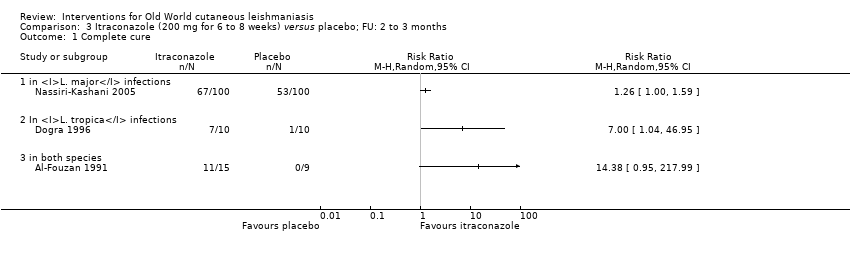
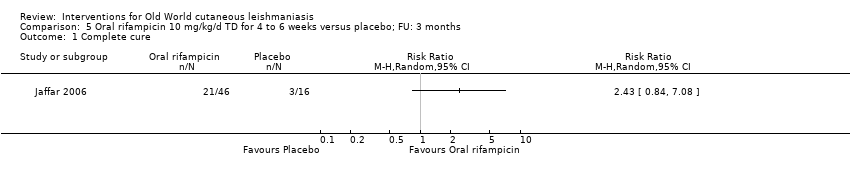
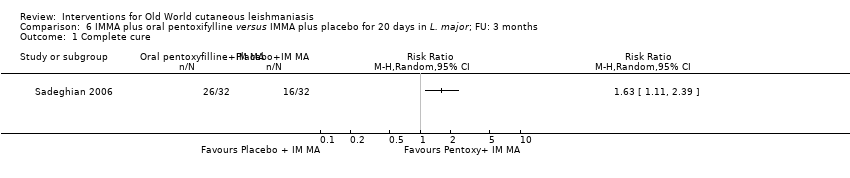
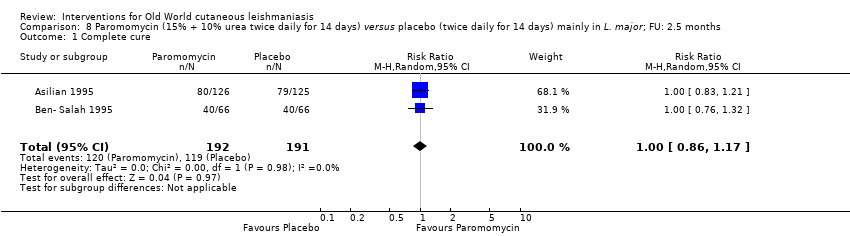
| Authors | Title | Journal | Observations | Number of participants |
| Akuffo H, Dietz M, Teklemariam S, Tadesse T, Amare G, Yermane Berhan T | The use of itraconazole in the treatment of leishmaniasis caused by Leishmania aethiopica | Trans R Soc Trop Med & Hyg 1990; 84: 532‐534 | Quasi‐randomised trial | 14 |
| Al‐Waiz M, Sharique KE, Al‐Assir M | Treatment of cutaneous leishmaniasis by intralesional metronidazole | Saudi Med J 2004; 25 (10): 1512‐1513 | Non‐randomised trial | 73 |
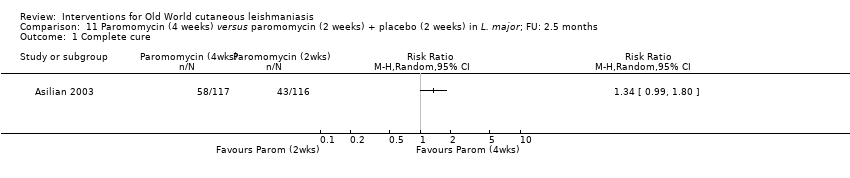
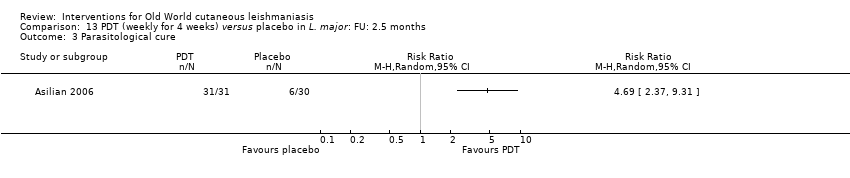
| Asilian A, Sadeghinia A, Faghihi G, Momeni A, Amini Harandi A | The efficacy of treatment with intralesional meglumine antimoniate alone, compared with that of cryotherapy combined with the meglumine antimoniate or intralesional sodium stibogluconate, in the treatment of cutaneous leishmaniasis | Ann Trop Med Parasitol 2003; 97 (5): 493‐498 | Non‐randomised trial | 180 |
| Daoud S, Boushi L | Azithromycin, ineffective in the treatment of old‐world cutaneous leishmaniasis | Int J Dermatol 2006; 45: 1126‐1128 | Non‐randomised trial | 45 |
| El‐Darouti MA, Al‐Rubaie SM | Cutaneous leishmaniasis: treatment with combined cryotherapy and intralesional stibogluconate injection | Int J Dermatol 1990; 29(1): 56‐9 | Non‐randomised trial | 44 |
| El‐Safi SH, Murphy AG, Bryceson ADM, Neal RA | A double‐blind clinical trial of the treatment of cutaneous leishmaniasis with paromomycin ointment | Trans R Soc Trop Med & Hyg 1990; 84: 690‐691 | Quasi‐randomised trial (double‐blinded study) | 40 |
| Gurei MS, Tatli N, Ozbilge H, Erel O, Seyrek A, Kocygit A, Ulukanligil M | Efficacy of cryotherapy and intralesional pentostam in treatment of cutaneous leishmaniasis | J Egypt S Parasitol 2000; 30 (1): 169‐176 | Non‐randomised trial | 97 |
| Hellier I, Dereure O, Tournillac I, Pratlong F, Guillot B, Dedet JP, Guilhou JJ | Treatment of old world cutaneous leishmaniasis by pentamidine isethionate | Dermatology 2000; 200 (2): 120‐123 | Non‐randomised trial | 11 |
