12 months of age. We defined severe neurodevelopmental disability as any one or a combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70), auditory and visual impairment. Development should have been assessed by means of a previously validated tool, such as Bayley Scales of Infant Development (Psychomotor Developmental Index and Mental Developmental Index (Bayley 1993)).
-
Incidence of continuous EFM during labour.
-
Incidence and severity of hypoxic ischaemic encephalopathy (incidence only reported)
-
Incidence of seizures in the neonatal period, either apparent clinically or detected by electro‐encephalographic recordings.
GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from high quality by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not find any cluster‐randomised trials from our search. In future updates, if we identify cluster‐randomised trials we will include them in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not plan to include cross‐over design trials in this review.
Other unit of analysis issues
Multiple pregnancies
As this review is based on women experiencing low‐risk pregnancies, we did not plan to include women with multiple pregnancies.
Multiple‐armed studies
No multiple‐armed studies have been included in this update. In future updates, if multiple‐armed trials are identified, we will combine all relevant intervention and control groups together to create a single pair‐wise comparison (see section 16.5.4 of Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011).
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (< 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (> 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analyses using the Review Manager software (RevMan 2014). The largest of the four included trials (Impey 2003) included women in whom the liquor was known to be clear (i.e. only women who had either a spontaneous rupture of the membranes or an amniotomy were included in the study). This knowledge of the presence of clear liquor would have given clinicians an additional clinical feature used in the assessment of fetal well being that would not have been available for all women included in the other three trials (Cheyne 2003; Mires 2001; Mitchell 2008) where membrane rupture and clear liquor were not inclusion criteria. Because of this, we believed that there was clinical heterogeneity sufficient to expect that the underlying treatment effects would differ between the included trials (and in particular between the Impey 2003 trial and the other three trials (Cheyne 2003; Mires 2001; Mitchell 2008)). We therefore used random‐effects meta‐analysis to produce an overall summary of the average treatment effect across the four included trials. We have treated this random‐effects summary as the average range of possible treatment effects. For each outcome reported, we present the results of the random‐effects analyses as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
We planned to carry out the following subgroup analysis using a priori outcomes.
-
Women in‐labour versus women not in‐labour on clinical assessment post admission CTG.
However, all four studies included only women in labour (at point of intervention) and therefore this subgroup analysis was not possible.
In future updates, if subgroup analysis is possible, we will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates of the review, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. If we include cluster‐RCTs, along with the individually‐randomised trials, we will carry out sensitivity analysis to investigate the effect of the variation randomisation unit.
Results
Description of studies
Results of the search
An updated search of the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2016) found no new studies for consideration. The original search in Devane 2012 found seven reports and our search of the other databases did not identify any additional reports. These seven reports related to four completed (Cheyne 2003; Impey 2003; Mires 2001; Mitchell 2008) and one ongoing trial (Devane 2008). At the time of this update, Devane 2008 is ongoing (see Figure 1).

Study flow diagram
Included studies
Methods
We included four randomised controlled trials (RCTs) with 13,296 women (Cheyne 2003; Impey 2003; Mires 2001; Mitchell 2008) (see Characteristics of included studies). We did not exclude any study and found one ongoing study (Devane 2008, see Characteristics of ongoing studies).
Settings
The studies were conducted in hospitals in Scotland (Cheyne 2003; Mires 2001), Ireland (Impey 2003) and England (Mitchell 2008).
Participants
The number of pregnant women included in each study ranged from 334 (Cheyne 2003) to 8628 (Impey 2003). All four studies included women in labour. Therefore, we were unable to perform our planned subgroup analysis by whether or not women were in labour or not on clinical assessment post the admission cardiotocograph (CTG) (see Subgroup analysis and investigation of heterogeneity).
