Intervencije za liječenje kožnih zaraznih moluski.
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | 74 children 2 to 12 years of age, hospital outpatient clinic in Kuwait | |
| Interventions | Imiquimod 5% for up to 16 weeks versus cryospray for up to 2 weeks | |
| Outcomes | Cure at 3, 6, 12, and 16 weeks; cosmetic outcome; adverse effects | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details except 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Probably not blinded: cream versus cryospray |
| Blinding of outcome assessment (detection bias) | High risk | Probably not blinded: cream versus cryospray |
| Incomplete outcome data (attrition bias) | Low risk | All participants had complete follow‐up. |
| Incomplete outcome data (attrition bias) | Low risk | All participants had complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No baseline imbalance for gender, MC area, morphology, MC location, or baseline lesion count, but no data on compliance |
| Methods | Double‐blind randomised placebo‐controlled trial | |
| Participants | 38 patients (1 to 16 years; M/F: 18/20) were enrolled, Dept of Dermatology, UK. | |
| Interventions | 35 mg/kg/day cimetidine, given once daily as oral suspension versus a matching placebo for 3 months | |
| Outcomes | Complete clearance after 4 months of treatment. Reduction of lesions. Adverse events: not mentioned | |
| Notes | 50% dropout rate. Published abstract only. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". No details in abstract |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described in the abstract. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind placebo‐controlled"; "The dose of cimetidine was 35 mg/kg‐1/day‐1"; "The placebo group received a manufactured placebo". Probably done, placebo controlled, both suspensions |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Double‐blind placebo‐controlled"; "The dose of cimetidine was 35 mg/kg‐1/day‐1"; "The placebo group received a manufactured placebo". Probably done, placebo controlled, both suspensions |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported in the abstract |
| Incomplete outcome data (attrition bias) | High risk | 4 months: 19/35 completed the treatment course. Quote: "The number of patients who received placebo or cimetidine was similar in the groups that did not attend or withdrew." > 30% withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The mean age and sex of the patients and incidence of atopic disease in each treatment group was similar." No compliance data |
| Methods | Randomised controlled trial. Body sides were randomised left‐right. | |
| Participants | 30 children (2 to 12 years of age; M/F: 18/12) were recruited, Dept of Dermatology, UK. | |
| Interventions | Sterile normal 0.9% saline versus 5% potassium hydroxide for 3 weeks | |
| Outcomes | Complete clearance of lesions and side effects after 12 weeks | |
| Notes | Unpublished, year of study unclear. Unpublished paper obtained in 2007. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomised to right or left side of body". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomised to right or left side of body". Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "30 patients were recruited in this double‐blind study". "All subjects were given seven bottles clearly labelled R and seven bottles labelled L, for use on the right and left side of the body respectively (patient and investigator did not know which is active site)" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "30 patients were recruited in this double‐blind study". "All subjects were given seven bottles clearly labelled R and seven bottles labelled L, for use on the right and left side of the body respectively (patient and investigator did not know which is active site)" |
| Incomplete outcome data (attrition bias) | High risk | 12 weeks: 10/30 did not complete study, 2 withdrew due to severe stinging from KOH, and 8 children were lost to follow‐up. > 30% dropouts |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 31 children, mean age 4.6 years. Sex not reported. USA, outpatient clinic | |
| Interventions | 10% lemon myrtle oil or vehicle (olive oil) for 3 weeks | |
| Outcomes | Complete clearance or > 90% reduction in number of lesions after 3 weeks | |
| Notes | Funding: Center for Biomedical Research, a commercial institute involved in drug research and sale. Partner of Naturopathix (ZymaDerm for molluscum) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomised to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100. Odd numbers were assigned to active treatment even numbers to vehicle" |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were randomised to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100." "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion." "A mild synthetic lemon fragrance not containing citral was added to scent the control olive oil preparation. This fragrance by itself had no therapeutic effect." Vehicle controlled. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion." "A mild synthetic lemon fragrance not containing citral was added to scent the control olive oil preparation. This fragrance by itself had no therapeutic effect." Vehicle controlled. |
| Incomplete outcome data (attrition bias) | Low risk | 21 days: 4/31 withdrew: 1/16 in lemon myrtle oil group lost to follow‐up; 3/15 missing in vehicle group, withdrew because of worsening of the molluscum. Withdrawn participants included in analysis as failures. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address medium‐ and long‐term outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The mean number of lesions at enrolment did not differ between treatment groups. No sex or age comparison between groups. No compliance data |
| Methods | Randomised trial | |
| Participants | Children 1 to 18 years with a minimum of 3 molluscum lesions, target sample size 40, Karnataka, India | |
| Interventions | Imiquimod 5% cream application alternate nights versus 10% potassium hydroxide solution applied alternate nights for 12 weeks | |
| Outcomes | Complete clearance, time points 4, 8, and 12 weeks | |
| Notes | Funding: this study reported that they had no support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The lottery method"; no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Different instructions, therefore not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Different instructions, therefore not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ and long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalance in terms of age, gender, and number of lesions. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 32 children, 5 to 10 years of age, recruited in local paediatricians' offices, university clinics and through mass emails to university students and staff, North Carolina, USA | |
| Interventions | Cantharidin collodion 0.7% versus vehicle collodion for approximately 8 weeks | |
| Outcomes | Complete clearance, lesion counts, adverse effects, approximately 8 weeks after start of treatment | |
| Notes | Funding: Doris Duke Charitable Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization schedule was prepared before first recruitment using permuted blocks of size 2" |
| Allocation concealment (selection bias) | Low risk | After eligibility was assessed, study personnel "assigned the next unique subject identification number and dispensed the appropriate drug" |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients, parents and investigators were blinded to treatment assignment" and "placebo was identical in texture and smell" |
