Vacunas para la prevención de la gripe en personas de edad avanzada
Resumen
Antecedentes
Las consecuencias de la gripe en las personas de edad avanzada (a partir de los 65 años de edad) son complicaciones, hospitalizaciones y muerte. El objetivo principal de la vacunación contra la gripe en las personas de edad avanzada es reducir el riesgo de muerte en las que son más vulnerables. Ésta es una actualización de una revisión publicada en 2010. Esta revisión se actualizará en el futuro solo cuando se disponga de nuevos ensayos o vacunas. Los datos observacionales incluidos en las versiones anteriores de la revisión se han mantenido por razones históricas, pero no se han actualizado debido a que no han influido en las conclusiones de la revisión.
Objetivos
Evaluar los efectos (eficacia, efectividad y efectos perjudiciales) de las vacunas contra la gripe en las personas de edad avanzada.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) (The Cochrane Library 2016, Número 11) que contiene el registro especializado del Grupo Cochrane de Infecciones Respiratorias Agudas (Cochrane Acute Respiratory Infections Group); MEDLINE (1966 hasta 31 de diciembre 2016); Embase (1974 hasta 31 de diciembre 2016); Web of Science (1974 hasta 31 de diciembre 2016); CINAHL (1981 hasta 31 de diciembre 2016); LILACS (1982 hasta 31 de diciembre 2016); la WHO International Clinical Trials Registry Platform (ICRTP; 1 de julio 2017); y ClinicalTrials.gov (1 de julio 2017).
Criterios de selección
Ensayos controlados aleatorizados (ECA) y ensayos controlados cuasialeatorizados que evaluaron la eficacia contra la gripe (casos confirmados por laboratorio) o la efectividad contra la enfermedad similar a la gripe (ESG), o la seguridad. Se consideró cualquier vacuna para la gripe administrada de forma independiente, en cualquier dosis, preparación o esquema de administración, comparada con placebo o ninguna intervención. Las versiones anteriores de esta revisión incluyeron 67 estudios de cohortes y de casos y controles. Las búsquedas de estos diseños de ensayo se dejaron de actualizar.
Obtención y análisis de los datos
Dos autores de manera independiente evaluaron el riesgo de sesgo y extrajeron los datos. La certeza de la evidencia se calificó según los criterios GRADE para los resultados fundamentales de gripe, ESG, complicaciones (hospitalización, neumonía) y eventos adversos. Se presentaron los riesgos del grupo control agrupados para ilustrar el efecto en términos absolutos. Se les utilizó como base para calcular el número necesario a vacunar para prevenir un caso de cada evento para los resultados de gripe y ESG.
Resultados principales
Se identificaron ocho ECA (más de 5000 participantes), de los cuales cuatro evaluaron los efectos perjudiciales. Los estudios se realizaron en ámbitos comunitarios y geriátricos de Europa y los EE.UU. entre 1965 y 2000. El riesgo de sesgo redujo la certeza de los hallazgos para la gripe y la ESG, pero no para otros resultados.
Los adultos mayores que reciben la vacuna contra la gripe pueden presentar menos gripe durante una única estación en comparación con placebo, del 6% al 2,4% (riesgo relativo [RR] 0,42; intervalo de confianza [IC] del 95%: 0,27 a 0,66; evidencia de certeza baja). La certeza de la evidencia se consideró baja por la falta de certeza en cuanto a la manera en la que se diagnosticó la gripe. Los adultos mayores probablemente presentan menos ESG en comparación con los que no reciben una vacunación en el curso de una única temporada de gripe (3,5% versus 6%; RR 0,59; IC del 95%: 0,47 a 0,73; evidencia de certeza moderada). Estos resultados indican que 30 personas necesitarían ser vacunadas para prevenir que una persona presente gripe, y 42 necesitarían ser vacunadas para prevenir que una persona presente una ESG.
El estudio que proporcionó datos de la mortalidad y la neumonía tuvo poco poder estadístico para detectar diferencias en estos resultados. Hubo tres muertes en 522 participantes en el brazo de vacunación y una muerte en 177 participantes en el brazo placebo, lo que proporciona evidencia de certeza muy baja del efecto sobre la mortalidad (RR 1,02; IC del 95%: 0,11 a 9,72). No se registraron casos de neumonía en un estudio que informó de este resultado (evidencia de certeza muy baja). No se informaron datos sobre las hospitalizaciones. Los intervalos de confianza alrededor del efecto de las vacunas sobre la fiebre y las náuseas fueron amplios, y no existe información suficiente acerca de estos efectos perjudiciales en los pacientes mayores (fiebre: 1,6% con placebo comparado con 2,5% después de la vacunación [RR 1,57; 0,92 a 2,71; evidencia de certeza moderada]); náuseas (2,4% con placebo en comparación con 4,2% después de la vacunación [RR 1,75; IC del 95%: 0,74 a 4,12; evidencia de certeza baja]).
Conclusiones de los autores
Los adultos mayores que reciben la vacuna contra la gripe pueden presentar un riesgo menor de gripe (del 6% al 2,4%) y probablemente tienen un riesgo menor de ESG en comparación con los que no reciben la vacunación en el curso de una única estación de gripe (del 6% al 3,5%). No es posible precisar la magnitud del efecto de estas vacunas en diferentes estaciones. Ocurrieron muy pocas muertes y no se informaron datos sobre la hospitalización. No se registraron casos de neumonía en un estudio que informó de este resultado. No se cuenta con suficiente información para evaluar los efectos perjudiciales en relación con la fiebre y las náuseas en esta población.
La evidencia sobre el riesgo menor de gripe y ESG con la vacunación es limitada por los sesgos en el diseño o la realización de los estudios. La falta de detalles con respecto a los métodos utilizados para confirmar el diagnóstico de la gripe limita la aplicabilidad de este resultado. La evidencia disponible en relación con las complicaciones es de calidad deficiente, es insuficiente o antigua y no proporciona una orientación clara para la salud pública con respecto a la seguridad, la eficacia o la efectividad de las vacunas contra la gripe para las personas a partir de los 65 años de edad. La sociedad debe invertir en estudios de investigación sobre una generación nueva de vacunas contra la gripe para las personas de edad avanzada.
PICO
Resumen en términos sencillos
Vacunas para la prevención de la gripe estacional y sus complicaciones en personas a partir de los 65 años de edad
Objetivo de la revisión
El objetivo de esta revisión Cochrane, publicada por primera vez en 2006, fue resumir los estudios de investigación que examinan los efectos de la inmunización de las personas de edad avanzada (a partir de los 65 años de edad) con la vacuna contra la gripe durante las estaciones en las que ocurre la enfermedad. Se utilizó la información de los ensayos aleatorizados que compararon la vacuna contra la gripe versus una vacuna simulada o ninguna vacuna. Las vacunas contra la gripe se prepararon mediante el tratamiento de los virus de la gripe con un producto químico que mata el virus (virus inactivado) y la vacunación se administró mediante una inyección a través de la piel. El interés se centró en demostrar los efectos de las vacunas en cuanto a la reducción del número de personas de edad avanzada con gripe confirmada, el número que presentó síntomas similares a la gripe como cefalea, temperatura alta, tos y dolor muscular (enfermedad similar a la gripe o ESG) y los efectos perjudiciales de la vacunación. Se buscó evidencia de la repercusión de la gripe o la ESG, como el ingreso al hospital, las complicaciones y la muerte. Esta revisión se actualizará en el futuro solo cuando se disponga de nuevos ensayos o vacunas.
Los datos observacionales de 67 estudios incluidos en las versiones anteriores de la revisión se han mantenido por razones históricas, pero no se han actualizado debido a que no han influido en las conclusiones de la revisión.
¿Qué se estudió en esta revisión?
Más de 200 virus causan ESG y producen los mismos síntomas (fiebre, cefalea, malestar, dolores, tos y rinorrea). Sin pruebas de laboratorio los médicos no pueden distinguir entre estos virus, ya que duran días y pocas veces provocan la muerte o una enfermedad grave. En el mejor de los casos, las vacunas son efectivas solo contra la gripe A y B, que representan cerca del 5% de todos los virus circulantes. La vacuna inactivada se prepara mediante el tratamiento de los virus de la gripe con un agente químico específico que "mata" el virus. Las preparaciones finales pueden contener virus completos (vacuna entera) o la parte viral activa (vacunas fraccionadas o de subunidades). Estas vacunas se administran habitualmente mediante una inyección a través de la piel. Habitualmente las cepas de los virus contenidas en la vacuna son las que se espera que circulen en las siguientes temporadas epidémicas (dos cepas tipo A y una o dos cepas tipo B), según las recomendaciones de la Organización Mundial de la Salud (vacuna estacional). La vacuna pandémica solo contiene la cepa del virus que es responsable de la pandemia (p.ej., el tipo A H1N1 para la pandemia de 2009 a 2010).
Mensajes clave
Las vacunas inactivadas pueden reducir la proporción de personas de edad avanzada que tienen gripe y ESG. Los datos sobre las muertes fueron escasos y no se encontraron datos sobre las hospitalizaciones debido a complicaciones. Sin embargo, la variación en los resultados de los estudios significa que no es posible tener seguridad acerca de cuán grande será la diferencia que causarán estas vacunas a través de diferentes estaciones.
Resultados principales
Se encontraron ocho ensayos controlados aleatorizados (más de 5000 personas), de los cuales cuatro evaluaron los efectos perjudiciales. Los estudios se realizaron en ámbitos comunitarios y geriátricos de Europa y los EE.UU. entre 1965 y 2000.
Los adultos mayores que reciben la vacuna contra la gripe pueden presentar menos gripe durante una única estación, del 6% al 2,4%, lo que significa que 30 personas necesitarían ser vacunadas con vacunas inactivadas contra la gripe para prevenir un caso de gripe. Los adultos mayores probablemente también presentan menos ESG, del 6% al 3,5%, lo que significa que 42 personas necesitarían ser vacunadas para prevenir un caso de ESG. La cantidad de información sobre la neumonía y la mortalidad fue limitada. Los datos no fueron suficientes para tener certeza acerca del efecto de las vacunas sobre la mortalidad. No hubo casos de neumonía en un estudio que informó de este resultado y no hubo datos sobre las hospitalizaciones. No se cuenta con suficiente información para evaluar los efectos perjudiciales en relación con la fiebre y las náuseas en esta población.
La repercusión de las vacunas contra la gripe en las personas mayores es moderada, independientemente del ámbito, el resultado, la población y el diseño del estudio.
¿Cuán actualizada está esta revisión?
La evidencia está actualizada hasta el 31 de diciembre 2016.
Authors' conclusions
Summary of findings
| Influenza vaccine compared to placebo for preventing influenza in the elderly | ||||||
| Patient or population: people aged over 65 years | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with influenza vaccine | |||||
| Influenza assessed with: laboratory confirmation Follow‐up was conducted over an influenza season. | Study population1 | RR 0.42 | 2217 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 24 per 1000 | |||||
| Influenza‐like illness Follow‐up was conducted over an influenza season. | Study population1 | RR 0.59 | 6894 | ⊕⊕⊕⊝ | ||
| 59 per 1000 | 35 per 1000 | |||||
| Pneumonia Follow‐up was conducted over an influenza season. | No events occurred in 1 study of 699 people. | ‐ | 699 | ⊕⊝⊝⊝ | ||
| Hospitalisations ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| All deaths Follow‐up was conducted over an influenza season. | Study population1 | RR 1.02 | 699 | ⊕⊝⊝⊝ | ||
| 6 per 1000 | 6 per 1000 | |||||
| Fever Follow‐up was conducted over an influenza season. | Study population1 | RR 1.57 | 2519 | ⊕⊕⊕⊝ | ||
| 16 per 1000 | 25 per 1000 | |||||
| Nausea Follow‐up was conducted over an influenza season. | Study population1 | RR 1.75 | 672 | ⊕⊕⊝⊝ | ||
| 24 per 1000 | 42 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk taken as aggregate of the study control group risks. | ||||||
Background
Description of the condition
Viral respiratory disease imposes a heavy burden on society. The majority of viral respiratory disease (influenza‐like illness (ILI)) is caused by many different agents that are not clinically distinguishable from one another. A variable proportion of ILI (7% to 15% on average) is caused by influenza viruses and is known as influenza (Jefferson 2009a).
Influenza is an acute respiratory infection caused by a virus of the Orthomyxoviridae family. Three serotypes are known (A, B, and C). Influenza causes an acute febrile illness with muscle ache, headache, and cough. Although the median duration of the acute illness is three days, cough and malaise can persist for weeks. Complications of influenza include otitis media, pneumonia, secondary bacterial pneumonia, exacerbations of chronic respiratory disease, and bronchiolitis in children. Additionally, influenza can cause a range of non‐respiratory complications including febrile convulsions, Reye's syndrome, and myocarditis (Treanor 2016; Wiselka 1994). Efforts to prevent or minimise the impact of seasonal influenza in the second part of the 20th century centred on the use of vaccines. Due to the yearly changes in viral antigenic configuration and the lack of carry‐over protection from year to year, a new vaccination campaign needs to be organised annually, with a huge scientific and logistic effort to ensure production and delivery of the vaccines.
Description of the intervention
Currently there are three types of influenza vaccines:
-
whole‐virion vaccines, which consist of complete viruses that have been 'killed' or inactivated, so that they are not infectious but retain their strain‐specific antigenic properties;
-
subunit virion vaccines, which are made of surface antigens (H and N) only; and
-
split‐virion vaccines, in which the viral structure is broken up by a disrupting agent.
These vaccines contain both surface and internal antigens. In addition, a variety of non‐European manufacturers produce live attenuated vaccines. Traditionally, whole‐virion vaccines are thought to be less well tolerated due to the presence of a lipid stratum on the surface of the viral particles (a remnant of the host cell membrane coating the virion, when budding from the host cell).
Influenza vaccines are produced worldwide. Periodic antigenic drifts and shifts pose problems for vaccine production and procurement, as a new vaccine closely matching the circulating antigenic configuration must be produced and procured for the beginning of each new influenza 'season'. To achieve this, the World Health Organization (WHO) has established a worldwide surveillance system, allowing for the identification and isolation of viral strains circulating the different parts of the globe. Sentinel practices (designated primary care points) recover viral particles from the nasopharynx of people with influenza‐like symptoms, and the samples are swiftly sent to the laboratories of the national influenza centres (110 laboratories in 79 countries). When new strains are detected, the samples are sent to one of the four WHO reference centres (London, Atlanta, Tokyo, and Melbourne) for antigenic analysis. Information on the circulating strain is then sent to the WHO, which in February of each year recommends, through a committee, the strains to be included in the vaccine for the forthcoming 'season'. Individual governments may or may not follow the WHO recommendations. Surveillance and early identification thus play a central part in the composition of the vaccine.
The global influenza spread and burden can be assessed by consulting the WHO Global Influenza Surveillance and Response System (GISRS) web page, which provides an overview and country comparison options (www.who.int/influenza/resources/charts/en/).
How the intervention might work
Vaccines work by simulating an infection and stimulating the body to produce antibodies against the threat and activate other defence mechanisms.
Vaccines have been the main global weapon to minimise the impact of influenza in the elderly for the last four decades, as people aged 65 and older are at higher risk of complications, hospitalisations, and death from influenza. According to the Centers for Disease Control and Prevention, the primary goal of influenza vaccination in the elderly is to reduce the risk of complications among people who are most vulnerable (ACIP 2005; CDC 2004). Vaccines containing yearly WHO recommended influenza strains are used worldwide (Grohskopf 2016; Treanor 2016; WHO 2016).
The European Medicines Agency (EMA) recently made changes to the registration of seasonal, pre‐pandemic, and pandemic influenza vaccines (EMA 2014; Wijnans 2016). Changes were introduced in 2014, triggered by the realisation that antibody responses are not sufficient predictors of field protection, as our reviews have consistently shown over the years. Most of the data for influenza vaccines included in our reviews are from registered vaccines, and yet the field protection afforded is modest or negligible. In addition, the methods of standardisation of antibody titres were lacking. The new rules for adults and elderly require demonstration of non‐inferiority of antibody response (immunogenicity) by a candidate seasonal influenza vaccine compared to an established one. In addition, whenever a demonstration of clinical efficacy is necessary, EMA encourages minimal use of placebo and encourages the use of active controls (such as non‐influenza vaccines) with ILI (and relevant polymerase chain reaction (PCR) results) as a primary endpoint. Clinical effectiveness should be tested by carrying out (preferably prospective) cohort studies or nested so‐called test negative case‐control studies following the European Centre for Disease Prevention and Control protocol (ECDC 2009a; ECDC 2009b).
Harms surveillance is now required with a follow‐up of at least 6 months’ duration and in the general elderly population a database of at least 3000 people exposed to the vaccine. Enhanced vaccine vigilance data should be collected as soon as possible at the beginning of the vaccination campaign each year.
Why it is important to do this review
Due to the unique production cycle of influenza vaccines (they are tested using surrogate outcomes ‐ antibody stimulation ‐ ahead of each influenza 'season'), past performance is probably the only reliable way to predict future performance.
An accurate assessment of the effects (efficacy, effectiveness, and safety profile) of influenza vaccines is essential to allow for rational choice between alternative strategies. This review, with its two companion reviews (Demicheli 2014; Jefferson 2012), are long‐running reviews. They are among the most consistently accessed in the whole Cochrane Database of Systematic Reviews, confirming the importance of the topic and interest in it. Periodic updates, some stretching back almost two decades, have allowed us to include an increasing number of studies on the effects of influenza vaccines and monitor their impact on our reviews (Table 1).
| Review version (searches date) | Number of included trials (RCTs/CCTs) | Number of included observational studies | Estimates of effect (RCTs/CCTs only) | Conclusions (1 to 2 lines from abstract) |
|---|---|---|---|---|
| Version 1 (24 May 2006) | 9 | 621 | Influenza‐like illness LAIV = no data TIV = 41% (95% CI 27% to 53%) IAV = n.s. Influenza LAIV = n.s. TIV = 58% (95% CI 34% to 73%) IAV = n.s. | In long‐term care facilities, where vaccination is most effective against complications, the aims of the vaccination campaign are fulfilled, at least in part. However, according to reliable evidence the usefulness of vaccines in the community is modest. The apparent high effectiveness of the vaccines in preventing death from all causes may reflect a baseline imbalance in health status and other systematic differences in the 2 groups of participants. |
| Version 2 (20 January 2010) | 9 | 662 | Influenza‐like illness LAIV = no data TIV = 41% (95% CI 27% to 53%) IAV = n.s. Influenza LAIV = n.s. TIV = 58% (95% CI 34% to 73%) IAV = n.s. | The available evidence is of poor quality and provides no guidance regarding the safety, efficacy, or effectiveness of influenza vaccines for people aged 65 years or older. To resolve the uncertainty, an adequately powered publicly‐funded randomised, placebo‐controlled trial run over several seasons should be undertaken. |
1These include 49 cohort studies for efficacy/effectiveness (79 data sets); 10 case‐control studies for efficacy/effectiveness (12 data sets); 3 studies (cohorts) for Guillain‐Barré syndrome.
2For this update, two cohort studies and two case‐control studies were added to the review (all assessing efficacy/effectiveness).
Key: CCT = controlled clinical trial; CI = confidence interval; IAV = inactivated aerosol vaccines; LAIV = live attenuated vaccines; n.s. = not significant; RCT = randomised controlled trial; TIV = trivalent inactivated vaccines
The reviews are not methodologically homogeneous, as their methods reflect the history and development of Cochrane Reviews. In particular, the inclusion of observational studies, which was initially favoured for the assessment of harms, has been a source of discussion. In the elderly, randomised evidence represents 11% of included studies because no eligible trials have been completed in the past three decades, while numerous observational studies are completed each year.
Historically, observational studies have been of poor methodological quality, often reporting conflicting or paradoxical results, preventing the drawing of firm conclusions. However, inclusion of particular study types and increasing size of the data sets has not led to a change in the conclusion of the reviews, while leading to a greatly increased workload. This is the main reason why the authors, the review group, and the Cochrane editors have decided to stabilise all three reviews, that is to not carry out routine updates of the observational data set and to update the randomised data set if certain conditions are fulfilled in the future.
For the same reason, the observational content of this review and its companions have been retained as historical evidence of the life cycle of the reviews.
We plan to update the randomised evidence in this review if any or all of the following conditions are fulfilled in the future:
-
a trial assessing the clinical effects of the evolution of current technologies becomes available;
-
a new type of vaccine is developed; or
-
a new credible causal paradigm for influenza is put forward.
For an overview of the three reviews, see the covering editorial at https://community.cochrane.org/news/why-have-three-long-running-cochrane-reviews-influenza-vaccines-been-stabilised.
Objectives
To assess the effects (efficacy, effectiveness, and harm) of vaccines against influenza in the elderly.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) or quasi‐RCTs. We have not sought new cohort and case‐control studies for the update of this review, but we have retained the data from previous versions for historical reasons. For study design definitions, see Appendix 1.
Types of participants
Elderly participants aged 65 years or older, irrespective of setting. We excluded studies assessing efficacy in selected groups affected by a specific chronic pathology (i.e. diabetes or cardiac disease), as we were interested in the whole population. The question of whether these vaccines are effective in specific at‐risk populations is the topic of other reviews.
Types of interventions
-
Vaccination with any influenza vaccine given independently, in any dose, preparation, or time schedule, compared with placebo or with no intervention.
-
We also considered new, as yet unlicensed vaccine types (e.g. live attenuated and DNA vaccines).
-
Vaccination of staff in order to protect patients and residents admitted into hospitals, nursing homes, and long‐term care facilities has been assessed by a separate review (Thomas 2010).
-
We excluded studies in which a vaccine was administered after the beginning of the epidemic period.
-
We excluded old oil adjuvant vaccine or vaccines with a content greater than 15 µg of haemagglutinin/strain/dose from the safety assessment.
Types of outcome measures
Primary outcomes
For treatment efficacy and effectiveness
We included outcomes occurring within the epidemic period (the six‐month winter period, if not better specified). When trial authors presented data according to different levels of viral circulation, we only included data restricted to higher viral circulation.
-
Cases of influenza, laboratory‐confirmed (by means of viral isolation, serological supporting evidence), or both.
-
Cases of influenza, clinically defined from a list of likely respiratory and systemic signs and symptoms. We accepted the trial authors' definition of clinical illness because some countries have their own official definition.
-
Cases of influenza (as defined above) admitted to hospital.
-
Deaths (total).
-
Deaths due to influenza (as defined above) or to its complications.
-
Other direct or indirect indicator of disease impact: pneumonia; hospitalisation due to any respiratory disease, and hospitalisation due to heart disease.
We excluded studies with generic outcomes (e.g. deaths from all causes) and long‐term (one‐year) follow‐up, as most illnesses were most likely due to causes other than influenza. We excluded studies reporting only serological outcomes.
Secondary outcomes
For adverse events
-
Local events for aerosol vaccines (upper respiratory tract infection symptoms such as cough, coryza, sore throat, and hoarseness) within seven days of vaccination.
-
Local events for parenteral vaccines (tenderness/soreness, erythema, induration, and arm stiffness) within seven days from vaccination.
-
Systemic events (myalgia, fever, headache, fatigue, indisposition, rash, angioedema, and asthma) within seven days from vaccination.
-
Rare events (thrombocytopenia, neurological disorders, and Guillain‐Barré syndrome.
Search methods for identification of studies
Electronic searches
For this 2016 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11, searched 31 December 2016 via the Cochrane Library), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (Ovid) (1966 to 31 December 2016); Embase (Elsevier) (1974 to 31 December 2016); Web of Science (Thomson Reuters) (1974 to 31 December 2016); Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) (1981 to 31 December 2016); Latin American and Caribbean Health Sciences Information Database (LILACS) (Bireme) (1982 to 31 December 2016); World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en, 1 July 2017); and ClinicalTrials.gov (www.clinicaltrials.gov, 1 July 2017).
The MEDLINE search (Appendix 2) was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008) revision; Ovid format (Lefebvre 2011), and these search terms were adapted to search Embase (Appendix 3), Web of Science (Appendix 4), CINAHL (Appendix 5), LILACS (Appendix 6), WHO ICTRP (Appendix 7), and ClinicalTrials.gov (Appendix 7).
There were no language or publication restrictions.
Searching other resources
The 2016 update included searches of the databases just listed to identify trials only. For details of other resources searched for previous versions of the review, see Appendix 8.
Data collection and analysis
Selection of studies
Two review authors (TOJ, EF) independently applied inclusion criteria to all identified and retrieved articles.
Data extraction and management
Two review authors (EF and LAA) independently performed data extraction using a data extraction form (Appendix 9). Two review authors (TOJ, CDP) checked data and entered the data into Review Manager 5 (RevMan 2014).
We extracted data on the following.
-
Methodological quality of studies
-
Study design (Appendix 1)
-
Description of setting
-
Characteristics of participants
-
Description of vaccines (content and antigenic match)
-
Description of viral circulation degree
-
Description of outcomes
-
Length of follow‐up
-
Publication status
-
Date of study
-
Location of study
Assessment of risk of bias in included studies
Experimental studies
All review authors independently assessed the methodological quality of the included studies using criteria from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and results were introduced into the sensitivity analysis.
We classified studies according to the following criteria.
Randomisation
A = individual participants allocated to vaccine or control group.
B = groups of participants allocated to vaccine or control group.
Generation of the allocation sequence
A = adequate, e.g. table of random numbers or computer‐generated random numbers.
B = inadequate, e.g. alternation, date of birth, day of the week, or case record number.
C = not described.
Allocation concealment
A = adequate, e.g. numbered or coded identical containers administered sequentially, on‐site computer system that can only be accessed after entering the characteristics of an enrolled participant, or serially numbered, opaque, sealed envelopes.
B = possibly adequate, e.g. sealed envelopes that are not sequentially numbered or opaque.
C = inadequate, e.g. open table of random numbers.
D = not described.
Blinding
A = adequate double‐blinding, e.g. placebo vaccine.
B = single‐blind, e.g. blinded outcome assessment.
C = no blinding.
Follow‐up
Average duration of follow‐up and number of losses to follow‐up.
Non‐experimental studies
In previous versions of this review we carried out quality assessment of non‐RCTs in relation to the presence of potential confounders that could make interpretation of the results difficult. We evaluated the quality of case‐control and cohort studies (prospective and retrospective) using the appropriate Newcastle‐Ottawa Scales (NOS) (Appendix 10). Due to the lack of empirical evidence on the impact that the methodological quality has on the results of non‐RCTs, this evaluation was only used at the analysis stage as a mean of interpretation of the results, and a set of sensitivity analyses was performed for this scope. We classified studies as at low risk of bias (up to one inadequate item in the NOS), medium risk of bias (up to three inadequate items), high risk of bias (more than three inadequate items), and very high risk of bias (when there was no description of methods).
In case of disagreement between the review authors, TOJ arbitrated.
Measures of treatment effect
We summarised efficacy (against influenza) and effectiveness (against influenza‐like illness) estimates as risk ratio (RR) (using a 95% confidence interval (CI)) or odds ratio (OR) (using a 95% CI). Absolute vaccine efficacy (VE) is expressed as a proportion, using the formula VE = 1 ‐ RR or VE = 1 ‐ OR whenever significant. When not significant, we reported the relevant RR or OR.
We have calculated the number needed to vaccinate (NNV) as the reciprocal of the risk difference. Rather than use the pooled risk difference, we have multiplied an illustrative control group risk by the RR to generate a difference in risk.
Unit of analysis issues
We identified no studies with a cluster design, and no studies contributed more than one treatment comparison to the analyses.
Dealing with missing data
In earlier versions of the review, we considered contacting the first or corresponding author of the study to request missing data whenever we identified non‐reporting or partial reporting of data. This proved to be a major task with few returns. It was not carried out for this update as we are stabilising this review, that is we will update the randomised data set if certain conditions are fulfilled in the future.
Assessment of heterogeneity
We calculated the I2 statistic for every pooled estimate to assess the effect on statistical heterogeneity. The I2 statistic can be interpreted as the proportion of total variation among effect estimates that is due to heterogeneity rather than sampling error, and it is intrinsically independent of the number of studies. When the I2 statistic is less than 30%, there is little concern about statistical heterogeneity (Higgins 2002; Higgins 2003).
Assessment of reporting biases
Prior to the 2010 update, we carried out a complete re‐extraction of all studies and reassessed their methodological quality. We also assessed concordance between data presented and conclusions and direction of conclusions (in favour or not of the performance of influenza vaccines). We also looked at the relationship between these variables and study funding and journal of publication (see 'Potential biases in the review process' in the Discussion and Jefferson 2009b).
Data synthesis
Aggregation of data was dependent on the sensitivity and homogeneity of definitions of exposure, populations, and outcomes used. Where we found studies to be homogenous, we carried out a meta‐analysis of these studies within each design category. We pooled whole, split, and subunit vaccines, as in community studies this information was not reported. When a study reported data for more than one influenza season or for more than one setting, we considered these separately, creating separate data sets. Within the data sets, we used the term 'observation' to describe an occurrence (i.e. a particular outcome such as fever) and not number of participants, as multiple outcomes were sometimes recorded for the same participant. We used random‐effects models throughout to take account of the between‐study variance in our findings (DerSimonian 1986).
We analysed non‐RCT separately from RCT evidence.
We grouped reports first according to the setting of the study (community or long‐term care facilities) and then by level of viral circulation and vaccine matching (when trial authors presented data according to different levels of viral circulation, we included only data relating to higher viral circulation). We considered a period 'epidemic' when the weekly incidence rate exceeded the seasonal threshold. A vaccine was defined as 'matching' when the vaccine strains were antigenically similar to the wild circulating strains. We further stratified by co‐administration of pneumococcal polysaccharide vaccine (PPV) and by different types of influenza vaccines (live, inactivated, with adjuvant).
Wherever possible, we performed a quantitative analysis adjusted for confounders if the cohort or case‐control studies used the same methods of adjustment (logistic regression) for the same confounders (sex, age, smoking, and comorbidities). We constructed a comparison with effect sizes adjusted for the effects of possible known confounders and their standard error, which we derived from the reported confidence intervals (Greenland 1987), and performed quantitative analysis with the inverse of the variance (Higgins 2011).
We included the findings of one case‐control study reporting data stratified by risk factors for influenza by use of the inverse variance combining stratum‐specific effect size and overall effect size (Mullooly 1994).
Subgroup analysis and investigation of heterogeneity
We did a further analysis to investigate the causes of heterogeneity. To assess the effect of viral circulation and vaccine matching on overall heterogeneity, we calculated heterogeneity within each grouping and compared its sum with the overall heterogeneity (Greenland 1987).
Sensitivity analysis
We performed a subanalysis of studies describing a better defined epidemic period for most significant comparisons. We then tested effect size from cohort studies conducted in long‐term care facilities (where data are more plentiful), stratified by methodological quality of the studies.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings Table 1 using the following outcomes: influenza, ILI, pneumonia, hospitalisation, all deaths, fever, and nausea. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid readers’ understanding of the review where necessary. We have restricted our focus in the 'Summary of findings' table to evidence from randomised studies comparing influenza vaccine with placebo, which was the most commonly adopted strategy.
Results
Description of studies
Results of the search
In the first publication of this review (Rivetti 2006), we identified 4400 titles of reports of potentially relevant studies and screened these for retrieval. We excluded 4088 reports by screening titles and abstracts and retrieved 312 reports for detailed assessment; 241 reports did not fulfil our inclusion criteria.
In the 2010 review update (Rivetti 2010), we identified 1435 reports of potentially relevant studies. We retrieved 18 studies for further evaluation; we included four and excluded 14 for various reasons. We identified two case‐control studies, Jordan 2007 and Puig‐Barbera 2007, and two cohort studies, Hara 2006 and Leung 2007, fulfilling the inclusion criteria.
For this 2016 update we did not identify new randomised evidence.
Included studies
We included 75 studies in previous versions of the review: 68 studies were used to assess efficacy/effectiveness, and 8 were included in the safety assessment (one RCT was included in both assessments). See Figure 1 for study flow.