Three studies included women in spontaneous labour only (Cheyne 2003; Mitchell 2008; Mires 2001) and one included women who were in spontaneous or induced labour (Impey 2003). All studies included women who were regarded as being at "low risk" of maternal and fetal complications with the exception of Impey 2003 who included a relatively small (approximately 5%) proportion of women with a previous caesarean section and prior to 37 completed weeks' gestation. Details on participant inclusion criteria, including what constituted low risk are given in Characteristics of included studies.
Interventions and controls
Women allocated to admission CTG received a routine 15‐minute (Mitchell 2008) or 20‐minute (Cheyne 2003; Impey 2003; Mires 2001) tracing. Women allocated to intermittent auscultation received intermittent auscultation of the fetal heart for at least one full minute (Cheyne 2003; Impey 2003; Mires 2001; Mitchell 2008) during and after a contraction (Cheyne 2003; Mires 2001) or after a contraction only (Impey 2003; Mitchell 2008).
Outcomes
Outcomes reported were: caesarean section; instrumental vaginal birth; continuous electronic fetal monitoring (EFM) during labour; amniotomy; oxytocin for augmentation of labour; epidural; fetal blood sampling; fetal and neonatal deaths; Apgar score less than seven at or after five minutes; admission to neonatal intensive care; neonatal seizures; length of stay in neonatal intensive care (hours); hypoxic ischaemic encephalopathy; evidence of fetal multi‐organ compromise within the first 24 hours after birth.
Funding
Cheyne 2003, Impey 2003 and Mitchell 2008 were funded by the hospitals or NHS Trusts where the trials took place (North Glasgow University Hospitals NHS Trust, Research Committee of the National Maternity Hospital, Holles St, Dublin, Ireland, and Buckinghamshire Hospitals NHS Trust's Research Department, respectively). Mires 2001 was funded by Chief Scientists Office of the Scottish Executive, Edinburgh.
Impey 2003 and Mires 2001 declared no conflicts of interest. The remaining two trials (Cheyne 2003; Mitchell 2008) did not include declarations of interest.
Excluded studies
We did not exclude any studies.
Risk of bias in included studies
We assessed the risk of bias in included studies within the domains of (i) random sequence generation (selection bias) (ii) allocation concealment (selection bias) (iii) blinding of participants and personnel (performance bias)(iv) blinding of outcome assessment (detection bias) (v) incomplete outcome data (attrition bias) (vi) selective reporting (reporting bias) and (vii) other bias (see Assessment of risk of bias in included studies above). Overall, the studies were assessed at low risk of bias across most domains with some exceptions, which are detailed below.
Allocation
We assessed all four included studies as having low risk of bias in random sequence generation and in allocation concealment.
Blinding
We felt it unreasonable to expect blinding of participants and professionals providing care (see Assessment of risk of bias in included studies). Nevertheless, due to knowledge of the allocated interventions by participants and personnel during the study, all four studies were rated high risk for performance bias. Risk of bias for blinding for outcome assessors was assessed as low for two studies (Impey 2003; Mires 2001), unclear for one (Mitchell 2008) and high risk in one where outcome assessment was not blinded (Cheyne 2003).
Incomplete outcome data
Overall, loss to follow‐up was low across all outcomes for all four studies with the exception of umbilical cord blood gas analyses (arterial pH, venous pH and base deficit/base excess (BD/BE)). Two studies included this outcome (Impey 2003; Mires 2001) but the range of values used for this outcome in both these studies differed from that prespecified in this review, and therefore, we have not used these data. For information, Impey 2003 reports missing data for the outcome "pH less than seven or BD/E greater than 12 mmol/L" of 7.5% and 7.8% for admission CTG and intermittent ausculation respectively. Mires 2001 reports missing data for their primary outcome of metabolic acidosis defined as "pH less than 7.20 or BD greater than 8 mmol/L" of 26% and 27% for admission CTG and intermittent ausculation respectively. One study reported a loss to follow‐up of 7% (N = 22) of women (Cheyne 2003). However, data were identified and extracted subsequently for 21 of these 22 women by the trial author and kindly provided to the review team.
Selective reporting
All four studies reported all outcomes mentioned in the methods section in the results section of the trial publication(s) and were therefore assessed as being at low risk of selective reporting.