| Blinding of outcome assessment (detection bias) | Low risk | "Patients, parents and investigators were blinded to treatment assignment" and "placebo was identical in texture and smell" |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants dropped out immediately after randomisation because they did not meet all eligibility criteria (< 20%). |
| Incomplete outcome data (attrition bias) | Unclear risk | No long‐term data |
| Selective reporting (reporting bias) | Low risk | Reported outcomes similar to those in trial register resume. |
| Other bias | Unclear risk | Imbalance in dry skin. Allocation bias was due to dropout. |
| Methods | Randomised controlled trial | |
| Participants | 323 children, 2 to 12 years of age, with molluscum contagiosum in 19 outpatient clinics in the USA were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream 3 times weekly for 16 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects after 4, 8, 12, 16, 18, and 28 weeks | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to a treatment arm in blocks of 6 according to a computer‐generated randomizations schedule. Randomization was 2:1 (active:vehicle) for a planned number of 300 subjects to be randomised into the study" (p.40) |
| Allocation concealment (selection bias) | Low risk | "The treatment assignments were concealed from the subjects, investigators and study staff, and the 3M clinical research team. The clinical packaging group at 3M Pharmaceuticals held the master code for the treatment randomizations schedule, and supplied the investigators with each subject’s treatment assignment as a hidden (tear‐off) panel on the study cream label, which was affixed to the blinded Drug Label page" (p.43) |
| Blinding of participants and personnel (performance bias) | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear when participants dropped out, so impossible to distinguish short‐term from long‐term. Primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) | Low risk | 53/323 participants dropped out, reasons mentioned (< 30%). |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalance, compliance data available, primary analysis by intention‐to‐treat |
| Methods | Open randomised trial | |
| Participants | 30 people with molluscum contagiosum in Iran | |
| Interventions | 10% potassium hydroxide solution versus cryotherapy | |
| Outcomes | Lesion response and side effects 4 weeks after start of treatment | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the simple randomization method"; no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Different administration, so could not be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Different administration, so could not be blinded |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable, no medium‐ and long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline comparison; no compliance data |
| Methods | Randomised controlled trial | |
| Participants | 124 children, 1 to 16 years of age, dermatology clinic, Montreal, Canada M/F: 57/67 | |
| Interventions | 4 arms: curettage, topical cantharidin 0.7%, topical salicylic acid 16.7% + lactic acid 16.7%, topical imiquimod cream 5% | |
| Outcomes | Number of visits required. Intervals between study visits not reported, so outcome data not suitable for inclusion. | |
| Notes | Total number of participants unclear. Percentage of group 3 in Table 1 does not correspond to number mentioned in text. Funding: 3 pharmaceutical companies provided treatments for free. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomizations list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomizations list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)." Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This is not a double‐blind study." Physical versus topical treatment |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This is not a double‐blind study." Physical versus topical treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 114 children, 1 to 15 years of age, sex not reported, UK, outpatient departments of teaching hospital and district general hospital | |
| Interventions | Topical salicylic acid 12%, or phenol 10% + 70% alcohol, or 70% alcohol at monthly visits for a maximum of 6 months | |
| Outcomes | Complete clearance of lesions after 6 months | |
| Notes | Funding: pharmaceutical company provided medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomised according to a random number table" |
| Allocation concealment (selection bias) | High risk | Quote: "The investigators were not blinded to randomizations" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The patients in the salicylic acid groups were aware of their treatments. The other two groups treated with vehicle or phenol were single‐blinded, as the patients/parents were unaware of which treatment they received." "The vehicle and diluted phenol were prepared by the hospital pharmacy and labelled with a letter." "The investigators were not blinded for the randomization" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The patients in the salicylic acid groups were aware of their treatments. The other two groups treated with vehicle or phenol were single‐blinded, as the patients/parents were unaware of which treatment they received." "The vehicle and diluted phenol were prepared by the hospital pharmacy and labelled with a letter." "The investigators were not blinded for the randomization" |
| Incomplete outcome data (attrition bias) | Unclear risk | No short‐term outcomes reported. |
| Incomplete outcome data (attrition bias) | High risk | Up to 6 months: 31/114 lost to follow‐up: 13/37 in salicylic acid arm, 9/41 in dilute phenol arm, 9/36 in alcohol arm. > 30% dropouts |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The baseline characteristics of the three groups were similar." See also Table I, Baseline characteristics. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 50 children, 3 to 15 years of age, recruited in a hospital outpatient clinic in Brazil | |
| Interventions | (1) potassium hydroxide 10% in aqueous solution; (2) 14% salicylic acid + 14% lactic acid in collodion; both for 3 months or (3) curettage once | |
| Outcomes | Cure, adverse effects 90 days after start of treatment | |
| Notes | 2 groups applied medication at home; treatment duration was not reported for these groups. These participants were seen every 15 days until day 90 after start of treatment. The third group underwent curettage (once). These participants were seen day 7 and 90 after treatment. Outcomes were reported at 90 days, but as we do not know how long topical treatments were applied and assuming that parents were instructed to stop treatment when lesions had resolved, it is hard to say whether these are short‐ or long‐term outcomes (apart from the curettage group). Funding: 2 pharmaceutical companies provided medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were allocated randomly into three study groups”; insufficient information |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Different treatments: topical treatment versus curettage |
| Blinding of outcome assessment (detection bias) | High risk | Different treatments: topical treatment versus curettage. Also, follow‐up moments differed between treatment groups (topical: every 15 days for 90 days; curettage: days 7 and 90 after the procedure) |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions in table and text show that these were not based on number of participants randomised, so there must have been loss to follow‐up; magnitude unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline comparison. Imbalance in treatment adherence (not statistically significant) |