Study flow. We identified no new randomised controlled trials for the 2016 update and stabilisation.
The 65 studies included in the efficacy/effectiveness assessment were split into subsets by influenza season or setting or vaccine type, resulting in 100 data sets.
We identified eight published RCTs published over four decades involving just over 5000 participants (Allsup 2004; Edmondson 1971; Govaert 1993; Govaert 1994a; Keitel 1996; Margolis 1990a; Rudenko 2001; Stuart 1969; Treanor 1994). Four of these RCTs only reported safety outcomes (Govaert 1993; Keitel 1996; Margolis 1990a; Treanor 1994).
Fifty‐one cohort studies resulted in 80 data sets (Arden 1988; Arroyo 1984; Aymard 1979a; Aymard 1979b; Caminiti 1994; Cartter 1990a; Cartter 1990b; Cartter 1990c; Christenson 2001a; Christenson 2001b; Christenson 2004a; Christenson 2004b; Coles 1992; Comeri 1995; Consonni 2004a; Consonni 2004b; Cuneo Crovari 1980; Currier 1988; D'Alessio 1969; Davis 2001a; Davis 2001b; Deguchi 2001; Feery 1976; Fleming 1995; Fyson 1983a; Fyson 1983b; Gavira Iglesias 1987; Gené Badia 1991; Goodman 1982; Gross 1988; Hak 2002a; Hak 2002b; Hara 2006; Horman 1986; Howarth 1987a; Howarth 1987b; Howells 1975a; Howells 1975b; Howells 1975c; Isaacs 1997; Kawai 2003; Leung 2007; Lopez Hernandez 1994; Mangtani 2004b; Mangtani 2004c; Mangtani 2004d; Mangtani 2004e; Mangtani 2004f; Mangtani 2004g; Mangtani 2004h; Mangtani 2004i; Mangtani 2004j; Meiklejohn 1987; Monto 2001; Morens 1995; Mukerjee 1994; Murayama 1999; Nichol 1994a; Nichol 1994b; Nichol 1994c; Nichol 1998a; Nichol 1998b; Nichol 2003a; Nichol 2003b; Nicholson 1999; Nordin 2001a; Nordin 2001b; Patriarca 1985a; Patriarca 1985b; Pregliasco 2002; Ruben 1974; Saah 1986a; Saah 1986b; Saah 1986c; Saito 2002a; Saito 2002b; Shapiro 2003; Strassburg 1986; Taylor 1992; Voordouw 2003).
Twelve case‐control studies resulted in 14 data sets (Ahmed 1995; Ahmed 1997; Crocetti 2001; Fedson 1993a; Fedson 1993b; Foster 1992; Jordan 2007; Mullooly 1994; Ohmit 1995a; Ohmit 1995b; Ohmit 1999; Puig‐Barberà 1997; Puig‐Barberà 2004; Puig‐Barbera 2007).
Roughly half (n = 52) the data sets reported A/H3N2 virus circulating; 4% (n = 4) B viruses, 1% (n = 1) A/H1N1, 1% (n = 1) A/H2N2, and 7% (n = 7) reported A/H3N2 and A/H1N1 circulating at the same time. The remaining 37% (n = 35) of the data sets provided insufficient information on circulating subtypes.
Twenty‐four studies resulting in 39 data sets collected information about the health conditions of vaccinated and unvaccinated people and reported stratified results or adjusted rates. Participants suffering from lung disease, heart disease, renal disease, diabetes and other endocrine disorders, immunodeficiency or immunosuppressive diseases, cancer, dementia or stroke, vasculitis, and rheumatic disease were considered as belonging to risk groups.
The included studies used the recommended and licenced vaccine formulation, even if some authors did not declare vaccine composition.
In the RCTs, the comparison was placebo. All cohort studies compared the effects of vaccination against no vaccination.
In our safety assessment, we included four RCTs (Govaert 1993; Keitel 1996; Margolis 1990a; Treanor 1994), and we commented on three surveillance studies with a non‐comparative design assessing rare events (Guillain‐Barré syndrome) in the text but did not include them in our meta‐analysis (Kaplan 1982; Lasky 1998; Schonberger 1979). One RCT assessed a vaccine that has not been in production for decades (Stuart 1969); its harms data were not extracted.
Excluded studies
The most frequent reasons for study exclusion were lack of presentation of original data, lack of placebo or standard care comparator, and presence of antibody titres as outcomes. A complete list with reasons for exclusion is provided in the Characteristics of excluded studies table.
Risk of bias in included studies
The results of our 'Risk of bias' assessment were as follows.
Cohort/case‐control studies (from previous versions of the review)
-
Low risk of bias: 18
-
Medium risk of bias: 31
-
High risk of bias: 11
-
Very high risk of bias: 3
Surveillance studies (from previous versions of the review)
For three surveillance studies assessing rare harms, we did not perform quality evaluation. All studies were population based with good case findings and case definitions.
Allocation
Experimental studies
-
Allocation concealment: adequate 3
-
Allocation concealment: unclear 1
-
Allocation concealment: inadequate 0
-
Allocation concealment: not described 5
Blinding
See 'Potential biases in the review process' in the Discussion.
Incomplete outcome data
The vast majority of evidence for our review stems from non‐RCTs. In most of the trials, the quality of the text was such that we could not assess the impact of any incomplete outcome data (Jefferson 2009b).
Selective reporting
Selective reporting including major inconsistencies between different parts of the text was a common feature. See 'Potential biases in the review process' in the Discussion.
Other potential sources of bias
See 'Potential biases in the review process' in the Discussion.
Effects of interventions
See: Summary of findings 1 Influenza vaccine compared to placebo for preventing influenza in the elderly
Randomised controlled trials
Given the heterogeneous nature of the vaccines tested (monovalent, trivalent, live, or inactivated aerosol vaccines), setting, follow‐up, and outcome definition, we could draw no firm conclusions from this body of evidence. Only three trials specified follow‐up (Govaert 1994a; Rudenko 2001; Stuart 1969), which ranged from 42 to 180 days.
We have presented the findings of the most important outcomes in summary of findings Table 1.
Influenza vaccines may reduce the risk of influenza from 6% in unvaccinated people to 2.4% following vaccination (vaccine efficacy (VE) 58%, risk ratio (RR) 0.42, 95% confidence interval (CI) 0.27 to 0.66; low‐certainty evidence) (summary of findings Table 1). These results gave a number needed to vaccinate (NNV) of 30 (Analysis 1.1).
Based on a meta‐analysis of four trials of inactivated vaccines (Allsup 2004; Edmondson 1971; Govaert 1994a; Stuart 1969), vaccines probably reduce the risk of ILI from 6% in unvaccinated people to 3.5% following vaccination (VE 41%, RR 0.59 95% CI 0.47 to 0.73; moderate‐certainty evidence). These results gave a NNV of 42 (Analysis 1.2).
There was very limited information available regarding the effects of vaccines on risk of hospitalisation, pneumonia, and death. No study reported hospitalisations. One study of 699 participants reported no cases of pneumonia (very low‐certainty evidence), and that 4 deaths occurred at comparable rates between the groups (RR 1.02, 95% CI 0.11 to 9.72; very low‐certainty evidence).
Although small increases in fever (from 1.6% to 2.5%; RR 1.57, 95% CI 0.92 to 2.71; moderate‐certainty evidence) and nausea (from 2.4% to 4.2%; RR 1.75, 95% CI 0.74 to 4.12; low‐certainty evidence) occurred following influenza vaccination, CIs for these results are wide, and we downgraded the certainty of evidence in both cases for imprecision (summary of findings Table 1).
Increased risks of general malaise, upper respiratory tract symptoms, and headache following vaccination ranged between 1.1 and 1.57, although the CIs are wide for all of these outcomes (see Analysis 2.1; Analysis 2.3; Analysis 2.4). Following vaccination there were increased risks of sore arm (RR 3.56, 95% CI 2.61 to 4.87) (Analysis 2.6) and swelling (RR 8.23, 95% CI 3.98 to 17.05) (Analysis 2.7) compared with placebo.
Three studies assessed the effects of inactivated aerosol vaccine (Edmondson 1971; Rudenko 2001; Treanor 1994). There was no evidence of lower risk of influenza or ILI following vaccination compared with placebo with vaccine matching in the presence of an outbreak (Analysis 3.1; Analysis 3.2), or with vaccine matching outside of an outbreak (influenza: Analysis 4.1). Effect sizes for harms (namely malaise, fever, upper and lower respiratory tract symptoms) were all higher with vaccines, but the size of the study contributing data to these outcomes was small, and the confidence interval includes there being no increase in these events with the vaccines (see Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4).
Cohort studies in long‐term care facilities (from previous versions of the review)
Thirty cohort studies in long‐term care facilities contributed data to 41 data sets and over 34,000 observations (Arden 1988; Arroyo 1984; Aymard 1979a; Aymard 1979b; Cartter 1990a; Cartter 1990b; Cartter 1990c; Coles 1992; Cuneo Crovari 1980; Currier 1988; Deguchi 2001; Feery 1976; Fyson 1983a; Fyson 1983b; Goodman 1982; Gross 1988; Horman 1986; Howarth 1987a; Howarth 1987b; Howells 1975a; Howells 1975b; Howells 1975c; Isaacs 1997; Leung 2007; Meiklejohn 1987; Monto 2001; Morens 1995; Mukerjee 1994; Murayama 1999; Patriarca 1985a; Patriarca 1985b; Ruben 1974; Saah 1986a; Saah 1986b; Saah 1986c; Saito 2002a; Saito 2002b; Strassburg 1986; Taylor 1992). These studies were very focused and were fairly well resourced: 35 data sets reported virologic surveillance that confirmed influenza virus circulation, and 22 data sets had short follow‐up (less than three months). The studies assessed the effects of vaccines in residential communities. The resident population is described in about half of the included data sets as predominantly aged older than 75 years, with multiple chronic pathologies and a high dependency level. However, breakdown of potential confounding factors (such as age, sex, smoking status, and underlying chronic disease) is rarely reported by vaccine exposure, making correction of confounders impossible.
Studies recorded during outbreaks or periods of high viral circulation (from previous versions of the review)
Of the 41 data sets, 30 data sets with a total of 9879 observations were recorded during outbreaks or periods of high viral circulation (Arden 1988; Arroyo 1984; Aymard 1979a; Aymard 1979b; Cartter 1990a; Cartter 1990b; Cartter 1990c; Coles 1992; Cuneo Crovari 1980; Currier 1988; Feery 1976; Fyson 1983a; Fyson 1983b; Goodman 1982; Gross 1988; Horman 1986; Isaacs 1997; Leung 2007; Meiklejohn 1987; Monto 2001; Morens 1995; Mukerjee 1994; Murayama 1999; Patriarca 1985a; Ruben 1974; Saah 1986a; Saah 1986b; Strassburg 1986; Taylor 1992). The influenza virus subtype is positively identified in 28 data sets (A/H3N2 in 25 data sets). The focus of 22 data sets from 19 studies was on assessment of the effect of vaccination on single epidemic sources (Arden 1988; Arroyo 1984; Cartter 1990a; Cartter 1990b; Cartter 1990c; Coles 1992; Cuneo Crovari 1980; Currier 1988; Feery 1976; Fyson 1983a; Fyson 1983b; Goodman 1982; Horman 1986; Isaacs 1997; Meiklejohn 1987; Morens 1995; Murayama 1999; Ruben 1974; Saah 1986a; Saah 1986b; Strassburg 1986; Taylor 1992). Viral circulation was confirmed by isolates, increases in antibody titres, or observation of an epidemic of ILI in an institution at the same time as influenza A or B circulation in the surrounding community. A high proportion of cases classified as ILI were probably influenza cases. Twenty‐two data sets from 18 studies provided information about vaccine content match with circulating influenza viruses (Arden 1988; Aymard 1979a; Cartter 1990a; Cartter 1990b; Cartter 1990c; Feery 1976; Fyson 1983a; Fyson 1983b; Goodman 1982; Gross 1988; Hara 2006; Horman 1986; Isaacs 1997; Meiklejohn 1987; Monto 2001; Morens 1995; Mukerjee 1994; Murayama 1999; Patriarca 1985a; Saah 1986b; Strassburg 1986; Taylor 1992). We thus grouped our analyses by viral circulation and vaccine match.
Efficacy of the vaccines against influenza was tested in only six data sets (1250 observations) (Cuneo Crovari 1980; Feery 1976; Gross 1988; Morens 1995; Ruben 1974; Taylor 1992), and was not significant both for vaccine matching (RR 1.04, 95% CI 0.43 to 2.51; Analysis 6.1.1) and when matching was absent or unknown (RR 0.47, 95% CI 0.22 to 1.04; Analysis 6.1.2).
Twenty‐two data sets assessed the effectiveness of influenza vaccines in preventing ILI (Analysis 6.1; Analysis 6.2). In these data sets, follow‐up was restricted to an outbreak period, and the authors reported a virologic surveillance that confirmed influenza virus circulation.
The overall effectiveness of vaccines (VE) against ILI was 23% (6% to 36%; RR 0.77, 95% CI 0.64 to 0.94; Analysis 6.2.1) when vaccine matching was good and not significantly different from no vaccination (RR 0.80, 95% CI 0.60 to 1.05; Analysis 6.2.2) when matching was poor or unknown. Heterogeneity was high, even within the same influenza season and within the same institution when data from different accommodation blocks were analysed. We noted no association (correlation coefficient 0.09) between vaccine coverage and attack rate of ILI (Figure 2).
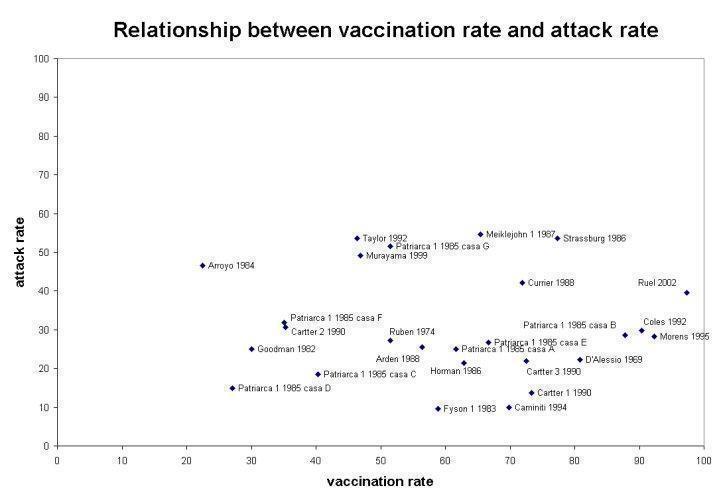
Relationship between vaccination rate and attack rate
The effectiveness of the vaccines in preventing pneumonia was assessed in 13 data sets (Analysis 6.3.1 and Analysis 6.3.2; 8446 observations). All of them reported virologic surveillance, and eight had follow‐ups shorter than three months (Arroyo 1984; Coles 1992; Currier 1988; Horman 1986; Meiklejohn 1987; Morens 1995; Patriarca 1985a; Taylor 1992). Well‐matched vaccines were 46% (30% to 58%; Analysis 6.3.1) effective in preventing pneumonia (Gross 1988; Horman 1986; Meiklejohn 1987; Monto 2001; Morens 1995; Patriarca 1985a; Saah 1986b; Taylor 1992). When matching was poor or unknown (Arroyo 1984; Coles 1992; Currier 1988; Leung 2007; Saah 1986a), vaccines had no effect (RR 0.68, 95% CI 0.39 to 1.21; Analysis 6.3.2). Excluding studies with the longest follow‐up, that is six months, did not affect our conclusions (Gross 1988; Saah 1986a; Saah 1986b).
Eight data sets assessed the effectiveness of well‐matched vaccines in preventing hospitalisation for influenza or pneumonia (Arden 1988; Cartter 1990a; Cartter 1990b; Cartter 1990c; Meiklejohn 1987; Murayama 1999; Patriarca 1985a; Taylor 1992). All of them had a brief and well‐defined follow‐up; effectiveness was 45% (16% to 64%; Analysis 6.4.1). Two studies reported a non‐significant effect when the vaccine did not match the circulating strain or was not reported (Analysis 6.4.2) (Coles 1992; Leung 2007).
Vaccination had a significant effect on the prevention of deaths due to influenza or pneumonia, though this was in the presence of considerable heterogeneity between the 20 data sets (Analysis 6.5.1 and Analysis 6.5.2) (Arroyo 1984; Cartter 1990a; Cartter 1990b; Cartter 1990c; Coles 1992; Feery 1976; Fyson 1983a; Fyson 1983b; Goodman 1982; Horman 1986; Meiklejohn 1987; Monto 2001; Morens 1995; Murayama 1999; Patriarca 1985a; Ruben 1974; Saah 1986a; Saah 1986b; Strassburg 1986; Taylor 1992). Eighteen studies reported virologic surveillance to confirm influenza virus circulation; of these, 16 had a follow‐up shorter than three months, and two had a four‐month follow‐up (Feery 1976; Monto 2001). Two studies lacked virologic surveillance and had a follow‐up of six months (Saah 1986a; Saah 1986b).
The vaccine was effective if it was a good match (VE 42%; 17% to 59%; Analysis 6.5.1), otherwise it was not effective (RR 0.34, 95% CI 0.11 to 1.02; Analysis 6.5.2).
Excluding two studies with a six‐month follow‐up and absence of viral surveillance affects the summary estimate more than the efficacy in the 'epidemic‐matching' group, which drops from 42% to 39% (95% CI 12 to 58) (Saah 1986a; Saah 1986b).
Only one small study with a six‐month follow‐up assessed the effectiveness in reducing all‐cause mortality (Gross 1988), which was found to be significant (60%; 23% to 79%; Analysis 6.6.1).
Studies carried out during low viral circulation (from previous versions of the review)
Eleven data sets (27,283 observations) assessed the effects of influenza vaccines in 350 institutional facilities during low viral circulation (Caminiti 1994; Deguchi 2001; Howarth 1987a; Howarth 1987b; Howells 1975a; Howells 1975b; Howells 1975c; Patriarca 1985b; Saah 1986c; Saito 2002a; Saito 2002b). Apart from Patriarca 1985, in this subgroup we found studies with the longest (five to six months) and most poorly defined follow‐up. Two of these studies did not report virologic surveillance (Deguchi 2001; Saah 1986c).
