Administración prenatal de progesterona para la prevención del parto prematuro en pacientes consideradas en riesgo de parto prematuro
Resumen
Antecedentes
El parto prematuro es una complicación grave del embarazo asociado con mortalidad y morbilidad perinatales. Se ha recomendado la administración de progesterona para la prevención del trabajo de parto prematuro.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de la progesterona para la prevención del parto prematuro en pacientes consideradas con mayor riesgo de parto prematuro y en los lactantes.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (14 de enero de 2013) y se revisaron las listas de referencias de todos los artículos.
Criterios de selección
Ensayos controlados aleatorios en los que se proporcionó progesterona para prevenir el parto prematuro.
Obtención y análisis de los datos
Dos revisores evaluaron de forma independiente los ensayos para determinar su calidad metodológica y extrajeron los datos.
Resultados principales
Se incluyeron 36 ensayos controlados aleatorios (8523 mujeres y 12 515 lactantes).
Progesterona versus placebo en pacientes con antecedentes de parto prematuro espontáneo
La progesterona se asoció con una reducción estadísticamente significativa del riesgo de mortalidad perinatal (seis estudios; 1453 mujeres; cociente de riesgos [CR] 0,50; intervalo de confianza [IC] del 95%: 0,33 a 0,75), parto prematuro antes de las 34 semanas (cinco estudios; 602 mujeres; CR promedio 0,31; IC del 95%: 0,14 a 0,69), peso al nacer del lactante menor de 2500 g (cuatro estudios; 692 lactantes; CR 0,58; IC del 95%: 0,42 a 0,79), uso de asistencia respiratoria (tres estudios; 633 mujeres; CR 0,40; IC del 95%: 0,18 a 0,90), enterocolitis necrosante (tres estudios; 1170 mujeres; CR 0,30; IC del 95%: 0,10 a 0,89), muerte neonatal (seis estudios; 1453 mujeres; CR 0,45; IC del 95%: 0,27 a 0,76), ingreso a la unidad de cuidados intensivos neonatales (tres estudios; 389 mujeres; CR 0,24; IC del 95%: 0,14 a 0,40), parto prematuro antes de las 37 semanas (diez estudios; 1750 mujeres; CR promedio 0,55; IC del 95%: 0,42 a 0,74) y un aumento estadísticamente significativo en la prolongación del embarazo en semanas (un estudio; 148 mujeres; diferencia de medias [DM] 4,47; IC del 95%: 2,15 a 6,79). En la mayoría de los resultados examinados, no se observaron efectos diferenciales en cuanto a la vía de administración, el momento de comienzo del tratamiento ni la dosis de progesterona.
Progesterona versus placebo en pacientes con un cuello uterino corto identificado mediante ecografía
En comparación con placebo, la progesterona se asoció con una reducción estadísticamente significativa del riesgo del parto prematuro antes de las 34 semanas (dos estudios; 438 mujeres; CR 0,64; IC del 95%: 0,45 a 0,90), parto prematuro antes de las 28 semanas de gestación (dos estudios; 1115 mujeres; CR 0,59; IC del 95%: 0,37 a 0,93) y un aumento del riesgo de urticaria en las pacientes (un estudio; 654 mujeres; CR 5,03; IC del 95%: 1,11 a 22,78). No fue posible evaluar el efecto de la vía de administración de la progesterona, la edad gestacional al comenzar el tratamiento ni la dosis acumulativa total de la medicación.
Progesterona versus placebo en pacientes con un embarazo múltiple
La progesterona no se asoció con diferencias estadísticamente significativas en los resultados informados.
Progesterona versus ningún tratamiento / placebo en pacientes después que presentaron amenaza de trabajo de parto prematuro
La progesterona se asoció con una reducción estadísticamente significativa en el riesgo del peso al nacer del lactante menor de 2500 g (un estudio; 70 lactantes; CR 0,52; IC del 95%: 0,28 a 0,98).
Progesterona versus placebo en pacientes con "otros" factores de riesgo de parto prematuro
La progesterona se asoció con una reducción estadísticamente significativa en el riesgo de peso al nacer del lactante inferior a 2500 g (tres estudios; 482 lactantes; CR 0,48; IC del 95%: 0,25 a 0,91).
Conclusiones de los autores
El uso de progesterona se asocia con efectos beneficiosos en la salud del lactante después de la administración a las pacientes consideradas con mayor riesgo de parto prematuro debido a un parto prematuro previo o si se ha identificado en el examen ecográfico que presentan un cuello uterino corto. Sin embargo, existe información limitada disponible en relación con los resultados del lactante a más largo plazo y en la niñez, por lo que su evaluación todavía es una prioridad.
Se necesitan ensayos adicionales para evaluar el momento adecuado, la forma y la dosis de administración del tratamiento con progesterona cuando se proporciona a pacientes consideradas con mayor riesgo de parto prematuro.
PICO
Resumen en términos sencillos
Administración prenatal de progesterona para prevenir el parto prematuro en pacientes consideradas en riesgo de tener un recién nacido temprano
Los recién nacidos antes de las 37 semanas, y en particular, los nacidos antes de las 34 semanas, se encuentran en mayor riesgo de presentar problemas al nacer y complicaciones en la infancia. Los lactantes que nacen prematuros tienen mayor riesgo de muerte en el primer año de vida, y los que sobreviven tienen un mayor riesgo de ingreso hospitalario repetido y resultados adversos que incluyen parálisis cerebral y discapacidad a largo plazo. La progesterona es una hormona que reduce las contracciones del útero, tiene una función importante para mantener el embarazo y se indica para la prevención del trabajo de parto prematuro. Los efectos secundarios maternos del tratamiento con progesterona incluyen cefalea, sensibilidad mamaria, náuseas, tos e irritación local si se administra por vía intramuscular. Actualmente, es escasa la información disponible sobre la dosis adecuada de progesterona, la forma de administración, la edad gestacional para comenzar el tratamiento o la duración del mismo.
La revisión de 36 ensayos controlados aleatorios con 8523 mujeres consideradas con mayor riesgo de parto prematuro y 12 515 lactantes encontró que cuando se proporcionó progesterona (mediante inyección en el músculo en algunos estudios y como un pesario en la vagina en otros), hubo efectos beneficiosos que incluyeron la reducción en el riesgo de muerte del lactante después del parto, presentar complicaciones como necesitar asistencia respiratoria, enterocolitis necrosante o necesitar el ingreso a cuidados intensivos neonatales, se prolongó el embarazo y disminuyeron las probabilidades de ingreso en cuidados intensivos neonatales.
La información relacionada con los resultados del lactante a más largo plazo y en la niñez fue limitada. En general, los ensayos incluidos en esta revisión se consideraron de calidad buena a aceptable. Se necesitan más ensayos para evaluar el momento adecuado, la vía y la dosis de administración de la progesterona.
Authors' conclusions
Background
Description of the condition
Preterm birth before 37 weeks' gestation is a common problem in obstetric care, with estimates ranging from 5% in several European countries to 18% in some African countries (Blencowe 2012). In Australia, approximately 8% of all infants were born preterm in 2000, with 2.7% of these births occurring prior to 34 weeks' gestation (AIHW 2003). Figures are similar for the United States, with a preterm birth rate of 12.0% (Blencowe 2012). While less than 2% of these infants were born prior to 32 weeks' gestation (Martin 2003), they are at increased risk of complications in infancy, and contribute in excess of 50% of the overall perinatal mortality (AIHW 2003). Infants who are born preterm are at greater risk of dying in their first year of life (Martin 2003), and of those infants who survive, there is an increased risk of repeated admission to hospital (Elder 1999) and adverse outcomes including cerebral palsy and long‐term disability (Hack 1999; Stanley 1992), creating a significant burden upon the community (Kramer 2000).
The 'cause' of preterm labour is multifactorial in origin, and it is important to consider the role of any identifiable risk factors in a woman's pregnancy.
The most significant and consistently identified risk factor for preterm birth, is a woman's history of previous preterm birth (Adams 2000; Bakketeig 1979; Berkowitz 1993; Bloom 2001; Goldenberg 1998; Kaminski 1973; Kistka 2007; Papiernik 1974; Petrini 2005; Robinson 2001). Estimates suggest the rate of recurrent preterm birth in this group of women to be 22.5% (Petrini 2005), a 2.5 times increased risk ratio when compared with women with no previous spontaneous preterm birth (Mercer 1999). For women with a history of a single preterm birth, the recurrence risk in a subsequent pregnancy is approximately 15%, increasing to 32% where there have been two previous preterm births (Carr‐Hill 1985). Information derived from population‐based cohort data suggests that for women who give birth between 20 and 31 weeks' gestation in one pregnancy, 29.3% will give birth prior to 37 weeks in a subsequent pregnancy (Adams 2000). For approximately 10% of these women, the preterm birth will occur at a similar gestational age (Adams 2000; Kistka 2007). In up to 50% of cases of preterm birth, the cause is spontaneous onset of labour or preterm premature rupture of membranes (PPROM) (Hewitt 1988; Mattison 2001; McLaughlin 2002).
Other characteristics in a woman's current pregnancy may place her at increased risk of preterm birth, including women with a short cervix identified by ultrasound assessment, the presence of fetal fibronectin in the vaginal secretions, and presentation with symptoms or signs of threatened preterm labour.
The identification of a short cervix (considered to be less than 2.5cm) on ultrasound examination has been associated with an increased likelihood of preterm birth before 34 weeks’ gestation (Smith 2007). Identification of fetal fibronectin present in cervico‐vaginal secretions has also been proposed as a means of identifying women at risk of preterm birth. Overall, the value of fetal fibronectin in women presenting with symptoms of threatened preterm labour, is a negative test, where women are unlikely to proceed to preterm birth before 34 weeks’ gestation or within seven days of testing (Smith 2007).
Multiple pregnancy is a strong risk factor for preterm birth though the mechanisms may be different to those operating in women with a singleton pregnancy. Up to 50% of women with a twin pregnancy will give birth prior to 37 weeks' gestation (AIHW 2003). The preterm birth risk of early birth before 37 weeks for women with a singleton pregnancy is 6.3% compared with 97% for women with a triplet pregnancy (AIHW 2003).
Description of the intervention
Progesterone may be administered in various forms and by various routes. These different formulations and modes of administration will have different absorption patterns and potentially have differing bio effects. Whilst no teratogenic effects have been described with most progesterones, there is little in the way of long‐term safety data. Maternal side‐effects from progesterone therapy include headache, breast tenderness, nausea, cough and local irritation if administered intramuscularly. At present, there is little information available regarding the optimal dose of progesterone, mode of administration, gestation to commence therapy, or duration of therapy (Greene 2003; Iams 2003).
How the intervention might work
Progesterone has a role in maintaining pregnancy (Haluska 1997; Pepe 1995; Pieber 2001) and is thought to act by suppressing smooth muscle activity in the uterus (Astle 2003; Grazzini 1998). In many animal species, there is a reduction in the amount of circulating progesterone before the onset of labour. While these changes have not been shown to occur in women (Astle 2003; Block 1984; Lopez‐Bernal 2003; Pieber 2001; Smit 1984), it has been suggested that there is a 'functional' withdrawal of progesterone related to changes in the expression of progesterone receptors in the uterus (Astle 2003; Condon 2003; Haluska 2002; Pieber 2001). There have been recent reports in the literature advocating the use of progesterone to reduce the risk of preterm birth (da Fonseca 2003; Meis 2003), rekindling interest that dates back to the 1960s (Le Vine 1964).
This review was modified in 2006, from the original protocol published in The Cochrane Library in Issue 4, 2004, in order to clarify the scope of the review. The title and objectives changed, and the description of participants expanded to include the reason the women were considered to be at increased risk of preterm birth. The primary outcome measure of preterm birth less than 32 weeks' gestation has been changed to preterm birth less than 34 weeks' gestation to be consistent with World Health Organization definitions of preterm birth. Secondary outcome measures reflecting childhood developmental assessment have been added, reflecting the need for ongoing evaluation of children participating in randomised trials.
Why it is important to do this review
Preterm birth and its consequences for women and their babies is a significant health problem in pregnancy and childbirth. While the suppression or prevention of preterm labour should lead to improved survival through a lower incidence of premature delivery, there are theoretical reasons why a fetus may not survive without disability. It is possible that an intrauterine mechanism that would trigger preterm labour could also cause neurological injury to the fetus and that progesterone may prevent labour but not fetal injury. The purpose of this review is to assess the benefits and harms of progesterone administration for the prevention of preterm birth for both women and their infants, when considering the risk factors present for preterm birth.
Objectives
To assess the benefits and harms of progesterone administration for the prevention of preterm birth in women and their infants.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials, in which progesterone was administered for the prevention of preterm birth, subdivided by the reason women were considered to be at risk for preterm birth.
Trials were excluded if:
-
they utilised quasi‐randomised methodology; cross‐over design;
-
progesterone was administered for the acute treatment of actual or threatened preterm labour (that is, where progesterone was administered as an acute tocolytic medication); or
-
progesterone was administered in the first trimester only for preventing miscarriage.
Types of participants
Pregnant women considered to be at increased risk of preterm birth. These reasons include:
-
past history of spontaneous preterm birth (including preterm prelabour rupture of membranes);
-
multiple pregnancy;
-
ultrasound identified short cervical length;
-
fetal fibronectin testing;
-
following acute presentation with symptoms or signs of threatened preterm labour (where a tocolytic medication may have been administered);
-
other reason considered to be at increased risk of preterm birth.
Types of interventions
Administration of progesterone by any route for the prevention of preterm birth.
Types of outcome measures
Primary outcomes
-
Perinatal mortality
-
Preterm birth (less than 34 weeks' gestation)
-
Major neurodevelopmental handicap at childhood follow‐up
Secondary outcomes
Maternal
-
Threatened preterm labour (as defined by trial authors)
-
Prelabour spontaneous rupture of membranes
-
Adverse drug reaction
-
Pregnancy prolongation (interval between randomisation and birth)
-
Mode of birth
-
Number of antenatal hospital admissions
-
Satisfaction with the therapy
-
Use of tocolysis
-
Antenatal corticosteroids (not a prespecified outcome)
-
Maternal quality of life (not a prespecified outcome)
Infant
-
Birth before 37 completed weeks
-
Birth before 28 completed weeks
-
Birthweight less than the third centile for gestational age
-
Birthweight less than 2500 g
-
Apgar score of less than seven at five minutes
-
Respiratory distress syndrome
-
Use of mechanical ventilation
-
Duration of mechanical ventilation
-
Intraventricular haemorrhage ‐ grades III or IV
-
Periventricular leucomalacia
-
Retinopathy of prematurity
-
Retinopathy of prematurity ‐ grades III or IV
-
Chronic lung disease
-
Necrotising enterocolitis
-
Neonatal sepsis
-
Fetal death
-
Neonatal death
-
Admission to neonatal intensive care unit
-
Neonatal length of hospital stay
-
Teratogenic effects (including virilisation in female infants)
-
Patent ductus arteriosis (not a prespecified outcome)
Child
-
Major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than ‐2 standard deviations below mean))
-
Developmental delay (however defined by the authors)
-
Intellectual impairment
-
Motor impairment
-
Visual impairment
-
Blindness
-
Deafness
-
Hearing impairment
-
Cerebral palsy
-
Child behaviour
-
Child temperament
-
Learning difficulties
-
Growth assessments at childhood follow‐up (weight, head circumference, length, skin fold thickness)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (14 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of searching carried out for the initial version of the review, please see Appendix 1.
Searching other resources
We also manually cross‐referenced key publications.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Appendix 2.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted third author. We entered data into Review Manager software (RevMan 2012) and checked for accuracy. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was resolved by discussion or by involving the third author.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; or less than 20% losses to follow‐up);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There are several ways this can be done, and these studies should present something like an odds ratio adjusted for non‐independence. If adjustment was not done, we assessed the potential for bias i.e. if multiples only made up a small proportion of the total then there is probably not much potential for bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods, if required.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include trials of this type in future updates. If we do, we plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not included.
Other unit of analysis issues
The analysis in this review involves multiple pregnancies, therefore, wherever possible, analyses should be adjusted for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004). Treating babies from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than babies from different pregnancies, will overestimate the sample size and give confidence intervals that are too narrow. Each woman can be considered a cluster in multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of relative risk and adjustment of confidence intervals. Usually this will mean that the confidence intervals get wider. Although this may make little difference to the conclusion of a trial, it avoids misleading results in those trials where the difference may be substantial.
We planned to adjust for clustering in the analyses, wherever possible, and to use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, due to insufficient information in the included trials, we were not able to adjust our analyses. In future updates, if possible, we will adjust for clustering in the analyses.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If 10 or more studies contributed data to meta‐analysis for any particular outcome, we investigated reporting biases (such as publication bias) using funnel plots. We assessed possible asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it. In this version of the review insufficient data were available to allow us to carry out this planned analysis.
Data synthesis
We carried out statistical analysis using the RevMan software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and where we judged the trials’ populations and methods to be sufficiently similar. If we suspected clinical heterogeneity sufficient to expect the underlying treatment effects to differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary provided that we considered an average treatment effect across trials was clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and the clinical implications of treatment effects differing between trials is discussed. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Results were analysed according to the reason women were considered to be at risk of preterm birth, including:
-
past history of spontaneous preterm birth (including preterm prelabour rupture of membranes);
-
multiple pregnancy;
-
ultrasound identified short cervical length;
-
fetal fibronectin testing;
-
presentation with symptoms or signs of threatened preterm labour;
-
other reason for risk of preterm birth.
For analyses where there are high levels of heterogeneity we have provided an estimate of the 95% range of underlying intervention effects (prediction interval).
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We planned, where possible, to carry out the following subgroup analyses:
-
time of treatment commencing (before 20 weeks' gestation versus after 20 weeks' gestation);
-
route of administration (intramuscular, intravaginal, oral, intravenous);
-
different dosage regimens (divided arbitrarily into a cumulative dose of less than 500 mg per week and a dose of greater than or equal to 500 mg per week).
All outcomes were considered in subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012).
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
In the 2006 update, our search strategy identified 22 studies for consideration. Eleven studies met the inclusion criteria stated (Borna 2008; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Hartikainen 1980; Hauth 1983; Johnson 1975; Meis 2003; O'Brien 2007; Papiernik 1970; Rouse 2007) involving a total of 2714 women and 3452 infants. The study by Northern (Northen 2007) reports the follow‐up of children involved in the Meis study (Meis 2003).
Sixty‐four reports from an updated search in January 2013 have been assessed for this update. Of these 64 reports, an additional 25 studies (33 reports) to the original 11 studies, were included (Aboulghar 2012; Akbari 2009; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; Elsheikhah 2010; Glover 2011; Grobman 2012; Hassan 2011; Ibrahim 2010; Lim 2011; Majhi 2009; Moghtadaei 2008; Ndoni 2010; Norman 2009; Rai 2009; Rode 2011; Rozenberg 2012; Saghafi 2011a; Senat 2012; Serra 2013; Sharami 2010); seven studies (seven reports) were excluded (Abbott 2012; Arikan 2011; Berghella 2010; Chandiramani 2012; Ionescu 2012; Keeler 2009; Rust 2006); two studies (two reports) are ongoing studies (Coomarasamy 2012; van Os 2011); one is an additional report of an ongoing study (Crowther 2007); one additional report was added to each of the following included studies (Combs 2010; Combs 2011; Facchinetti 2007; Lim 2011; Nassar 2007). Two additional reports were identified for the Norman 2009; O'Brien 2007; Rode 2011 and Rouse 2007 included studies. Three additional reports were identified for the Meis 2003 included study.
A total of 36 studies are included in this update.
Included studies
Refer to table Characteristics of included studies for further details.
Use of progesterone in women with a history of prior spontaneous preterm birth
Description of studies
Eleven studies were included involving a total of 1936 women with a past history of spontaneous preterm birth (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Glover 2011; Johnson 1975; Ibrahim 2010; Majhi 2009; Meis 2003; O'Brien 2007; Rai 2009; Saghafi 2011a). Four studies compared weekly intramuscular injection with placebo (Ibrahim 2010; Johnson 1975; Meis 2003) or routine care (Saghafi 2011a); five studies compared daily vaginal progesterone, three with placebo (Cetingoz 2011; da Fonseca 2003; O'Brien 2007) and two with routine care (Akbari 2009; Majhi 2009); and two studies compared daily oral progesterone with placebo (Glover 2011; Rai 2009). Dose of progesterone administered varied from 90 mg daily (O'Brien 2007), to 100 mg daily (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Majhi 2009), to 200 mg daily (Rai 2009), to 400 mg daily (Glover 2011), to 200 mg weekly (Rai 2009), to 250 mg weekly (Johnson 1975; Meis 2003; Saghafi 2011a). Supplementation commenced prior to 20 weeks' gestation in four trials (Glover 2011; Johnson 1975; Meis 2003; O'Brien 2007), and continued up to a gestational age varying from 24 weeks (Johnson 1975; Majhi 2009; Rai 2009), to 28 weeks (da Fonseca 2003), to 34 weeks (Akbari 2009; Cetingoz 2011), to 36 weeks (Ibrahim 2010; Meis 2003), and to 37 weeks (O'Brien 2007; Saghafi 2011a) gestation.
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 28 weeks' gestation (Rai 2009), 32 weeks' gestation (O'Brien 2007), 34 weeks' gestation (Akbari 2009; Majhi 2009), and 37 weeks' gestation (Akbari 2009; Cetingoz 2011; da Fonseca 2003; Ibrahim 2010; Johnson 1975; Majhi 2009; Meis 2003; Saghafi 2011a). Eight trials involved single centres (Akbari 2009; da Fonseca 2003; Glover 2011; Ibrahim 2010; Johnson 1975; Majhi 2009; Rai 2009; Saghafi 2011a), and two were multicentre trials (Meis 2003; O'Brien 2007), conducted principally from the United States of America (Glover 2011; Johnson 1975; Meis 2003; O'Brien 2007), India (Majhi 2009; Rai 2009), Iran (Akbari 2009; Saghafi 2011a), Egypt (Ibrahim 2010), Istanbul (Cetingoz 2011), and Brazil (da Fonseca 2003). The report by Northen 2007 reports childhood follow‐up of 348 participants in the Meis randomised trial (Meis 2003).
One study (Cetingoz 2011) included a mix of women with a history of prior preterm birth (n = 71) and women with a multiple pregnancy (n = 67) and the results for this study have been analysed separately for the two risk groups.
Use of progesterone in women with a short cervix identified on transvaginal ultrasound examination
Description of studies
Four studies were included involving 1560 women who were identified with a short cervix (various definitions: less than 15 mm (Fonseca 2007); less than 30 mm (Grobman 2012); between 10 and 20mm (Hassan 2011); and less than 25 mm (Rozenberg 2012)) at the time of transvaginal ultrasound examination. One study compared weekly intramuscular injection with placebo (Grobman 2012); one study compared twice weekly intramuscular injection with no treatment (Rozenberg 2012) and two studies compared daily intravaginal progesterone with placebo (Fonseca 2007; Hassan 2011). Dose of progesterone administered varied from 90 mg daily in the morning (Hassan 2011), to 200 mg nightly (Fonseca 2007), to 250 mg weekly (Grobman 2012), to 500 mg twice weekly (Rozenberg 2012). Supplementation commenced from 16 to 22 weeks' gestation in one study (Grobman 2012), from 19 to 23 weeks in another study (Hassan 2011), from 24 to 31 weeks in another study (Rozenberg 2012), and from 24 to 33 completed weeks of gestation in another study (Fonseca 2007).
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 33 weeks' gestation (Hassan 2011), 34 weeks' gestation (Fonseca 2007), 35 weeks' gestation (Grobman 2012), or 37 weeks' gestation (Grobman 2012) and time from randomisation to delivery in one study (Rozenberg 2012). All trials were multicentre conducted in centres worldwide, including the United Kingdom, USA, France, Greece, Chile and Brazil.
One study (Fonseca 2007) included a mix of singleton and twin pregnancies (226 singleton and 24 twin pregnancies), but due to the small proportion of twin pregnancies in this study, we have analysed all of this data within the short cervix subgroup.
Use of progesterone in women with a multiple pregnancy
Description of studies
Fourteen studies were included involving 3792 women; 11 trials with a twin pregnancy (Aboulghar 2012; Cetingoz 2011; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013), two trials with a triplet pregnancy (Caritis 2009; Combs 2010) or one trial with any multiple pregnancy, e.g. twins, triplets or quadruplets (Lim 2011). Six studies compared 250 mg weekly intramuscular progesterone injections with placebo (Caritis 2009; Combs 2010; Combs 2011; Hartikainen 1980; Lim 2011; Rouse 2007); one study compared 1000 mg weekly intramuscular progesterone injections with no treatment (Senat 2012); three studies compared intravaginal progesterone with placebo (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010), one at a daily dose of 100 mg (Cetingoz 2011), one at a daily dose of 200 mg (Elsheikhah 2010) and one at a daily dose of 400 mg Aboulghar 2012); one study compared 90 mg daily intravaginal gel with placebo (Norman 2009); and one study compared 100 mg daily oral progesterone with placebo (Rode 2011). One trial (Serra 2013) consisted of three groups and compared 200 mg daily intravaginal progesterone with 400 mg daily intravaginal progesterone with placebo. Supplementation commenced from 16 to 20 weeks' gestation in three studies (Caritis 2009; Lim 2011; Rouse 2007), from 16 to 22 weeks in one study (Combs 2010), from 16 to 24 weeks in one study (Combs 2011), from 18 to 24 weeks in one study (Aboulghar 2012; Rode 2011), from 24 to 34 weeks in two studies (Cetingoz 2011; Elsheikhah 2010), from 24 weeks' gestation in one study (Norman 2009), and from 28 completed weeks' of gestation in one study (Hartikainen 1980).
The primary outcomes reported by the trials related to the occurrence of preterm birth prior to 34 weeks' gestation (Aboulghar 2012; Cetingoz 2011; Rode 2011; Senat 2012) or 37 weeks' gestation (Aboulghar 2012; Cetingoz 2011; Serra 2013), delivery or fetal loss before 34 weeks' gestation (Norman 2009), delivery or fetal loss before 35 weeks' gestation (Caritis 2009), mean cervical length and mean gestational age at delivery (Elsheikhah 2010), perinatal death (Hartikainen 1980) or composite neonatal morbidity (Combs 2010; Combs 2011; Lim 2011; Senat 2012). Five trials were multicentre conducted in centres worldwide, including the United Kingdom, USA, the Netherlands, Denmark, Austria and France (Caritis 2009; Combs 2010; Combs 2011; Lim 2011; Norman 2009; Rode 2011; Rouse 2007) and four were single‐centre trials conducted in Istanbul, Egypt and Finland (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980).
Two studies included a mix of women with multiple and singleton pregnancies (Aboulghar 2012; Cetingoz 2011). One study (Aboulghar 2012) included a mix of women with singleton pregnancies (n = 215) and women with a multiple pregnancy (n = 91) all conceived by IVF/ICSI (in vitro fertilisation/intracytoplasmic sperm injection) and the results for this study have been analysed separately for the two risk groups: women at risk of preterm birth for 'other' reasons; and women with a multiple pregnancy. One study (Cetingoz 2011) included a mix of women with a history of prior preterm birth (n = 71) and women with a multiple pregnancy (n = 67) and the results for this study have been analysed separately for the two risk groups.
Use of progesterone in women following symptoms or signs of threatened preterm labour
Description of studies
Six small studies were included involving a total of 505 women presenting with symptoms or signs of threatened preterm labour (Borna 2008; Briery 2011; Combs 2011a; Facchinetti 2007; Ndoni 2010; Sharami 2010). Two studies compared 250 mg weekly progesterone injections with placebo (Briery 2011; Combs 2011a), one study compared vaginal progesterone pessaries on a daily basis (400 mg) with no treatment (Borna 2008), one study compared 341 mg intramuscular progesterone injection every four days with no treatment (Facchinetti 2007), one study had three arms and compared intramuscular progesterone with oral progesterone with placebo (Ndoni 2010) and one study compared vaginal pessaries on a daily basis (200 mg) with placebo pessaries (Sharami 2010). Women presented with symptoms and signs between 24 and 34 weeks' gestation (Borna 2008), between 25 and 33 weeks' gestation (Facchinetti 2007), between 20 and 30 weeks' gestation (Briery 2011), between 23 and 31.9 weeks' gestation (Combs 2011a), between 15 and 22 weeks' gestation (Ndoni 2010) and between 28 and 36 weeks' gestation (Sharami 2010). The primary outcomes reported included the interval from randomisation to birth in one study (Borna 2008), transvaginal ultrasound assessment of cervical length in one study (Facchinetti 2007), gestational age at birth in one study (Briery 2011), prolongation of pregnancy and composite neonatal morbidity in one study (Combs 2011a) and time until delivery and birth before 34 or 37 completed weeks in one study (Sharami 2010). In one study, reported only in abstract form, data relating to outcomes was not reported (Ndoni 2010). One study was a multicentre study conducted in the USA (Combs 2011a) and the remaining five studies were single‐centre studies conducted in Iran, the USA, Italy, and Albania (Borna 2008; Briery 2011; Facchinetti 2007; Ndoni 2010; Sharami 2010).
Use of progesterone in women at risk of preterm birth for 'other' reasons
Description of studies
Papiernik 1970 recruited 99 women from Paris, France, in a single centred trial, with a 'high preterm risk score'. Women were allocated to receive intramuscular progesterone three times per week or placebo, from 28 to 32 weeks' gestation.
Hauth 1983 involved 168 women from the United States of America who were considered to be at risk of preterm birth due to active military service. Women received 1000 mg of progesterone weekly or placebo, from 16 to 20 weeks' gestation, up until 36 weeks' gestation. The primary outcome for the study related to the incidence of preterm birth at less than 37 weeks' gestation.
Moghtadaei 2008 involved 260 women from Iran, in a single centre trial, who were considered to be at risk of preterm birth due to advanced maternal age (greater than 35 years). Women received weekly injections of 17P (250 mg) starting at 16 to 20 weeks' gestation until 34 weeks or matching placebo. The main outcomes for the study included delivery before 37, 35 or 32 weeks' gestation, hypertension, diabetes, intrauterine growth restriction or side effects at the injection site. Data from this study could not be included in a meta‐analysis because the number of women randomised to each group was not reported in the brief abstract report of the study.
Aboulghar 2012 recruited 313 women from Egypt who were considered to be at high risk of preterm birth because all the pregnancies were conceived by IVF or ICSI. Women received vaginal progesterone 200 mg twice daily from randomisation until delivery or 37 weeks’ gestation or matching placebo. The primary outcomes included preterm birth of singleton and twin pregnancies before 37 completed weeks and before 34 completed weeks. This study contains a mix of singleton pregnancies (n = 215) and twin pregnancies (n = 91) and presented some outcome data separately, as well as for the whole group. The results for this study have been analysed separately for the two risk groups: multiple pregnancies and women at risk for 'other' reasons.
Excluded studies
In total, 16 studies were excluded (Abbott 2012; Arikan 2011; Berghella 2010; Breart 1979; Brenner 1962; Chandiramani 2012; Corrado 2002; Hobel 1986; Ionescu 2012; Keeler 2009; Le Vine 1964; Rust 2006; Suvonnakote 1986; Turner 1966; Walch 2005; Yemini 1985). Three studies were excluded as they used a quasi‐randomised method of treatment allocation (Le Vine 1964; Suvonnakote 1986; Yemini 1985). One study (Hobel 1986) compared an oral progestogen with placebo, but presented outcomes only as percentages. Five studies were excluded as progesterone was administered in the first trimester to prevent miscarriage (Breart 1979; Brenner 1962; Corrado 2002; Turner 1966; Walch 2005), and are covered by the Cochrane review relating to the use of progesterone for prevention of miscarriage (Haas 2008). One study was excluded because progesterone was administered as an acute tocolytic medication (Arikan 2011). A further six studies were excluded because they compared progesterone with cerclage (Abbott 2012; Chandiramani 2012; Ionescu 2012; Keeler 2009; Rust 2006) or compared cerclage with no cerclage (Berghella 2010), and are covered by other Cochrane reviews (Alfirevic 2012; Rafael 2011).
Refer to table Characteristics of excluded studies for further details.
Studies awaiting assessment
There are 11 ongoing studies awaiting assessment (Coomarasamy 2012; Creasy 2008; Crowther 2007; Martinez 2007; Nassar 2007; Norman 2012; Perlitz 2007; Starkey 2008; Swaby 2007; van Os 2011; Wood 2007).
Risk of bias in included studies
The overall quality of the included trials varied from good to fair. Refer to table Characteristics of included studies for further details and to Figure 1; Figure 2, for a summary of 'Risk of bias' assessments.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
While all trials were stated to be randomised and placebo controlled, the method of randomisation was only described in 23 trials (Borna 2008; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Facchinetti 2007; Glover 2011; Grobman 2012; Hassan 2011; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013). Allocation concealment was assessed as low risk of bias in 23 trials (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013); and unclear in 13 trials (Akbari 2009; Borna 2008; Elsheikhah 2010; Facchinetti 2007; Hartikainen 1980; Hauth 1983; Ibrahim 2010; Moghtadaei 2008; Ndoni 2010; Papiernik 1970; Rozenberg 2012; Saghafi 2011a; Sharami 2010).
Blinding
Twenty‐five of the 32 included trials were placebo controlled, with blinding of caregivers and participants (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Rouse 2007; Serra 2013; Sharami 2010).
Blinding of outcome assessment was evident in 15 of the trials (Aboulghar 2012; Combs 2010; da Fonseca 2003; Fonseca 2007; Grobman 2012; Hartikainen 1980; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; O'Brien 2007; Papiernik 1970; Rode 2011; Rouse 2007; Serra 2013).