| Methods | Double‐blind randomised controlled trial, addressing various types of warts (n = 124), including molluscum contagiosum (n = 20) | |
| Participants | 14 molluscum patients (sex distribution unknown) randomised to the treatment arm, 6 patients were randomised to receive plain sugar globules as a placebo (personal communication Dr Manchanda). 10 participants were aged below 10 years; 7 from 10 to 20 years; and 3 from 21 to 30 years (personal communication with Dr Manchanda). India, Homoeopathic Medical College & Hospital, New Delhi | |
| Interventions | Different potencies of homeopathic drug calcarea carbonica daily for 15 days (n = 14) versus sugar globules (placebo). Unclear which participants received what potency | |
| Outcomes | Improvement (not clear after what period) | |
| Notes | Paper reports on (1) cross‐over study and (2) parallel study. We excluded the cross‐over study because 1 arm had fewer than 5 participants. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." Randomisation not mentioned in paper, "sequence was generated manually" (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." "Therefore it was found that after decoding method of concealment is not described" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Two types of placebo controlled double‐blind clinical trials were undertaken." "The subjects were given both drug and placebo." Quote (personal communication): “The identity of the drugs was kept secret in a sealed cover which was opened only at the time un‐blinding the experiment." "The plain sugar globules looks like homoeopathic drug Calcerea carbonica was used as placebo." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Two types of placebo controlled double‐blind clinical trials were undertaken." "The subjects were given both drug and placebo." Quote (personal communication): “The identity of the drugs was kept secret in a sealed cover which was opened only at the time un‐blinding the experiment." "The plain sugar globules looks like homoeopathic drug Calcerea carbonica was used as placebo." |
| Incomplete outcome data (attrition bias) | Low risk | 15 days: 20/124 dropouts, unclear what skin disease and group assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | No long‐term outcome |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 53 children (aged 9 months to 15 years according to trial register), recruited in outpatient clinic in Idaho, USA | |
| Interventions | (1) iodine; (2) tea tree oil (Melaleuca alternifolia); (3) tea tree oil + iodine for 30 days | |
| Outcomes | Cure or reduction in the number of lesions > 90%, adverse effects, 30 days after start of treatment | |
| Notes | Funding: Center for Biomedical Research, a commercial institute involved in drug research and sale. Partner of Naturopathix (ZymaDerm for molluscum) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to trial register: "Subject or subject's parent blindly chose a numbered token from an opaque container containing tokens numbered 1‐99. Numbers 1‐33 assigned to iodine treatment, numbers 34‐66 assigned to tea tree oil treatment, numbers 67‐99 assigned to tea tree oil + iodine treatment" and "Randomization by sealed container" |
| Allocation concealment (selection bias) | Low risk | According to trial register: "Subject or subject's parent blindly chose a numbered token from an opaque container containing tokens numbered 1‐99. Numbers 1‐33 assigned to iodine treatment, numbers 34‐66 assigned to tea tree oil treatment, numbers 67‐99 assigned to tea tree oil + iodine treatment" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Patients and physicians were blinded to treatment protocol.” “A mild synthetic lemon fragrance not containing citral was added to scent the iodine olive oil preparation.” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Patients and physicians were blinded to treatment protocol.” “A mild synthetic lemon fragrance not containing citral was added to scent the iodine olive oil preparation.” |
| Incomplete outcome data (attrition bias) | Low risk | 5/53 participants (less than 20%) were lost to follow‐up (Markum 2012, from Table 1 of publication). |
| Incomplete outcome data (attrition bias) | Unclear risk | No long‐term data |
| Selective reporting (reporting bias) | Low risk | Reported outcomes similar to those mentioned in trial register |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial (written correspondence Dr Ohkuma), the method of generation of randomisation sequence remained unclear, as was the concealment of allocation. It was also unclear if participants were analysed according to the group to which they were randomised (intention‐to‐treat analysis) and how blinding was performed. | |
| Participants | 35 patients with molluscum contagiosum, aged between 2 and 9 years (M/F: 21/14), Japan, Department of Dermatology | |
| Interventions | 3 interventions were compared: 10% povidone iodine solution combined with 50% salicylic acid plaster (n = 20), iodine alone (n = 5), and salicylic plaster alone (n = 10). | |
| Outcomes | Time to cure. Study duration unknown, but paper indicated that treatment continued as long as necessary; range was 7 to 64 days; mean 26 days for iodine + plaster, 86 days for iodine only, and 47 days for plaster only . | |
| Notes | No baseline comparison, no compliance data Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (personal communication, not in paper). Insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Iodine versus salicylic plaster: hard to mask |
| Blinding of outcome assessment (detection bias) | High risk | Iodine versus salicylic plaster: hard to mask |
| Incomplete outcome data (attrition bias) | Low risk | No loss reported, all participants in outcome table. Follow‐up period unclear. Duration of treatment ranged from 7 to 64 days. |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "In the former, two girls and three boys between the age of 3 and 5 were included and 4 girls and 6 boys between 2 and 9 comprised the latter control group." No imbalance for sex. No compliance data |
| Methods | Group sequential double‐blind randomised trial | |
| Participants | 30 molluscum patients were enrolled, 16 in the acidified nitrite group and 14 controls, with a median age of 6 years, 22 girls and 8 boys. UK, Department of Dermatology | |
| Interventions | 5% sodium nitrite co‐applied daily with 5% salicylic acid under occlusion versus identical cream with 5% salicylic acid omitting sodium nitrite, for 3 months | |
| Outcomes | Time to complete resolution, adverse events, study duration 3 months | |
| Notes | No baseline imbalance for duration and number of lesions, no compliance data. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Group sequential design in which subjects were randomised to receive either"; insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Double‐blind, group sequential design in which subjects were randomised to receive either 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, or identical cream with 5% salicylic acid but omitting sodium nitrite, as a control." Not done, active intervention was associated with brown staining. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Double‐blind, group sequential design in which subjects were randomised to receive either 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, or identical cream with 5% salicylic acid but omitting sodium nitrite, as a control." Not done, active intervention was associated with brown staining. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only long‐term data |