Other potential sources of bias
We identified no other sources of potential bias in three of the four studies (Cheyne 2003; Impey 2003; Mitchell 2008). One study (Mires 2001) recruited women (N = 3752) to the study and randomised them to admission CTG or intermittent auscultation during the third trimester. However, some women developed an obstetric complication between randomisation and admission in labour that warranted continuous fetal heart rate (FHR) monitoring in labour, such that only 2367 women were judged to be low risk when in labour (1186 admission CTG, 1181 intermittent auscultation). Of the 1885 women randomised to intermittent auscultation in the third trimester, 704 (37%) developed complications during pregnancy and required admission CTG on admission. This is addressed further under Sensitivity analysis.
Effects of interventions
Admission cardiotocography versus intermittent auscultation (low‐risk women, four studies, 11,339 women)
For this comparison, we included all women as randomised in the Cheyne 2003 and Mitchell 2008 studies and the subgroups of low‐risk women in the Impey 2003; Mires 2001 studies (see Characteristics of included studies and Sensitivity analysis for details).
Main outcomes
The difference in the average treatment effect across included trials between women allocated to admission CTG and women allocated to intermittent auscultation in caesarean section has a risk ratio (RR) of 1.20 and a 95% confidence interval (CI) of 1.00 to 1.44, four trials, 11,338 women (Analysis 1.1). Given that the 95% CI just reaches 1.00 and the absence of measurable heterogeneity in this outcome analysis (T² = 0.00, I² = 0%), the probability is that admission CTG increases the caesarean section rate by approximately 20%. There was no significant difference in the average treatment effect across included trials between women allocated to admission CTG and women allocated to intermittent auscultation in instrumental vaginal birth (RR 1.10, 95% CI 0.95 to 1.27, 4 trials, 11,338 women, T² = 0.01, I² = 38%, Analysis 1.2) and fetal and neonatal deaths (RR 1.01, 95% CI 0.30 to 3.47, 4 trials, 11,339 infants, T² = 0.00, I² = 0%, Analysis 1.8). None of the included studies reported data for the outcome 'Severe neurodevelopmental disability assessed at greater than, or equal to 12 months of age'.
Other important outcomes
Women allocated to admission CTG had, on average, significantly higher rates of continuous EFM during labour (RR 1.30, 95% CI 1.14 to 1.48, 3 trials, 10,753 women, T² = 0.01, I² = 79%, Analysis 1.3) and fetal blood sampling (RR 1.28, 95% CI 1.13 to 1.45, 3 trials, 10,757 women, T² = 0.00, I² = 0%, Analysis 1.7) than women allocated to intermittent auscultation.
There was no significant difference in the average treatment effect across included trials between women allocated to admission CTG and women allocated to intermittent auscultation in amniotomy (RR 1.04, 95% CI 0.97 to 1.12, 2 trials, 2694 women, T² = 0.00, I² = 0%, Analysis 1.4), oxytocin for augmentation of labour (RR 1.05, 95% CI 0.95 to 1.17, 4 trials, 11,324 women, T² = 0.00, I² = 34%, Analysis 1.5), epidural (RR 1.11, 95% CI 0.87 to 1.41, 3 trials, 10,757 women, T² = 0.03, I² = 86%, Analysis 1.6), Apgar score less than seven at or after five minutes (RR 1.00, 95% CI 0.54 to 1.85, 4 trials, 11,324 infants, T² = 0.10, I² = 25%, Analysis 1.11), hypoxic ischaemic encephalopathy (RR 1.19, 95% CI 0.37 to 3.90, 1 trial, 2367 infants, heterogeneity not applicable, Analysis 1.12), admission to neonatal intensive care units (RR 1.03, 95% CI 0.86 to 1.24, 4 trials, 11,331 infants, T² = 0.00, I² = 0%, Analysis 1.10), neonatal seizures (RR 0.72, 95% CI 0.32 to 1.61, 1 trial, 8056 infants, heterogeneity not applicable, Analysis 1.13), evidence of fetal multi‐organ compromise within the first 24 hours after birth (RR 0.56, 95% CI 0.19 to 1.67, 1 trial, 8056 infants, heterogeneity not applicable, Analysis 1.9), length of stay in neonatal intensive care (hours) (mean difference (MD) 6.20 hours, 95% CI ‐8.70 to 21.10, 1 trial, 318 infants, heterogeneity not applicable, Analysis 1.15) and length of stay in neonatal intensive care (days) (MD 1.80, 95% CI ‐0.59 to 4.19, 1 trial, 91 infants, heterogeneity not applicable, Analysis 1.14).
