Aspirin für in‐vitro Fertilisation
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomised, double‐blind placebo controlled trial | |
| Participants | Inclusion criteria: unselected women undergoing IVF cycles | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES Live birth rate (per woman/couple) in intervention group: NS Live birth rate (per woman/couple) in control group: NS SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Hôpital Edouard Herriot, Médecine de la Reproduction, Lyon and Institut Rhônalpin, Bron, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Clinical pregnancy rates are reported as percentages |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: parallel randomised controlled trial | |
| Participants | Inclusion criteria: women undergoing a frozen embryo cycle, using their own oocytes | |
| Interventions | Dose of aspirin given to intervention group: 81 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Assisted Conception Unit, Cooper Hospital University Medical Centre, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Participants were not blinded; it was not stated whether the individuals who administered the intervention were blinded; assessors of the outcomes were blinded |
| Incomplete outcome data (attrition bias) | High risk | Reporting clinical pregnancy rates only |
| Selective reporting (reporting bias) | Unclear risk | No reports |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: randomized, double‐blind placebo controlled trial | |
| Participants | Inclusion criteria: Dutch‐speaking women starting a first or second IVF/ICSI cycle | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Centre for Reproductive Medicine of the University Hospital of Ghent, Belgium Source of funding: One of the authors is holder of a fundamental clinical research mandate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized |
| Allocation concealment (selection bias) | Low risk | By the central pharmacy of the hospital |
| Blinding (performance bias and detection bias) | Low risk | Stated as being double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Reporting of primary and some of the secondary measures of the review |
| Selective reporting (reporting bias) | Low risk | No reports |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: randomized study | |
| Participants | Inclusion criteria: nonselected infertile patients who were undergoing their first ICSI cycle | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Faculty of Medicine, Department of Obstetrics and Gynecology, Ankara University, Turkey Source of funding: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lottery randomisation |
| Allocation concealment (selection bias) | Unclear risk | Envelopes and initial letters of the groups |
| Blinding (performance bias and detection bias) | Low risk | Participants and the individuals providing the intervention were blinded |
| Incomplete outcome data (attrition bias) | High risk | Not addressing live birth rate, intention to treat or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No reports |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: randomized placebo‐controlled trial | |
| Participants | Inclusion criteria: (i) age < 40 years (ii) < 4 previous ovarian stimulations and (iii) no contraindications for aspirin | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Ectopic rate in intervention group: 4/242 (1.6%) | |