| Incomplete outcome data (attrition bias) | High risk | 21/30 dropouts after 3 months (> 30%) |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No compliance data. Duration and number of lesions were very similar (communication with author). |
| Methods | Randomised controlled trial | |
| Participants | 125 children, 1 to 12 years of age, with molluscum contagiosum in 9 outpatient clinics in the USA and Canada were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream daily for 8 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects, 12 weeks after start of treatment | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomly assigned to a treatment arm in blocks of 4 according to a computer‐generated randomizations.” (p.32) |
| Allocation concealment (selection bias) | Low risk | “Subjects/legal parental custodian(s) and investigators were unaware of the study assignment” (p.25) "This was a double‐blind, vehicle‐controlled study. 3M held the master code for the treatment randomizations schedule and supplied the investigators with each subject’s randomizations code as a hidden (tear‐off) disclosure panel on the study cream carton label. The randomizations code for an individual subject was to be broken only in case of an emergency, such as a serious adverse event (SAE)." (p.34) |
| Blinding of participants and personnel (performance bias) | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) | Low risk | Not applicable: primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) | Unclear risk | No long‐term outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalance, compliance data available, primary analysis by intention‐to‐treat |
| Methods | Randomised controlled trial | |
| Participants | 379 children, 2 to 12 years of age, with molluscum contagiosum in 19 outpatient clinics in the USA were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream 3 times weekly for 16 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects, 4, 8, 12, 16, 18, and 28 weeks after start of treatment | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to a treatment arm in blocks of 6 according to a computer‐generated randomizations schedule. Randomization was 2:1 (active:vehicle)" (p.40) |
| Allocation concealment (selection bias) | Low risk | "This was a double‐blind, vehicle‐controlled study; accordingly, the treatment assignments were concealed from the subjects, investigators and study staff, and the 3M clinical research team. The clinical packaging group at 3M Pharmaceuticals held the master code for the treatment randomizations schedule, and supplied the investigators with each subject’s treatment assignment as a hidden (tear‐off) panel on the study cream label, which was affixed to the blinded Drug Label page" (p.43) |
| Blinding of participants and personnel (performance bias) | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) | Low risk | Primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) | Low risk | 48/379 discontinued, reasons were mentioned (< 30%). |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalances, reported on compliance, primary analysis by intention‐to‐treat |
| Methods | Randomised trial | |
| Participants | 30 children, age and sex unknown, Iran, hospital dermatology clinic | |
| Interventions | Topical benzoyl peroxide 10% cream versus tretinoin 0.05% cream, 2 times daily for 4 weeks | |
| Outcomes | Lesion count, lesion free, and side effects, 6 weeks after start of treatment | |
| Notes | Information based on abstract; proportions cured used to estimate absolute numbers. Abstract published in 2004; unclear when study was carried out. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Investigator masked"; no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Investigator masked"; no further details |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Efficacy was assessed at 2, 4, and 6 weeks, but the paper only reports results at week 6. |
| Other bias | Unclear risk | No baseline characteristics or compliance data |
| Methods | Randomised controlled trial | |
| Participants | 30 patients, 1 to 36 years of age, setting unclear, Korea | |
| Interventions | Imiquimod cream 5% versus potassium hydroxide solution 10% "for 3 months" (see Notes) | |
| Outcomes | Cure, adverse effects | |
| Notes | Applied medication until all lesions were cleared. Mean duration of treatment > 4 months; this is inconsistent with 'time after treatment'. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Open study, method of application differed between treatment arms. |
| Blinding of outcome assessment (detection bias) | High risk | Open study, so investigators were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants lost to follow‐up (< 20%) |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants lost to follow‐up (< 30%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Imbalance in sex. No compliance data |
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 20 children from a paediatric dermatology clinic, age range 2 to 12 years, M/F 6/14. UK, Department of Dermatology, London | |
| Interventions | Application of 10% potassium hydroxide solution twice daily applied with a cotton swab, continued until the lesions showed signs of inflammation (n = 10). The control group received saline (n = 10), for a maximum of 3 months. | |
| Outcomes | Time to resolution, adverse events 3 months after start of treatment | |
| Notes | Number of participants who completed the study differs between unpublished paper (18/20) and published paper (19/20). Latter number included in corrected version of 2009 update (December 2009). Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments". Insufficient information |
| Allocation concealment (selection bias) | Low risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments." Central allocation: pharmacy controlled |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both the patients and the observer were blinded". "Both solutions were dispensed in identical, unlabeled bottles. The sequence was not revealed until the end of the study." Staining and stinging reported in the potassium hydroxide group. Participant, care provider, and outcome assessor probably blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Both the patients and the observer were blinded". "Both solutions were dispensed in identical, unlabeled bottles. The sequence was not revealed until the end of the study." Staining and stinging reported in the potassium hydroxide group. Participant, care provider, and outcome assessor probably blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 2 weeks: 1/20 did not complete study. 1/10 in the potassium hydroxide group withdrew after 2 weeks because of discomfort of the skin localised to the application site. |
| Incomplete outcome data (attrition bias) | Low risk | Not applicable: no long‐term results |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance for sex, lesion site, and numbers. No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 23 children, 1 to 9 years of age, M/F 12/11, USA, Alabama, Illinois, New York | |
| Interventions | Imiquimod cream 5% or vehicle 3 times a week for 12 weeks | |
| Outcomes | Complete or partial clearance (> 30% decrease from baseline lesion count), adverse events after 4, 8, and 12 weeks | |