Data were not reported, were unavailable or were unavailable in a format that could be used in this review for the following other important outcomes.
Maternal
-
Incidence of serious maternal complications (e.g. admission to intensive care unit, septicaemia (a form of blood infection), organ failure).
-
Mobility during labour.
-
Perceived control and self‐confidence or both during labour.
-
Incidence of use of non‐pharmacological methods of coping with labour, e.g. transcutaneous electrical nerve stimulation, hydrotherapy.
-
Satisfaction with labour experience.
-
Length of hospital stay.
Sensitivity analyses
One study (Mires 2001) recruited women (N = 3752) to the study and randomised them to admission CTG or intermittent auscultation during the third trimester. However, some women developed an obstetric complication between randomisation and admission in labour that warranted continuous FHR monitoring in labour, such that only 2367 women were judged to be at low risk when in labour (1186 admission CTG, 1181 intermittent auscultation). Of the 1881 women randomised to intermittent auscultation in the third trimester, 704 (37%) developed complications during pregnancy and required an admission CTG on admission to the labour ward. However, the proportion of women who developed complications were similar in each group, suggesting an absence of differential treatment of women post‐randomisation. The trial author kindly provided data separately for the outcomes in this subgroup of women, and we have included these data in the main analyses in this review (Characteristics of included studies).
A second study (Impey 2003) randomised women at the point of labour. However, this study included a relatively small number (fewer than 5%) of women who had a previous lower segment caesarean section and who went into labour prior to 37 completed weeks' gestation. The trial author kindly provided data separately for the outcomes for women between 37 and 42 completed weeks with no previous caesarean section and we have included these data in the main analyses in this review. We explored the dependency of the findings of this review on the decision to use data from the low‐risk subgroups of women in both the Impey 2003 and Mires 2001 studies through a post‐hoc sensitivity analysis in which the primary analysis was repeated with data from the whole groups as randomised in both studies. Results for this were consistent with primary comparison effects for the low‐risk subgroup of women with the exception of two outcomes. Caesarean section became statistically significant, with significantly more women allocated to admission CTG having, on average, a caesarean section compared with women allocated to intermittent auscultation (RR 1.17, 95% CI 1.02 to 1.34, 4 trials, 13,247 women, T² = 0.00, I² = 0%, Analysis 2.1). Epidural also became significant, with significantly more women allocated to intermittent auscultation having, on average, an epidural compared with women allocated to admission CTG (RR 1.11, 95% CI 1.01 to 1.22, 2 trials, 4085 women, T² = 0.00, I² = 0%, Analysis 2.6).
In the main comparison, three outcomes (instrumental vaginal birth, continuous EFM during labour and epidurals) had significant statistical heterogeneity where T² was greater than zero and either I² was greater than 30% or there was a low P value (< 0.10) in the Chi² test for heterogeneity. On investigating this heterogeneity, we found that the Mires 2001 study appeared to drive the heterogeneity for instrumental vaginal birth and continuous EFM during labour. When Mires 2001 was removed from analyses for each of these two outcomes, the heterogeneity was no longer substantial. Removal of Mires 2001 from analyses for each of these two outcomes did not alter the direction or significance of the effect. Heterogentity for the third outcome, epidural, seemed to be driven by Impey 2003, which in contrast to the direction of effect of the other two studies included in this outcome, found a non‐significant reduction in epidurals in women allocated to admission CTG.
