Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised controlled trial; Randomization: minimum likelihood allocation. | |
| Participants | 39 opiate dependent, (DSM‐III‐R), stable, residing in USA, age 18 or older, eligible for MMT according to FDA requirements. (1)19 (2)20. Average age 33.5; 64% men; 97% White; mean use of heroin 10 years; mean age at the first use 20; 41% never married; 92% high school; 41% employed. Ex C: Psychosis, dementia, major medical disorder, pregnancy. | |
| Interventions | For all BDT, dose‐taper 4 mg/70 kg, dose increased to 8 mg if withdrawal, after the first week patients were maintained for an additional 42 hours, 72 hours or 7 days for the 2, 4, or 8 mg/70 kg dose respectively; then the dose was decreased gradually 10% every 5 days for the remainder 160 days. (1) Behavioural Therapy. (2) Standard counselling sessions once per week for 37 min. Duration 26 weeks. | |
| Outcomes | Retention in treatment as % of participants that completed the treatment. Use of primary substance of abuse as % of continued abstinent at 4, 8, 12 and 16 weeks and as % of abstinent from opioids at 23 and 26 weeks. Use of other drug as n. of positive participants (at least 1 positive urine specimen during the 26 weeks). Results at follow‐up as no. of opioid abstinent at 29 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; Randomization:method not reported. | |
| Participants | 81 opiate users, no detail of use, (1)41 (2)40. Average age 28; 65% men; 53% Caucasian, 12% African‐American, 24% Hispanic; 27% treated previously. | |
| Interventions | For all methadone detoxification, starting from 40 mg/day and tapered from day 3 of 5 mg every second day, the final dose on day 16 was 5 mg. (1) Contingency Management, participants paid for drug‐free urine 6 times during treatment. (2) Control , participants paid for each urine given. Duration 16 days. | |
| Outcomes | Use of primary substance of abuse as % of positive urine samples. Retention in treatment as days in treatment but only statistical test results reported. Psychiatric symptoms/psychological distress, no data only conclusions of the authors. | |
| Notes | Community Oriented Program Environment Scale (COPES) on days 3‐5 and 11‐13. Participants also completed Client Satisfaction Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; | |
| Participants | 27 opiate dependent, had to provide 50% or more opiate‐free urine during the first 3 weeks of the detoxification before the start of the trial. (1)9 (2)8 (3)10. 100% men; no other information available on the characteristics of the participants. | |
| Interventions | For all methadone detoxification, all stabilized on 30 mg/day during 21 days, trial starts on day 22; methadone dose was reduced in alternating 2 and 3 mg/day steps until 0 mg reached at the end of 63 days (week 9). (1) Contingency Management, participants could increase their clinic dose of methadone if their most recent urine sample was opioid free. (2) Non Contingency Management, the same amount of extra methadone available as contingent group but the dose increase is independent of the urinalysis results. Duration 13 weeks. | |
| Outcomes | Retention in treatment as % of participants terminating the treatment. Use of primary substance of abuse as average % of positive tests (3 tests per participant per week). Compliance as % of clinic absence. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; | |
| Participants | 39 opiate dependent, had to provide 50% or more opiate free urines during the first 3 weeks after treatment enrolment. (1)13, (2)13, (3)13. Average age 32; 100% men; 51% White; 49% African‐American; mean years of continuous opiate use: 9.2; average years of educational level 11.6; 46% employed; legal state free 69%, parole/probation/pending trial 31%. | |
| Interventions | For all: During the first 3 weeks, patients were stabilized on 30 mg/day of methadone; from week 4, methadone dose decreased in alternating 2 mg and 3 mg steps until 0 mg was reached on week 10. (1) Contingency Management, participants could increase their methadone dose by 5, 10, 15 or 20 mg on a daily basis from day 22‐77 of detoxification but only if their most recent urine sample was opiate free. (2) Non Contingency Management , participants could increase their methadone dose by 5, 10, 15 or 20 mg on a daily basis from day 22‐77 of detoxification independent of their urinalysis results. (3) Control, participants did not receive dose increase. Duration 13 weeks.Retention in treatment as average number of days in treatment. Compliance as % of missing clinic visits and as withdrawal symptoms (scores). Use of primary substance of abuse as % of opiate positive urine samples and as average daily amount of supplemental methadone received. | |
| Outcomes | Retention in treatment as average number of days in treatment. Compliance as % of missing clinic visits and as withdrawal symptoms (scores). Use of primary substance of abuse as % of opiate positive urine samples and as average daily amount of supplemental methadone received. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; Blinding not possible and blinding of outcome assessor unclear | |