The vaccines were 33% effective (2% to 54%; Analysis 6.2.3) in preventing ILI (Caminiti 1994; Patriarca 1985b; Saito 2002a; Saito 2002b), but had no significant effects in preventing influenza (RR 0.23, 95% CI 0.05 to 1.03; Analysis 6.1.3). This observations is based on two data sets from a single, relatively small study (691 observations) (Howarth 1987a; Howarth 1987b). Both comparisons are from well‐matched vaccines.
We identified a few data sets that assessed the effectiveness of vaccines in preventing complications. Four briefly reported data sets from two studies carried out in situations of low viral circulation and poor vaccine matching reported a combined effectiveness of 65% (32% to 82%; Analysis 6.3.4) in preventing pneumonia (Howells 1975a; Howells 1975b; Howells 1975c; Saah 1986c).
During periods of low viral circulation, vaccines did prevent hospital admission for pneumonia or influenza (VE 68%; 24% to 86%; Analysis 6.4.3). However, one of the included studies was at high risk of bias, meaning that this outcome may not be accurate (Deguchi 2001). The study was set in 301 nursing homes comprising 22,462 elderly participants during the non‐epidemic 1998 to 1999 season in Japan. The same study has a large weight in the analysis of effectiveness against deaths by influenza and pneumonia (VE 71%; 43% to 85%; Analysis 6.5.3 and Analysis 6.5.4) (Caminiti 1994; Deguchi 2001; Howells 1975a; Howells 1975b; Howells 1975c; Patriarca 1985b; Saah 1986c).
Cohort studies in community‐dwelling elderly (from previous versions of the review)
We included 21 studies with 40 data sets in elderly participants living in open communities (Christenson 2001a; Christenson 2001b; Christenson 2004a; Christenson 2004b; Comeri 1995; Consonni 2004a; Consonni 2004b; Davis 2001a; Davis 2001b; Davis 2001c; Fleming 1995; Gavira Iglesias 1987; Gené Badia 1991; Hak 2002a; Hak 2002b; Hara 2006; Kawai 2003; Lopez Hernandez 1994; Mangtani 2004b; Mangtani 2004c; Mangtani 2004d; Mangtani 2004e; Mangtani 2004f; Mangtani 2004g; Mangtani 2004h; Mangtani 2004i; Mangtani 2004j; Nichol 1994a; Nichol 1994b; Nichol 1994c; Nichol 1998a; Nichol 1998b; Nichol 2003a; Nichol 2003b; Nicholson 1999; Nordin 2001a; Nordin 2001b; Pregliasco 2002; Shapiro 2003; Voordouw 2003). The studies contained over 3 million observations, mainly collected using data‐linkage from insurance reimbursement, hospital, or primary care databases; 13 of these studies reported data stratified or adjusted by risk factors and other potential confounders. These studies had long follow‐ups: 12 data sets had a follow‐up =< 3 months; 13 data sets had a follow‐up ranging from 4 to 5 months; 8 data sets had a follow‐up ranging from 6 to 7 months; 4 data sets had a follow‐up ranging from 8 to 12 months; and 2 data sets were without a well‐defined follow‐up. In nine data sets, follow‐up was defined by relying on virologic surveillance, and three data sets had laboratory confirmation of cases. On the basis of this large body of evidence, we divided our analysis into six separate comparisons.
Inactivated influenza vaccines in all community‐dwelling elderly (from previous versions of the review)
Our second comparison relied on 1 million observations in 20 data sets from 16 studies (Christenson 2001a; Christenson 2004a; Comeri 1995; Davis 2001c; Fleming 1995; Gavira Iglesias 1987; Gené Badia 1991; Hara 2006; Kawai 2003; Lopez Hernandez 1994; Mangtani 2004a; Nichol 1994a; Nichol 1994b; Nichol 1994c; Nichol 1998b; Nichol 2003a; Nichol 2003b; Nicholson 1999; Shapiro 2003; Voordouw 2003).
In elderly individuals living in the community, inactivated influenza vaccines were not effective against ILI, influenza, or pneumonia. No comparison provided enough data for stratification by viral circulation and vaccine matching.
Eight data sets (784,643 observations) with medium to long follow‐up (135 to 365 days) addressed vaccine effectiveness against hospitalisations for influenza or pneumonia (Christenson 2001a; Christenson 2004a; Nichol 1994a; Nichol 1994b; Nichol 1994c; Nichol 1998b; Nichol 2003a; Nichol 2003b). Well‐matched vaccines prevented hospital admissions for these illnesses (VE 26%; 12% to 38%; Analysis 8.4.1) but not for cardiac disease (RR 0.87, 95% CI 0.67 to 1.12; Analysis 8.9). Excluding the only study with a one‐year follow‐up (Christenson 2004a), effectiveness in preventing hospital admissions was increased to 29% (95% CI 14 to 42).
Inactivated influenza vaccines in all community‐dwelling elderly (adjusted for confounders) (from previous versions of the review)
This is another data set with seven studies contributing 19 data sets with over a million observations from several consecutive influenza seasons (Davis 2001a; Davis 2001b; Davis 2001c; Fleming 1995; Mangtani 2004b; Mangtani 2004c; Mangtani 2004d; Mangtani 2004e; Mangtani 2004f; Mangtani 2004g; Mangtani 2004h; Mangtani 2004i; Mangtani 2004j; Nichol 1998a; Nichol 2003a; Nichol 2003b; Nordin 2001a; Nordin 2001b; Voordouw 2003). Most of the studies included in this analysis used data linkage and adjusted their odds ratio (OR) calculations to allow for the effect of confounding of several variables (sex, age, smoking, comorbidities). The effects of the vaccines were all significant.
Hospitalisations for influenza or pneumonia: eight data sets (based on 949,215 observations), all but one with a follow‐up lasting 135 days (OR 0.73, 95% CI 0.67 to 0.79; Analysis 9.1) (Davis 2001a; Davis 2001b; Davis 2001c; Nichol 1998a; Nichol 2003a; Nichol 2003b; Nordin 2001b). Excluding the data set with the longest follow‐up (eight months), Nordin 2001a, did not change the result.
Hospitalisations for respiratory diseases: OR 0.78, 95% CI 0.72 to 0.85 (Analysis 9.2). Data sets have a follow‐up of 135 days or less, so a sensitivity analysis appears to be superfluous.
Hospitalisation for cardiac disease: OR 0.76, 95% CI 0.70 to 0.82 (Analysis 9.3). Data sets have a follow‐up of 135 days or less, so a sensitivity analysis appears to be superfluous.
Mortality due to all causes: seven data sets with follow‐up ranging from 75 to 240 days: OR 0.53, 95% CI 0.46 to 0.61 (Analysis 9.4) (Fleming 1995; Nichol 1998a; Nichol 2003a; Nichol 2003b; Nordin 2001a; Nordin 2001b; Voordouw 2003). Excluding data sets with a follow‐up period equal to or longer than six months, Nordin 2001a and Voordouw 2003, did not change the final result.
Death from respiratory disease was not significantly affected. Seven data sets with a follow‐up ranging from 75 to 210 days assessed the effect on mortality due to all causes (VE 42%; 24% to 55%; Analysis 8.8) (Fleming 1995; Gené Badia 1991; Lopez Hernandez 1994; Nichol 2003a; Nichol 2003b; Shapiro 2003; Voordouw 2003). Excluding four data sets with a follow‐up equal to or longer than six months, Gené Badia 1991, Lopez Hernandez 1994, Voordouw 2003, or a non‐defined follow‐up (Shapiro 2003), the efficacy falls from 42% to 39% (95% CI 28 to 49).
Inactivated influenza vaccines in community‐dwelling elderly at risk of influenza complications (from previous versions of the review)
In the third comparison, we assessed the effectiveness of inactivated influenza vaccines in elderly individuals living in the community and at risk of complications associated with influenza. People with any of the following underlying conditions were considered at risk of complications: lung disease, heart disease, renal disease, diabetes and other endocrine disorders, immunodeficiency or immunosuppressive diseases, cancer, dementia or stroke, vasculitis, or rheumatic disease. Seven data sets from six studies were relevant. The only significant effect was that for deaths from all causes (VE 61%; 3% to 84%; Analysis 10.6) from 68,032 observations with high heterogeneity (I2 = 94.1%) (Fleming 1995; Shapiro 2003; Voordouw 2003).
Inactivated influenza vaccines in community‐dwelling elderly without risk of influenza complications (from previous versions of the review)
In this stratum, six studies with seven data sets contributed several hundred thousand observations (Fleming 1995; Hak 2002a; Hak 2002b; Mangtani 2004a; Nichol 1998a; Shapiro 2003; Voordouw 2003). However, most outcomes were only assessed by one study. The only notable results were the vaccines' effectiveness in preventing hospital admission for influenza or pneumonia (VE 50%; 37% to 60%; Analysis 11.3), although this observation was based on only one data set with 101,619 observations (Nichol 1998a), and there was a lack of effect on all‐cause mortality (RR 0.65, 95% CI 0.33 to 1.29; 43,821 observations; Analysis 11.6) (Fleming 1995; Shapiro 2003; Voordouw 2003).
Inactivated influenza and polysaccharide vaccine (PPV) on community‐dwelling elderly (from previous versions of the review)
Three studies assessed the impact of inactivated influenza and concomitant PPV on hospitalisations for influenza or pneumonia or respiratory diseases (VE 33%; 30% to 36%, based on 518,748 observations; Analysis 12.2) (Christenson 2001b; Christenson 2004b; Consonni 2004b), and two data sets assessed the effect on all‐cause mortality (VE 56%; 54% to 59%; Analysis 12.4) (Christenson 2001b; Consonni 2004b).
The addition of PPV did not appear to improve the performance of influenza vaccines significantly.
Adjuvant influenza vaccines in all community‐dwelling elderly (from previous versions of the review)
Two small studies with a combined denominator of 498 assessed the impact of vaccines containing a virosomal adjuvant in preventing ILI (VE 70%; 44% to 84%; Analysis 13.1) and hospitalisations (RR 0.17, 95% CI 0.02 to 1.28; Analysis 13.2.3) during a year of low viral circulation but with a vaccine with a good match (Consonni 2004a; Pregliasco 2002). The study by Consonni 2004a also assessed the impact on all‐cause mortality and found no effect (RR 2.10, 95% CI 0.10 to 43.10; Analysis 13.3.3). This is not surprising given its population size of 129 people (too small for any significant effect to be evident).
Case‐control studies (from previous versions of the review)
We included 12 studies contributing 14 data sets (Ahmed 1995; Ahmed 1997; Crocetti 2001; Fedson 1993a; Fedson 1993b; Foster 1992; Jordan 2007; Mullooly 1994; Ohmit 1995a; Ohmit 1995b; Ohmit 1999; Puig‐Barberà 1997; Puig‐Barberà 2004; Puig‐Barbera 2007). Eight data sets from seven studies assessed the effects of inactivated influenza vaccines on community‐dwelling elderly (Ahmed 1995; Ahmed 1997; Crocetti 2001; Fedson 1993a; Fedson 1993b; Jordan 2007; Puig‐Barberà 1997; Puig‐Barbera 2007); five looked at the co‐administration of inactivated influenza with PPV on institutionalised elderly (Foster 1992; Mullooly 1994; Ohmit 1995a; Ohmit 1995b; Ohmit 1999); one study was of adjuvant influenza with PPV on community‐dwelling elderly (Puig‐Barberà 2004); and one was of adjuvanted influenza vaccines (MF59) alone (Puig‐Barbera 2007). Only three of these studies, all of which assessed influenza and pneumococcal vaccines, had a long follow‐up (six months). Since all data sets adjusted their ORs for likely confounding factors, we structured our analysis on five strata, further subdividing each analysis by viral circulation and vaccine matching whenever possible.
Inactivated influenza vaccines on community‐dwelling elderly (from previous versions of the review)
Before adjustment, inactivated influenza vaccines were associated with an increased risk of admission for any respiratory disease (OR 1.08, 95% CI 0.92 to 1.26; 20,582 observations; Analysis 14.2.1) (Ahmed 1997; Fedson 1993a; Fedson 1993b), and did not prevent hospital admission for influenza and pneumonia in elderly individuals living in the community (OR 0.89, 95% CI 0.69 to 1.15; 1074 observations; Analysis 14.1) (Crocetti 2001; Puig‐Barberà 1997), or affect hospitalisation for ILI (Analysis 14.2.2), Jordan 2007, or affect mortality from influenza and pneumonia, though this conclusion was based on a relatively small data set of 1092 observations (Analysis 14.3.1) (Ahmed 1995). The single study on adjuvanted vaccines showed no effect on pneumonia no better defined (Analysis 14.4.1) (Puig‐Barbera 2007).
Inactivated influenza vaccines on community‐dwelling elderly ‐ adjusted analysis (from previous versions of the review)
After adjustment, however, the vaccines did reduce the risk of death from influenza and pneumonia (OR 0.74, 95% CI 0.60 to 0.92; Analysis 15.3) (Ahmed 1995; Mullooly 1994), and prevent admission for influenza and pneumonia (OR 0.59, 95% CI 0.47 to 0.74; Analysis 15.1), Crocetti 2001, Foster 1992, Mullooly 1994, Puig‐Barberà 1997, Puig‐Barberà 2004, and for all respiratory diseases (OR 0.71, 95% CI 0.56 to 0.90; Analysis 15.2) (Ahmed 1997; Fedson 1993a; Fedson 1993b).
Possible causes of observed heterogeneity ‐ post hoc analysis
Of the 15 main comparisons with 61 outcome combinations, we noted in a subsequent analysis that seven comparisons with 20 outcome combinations had an I2 statistic greater than 30% and that the heterogeneity of these studies could be explained by grouping by viral circulation and vaccine matching.
Safety
We included data on local and systemic harms. For local harms, we included tenderness, sore arm, swelling, erythema, and induration. Similar local symptoms were pooled in the analysis due to small data sets. Systemic symptoms were general malaise, fever, headache, nausea, and respiratory tract symptoms.
The only studies evaluating rare adverse events were three surveillance studies assessing Guillain‐Barré syndrome with neither cohort nor case‐control design (Table 2) (Kaplan 1982; Lasky 1998; Schonberger 1979). Case finding was carried out by interviewing neurologists or by searching discharge diagnoses databases. Vaccination rates in the relevant populations were estimated from specific survey or from national immunisation survey. All studies were conducted in the USA and assessed the entire population irrespective of age. Lasky 1998 and Schonberger 1979 reported outcome stratified by age, allowing data extraction for elderly people. We reported the results of these studies in Table 2. The strong and significant association between A/New Jersey/76 swine vaccine and Guillain‐Barré syndrome during the 1976 to 1977 influenza season was not confirmed in subsequent seasons, when other vaccines not containing A/New Jersey/76 were used.
| Study | Influenza season | Vaccine | Population | Age | RR (95% CI) |
|---|---|---|---|---|---|
| 1976 to 1977 | A/New Jersey/76 or A/New Jersey/76 and A/Victoria/75 swine vaccine | All the USA population | > 64 years | 5.2 (3.9 to 7.0) | |
| 1979 to 1980 | Inactivated trivalent | All the USA population | > 18 years | 0.6 (0.45 to 1.32) | |
| 1980 to 1981 | Inactivated trivalent | All the USA population | > 18 years | 1.4 (0.80 to 1.76) | |
| 1992 to 1994 | Inactivated trivalent | 21 million | > 64 years | 1.5 (0.7 to 3.3) |
Key: CI = confidence interval; RR = risk ratio
Discussion
Summary of main results
Our findings show that according to randomised evidence, the effectiveness of trivalent inactivated influenza vaccines in elderly individuals, when considered in absolute terms, is modest irrespective of setting, outcome, population, and study design. The certainty of the evidence was low for influenza and moderate for ILI. Our estimates were consistently below those usually quoted for economic modelling or decision making. In view of the known variability of incidence and effect of influenza, we constructed a large number of comparisons and strata to minimise possible heterogeneity between studies and to aid comparability. We also performed subanalysis of studies describing better defined epidemic periods. Despite our attempts, we noted significant residual heterogeneity among studies that could be explained only in part by different study designs, methodological quality, settings, viral circulation, vaccine types and matching, age, population types, and risk factors. We think the residual heterogeneity could be the result of the unpredictable nature of the spread of influenza and ILI and the bias caused by the non‐randomised nature of our evidence base. Our sensitivity analysis did not affect the final result.