| Notes | Setting of trial: Four fertility centres situated in Finland: Oulu University Hospital; The Family Federation of Finland, Oulu; Tampere University Hospital; and Kuopio University Hospital. Source of funding: stated (supported by the University of Oulu, Bayer AG, The Academy of Finland, Sigrid Jusélius Foundation, Maud Kuistila Foundation and Paavo Ilmari Ahvenainen Foundation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By means of computer‐generated random numbers in blocks of four by the pharmacist (third‐party administrator) |
| Allocation concealment (selection bias) | Low risk | By using opaque sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the participants and investigators was ensured by treating the women with tablets of aspirin or placebo of identical appearance |
| Incomplete outcome data (attrition bias) | Low risk | Reporting of the primary outcome/clinical pregnancy as rates ‒ percentages per embryo transfer |
| Selective reporting (reporting bias) | Low risk | No reports to suggest selective reporting |
| Other bias | High risk | Trial was partly funded by a pharmaceutical company producing aspirin |
| Methods | Study design: double blind, parallel randomised controlled trial | |
| Participants | Inclusion criteria: women undergoing IVF who were described as poor responders due to either poor response in previous cycles, or high basal levels of FSH (> 10 IU/L) | |
| Interventions | Dose of aspirin given to intervention group: 80 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Assisted Conception Unit, Hong Kong | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Coordinated by a research nurse |
| Blinding (performance bias and detection bias) | Low risk | Participants and the individuals providing the intervention were blinded |
| Incomplete outcome data (attrition bias) | High risk | Only reporting of clinical pregnancy rates |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No data |
| Methods | Study design: double blind, placebo controlled trial for patients with previous failed conception | |
| Participants | Inclusion criteria: patients < 39 years of age at the start of treatment, with serum FSH level ≤10 IU/L on cycle day 3 and with at least one previous IVF or intracytoplasmic sperm injection (ICSI) treatment with failed conception. Patients did not have a previous ongoing pregnancy, both ovaries were present, and there was no contraindication for aspirin | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: University Fertility Clinic, Department of Obstetrics, Gynecology and Reproductive Medicine Free University Medical Center, Amsterdam, The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized tables |
| Allocation concealment (selection bias) | Low risk | Performed by an independent pharmacist of the hospital pharmacy |
| Blinding (performance bias and detection bias) | Low risk | Participants and the individuals providing the intervention were blinded |
| Incomplete outcome data (attrition bias) | High risk | No reporting of live birth rate |
| Selective reporting (reporting bias) | Low risk | No reports for selective reporting |
| Other bias | Low risk | Free from other problems |
| Methods | Study design: parallel randomised controlled trial | |
| Participants | Inclusion criteria: women undergoing IVF | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Preterm birth: NS | |