Four trials were assessed as high risk of bias for both blinding of caregivers and participants and outcome assessment as no blinding was attempted (Borna 2008; Facchinetti 2007; Rozenberg 2012; Senat 2012).
Incomplete outcome data
Thirty‐one studies were assessed as being at low risk of bias for attrition bias. Thirteen studies reported no losses to follow‐up (Borna 2008; Caritis 2009; Combs 2010; Combs 2011a; Facchinetti 2007; Fonseca 2007; Hartikainen 1980; Hauth 1983; Ibrahim 2010; Majhi 2009; Meis 2003; Papiernik 1970; Saghafi 2011a) and 18 studies reported less than 20% loss to follow‐up (Aboulghar 2012; Briery 2011; Cetingoz 2011; Combs 2011; da Fonseca 2003; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Serra 2013; Sharami 2010). In five studies reported only in abstract form, it was unclear whether attrition bias was present (Elsheikhah 2010; Grobman 2012; Moghtadaei 2008; Ndoni 2010; Senat 2012) and in one study details were insufficient to make a judgement (Akbari 2009).
Selective reporting
Twenty‐five studies were assessed as being at low risk of bias for selective reporting (Aboulghar 2012; Akbari 2009; Borna 2008; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Ibrahim 2010; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012) as all expected outcomes were reported. One study was assessed as being at high risk of bias, because one of the outcomes was incompletely reported on (Facchinetti 2007) and in one study it was difficult to assess selective reporting based on a translation of the original report (Papiernik 1970). In all the other study reports, it was not possible to determine whether or not selection bias was present (Briery 2011; Elsheikhah 2010; Glover 2011; Moghtadaei 2008; Ndoni 2010; Rozenberg 2012; Saghafi 2011a; Senat 2012; Serra 2013; Sharami 2010).
Other potential sources of bias
Twenty‐one studies were assessed as being at low risk of bias for other potential sources of bias based on baseline characteristics being similar between groups and no other bias apparent (Borna 2008; Briery 2011; Caritis 2009; Combs 2010; Combs 2011; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Glover 2011; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Saghafi 2011a; Sharami 2010). In the remaining studies, it was not possible to determine whether or not other sources of bias were present (Aboulghar 2012; Akbari 2009; Cetingoz 2011; Combs 2011a; Elsheikhah 2010; Grobman 2012; Hartikainen 1980; Ibrahim 2010; Majhi 2009; Moghtadaei 2008; Ndoni 2010; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013).
Assessment of studies that included multiple pregnancies
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There were 14 studies that included a multiple pregnancy (Aboulghar 2012; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013) and in seven studies adjustment appears to have been made in the analysis (Caritis 2009; Combs 2010; Combs 2011; Fonseca 2007; Lim 2011; Norman 2009; Rode 2011). In the remaining seven studies (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980; Rouse 2007; Senat 2012; Serra 2013), it is not clear that any adjustment was made.
There were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way.
Effects of interventions
Thirty‐six randomised controlled trials (8523 women and 12,515 infants) in total were included in this review.
Data were only available in a suitable format from 30 randomised controlled trials involving a total of 7561 women and 10,114 infants. Data from these 30 trials contributed data that were included in meta‐analyses. As the aetiology of preterm birth is multifactorial, results are presented according to the reason considered to be at risk for preterm birth (past history of spontaneous preterm birth (including preterm premature rupture of membranes), ultrasound identified short cervical length, multiple pregnancy, prior presentation with threatened preterm labour, and other reason for risk of preterm birth).
Progesterone versus placebo/no treatment for women with a past history of spontaneous preterm birth
Eleven randomised controlled trials involving a total of 1899 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, there was a statistically significant reduction in perinatal mortality overall (six studies; 1453 women; risk ratio (RR) 0.50, 95% confidence interval (CI) 0.33 to 0.75), Analysis 1.1. For the primary outcome preterm birth less than 34 weeks' gestation, there was also a statistically significant difference between progesterone when compared with placebo (five studies; 602 women; average RR 0.31, 95% CI 0.14 to 0.69), Analysis 1.2. Substantial heterogeneity was evident for Analysis 1.2 (heterogeneity: Tau² = 0.45, Chi² = 9.15, df = 4 (P = 0.06), I² = 56%) and so a random‐effects model was used. Major neurodevelopmental handicap in childhood was not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, the results showed:
-
preterm birth less than 37 weeks' gestation (10 studies; 1750 women; average RR 0.55, 95% CI 0.42 to 0.74); considerable heterogeneity was identified, and a random‐effects model was used (heterogeneity: Tau² = 0.11; Chi² = 29.60, df = 9 (P = 0.0005); I² = 70%), Analysis 1.3. This was also evident for the intramuscular subgroup (four studies; 652 women; average RR 0.62, 95% CI 0.52 to 0.75), Analysis 1.3.1. However, for the oral subgroup, no statistically significant differences were observed, Analysis 1.3.3. A funnel plot for this analysis (Figure 3), including the 10 studies was very asymmetrical. This suggests that there may be some important biases or small‐study effects in the set of studies in this analysis and so these results should be viewed with caution.
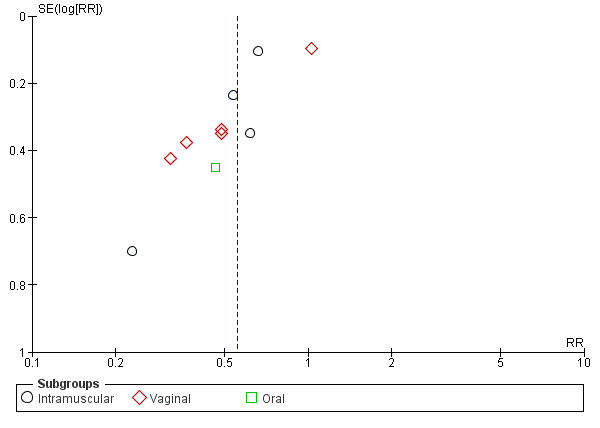
Funnel plot of comparison: 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, outcome: 1.3 Preterm birth less than 37 weeks.
There was also a statistically significant reduction in the risk of:
-
infant birthweight less than 2500 g (four studies; 692 infants; RR 0.58, 95% CI 0.42 to 0.79), Analysis 1.9;
-
use of assisted ventilation (three studies; 633 women; RR 0.40, 95% CI 0.18 to 0.90), Analysis 1.11;
-
necrotising enterocolitis (three studies; 1170 infants; RR 0.30, 95% CI 0.10 to 0.89), Analysis 1.16;
-
neonatal death (six studies; 1453 women; RR 0.45, 95% CI 0.27 to 0.76), Analysis 1.20;
-
admission to neonatal intensive care unit (three studies; 389 women; RR 0.24, 95% CI 0.14 to 0.40), Analysis 1.33.
For infant outcomes Apgar score less than seven at five minutes Analysis 1.32, respiratory distress syndrome Analysis 1.10, intrauterine fetal death Analysis 1.19, intraventricular haemorrhage (all grades) Analysis 1.12, intraventricular haemorrhage (grade III or IV) Analysis 1.13, periventricular leucomalacia Analysis 1.14, retinopathy of prematurity Analysis 1.15, neonatal sepsis Analysis 1.17, patent ductus arteriosus Analysis 1.18, intrauterine fetal death Analysis 1.19, or neonatal length of hospital stay Analysis 1.34, there were no statistically significant differences identified.
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant increase in:
-
pregnancy prolongation in weeks (one study; 148 women; mean difference (MD) 4.47, 95% CI 2.15 to 6.79), Analysis 1.31.
There were no statistically significant differences for the outcomes threatened preterm labour Analysis 1.4, spontaneous vaginal birth Analysis 1.5, adverse drug reaction Analysis 1.30, caesarean birth Analysis 1.6, use of antenatal corticosteroids Analysis 1.7, or the use of antenatal tocolysis Analysis 1.8.
Secondary childhood outcomes
There were no statistically significant differences identified for the outcomes developmental delay Analysis 1.21, intellectual impairment Analysis 1.22, motor impairment Analysis 1.23, visual impairment Analysis 1.24, hearing impairment Analysis 1.25, cerebral palsy Analysis 1.26, learning difficulties Analysis 1.27, height less than fifth centile Analysis 1.28 , weight less than the fifth centile Analysis 1.29, infant weight at six, 12 and 24 months' follow‐up Analysis 1.34; Analysis 1.35; Analysis 1.36, infant length (cm) at six, 12 and 24 months' follow‐up Analysis 1.38; Analysis 1.39; Analysis 1.40, and infant head circumference (cm) at six, 12 and 24 months' follow‐up Analysis 1.41; Analysis 1.42; Analysis 1.43.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on the majority of outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal versus oral). However, for respiratory distress syndrome, the subgroup analysis indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.001, I² = 84.8%, Analysis 1.10), although only one trial was included in each subgroup of intramuscular versus vaginal versus oral, Analysis 1.10.
We performed subgroup analysis to investigate the differential effect of time of commencement of supplementation (prior to 20 weeks' gestation versus after 20 weeks' gestation) where outcome data allowed, and found no subgroup differences (test for subgroup differences: P = 0.28, I² = 15.9%, Analysis 2.1).
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for the majority of outcomes examined: Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.9; Analysis 3.10; Analysis 3.11; Analysis 3.12. However, for intraventricular haemorrhage (all grades), the subgroup analysis indicated a differential effect between the different doses of progesterone (test for subgroup differences: P = 0.04, I² = 76.2%, Analysis 1.38), although only one trial was included in each subgroup of intramuscular versus vaginal versus oral, Analysis 1.10.
Progesterone versus placebo for women with a short cervix identified on ultrasound
Four randomised controlled trials involving a total of 1556 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcome perinatal death, there were no statistically significant differences identified when compared with placebo, Analysis 4.1. Women administered progesterone were significantly less likely to have a preterm birth at less than 34 weeks' gestation (two studies; 438 women; RR 0.64, 95% CI 0.45 to 0.90), Analysis 4.2. Major neurodevelopmental handicap in childhood was not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, for the outcome preterm birth at less than 37 weeks' gestation, there were no statistically significant differences identified when compared with placebo, Analysis 4.12. However, women administered progesterone were significantly less likely to have a preterm birth at less than 28 weeks' gestation (two studies; 1115 women; RR 0.59, 95% CI 0.37 to 0.93), Analysis 4.13.
For infant outcomes infant birthweight less than 2500 g Analysis 4.14, respiratory distress syndrome Analysis 4.15, Apgar score less than seven at five minutes Analysis 4.16, need for assisted ventilation Analysis 4.17, intraventricular haemorrhage (all grades) Analysis 4.18, intraventricular haemorrhage (grades III or IV) Analysis 4.19, periventricular leucomalacia Analysis 4.20, retinopathy of prematurity Analysis 4.21, necrotising enterocolitis Analysis 4.22, neonatal sepsis Analysis 4.23, intrauterine fetal death Analysis 4.24, neonatal death Analysis 4.25 or admission to neonatal intensive care unit Analysis 4.26, there were no statistically significant differences identified.
Secondary maternal outcomes
Women administered progesterone were significantly more likely to experience the adverse drug reaction urticaria (one study; 654 women; RR 5.03, 95% CI 1.11 to 22.78), Analysis 4.7. For all other maternal outcomes, threatened preterm labour Analysis 4.3, prelabour spontaneous rupture of membranes Analysis 4.4, adverse drug reactions (any, injection site, nausea) Analysis 4.5; Analysis 4.6; Analysis 4.8, pregnancy prolongation Analysis 4.9, caesarean section Analysis 4.10, or antenatal tocolysis Analysis 4.11, there were no statistically significant differences identified.
Secondary childhood outcomes
None of the secondary childhood outcomes were reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on the outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal), Analysis 4.20; Analysis 4.21; Analysis 4.23. It was not possible to assess the effect of gestational age at commencing therapy.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for the two outcomes examined: Analysis 5.1; Analysis 5.2.
Progesterone versus placebo for women with a multiple pregnancy
Ten randomised controlled trials involving a total of 3395 women and 6178 infants were included in the meta‐analysis.
One trial (Serra 2013) consisted of three groups: progesterone 200 mg versus progesterone 400 mg versus placebo. This trial has been analysed as separate pair‐wise comparisons, with separate analyses for progesterone 200 mg versus placebo (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.5; Analysis 6.9; Analysis 6.10; Analysis 6.11; Analysis 6.12; Analysis 6.13; Analysis 6.14; Analysis 6.21; Analysis 6.24; Analysis 6.25; Analysis 6.26) and progesterone 400 mg versus placebo (Analysis 6.27; Analysis 6.28; Analysis 6.29; Analysis 6.30; Analysis 6.31; Analysis 6.32; Analysis 6.33; Analysis 6.34; Analysis 6.35; Analysis 6.36; Analysis 6.37; Analysis 6.38; Analysis 6.39; Analysis 6.40; Analysis 6.41; Analysis 6.42; ; Analysis 7.1; Analysis 7.2; Analysis 7.3).
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 6.1, Analysis 6.27, and preterm birth less than 34 weeks' gestation Analysis 6.2; Analysis 6.28, there were no statistically significant differences identified when compared with placebo. Major neurodevelopmental handicap in childhood was not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences identified in the risk of birth before 37 Analysis 6.11; Analysis 6.33 or 28 weeks Analysis 6.12; Analysis 6.34, infant birthweight less than 2500 g Analysis 6.13; Analysis 6.35, Apgar score less than seven at five minutes Analysis 6.14; Analysis 6.36, respiratory distress syndrome Analysis 6.15, need for ventilation Analysis 6.16; Analysis 6.37, intraventricular haemorrhage Analysis 6.17; Analysis 6.18, periventricular leucomalacia Analysis 6.19, retinopathy of prematurity Analysis 6.20, chronic lung disease Analysis 6.21, necrotising enterocolitis Analysis 6.22, neonatal sepsis Analysis 6.23, fetal death Analysis 6.24; Analysis 6.38, neonatal death Analysis 6.25; Analysis 6.39, or admission to neonatal intensive care unit (NICU) Analysis 6.26; Analysis 6.40. Due to extreme heterogeneity, we did not combine data from trials for the outcomes neonatal length of hospital stay or patent ductus arteriosus.
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences identified in any of the following maternal outcomes: prelabour spontaneous rupture of membranes Analysis 6.3, adverse drug reaction Analysis 6.4, caesarean section Analysis 6.5, spontaneous birth Analysis 6.6, assisted birth Analysis 6.7, satisfaction with the therapy Analysis 6.8, antenatal tocolysis Analysis 6.9, or antenatal corticosteroids Analysis 6.10.
Secondary childhood outcomes
None of the secondary childhood outcomes were reported.
Sensitivity analyses to account for multiple pregnancies
For multiple pregnancies we had planned to analyse neonatal data as 'clusters' to account for dependency between twins and triplets. We anticipated that the degree of dependence between twins would vary at the outcome level i.e. in the case of preterm birth the dependency (ICC) is likely to be high, whereas in some outcomes, such as perinatal death or morbidity, the ICC may be much lower. However, there were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way.
For the primary outcome perinatal death, we therefore carried out a sensitivity analysis assuming two extremes. In the first we assumed complete dependence between infants in multiple pregnancies, i.e. we assumed outcomes were the same for all infants within that pregnancy; in this case the effective sample size for twin pregnancies would be the total number of women rather than infants, and for infant outcomes all event rates and sample sizes were therefore divided by two. In the second sensitivity analyses we assumed very limited dependence (1%) and in this case event rates and sample sizes were divided by 1.01. Results from sensitivity analyses were very similar to those from the unadjusted analyses although the effect estimates changed slightly due to rounding up of event rates and sample sizes, and the 95% CIs were generally slightly wider. Adjusted analyses showed that for women administered progesterone during pregnancy, for the primary outcome perinatal death, there were no statistically significant differences identified when compared with placebo. (Data not shown, available from the authors on request).
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses and found no differential effect on the majority of outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal). However, for spontaneous birth, infant birthweight less than 2500 g and admission to NICU, the subgroup analyses indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.0008, I² = 91.1%, Analysis 6.6; P = 0.02, I² = 80.8% Analysis 6.13; P = 0.009, I² = 85.5%, Analysis 6.26) although it must be noted that in Analysis 6.6; Analysis 6.26, some of the subgroups contained only one trial.
We performed subgroup analysis to investigate the differential effect of time of commencement of supplementation (prior to 20 weeks' gestation versus after 20 weeks' gestation) where outcome data allowed, and found no subgroup differences for the outcomes examined, Analysis 7.1; Analysis 7.2; Analysis 7.3.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 per week mg versus greater than 500 mg per week) and found no differential effect for the majority of outcomes examined, Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5; Analysis 8.6; Analysis 8.7. However, for infant birthweight less than 2500 g and admission to NICU, the subgroup analyses indicated a differential effect between the cumulative doses of progesterone (test for subgroup difference: P = 0.02, I² = 80.8%, Analysis 8.5; P = 0.009, I² = 85.5%, Analysis 8.8).
Progesterone versus placebo/no treatment for women following presentation with threatened preterm labour
Five randomised controlled trials involving a total of 384 women and infants were included in the meta‐analysis.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 9.1, and preterm birth less than 34 weeks' gestation Analysis 9.2, there were no statistically significant differences identified when compared with placebo. Major neurodevelopmental handicap in childhood was not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant reduction in the risk of:
-
infant birthweight less than 2500 g (one study; 70 infants; RR 0.52, 95% CI 0.28 to 0.98), Analysis 9.11.
There were no statistically significant differences for any of the other outcomes analysed: preterm birth less than 37 weeks' gestation Analysis 9.10, respiratory distress syndrome Analysis 9.12, intraventricular haemorrhage grade III or IV Analysis 9.13, periventricular leucomalacia Analysis 9.14, needed for mechanical ventilation Analysis 9.15, necrotising enterocolitis Analysis 9.16, neonatal sepsis Analysis 9.17, fetal death Analysis 9.18, neonatal death Analysis 9.19, or neonatal length of hospital stay Analysis 9.20.
Secondary maternal outcomes
For women administered progesterone during pregnancy, when compared with placebo, there were no statistically significant differences for any of the outcomes analysed: pregnancy prolongation Analysis 9.3; Analysis 9.4; Analysis 9.5; Analysis 9.6, spontaneous vaginal birth Analysis 9.7, caesarean section Analysis 9.8, or use of tocolysis Analysis 9.9.
Secondary childhood outcomes
There were no secondary childhood outcomes reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analyses where possible for all outcomes and found no differential effect on some of the outcomes examined when considering route of administration of progesterone (intramuscular versus vaginal), Analysis 9.2; Analysis 9.9; Analysis 9.12; Analysis 9.17; Analysis 9.19. However, for pregnancy prolongation and preterm birth less than 37 weeks' gestation, the subgroup analyses indicated a differential effect between the different routes of administration (test for subgroup differences: P = 0.001, I² = 90.5%, Analysis 9.3; P = 0.04, I² = 75.6%, Analysis 9.10), although it must be noted that in both analyses the subgroups contained only one trial.
It was not possible to assess the effect of gestational age at commencing therapy.
We also performed subgroup analysis by total weekly cumulative dose of progesterone (less than 500 mg versus greater than 500 mg) and found no differential effect for three outcomes examined: Analysis 10.3; Analysis 10.4; Analysis 10.5. However, for the two outcomes, pregnancy prolongation and preterm birth less than 37 weeks' gestation, the subgroup analyses indicated a differential effect between the different drug doses (test for subgroup differences: P = 0.001, I² = 90.5%, Analysis 10.1; P = 0.04, I² = 75.6% , Analysis 10.2).
Progesterone versus placebo for women with 'other' risk factors for preterm birth
Three randomised controlled trials involving a total of 482 women and infants were included in the meta‐analysis. Data from a fourth study (Moghtadaei 2008), could not be included in the meta‐analysis because the number of women randomised to each group was not reported in the brief abstract report of the study.
Primary outcomes
For women administered progesterone during pregnancy, for the primary outcomes perinatal death Analysis 11.1 and preterm birth less than 34 weeks' gestation Analysis 11.2, there were no statistically significant differences identified when compared with placebo. The outcome major neurodevelopmental handicap in childhood were not reported.
Secondary infant outcomes
For women administered progesterone during pregnancy, when compared with placebo, there was a statistically significant reduction in the risk of:
-
infant birthweight less than 2500 g (three studies; 482 infants; RR 0.48, 95% CI 0.25 to 0.91) Analysis 11.4.
In addition, for women administered progesterone who were considered to be at risk of preterm birth for 'other' reasons, when compared with placebo, there were no statistically significant differences for the outcomes preterm birth less than 37 weeks' gestation Analysis 11.3., perinatal death Analysis 11.1, intrauterine fetal death Analysis 11.5 or neonatal death Analysis 11.6.
Secondary childhood outcomes
There were no secondary childhood outcomes reported.
Effect of route of administration, time of commencing therapy, and dose of progesterone
We investigated statistical heterogeneity (I² > 30%) by performing subgroup analysis for perinatal death Analysis 12.1, preterm birth less than 37 weeks Analysis 12.2, and infant birthweight less than 2500 g, Analysis 12.3, and no differential effect was observed related for gestational age at commencing therapy, (test for subgroup differences: P = 0.10, I² = 63.9%, Analysis 12.2; P = 0.19, I² = 42.2%, Analysis 12.3).
Discussion
The randomised trials identified assessed the use of progesterone in women considered to be at increased risk of preterm birth by virtue of history of spontaneous preterm birth, ultrasonographic evaluation of cervical length, presentation in threatened preterm labour, multiple pregnancy, or other reasons (including 'high preterm risk score' and active military duty).
Summary of main results
Progesterone for women with a past history of spontaneous preterm birth
For women with a past history of spontaneous preterm birth, there was a statistically significant reduction in the risk of the primary outcomes, perinatal death and in the risk of preterm birth less than 34 weeks' gestation. The reduction in risk of perinatal death is confined to the subgroup of women receiving intramuscular progesterone. There was significant heterogeneity identified for the outcomes preterm birth before 34 and 37 weeks’ gestation, with evidence of funnel plot asymmetry, raising questions about potential bias and therefore caution in interpretation. For the secondary infant and maternal outcomes, the use of progesterone was associated with a reduction in the risk of infant birthweight less than 2500 g, use of assisted ventilation, necrotising enterocolitis, neonatal death, admission to neonatal intensive care unit, preterm birth less than 37 weeks' gestation and a significant increase in prolongation in pregnancy prolongation. There were no significant differences identified for other secondary infant and maternal health outcomes with the use of progesterone. Information related to childhood health and well being is limited, with only two trials reporting two‐year follow‐up results to date (Northen 2007; O'Brien 2007), in which there were no documented differences in growth or developmental outcomes between those infants exposed in utero to progesterone and those to placebo. There was no differential effect on outcomes in terms of time of commencing therapy and dose of progesterone from the available evidence.
Further information is required about the optimal route of administration of progesterone, with the largest study to date using vaginal progesterone gel suggesting no benefit in this group of women (O'Brien 2007).
Progesterone for women with a short cervix identified on ultrasound
In the trials to date assessing the role of progesterone in women with a short cervix identified on ultrasound (Fonseca 2007; Grobman 2012; Hassan 2011; Rozenberg 2012), there were no statistically significant differences identified for the primary outcome perinatal death. Women administered progesterone were significantly less likely to have preterm birth less than 34 weeks' gestation. For the secondary infant outcomes, the use of progesterone was associated with a reduction in risk of preterm birth at less than 28 weeks' gestation. There was also a significant increase in the risk urticaria in women receiving progesterone. Further information is required about the risk of other infant health outcomes, and maternal health outcomes in this group of women. Reporting of childhood outcomes is lacking, with no trials reporting this information to date. The relative efficacy of progesterone compared with cerclage for women with a short cervix remains uncertain, as does the use of progesterone as an adjunct therapy following cerclage placement (Conde‐Agudelo 2013).
Progesterone for women with a multiple pregnancy
The role of progesterone in women with a multiple pregnancy is less clear, with no identified differences in the primary outcomes perinatal death, and preterm birth less than 34 weeks' gestation. There were also no differences identified for the other secondary infant and maternal health outcomes. Information relating to long‐term childhood health outcomes is unavailable to date. There are several ongoing randomised trials assessing the role of intramuscular (Nassar 2007) and vaginal (Wood 2007) progesterone in women with a multiple pregnancy which will contribute information about the role of progesterone in this group of women.
For multiple pregnancies, we had planned to analyse neonatal data as 'clusters' to account for dependency between twins and triplets. We anticipated that the degree of dependence between twins would vary at the outcome level i.e. in the case of preterm birth the dependency (ICC) is likely to be high, whereas in some outcomes such as perinatal death or morbidity, the ICC may be much lower. However, there were insufficient data presented in the trial reports to allow us to carry out necessary adjustment for cluster design effect ourselves and, although in several trials results had already been adjusted, we were not able to present these data in our data and analyses tables because they were not reported in a consistent way. In future updates, we will contact trial authors for additional information to allow us to carry out necessary adjustments in the analysis of the neonatal data.
Progesterone for women following presentation with threatened preterm labour
The role of progesterone for women following presentation with threatened preterm labour remains uncertain. The identified randomised trials indicate a reduction in only the risk of infant birthweight less than 2500 g. However, the outcomes have been reported in only five small trials (332 women), with only four contributing data to meta‐analysis (211 women) and are underpowered to detect differences in both maternal and infant health outcomes. There is an ongoing randomised trial assessing the role of vaginal progesterone in women presenting with symptoms or signs of threatened preterm labour which will contribute information about the role of progesterone in this group of women (Martinez 2007).
Progesterone versus placebo for women with 'other' risk factors for preterm birth
The role of progesterone in women considered to be at risk of preterm birth for 'other reasons' is uncertain, with the three randomised trials that contributed data to the meta‐analysis to date indicating no benefit in terms of perinatal death or preterm birth less than 37 weeks' gestation. However, the combined sample size of these trials is significantly underpowered to detect all but large differences in these outcomes.
There is evidence that progesterone for women with a history of previous preterm birth is associated with a reduction in preterm birth before 34 and 37 weeks gestation. As indicated earlier, there was considerable heterogeneity identified, in addition to evidence of funnel plot asymmetry, raising concern about potential bias. The observed reduction in perinatal mortality appears confined to the use of intramuscular progesterone.
However, information relating to longer‐term infant and childhood outcomes is currently insufficient, with only two randomised trials reporting to date. Ongoing follow‐up of children exposed to progesterone in utero remains a priority. Maternal outcomes following antenatal progesterone therapy were poorly reported in the available literature, including treatment side‐effects, preferences of mode of administration and satisfaction of care. Further information is required on these important issues (Greene 2003; Iams 2003). In addition, there remains uncertainty as to the optimal dose of progesterone to be administered, the optimal route of administration, and the optimal gestational age at which to commence therapy. The American College of Obstetricians and Gynecologists have issued a committee opinion relating to the use of progesterone for the prevention of preterm birth, indicating the need for further information about the optimal mode of administration (ACOG 2003), including studies directly comparing the relative efficacy of vaginal and intramuscular preparations.
Overall completeness and applicability of evidence
The majority of studies to date have reported short‐term maternal and infant outcomes, with only two studies reporting longer‐term childhood outcomes (Northen 2007; O'Brien 2007).
As outlined above, there are a number of ongoing studies which will contribute to the evidence relating to the use of progesterone for women considered at risk of preterm birth. The trials by Crowther (Crowther 2007), Norman (Norman 2012), and Perlitz (Perlitz 2007) will contribute data from women with a prior preterm birth; van Os (van Os 2011) will contribute data from women with a short cervix identified by ultrasound; Nassar (Nassar 2007), and Wood (Wood 2007) will contribute data from women with a multiple pregnancy; and Martinez (Martinez 2007) will contribute data from women who present with symptoms or signs of threatened preterm labour.
Quality of the evidence
Overall, the trials included in this review were considered to be of good to fair quality. Of the 36 included trials, adequate randomisation methods were described in 23 (Borna 2008; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Facchinetti 2007; Glover 2011; Grobman 2012; Hassan 2011; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Rozenberg 2012; Senat 2012; Serra 2013), allocation concealment was assessed to be at low risk of bias in 23 (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hassan 2011; Johnson 1975; Lim 2011; Majhi 2009; Meis 2003; Norman 2009; O'Brien 2007; Rai 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013), and blinding was achieved with the use of a placebo agent in 25 (Aboulghar 2012; Briery 2011; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Combs 2011a; da Fonseca 2003; Fonseca 2007; Glover 2011; Grobman 2012; Hartikainen 1980; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Rouse 2007; Serra 2013; Sharami 2010).Twenty‐one of the 36 included studies were assessed as being at low risk of bias for other potential sources of bias (Borna 2008; Briery 2011; Caritis 2009; Combs 2010; Combs 2011; da Fonseca 2003; Facchinetti 2007; Fonseca 2007; Glover 2011; Hassan 2011; Hauth 1983; Johnson 1975; Lim 2011; Meis 2003; Norman 2009; O'Brien 2007; Papiernik 1970; Rai 2009; Rode 2011; Saghafi 2011a; Sharami 2010).
We assessed whether studies that included multiple pregnancies accounted appropriately for non‐independence of babies from the same pregnancy in the analysis. There were 14 studies that included a multiple pregnancy (Aboulghar 2012; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Elsheikhah 2010; Fonseca 2007; Hartikainen 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2012; Serra 2013) and in seven studies adjustment appears to have been made in the analysis (Caritis 2009; Combs 2010; Combs 2011; Fonseca 2007; Lim 2011; Norman 2009; Rode 2011). In the remaining seven studies (Aboulghar 2012; Cetingoz 2011; Elsheikhah 2010; Hartikainen 1980; Rouse 2007;Senat 2012; Serra 2013), it is not clear that any adjustment was made. There was not enough information in the trial reports for us to carry out the necessary adjustments ourselves in the analysis of neonatal outcomes. This information will be sought for future updates.
Potential biases in the review process
The possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways: two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
Despite the differences in methodology and included studies, our findings of a reduction in preterm birth before 34 weeks' and 28 weeks' gestation for women with a short cervix identified on ultrasound examination, and a reduction in infant respiratory distress syndrome are consistent with those reported in an individual patient data meta‐analysis by Romero and colleagues (Romero 2012). Similarly, the findings of a proposed individual patient data meta‐analysis for women with a multiple pregnancy are awaited to evaluate if there are specific subgroups of women with a multiple pregnancy who may receive benefit from progesterone therapy (Schuit 2012).
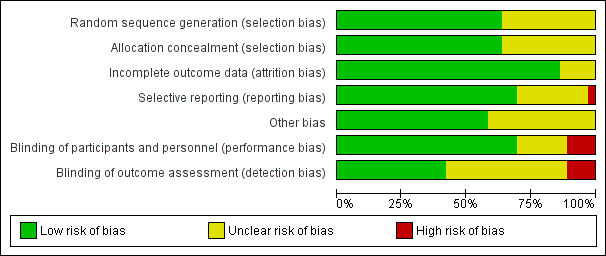
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, outcome: 1.3 Preterm birth less than 37 weeks.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 1 Perinatal mortality.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 2 Preterm birth less than 34 weeks.
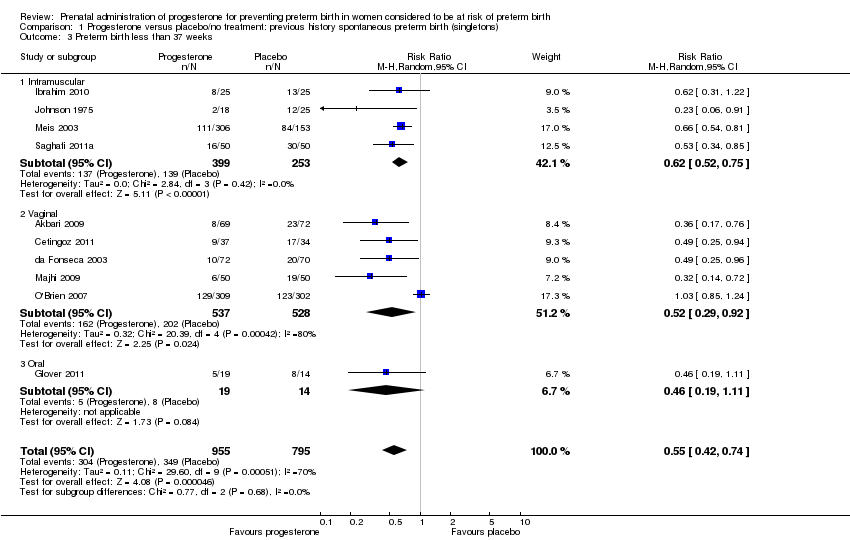
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 3 Preterm birth less than 37 weeks.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 4 Threatened preterm labour.
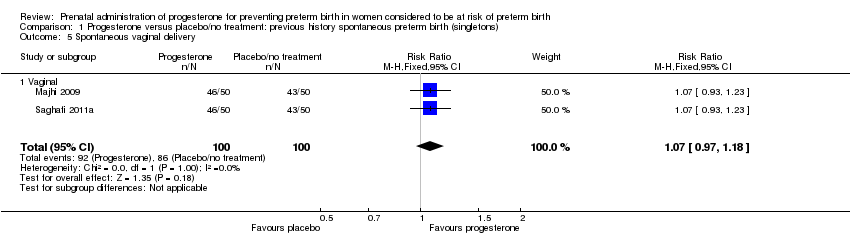
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 5 Spontaneous vaginal delivery.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 6 Caesarean section.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 7 Antenatal corticosteroids.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 8 Antenatal tocolysis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 9 Infant birthweight less than 2500 g.
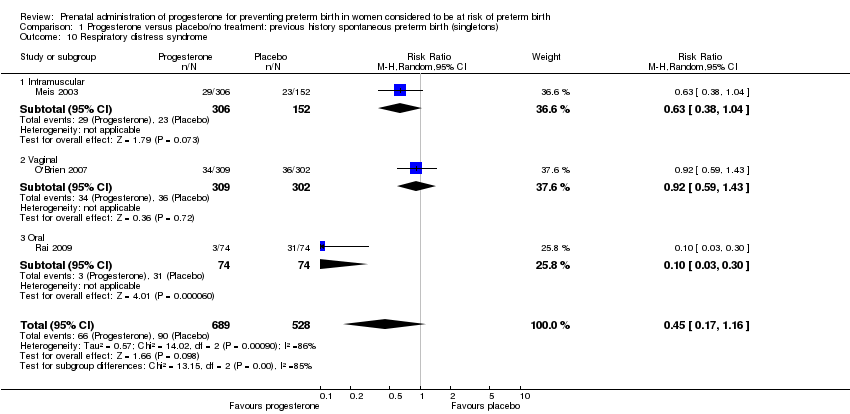
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 10 Respiratory distress syndrome.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 11 Use of assisted ventilation.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 12 Intraventricular haemorrhage ‐ all grades.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 13 Intraventricular haemorrhage ‐ grade III or IV.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 14 Periventricular leucomalacia.
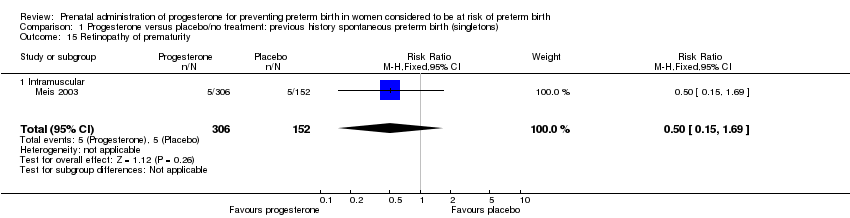
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 15 Retinopathy of prematurity.
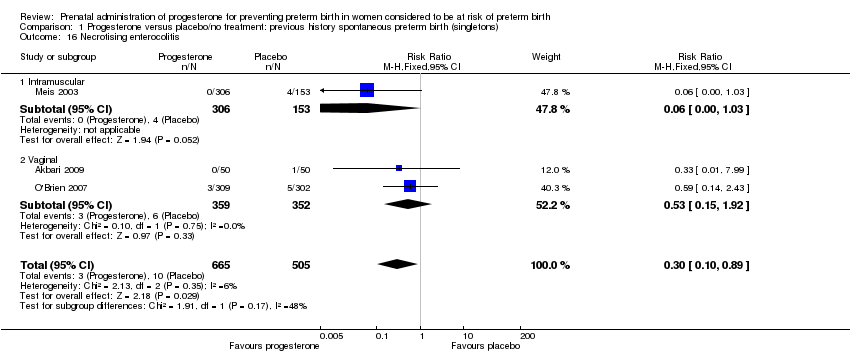
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 16 Necrotising enterocolitis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 17 Neonatal sepsis.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 18 Patent ductus arteriosus.
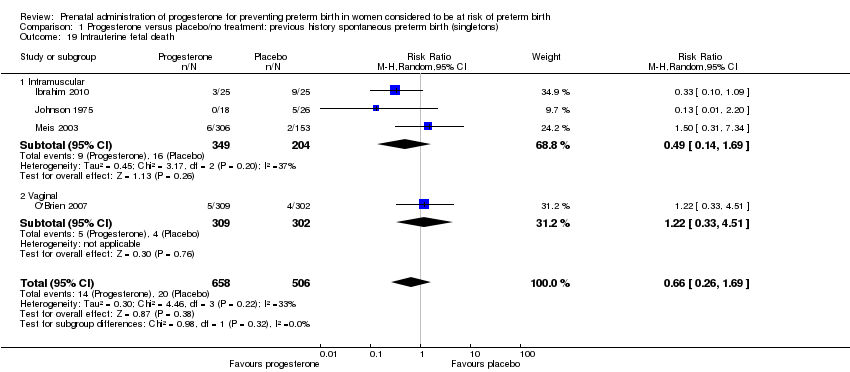
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 19 Intrauterine fetal death.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 20 Neonatal death.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 21 Developmental delay.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 22 Intellectual impairment.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 23 Motor Impairment.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 24 Visual Impairment.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 25 Hearing Impairment.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 26 Cerebral palsy.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 27 Learning difficulties.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 28 Height less than 5th centile.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 29 Weight less than 5th centile.
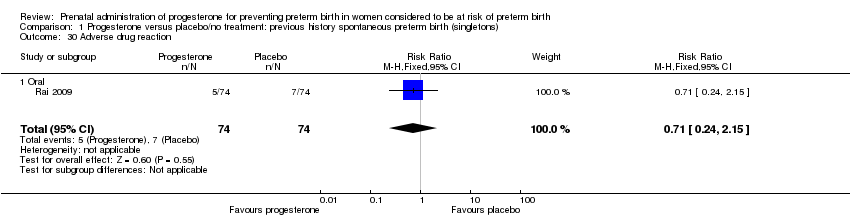
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 30 Adverse drug reaction.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 31 Pregnancy prolongation (weeks).
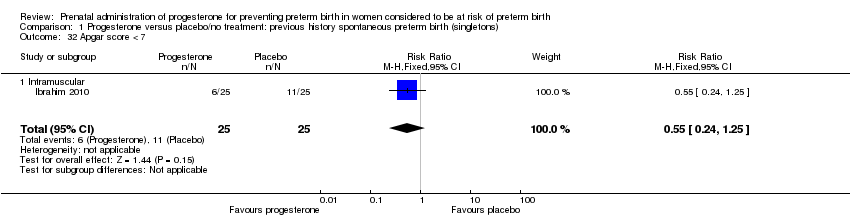
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 32 Apgar score < 7.