Overall completeness and applicability of evidence
Whatever the causes of observed variability, we believe that the decision to vaccinate against influenza cannot be made based on the results of single studies, or reporting observations from a few seasons; rather, it should be taken on the basis of all available evidence. The conclusions drawn from studies done in individuals living in long‐term care facilities differ from those drawn from studies in individuals living in the community. Studies done in residents of care homes often indicate the inevitably improvised nature of efforts to study the effect of vaccination during an epidemic. The resident population is usually more homogeneous than that in the community: older, with similar viral exposure and risk levels. Despite remaining heterogeneity and an overestimation of the effects as a result of study design, it is possible to detect a gradient of effectiveness, in which vaccines have little effect on cases of ILI. This finding suggests that control of influenza through vaccination is a possibility. However, the effectiveness of vaccines in the community is modest, irrespective of adjustment for systematic differences between vaccine recipients and non‐recipients. The difficulties of achieving good coverage in those who need it most or the diluting effect on vaccines for influenza of other agents circulating in the community (causing ILI, clinically indistinguishable from influenza) might be to blame. We noted empirical proof of both these possibilities, with differential vaccine uptake among the same population (linked to age, sex, and health status) and a low effect on ILI throughout our data sets, even in periods of supposedly high influenza viral circulation, when the proportion of cases of ILI caused by influenza are highest and the possible benefits of vaccination should be greatest.
The impact of vaccines on pneumonia, mortality, and hospitalisations from the randomised evidence available is insufficient to draw conclusions. Very few studies have captured data on these outcomes, and where we have obtained data, the rate of events was too low to be able to determine the size of effect (summary of findings Table 1).
Safety does not appear to be a particular problem: the public health safety profile of the vaccines is acceptable. However, relatively few studies assessed safety outcomes.
Quality of the evidence
We rated the quality of evidence to be moderate for ILI and fever, low for influenza and nausea, and very low for hospitalisations and death (summary of findings Table 1). Most of the available evidence for these outcomes is from studies with unclear risk of bias across multiple domains. Fever was the only outcome where we did not consider bias to be influential due to consistency in direction and size of effect with the low risk of bias study (Govaert 1994a). The lack of detail regarding the diagnosis of influenza limits the applicability of our findings to laboratory‐confirmed influenza and is a source of indirectness.
For other outcomes (e.g. nausea and fever), the method of ascertainment is less uncertain due to the expected mechanism of action of vaccines.
Imprecision arising from wide CIs or low event rates, or both, affects the evidence for adverse events, death, and pneumonia to varying degrees. The CIs for fever and nausea were wide (serious imprecision), but death and pneumonia were rare events in the studies, prompting us to downgrade two levels for very serious imprecision. The rating of very low quality for the latter two outcomes reflects the sparse nature of the data available for analysis.
The main problem with interpreting our substantial data set is caused by the relative scarcity of randomised controlled trials (RCTs). Only one trial assessed currently available vaccines and reached satisfactory completion (Govaert 1994a). The remainder of the data set consists of evidence from non‐RCTs.
Our main concern was the quality of the non‐RCTs, which likely affected the estimates of effect reported in our review. The findings of the included cohort studies are likely to have been affected to varying degrees by selection bias. Differential uptake of influenza vaccines is linked to several factors (anxiety over unwanted effects, disease threat perception, societal and economic conditions, education, health status) and hence to outcome. Confounding by indication (people with chronic illness or people who are perceived to be frailer than others are more likely to be vaccinated) might reduce the estimated vaccine efficacy. People with terminal illness or with socio‐economic disadvantages are less likely to be vaccinated, and this fact might enhance vaccine efficacy. Both these interpretations are based on empirical evidence. For example, one cohort study had difficulty achieving high coverage in those most at need (Gené Badia 1991). Differential vaccine uptake and the resulting selection bias is the most likely explanation for the high effectiveness of influenza vaccines in preventing deaths from all causes. A good example of the potential effect of such confounders is the apparently counterintuitive effectiveness of the vaccines in elderly individuals living in the community. In this population, vaccine effectiveness shows an implausible sequence: the vaccines are apparently ineffective in the prevention of influenza, ILI, pneumonia, hospital admissions, or deaths from any respiratory disease, but are effective in the prevention of hospital admission for influenza and pneumonia and in the prevention of deaths from all causes.
Non‐RCT evidence in this review is open to any alternative interpretation and consistently fails to give satisfactory answers. Since the publication of our 2006 review (Rivetti 2006), several empirical studies looking at the effect of selection bias in retrospective cohorts (variously called selection bias, confounding by indication, or healthy user effect) have been published. Some confirmed the presence and effect of confounders (Eurich 2008; Fukushima 2008; Glezen 2006; Hirota 2008; Jackson 2006a; Jackson 2006b; Jackson 2006c; Jackson 2006d; Jackson 2006e). Other studies, mainly carried out by the authors of cohort studies in question, failed to find any effect of confounding on mortality once adjustment had been carried out (Groenwold 2008; Groenwold 2009; Hak 2006; Nichol 2007). For example, proof of bias was provided by a study evaluating the risk of hospitalisation and death in vaccinated compared with unvaccinated seniors during influenza and non‐influenza periods (Jackson 2006a). Consistent with other published studies, during influenza season, vaccination was associated with a 44% reduction in risk of all‐cause mortality. However, in the period before the influenza season, vaccination was associated with a 61% reduction in risk of this outcome. The reduction in risk before the influenza season indicates the presence of bias due to preferential selection of vaccination by relatively healthy seniors, and the strength of that bias is sufficient to account entirely for the association found during the influenza season. In a second, nested case‐control study, seniors with functional markers of frailty (such as dependence on washing) were found to be at a greatly increased risk of death and were less likely to have received influenza vaccine, indicating that these factors are important sources of bias in assessment of influenza vaccine effectiveness (Jackson 2006b).
Regardless of the results of empirical studies, the sheer implausibility of the effectiveness sequence which ends with high estimates of effect against mortality from all causes points to considerable confounding and calls into question the reliability of using such non‐specific outcomes. Systematic differences between the intervention and control arms of cohort studies are likely to be the result of a baseline imbalance in health status and other known and unknown systematic differences in the two groups of participants. The rationale of the work starts from the observation that the 47% reduction in risk of all‐cause mortality in elderly community dwellers observed in our review exceeds by far the estimated possible impact of influenza on winter‐seasonal mortality of 5% in an average season (Glezen 2006). Until improvement of cohort study design is available, the use in non‐RCTs of highly non‐specific outcome indicators, such as all‐cause mortality, is likely to lead to unrealistic estimates of the effects of the vaccines.
Evidence from RCTs, in which bias is reduced to a minimum, is scant and badly reported. Unfortunately, because of the global recommendations on influenza vaccination, placebo‐controlled trials, which could clarify the effects of influenza vaccines in individuals, are no longer considered possible on ethical grounds.
Since publication of the first version of this review, we have noted increased reliance of the case test‐negative study design to assess influenza vaccine effectiveness post hoc, that is after the influenza 'season'. The case test‐negative design is described in detail elsewhere (Foppa 2013; Valenciano 2012).
The data included in a case test‐negative design are sometimes harvested from an ongoing networked surveillance cohort. In this case, the case test‐negative design is described as 'nested' within the cohort. The source cohort can include community and hospitalised cases and controls, allowing flexibility. In Europe, the surveillance programme has a formal structure and is known as Influenza ‐ Monitoring Vaccine Effectiveness (I‐MOVE). I‐MOVE is partly funded and co‐ordinated by the European Centre for Disease Prevention and Control (sites.google.com/site/epiflu/Home) (Kissling 2017).
Briefly, the study design, which is similar to a case‐control design, consists of selecting influenza cases (e.g. cases of ILI who have tested positive for influenza) and controls (cases of ILI who have tested negative) and calculating the relevant odds ratio (OR). Cases and controls are subsequently stratified by vaccination status. An estimate of vaccines effectiveness (VE) is derived from the OR of influenza in vaccinated/unvaccinated participants using the standard formula VE = 1 ‐ OR%. The practice of using the OR estimate as an approximation of the risk ratio was first used in a 1980 study on pneumococcal vaccine (Broome 1980).
Despite their popularity, test‐negative designs have limited public health significance.
The design does not test field effectiveness, but the capacity of the vaccines to generate a negative polymerase chain reaction result (what we would call laboratory efficacy). Both cases and controls are symptomatic, so any prevention is solely focused on the negativity of laboratory tests. In addition, no useful public health absolute measures of effect can be derived (such as absolute risk reduction and its reciprocal number needed to vaccinate) by such designs, as the background rates of infection and viral circulation are not part of the calculation of the estimates of effect. The mathematical method first used by Broome and colleagues is correct if three key assumptions are met:
-
the risk of non‐influenza ILI is the same in vaccinated and non‐vaccinated individuals (a factor called “k” by Broome and colleagues) (Broome 1980);
-
the attack rate in the vaccinated population is low; and
-
the circulating serotypes are similar to those in the selected population within the case‐control studies based on test‐negative design.
All assumptions are unlikely to be fulfilled at the same time, especially in multicentre/multi country surveillance cohorts with a non‐random sampling frame. For example, Cowling and colleagues reported an increased risk of non‐influenza respiratory virus infections associated with receipt of inactivated influenza vaccine. In addition, the OR will give falsely high VE if the attack rate in the vaccinated population is high (Cowling 2012; Orenstein 1985). Apart from these fundamental design problems, case test‐negative studies are also affected by poor reporting. (See also Perencevich 2013a; Perencevich 2013b).
Potential biases in the review process
The publication of our 2006 review sparked a discussion that continues to this day (Rivetti 2006). Because we are conscious that (despite the inconclusive evidence) we could have introduced our own biases into the reviewing process, we re‐extracted and reassessed all studies included in this and all other reviews of influenza vaccine studies (259 primary studies, reporting 274 data sets). We worked independently in two teams of two, extracting directly into pre‐set forms with rigid criteria but using the same quality assessment scales used in the original version of the review. As well as assessing quality of study design, we assessed concordance between data presented and conclusions and direction of conclusions (in favour or not of the performance of influenza vaccines). We also looked at the relationship between these variables and study funding and journal of publication. We found that higher‐quality studies were significantly more likely to show concordance between data presented and conclusions (OR 16.35, 95% CI 4.24 to 63.04) and less likely to favour effectiveness of vaccines (0.04, 0.02 to 0.09). Government‐funded studies were less likely to have conclusions favouring the vaccines (0.45, 0.26 to 0.90). A higher mean journal impact factor was associated with complete or partial industry funding compared with government or private funding and no funding (differences between means 5.04). Study size was not associated with concordance, content of take‐home message, funding, or study quality. Higher citation index factor was associated with partial or complete industry funding (Jefferson 2009b).
We concluded that the general quality of influenza vaccines studies is very low and that publication in prestigious journals is associated with partial or total industry funding. We could not explain this association with study quality, size or study status (registration trials using surrogate outcomes such as antibody titres were not included in the review).
Agreements and disagreements with other studies or reviews
Nichol provides a useful overview of reviews of influenza vaccines in all age groups (Nichol 2008). For the elderly, she identified our review and a review by Vu 2002. Although the point estimates appear approximately similar across the reviews, both Vu and Nichol fail to assess study quality and interpret results accordingly. We have summarised other main reviews identified below.
The review Belongia 2016 includes 56 case test‐negative studies. The authors briefly list five methodological quality criteria which do not appear to have been validated. The authors do not list blinding of assessors to vaccination status, a very well‐known source of bias in case‐control studies. The authors report substantial variation in vaccine effectiveness, but mention none of the limitations of test‐negative designs.
The review by Darvishian 2017 includes data from 4975 elderly who had been included in previous test‐negative studies. The text refers to “individual participant data meta‐analysis”, but the basic problems with test‐negative designs are not addressed, and previous study authors were asked to extract their own study data on provided forms with no methodological quality assessment. The authors conclude that "Influenza vaccination is moderately effective against laboratory‐confirmed influenza in elderly people during epidemic seasons".
The review by Osterholm 2012 included comparative studies with data from the USA and influenza diagnosed by polymerase chain reaction. The authors found variable effectiveness but a lack of evidence in the elderly.

