皮肤传染性软疣的干预措施
摘要
研究背景
传染性软疣是一种常见的皮肤感染疾病,它由痘病毒引起,主要发生在儿童之中。在没有免疫缺陷的人群中,感染通常在几个月内消退;但因社交和美容的原因,或出于避免疾病传染,治疗也许更受青睐。目前还缺乏能支持各种治疗方法的明确证据基础。
这是对一个2006年首次发表的Cochrane系统综述的更新,上一次更新是在2009年。
研究目的
评估特定的治疗与管理策略的效果,纳入了等待自然消退、患皮肤、非生殖器传染性软疣的无免疫缺陷人群。
检索策略
截至2016年7月,我们更新了对如下数据库的检索:Cochrane皮肤组专业注册库(the Cochrane Skin Group Specialised Register),Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL),国际性综合生物医学信息书目数据库(MEDLINE),生物医学与药理学文摘数据库(Embase),拉丁美洲和加勒比国家健康信息(LILACS)。我们检索了6个试验注册库并筛选了纳入研究和综述文章的参考文献列表,以进一步参考相关的随机对照试验。我们与该领域的制药公司和专家取得联系,以进一步确定相关的随机对照试验。
纳入排除标准
对无免疫缺陷的传染性软疣患者的所有治疗的随机对照实验。我们排除了针对性传播型传染性软疣和免疫缺陷人群(包括艾滋病感染者)的试验。
资料收集与分析
两名综述作者独立筛选研究,评估方法学质量,并从所筛选研究中提取资料。我们尽可能从研究作者那里获取缺失的资料。
主要结果
我们在这此次更新中发现了11项新研究,总共纳入了22项研究与1650名受试者。这些研究探讨了局部(20项研究)和全身干预治疗(2项研究)的效果。
一位该领域的专家让我们注意到,在新纳入的研究中,有三项未发表的大型研究的完整试验报告。它们都提供了中等质量证据,证明与赋形药(安慰剂)的短期临床治疗(4项研究,850名受试者,治疗开始后的12周,风险比(risk ratio, RR)=1.33, 95%置信区间(confidence interval, CI) 0.92至1.93)、中期临床治疗(2项研究,702名受试者,治疗开始后的18周,风险比=0.88,95%置信区间 0.67至1.14)和长期临床治疗(2项研究),702名受试者,治疗开始后的28周,风险比=0.97,95%置信区间 0.79至1.17)相比,5%咪喹莫特缺乏疗效。我们发现了类似但更确定的短期改善结果(4项研究,850名受试者,治疗开始后的12周,风险比=1.14, 95%置信区间 0.89至1.47;高质量证据)。对于“任何不良反应”这一结局,我们发现了高质量证据,表明局部使用5%咪喹莫特和赋形药之间几乎无差异或没有差异(3项研究,827名受试者,风险比=0.97,95%置信区间 0.88至1.07);但在使用咪喹莫特治疗的组别中,用药部位反应更常见(中等质量证据):任何用药部位反应(3项研究,827名受试者,风险比=1.41,95%置信区间 1.13至1.77,需要治疗额外有害结局(NNTH)的人数为11);严重的用药部位反应(3项研究,827名受试者,风险比=4.33,95%置信区间 1.16至16.19,需要额外治疗有害结局的人数超过40)。
在以下11项比较中,只有有限的证据表明哪种治疗在实现短期临床治愈方面效果更优(低质量证据):5%咪喹莫特的疗效低于冷冻喷雾(1项研究,74名受试者,风险比=0.60,95%置信区间 0.46至0.78)和10%氢氧化钾(2 项研究,67名受试者,风险比=0.65,95%置信区间 0.46至0.93);10%澳大利亚柠檬香桃木油比橄榄油更有效(1项研究,31名受试者,风险比=17.88,95%置信区间 1.13至282.72);10%过氧苯甲酰乳膏比0.05%维A酸更有效(1项研究,30名受试者,风险比=2.20,95%置信区间 1.01至4.79);5%亚硝酸钠与5%水杨酸联合使用比单独使用5%水杨酸更有效(1项研究,30名受试者,风险比=3.50,95%置信区间 1.23至9.92);碘加茶树油比单独使用茶树油(1项研究,37名受试者,风险比=0.20,95%置信区间 0.07至0.57)或单独使用碘(1项研究,37名受试者,风险比=0.07,95%置信区间 0.01至0.50)更有效。尽管存在不确定性,但10%氢氧化钾似乎比生理盐水更有效(1 项研究,20名受试者,风险比=3.50,95%置信区间 0.95至12.90);顺势疗法的碳酸钙似乎比安慰剂更有效(1项研究,20名受试者,风险比=5.57,95%置信区间 0.93至33.54);2.5%氢氧化钾溶液疗效低于5%氢氧化钾溶液(1项研究,25名受试者,风险比=0.35,95%置信区间 0.12至1.01);10%聚维酮碘溶液加50%水杨酸软膏似乎比单独使用水杨酸软膏更为有效(1项研究,30名受试者,风险比=1.43,95%置信区间 0.95至2.16)。