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 33 Admission to neonatal intensive care unit.
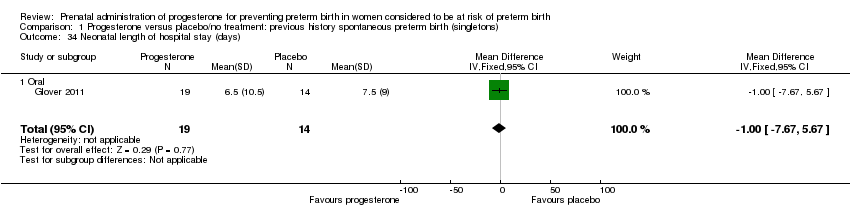
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 34 Neonatal length of hospital stay (days).
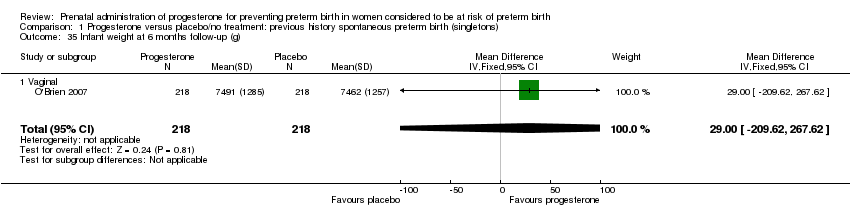
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 35 Infant weight at 6 months follow‐up (g).