| Participants | 211 indigent opiate abusers; mean age (1)35.7 (2)36.5 years; male (1)40% (2)37%; African American (1)62% (2)74%, Caucasian (1)32% (2)25%, Other (1)6% (2)1%; Mean education years (1)11.3 (2)11.4; Employed (1)19.3% (2)26.9%; Married (1)19.8% (2)15.1%, Single (1)80.2% (2)84.9% | |
| Interventions | For all 0.3 mg/day intramuscular buprenorphine administered for 4 days; in addition all patients who were still enrolled on Friday received a 7 day clonidine patch to wear during the following week, group counselling was held on a daily basis (1) Contingent n. 109, vouchers $100 if urine tested negative for both opiates and cocaine on Friday; (2) Non contingent n.102, vouchers delivered independent of their urine test results | |
| Outcomes | Use of opioids | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; | |
| Participants | 102 opiate dependent. (1)35 (2)32 (3)35. Results on 92: (1)31, (2)29, (3)32.Average age 41; 100% men; 74% African‐American; 27% married; average years of educational level 12; 47% employed; mean use of heroin 11 years, mean use of cocaine 3 years, mean problematic alcohol use 7 years. Ex C: Need for medical or psychiatric hospitalisation at the time of admission, plan for an imminent move from the Philadelphia area. | |
| Interventions | For all MMT, 60 to 90 mg/day. (1) Enhanced Methadone Services, on site medical, psychiatric, employment and family therapies services. (2) Standard Methadone Services, counseling sessions 1 per week. (3) Only methadone (especially permitted by FDA). Duration 24 weeks. | |
| Outcomes | Use of primary substance of abuse as % of opiate positive urine samples and as % of participants with opiate free urine samples per 8, 12, 16 consecutive weeks. Use of other drugs as % of cocaine positive urine samples. Severity of dependence as ASI (composite scores). | |
| Notes | Results on 92 participants who completed at least 2 weeks of the protocol and who were contacted at 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial. | |
| Participants | 50 heroin dependents, (1)25 (2)25. Average age 30; 66% men; mean use of heroin 8.8 years, mean number of previous detoxification treatments 4. | |
| Interventions | For all methadone detoxification, 35 mg on day 1 tapered to zero on day 21. (1) Counseling Treatment, mandatory psychotherapeutic counseling session on the second dosing day. Subsequent non mandatory sessions were scheduled during the second and the third weeks of treatment. (2) Control. Duration 21 days, follow‐up at 6 months. | |
| Outcomes | Retention in treatment as no. completed, no. of mean days in treatment, no. of drop‐outs. Use of primary substance of abuse as no. of participants with morphine negative samples. Compliance as no. visits attended while in treatment. Results at follow‐up as no. of participants transferred to MMT, no. in continued treatment for 6 months, no. re‐addicted and no. lost | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised controlled trial; | |
| Participants | 48 opiate dependent, age between 18‐65 years, eligible for MMT according to FDA guidelines, reported intravenous opiate use during the past 30 days. (1)26, (2)22. Mean age 40.7; 64.5% men; 48% White; 41.6%; 19% employed part time; 31% employed full time; 50% unemployed; 66% HIV positive; 66% reporting needle sharing; 66% reporting use of condom. Ex cr: pregnant women, current major psychiatric disorders other than drug abuse, unstable serious medical illness. | |
| Interventions | For all: Methadone detoxification after maintenance treatment. During weeks 1‐4 MMT then randomisation, MMT continue during weeks 5‐10 then methadone detoxification during weeks 11‐23. In the weeks 24‐26 no medication. (1) Contingency management, methadone mean dose 76.4, patients could obtain vouchers 3 times a week by providing opiate urine specimens. Upon providing the first opiate free urine specimen, participants received a voucher of $2.50, thereafter the value of the voucher increased by $1.50 with every consecutive opiate free urine to a maximum of $40. A maximum of $2232 could be earned. (2) Control, methadone mean dose 70.3, patients did not receive vouchers. Duration: 26 weeks | |
| Outcomes | Retention in treatment as no. retained. Use of primary substance of abuse as % of opiate negative urine samples, % of repeated opiate negative specimens. Severity of dependence as average number of intravenous drug injection per week. Compliance as withdrawal symptoms (scores of Visual Analog Scale) as no. lost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Allocation: randomised controlled trial; | |
| Participants | 119 opiate dependent, age over 18, use of opiate more than 6 months, agree to be seen with their partner or family if required. (1)41 (2)38 (3)40. Average age 28; 63% men; 80% living with a partner, of those 53% with a drug using partner, 14% living with the family of origin, 6% living alone; 27% employed full time, 20% employed part time 53% unemployed; 59% had criminal convictions, 18% refused to answer, 23% never charged with criminal offence. Ex C: History of psychiatric treatments, currently dependent on alcohol | |
