Bain ou douche pré‐opératoire avec utilisation d'antiseptiques cutanés pour prévenir l'infection du site opératoire
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT. | |
| Participants | 3733 patients undergoing elective or potentially contaminated surgery. | |
| Interventions | All patients showered 3 times (on admission, the night before surgery and the morning of surgery) using 50 ml of either: | |
| Outcomes | Primary outcome: | |
| Notes | Data were extracted from 3 papers reporting results from 1 study (see Lynch 1992 & Byrne 1994). There were minor discrepancies in numbers reported between the 3 studies. The version reported is the definitive study (personal correspondence with author). The abstract stated there were 1753 patients in the placebo group but this should have been 1735 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 6. Randomisation in each group of 6 was by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Personal communication with author. Allocation in sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | 2 bottles of either 4% chlorhexidine detergent solution or a physically‐identical placebo detergent were given to the patient. Neither the investigators nor the patient were aware of the allocation. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessment blinded |
| Incomplete outcome data (attrition bias) | Low risk | Of 3733 patients randomised, 244 (6.5%) were excluded from the outcome analysis. The majority of these, 219 (5.9%) were because the operation was cancelled after randomisation. 4 patients in the chlorhexidine group and 9 patients in the control group were excluded for protocol violations. However, as these numbers are extremely small (0.4% of those randomised), it is unlikely that results were compromised. |
| Selective reporting (reporting bias) | Low risk | The study reported on all of the stated outcomes. |
| Other bias | Low risk | The trial was supported by ICI Pharmaceuticals. However, as results did not support the use of chlorhexidine, it is unlikely that results were compromised. |
| Methods | RCT. | |
| Participants | 66 patients undergoing vascular reconstruction surgery. | |
| Interventions | All patients had 2 baths. | |
| Outcomes | Primary outcome: | |
| Notes | Different washing information provided to participants in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Investigator contacted ‐ no further information available. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Investigator contacted ‐ no further information available. |
| Blinding (performance bias and detection bias) | High risk | Both the investigator and the participant were aware of the allocation. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessment blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Results were available for all those enrolled. No drop outs or exclusions were described. |
| Selective reporting (reporting bias) | Low risk | The study reported on wound infection. A number of post hoc analyses were also conducted. |
| Other bias | High risk | Baseline data tables were not provided but the author stated that "clinical details of the two groups were similar". |
| Methods | Cluster RCT. | |
| Participants | 2015 patients undergoing routine surgery. | |
| Interventions | All patients had either a shower or bath on the day before and morning of their operation. | |
| Outcomes | Primary outcome: | |
| Notes | Data were extracted from 2 papers reporting results from 1 study (Hayek 1988). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | 3 arms to the study. "None of the ward staff or those assessing the wounds were aware of whether placebo or active compound was being used as these were issued in identical coded sachets, though no form of double blind was possible for the bar soap group". |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessment blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | It appears from the text that results were available from all patients who entered the study. However, as patients were followed up for 6 weeks postoperatively; it seems unusual that none were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | High risk | No baseline data presented. The authors state that "The three groups are comparable except for one interesting facet; only 14% washed their hair with bar soap against 28% with either of the liquids. |
| Methods | RCT. | |
| Participants | 94 patients undergoing vasectomy. | |
| Interventions | Group 1: 1 preoperative shower with chlorhexidine 4%, | |
| Outcomes | Primary outcome: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with study authors confirmed that trialists used computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding of the intervention was not possible. |
| Blinding (performance bias and detection bias) | Unclear risk | It is unclear if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All but 1 enrolled patient were assessed 7 days postoperatively; 83 returned to the ward, 10 were contacted at home and 1 was lost to follow‐up (it is unclear which group this patient was in); results were presented for the total number enrolled. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | No baseline data presented. |