| Notes | Setting of trial: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women undergoing IVF/not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | No reporting of live birth or clinical pregnancy rate per woman |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: randomised, double‐blind placebo‐controlled study | |
| Participants | Inclusion criteria: women undergoing conventional IVF randomly referred to the Institute | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: Department of Endocrinology and Female Infertility (Moini, Zafarani, Haddadian, Ahmadi), Royan Institute, and the Department of Obstetrics and Gynecology (Honar, Riazi), Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Reporting of clinical pregnancy rate per embryo transfer |
| Selective reporting (reporting bias) | Unclear risk | No data |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: parallel randomised controlled trial | |
| Participants | Inclusion criteria: women less than 40, with fewer than four previous ovarian stimulations and no contraindications for aspirin. | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Complications with IVF/ICSI procedure (per woman/couple) in intervention group: NS | |
| Notes | Setting of trial: Four fertility centres situated in Finland: Oulu University Hospital; The Family Federation of Finland, Oulu; Tampere University Hospital; and Kuopio University Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes prepared by pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Participants and the individuals providing the intervention were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Reporting most of the outcome measures of the review |
| Selective reporting (reporting bias) | Low risk | No data for selective reporting |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: double blind, parallel, randomised placebo‐controlled trial | |
| Participants | Inclusion criteria: Women undergoing IVF with tubal factor infertility | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Both participants and individuals who provided the treatment were blinded |
| Incomplete outcome data (attrition bias) | High risk | Not reporting live birth rate |
| Selective reporting (reporting bias) | Unclear risk | No data |
| Other bias | Unclear risk | No data |
| Methods | Study design: single blind, parallel randomised controlled trial | |
| Participants | Inclusion criteria: couples undergoing ICSI for male factor infertility | |
| Interventions | Dose of aspirin given to intervention group: 80 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Ectopic pregnancy in 5/139 (3.6%) Preterm birth: NS Complications during pregnancy/birth (per woman/couple) in intervention group: NS | |
| Notes | Setting of trial: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Coordinated by a research nurse / no further data |
| Blinding (performance bias and detection bias) | Low risk | Only the individuals providing the treatment were blinded |
| Incomplete outcome data (attrition bias) | High risk | No reporting of live birth rate |
| Selective reporting (reporting bias) | Unclear risk | No data |
| Other bias | Unclear risk | Unclear |
| Methods | Study design: double blind, parallel randomised controlled trial | |
| Participants | Inclusion criteria: women less than 39 years old with regular cycles undergoing first IVF/ICSI cycle | |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day | |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES | |
| Notes | Setting of trial: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified according to primary/secondary infertility |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | Participants and providers of treatment were blinded |
| Incomplete outcome data (attrition bias) | High risk | No reporting of live birth rate |
| Selective reporting (reporting bias) | Unclear risk | No data for selective reporting |
| Other bias | Unclear risk | No data |
NS = not stated
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Retrospective cohort study | |
| Retrospective cohort analysis | |
| ASP and prednisone; not RCT | |
| Not RCT | |
| Not RCT; ASP and heparin | |
| ASP and growth hormone | |
| Uterine artery haemodynamics was the primary outcome after aspirin treatment | |
| Combination of ASP and terbutaline | |
| Not RCT | |
| Not IVF or ICSI | |
| Not RCT | |
| Not RCT, no IVF | |
| Not RCT | |
| Not RCT | |
| Follow‐up through a questionnaire/original study Lambers 2008 | |
| No reply to consecutive emails sent. Not enough information to determine eligibility | |
| No reply to consecutive emails sent. No available data (presented in conference proceedings) to perform analysis. | |
| No reply to emails sent. Not enough information to determine eligibility | |
| ASP and prednisone | |
| Quasi‐randomised study | |
| Compared different regimens of aspirin in IVF | |
| ASP and prednisone | |
| Oocyte donation programme | |
| ASP and heparin | |
| Not RCT | |
| ASP and heparin; cross‐over trial | |
| ASP and heparin | |
| Not RCT | |
| ASP and prednisone | |
| ASP and prednisone | |
| No reply to consecutive emails sent. No available data (presented in conference proceedings) to perform analysis | |
| Not RCT | |
| Quasi‐randomised study | |
| Not RCT | |
| Quasi‐RCT | |
| Not RCT | |
| Oocyte donation programme, quasi‐randomised study | |
| Retrospective analysis | |
| Ovulation induction, not IVF | |
| Retrospective analysis, ASP and prednisone | |
| Not investigating outcome of interest. E‐mail sent to verify this. |
ASP = aspirin treatment
RCT = randomised controlled trial
IVF = in vitro fertilisation
ICSI = intracytoplasmic sperm injection
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Effect of Low Dose Aspirin in Increasing the Chance of Pregnancy |
| Methods | Study design: randomised‐controlled clinical trial |
| Participants | Inclusion criteria: women 18 to 40 years, undergoing IVF with long or antagonist protocol, who did not achieve a pregnancy following a fresh embryo transfer or women whose embryos had not been transferred due to OHSS, or had frozen embryos available for another transfer |
| Interventions | Dose of aspirin given to intervention group: 100 mg per day |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Preterm birth: NS Complications during pregnancy/birth (per woman/couple) in intervention group: NS |
| Starting date | May 2012 to October 2012 |
| Contact information | |
| Notes | http://clinicaltrials.gov/show/NCT01633528 Setting of trial: Royan Institute, Tehran, Iran |
| Trial name or title | |
| Methods | Study design: randomised‐controlled clinical trial |
| Participants | Inclusion criteria: Women 19 to 35 years undergoing IVF, with FSH levels of ≤8 IU/l and BMI between 19 kg/m² and 25 kg/m², with presence of both ovaries; ≥ 2 previous IVF failures, good‐quality embryos for transfer and endometrial thickness between 10 mm and 14 mm |
| Interventions | Dose of aspirin given to intervention group: 75 mg per day |