| Notes | Presented as a pilot study. Funding: not mentioned, but 1 of the authors was reported to be a consultant for 3M Pharmaceuticals, and was also the principal investigator of 2 other unpublished studies funded by this company (Paller 2005a; Paller 2005b). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomised to either imiquimod or vehicle". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomised to either imiquimod or vehicle". Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "In a Double Blind, Randomized Pilot Trial"; "imiquimod vs vehicle". Only participants and physicians involved, so probably at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "In a Double Blind, Randomized Pilot Trial"; "imiquimod vs vehicle". Only participants and physicians involved, so probably at low risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | 2 weeks: 2/23 did not complete the study (discontinued treatment) |
| Incomplete outcome data (attrition bias) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Baseline imbalance for mean lesion count, imiquimod: 27.0 versus vehicle: 19.4 (not statistically significant). No compliance data |
| Methods | Randomised controlled trial | |
| Participants | 29 children, 15 months to 18 years of age, outpatient clinic, Turkey | |
| Interventions | Potassium hydroxide 2.5% versus potassium hydroxide 5% twice daily for 60 days | |
| Outcomes | Cure, adverse effects after 15, 30, 45, and 60 days | |
| Notes | Funding: not mentioned, but authors report having no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details except "randomised study" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | "The solution vials were indistinguishable, regardless of content, and were stored at room temperature.” But paper does not use the word 'blinded'. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether outcome assessors were aware of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant in potassium hydroxide 2.5% group and 2 participants in potassium hydroxide 5% group "removed from study" due to irregular attendance at follow‐up visits. 1 further participant in potassium hydroxide 5% group quit study due to excessive burning. Total number of loss to follow‐up: 4/29 (< 20%) |
| Incomplete outcome data (attrition bias) | Unclear risk | No long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline comparison, no compliance data |
KOH: potassium hydroxide
MC: molluscum contagiosum
NS: normal saline
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Open‐label study, imiquimod 5% cream (n = 12) | |
| HIV‐infected patients (n = 40) | |
| Open‐label study, imiquimod 5% cream (n = 13) | |
| RCT comparing 2 types of cryotherapy for cutaneous skin lesions; 124 participants, of which 10 were molluscum patients, distributed 9:1 over 2 arms | |
| Patient series, 10% potassium hydroxide (n = 40) | |
| Patient series, topical cantharidin (n = 54) | |
| Pulsed dye laser (n = 20), not randomised (personal communication) | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 83) | |
| Large parallel controlled study (n = 1656), with 4 arms, no randomisation (personal communication with Dr He through Taixiang Wu) | |
| Open‐label study, imiquimod 5% cream, no control group, patients with common warts or molluscum contagiosum (n = 65) | |
| Patient series, topical treatment with Manuka honey (n = 15) | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 24) | |
| Patient series of topical 5% imiquimod (n = 4) | |
| Cross‐over study of patients with different types of warts (n = 43), 10 molluscum patients. 1 of the treatment arms (placebo first?) had fewer than 2 participants. | |
| Non‐randomised, comparative study of imiquimod 5% cream versus 10% potassium hydroxide (n = 40) | |
| Open‐label study, imiquimod 5% cream, no control group (n = 22) | |
| RCT of people with sexually transmitted disease, not a focus of this review | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 55) | |
| Randomised split‐face study in 20 patients with disseminated facial molluscum contagiosum and HIV infection, not a focus of this review | |
| Not randomised but alternate assignment (personal communication, Alireza Firooz) | |
| RCT comparing two topical analgesics before curettage (n = 40). Not a focus of this review | |
| Not a randomised trial, curettage (n = 73) | |
| Case report, 3 children, topical imiquimod 5% | |
| RCT, n = 150, mainly genital lesions, not a focus of this review | |
| RCT, n = 100, mainly genital lesions, not a focus of this review | |
| Controlled trial (n = 16) comparing phenol ablation and physical expression. Lesions were unit of treatment and analysis. No randomisation | |
| Controlled trial (n = 34) of children aged 2 to 12 years. 10% potassium hydroxide versus placebo. Not randomised, but alternate assignment |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unknown, no full‐text paper and no abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial |
| Participants | Patients with molluscum contagiosum in Turkey |
| Interventions | 10% potassium hydroxide solution versus salicylic and lactic acid combination |
| Outcomes | Lesion response and side effects |
| Notes | Full text obtained August 2016. Email contact about unclear randomisations ("Patients were randomised into two treatment groups according to appealing number." "The treatment groups were not matched with the baseline characteristics because of the randomizations by application rank." (p.301) (8 August 2016) |
| Methods | Possibly randomised (participants were divided into 2 groups) |
| Participants | Children aged 2 to 14 years with molluscum contagiosum in Pakistan |
| Interventions | 5% or 10% potassium hydroxide solution |
| Outcomes | Complete clearance, partial clearance, adverse effects |
| Notes | Summer 2016: asked author for additional information regarding randomisation (unclear from paper) |
| Methods | Double‐blind, randomised clinical trial, in 3 groups |
| Participants | Children aged 2 to 6 years with molluscum contagiosum in Spain. Planned number of participants: 60 |
| Interventions | Application of topical 10% potassium hydroxide in an aqueous solution; 15% potassium hydroxide; placebo |
| Outcomes | Primary outcome: efficacy (disappearance of lesions) after 60 days |
| Notes | Email correspondence in January 2015: results are expected soon |
| Methods | From trial register: Allocation: Randomized Endpoint Classification: Safety/Efficacy Study Intervention Model: Crossover Assignment Masking: Double Blind (Subject, Caregiver, Investigator) Primary Purpose: Treatment |
| Participants | n=100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cantharidin 0.7% topical, cantharidin 0.7% topical with occlusion, placebo, or placebo with occlusion. Treatments were applied at weeks 0 and 3 (blinded phase). At week 6, all participants were treated with open‐label, topical cantharidin 0.7% without occlusion every 3 weeks until all lesions resolved (open‐label phase). |
| Outcomes | Primary outcome measures: Percentage of participants who achieve complete clearance at 6 weeks and 33 weeks [Time Frame: 33 weeks] [Designated as safety issue: No] Assess percentage of participants who achieve a lesion count of zero at 6 weeks (end of blinded phase) and 33 weeks (end of open‐label phase) |
| Notes | Study completion date: January 2016 |
| Methods | Randomised (abstract) or non‐randomised (methods) controlled clinical trial |
| Participants | Children with molluscum contagiosum in tertiary care centre in Nepal |
| Interventions | 5% potassium hydroxide solution versus 0.05% tretinoin cream |
| Outcomes | Number of lesions, local and systemic side effects |
| Notes | February 2015: asked author for additional information regarding randomisation (conflicting statements in paper) |