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 36 Infant weight at 12 months follow‐up (g).
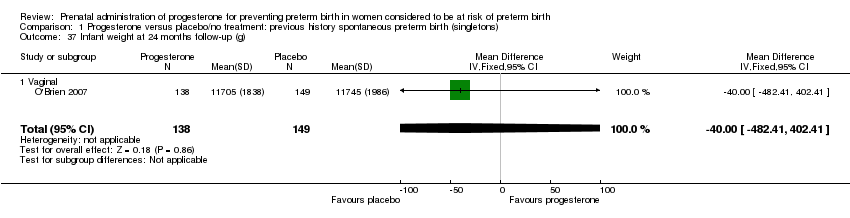
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 37 Infant weight at 24 months follow‐up (g).

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 38 Infant length at 6 months follow‐up (cm).
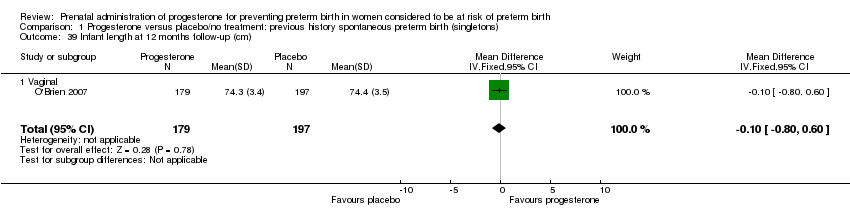
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 39 Infant length at 12 months follow‐up (cm).
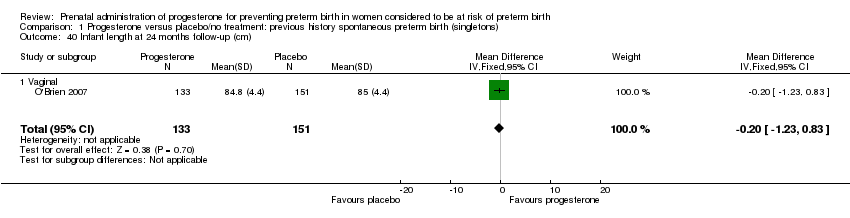
Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 40 Infant length at 24 months follow‐up (cm).