Discussion
Summary of main results
This updated review included four trials (Cheyne 2003; Impey 2003; Mires 2001; Mitchell 2008) involving more than 13,000 women. All four studies included women in labour. No new studies were included in the update.
The admission cardiotocograph (CTG) was introduced as a means of attempting to identify those fetuses at greatest risk of intrapartum hypoxia (Arulkumaran 2000; RCOG 2001) who might benefit from more intensive monitoring by continuous electronic fetal monitoring and fetal scalp blood gas analysis, or both, or from immediate intervention (e.g. expedited birth). Although there was no significant difference in caesarean sections (using a strict P = 0.05 criterion) between women allocated to admission CTG and women allocated to intermittent auscultation, on average the probability is that admission CTG increases the caesarean section rate by approximately 20%. This is reinforced by the 95% confidence interval (CI) just reaching 1.00 and by the absence of measurable heterogeneity in this outcome analysis. Further, all four included studies found fewer caesarean sections associated with intermittent auscultation, although no individual study showed a statistically significant difference. Although numbers needed to treat/harm (NNT/H) analyses remain controversial in the context of meta‐analysis, and should be interpreted with caution, we estimated that overall, one additional caesarean section was performed for every 136 women monitored continuously (risk difference (RD) (controls‐treated) = ‐0.0074 (‐0.015 to ‐0.0002), 95% CI 69 to 5641).
On average, women allocated to admission CTG had a significantly higher rate of continuous electronic fetal monitoring during labour and fetal blood sampling than women allocated to intermittent auscultation.
Overall completeness and applicability of evidence
All four included studies provide relevant evidence on the effects of the admission CTG compared with intermittent auscultation on maternal and infant outcomes for pregnant women without risk factors on their admission to the labour ward. There are three important points in discussing how the results of the review fit into the context of current practice. Firstly, the largest study in this review (Impey 2003) included women in which the colour of the liquor was known to be clear. As such, clinicians caring for these women had an additional, and important, feature used in the overall assessment of fetal wellbeing. Secondly, all four studies included women in either spontaneous or induced labour. In some practice contexts, the admission CTG is performed in the absence of a diagnosis of labour, that is, an admission CTG is done before an assessment to diagnose labour is made. Thirdly, in Mitchell 2008, women allocated to admission CTG received a routine 15‐minute CTG. This is less than the 20 minutes recommended for visual assessment of fetal heart rate (FHR) reactivity by some guidelines (RCOG 2001). These points should be considered in determining the applicability of the evidence presented here to different practice contexts.
It is reasonable to assume that outcomes related to perinatal death are perhaps those of most importance to women and maternity care professionals. In this review, there was no significant difference in perinatal mortality between admission CTG and intermittent auscultation. However, to identify correctly a 20% reduction in proportion of perinatal deaths (assuming a developed world rate of seven per 1000) between admission CTG and intermittent auscultation, a sample size of more than 100,000 is required (with α = 0.05, β‐1 = 20%) and even then a 20% reduction might be regarded as optimistic, with lower effect sizes requiring higher sample sizes. Such sample sizes are unlikely, except perhaps in the largest of mega‐trials, and therefore, typical randomised trials and systematic reviews of these trials, including this review, are insufficiently powered to evaluate the effects of different fetal monitoring modalities on fetal and neonatal mortality measures. Therefore, while this review found no evidence of an effect for admission CTG on perinatal mortality, this should not be confused with evidence of no effect.
There are other important outcomes, which are not reported, are unavailable or are not in a suitable format to be included in the analysis; these include perceived control and satisfaction with labour. This reflects a widespread tendency among the clinical and research community to frame outcomes in a non‐salutogenic or pathological manner (e.g. operative birth) rather than in a salutogenic, wellbeing‐orientated manner (e.g. normal birth). It may also reflect the relative difficulty of quantifying outcomes that are subjective and difficult, although important, to measure.