| Methods | Unknown, no full‐text paper or abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Unknown, no full‐text paper or abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Dose Range‐Finding Phase 2 Trial of a Botanical Drug for the Treatment of Molluscum Contagiosum in Pediatric Subjects (original title, later changed to: A Single‐center, Double‐blind, Placebo‐controlled, Randomized Safety and Efficacy Trial of a Botanical Drug Product, East Indian Sandalwood Oil (EISO), at One Dose Level for the Treatment of Molluscum Contagiosum in Pediatric Subjects |
| Methods | Double‐blind, randomised controlled trial |
| Participants | Children 2 to 17 years of age with molluscum contagiosum, in Texas USA, planned number of participants: 60 |
| Interventions | 10% East Indian sandalwood oil cream administered twice a day for 90 days versus placebo cream |
| Outcomes | The primary purpose of this study is to determine the safety profile of East Indian sandalwood. Safety will be assessed by evaluating adverse events with respect to severity, duration, and relationship to study drug compared to placebo. Secondary outcomes: change in lesion count; improvement in Global Aesthetic Improvement Scale score; complete resolution of lesions; improvement in Evaluator's Global Severity Score. |
| Starting date | September 2015, estimated study completion date September 2016 |
| Contact information | Dr John C Browning, [email protected] |
| Notes | See History of Changes in trial register (duration changed from 60 to 90 days; upper age limit changed; 3 strengths changed into 1; dates; title changed). Results of previously announced dose‐finding study unknown, asked by email July 2015. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.92, 1.93] |
| Analysis 1.1 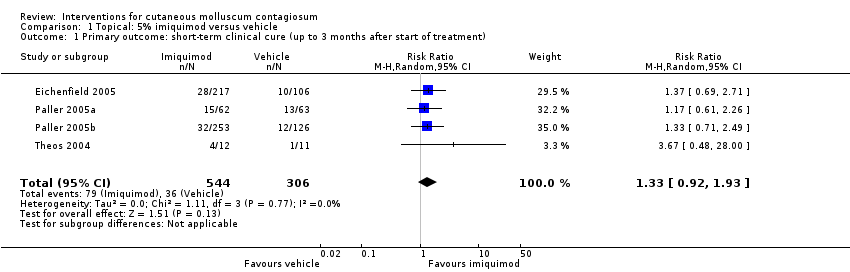 Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.14] |
| Analysis 1.2  Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment). | ||||
| 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.17] |
| Analysis 1.3 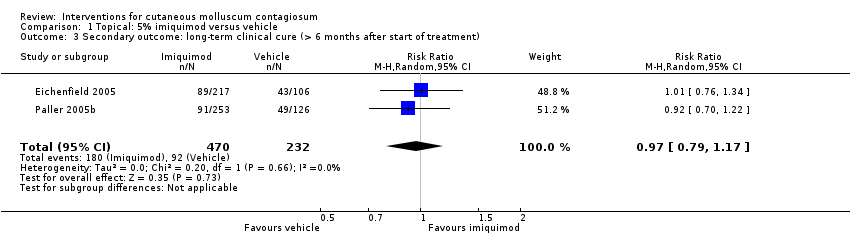 Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment). | ||||
| 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.89, 1.47] |
| Analysis 1.4  Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment). | ||||
| 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| Analysis 1.5 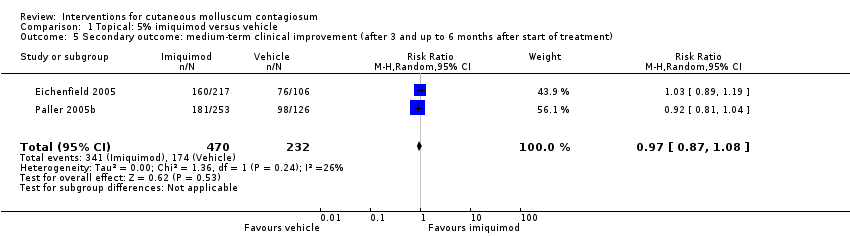 Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment). | ||||
| 6 Secondary outcome: recurrence Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.31, 23.23] |
| Analysis 1.6 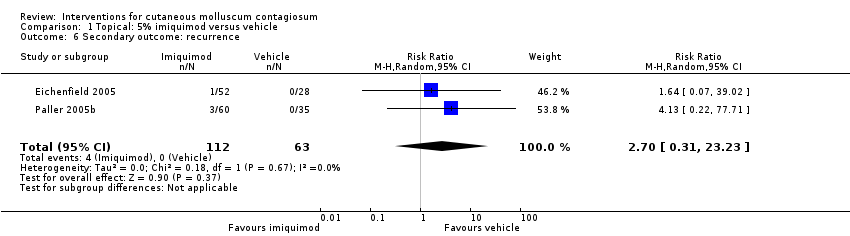 Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 6 Secondary outcome: recurrence. | ||||
| 7 Secondary outcome: any side effect Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| Analysis 1.7  Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 7 Secondary outcome: any side effect. | ||||
| 8 Secondary outcome: application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.13, 1.77] |
| Analysis 1.8  Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 8 Secondary outcome: application site reaction. | ||||
| 9 Secondary outcome: severe application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [1.16, 16.19] |
| Analysis 1.9  Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 9 Secondary outcome: severe application site reaction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.1 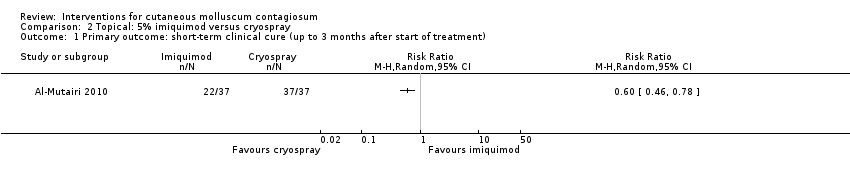 Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 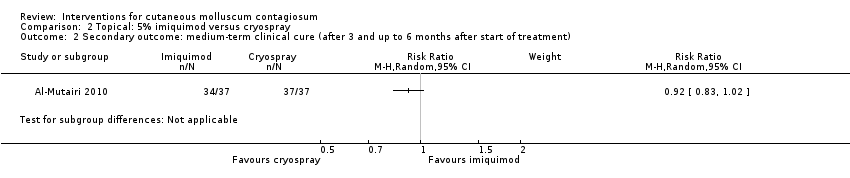 Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment). | ||||
| 3 Secundary outcome: recurrence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 3 Secundary outcome: recurrence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.93] |
| Analysis 3.1  Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: any side effect Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.81] |
| Analysis 3.2  Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 2 Secondary outcome: any side effect. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Topical: 10% lemon myrtle oil versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Topical: 10% benzoyl peroxide cream versus 0.05% tretinoin cream, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.1 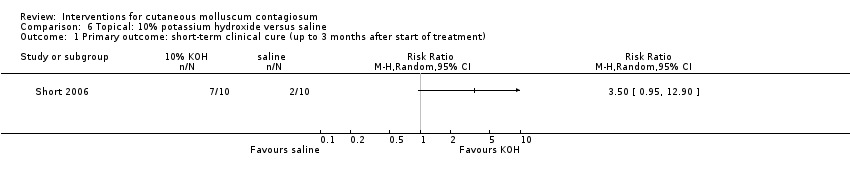 Comparison 6 Topical: 10% potassium hydroxide versus saline, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.1 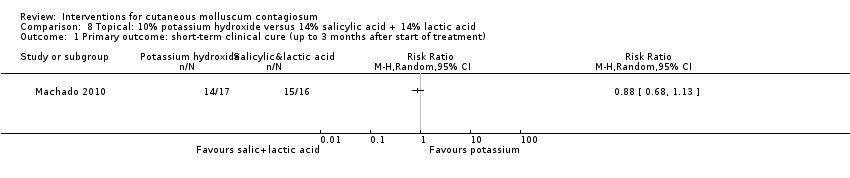 Comparison 8 Topical: 10% potassium hydroxide versus 14% salicylic acid + 14% lactic acid, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Topical: 10% potassium hydroxide versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 11.1 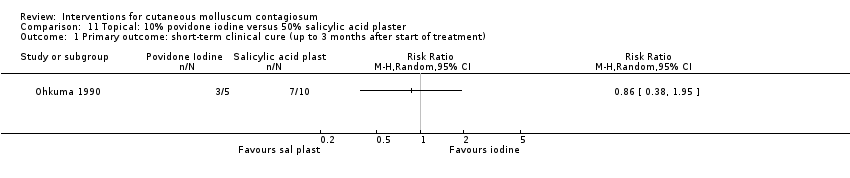 Comparison 11 Topical: 10% povidone iodine versus 50% salicylic acid plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 12.1 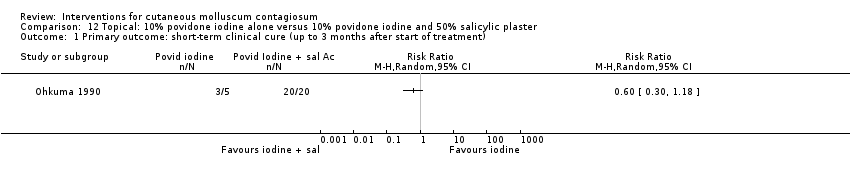 Comparison 12 Topical: 10% povidone iodine alone versus 10% povidone iodine and 50% salicylic plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 13.1  Comparison 13 Topical: 10% povidone iodine and 50% salicylic acid plaster versus 50% salicylic plaster alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.1 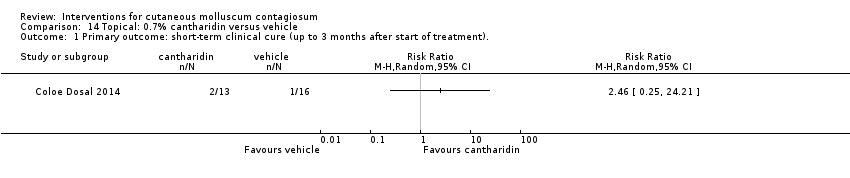 Comparison 14 Topical: 0.7% cantharidin versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 15.1 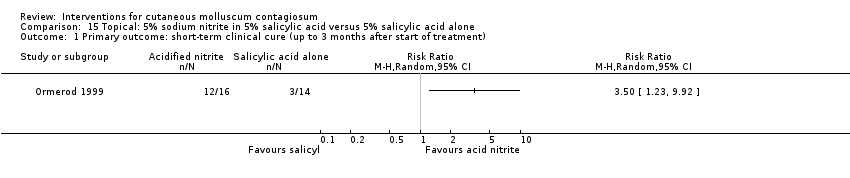 Comparison 15 Topical: 5% sodium nitrite in 5% salicylic acid versus 5% salicylic acid alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 16.1 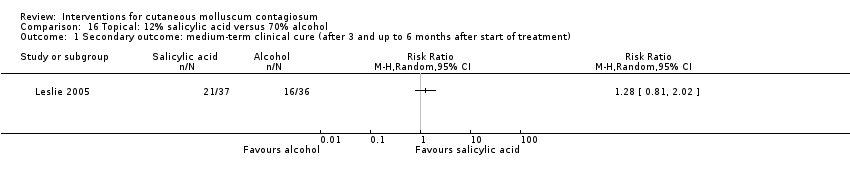 Comparison 16 Topical: 12% salicylic acid versus 70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 17.1  Comparison 17 Topical: 12% salicylic acid versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 18.1 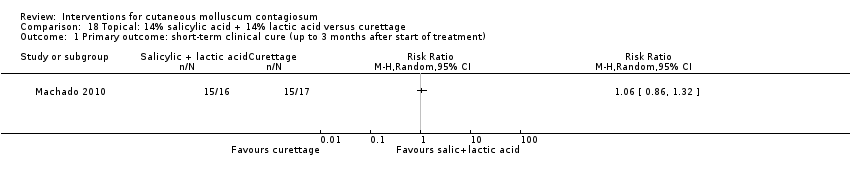 Comparison 18 Topical: 14% salicylic acid + 14% lactic acid versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 19.1  Comparison 19 Topical: 70% alcohol versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 20.1 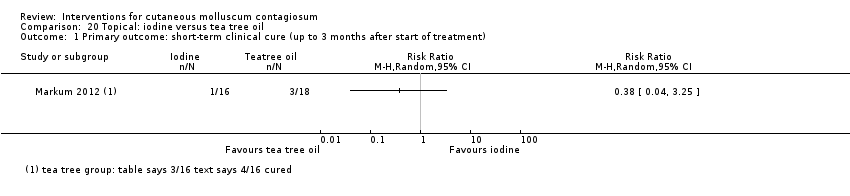 Comparison 20 Topical: iodine versus tea tree oil, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 20.2 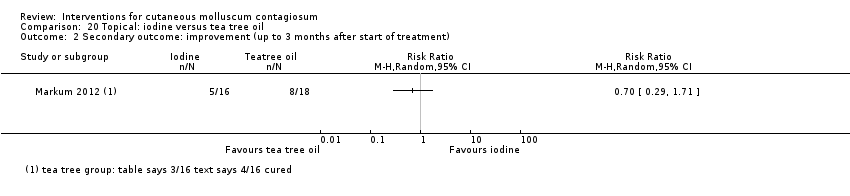 Comparison 20 Topical: iodine versus tea tree oil, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 21.1  Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 21.2  Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 22.1  Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 22.2 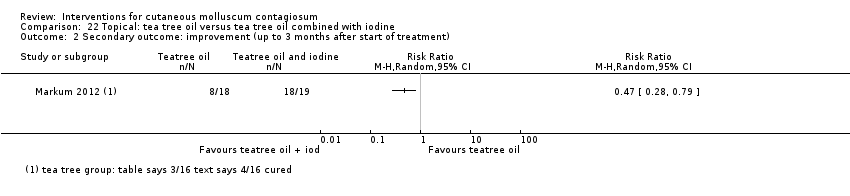 Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 23.1  Comparison 23 Systemic: cimetidine versus placebo, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment). | ||||
| 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 23.2 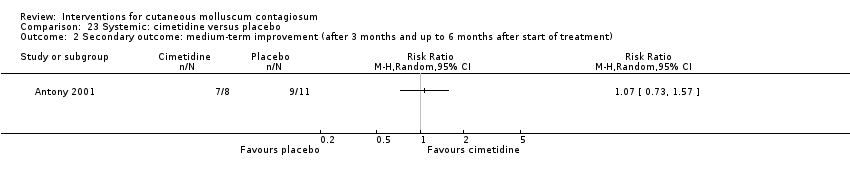 Comparison 23 Systemic: cimetidine versus placebo, Outcome 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 24.1  Comparison 24 Systemic: calcarea carbonica versus placebo, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | ||||

Flow diagram of inclusion for this update.

Risk of bias table: review authors' judgements about each methodological quality item for each included study.
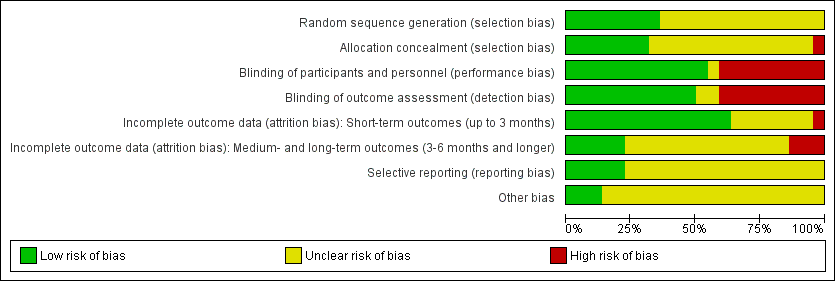
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
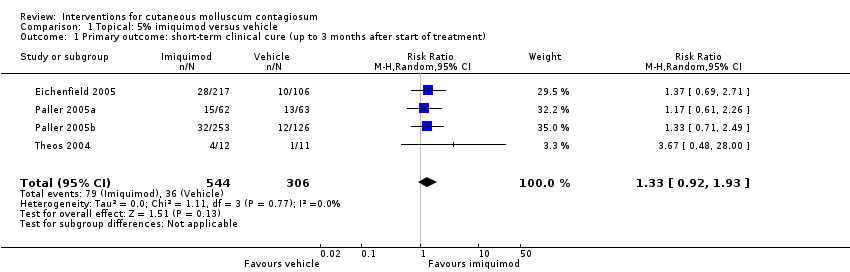
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
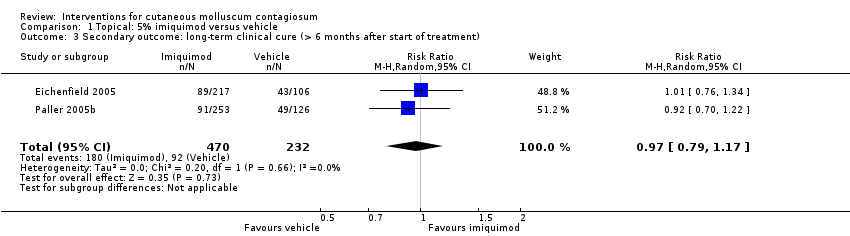
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment).
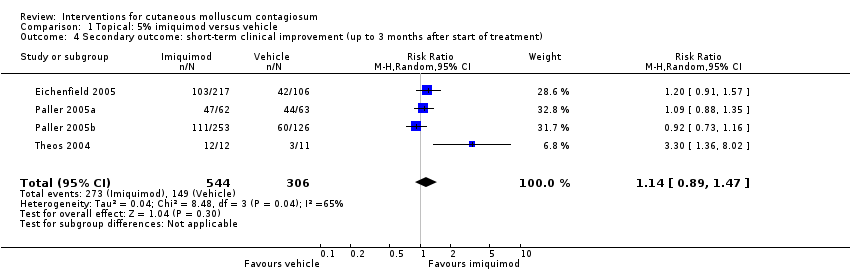
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
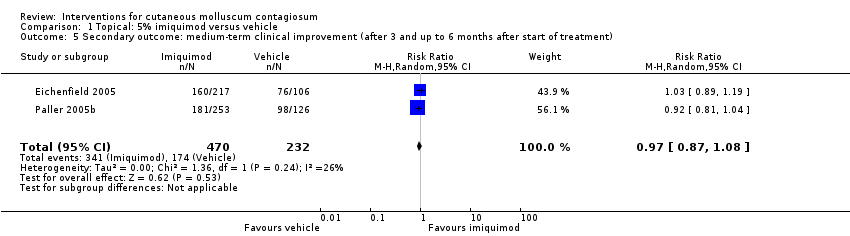
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment).