我们发现其他比较没有显著的统计学差异(其中大多数涉及两种不同的局部治疗)。我们没有发现表达病变或局部使用过氧化氢的随机对照试验证据。
研究局限性包括未采用盲法,脱落数量多以及未采用意向治疗分析。除了使用咪喹莫特的用药部位严重反应外,上述评价治疗均与严重不良反应无关(低质量证据)。最常见的不良事件是用药疼痛、红斑和瘙痒。以下比较所纳入的研究并未报告不良反应:碳酸钙与安慰剂相比、10%聚维酮碘加50%水杨酸软膏与水杨酸软膏相比,以及10%过氧化苯甲酰与0.05%维A酸相比。
由于信息不足,我们无法判断大多数研究,特别是关于分配方案隐藏和可能的选择性报告的偏倚风险。我们认为有五项研究偏倚风险低。
作者结论
在治疗传染性软疣方面,没有一种干预措施具有令人信服的疗效。我们发现,中等质量证据表明,就临床治愈效果而言,局部使用5%咪喹莫特并不比赋形药有效,但会导致更多的用药部位反应;而高质量的证据表明,在短期改善方面,两种治疗之间没有差异。然而,高质量的证据表明,两组中一般不良反应的数量相似。因为发现的证据并不支持任何一种治疗方法,所以等待传染性软疣自然消退仍然是治疗这种疾病的有效方法。
PICO
简语概要
传染性软疣(儿童常见的病毒性皮肤感染疾病)的治疗
综述问题
我们评价了所有治疗常见病毒性皮肤感染传染性软疣的效果的证据。我们排除了免疫系统受到抑制或者患有性传播传染性软疣的人群。
研究背景
在健康人群中,传染性软疣是一种自限性的、相对无害的病毒性皮肤传染病。它多发于儿童和青少年,在成人中很少见。它在世界范围内均有发生,但在气候温暖的地区似乎更为常见。传染性软疣通常表现为单个或多个充满油性物质的丘疹。人们寻求治疗可能是出于社交和美容原因,也可能是担心将疾病传染给他人。治疗的目的是加快康复过程。
研究特征
我们检索了截至2016年7月的文献。我们纳入了22项试验(共纳入1650名受试者)。其中20项研究评价了局部治疗,两项研究评价了口服治疗(口服)。比较纳入了物理疗法,局部治疗以及口服治疗。大多数研究在北美、英国、亚洲或南美这些地区或国家的医院门诊或急诊进行。受试者包括男性和女性,主要是儿童或年轻人。在随机分组后,随访持续时间从3周到28周不等。只有5项研究的随访时间超过3个月。
5项研究报告了商业资金资助,3项研究获取了由制药公司提供的免费药物,12项研究并未提及资金来源,一项研究报告了慈善资金,一项研究报告称其未获得资金支持。
主要结果
我们发现,许多常见的软疣治疗方法(如物理破坏)并未得到充分评价。一些纳入的治疗方法并不属于标准治疗。
我们发现,中等质量证据表明,在实现短期、中期和长期临床治愈效果方面,局部使用5%咪喹莫特可能并不比使用赋形药(即不含咪喹莫特的相同乳膏)更有效。高质量(因此更为确定)证据表明,治疗开始后的3个月内,局部使用5%咪喹莫特改善软疣的疗效并不比安慰剂好。
高质量证据表明,与赋形药组对比,使用5%咪喹莫特的不良反应数量差异很小或根本没有差异。然而,中等质量的证据表明,与赋形药组相比,局部使用5%咪喹莫特可能会产生更多的用药部位反应。
基于一两项大多为小型研究的低质量证据揭示了短期临床治愈结局的如下结果:5%咪喹莫特的疗效低于冷冻喷雾或10%氢氧化钾;10%澳洲柠檬香桃木油比橄榄油更有效;10%过氧化苯甲酰乳膏比0.05%维A酸更有效;5%亚硝酸钠与5%水杨酸并用比单独使用5%水杨酸疗效更好;碘加茶树油比单独使用茶树油或碘更有效。我们发现更多不确定(低质量)的证据表明,10%氢氧化钾比盐水更有效;顺势疗法的碳酸钙比安慰剂更有效;2.5%氢氧化钾溶液的疗效低于5%氢氧化钾溶液;10%聚维酮碘溶液和50%水杨酸软膏比单独使用水杨酸软膏疗效更佳。
除了使用咪喹莫特的用药部位严重反应之外,这些治疗均未导致严重的不良反应(低质量证据)。最常报告的的不良反应是治疗过程中的疼痛、发红和瘙痒。
我们发现,在其他比较中评估的治疗之间没有差异。
我们没有发现几种常用治疗方法的随机试验,比如利用橙棒(指甲棒)或局部使用过氧化氢指示损伤部位。因为大多数损伤能够在数月中消退,所以,除非出现更好的证据证明积极治疗的优越性,传染性软疣可以留待其自然愈合。
证据质量
对于局部使用咪喹莫特,临床治愈、短期改善和不良反应的证据质量为从中等到高。对于所有其他的比较,短期临床治愈效果和不良反应的证据质量都低。所纳入研究的共同局限性在于受试者人数少、研究人员未设盲,以及未完成研究的受试者(在一些研究中数量众多)未被纳入分析。
Authors' conclusions
Summary of findings
| Imiquimod versus vehicle for cutaneous molluscum contagiosum | ||||||
| Patient or population: molluscum contagiosum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical vehicle | Risk with topical imiquimod | |||||