In addition to statistical heterogeneity, there was evidence of clinical heterogeneity between studies in the numbers of women having an epidural. In Impey 2003, significantly more women allocated to intermittent auscultation had an epidural compared with women allocated to admission CTG. This contrasts with Mires 2001, who found significantly fewer epidurals in women allocated to intermittent auscultation. The third study reporting on this outcome, Cheyne 2003, found no significant difference in epidurals between groups. It is difficult to explain such heterogeneity. All three studies found an increased rate of continuous electronic fetal monitoring for women allocated to admission CTG, making it unlikely that differing practices in use of continuous electronic fetal monitoring indications give rise to differential effects on epidural use. Futhermore, although the labours of nulliparous women in Impey 2003 were managed actively, the package of care for active management in labour has not been shown to impact on epidural rates (Brown 2008).
Quality of the evidence
Overall, risk of bias of the four included studies was assessed as low across all domains (Figure 2) with the exception of performance bias, which was judged to be high risk across all included outcomes for all studies, and blinded outcome assessment (detection bias), which was unclear in Mitchell 2008 and not carried out in Cheyne 2003. Of the 3752 women randomised during the third trimester in the study by Mires 2001, 37% developed an obstetric complication between randomisation and admission in labour that warranted continuous FHR monitoring in labour. Specific complications are given and these are in line with clinical norms reported in the literature. The study by Impey 2003 also included a small proportion of women with risk factors. Both Impey 2003 and Mires 2001 provided data for the subgroup of low‐risk women, and these data were used in the main analyses in this review. Sensitivity analyses were done in which the outcomes for all randomised women were used. Results were consistent with the main comparison effects, with the exception of two outcomes. Caesarean section became statistically significant, with significantly more women allocated to admission CTG having, on average, a caesarean section compared with women allocated to intermittent auscultation. Epidural also became significant, with significantly more women allocated to intermittent auscultation having, on average, an epidural compared with women allocated to admission CTG. However, these findings should be interpreted with caution. For the outcome caesarean section in whole‐group comparison, Mires 2001 contributes most weight to the meta‐analysis. However, in this study 37% (N = 704) of women randomised to intermittent auscultation developed complications during pregnancy and required admission CTG on admission.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Funding was provided by the hospitals where the trials took place (Cheyne 2003; Impey 2003; Mitchell 2008) or by government grants (Mires 2001). There were no declarations of interest made in Impey 2003 and Mires 2001. The other two trials (Cheyne 2003; Mitchell 2008) did not mention any declarations of interest.
The GRADE approach was used to assess evidence quality. All outcomes, with the exception of perinatal mortality rate, were downgraded for lack of blinding because it was felt that knowledge of allocation could affect the outcomes. The level of evidence for incidence of caesarean section was graded moderate. The other maternal outcomes (incidence of operative vaginal birth and of continuous electronic fetal monitoring during labour) were downgraded for imprecision and inconsistency respectively; the level of evidence for both outcomes was graded low. The evidence for perinatal mortality rate was graded moderate for imprecision due to wide confidence intervals crossing the line of no effect. Incidence of seizures in the neonatal period was graded low for imprecision. Incidence of hypoxic ischaemic encephalopathy was downgraded for wide confidence intervals crossing the line of no effect, few events and having data contributed from one small study meaning its level of evidence was graded very low. One main outcome (severe neurodevelopmental disability assessed at 12 months of age or more) selected for the 'Summary of findings' table was not reported. See summary of findings Table for the main comparison.
Potential biases in the review process
It is possible that we introduced bias during the review process. However, we attempted to minimise bias by applying the following approaches: two review authors (DD, JGL) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We attempted to identify all relevant trials by conducting a comprehensive search of the literature.
Declan Devane and Valerie Smith are currently conducting a trial, known as the ADCAR Trial, evaluating the effectiveness of the admission CTG compared with intermittent auscultation. This study is ongoing; however, if it is completed for future review updates, neither author will be involved in assessing the trial for inclusion, assessing risk of bias, or data extraction.