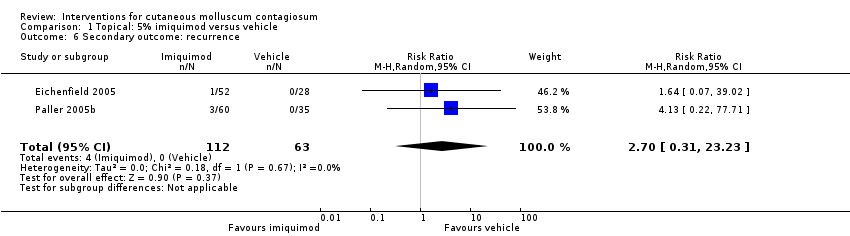
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 6 Secondary outcome: recurrence.
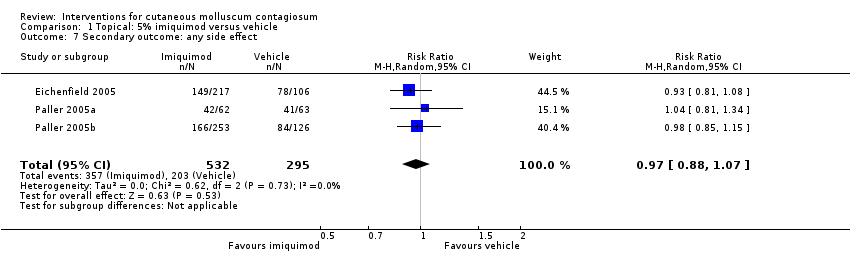
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 7 Secondary outcome: any side effect.
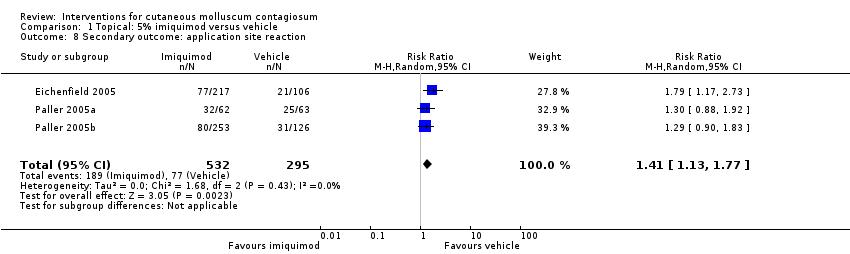
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 8 Secondary outcome: application site reaction.

Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 9 Secondary outcome: severe application site reaction.
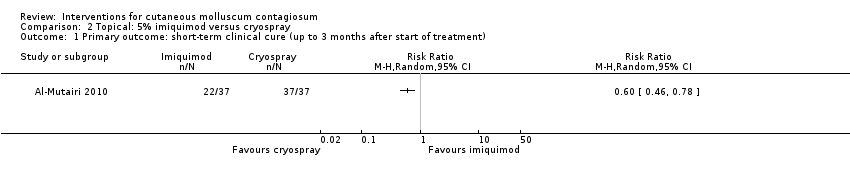
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
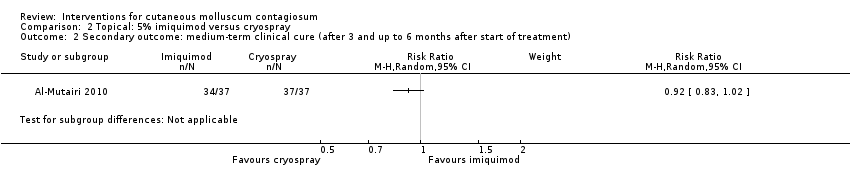
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
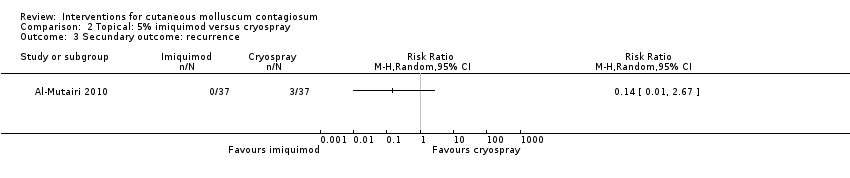
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 3 Secundary outcome: recurrence.
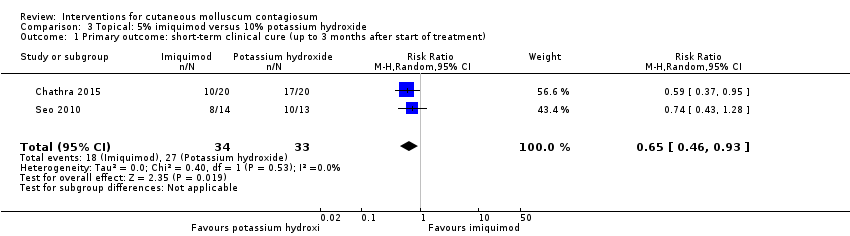
Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 2 Secondary outcome: any side effect.
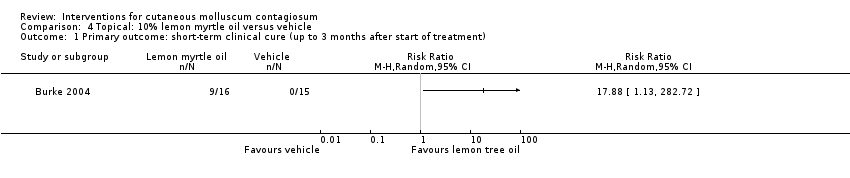
Comparison 4 Topical: 10% lemon myrtle oil versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
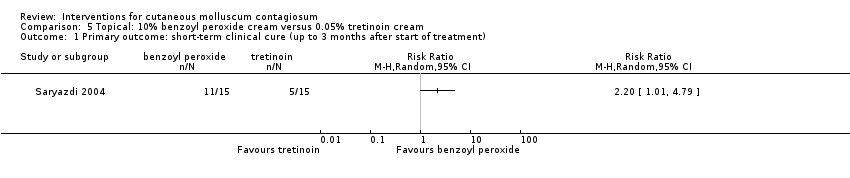
Comparison 5 Topical: 10% benzoyl peroxide cream versus 0.05% tretinoin cream, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
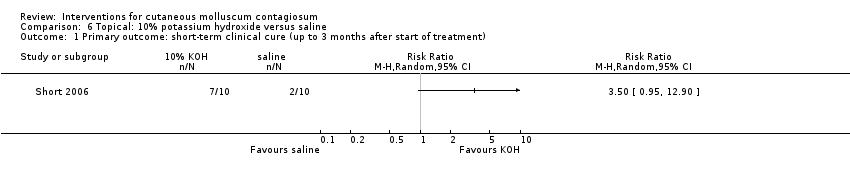
Comparison 6 Topical: 10% potassium hydroxide versus saline, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
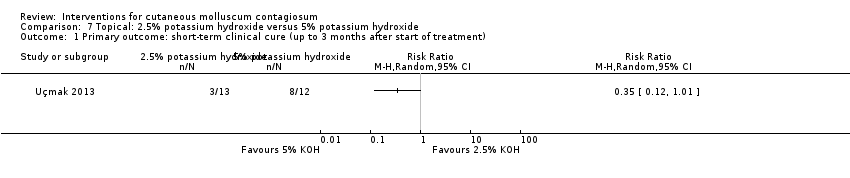
Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment).

Comparison 8 Topical: 10% potassium hydroxide versus 14% salicylic acid + 14% lactic acid, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 9 Topical: 10% potassium hydroxide versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
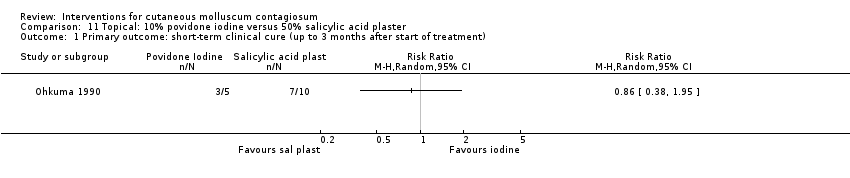
Comparison 11 Topical: 10% povidone iodine versus 50% salicylic acid plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 12 Topical: 10% povidone iodine alone versus 10% povidone iodine and 50% salicylic plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 13 Topical: 10% povidone iodine and 50% salicylic acid plaster versus 50% salicylic plaster alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 14 Topical: 0.7% cantharidin versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment)..