Study flow. We identified no new randomised controlled trials for the 2016 update and stabilisation.
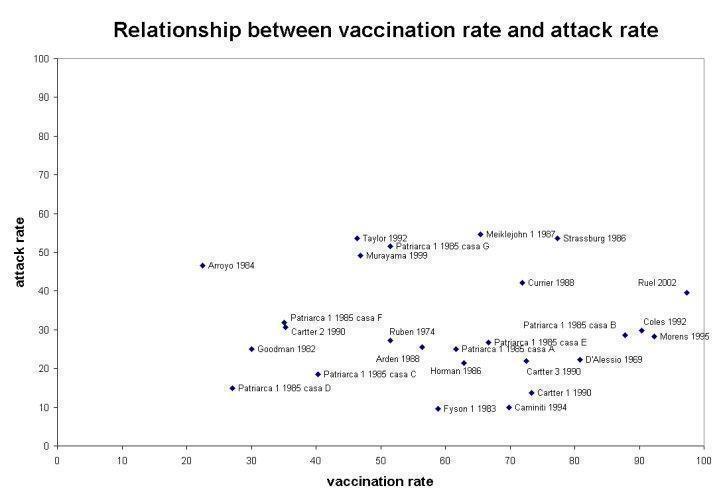
Relationship between vaccination rate and attack rate