Agreements and disagreements with other studies or reviews
An earlier review on the effects of admission CTG compared with intermittent auscultation of the fetal heart rate (Gourounti 2007), which included three (Cheyne 2003; Impey 2003; Mires 2001) of the four trials included in our review found an increased relative risk of caesarean section and instrumental delivery associated with admission CTG group. Our finding supports the likelihood of an increased risk for caesarean section associated with admission CTG but we did not find a significant increase in instrumental delivery with admission CTG.
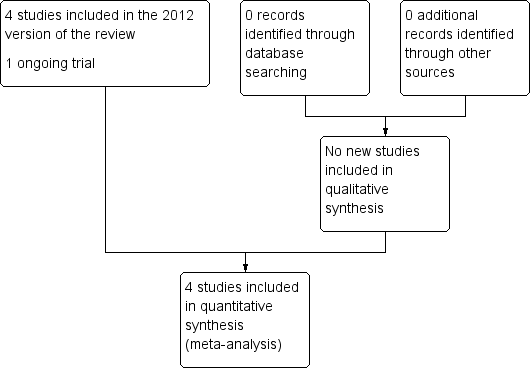
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 1 Caesarean section.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 2 Instrumental vaginal birth.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 3 Continuous EFM during labour.
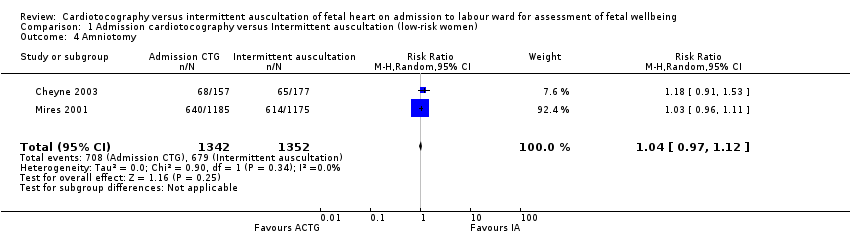
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 4 Amniotomy.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 5 Oxytocin for augmentation of labour.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 6 Epidural.
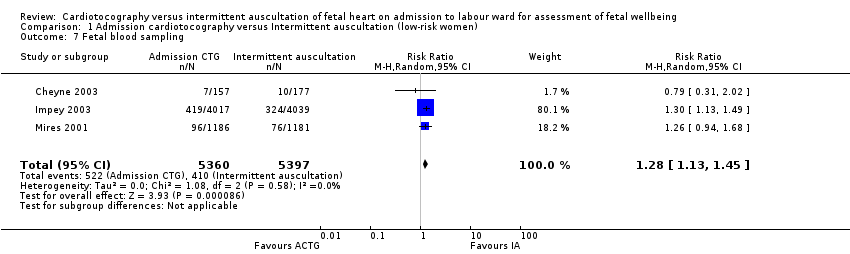
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 7 Fetal blood sampling.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 8 Fetal and neonatal deaths.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 10 Admission to neonatal intensive care.
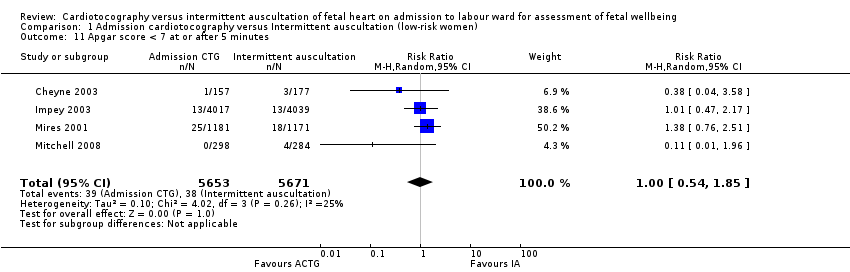
Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 11 Apgar score < 7 at or after 5 minutes.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 12 Hypoxic ischaemic encephalopathy.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 13 Neonatal seizures.