| Short‐term clinical cure (up to 3 months after start of treatment) (completely cleared short term) | Study population | RR 1.33 | 850 | ⊕⊕⊕○ | Analysis 1.1 | |
| 118 per 1000 | 156 per 1000 | |||||
| Medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) (completely cleared medium term) | Study population | RR 0.88 | 702 | ⊕⊕⊕○ | Analysis 1.2 | |
| 272 per 1000 | 239 per 1000 | |||||
| Long‐term clinical cure (beyond 6 months after start of treatment) (completely cleared long term) | Study population | RR 0.97 | 702 | ⊕⊕⊕○ | Analysis 1.3 | |
| 401 per 1000 | 389 per 1000 | |||||
| Short‐term clinical improvement (up to 3 months after start of treatment) | Study population | RR 1.14 | 850 | ⊕⊕⊕⊕ | Analysis 1.4 | |
| 487 per 1000 | 555 per 1000 | |||||
| Any adverse effect | Study population | RR 0.97 | 827 | ⊕⊕⊕⊕ | Analysis 1.7 | |
| 688 per 1000 | 667 per 1000 | |||||
| Application site reactions | Study population | RR 1.41 | 827 | ⊕⊕⊕○ | Analysis 1.8. This outcome was not prespecified in our protocol. | |
| 261 per 1000 | 368 per 1000 | |||||
| Severe application site reactions | Study population | RR 4.33 | 827 | ⊕⊕⊕○ | Analysis 1.9. This outcome was not prespecified in our protocol. | |
| 7 per 1000 | 29 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. 2Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as both studies were judged to be at low risk of bias. 3We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. We also decided not to downgrade for inconsistency as removing one outlier eliminated inconsistency but hardly affected pooled estimate. 4We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. 5Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. | ||||||
Background
Description of the condition
Molluscum contagiosum is a viral skin infection most frequently encountered in children (Chen 2013). The infection is caused by the molluscum contagiosum virus, which is classified within the family of poxviruses (Poxviridae) (Buller 1991). The virus is assumed to be the only remaining poxvirus that specifically affects human beings (Chen 2013).
