| Name of rating scale | Description |
| Bech and Refaelsen mania rating scale (BRMAS) Bech 1979 | This is an 11 item scoring scale: each symptom is measured from 0 to 4, increasing from not present to severe. The items included activity (motor and verbal), flight of thought, voice level, hostility/destructiveness, mood, self esteem, contact, sleep, sexual interest and work. |
| Young Mania Rating Scale (YMRS) Young 1978 | This scale has 11 items which are rated after a clinical interview. Irritability, speech rate and amount, content of thought and disruptive behaviour items are given extra weight in the total by being scored from 0‐8, whereas the remaining items are scored from 0‐4. Higher scores indicate more symptoms. |
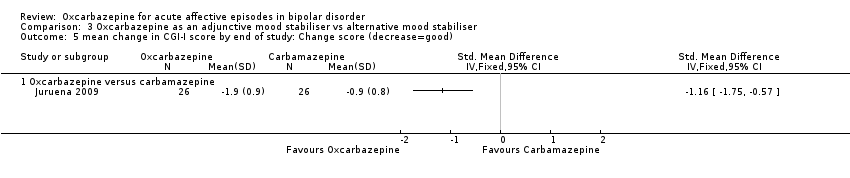
| Clinical Global Improvement (CGI) Guy 1976 | The scale assesses both severity of illness and clinical improvement by comparing the condition of the subject standardised against others with the same diagnosis. The seven point scoring system is usually employed, with low scores showing decreased severity and overall improvement. |
| Clinical Global Improvement, Bipolar Version (CGI‐BP) Spearing 1997 | This is a modified version of the scale which includes the correction of perceived inconsistencies in scaling, detailed definitions of illness severity and change, the inclusion of time frames and the separation of the assessment of improvement in illness from the assessment of the adverse effects of treatment. Previous phases of illness are also used as comparators for the assessed period. A CGI score of greater than three is often taken to indicate a clinical response. |
| Child Health Questionnaire (CHQ) Landgraf 1999 | The scale is a quality of life questionnaire which has both physical and psychosocial components. It assesses a child's physical, emotional, and social well‐being from the perspective of a parent or guardian {CHQ‐PF50 and PF‐28 (short form)} or, in some instances, the child directly (CHQ‐CF87, for children ten years of age and older). There are 14 domains, with scores aggregated to derive two summary component scores of physical and psychosocial health. Scores can then be transformed to a 0‐100 scale. |
| Hamilton Depression Rating Scale‐21 (HDRS) Hamilton 1960 | The HDRS (also known as the Ham‐D) is a widely used clinician‐administered depression assessment scale. The original version contains 17 items (HDRS17) pertaining to symptoms of depression experienced over the past
week. The HDRS17 has 17 items which can be graded from 0 to 4 based on a semi structured interview. A later 21‐item version (HDRS21) included four items for definition of subtype of depression. |
| Montgomery‐Asberg Depression Rating Scale (MADRS) Montgomery 1979 | This scale is a 10 item depression rating score which has a fixed scaling of seven points (from 0 through 6). Compared to the HAM‐D the MADRS is supposed to capture pure psychiatric symptoms better. The HAM‐D and MADRS have been found to have high level of correlation with each other and hence they are both usually applied together. |
| Brief Psychiatric Rating Scale (BPRS) Overall 1962 | The Brief Psychiatric rating scale (BPRS) assesses the level of 18 symptom constructs of psychosis such as hostility, suspicion, hallucination and grandiosity. It is based on the clinician's interview with the patient and observations of the patient's behavior over the previous 2–3 days. |
| Children's depression rating scale (CDRS) Poznaski 1984 | The Children’s Depression Rating Scale (CDRS) is a 16‐item measure used to determine the severity of depression in children 6‐12 years of age.Items are measured on 3‐, 4‐, 5‐, and 6‐point scales. The CDRS is derived from the Hamilton Rating Scale for Depression (HAM‐D); a score of 15 on the CDRS is equivalent to a score of 0 on the HAM‐D. Assessment information is based on parent, child and schoolteacher interviews. |