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 14 Length of stay in neonatal intensive care (days).

Comparison 1 Admission cardiotocography versus Intermittent auscultation (low‐risk women), Outcome 15 Length of stay in neonatal intensive care (hours).
| Admission cardiotocography compared to Intermittent auscultation (low‐risk women) for assessment of fetal wellbeing | ||||||
| Patient or population: Low risk pregnant women. All of the women were in labour. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Intermittent auscultation (low‐risk women) | Risk with admission cardiotocography | |||||
| Incidence of caesarean section | Study population | RR 1.20 | 11338 | ⊕⊕⊕⊝ | ||
| 36 per 1000 | 44 per 1000 | |||||
| Incidence of operative vaginal birth | Study population | RR 1.10 | 11338 | ⊕⊕⊝⊝ | ||
| 126 per 1000 | 139 per 1000 | |||||
| Perinatal mortality rate (fetal and neonatal deaths excluding lethal congenital anomalies) | Study population | RR 1.01 | 11339 | ⊕⊕⊕⊝ | ||
| 1 per 1000 | 1 per 1000 | |||||
| Severe neurodevelopmental disability assessed ≥ 12 months of age | Study population | ‐ | (0 RCTs) | ‐ | None of the included studies reported data for the outcome | |
| see comment | see comment | |||||
| Incidence of continuous electronic fetal monitoring during labour | Study population | RR 1.30 | 10753 | ⊕⊕⊝⊝ | ||
| 417 per 1000 | 542 per 1000 | |||||
| Incidence and severity of hypoxic ischaemic encephalopathy (incidence only reported) | Study population | RR 1.19 | 2367 | ⊕⊝⊝⊝ | ||
| 4 per 1000 | 5 per 1000 | |||||
| Incidence of seizures in the neonatal period | Study population | RR 0.72 | 8056 | ⊕⊕⊝⊝ | ||
| 3 per 1000 | 2 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations: outcome may have been affected by lack of blinding as all studies judged to be at high risk of performance bias (‐1) 2 Good sample size (> 3000), no measurable heterogeneity (I² = 0%), however 95% confidence interval touches the line of no effect (not downgraded) 3 Good sample size (> 3000), though wide confidence intervals cross the line of no effect (‐1) 4 Studies contributing data had design limitations: unlikely this outcome was affected by lack of blinding (not downgraded) 5 Few events but good sample size (not downgraded) 6 Very wide confidence intervals crossing the line of no effect (‐1) 7 Statistical heterogeneity (I² = 79%) (‐1) 8 Wide confidence interval crossing the line of no effect, few events & small sample size (based on one study) (‐2) 9 Wide confidence intervals crossing the line of no effect, large sample size with data from one study (‐1) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.00, 1.44] |
| 2 Instrumental vaginal birth Show forest plot | 4 | 11338 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| 3 Continuous EFM during labour Show forest plot | 3 | 10753 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.48] |
| 4 Amniotomy Show forest plot | 2 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 5 Oxytocin for augmentation of labour Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.17] |
| 6 Epidural Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.87, 1.41] |
| 7 Fetal blood sampling Show forest plot | 3 | 10757 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.13, 1.45] |
| 8 Fetal and neonatal deaths Show forest plot | 4 | 11339 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.30, 3.47] |
| 9 Evidence of fetal multi‐organ compromise within the first 24 hours after birth Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.67] |
| 10 Admission to neonatal intensive care Show forest plot | 4 | 11331 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.24] |
| 11 Apgar score < 7 at or after 5 minutes Show forest plot | 4 | 11324 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.85] |
| 12 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.90] |
| 13 Neonatal seizures Show forest plot | 1 | 8056 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.61] |
| 14 Length of stay in neonatal intensive care (days) Show forest plot | 1 | 91 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.59, 4.19] |
| 15 Length of stay in neonatal intensive care (hours) Show forest plot | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 6.20 [‐8.70, 21.10] |