| Outcomes | PRIMARY OUTCOME MEASURES SECONDARY OUTCOME MEASURES Preterm birth: NS Complications during pregnancy/birth (per woman/couple) in intervention group: NS |
| Starting date | |
| Contact information | |
| Notes | http://clinicaltrials.gov/show/NCT01924104 Setting of trial: Jeevan Jyoti Hospital, India |
NS = not stated
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman or couple Show forest plot | 3 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| Analysis 1.1 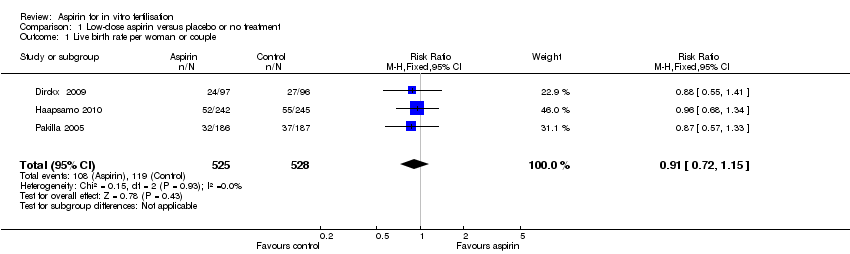 Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 1 Live birth rate per woman or couple. | ||||
| 2 Clinical pregnancy rate per woman or couple Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| Analysis 1.2 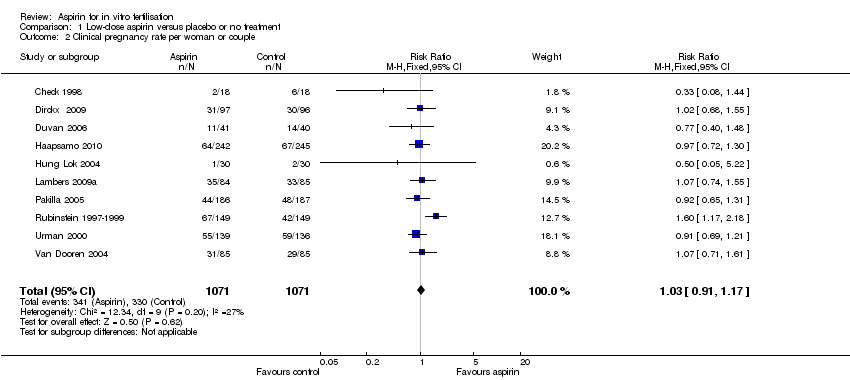 Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman or couple. | ||||
| 3 Ongoing pregnancy rate (beyond 12 weeks) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| Analysis 1.3  Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 3 Ongoing pregnancy rate (beyond 12 weeks). | ||||
| 4 Multiple pregnancy rate per woman per couple (on ultrasound) Show forest plot | 2 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.25] |
| Analysis 1.4  Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 4 Multiple pregnancy rate per woman per couple (on ultrasound). | ||||
| 5 Multiple pregnancy rate per woman per couple at birth Show forest plot | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.46] |
| Analysis 1.5 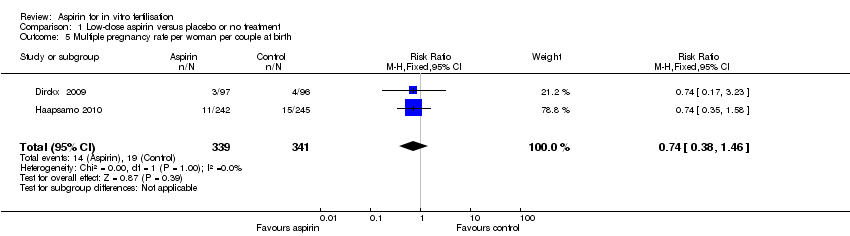 Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 5 Multiple pregnancy rate per woman per couple at birth. | ||||
| 6 Miscarriage rate per woman per couple Show forest plot | 5 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.68, 1.77] |
| Analysis 1.6  Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 6 Miscarriage rate per woman per couple. | ||||
| 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy Show forest plot | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.75, 4.63] |
| Analysis 1.7 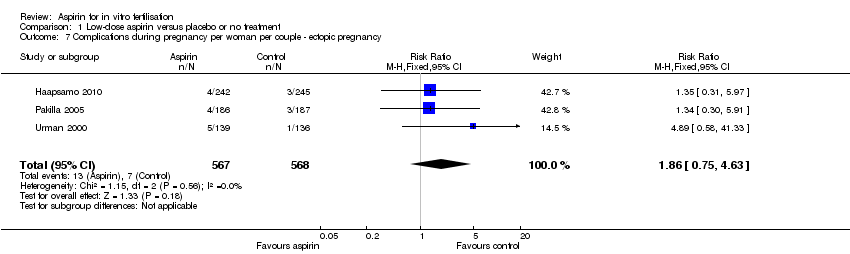 Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy. | ||||
| 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy Show forest plot | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| Analysis 1.8  Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy. | ||||
| 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| Analysis 1.9 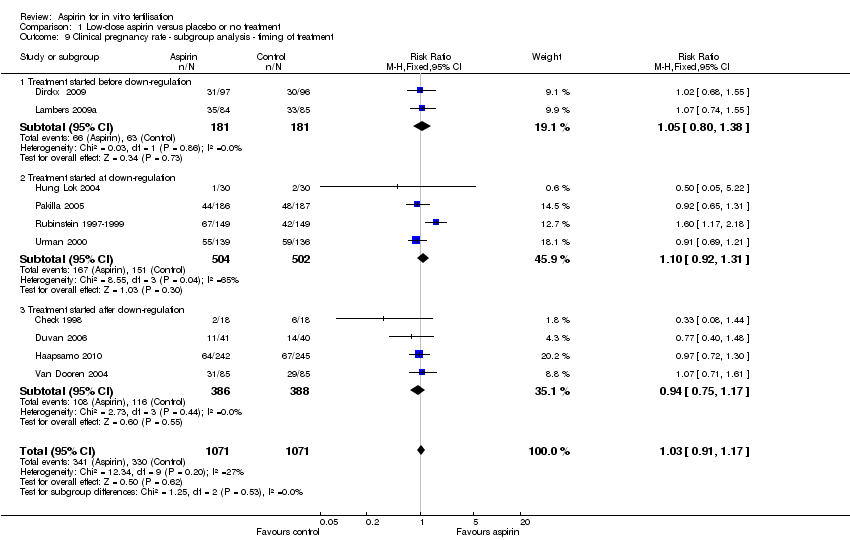 Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment. | ||||
| 9.1 Treatment started before down‐regulation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 9.2 Treatment started at down‐regulation | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 9.3 Treatment started after down‐regulation | 4 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.17] |
| 10 Clinical pregnancy rate ‐ sensitivity analysis Show forest plot | 3 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.23] |
| Analysis 1.10  Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 10 Clinical pregnancy rate ‐ sensitivity analysis. | ||||
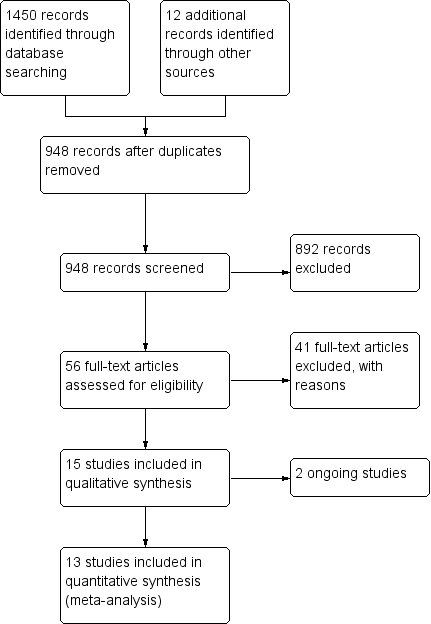
Study flow diagram
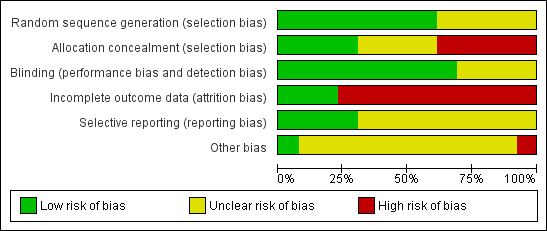
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
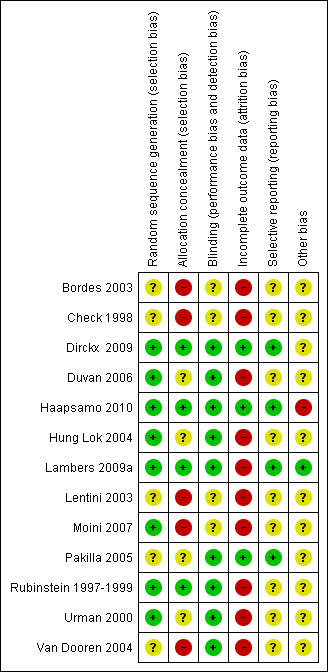
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman or couple.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 1 Live birth rate per woman or couple.