Comparison 1: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine, Outcome 1: Influenza

Comparison 1: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine, Outcome 2: Influenza‐like illness

Comparison 1: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine, Outcome 3: Pneumonia

Comparison 1: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine, Outcome 4: All deaths

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 1: General malaise

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 2: Nausea

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 3: Upper respiratory tract symptoms

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 4: Headache

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 5: Fever

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 6: Local tenderness/sore arm

Comparison 2: Influenza vaccines versus placebo: randomised controlled trials ‐ parenteral vaccine ‐ adverse events, Outcome 7: Swelling ‐ erythema ‐ induration

Comparison 3: Influenza vaccines versus placebo: randomised controlled trials ‐ inactivated aerosol vaccine, Outcome 1: Influenza

Comparison 3: Influenza vaccines versus placebo: randomised controlled trials ‐ inactivated aerosol vaccine, Outcome 2: Influenza‐like illness

Comparison 4: Influenza vaccines versus placebo: randomised controlled trials ‐ live aerosol vaccine, Outcome 1: Influenza

Comparison 5: Influenza vaccines versus placebo: randomised controlled trials ‐ live aerosol vaccine ‐ adverse events, Outcome 1: General malaise

Comparison 5: Influenza vaccines versus placebo: randomised controlled trials ‐ live aerosol vaccine ‐ adverse events, Outcome 2: Fever

Comparison 5: Influenza vaccines versus placebo: randomised controlled trials ‐ live aerosol vaccine ‐ adverse events, Outcome 3: Upper respiratory tract symptoms

Comparison 5: Influenza vaccines versus placebo: randomised controlled trials ‐ live aerosol vaccine ‐ adverse events, Outcome 4: Lower respiratory tract symptoms

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 1: Influenza

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 2: Influenza‐like illness

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 3: Pneumonia

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 4: Hospitalisation for influenza‐like illness or pneumonia

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 5: Deaths from flu or pneumonia

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 6: All deaths

Comparison 6: Influenza vaccines versus no vaccination: cohort studies in nursing homes, Outcome 7: Influenza cases (clinically defined without clear definition)

Comparison 7: Influenza and pneumococcal vaccines versus no vaccination: case‐control studies in nursing homes, Outcome 1: Influenza‐like illness

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 1: Influenza

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 2: Influenza‐like illness

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 3: Pneumonia

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 4: Hospitalisation for flu or pneumonia

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 5: Hospitalisation for any respiratory disease

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 6: Deaths from flu or pneumonia

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 7: Deaths from respiratory disease

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 8: All deaths

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 9: Hospitalisation for heart disease

Comparison 8: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 10: Combined outcome: all deaths or severe respiratory illness

Comparison 9: Influenza vaccines versus no vaccination: cohort studies in community ‐ adjusted rates, Outcome 1: Hospitalisation for influenza or pneumonia

Comparison 9: Influenza vaccines versus no vaccination: cohort studies in community ‐ adjusted rates, Outcome 2: Hospitalisation for any respiratory disease

Comparison 9: Influenza vaccines versus no vaccination: cohort studies in community ‐ adjusted rates, Outcome 3: Hospitalisation for heart disease

Comparison 9: Influenza vaccines versus no vaccination: cohort studies in community ‐ adjusted rates, Outcome 4: All deaths