| Methods | Cluster RCT. | |
| Participants | 2953 patients undergoing elective clean surgery. | |
| Interventions | All patients had 2 showers;1 the day before surgery and 1 on the day of surgery. | |
| Outcomes | Primary outcome: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out for each surgical unit by means of computer‐generated patient trial numbers. |
| Allocation concealment (selection bias) | Low risk | Bottles of solution were contained in boxes of 10, each holding 5 chlorhexidine and 5 placebo in random sequence. Unless the code was broken, it was impossible to know which preparation the patient had used. |
| Blinding (performance bias and detection bias) | Low risk | Neither patients nor investigators were aware of group allocation. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessment was by routine surveillance methods employed by individual hospitals (infection control nurse or surgeon). They were unaware of group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | 140 (4.7%) patients were withdrawn after randomisation either because the patient was not operated on (62 patients), had had 1 bath only (26), operation not stated 'clean' (14) or had a current infection (38). The withdrawals were evenly distributed between the chlorhexidine and placebo groups. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | ICI supplied the bathing materials. However, as results favoured the placebo group, this is unlikely to have affected results. |
| Methods | RCT. | |
| Participants | Adult patients, scheduled for plastic surgery at a University‐affiliated hospital in Brazil. Exclusions: hypersensitivity to chlorhexidine, presence of skin lesions, antibiotic use at time of surgery, diabetes, heavy smoking, immunosuppression. | |
| Interventions | Group 1: shower with liquid‐based detergent containing 4% chlorhexidine; | |
| Outcomes | Primary outcome: Secondary outcome: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Surgeons and patients were all blinded to the allocation group. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessors and microbiologists were all blinded to the allocation group. |
| Incomplete outcome data (attrition bias) | Low risk | All patients were followed for the nominated 30 days. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported. |
| Other bias | Low risk | The placebo solution was provided by Rioquimica Industria Farmaceutica, the manufacturer of the intervention product. However, as results in different groups were similar, this is unlikely to have affected results. |
| Methods | RCT. | |
| Participants | 1530 patients undergoing elective surgery of the biliary tract, inguinal hernia and breast cancer. | |
| Interventions | Group 1: patients washed their entire body with chlorhexidine on the day before surgery using 2 consecutive applications followed by rinsing under the shower; | |
| Outcomes | Primary outcome: | |
| Notes | This study was conducted over a 7 year period from 1978 to 1984. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with study authors confirmed that they used a randomisation list. |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to 1 of 3 wards in which different preoperative preparation schedules were used. The author states that "The randomisation was done by the Chief surgeon in such a way that he did not know the identity of the patients allocated to each ward." |
| Blinding (performance bias and detection bias) | High risk | Blinding would have been impossible because of the allocation method. |
| Blinding (performance bias and detection bias) | High risk | Outcome assessment was not blinded (personal correspondence with study authors). |
| Incomplete outcome data (attrition bias) | Low risk | 3 patients died in the few days following surgery, these were not identified by group. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Unclear risk | Baseline risk factors were similar across groups. |
Abbreviations
> = more than
ASEPSIS = a scoring method for postoperative wounds infections for use in clinical trials of antibiotic prophylaxis (Wilson 1986).
RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised controlled trial. | |
| No data on wound infection. Not a randomised controlled trial. | |
| Co‐intervention (mupirocin and chlorhexidine soap). Also included patients who did not undergo surgery. | |
| Not a randomised controlled trial. Local wash versus full body wash with chlorhexidine. | |
| Retrospective review comparing two hospitals. One hospital had a pre‐operative showering policy using an antiseptic solution; the other hospital did not. | |
| Not a clinical trial. | |
| Healthy volunteers used to compare skin concentration levels of chlorhexidine gluconate (CHG) following washing with various CHG products. | |
| Not a randomised controlled trial. Used a pre and post intervention design. | |
| Not a randomised controlled trial. | |
| No no‐antiseptic group. Did not report infection rates by group. | |
| Systematic review. | |
| Did not report infection rates by group. | |
| Patients in both groups showered with water. The trial compared methods of skin preparation prior to surgery. | |
| Not a randomised controlled trial. | |