| Kocygit A, Gur S, Gurel MS, Bulut V, Ulukanligil M | Antimonial therapy induces circulating pro inflammatory cytokines in participants with cutaneous leishmaniasis | IAI 2002; 70 (12): 6589‐6591 | Non‐randomised trial | 52 |
| Livshin R, Weinrauch L, Even‐Paz Z, El‐On J | Efficacy of rifampicin and isoniazid in cutaneous leishmaniasis | Int J Dermatol 1987; 26 (1): 55‐9 | Non‐randomised trial | 39 |
| Meymandi S, Holmes D, Crawford RI | Comparative study of the efficacy of combined imiquimod (Aldara T) 5% cream and intralesional meglumine antimoniate (Glucantime) | J Eur Acad Dermatol Venereol 2005; 19 (2): 38 | Non‐randomised trial | 99 |
| Ozgostasi O, Kirtak N, Erbagci Z | Cryotherapy in the treatment of cutaneous leishmaniasis | J Dermatol Treat 1997; 8: 179‐182 | Non‐randomised trial | 64 |
| Pareek SS | Combination therapy of sodium stibogluconate and rifampicin in cutaneous leishmaniasis | Int J Dermatol 1984; 23(1): 70‐1 | Non‐randomised trial | 32 |
| Sharquie KE, Al‐Hamamy H, El‐Yassin D | Treatment of cutaneous leishmaniasis by direct current electrotherapy: the baghdadin device | J Dermatol 1998; 25: 234‐237 | Non‐randomised trial | 69 |
| Vardy D, Barenholz Y, Cohen R, Zvulunov A, Biton A, Klaus S, Frankenburg S | Topical amphotericin B for cutaneous leishmaniasis | Arch Dermatol 1999; 135 (7): 856‐857 | Non‐randomised trial | 13 |
| Vardy D, Barenholz Y, Naftoliev N, Kaus S, Gilead L, Frankenburg S | Efficacious topical treatment for human cutaneous leishmaniasis with ethanolic lipid amphotericin B | Trans R Soc Trop Med & Hyg 2001; 95: 184‐186 | Non‐randomised trial (but single‐blinded for the participant) | 19 |
| Radmanesh M, Falahzadeh A, Dowraghi HF | Weekly versus every 3 days intralesional meglumine antimoniate therapy for cutaneous leishmaniasis | J Dermatolog Treat 1998; 9(4): 231‐233 | Non‐randomised trial | 70 |
| Mapar MA, Kavoosi H, Dabbagh MA | Assessment of the effect of topical opium in treatment of cutaneous leishmaniasis | Iranian J Dermatol 2001; 4(4): 23‐28. | Non‐randomised trial | 96 |
| Seeberger J, Daoud S, Pammer J | Transient effect of topical treatment of cutaneous leishmaniasis with imiquimod. | Int J Dermatol. 2003;42(7): 576‐9 | Non‐randomised trial | 15 |