Comparison 9: Influenza vaccines versus no vaccination: cohort studies in community ‐ adjusted rates, Outcome 5: Combined outcome: all deaths or severe respiratory illness

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 1: Influenza

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 2: Pneumonia

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 3: Hospitalisation for influenza or pneumonia

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 4: Hospitalisation for any respiratory disease

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 5: Deaths from respiratory disease

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 6: All deaths

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 7: Hospitalisation for heart disease

Comparison 10: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ risk groups, Outcome 8: Combined outcome: all deaths or severe respiratory illness

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 1: Influenza

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 2: Pneumonia

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 3: Hospitalisation for influenza or pneumonia

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 4: Hospitalisation for any respiratory disease

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 5: Deaths from respiratory disease

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 6: All deaths

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 7: Hospitalisation for heart disease

Comparison 11: Influenza vaccines versus no vaccination: cohort studies in community‐dwellers ‐ no risk groups, Outcome 8: Combined outcome: all deaths or severe respiratory illness

Comparison 12: Influenza and pneumococcal vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 1: Influenza‐like illness

Comparison 12: Influenza and pneumococcal vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 2: Hospitalisation for influenza or pneumonia or respiratory disease

Comparison 12: Influenza and pneumococcal vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 3: Deaths from influenza or pneumonia

Comparison 12: Influenza and pneumococcal vaccines versus no vaccination: cohort studies in community‐dwellers, Outcome 4: All deaths

Comparison 13: Influenza vaccines with adjuvant versus no vaccination: cohort studies in community‐dwellers, Outcome 1: Influenza‐like illness

Comparison 13: Influenza vaccines with adjuvant versus no vaccination: cohort studies in community‐dwellers, Outcome 2: Hospitalisation for influenza or pneumonia or respiratory disease

Comparison 13: Influenza vaccines with adjuvant versus no vaccination: cohort studies in community‐dwellers, Outcome 3: All deaths

Comparison 14: Influenza vaccines versus no vaccination: case‐control studies in community, Outcome 1: Hospitalisations for influenza or pneumonia

Comparison 14: Influenza vaccines versus no vaccination: case‐control studies in community, Outcome 2: Hospitalisations for any respiratory disease

Comparison 14: Influenza vaccines versus no vaccination: case‐control studies in community, Outcome 3: Deaths from influenza or pneumonia

Comparison 14: Influenza vaccines versus no vaccination: case‐control studies in community, Outcome 4: Pneumonia (no better defined)

Comparison 15: Influenza vaccines versus no vaccination: case‐control studies in community ‐ adjusted rates, Outcome 1: Hospitalisations for influenza or pneumonia

Comparison 15: Influenza vaccines versus no vaccination: case‐control studies in community ‐ adjusted rates, Outcome 2: Hospitalisations for any respiratory disease

Comparison 15: Influenza vaccines versus no vaccination: case‐control studies in community ‐ adjusted rates, Outcome 3: Deaths from pneumonia or influenza

Comparison 16: Influenza and pneumococcal vaccines versus no vaccination: case‐control studies in community, Outcome 1: Hospitalisations for influenza or pneumonia

Comparison 17: Influenza and pneumococcal vaccines versus no vaccination: case‐control studies in community ‐ adjusted rates, Outcome 1: Hospitalisations for influenza or pneumonia