| Interventions | For all methadone detoxification. (1) Family Therapy, methadone in a strict reduction regime non negotiable reducing daily dose 5 mg every 2 weeks plus 16 session of 1 hour every 2 weeks and then monthly. (2) Standard Clinic, | |
| Outcomes | Results at follow‐up as % of participants followed at 6 and 12 months, no. of heroin‐free, occasional use, regular use, in prison or unavailable, mortality rates as no. of deaths. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Footnotes
BDT: Buprenorphine Detoxification Treatment
COPES: Community Oriented Program Environment Scale
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association Washington DC
Ex C: Exclusion Criteria
FDA: Food and Drug Administration
HIV: Human Immunodeficency Virus
MMT: Methadone Maintenance Treatment
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Excluded as the study design was not in the inclusion criteria: review article. | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological treatment associated with psychosocial | |
| Excluded as type of outcomes not in the inclusion criteria: no separate data for detoxification and maintenance pharmacological interventions | |
| Excluded as the study design was not in the inclusion criteria: prospective study | |
| Excluded as the study design was not in the inclusion criteria: experimental prospective study. | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological treatment alone | |
| Excluded because the type of pharmacological intervention (naltrexone) not in the inclusion criteria | |
| Excluded as the study design was not in the inclusion criteria of the review: review article | |
| Excluded as the type of participants was not in the inclusion criteria: females between 30 and 50 years of age and dependent on or abusing alcohol, a prescription drug or both. | |
| Excluded as the study design and the type of participants was not in the inclusion criteria of the review: prospective intervention study; participants were patients discharged from an inpatient psychiatric service, excluded only those whose Axis I diagnosis was substance abuse or organic mental disorder and who stayed in the hospital less than 7 days. | |
| Excluded as the type of participants and the intervention was not in the inclusion criteria: Participants were probationers drug dependent (any drug) and the two treatments compared were both psychosocial without pharmacological intervention. | |
| Excluded as the type of interventions were not in the inclusion criteria: after detoxification, participants were randomised in four groups all without pharmacological interventions. | |
| Excluded as the type of participants and of interventions were not in the inclusion criteria: Participants were substance abusers (any drug), the experimental intervention was "attrition prevention" compared to standard care while awaiting treatment admission. | |
| Excluded as the type of participants not in the inclusion criteria: substances abusers (any drug). | |
| Excluded as the design not in the inclusion criteria: review article. | |
| Excluded as the design was not in the inclusion criteria: review article. | |
| Excluded as the intervention was not in the inclusion criteria: comparison between network therapy without drugs and buprenorphine without psychosocial | |
| Excluded as type of intervention not in the inclusion criteria: no psychosocial treatment | |
| Excluded as type of intervention not in the inclusion criteria: the study evaluate the efficacy of fentanyl compared with naltrexone | |
| Excluded as the study design not in the inclusion criteria: overview | |
| Excluded as the type of participants and intervention was not in the inclusion criteria: participants were inner city opiate abusers discharged from detoxification unit; the interventions were (1) reinforcement‐based intensive outpatient treatment and (2) community treatment resources, none with pharmacological plus psychosocial programs. | |
| Excluded as type of outcomes not in the inclusion criteria: knowledge about drugs, satisfaction and motivation were the outcomes considered but no data were provided | |
| Excluded as type of intervention not in the inclusion criteria: strengths‐based case management compared with passive referral | |
| Excluded as the type of participants not in the inclusion criteria: participants were overdose patients. | |
| Excluded as the study design not in the inclusion criteria: review article. | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological treatment considered | |
| Excluded as the study design not in the inclusion criteria: cohort study | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological treatment considered | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological treatment considered | |
| Excluded as the type of intervention not in the inclusion criteria: comparison of two drug free programs in short or long version. | |