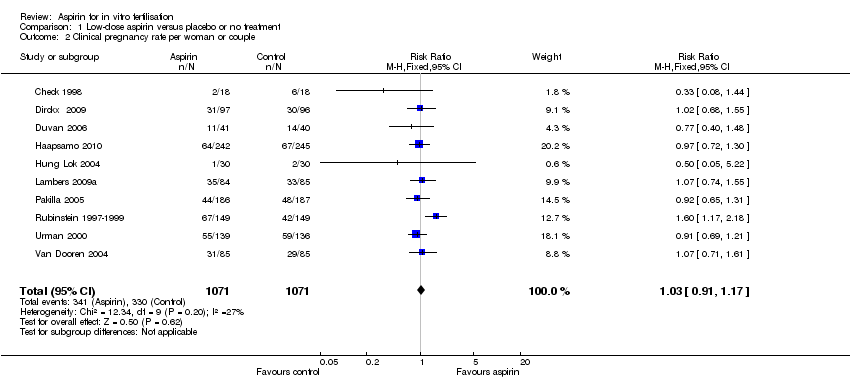
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 3 Ongoing pregnancy rate (beyond 12 weeks).
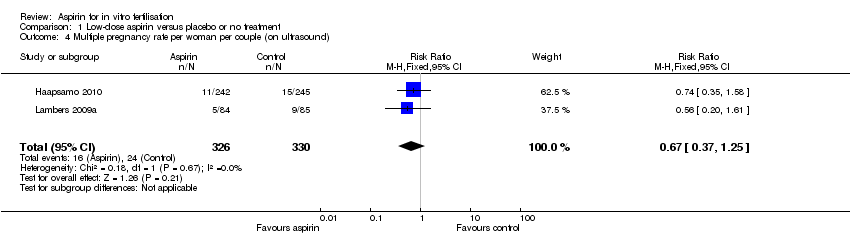
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 4 Multiple pregnancy rate per woman per couple (on ultrasound).
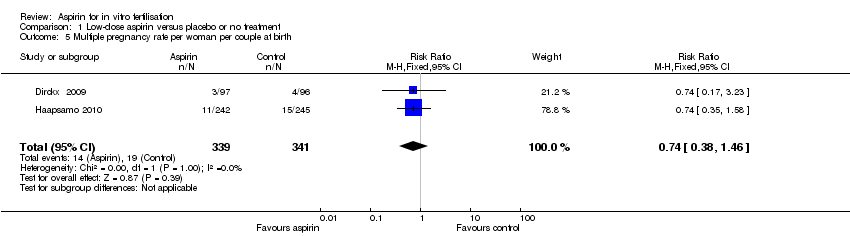
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 5 Multiple pregnancy rate per woman per couple at birth.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 6 Miscarriage rate per woman per couple.
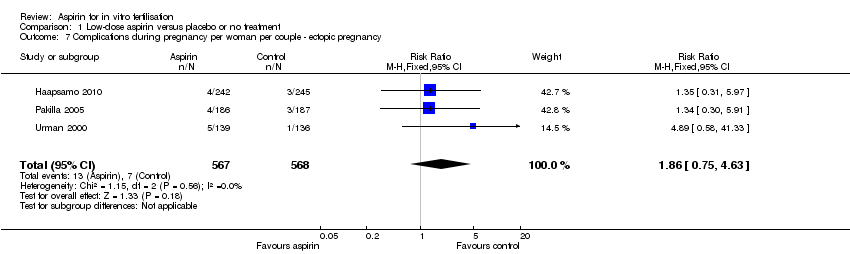
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy.