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 41 Infant head circumference at 6 months follow‐up (cm).

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 42 Infant head circumference at 12 months follow‐up (cm).

Comparison 1 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth (singletons), Outcome 43 Infant head circumference at 24 months follow‐up (cm).

Comparison 2 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 1 Preterm birth less than 37 weeks.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 1 Perinatal death.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 2 Preterm birth less than 37 weeks.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 3 Threatened preterm labour.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 4 Caesarean section.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 5 Antenatal corticosteroids.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 6 Need for tocolysis.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 7 Respiratory distress syndrome.
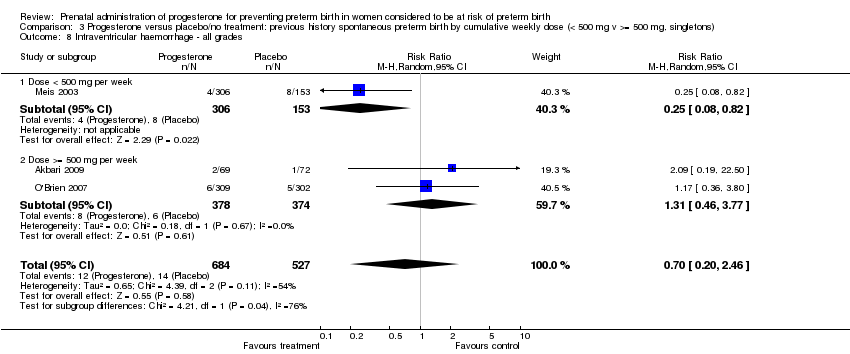
Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 8 Intraventricular haemorrhage ‐ all grades.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 9 Intraventricular haemorrhage ‐ grade III or IV.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 10 Necrotising enterocolitis.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 11 Intrauterine fetal death.

Comparison 3 Progesterone versus placebo/no treatment: previous history spontaneous preterm birth by cumulative weekly dose (< 500 mg v >= 500 mg, singletons), Outcome 12 Neonatal death.
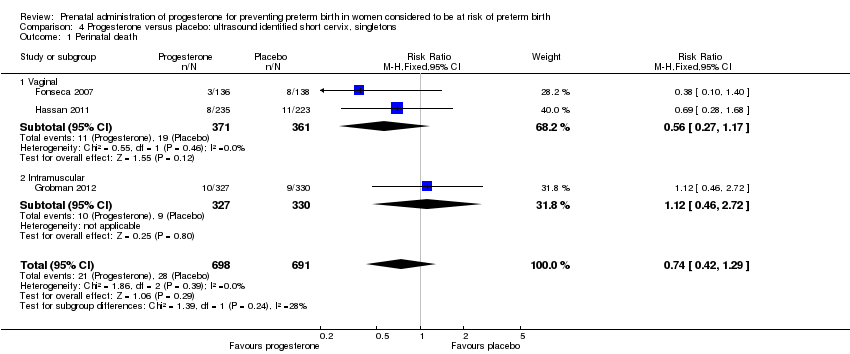
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 1 Perinatal death.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 2 Preterm birth less than 34 weeks.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 3 Preterm labour.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 4 Prelabour spontaneous rupture of membranes.
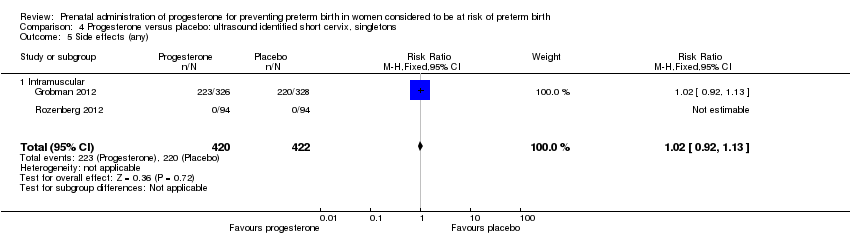
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 5 Side effects (any).

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 6 Side effects (injection site).

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 7 Side effects (urticaria).

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 8 Side effects (nausea).

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 9 Pregnancy prolongation (days).
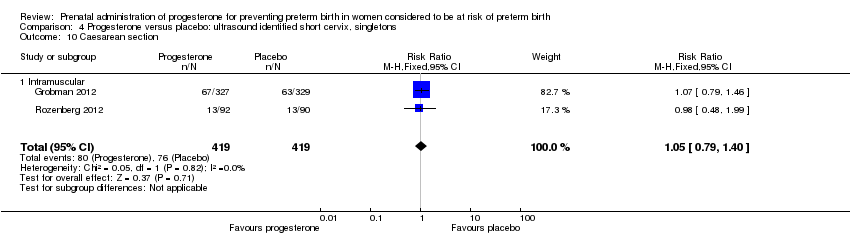
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 10 Caesarean section.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 11 Antenatal tocolysis.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 12 Preterm birth less than 37 weeks.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 13 Preterm birth less than 28 weeks.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 14 Infant birthweight less than 2500 g.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 15 Respiratory distress syndrome.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 16 Apgar score < 7.
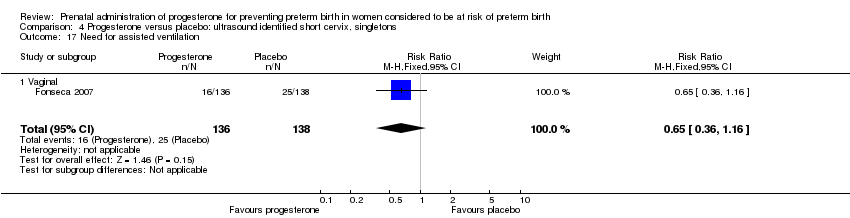
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 17 Need for assisted ventilation.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 18 Intraventricular haemorrhage ‐ all grades.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 19 Intraventricular haemorrhage ‐ grades III or IV.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 20 Periventricular leucomalacia.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 21 Retinopathy of prematurity.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 22 Necrotising enterocolitis.
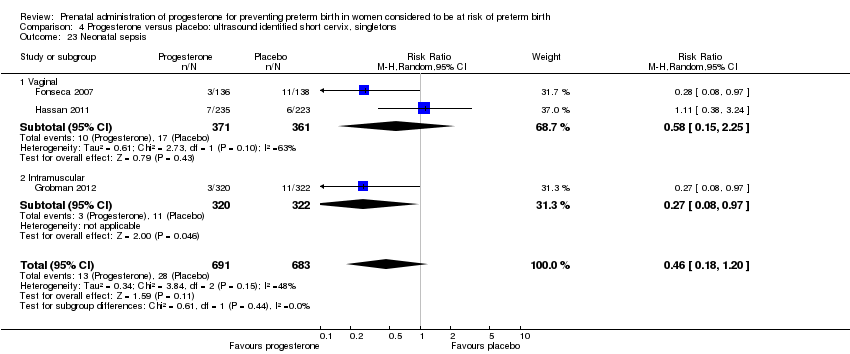
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 23 Neonatal sepsis.

Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 24 Intrauterine fetal death.
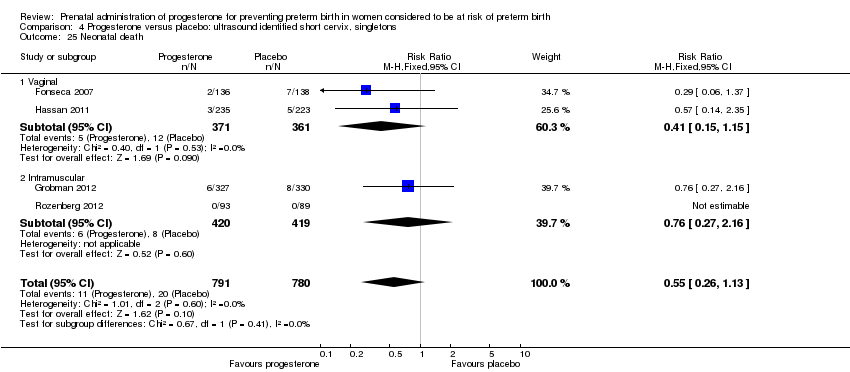
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 25 Neonatal death.
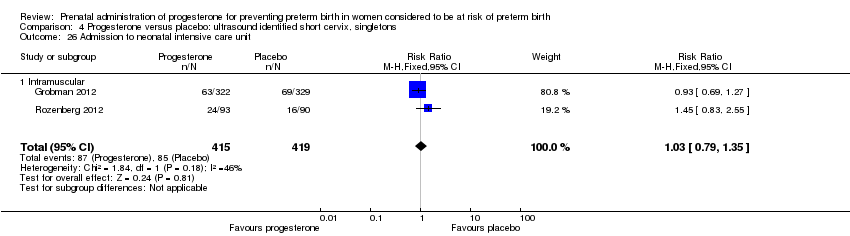
Comparison 4 Progesterone versus placebo: ultrasound identified short cervix, singletons, Outcome 26 Admission to neonatal intensive care unit.
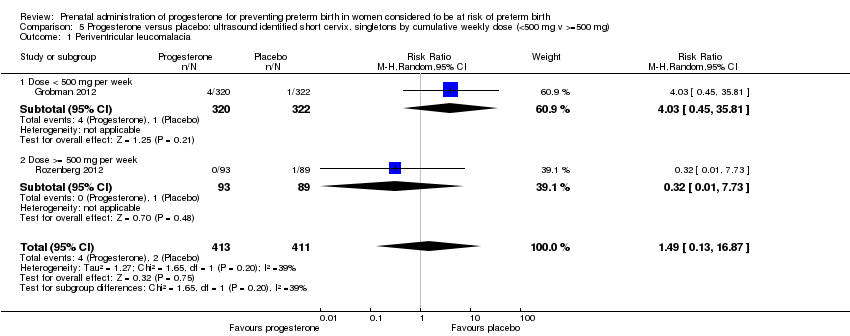
Comparison 5 Progesterone versus placebo: ultrasound identified short cervix, singletons by cumulative weekly dose (<500 mg v >=500 mg), Outcome 1 Periventricular leucomalacia.