| Excluded as the study design and the participants not in the inclusion criteria: research demonstration project and the participants were dually diagnosed homeless | |
| Excluded as the study design and the type of participants not in the inclusion criteria: cohort study and participants were substance abusers (any drug). | |
| Excluded as the study design and the type of participants not in the inclusion criteria: participants were substance abusers (any drug). | |
| Excluded as the study design not in the inclusion criteria: review article. | |
| Excluded as the type of participants and intervention not in the inclusion criteria: participants were substance dependent (any drug) and intervention was a comparisons between high standardization cognitive behavioural treatment, low standardization cognitive behavioural treatment, and treatment as usual. | |
| Excluded as type of outcomes reported not in the inclusion criteria: the outcomes were responses on interview containing15 agree/disagree questions tapping orientations to locus‐of‐control beliefs about drug misuse. | |
| Excluded as the study design not in the inclusion criteria: review article | |
| Excluded as the type of participants not in the inclusion criteria: participants were drug dependent (any drug). | |
| Excluded as the study design not in the inclusion criteria: review article | |
| Excluded as the study design not in the inclusion criteria: review | |
| Excluded because the type of intervention not in the inclusion criteria;: pharmacological intervention with naltrexone | |
| Excluded as the design not in the inclusion criteria: clinical not controlled study. | |
| Excluded as the study design not in the inclusion criteria: evaluation study. | |
| Excluded as the type of intervention not in the inclusion criteria: brief motivational intervention compared to a control group (education package), no information available on pharmacological intervention. | |
| Excluded as the type of participants not in the inclusion criteria: participants were substance dependent (any drug) | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological treatment | |
| Excluded as the study design not in the inclusion criteria: overview. | |
| Excluded as the type of participants and intervention not in the inclusion criteria: participants were double diagnosed homeless and two residential programs were compared. | |
| Excluded as type of intervention not in the inclusion criteria: no information on pharmacological treatment |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 5 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.11, 2.55] |
| Analysis 1.1  Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 1 Completion of treatment. | ||||
| 2 Use of primary substance Show forest plot | 4 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.93] |
| Analysis 1.2  Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 2 Use of primary substance. | ||||
| 3 Number of subjects abstinent at follow‐up Show forest plot | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.61, 3.66] |
| Analysis 1.3  Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 3 Number of subjects abstinent at follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 4 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.93, 2.35] |
| Analysis 2.1  Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 1 Completion of treatment. | ||||
| 2 Use of primary substance Show forest plot | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.07] |
| Analysis 2.2  Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 2 Use of primary substance. | ||||
| 3 Number of subjects abstinent at follow‐up Show forest plot | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.61, 3.76] |
| Analysis 2.3  Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 3 Number of subjects abstinent at follow‐up. | ||||
| 4 Compliance as clinic absences during the treatment Show forest plot | 3 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.59] |
| Analysis 2.4  Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 4 Compliance as clinic absences during the treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 3 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.93, 2.46] |
| Analysis 3.1  Comparison 3 Contingency Management Approaches plus MDT versus MDT alone, Outcome 1 Completion of treatment. | ||||
| 2 Compliance as clinical absences during the treatment Show forest plot | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.15, 0.56] |
| Analysis 3.2  Comparison 3 Contingency Management Approaches plus MDT versus MDT alone, Outcome 2 Compliance as clinical absences during the treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Use of primary substance Show forest plot | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.97] |
| Analysis 4.1  Comparison 4 Contingency Management Approaches plus BDT versus BDT alone, Outcome 1 Use of primary substance. | ||||
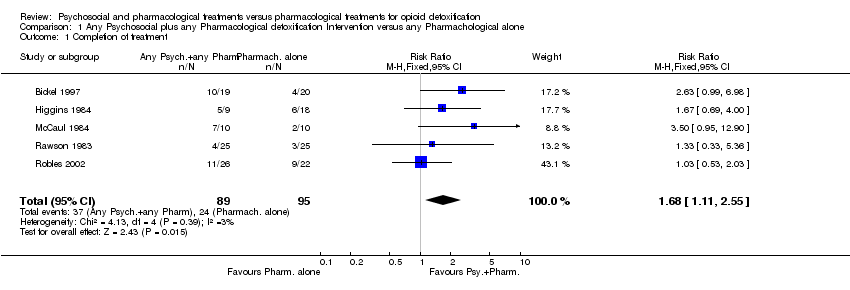
Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 1 Completion of treatment.

Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 2 Use of primary substance.
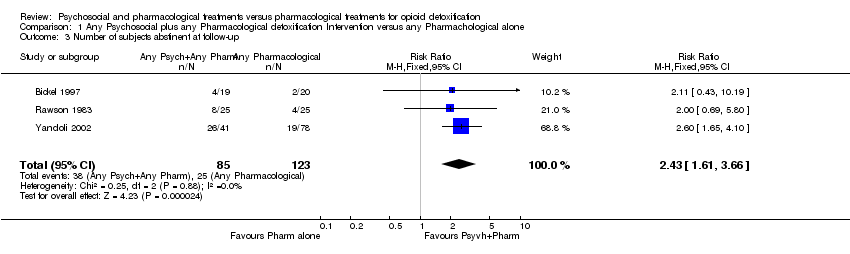
Comparison 1 Any Psychosocial plus any Pharmacological detoxification Intervention versus any Pharmachological alone, Outcome 3 Number of subjects abstinent at follow‐up.

Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 1 Completion of treatment.

Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 2 Use of primary substance.
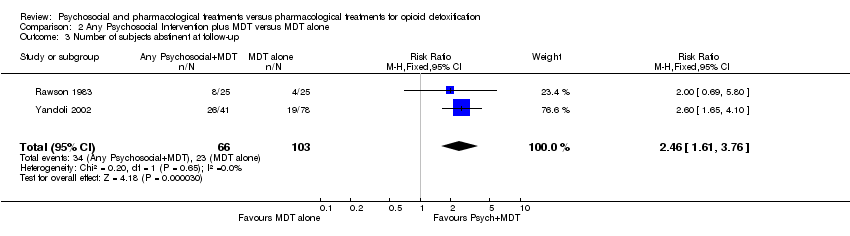
Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 3 Number of subjects abstinent at follow‐up.

Comparison 2 Any Psychosocial Intervention plus MDT versus MDT alone, Outcome 4 Compliance as clinic absences during the treatment.

Comparison 3 Contingency Management Approaches plus MDT versus MDT alone, Outcome 1 Completion of treatment.

Comparison 3 Contingency Management Approaches plus MDT versus MDT alone, Outcome 2 Compliance as clinical absences during the treatment.

Comparison 4 Contingency Management Approaches plus BDT versus BDT alone, Outcome 1 Use of primary substance.
| any pharmacological detoxification treatment plus psychosocial compared to any pharmacological treatment alone for opioid dependent requiring detoxification | ||||||
| Patient or population: patients with opioid dependent requiring detoxification Settings: outpatient and inpatient Intervention: any pharmacological detoxification treatment plus psychosocial Comparison: any pharmacological treatment alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| any pharmacological treatment alone | any pharmacological detoxification treatment plus psychosocial | |||||
| Completion of treatment | Low risk population | RR 1.68 | 184 | 1,2 | ||
| 253 per 1000 | 425 per 1000 | |||||
| use of opiate during treatment | Low risk population | RR 0.82 | 320 | ⊕⊕⊕⊝ | ||
| Medium risk population | ||||||
| 790 per 1000 | 648 per 1000 | |||||
| relapsed at follow‐up | Medium risk population | RR 0.41 | 208 | ⊕⊕⊕⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidance | ||||||
| 1 Four studies with unclear allocation concealment and one inadequate; 2 studies were single blind and 3 did not report data on blindness 2 All studies were conducted in USA 3 Four studies with unclear allocation concealment 4 All studies with unclear allocation concealment, 2 single blind, 1 not blind | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 5 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.11, 2.55] |
| 2 Use of primary substance Show forest plot | 4 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.93] |
| 3 Number of subjects abstinent at follow‐up Show forest plot | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.61, 3.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 4 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.93, 2.35] |
| 2 Use of primary substance Show forest plot | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.07] |
| 3 Number of subjects abstinent at follow‐up Show forest plot | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.61, 3.76] |
| 4 Compliance as clinic absences during the treatment Show forest plot | 3 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of treatment Show forest plot | 3 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.93, 2.46] |
| 2 Compliance as clinical absences during the treatment Show forest plot | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.15, 0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Use of primary substance Show forest plot | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.97] |


