Dilatación neumática endoscópica versus inyección de toxina botulínica en el tratamiento de la acalasia primaria
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT. | |
| Participants | 16 treatment naive adult participants. | |
| Interventions | 8 participants received 100U BTX and 8 participants underwent PD. | |
| Outcomes | Mean symptom score ‐ dysphagia (0‐3), chest pain (0‐3) , regurgitation (0‐3) | |
| Notes | No complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not stated |
| Allocation concealment? | Unclear risk | Method not stated |
| Blinding? | High risk | |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
| Methods | Double blind RCT. | |
| Participants | 34 treatment naive adults | |
| Interventions | BTX 80U 4 quadrants | |
| Outcomes | Mean symptom score ‐ dysphagia, chest pain, regurgitation (0‐9) | |
| Notes | 2 perforations in PD group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not stated |
| Allocation concealment? | Unclear risk | Method not stated |
| Blinding? | Low risk | |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
| Methods | RCT. | |
| Participants | 17 participants | |
| Interventions | BTX 60‐80 units | |
| Outcomes | Dysphagia (0‐3), chest pain (Y/N), regurgitation (Y/N) | |
| Notes | No complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? | High risk | |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
| Methods | RCT. | |
| Participants | 40 participants(>40yr) | |
| Interventions | BTX 200(80)U 4 quadrants | |
| Outcomes | Mean symptom (0‐15) 1, 6, 12 months | |
| Notes | No complication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? | High risk | |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
| Methods | RCT. | |
| Participants | 24 participants ‐ 15 had previously undergone PD | |
| Interventions | BTX 80 Units | |
| Outcomes | Median score (0‐20) for 4 symptoms ‐ dysphagia, regurgitation, chest pain and heartburn at 1 week, 1 month and 6 monthly for 30 months. | |
| Notes | No complications. Possible high risk of bias if not double blind. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not stated |
| Allocation concealment? | Unclear risk | Method not stated |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
| Methods | RCT. | |
| Participants | 47 treatment naive participants. | |
| Interventions | BTX 100U | |
| Outcomes | Median symptom score (0‐15) | |
| Notes | 1 perforation in PD group ‐ excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | |
| Allocation concealment? | Low risk | |
| Blinding? | High risk | |
| Incomplete outcome data addressed? | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Vs:versus
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT. | |
| Duplicate ‐ Meeting abstract. Full paper later published. | |
| Meeting abstract ‐ unable to contact author. | |
| Meeting abstract ‐ unable to contact author. | |
| Duplicate ‐ Meeting abstract. Full paper later published. | |
| Duplicate ‐ Meeting abstract. Full paper later published. | |
| Meeting abstract ‐ unable to contact author. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Initial remission Show forest plot | 4 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.38] |
| Analysis 1.1  Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 1 Initial remission. | ||||
| 2 Mean oesophageal pressure within first four weeks Show forest plot | 3 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.44, 0.91] |
| Analysis 1.2  Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 2 Mean oesophageal pressure within first four weeks. | ||||
| 3 Remission at six months Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.48, 5.67] |
| Analysis 1.3  Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 3 Remission at six months. | ||||
| 4 Remission at twelve months Show forest plot | 3 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.58, 4.52] |
| Analysis 1.4  Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 4 Remission at twelve months. | ||||
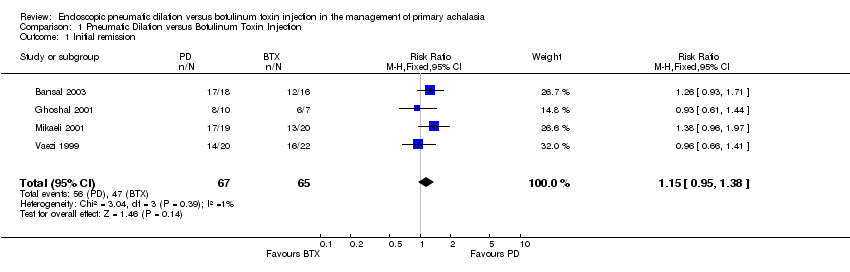
Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 1 Initial remission.
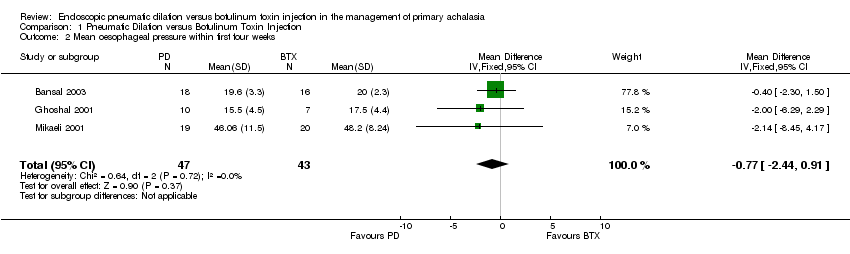
Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 2 Mean oesophageal pressure within first four weeks.
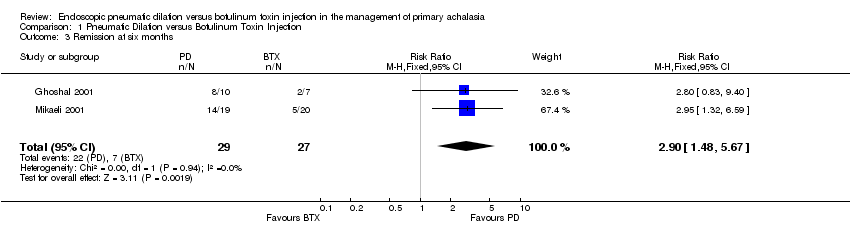
Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 3 Remission at six months.

Comparison 1 Pneumatic Dilation versus Botulinum Toxin Injection, Outcome 4 Remission at twelve months.
| Study | methods | Remission 1 month | Remission 6 months | Remission 12 months | Mean LOS pressure | Complications |
| 8 participants 100U BTX and 8 participants PD. | PD 8/8 | PD 8/8 | PD 8/8 | PD ‐72% | none | |
| 12 participants 80 Units BTX and 12 PD 40mm day 1 & 3. | PD 10/12 | PD 9/12 | PD 8/12 | PD ‐50% | none |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Initial remission Show forest plot | 4 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.38] |
| 2 Mean oesophageal pressure within first four weeks Show forest plot | 3 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.44, 0.91] |
| 3 Remission at six months Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.48, 5.67] |
| 4 Remission at twelve months Show forest plot | 3 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.58, 4.52] |