Comparison 5 Progesterone versus placebo: ultrasound identified short cervix, singletons by cumulative weekly dose (<500 mg v >=500 mg), Outcome 2 Admission to neonatal intensive care unit.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 1 Perinatal death.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 2 Preterm birth less than 34 weeks.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 3 Preterm PROM.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 4 Adverse drug reaction.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 5 Caesarean section.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 6 Spontaneous birth.
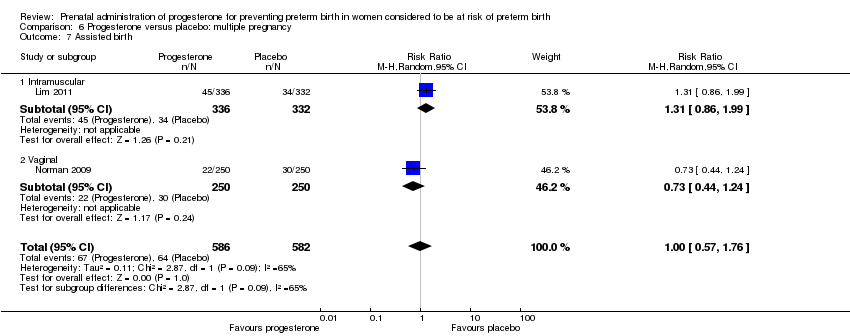
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 7 Assisted birth.
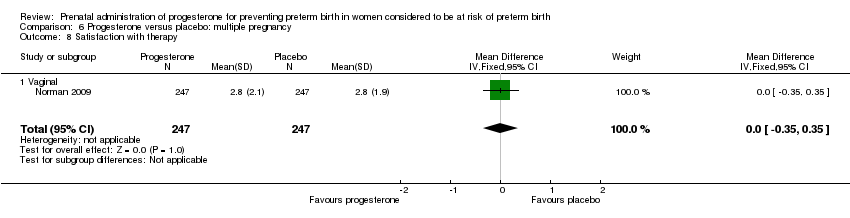
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 8 Satisfaction with therapy.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 9 Antenatal tocolysis.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 10 Antenatal corticosteroids.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 11 Preterm birth less than 37 weeks.
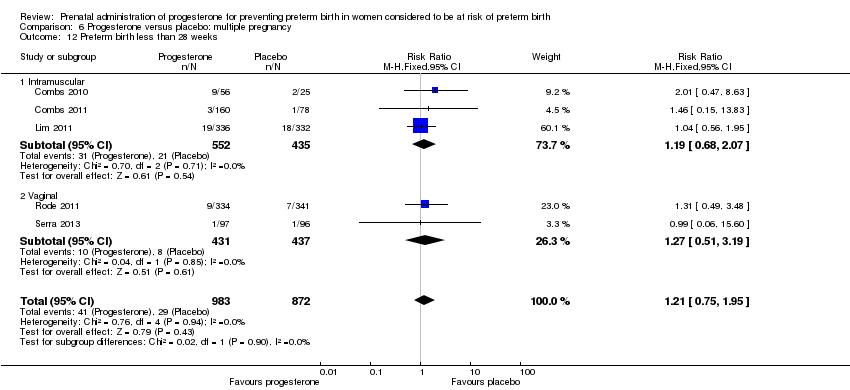
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 12 Preterm birth less than 28 weeks.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 13 Infant birthweight less than 2500 g.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 14 Apgar score < 7 at 5 minutes.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 15 Respiratory distress syndrome.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 16 Use of assisted ventilation.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 17 Intraventricular haemorrhage ‐ grades III or IV.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 18 Intraventricular haemorrhage ‐ all grades.
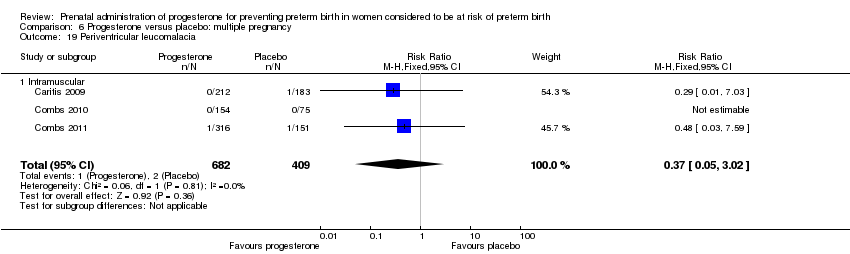
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 19 Periventricular leucomalacia.
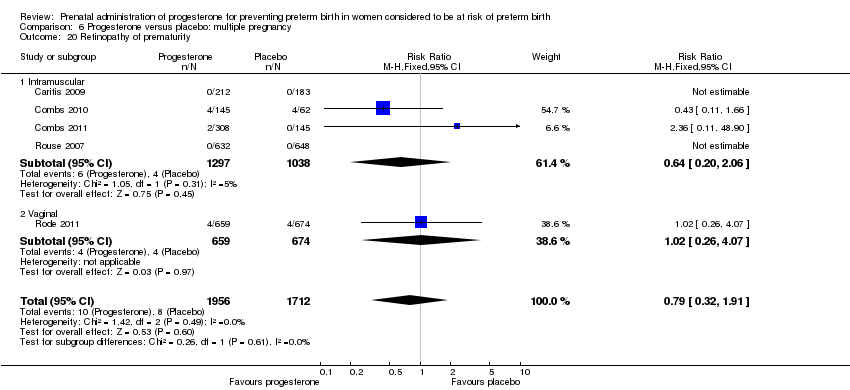
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 20 Retinopathy of prematurity.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 21 Chronic lung disease.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 22 Necrotising enterocolitis.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 23 Neonatal sepsis.
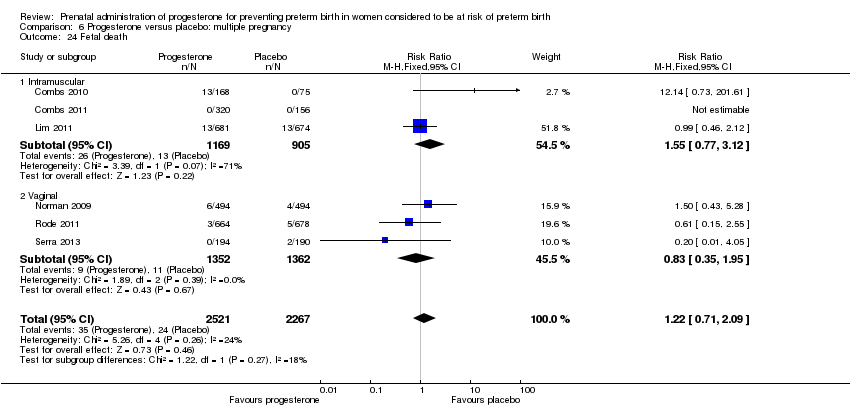
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 24 Fetal death.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 25 Neonatal death.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 26 Admission to NICU.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 27 Perinatal death.
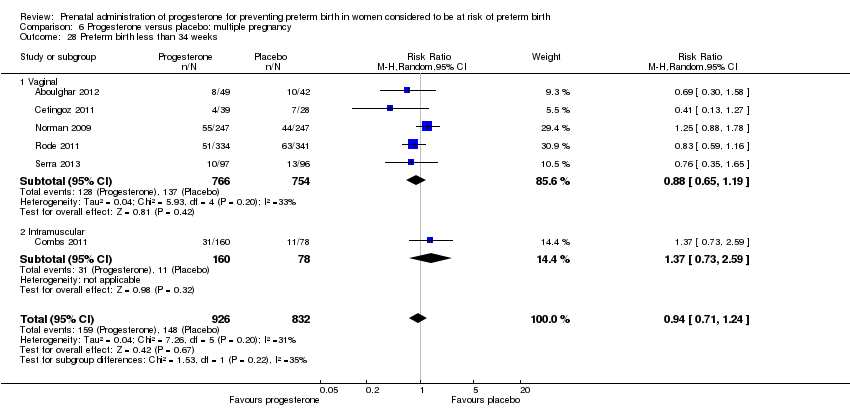
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 28 Preterm birth less than 34 weeks.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 29 Preterm PROM.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 30 Caesarean section.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 31 Antenatal tocolysis.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 32 Antenatal corticosteroids.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 33 Preterm birth less than 37 weeks.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 34 Preterm birth less than 28 weeks.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 35 Infant birthweight less than 2500 g.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 36 Apgar score < 7 at 5 minutes.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 37 Use of assisted ventilation.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 38 Fetal death.
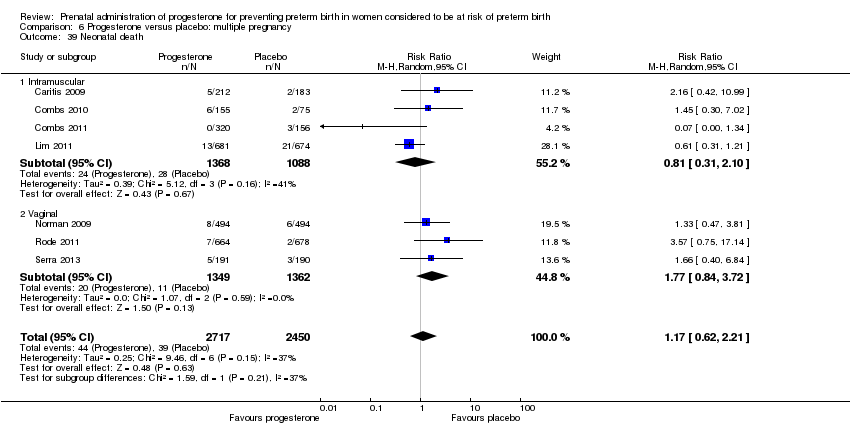
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 39 Neonatal death.

Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 40 Admission to NICU.
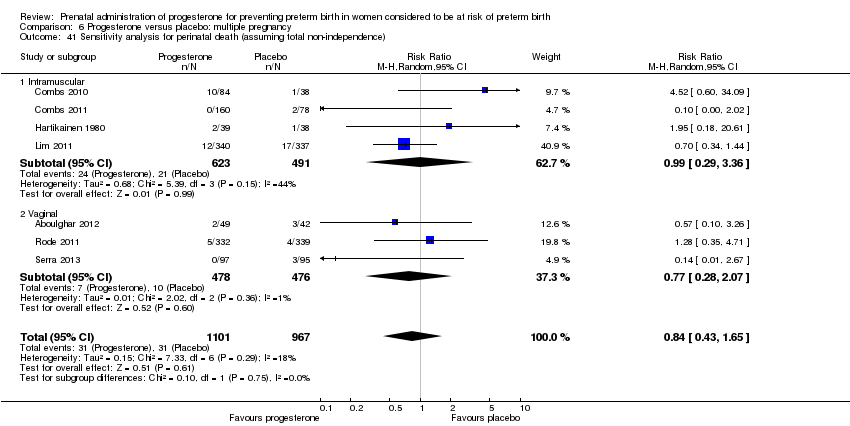
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 41 Sensitivity analysis for perinatal death (assuming total non‐independence).
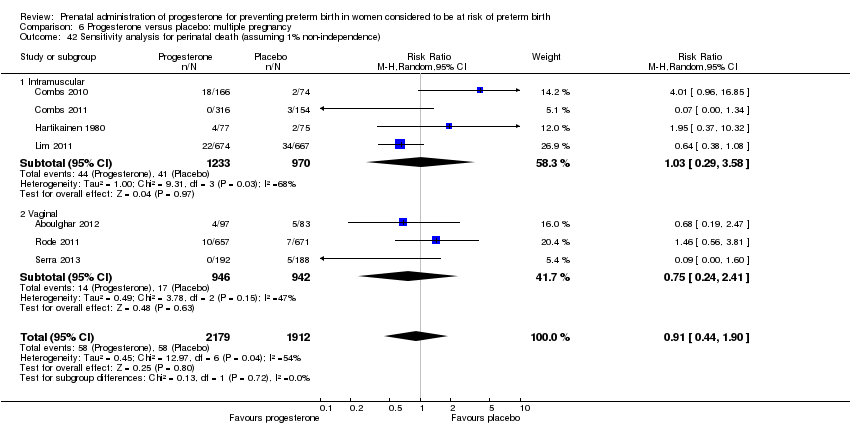
Comparison 6 Progesterone versus placebo: multiple pregnancy, Outcome 42 Sensitivity analysis for perinatal death (assuming 1% non‐independence).
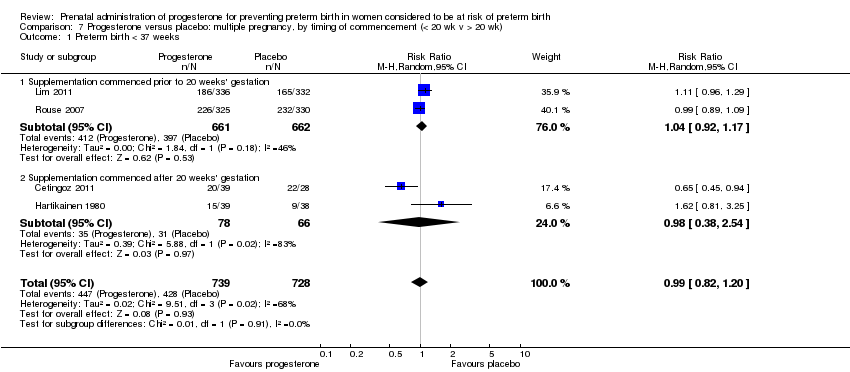
Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 1 Preterm birth < 37 weeks.
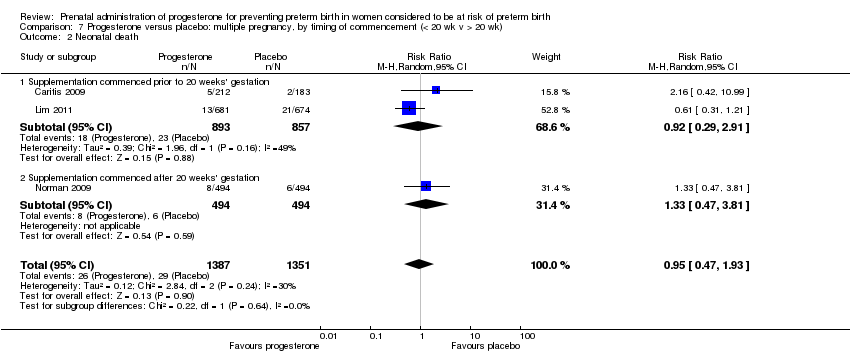
Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 2 Neonatal death.

Comparison 7 Progesterone versus placebo: multiple pregnancy, by timing of commencement (< 20 wk v > 20 wk), Outcome 3 Admission to NICU.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 1 Perinatal death.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 2 Preterm birth less than 34 weeks.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 3 Antenatal tocolysis.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 4 Preterm birth less than 37 weeks.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 5 Infant birthweight less than 2500 g.
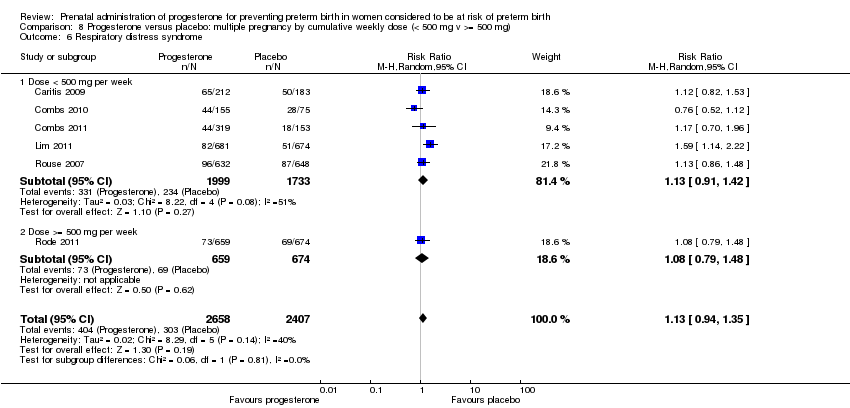
Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 6 Respiratory distress syndrome.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 7 Fetal death.

Comparison 8 Progesterone versus placebo: multiple pregnancy by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 8 Admission to NICU.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 1 Perinatal death.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 2 Preterm birth less than 34 weeks' gestation.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 3 Pregnancy prolongation (days).

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 4 Pregnancy prolongation ‐ less than 1 week.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 5 Pregnancy prolongation ‐ 1.0 to 1.9 weeks.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 6 Pregnancy prolongation ‐ 2 weeks or more.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 7 Spontaneous birth.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 8 Caesarean section.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 9 Use of tocolysis.
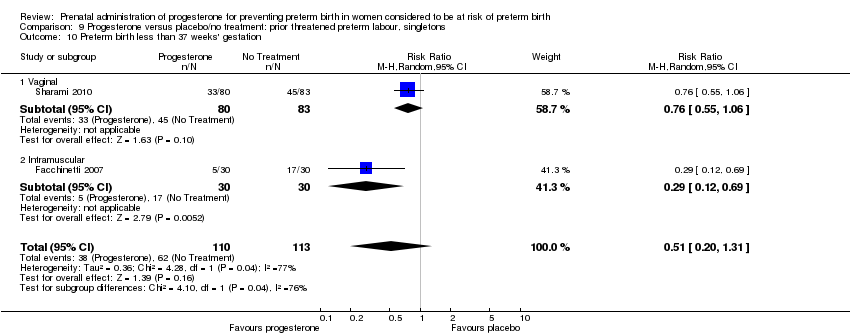
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 10 Preterm birth less than 37 weeks' gestation.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 11 Infant birthweight less than 2500 g.
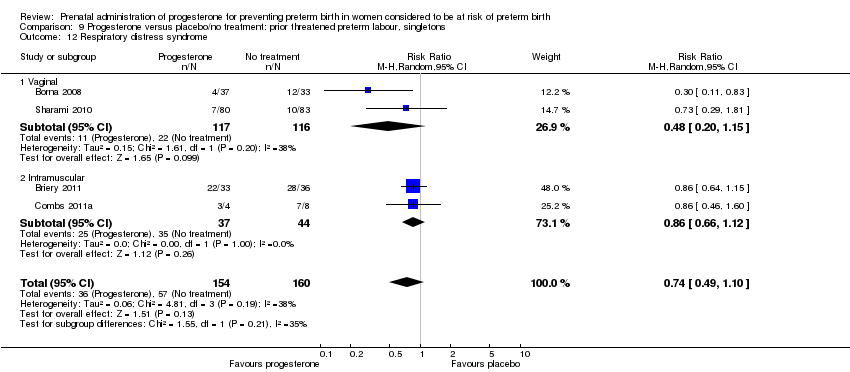
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 12 Respiratory distress syndrome.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 13 Intraventricular haemorrhage grade III or IV.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 14 Periventricular leucomalacia.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 15 Use of assisted ventilation.
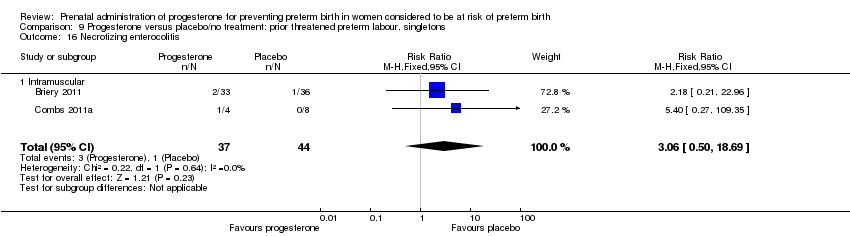
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 16 Necrotizing enterocolitis.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 17 Neonatal sepsis.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 18 Fetal death.
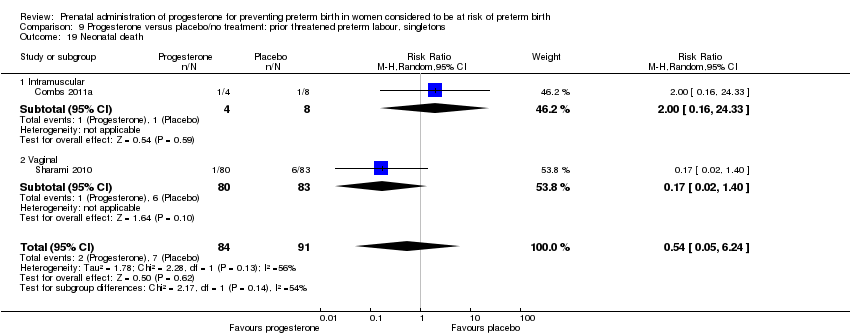
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 19 Neonatal death.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 20 Neonatal length of hospital stay (days).