| Bahamdan KA, Tallab TM, Johargi H, Nourad MM, Ibrahim K, el Sherbini AH, Karkashan E, Khare AK, Nauri MM | Terbinafine in the treatment of cutaneous leishmaniasis: a pilot study | Int J Dermatol. 1997; 36(1): 59‐60 | Uncontrolled | 27 |
| Morizot G, Delgiudice P, Caumes E, Laffitte E, Marty P, Dupuy A, Sarfati C, Hadj‐Rabia S, Darie H, LE Guern AS, Salah AB, Pratlong F, Dedet JP, Grogl M, Buffet PA | Healing of Old World cutaneous leishmaniasis in travelers treated with fluconazole: drug effect or spontaneous evolution? | Am J Trop Med & Hyg 2007; 76(1): 48‐52 | Uncontrolled | 35 |
| Leibovici V, Aram H | Cryotherapy in acute cutaneous leishmaniasis | Int J Dermatol. 1986;25(7): 473‐5 | Uncontrolled | 14 |
| Aram H, Leibovici V | Ultrasound‐induced hyperthermia in the treatment of cutaneous leishmaniasis | Cutis. 1987;40(4): 350‐3 | Uncontrolled | 18 |
| Junaid AJ | Treatment of cutaneous leishmaniasis with infrared heat | Int J Dermatol. 1986;25(7): 470‐2 | Uncontrolled | 178 |
| Faris RM, Jarallah JS, Khoja TA, al‐Yamani MJ | Intralesional treatment of cutaneous leishmaniasis with sodium stibogluconate antimony | Int J Dermatol. 1993;32(8): 610‐2 | Uncontrolled | 710 |
| Joshi RK, Nambiar PM | Dermal leishmaniasis and rifampicin | Int J Dermatol. 1989;28(9): 612‐4 | Uncontrolled | 15 |
| Abahusein A, Larbi EB, al‐Khawajah A, al‐Gindan Y, Jain S | Evaluation of topical ketoconazole in cutaneous leishmaniasis | East Afr Med J. 1992;69(1): 14‐7 | Uncontrolled | 10 |
| Sharquie KE, Al‐Talib KK, Chu C | Intralesional therapy of cutaneous leishmaniasis with sodium stibogluconate antimony | Br J Dermatol 1988; 119: 53‐57. | Non‐randomised trial | 60 |
| Van den Enden E, Van Gompel A, Stevens A, Vandeghinste N, Le Ray D, Gigase P, De Beule K, Van den Ende J | Treatment of cutaneous leishmaniasis with oral itraconazole | Int J Dermatol 1994; 33(4): 285‐286. | Uncontrolled | 22 |
| Tallab TM, Bahamdam KA, Mirdad S, Johargi H, Mourad MM, Ibrahim K, el Sherbini
AH, Karkashan E, Khare AK, Jamal A | Cutaneous leishmaniasis: schedules for intralesional treatment with sodium stibogluconate | Int J Dermatol 1996; 35(8): 594‐597. | Uncontrolled | 96 |
| Jabbar A, Junaid N | Treatment of cutaneous leishmaniasis with infrared heat | Int J Dermatol 1986; 25(7): 470‐472. | Uncontrolled | 178 |