| 2% chlorhexidine gluconate cloths were used to wipe over the entire body one hour after showering. | |
| Not a randomised controlled trial. Patients were allocated by month. | |
| Assessed reduction in microbial loads in healthy volunteers. | |
| Trial of healthy volunteers | |
| Assessed the influence of povidone‐iodine preoperative showers on skin colonization. Rates of wound infection not reported. | |
| Not a randomised controlled trial. Did not report infection rates by group. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
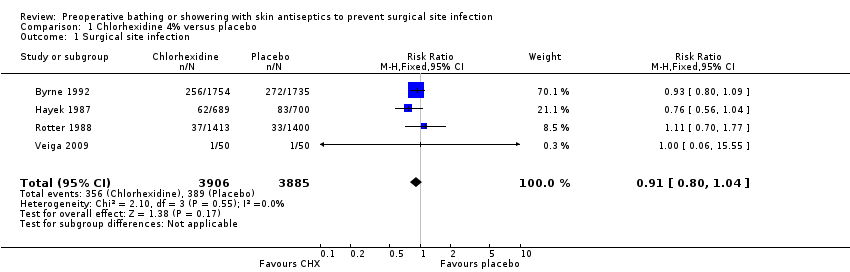
| 1 Surgical site infection Show forest plot | 4 | 7791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| Analysis 1.1  Comparison 1 Chlorhexidine 4% versus placebo, Outcome 1 Surgical site infection. | ||||
| 2 Surgical site infection (high quality studies) Show forest plot | 2 | 6302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| Analysis 1.2  Comparison 1 Chlorhexidine 4% versus placebo, Outcome 2 Surgical site infection (high quality studies). | ||||
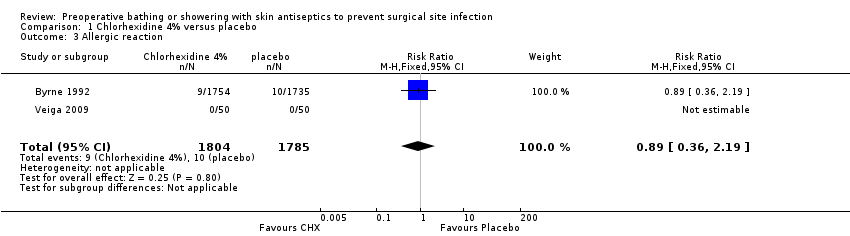
| 3 Allergic reaction Show forest plot | 2 | 3589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.36, 2.19] |
| Analysis 1.3  Comparison 1 Chlorhexidine 4% versus placebo, Outcome 3 Allergic reaction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 3 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
| Analysis 2.1  Comparison 2 Chlorhexidine 4% versus bar soap, Outcome 1 Surgical site infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.26, 2.62] |
| Analysis 3.1  Comparison 3 Chlorhexadine 4% versus no wash, Outcome 1 Surgical site infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | 1093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.85] |
| Analysis 4.1  Comparison 4 Chlorhexidine full wash versus partial wash, Outcome 1 Surgical site infection. | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
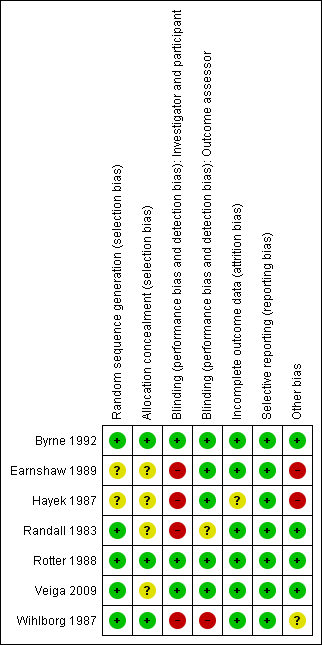
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Comparison 1 Chlorhexidine 4% versus placebo, Outcome 1 Surgical site infection.

Comparison 1 Chlorhexidine 4% versus placebo, Outcome 2 Surgical site infection (high quality studies).
Comparison 1 Chlorhexidine 4% versus placebo, Outcome 3 Allergic reaction.

Comparison 2 Chlorhexidine 4% versus bar soap, Outcome 1 Surgical site infection.

Comparison 3 Chlorhexadine 4% versus no wash, Outcome 1 Surgical site infection.
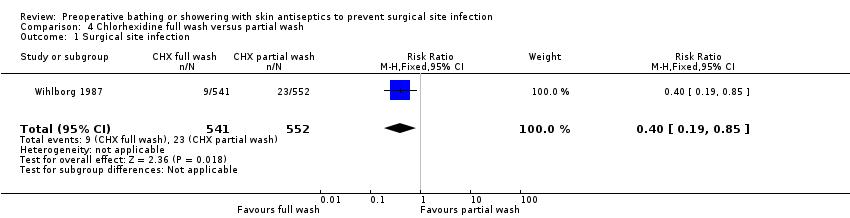
Comparison 4 Chlorhexidine full wash versus partial wash, Outcome 1 Surgical site infection.
| pre‐operative showering with Chlorhexidine 4% compared to placebo for surgical patients | ||||||
| Patient or population: surgical patients Settings: Hospitals Intervention: pre‐operative showering with Chlorhexidine 4% Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | pre‐operative showering with Chlorhexidine 4% | |||||
| Surgical site infection | Low risk population | RR 0.91 | 7791 | ⊕⊕⊕⊕ | ||
| 30 per 1000 | 27 per 1000 | |||||
| High risk population | ||||||
| 100 per 1000 | 91 per 1000 | |||||
| Allergic reaction | Study population | RR 0.89 | 3589 | ⊕⊕⊕⊝ | ||
| 6 per 1000 | 5 per 1000 | |||||
| Medium risk population | ||||||
| 3 per 1000 | 3 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some studies followed patients only until hospital discharge; however, as these studies are over 20 years old, we have assumed 7 days. | ||||||
| Chlorhexidine 4% compared to bar soap for Surgical patients | ||||||
| Patient or population: Surgical patients Settings: Intervention: Chlorhexidine 4% Comparison: bar soap | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| bar soap | Chlorhexidine 4% | |||||
| Surgical site infection | Study population | RR 1.02 | 1443 | ⊕⊝⊝⊝ | ||
| 136 per 1000 | 139 per 1000 | |||||
| Medium risk population | ||||||
| 128 per 1000 | 131 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The method of allocation was unclear in some studies and outcome assessment was not blinded. | ||||||
| Chlorhexadine 4% compared to no wash for surgical patients | ||||||
| Patient or population: surgical patients Settings: Hospital Intervention: Chlorhexadine 4% Comparison: no wash | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no wash | Chlorhexadine 4% | |||||
| Surgical site infection | Study population | RR 0.82 | 1142 | ⊕⊝⊝⊝ | ||
| 56 per 1000 | 46 per 1000 | |||||
| Medium risk population | ||||||
| 46 per 1000 | 38 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some studies followed patients only until hospital discharge; as the studies were over 20 years old, we have assumed this to be one week. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 4 | 7791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| 2 Surgical site infection (high quality studies) Show forest plot | 2 | 6302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 3 Allergic reaction Show forest plot | 2 | 3589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.36, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 3 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.26, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | 1093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.85] |