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 21 Apgar score less than seven at five minutes.
Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 22 Prelabour spontaneous rupture of membranes.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 23 Preterm birth less than 28 weeks' gestation.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 24 Apgar score less than seven at five minutes.

Comparison 9 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, Outcome 25 Admission to neonatal intensive care unit.
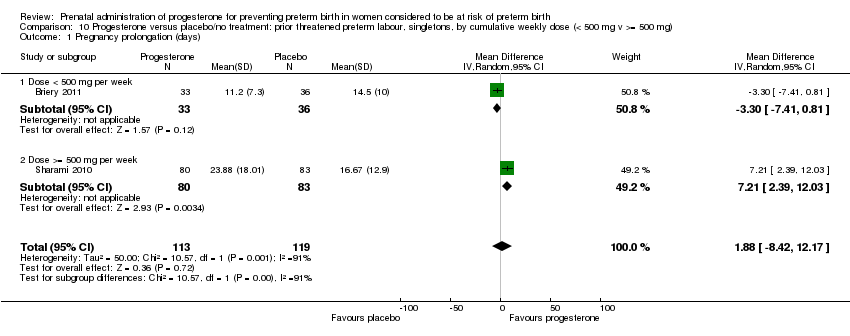
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 1 Pregnancy prolongation (days).

Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 2 Preterm birth less than 37 weeks' gestation.
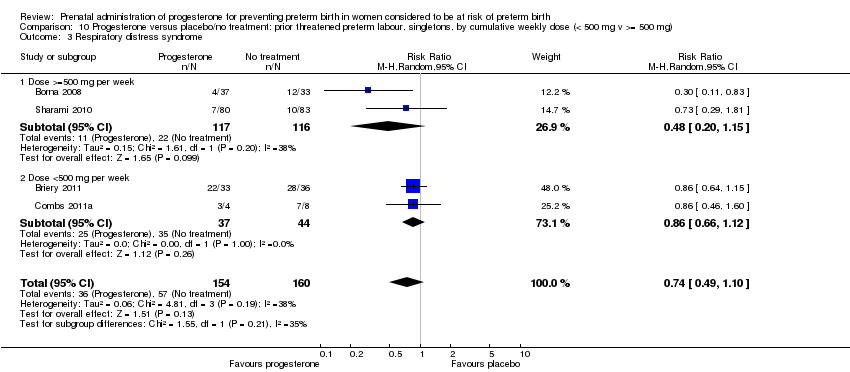
Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 3 Respiratory distress syndrome.

Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 4 Neonatal sepsis.

Comparison 10 Progesterone versus placebo/no treatment: prior threatened preterm labour, singletons, by cumulative weekly dose (< 500 mg v >= 500 mg), Outcome 5 Neonatal death.

Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 1 Perinatal death.
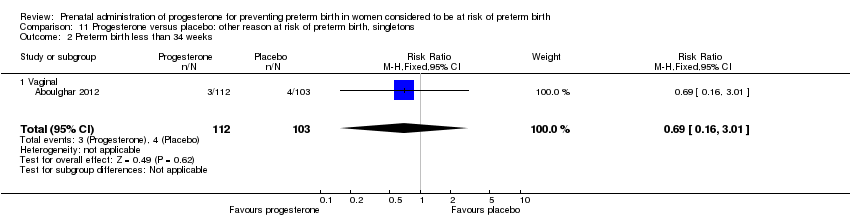
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 2 Preterm birth less than 34 weeks.
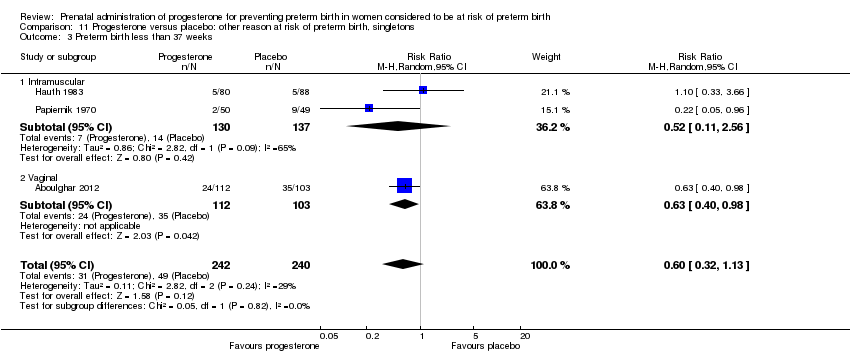
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 3 Preterm birth less than 37 weeks.
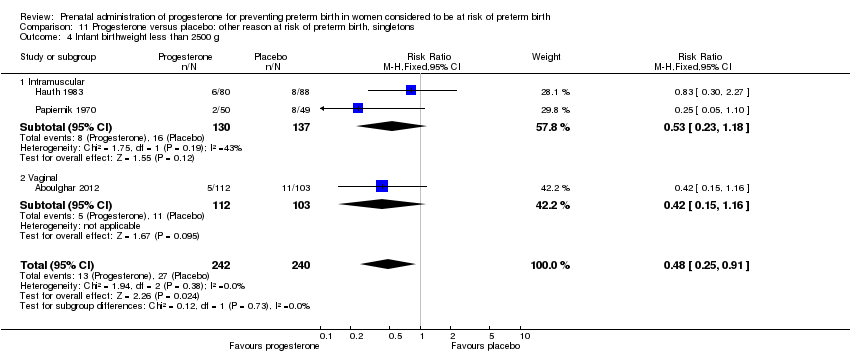
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 4 Infant birthweight less than 2500 g.

Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 5 Intrauterine fetal death.
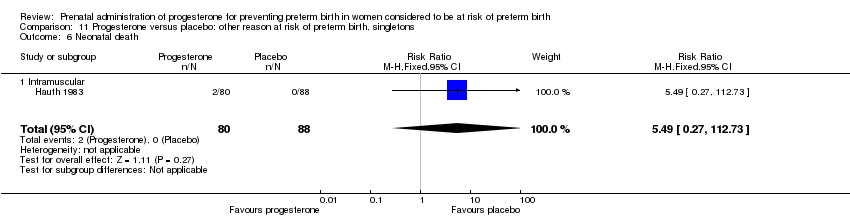
Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 6 Neonatal death.

Comparison 11 Progesterone versus placebo: other reason at risk of preterm birth, singletons, Outcome 7 Admission to neonatal intensive care unit.

Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 1 Perinatal death.
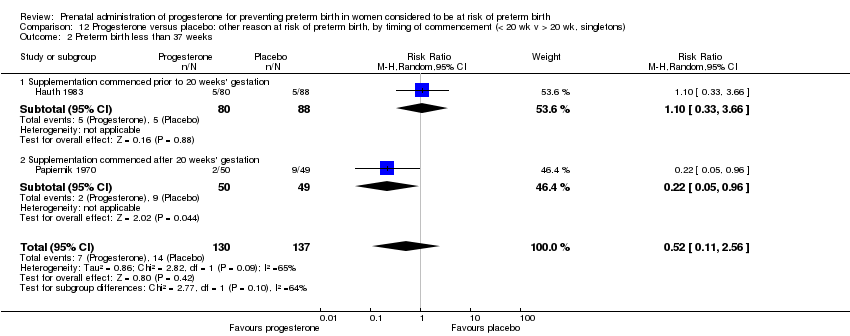
Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 2 Preterm birth less than 37 weeks.