Comparison 15 Topical: 5% sodium nitrite in 5% salicylic acid versus 5% salicylic acid alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
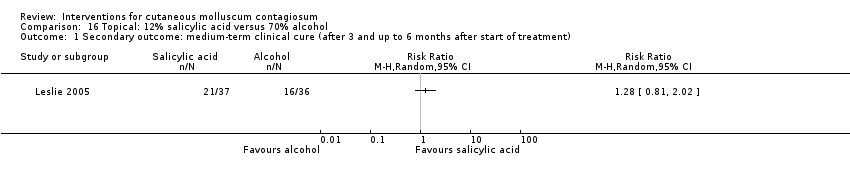
Comparison 16 Topical: 12% salicylic acid versus 70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
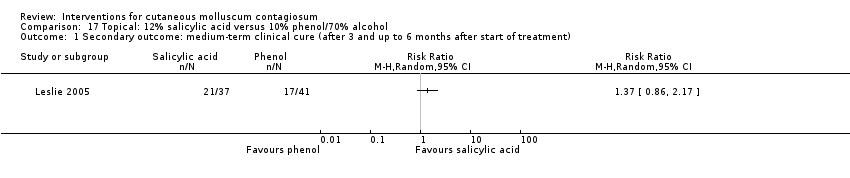
Comparison 17 Topical: 12% salicylic acid versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).

Comparison 18 Topical: 14% salicylic acid + 14% lactic acid versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 19 Topical: 70% alcohol versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).

Comparison 20 Topical: iodine versus tea tree oil, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
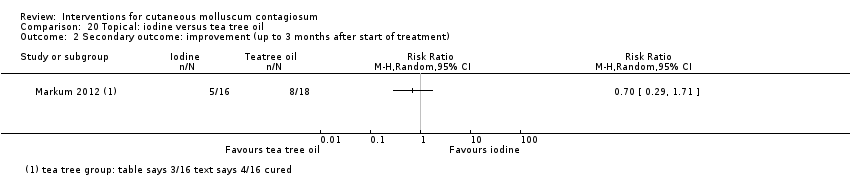
Comparison 20 Topical: iodine versus tea tree oil, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 23 Systemic: cimetidine versus placebo, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment).
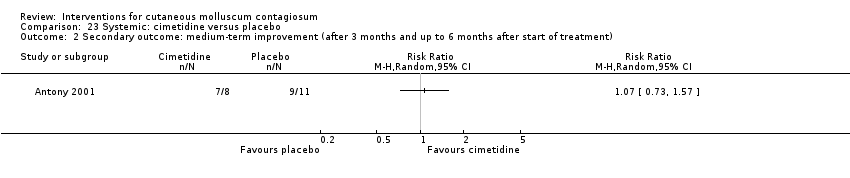
Comparison 23 Systemic: cimetidine versus placebo, Outcome 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment).
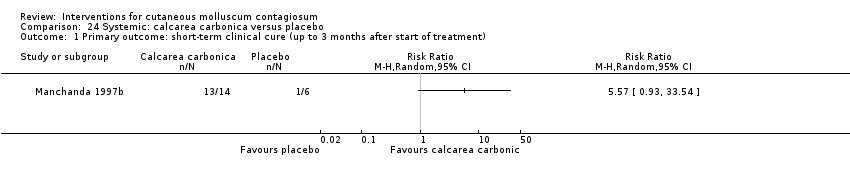
Comparison 24 Systemic: calcarea carbonica versus placebo, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
| Imiquimod versus vehicle for cutaneous molluscum contagiosum | ||||||
| Patient or population: molluscum contagiosum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical vehicle | Risk with topical imiquimod | |||||
| Short‐term clinical cure (up to 3 months after start of treatment) (completely cleared short term) | Study population | RR 1.33 | 850 | ⊕⊕⊕○ | Analysis 1.1 | |
| 118 per 1000 | 156 per 1000 | |||||
| Medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) (completely cleared medium term) | Study population | RR 0.88 | 702 | ⊕⊕⊕○ | Analysis 1.2 | |
| 272 per 1000 | 239 per 1000 | |||||
| Long‐term clinical cure (beyond 6 months after start of treatment) (completely cleared long term) | Study population | RR 0.97 | 702 | ⊕⊕⊕○ | Analysis 1.3 | |
| 401 per 1000 | 389 per 1000 | |||||
| Short‐term clinical improvement (up to 3 months after start of treatment) | Study population | RR 1.14 | 850 | ⊕⊕⊕⊕ | Analysis 1.4 | |
| 487 per 1000 | 555 per 1000 | |||||
| Any adverse effect | Study population | RR 0.97 | 827 | ⊕⊕⊕⊕ | Analysis 1.7 | |
| 688 per 1000 | 667 per 1000 | |||||
| Application site reactions | Study population | RR 1.41 | 827 | ⊕⊕⊕○ | Analysis 1.8. This outcome was not prespecified in our protocol. | |
| 261 per 1000 | 368 per 1000 | |||||
| Severe application site reactions | Study population | RR 4.33 | 827 | ⊕⊕⊕○ | Analysis 1.9. This outcome was not prespecified in our protocol. | |
| 7 per 1000 | 29 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. 2Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as both studies were judged to be at low risk of bias. 3We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. We also decided not to downgrade for inconsistency as removing one outlier eliminated inconsistency but hardly affected pooled estimate. 4We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. 5Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. | ||||||
| Treatment class | Treatment modality | Included studies | Other studies |
| 'Doing nothing' | Awaiting natural resolution | — | |
| Placebo | Antony 2001; Eichenfield 2005; Manchanda 1997b; Paller 2005a; Paller 2005b | — | |
| Surgical treatments | Cryotherapy | ||
| Curettage | |||
| Curettage with punch | — | ||
| Electric cauterisation | — | ||
| Physical expression (squeezing) | — | ||
| Pricking | — | ||
| Pulsed dye laser | — | ||
| Topical treatments | Acidified nitrite | ||
| Adapalene | — | ||
| Australian lemon myrtle oil | — | ||
| Benzoyl peroxide | — | ||
| Bromogeramine | — | ||
| Cantharidin | |||
| Cidofovir | — | ||
| Diphencyprone | — | ||
| Griseofulvin | — | ||
| Honey | — | ||
| Hydrogen peroxide cream | — | ||
| Hyperthermia | — | ||
| Imiquimod | Al‐Mutairi 2010; Eichenfield 2005; Hanna 2006; Paller 2005a; Paller 2005b; Seo 2010; Theos 2004 | Arican 2006; Barba 2001; Bayerl 2003; Hengge 2003; Lim 2003; Liota 2000; Metkar 2008; Skinner 2000; Skinner 2002; Syed 1998 | |
| Iodine | — | ||
| Iodine combined with tea tree oil | — | ||
| Milkweed | — | ||
| Povidone iodine plus salicylic acid | — | ||
| Phenol | |||
| Podophyllotoxin (HIV patients) | — | ||
| Potassium hydroxide | |||
| Retinoic acid | — | ||
| Salicylic acid | — | ||
| Salicylic acid combined with lactic acid | — | ||
| Salicylic acid combined with sodium nitrite | — | ||
| Silver nitrate | — | ||
| Tea tree oil | — | ||
| Tretinoin | — | ||
| Yellow oxide of mercury | — | ||
| Systemic treatments | Cimetidine | ||
| Calcarea carbonica (homeopathy) | |||
| Griseofulvin | — | ||
| Combinations of above | Potassium iodide followed by X‐rays | — |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.92, 1.93] |
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.14] |
| 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.17] |
| 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.89, 1.47] |
| 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| 6 Secondary outcome: recurrence Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.31, 23.23] |
| 7 Secondary outcome: any side effect Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 8 Secondary outcome: application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.13, 1.77] |
| 9 Secondary outcome: severe application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [1.16, 16.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Secundary outcome: recurrence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.93] |
| 2 Secondary outcome: any side effect Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