Comparison 18: Sensitivity analysis: comparison 01: subgroup analysis by study quality, Outcome 1: Influenza‐like illness
| Influenza vaccine compared to placebo for preventing influenza in the elderly | ||||||
| Patient or population: people aged over 65 years | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with influenza vaccine | |||||
| Influenza assessed with: laboratory confirmation Follow‐up was conducted over an influenza season. | Study population1 | RR 0.42 | 2217 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 24 per 1000 | |||||
| Influenza‐like illness Follow‐up was conducted over an influenza season. | Study population1 | RR 0.59 | 6894 | ⊕⊕⊕⊝ | ||
| 59 per 1000 | 35 per 1000 | |||||
| Pneumonia Follow‐up was conducted over an influenza season. | No events occurred in 1 study of 699 people. | ‐ | 699 | ⊕⊝⊝⊝ | ||
| Hospitalisations ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| All deaths Follow‐up was conducted over an influenza season. | Study population1 | RR 1.02 | 699 | ⊕⊝⊝⊝ | ||
| 6 per 1000 | 6 per 1000 | |||||
| Fever Follow‐up was conducted over an influenza season. | Study population1 | RR 1.57 | 2519 | ⊕⊕⊕⊝ | ||
| 16 per 1000 | 25 per 1000 | |||||
| Nausea Follow‐up was conducted over an influenza season. | Study population1 | RR 1.75 | 672 | ⊕⊕⊝⊝ | ||
| 24 per 1000 | 42 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk taken as aggregate of the study control group risks. | ||||||
| Review version (searches date) | Number of included trials (RCTs/CCTs) | Number of included observational studies | Estimates of effect (RCTs/CCTs only) | Conclusions (1 to 2 lines from abstract) |
|---|---|---|---|---|
| Version 1 (24 May 2006) | 9 | 621 | Influenza‐like illness LAIV = no data TIV = 41% (95% CI 27% to 53%) IAV = n.s. Influenza LAIV = n.s. TIV = 58% (95% CI 34% to 73%) IAV = n.s. | In long‐term care facilities, where vaccination is most effective against complications, the aims of the vaccination campaign are fulfilled, at least in part. However, according to reliable evidence the usefulness of vaccines in the community is modest. The apparent high effectiveness of the vaccines in preventing death from all causes may reflect a baseline imbalance in health status and other systematic differences in the 2 groups of participants. |
| Version 2 (20 January 2010) | 9 | 662 | Influenza‐like illness LAIV = no data TIV = 41% (95% CI 27% to 53%) IAV = n.s. Influenza LAIV = n.s. TIV = 58% (95% CI 34% to 73%) IAV = n.s. | The available evidence is of poor quality and provides no guidance regarding the safety, efficacy, or effectiveness of influenza vaccines for people aged 65 years or older. To resolve the uncertainty, an adequately powered publicly‐funded randomised, placebo‐controlled trial run over several seasons should be undertaken. |
| 1These include 49 cohort studies for efficacy/effectiveness (79 data sets); 10 case‐control studies for efficacy/effectiveness (12 data sets); 3 studies (cohorts) for Guillain‐Barré syndrome. Key: CCT = controlled clinical trial; CI = confidence interval; IAV = inactivated aerosol vaccines; LAIV = live attenuated vaccines; n.s. = not significant; RCT = randomised controlled trial; TIV = trivalent inactivated vaccines | ||||
| Study | Influenza season | Vaccine | Population | Age | RR (95% CI) |
|---|---|---|---|---|---|
| 1976 to 1977 | A/New Jersey/76 or A/New Jersey/76 and A/Victoria/75 swine vaccine | All the USA population | > 64 years | 5.2 (3.9 to 7.0) | |
| 1979 to 1980 | Inactivated trivalent | All the USA population | > 18 years | 0.6 (0.45 to 1.32) | |
| 1980 to 1981 | Inactivated trivalent | All the USA population | > 18 years | 1.4 (0.80 to 1.76) | |
| 1992 to 1994 | Inactivated trivalent | 21 million | > 64 years | 1.5 (0.7 to 3.3) | |
| Key: CI = confidence interval; RR = risk ratio | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Influenza Show forest plot | 3 | 2217 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.27, 0.66] |
| 1.1.1 Outbreak ‐ vaccine matching ‐ community ‐ healthy and ill | 1 | 1838 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.74] |
| 1.1.2 Outbreak ‐ vaccine matching ‐ psychiatric hospital | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.12, 1.06] |
| 1.1.3 No outbreak ‐ vaccine matching ‐ nursing home ‐ healthy and ill | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.20, 1.25] |
| 1.2 Influenza‐like illness Show forest plot | 4 | 6894 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.47, 0.73] |
| 1.2.1 Outbreak ‐ vaccine matching (circulating strains) ‐ community ‐ healthy | 2 | 2047 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.79] |
| 1.2.2 Outbreak ‐ vaccine matching ‐ community ‐ risk groups | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.53] |
| 1.2.3 Outbreak ‐ vaccine matching ‐ nursing home ‐ healthy | 1 | 4180 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.37, 0.80] |
| 1.2.4 Outbreak ‐ vaccine matching ‐ psychiatric hospital | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.92] |
| 1.3 Pneumonia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.1 Outbreak ‐ vaccine matching ‐ community ‐ healthy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 All deaths Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.1 Outbreak ‐ vaccine matching ‐ community ‐ healthy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 General malaise Show forest plot | 4 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.61] |
| 2.2 Nausea Show forest plot | 1 | 672 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.74, 4.12] |
| 2.3 Upper respiratory tract symptoms Show forest plot | 2 | 713 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.90, 2.01] |
| 2.4 Headache Show forest plot | 3 | 2519 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.76, 1.58] |
| 2.5 Fever Show forest plot | 3 | 2519 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.92, 2.71] |
| 2.6 Local tenderness/sore arm Show forest plot | 4 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [2.61, 4.87] |
| 2.7 Swelling ‐ erythema ‐ induration Show forest plot | 2 | 1847 | Risk Ratio (M‐H, Random, 95% CI) | 8.23 [3.98, 17.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Influenza Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.40, 1.99] |
| 3.1.1 Outbreak ‐ vaccine matching ‐ psychiatric hospital | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.40, 1.99] |
| 3.2 Influenza‐like illness Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.41, 1.71] |
| 3.2.1 Outbreak ‐ vaccine matching ‐ psychiatric hospital | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.41, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Influenza Show forest plot | 1 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.17] |
| 4.1.1 No outbreak ‐ vaccine matching ‐ nursing home ‐ healthy and ill | 1 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 General malaise Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.18, 53.20] |
| 5.2 Fever Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.09, 33.24] |
| 5.3 Upper respiratory tract symptoms Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.42, 6.29] |
| 5.4 Lower respiratory tract symptoms Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.41, 20.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Influenza Show forest plot | 8 | 1941 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.32, 1.29] |
| 6.1.1 Outbreak ‐ vaccine matching | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.43, 2.51] |
| 6.1.2 Outbreak ‐ vaccine matching absent or unknown | 2 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 1.04] |
| 6.1.3 No outbreak ‐ vaccine matching | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 1.03] |
| 6.1.4 No outbreak ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.2 Influenza‐like illness Show forest plot | 26 | 12388 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.66, 0.88] |
| 6.2.1 Outbreak ‐ vaccine matching (circulating strains) | 16 | 5963 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.94] |
| 6.2.2 Outbreak ‐ vaccine matching absent or unknown | 6 | 4096 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.05] |
| 6.2.3 No outbreak ‐ vaccine matching | 4 | 2329 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 6.2.4 No outbreak ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.3 Pneumonia Show forest plot | 17 | 10274 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.43, 0.66] |
| 6.3.1 Outbreak ‐ vaccine matching | 8 | 4482 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.70] |
| 6.3.2 Outbreak ‐ vaccine matching absent or unknown | 5 | 3991 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.21] |
| 6.3.3 No outbreak ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.3.4 No outbreak ‐ matching absent or unknown | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.68] |
| 6.4 Hospitalisation for influenza‐like illness or pneumonia Show forest plot | 12 | 28032 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.81] |
| 6.4.1 Outbreak ‐ vaccine matching | 8 | 2027 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 6.4.2 Outbreak ‐ vaccine matching absent or unknown | 2 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.43, 1.58] |
| 6.4.3 No outbreak ‐ vaccine matching | 2 | 22704 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.76] |
| 6.4.4 No outbreak ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.5 Deaths from flu or pneumonia Show forest plot | 27 | 32179 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.63] |
| 6.5.1 Outbreak ‐ vaccine matching | 16 | 6127 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.41, 0.83] |
| 6.5.2 Outbreak ‐ vaccine matching absent or unknown | 4 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.02] |
| 6.5.3 No outbreak ‐ vaccine matching | 3 | 23162 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.87] |
| 6.5.4 No outbreak ‐ vaccine matching absent or unknown | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.67] |
| 6.6 All deaths Show forest plot | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.77] |
| 6.6.1 Outbreak ‐ vaccine matching | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.77] |
| 6.6.2 Outbreak ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.6.3 No outbreak ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.6.4 No outbreak ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.7 Influenza cases (clinically defined without clear definition) Show forest plot | 7 | 24238 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 1.02] |
| 6.7.1 Outbreak ‐ vaccine matching | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.11, 4.56] |
| 6.7.2 Outbreak ‐ vaccine matching absent or unknown | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.59] |
| 6.7.3 No outbreak ‐ vaccine matching | 1 | 22462 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 6.7.4 No outbreak ‐ vaccine matching absent or unknown | 3 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Influenza‐like illness Show forest plot | 1 | 1198 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.40, 0.68] |
| 7.1.1 Outbreak ‐ vaccine matching | 1 | 1198 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.40, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Influenza Show forest plot | 2 | 18249 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 2.01] |
| 8.1.1 Epidemic year ‐ vaccine matching | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.37] |
| 8.1.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.1.3 Non‐epidemic year ‐ vaccine matching | 1 | 17822 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.91] |
| 8.1.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.2 Influenza‐like illness Show forest plot | 4 | 9613 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.33] |
| 8.2.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.2.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.2.3 Non‐epidemic year ‐ vaccine matching | 2 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.58, 2.03] |
| 8.2.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.16, 4.55] |
| 8.2.5 Epidemic year ‐ vaccine not matching | 1 | 4709 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.24, 0.81] |
| 8.3 Pneumonia Show forest plot | 2 | 18090 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
| 8.3.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3.3 Non‐epidemic year ‐ vaccine matching | 1 | 17822 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| 8.3.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.16, 57.42] |
| 8.4 Hospitalisation for flu or pneumonia Show forest plot | 9 | 784643 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.85] |
| 8.4.1 Epidemic year ‐ vaccine matching | 6 | 727776 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 8.4.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.4.3 Non‐epidemic year ‐ vaccine matching | 1 | 25532 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.83] |
| 8.4.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 1 | 26626 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.99] |
| 8.4.5 Epidemic year ‐ vaccine not matching | 1 | 4709 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.33, 2.40] |
| 8.5 Hospitalisation for any respiratory disease Show forest plot | 5 | 567299 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.43] |
| 8.5.1 Epidemic year ‐ vaccine matching | 3 | 515141 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.37, 1.64] |
| 8.5.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.5.3 Non‐epidemic year ‐ vaccine matching | 1 | 25532 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 8.5.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 1 | 26626 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.01, 1.34] |
| 8.6 Deaths from flu or pneumonia Show forest plot | 1 | 163391 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.09] |
| 8.6.1 Epidemic year ‐ vaccine matching | 1 | 163391 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.09] |
| 8.6.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.6.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.6.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.7 Deaths from respiratory disease Show forest plot | 1 | 426668 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.25, 1.39] |
| 8.7.1 Epidemic year ‐ vaccine matching | 1 | 426668 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.25, 1.39] |
| 8.8 All deaths Show forest plot | 8 | 409468 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.47, 0.80] |
| 8.8.1 Epidemic year ‐ vaccine matching | 4 | 300332 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.50, 0.70] |
| 8.8.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.8.3 Non‐epidemic year ‐ vaccine matching | 3 | 104427 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.39] |
| 8.8.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.8.5 Epidemic year ‐ vaccine not matching | 1 | 4709 | Risk Ratio (M‐H, Random, 95% CI) | 3.89 [0.90, 16.89] |
| 8.9 Hospitalisation for heart disease Show forest plot | 6 | 433934 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.12] |
| 8.9.1 Epidemic year ‐ vaccine matching | 4 | 381776 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.56, 0.97] |
| 8.9.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.9.3 Non‐epidemic year ‐ vaccine matching | 1 | 25532 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.81, 1.38] |
| 8.9.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 1 | 26626 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.07, 2.36] |
| 8.10 Combined outcome: all deaths or severe respiratory illness Show forest plot | 3 | 290819 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.85] |
| 8.10.1 Epidemic year ‐ vaccine matching | 2 | 132365 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.42, 1.55] |
| 8.10.2 Epidemic year ‐ vaccine matching absent or unknown | 1 | 158454 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.69, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Hospitalisation for influenza or pneumonia Show forest plot | 8 | Odds Ratio (IV, Random, 95% CI) | 0.73 [0.67, 0.79] | |
| 9.1.1 Epidemic ‐ vaccine matching | 6 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.65, 0.77] | |
| 9.1.2 Non‐epidemic ‐ vaccine not matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.90 [0.58, 1.38] | |
| 9.1.3 Epidemic year ‐ vaccine matching absent or unknown | 1 | Odds Ratio (IV, Random, 95% CI) | 0.82 [0.68, 0.98] | |
| 9.2 Hospitalisation for any respiratory disease Show forest plot | 13 | Odds Ratio (IV, Random, 95% CI) | 0.78 [0.72, 0.85] | |
| 9.2.1 Epidemic ‐ vaccine matching | 9 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.67, 0.74] | |
| 9.2.2 Non‐epidemic ‐ vaccine not matching | 2 | Odds Ratio (IV, Random, 95% CI) | 0.91 [0.76, 1.08] | |
| 9.2.3 Non‐epidemic year ‐ vaccine matching | 2 | Odds Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.06] | |
| 9.3 Hospitalisation for heart disease Show forest plot | 6 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.70, 0.82] | |
| 9.3.1 Epidemic year ‐ vaccine matching | 5 | Odds Ratio (IV, Random, 95% CI) | 0.75 [0.70, 0.82] | |
| 9.3.2 Non‐epidemic ‐ vaccine not matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.80 [0.55, 1.16] | |
| 9.4 All deaths Show forest plot | 7 | Odds Ratio (IV, Random, 95% CI) | 0.53 [0.46, 0.61] | |
| 9.4.1 Epidemic year ‐ vaccine matching | 5 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.42, 0.53] | |
| 9.4.2 Epidemic year ‐ vaccine matching absent or unknown | 1 | Odds Ratio (IV, Random, 95% CI) | 0.65 [0.57, 0.75] | |
| 9.4.3 Non‐epidemic year ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.60, 0.97] | |
| 9.5 Combined outcome: all deaths or severe respiratory illness Show forest plot | 1 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.37, 1.34] | |
| 9.5.1 Epidemic year ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.37, 1.34] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Influenza Show forest plot | 1 | 6423 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.17] |
| 10.1.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.1.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.1.3 Non‐epidemic year ‐ vaccine matching | 1 | 6423 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.17] |
| 10.1.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.2 Pneumonia Show forest plot | 1 | 6423 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.76, 1.94] |
| 10.2.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.2.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.2.3 Non‐epidemic year ‐ vaccine matching | 1 | 6423 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.76, 1.94] |
| 10.2.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.3 Hospitalisation for influenza or pneumonia Show forest plot | 1 | 45932 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.86] |
| 10.3.1 Epidemic year ‐ vaccine matching | 1 | 45932 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.86] |
| 10.3.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.3.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.3.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.4 Hospitalisation for any respiratory disease Show forest plot | 2 | 189004 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 10.4.1 Epidemic year ‐ vaccine matching | 2 | 189004 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 10.4.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.4.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.4.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.5 Deaths from respiratory disease Show forest plot | 1 | 142464 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 10.5.1 Epidemic year ‐ vaccine matching | 1 | 142464 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 10.6 All deaths Show forest plot | 3 | 68032 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.16, 0.97] |
| 10.6.1 Epidemic year ‐ vaccine matching | 1 | 2344 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.92] |
| 10.6.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.6.3 Non‐epidemic year ‐ vaccine matching | 2 | 65688 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.17, 1.28] |
| 10.6.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.7 Hospitalisation for heart disease Show forest plot | 1 | 45932 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.03] |
| 10.7.1 Epidemic year ‐ vaccine matching | 1 | 45932 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.03] |
| 10.7.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.7.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.7.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.8 Combined outcome: all deaths or severe respiratory illness Show forest plot | 2 | 146248 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.49, 0.74] |
| 10.8.1 Epidemic year ‐ vaccine matching | 1 | 54438 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.49, 0.60] |
| 10.8.2 Epidemic year ‐ vaccine matching absent or unknown | 1 | 91810 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.61, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Influenza Show forest plot | 1 | 11399 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.17] |
| 11.1.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.1.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.1.3 Non‐epidemic year ‐ vaccine matching | 1 | 11399 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.17] |
| 11.1.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.2 Pneumonia Show forest plot | 1 | 11399 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.92] |
| 11.2.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.2.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.2.3 Non‐epidemic year ‐ vaccine matching | 1 | 11399 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.92] |
| 11.2.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.3 Hospitalisation for influenza or pneumonia Show forest plot | 1 | 101619 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.40, 0.63] |
| 11.3.1 Epidemic year ‐ vaccine matching | 1 | 101619 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.40, 0.63] |
| 11.3.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.3.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.3.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4 Hospitalisation for any respiratory disease Show forest plot | 2 | 376324 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.27] |
| 11.4.1 Epidemic year ‐ vaccine matching | 2 | 376324 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.27] |
| 11.4.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.5 Deaths from respiratory disease Show forest plot | 1 | 281424 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.31, 1.53] |
| 11.5.1 Epidemic year ‐ vaccine matching | 1 | 281424 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.31, 1.53] |
| 11.6 All deaths Show forest plot | 3 | 43821 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.33, 1.29] |
| 11.6.1 Epidemic year ‐ vaccine matching | 1 | 7047 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.26, 4.49] |
| 11.6.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.6.3 Non‐epidemic year ‐ vaccine matching | 2 | 36774 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.27, 1.30] |
| 11.6.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.7 Hospitalisation for heart disease Show forest plot | 1 | 101619 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.01] |
| 11.7.1 Epidemic year ‐ vaccine matching | 1 | 101619 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.01] |
| 11.7.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.7.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.7.4 Non‐epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.8 Combined outcome: all deaths or severe respiratory illness Show forest plot | 2 | 135180 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.54, 0.70] |
| 11.8.1 Epidemic year ‐ vaccine matching | 1 | 68536 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.54, 0.78] |
| 11.8.2 Epidemic year ‐ vaccine matching absent or unknown | 1 | 66644 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Influenza‐like illness Show forest plot | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.64] |
| 12.1.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.1.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.1.3 Non‐epidemic year ‐ vaccine matching | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.64] |
| 12.2 Hospitalisation for influenza or pneumonia or respiratory disease Show forest plot | 3 | 518748 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.64, 0.70] |
| 12.2.1 Epidemic year ‐ vaccine matching | 2 | 518374 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.63, 0.71] |
| 12.2.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.2.3 Non‐epidemic year ‐ vaccine matching | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.10, 7.97] |
| 12.3 Deaths from influenza or pneumonia Show forest plot | 1 | 259627 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.33, 0.57] |
| 12.3.1 Epidemic year ‐ vaccine matching | 1 | 259627 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.33, 0.57] |
| 12.3.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.3.3 Non‐epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.4 All deaths Show forest plot | 2 | 260001 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.41, 0.46] |
| 12.4.1 Epidemic year ‐ vaccine matching | 1 | 259627 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.41, 0.46] |
| 12.4.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 12.4.3 Non‐epidemic year ‐ vaccine matching | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.08, 30.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Influenza‐like illness Show forest plot | 2 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.16, 0.56] |
| 13.1.1 Epidemic year ‐ vaccine matching | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.54] |
| 13.1.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.1.3 Non‐epidemic year ‐ vaccine matching | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.18, 0.82] |
| 13.2 Hospitalisation for influenza or pneumonia or respiratory disease Show forest plot | 2 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.28] |
| 13.2.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.2.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.2.3 Non‐epidemic year ‐ vaccine matching | 2 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.28] |
| 13.3 All deaths Show forest plot | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.10, 43.10] |
| 13.3.1 Epidemic year ‐ vaccine matching | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.3.2 Epidemic year ‐ vaccine matching absent or unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.3.3 Non‐epidemic year ‐ vaccine matching | 1 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.10, 43.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Hospitalisations for influenza or pneumonia Show forest plot | 2 | 1074 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 14.1.1 Outbreak ‐ vaccine matching (circulating strains) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 14.1.2 Outbreak ‐ vaccine matching absent or unknown | 1 | 825 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.22] |
| 14.1.3 No outbreak ‐ vaccine matching | 1 | 249 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.40] |
| 14.2 Hospitalisations for any respiratory disease Show forest plot | 4 | 21378 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.23] |
| 14.2.1 Outbreak ‐ vaccine matching | 3 | 20582 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.92, 1.26] |
| 14.2.2 No outbreak ‐ not matching | 1 | 796 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.52] |
| 14.3 Deaths from influenza or pneumonia Show forest plot | 1 | 1092 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.04] |
| 14.3.1 Outbreak ‐ vaccine matching | 1 | 1092 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.04] |
| 14.4 Pneumonia (no better defined) Show forest plot | 1 | 519 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 14.4.1 Outbreak ‐ partially matching | 1 | 519 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Hospitalisations for influenza or pneumonia Show forest plot | 5 | Odds Ratio (IV, Random, 95% CI) | 0.59 [0.47, 0.74] | |
| 15.1.1 Epidemic ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.85] | |
| 15.1.2 Non‐epidemic ‐ vaccine not matching | 0 | Odds Ratio (IV, Random, 95% CI) | Not estimable | |
| 15.1.3 Epidemic year ‐ vaccine matching absent or unknown | 2 | Odds Ratio (IV, Random, 95% CI) | 0.68 [0.58, 0.79] | |
| 15.1.4 Non‐epidemic ‐ vaccine matching | 2 | Odds Ratio (IV, Random, 95% CI) | 0.37 [0.16, 0.87] | |
| 15.2 Hospitalisations for any respiratory disease Show forest plot | 3 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.56, 0.90] | |
| 15.2.1 Epidemic ‐ vaccine matching | 3 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.56, 0.90] | |
| 15.2.2 Non‐epidemic ‐ vaccine matching | 0 | Odds Ratio (IV, Random, 95% CI) | Not estimable | |
| 15.2.3 Non‐epidemic year ‐ vaccine matching | 0 | Odds Ratio (IV, Random, 95% CI) | Not estimable | |
| 15.3 Deaths from pneumonia or influenza Show forest plot | 2 | Odds Ratio (IV, Random, 95% CI) | 0.74 [0.60, 0.92] | |
| 15.3.1 Epidemic year ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.60, 0.97] | |
| 15.3.2 Epidemic year ‐ vaccine matching absent or unknown | 1 | Odds Ratio (IV, Random, 95% CI) | 0.67 [0.42, 1.07] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Hospitalisations for influenza or pneumonia Show forest plot | 4 | 6629 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.09] |
| 16.1.1 Outbreak ‐ vaccine matching | 2 | 3617 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.31] |
| 16.1.2 No outbreak ‐ vaccine matching | 2 | 3012 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 Hospitalisations for influenza or pneumonia Show forest plot | 2 | Odds Ratio (IV, Random, 95% CI) | 0.68 [0.54, 0.86] | |
| 17.1.1 Epidemic ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.68 [0.50, 0.93] | |
| 17.1.2 Non‐epidemic ‐ vaccine not matching | 0 | Odds Ratio (IV, Random, 95% CI) | Not estimable | |
| 17.1.3 Epidemic year ‐ vaccine matching absent or unknown | 0 | Odds Ratio (IV, Random, 95% CI) | Not estimable | |
| 17.1.4 Non‐epidemic ‐ vaccine matching | 1 | Odds Ratio (IV, Random, 95% CI) | 0.69 [0.49, 0.97] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 Influenza‐like illness Show forest plot | 25 | 9211 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.65, 0.87] |
| 18.1.1 Quality A | 8 | 4502 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 18.1.2 Quality B | 13 | 3854 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
| 18.1.3 Quality C | 3 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.00] |
| 18.1.4 Quality D | 1 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.35, 0.57] |