Comparison 12 Progesterone versus placebo: other reason at risk of preterm birth, by timing of commencement (< 20 wk v > 20 wk, singletons), Outcome 3 Infant birthweight less than 2500 g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.33, 0.75] |
| 1.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.73] |
| 1.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.29] |
| 1.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.59] |
| 2 Preterm birth less than 34 weeks Show forest plot | 5 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.69] |
| 2.1 Intramuscular | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Vaginal | 4 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.44] |
| 2.3 Oral | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.90] |
| 3 Preterm birth less than 37 weeks Show forest plot | 10 | 1750 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.74] |
| 3.1 Intramuscular | 4 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.52, 0.75] |
| 3.2 Vaginal | 5 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.92] |
| 3.3 Oral | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 4 Threatened preterm labour Show forest plot | 2 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.62] |
| 4.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.87] |
| 4.2 Vaginal | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.11] |
| 5 Spontaneous vaginal delivery Show forest plot | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.18] |
| 5.1 Vaginal | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.18] |
| 6 Caesarean section Show forest plot | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.20] |
| 6.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 6.2 Vaginal | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 7 Antenatal corticosteroids Show forest plot | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 7.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 7.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.26] |
| 8 Antenatal tocolysis Show forest plot | 4 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 8.1 Intramuscular | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 8.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.74] |
| 8.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.35] |
| 9 Infant birthweight less than 2500 g Show forest plot | 4 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.79] |
| 9.1 Intramuscular | 3 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.81] |
| 9.2 Vaginal | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.74] |
| 10 Respiratory distress syndrome Show forest plot | 3 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.16] |
| 10.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.04] |
| 10.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 10.3 Oral | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.30] |
| 11 Use of assisted ventilation Show forest plot | 3 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.90] |
| 11.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 11.2 Oral | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.92] |
| 11.3 Vaginal | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.07, 0.81] |
| 12 Intraventricular haemorrhage ‐ all grades Show forest plot | 3 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.20, 2.46] |
| 12.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.82] |
| 12.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.46, 3.77] |
| 13 Intraventricular haemorrhage ‐ grade III or IV Show forest plot | 2 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.21, 11.75] |
| 13.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.12, 52.09] |
| 13.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.55] |
| 14 Periventricular leucomalacia Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.52] |
| 14.1 Vaginal | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.52] |
| 15 Retinopathy of prematurity Show forest plot | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.69] |
| 15.1 Intramuscular | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.69] |
| 16 Necrotising enterocolitis Show forest plot | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.89] |
| 16.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.03] |
| 16.2 Vaginal | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.92] |
| 17 Neonatal sepsis Show forest plot | 3 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.08, 2.23] |
| 17.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.35, 3.59] |
| 17.2 Vaginal | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 1.01] |
| 18 Patent ductus arteriosus Show forest plot | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.18] |
| 18.1 Intramuscular | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.18] |
| 19 Intrauterine fetal death Show forest plot | 4 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.26, 1.69] |
| 19.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.69] |
| 19.2 Vaginal | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.33, 4.51] |
| 20 Neonatal death Show forest plot | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.76] |
| 20.1 Intramuscular | 3 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.87] |
| 20.2 Vaginal | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.18] |
| 20.3 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.59] |
| 21 Developmental delay Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 2.04] |
| 21.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 2.04] |
| 22 Intellectual impairment Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.05, 31.34] |
| 22.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.05, 31.34] |
| 23 Motor Impairment Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.76] |
| 23.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.76] |
| 24 Visual Impairment Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.16, 4.57] |
| 24.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.16, 4.57] |
| 25 Hearing Impairment Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.24] |
| 25.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.24] |
| 26 Cerebral palsy Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.48] |
| 26.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.48] |
| 27 Learning difficulties Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.92] |
| 27.1 Intramuscular | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.92] |
| 28 Height less than 5th centile Show forest plot | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.49] |
| 28.1 Intramuscular | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.49] |
| 29 Weight less than 5th centile Show forest plot | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.05] |
| 29.1 Intramuscular | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.05] |
| 30 Adverse drug reaction Show forest plot | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.15] |
| 30.1 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.15] |
| 31 Pregnancy prolongation (weeks) Show forest plot | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 4.47 [2.15, 6.79] |
| 31.1 Oral | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 4.47 [2.15, 6.79] |
| 32 Apgar score < 7 Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.25] |
| 32.1 Intramuscular | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.25] |
| 33 Admission to neonatal intensive care unit Show forest plot | 3 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.14, 0.40] |
| 33.1 Oral | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.49] |
| 33.2 Vaginal | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.49] |
| 34 Neonatal length of hospital stay (days) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.67, 5.67] |
| 34.1 Oral | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.67, 5.67] |
| 35 Infant weight at 6 months follow‐up (g) Show forest plot | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 29.0 [‐209.62, 267.62] |
| 35.1 Vaginal | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 29.0 [‐209.62, 267.62] |
| 36 Infant weight at 12 months follow‐up (g) Show forest plot | 1 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐88.0 [‐381.48, 205.48] |
| 36.1 Vaginal | 1 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐88.0 [‐381.48, 205.48] |
| 37 Infant weight at 24 months follow‐up (g) Show forest plot | 1 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐482.41, 402.41] |
| 37.1 Vaginal | 1 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐40.0 [‐482.41, 402.41] |
| 38 Infant length at 6 months follow‐up (cm) Show forest plot | 1 | 430 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.59, 0.79] |
| 38.1 Vaginal | 1 | 430 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.59, 0.79] |
| 39 Infant length at 12 months follow‐up (cm) Show forest plot | 1 | 376 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 39.1 Vaginal | 1 | 376 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 40 Infant length at 24 months follow‐up (cm) Show forest plot | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.23, 0.83] |
| 40.1 Vaginal | 1 | 284 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.23, 0.83] |
| 41 Infant head circumference at 6 months follow‐up (cm) Show forest plot | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 41.1 Vaginal | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.23, 0.43] |
| 42 Infant head circumference at 12 months follow‐up (cm) Show forest plot | 1 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 42.1 Vaginal | 1 | 372 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 43 Infant head circumference at 24 months follow‐up (cm) Show forest plot | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.21, 0.61] |
| 43.1 Vaginal | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.21, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth less than 37 weeks Show forest plot | 6 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.89] |
| 1.1 Therapy commences before 20 weeks | 4 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 1.2 Therapy commences after 20 weeks | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 5 | 1403 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.35, 0.97] |
| 1.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.18] |
| 1.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.21] |
| 2 Preterm birth less than 37 weeks Show forest plot | 9 | 1700 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.74] |
| 2.1 Dose < 500 mg per week | 3 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.44, 0.80] |
| 2.2 Dose >= 500 mg per week | 6 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.85] |
| 3 Threatened preterm labour Show forest plot | 2 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.62] |
| 3.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.87] |
| 3.2 Dose >= 500 mg per week | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.11] |
| 4 Caesarean section Show forest plot | 3 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 4.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 4.2 Dose >= 500 mg per week | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 5 Antenatal corticosteroids Show forest plot | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 5.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.30] |
| 5.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.26] |
| 6 Need for tocolysis Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.52] |
| 6.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 6.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.74] |
| 7 Respiratory distress syndrome Show forest plot | 4 | 1359 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
| 7.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.05] |
| 7.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.23] |
| 8 Intraventricular haemorrhage ‐ all grades Show forest plot | 3 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.20, 2.46] |
| 8.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.82] |
| 8.2 Dose >= 500 mg per week | 2 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.46, 3.77] |
| 9 Intraventricular haemorrhage ‐ grade III or IV Show forest plot | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.21, 11.73] |
| 9.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.12, 51.92] |
| 9.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.55] |
| 10 Necrotising enterocolitis Show forest plot | 3 | 1170 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.25] |
| 10.1 Dose < 500 mg per week | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.03] |
| 10.2 Dose >= 500 mg per week | 2 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.15, 1.95] |
| 11 Intrauterine fetal death Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.32, 2.91] |
| 11.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.51] |
| 11.2 Dose >= 500 mg per week | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.33, 4.51] |
| 12 Neonatal death Show forest plot | 5 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.81] |
| 12.1 Dose < 500 mg per week | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.03] |
| 12.2 Dose >= 500 mg per week | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 3 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.29] |
| 1.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.17] |
| 1.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.46, 2.72] |
| 2 Preterm birth less than 34 weeks Show forest plot | 2 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.90] |
| 2.1 Vaginal | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.87] |
| 2.2 Intramuscular | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.43, 1.46] |
| 3 Preterm labour Show forest plot | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 3.1 Intramuscular | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 4 Prelabour spontaneous rupture of membranes Show forest plot | 2 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.62] |
| 4.1 Intramuscular | 2 | 845 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.62] |
| 5 Side effects (any) Show forest plot | 2 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 5.1 Intramuscular | 2 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 6 Side effects (injection site) Show forest plot | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.17] |
| 6.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.17] |
| 7 Side effects (urticaria) Show forest plot | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [1.11, 22.78] |
| 7.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [1.11, 22.78] |
| 8 Side effects (nausea) Show forest plot | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 8.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 9 Pregnancy prolongation (days) Show forest plot | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.29, 6.29] |
| 9.1 Intramuscular | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.29, 6.29] |
| 10 Caesarean section Show forest plot | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 10.1 Intramuscular | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 11 Antenatal tocolysis Show forest plot | 2 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.11] |
| 11.1 Intramuscular | 2 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.11] |
| 12 Preterm birth less than 37 weeks Show forest plot | 3 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 12.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 12.2 Intramuscular | 2 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.28] |
| 13 Preterm birth less than 28 weeks Show forest plot | 2 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.93] |
| 13.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 0.97] |
| 13.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 14 Infant birthweight less than 2500 g Show forest plot | 3 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 14.1 Vaginal | 2 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 14.2 Intramuscular | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.30] |
| 15 Respiratory distress syndrome Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 1.00] |
| 15.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.29, 0.85] |
| 15.2 Intramuscular | 2 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.58] |
| 16 Apgar score < 7 Show forest plot | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 16.1 Intramuscular | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 17 Need for assisted ventilation Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.16] |
| 17.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.16] |
| 18 Intraventricular haemorrhage ‐ all grades Show forest plot | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 18.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 19 Intraventricular haemorrhage ‐ grades III or IV Show forest plot | 2 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.17, 5.60] |
| 19.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.73] |
| 19.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.18, 22.08] |
| 20 Periventricular leucomalacia Show forest plot | 3 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.38, 8.24] |
| 20.1 Vaginal | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Intramuscular | 2 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.38, 8.24] |
| 21 Retinopathy of prematurity Show forest plot | 2 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.23, 4.42] |
| 21.1 Vaginal | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.25, 104.70] |
| 21.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.21] |
| 22 Necrotising enterocolitis Show forest plot | 3 | 1374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.78] |
| 22.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.30, 3.11] |
| 22.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 23 Neonatal sepsis Show forest plot | 3 | 1374 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.20] |
| 23.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.15, 2.25] |
| 23.2 Intramuscular | 1 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.97] |
| 24 Intrauterine fetal death Show forest plot | 3 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 24.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.28, 2.42] |
| 24.2 Intramuscular | 1 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.04 [0.45, 35.92] |
| 25 Neonatal death Show forest plot | 4 | 1571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.13] |
| 25.1 Vaginal | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.15, 1.15] |
| 25.2 Intramuscular | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.16] |
| 26 Admission to neonatal intensive care unit Show forest plot | 2 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 26.1 Intramuscular | 2 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Periventricular leucomalacia Show forest plot | 2 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.13, 16.87] |
| 1.1 Dose < 500 mg per week | 1 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.45, 35.81] |
| 1.2 Dose >= 500 mg per week | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.73] |
| 2 Admission to neonatal intensive care unit Show forest plot | 2 | 834 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.72, 1.65] |
| 2.1 Dose < 500 mg per week | 1 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.27] |
| 2.2 Dose >= 500 mg per week | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.83, 2.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 7 | 4136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 1.1 Intramuscular | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 1.2 Vaginal | 3 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
| 2 Preterm birth less than 34 weeks Show forest plot | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.27] |
| 2.1 Vaginal | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 2.2 Intramuscular | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 3 Preterm PROM Show forest plot | 3 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.70] |
| 3.1 Intramuscular | 2 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 3.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.74] |
| 4 Adverse drug reaction Show forest plot | 2 | 1162 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.19] |
| 4.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.01] |
| 4.2 Vaginal | 1 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.08] |
| 5 Caesarean section Show forest plot | 8 | 3136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.02] |
| 5.1 Intramuscular | 5 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 5.2 Vaginal | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.84, 0.98] |
| 6 Spontaneous birth Show forest plot | 2 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.38] |
| 6.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.75, 1.04] |
| 6.2 Vaginal | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.21, 2.49] |
| 7 Assisted birth Show forest plot | 2 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.76] |
| 7.1 Intramuscular | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 7.2 Vaginal | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.44, 1.24] |
| 8 Satisfaction with therapy Show forest plot | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 8.1 Vaginal | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 9 Antenatal tocolysis Show forest plot | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 9.1 Intramuscular | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 9.2 Vaginal | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10 Antenatal corticosteroids Show forest plot | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 10.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.17] |
| 10.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.81, 3.49] |
| 11 Preterm birth less than 37 weeks Show forest plot | 8 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.14] |
| 11.1 Intramuscular | 4 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.22] |
| 11.2 Vaginal | 4 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.13] |
| 12 Preterm birth less than 28 weeks Show forest plot | 5 | 1855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.95] |
| 12.1 Intramuscular | 3 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.68, 2.07] |
| 12.2 Vaginal | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.51, 3.19] |
| 13 Infant birthweight less than 2500 g Show forest plot | 7 | 5404 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 13.1 Intramuscular | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 13.2 Vaginal | 3 | 1902 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.94] |
| 14 Apgar score < 7 at 5 minutes Show forest plot | 4 | 3451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.21] |
| 14.1 Intramuscular | 2 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 14.2 Vaginal | 2 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.28, 1.23] |
| 15 Respiratory distress syndrome Show forest plot | 6 | 5065 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 15.1 Intramuscular | 5 | 3732 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.42] |
| 15.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.48] |
| 16 Use of assisted ventilation Show forest plot | 4 | 3392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.16] |
| 16.1 Intramuscular | 2 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 16.2 Vaginal | 2 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.36] |
| 17 Intraventricular haemorrhage ‐ grades III or IV Show forest plot | 4 | 2368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.92] |
| 17.1 Intramuscular | 4 | 2368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.92] |
| 18 Intraventricular haemorrhage ‐ all grades Show forest plot | 2 | 2688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.75, 4.21] |
| 18.1 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.77] |
| 18.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.62, 4.66] |
| 19 Periventricular leucomalacia Show forest plot | 3 | 1091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.05, 3.02] |
| 19.1 Intramuscular | 3 | 1091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.05, 3.02] |
| 20 Retinopathy of prematurity Show forest plot | 5 | 3668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 20.1 Intramuscular | 4 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.20, 2.06] |
| 20.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.26, 4.07] |
| 21 Chronic lung disease Show forest plot | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.13, 27.80] |
| 21.1 Intramuscular | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.13, 27.80] |
| 22 Necrotising enterocolitis Show forest plot | 6 | 5059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.50, 1.75] |
| 22.1 Intramuscular | 5 | 3726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.52, 1.88] |
| 22.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.63] |
| 23 Neonatal sepsis Show forest plot | 6 | 5065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.62] |
| 23.1 Intramuscular | 5 | 3732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.72] |
| 23.2 Vaginal | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.61, 2.13] |
| 24 Fetal death Show forest plot | 6 | 4788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.09] |
| 24.1 Intramuscular | 3 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.77, 3.12] |
| 24.2 Vaginal | 3 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.35, 1.95] |
| 25 Neonatal death Show forest plot | 7 | 5170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.48, 2.10] |
| 25.1 Intramuscular | 4 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.31, 2.10] |
| 25.2 Vaginal | 3 | 2714 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.36, 4.95] |
| 26 Admission to NICU Show forest plot | 5 | 4251 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.18] |
| 26.1 Vaginal | 4 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 26.2 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 27 Perinatal death Show forest plot | 7 | 4133 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.61, 2.08] |
| 27.1 Intramuscular | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 27.2 Vaginal | 3 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.35] |
| 28 Preterm birth less than 34 weeks Show forest plot | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 28.1 Vaginal | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 28.2 Intramuscular | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 29 Preterm PROM Show forest plot | 3 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.60] |
| 29.1 Intramuscular | 2 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 29.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.12] |
| 30 Caesarean section Show forest plot | 8 | 3136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.01] |
| 30.1 Intramuscular | 5 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 30.2 Vaginal | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.98] |
| 31 Antenatal tocolysis Show forest plot | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 31.1 Intramuscular | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 31.2 Vaginal | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.05] |
| 32 Antenatal corticosteroids Show forest plot | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 32.1 Intramuscular | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.17] |
| 32.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.76, 3.31] |
| 33 Preterm birth less than 37 weeks Show forest plot | 8 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 33.1 Intramuscular | 4 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.22] |
| 33.2 Vaginal | 4 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 34 Preterm birth less than 28 weeks Show forest plot | 5 | 1855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.80, 2.04] |
| 34.1 Intramuscular | 3 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.68, 2.07] |
| 34.2 Vaginal | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.63, 3.69] |
| 35 Infant birthweight less than 2500 g Show forest plot | 7 | 5401 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.89, 1.04] |
| 35.1 Intramuscular | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 35.2 Vaginal | 3 | 1899 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.96] |
| 36 Apgar score < 7 at 5 minutes Show forest plot | 4 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.27] |
| 36.1 Intramuscular | 2 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 36.2 Vaginal | 2 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.58] |
| 37 Use of assisted ventilation Show forest plot | 4 | 3389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 37.1 Intramuscular | 2 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 37.2 Vaginal | 2 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.59] |
| 38 Fetal death Show forest plot | 6 | 4785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.79, 2.29] |
| 38.1 Intramuscular | 3 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.77, 3.12] |
| 38.2 Vaginal | 3 | 2711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.49, 2.48] |
| 39 Neonatal death Show forest plot | 7 | 5167 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.62, 2.21] |
| 39.1 Intramuscular | 4 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.31, 2.10] |
| 39.2 Vaginal | 3 | 2711 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.84, 3.72] |
| 40 Admission to NICU Show forest plot | 5 | 4248 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 40.1 Vaginal | 4 | 2893 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 40.2 Intramuscular | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 41 Sensitivity analysis for perinatal death (assuming total non‐independence) Show forest plot | 7 | 2068 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.43, 1.65] |
| 41.1 Intramuscular | 4 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.29, 3.36] |
| 41.2 Vaginal | 3 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.28, 2.07] |
| 42 Sensitivity analysis for perinatal death (assuming 1% non‐independence) Show forest plot | 7 | 4091 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.44, 1.90] |
| 42.1 Intramuscular | 4 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.29, 3.58] |
| 42.2 Vaginal | 3 | 1888 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth < 37 weeks Show forest plot | 4 | 1467 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 1.1 Supplementation commenced prior to 20 weeks' gestation | 2 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 1.2 Supplementation commenced after 20 weeks' gestation | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.38, 2.54] |
| 2 Neonatal death Show forest plot | 3 | 2738 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.93] |
| 2.1 Supplementation commenced prior to 20 weeks' gestation | 2 | 1750 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.29, 2.91] |
| 2.2 Supplementation commenced after 20 weeks' gestation | 1 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.81] |
| 3 Admission to NICU Show forest plot | 2 | 2343 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.95, 1.43] |
| 3.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.88, 1.26] |
| 3.2 Supplementation commenced after 20 weeks' gestation | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 7 | 4136 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 1.1 Dose < 500 mg per week | 4 | 2228 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.30, 3.71] |
| 1.2 Dose >= 500 mg per week | 3 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.41] |
| 2 Preterm birth less than 34 weeks Show forest plot | 6 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.27] |
| 2.1 Dose < 500 mg per week | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.73, 2.59] |
| 2.2 Dose > 500 mg per week | 5 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Antenatal tocolysis Show forest plot | 7 | 2642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 3.1 Dose < 500 mg per week | 5 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 3.2 Dose >= 500 mg per week | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 4 Preterm birth less than 37 weeks Show forest plot | 8 | 3489 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.06] |
| 4.1 Dose < 500 mg per week | 6 | 2380 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.15] |
| 4.2 Dose >= 500 mg per week | 4 | 1109 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 5 Infant birthweight less than 2500 g Show forest plot | 7 | 5404 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 5.1 Dose < 500 mg per week | 4 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.14] |
| 5.2 Dose >= 500 mg per week | 3 | 1902 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.94] |
| 6 Respiratory distress syndrome Show forest plot | 6 | 5065 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 6.1 Dose < 500 mg per week | 5 | 3732 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.42] |
| 6.2 Dose >= 500 mg per week | 1 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.48] |
| 7 Fetal death Show forest plot | 6 | 4788 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.51, 2.23] |
| 7.1 Dose < 500 mg per week | 3 | 2074 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.19, 33.68] |
| 7.2 Dose >= 500 mg per week | 3 | 2714 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.36, 2.16] |
| 8 Admission to NICU Show forest plot | 5 | 4251 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.18] |
| 8.1 Dose < 500 mg per week | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.05, 1.62] |
| 8.2 Dose >= 500 mg per week | 4 | 2896 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 1.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 2 Preterm birth less than 34 weeks' gestation Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.65] |
| 2.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.72, 1.39] |
| 2.2 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.37, 2.27] |
| 3 Pregnancy prolongation (days) Show forest plot | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐8.42, 12.17] |
| 3.1 Intramuscular | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐7.41, 0.81] |
| 3.2 Vaginal | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 7.21 [2.39, 12.03] |
| 4 Pregnancy prolongation ‐ less than 1 week Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.07, 2.37] |
| 4.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.07, 2.37] |
| 5 Pregnancy prolongation ‐ 1.0 to 1.9 weeks Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.16, 24.33] |
| 5.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.16, 24.33] |
| 6 Pregnancy prolongation ‐ 2 weeks or more Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.42, 9.42] |
| 6.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.42, 9.42] |
| 7 Spontaneous birth Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.49] |
| 7.1 Intramuscular | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.49] |
| 8 Caesarean section Show forest plot | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.51, 1.60] |
| 8.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.51, 1.60] |
| 9 Use of tocolysis Show forest plot | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.58, 1.65] |
| 9.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.62] |
| 9.2 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.73] |
| 10 Preterm birth less than 37 weeks' gestation Show forest plot | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.31] |
| 10.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.06] |
| 10.2 Intramuscular | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.69] |
| 11 Infant birthweight less than 2500 g Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.98] |
| 11.1 Vaginal | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.98] |
| 12 Respiratory distress syndrome Show forest plot | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
| 12.1 Vaginal | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 12.2 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 13 Intraventricular haemorrhage grade III or IV Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 152.93] |
| 13.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 152.93] |
| 14 Periventricular leucomalacia Show forest plot | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Use of assisted ventilation Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.37] |
| 15.1 Vaginal | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.37] |
| 16 Necrotizing enterocolitis Show forest plot | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.50, 18.69] |
| 16.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.50, 18.69] |
| 17 Neonatal sepsis Show forest plot | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.17, 1.68] |
| 17.1 Vaginal | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.00] |
| 17.2 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.05] |
| 18 Fetal death Show forest plot | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.75] |
| 18.1 Intramuscular | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.75] |
| 19 Neonatal death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.24] |
| 19.1 Intramuscular | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 19.2 Vaginal | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.40] |
| 20 Neonatal length of hospital stay (days) Show forest plot | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐15.84, 11.53] |
| 20.1 Intramuscular | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐15.84, 11.53] |
| 21 Apgar score less than seven at five minutes Show forest plot | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 21.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 22 Prelabour spontaneous rupture of membranes Show forest plot | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.45] |
| 22.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.45] |
| 23 Preterm birth less than 28 weeks' gestation Show forest plot | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 23.1 Vaginal | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 24 Apgar score less than seven at five minutes Show forest plot | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 24.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 25 Admission to neonatal intensive care unit Show forest plot | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.27, 9.07] |
| 25.1 Vaginal | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.27, 9.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pregnancy prolongation (days) Show forest plot | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐8.42, 12.17] |
| 1.1 Dose < 500 mg per week | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐7.41, 0.81] |
| 1.2 Dose >= 500 mg per week | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 7.21 [2.39, 12.03] |
| 2 Preterm birth less than 37 weeks' gestation Show forest plot | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.31] |
| 2.1 Dose >=500 mg per week | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.06] |
| 2.2 Dose <500 mg per week | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.69] |
| 3 Respiratory distress syndrome Show forest plot | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
| 3.1 Dose >=500 mg per week | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 3.2 Dose <500 mg per week | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 4 Neonatal sepsis Show forest plot | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.17, 1.68] |
| 4.1 Dose >=500 mg per week | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.00] |
| 4.2 Dose <500 mg per week | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.05] |
| 5 Neonatal death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.24] |
| 5.1 Dose <500 mg per week | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.16, 24.33] |
| 5.2 Dose >=500 mg per week | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 3 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.09, 3.00] |
| 1.1 Intramuscular | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.23, 5.29] |
| 1.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.55] |
| 2 Preterm birth less than 34 weeks Show forest plot | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 3.01] |
| 2.1 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 3.01] |
| 3 Preterm birth less than 37 weeks Show forest plot | 3 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.13] |
| 3.1 Intramuscular | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.56] |
| 3.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.98] |
| 4 Infant birthweight less than 2500 g Show forest plot | 3 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.91] |
| 4.1 Intramuscular | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.18] |
| 4.2 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.16] |
| 5 Intrauterine fetal death Show forest plot | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.45] |
| 5.1 Intramuscular | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.45] |
| 6 Neonatal death Show forest plot | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [0.27, 112.73] |
| 6.1 Intramuscular | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [0.27, 112.73] |
| 7 Admission to neonatal intensive care unit Show forest plot | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.11] |
| 7.1 Vaginal | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.71, 4.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.23, 5.29] |
| 1.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.23, 5.29] |
| 1.2 Supplementation commenced after 20 weeks' gestation | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Preterm birth less than 37 weeks Show forest plot | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.56] |
| 2.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.33, 3.66] |
| 2.2 Supplementation commenced after 20 weeks' gestation | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 3 Infant birthweight less than 2500 g Show forest plot | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.65] |
| 3.1 Supplementation commenced prior to 20 weeks' gestation | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.30, 2.27] |
| 3.2 Supplementation commenced after 20 weeks' gestation | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.10] |