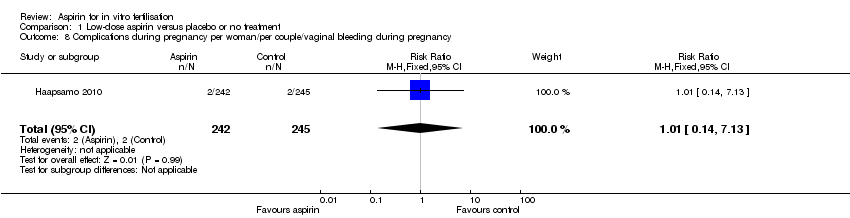
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment.
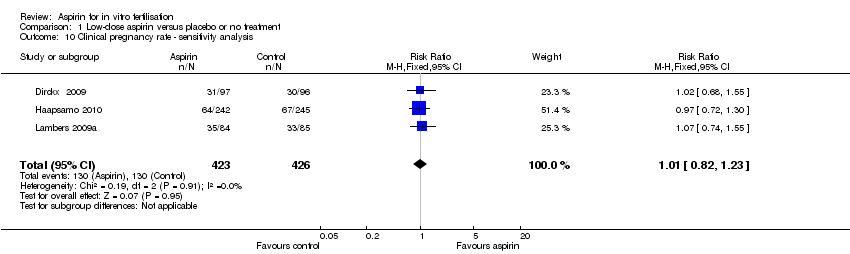
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 10 Clinical pregnancy rate ‐ sensitivity analysis.
| Low‐dose aspirin compared to placebo or no treatment for women undergoing ART | ||||||
| Population: Women with subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Low‐dose aspirin | |||||
| Live birth | 225 per 1000 | 204 per 1000 | RR 0.91 | 1053 | ⊕⊕⊕⊝ | |
| Clinical pregnancy | 337 per 1000 | 347 per 1000 | RR 1.03 | 2142 | ⊕⊕⊕⊝ | |
| Multiple pregnancy (on ultrasound) | 84 per 1000 | 56 per 1000 | RR 0.67 (0.37 to 1.25) | 656 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | 43 per 1000 | 47 per 1000 | RR 1.1 | 1497 | ⊕⊕⊝⊝ | |
| Ectopic pregnancy | 12 per 1000 | 23 per 1000 | RR 1.86 | 1135 | ⊕⊝⊝⊝ | |
| Vaginal bleeding | 8 per 1000 | 8 per 1000 (1 to 58) | RR 1.01 (0.14 to 7.13) | 487 (1 study) | ⊕⊝⊝⊝ | |
| Other adverse events | Other adverse events (such as preterm birth, antepartum haemorrhage, need for operative delivery) were not reported in the included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious imprecision with low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in the control group. 6 Single study. Very serious imprecision with very low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in either group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman or couple Show forest plot | 3 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 2 Clinical pregnancy rate per woman or couple Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 3 Ongoing pregnancy rate (beyond 12 weeks) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Multiple pregnancy rate per woman per couple (on ultrasound) Show forest plot | 2 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.25] |
| 5 Multiple pregnancy rate per woman per couple at birth Show forest plot | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.46] |
| 6 Miscarriage rate per woman per couple Show forest plot | 5 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.68, 1.77] |
| 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy Show forest plot | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.75, 4.63] |
| 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy Show forest plot | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 9.1 Treatment started before down‐regulation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 9.2 Treatment started at down‐regulation | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 9.3 Treatment started after down‐regulation | 4 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.17] |
| 10 Clinical pregnancy rate ‐ sensitivity analysis Show forest plot | 3 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.23] |