Infection follows after contact with infected people or contaminated objects (Chen 2013). Molluscum contagiosum usually presents as single or multiple (usually no more than 20) painless, spherical, shiny, pearly white papules that classically have a central dimple. Their size may vary from tiny 1 mm papules to large nodules over 1 cm in diameter. The lesions may itch (Rogers 1998).
In addition to the common form of benign skin tumours (mostly found in children), there is also a sexually transmitted variant of molluscum contagiosum that occurs on genital, perineal, pubic, and surrounding skin (Czelusta 2000). Molluscum contagiosum lesions may also appear in or around the mouth (Whitaker 1991). Molluscum contagiosum has also been observed with other diseases in people with immune deficiency (Gottlieb 1994; Mansur 2004). People with HIV infection are particularly prone to molluscum contagiosum; prevalence in this population has been reported to range from 5% to 18% (Hira 1988; Husak 1997; Matis 1987). The focus of this review was the common form of molluscum contagiosum only.
Epidemiology
Molluscum contagiosum occurs worldwide. Previous reviews have reported that it as more frequent in geographic areas with warm climates, but this may be due to selective publication of local outbreaks (Olsen 2014). Infection is rare in children under the age of 1 year, typically occurring in the 2‐ to 5‐year‐old age group (Rogers 1998). The age of peak incidence is reported to be between the ages of 2 and 3 years in Fiji (Postlethwaite 1967), and between 1 and 4 years in the Congo (formerly Zaire) (Torfs 1959). In Papua New Guinea the annual incidence rate for children under 10 years of age was 6% (Sturt 1971). Population‐based occurrence rates are scarce for high‐income countries. In a large questionnaire study among parents of children attending kindergartens and elementary schools, the reported prevalence of molluscum contagiosum was 5.6% and 7.4%, respectively (Niizeki 1984). Much higher prevalence rates have been reported during outbreaks in closed communities (Overfield 1966). In 1878 an outbreak in an English school was reported involving 9 children (Liveing 1878). A recent meta‐analysis of five cross‐sectional surveys among children (age range 0 to 16 years) resulted in a pooled prevalence rate of 2.8% (95% confidence interval 0.0 to 5.9) (Olsen 2014).
In the USA, the estimated number of physician visits for molluscum contagiosum from 1990 to 1999 was 280,000 per year (Molino 2004). One out of 6 Dutch children aged 15 years have visited their doctor for molluscum contagiosum at least once (Koning 1994). There is generally no difference in incidence between males and females (Koning 1994; Relyveld 1988; Sturt 1971); however, an unequal sex ratio was found in studies from Japan (Niizeki 1984), Alaska (Overfield 1966), and Fiji (Hawley 1970), where boys were affected more often. This is probably due to habits associated with the spread of the infection, such as swimming (Niizeki 1984; Postlethwaite 1967). Outbreaks may occur among children who bathe or swim together. A history of eczema was found in 62% of children with molluscum contagiosum in Australia (Braue 2005). In the adolescent and adult age groups sexual transmission becomes important.
Natural history
The estimated incubation period varies from 14 days to 6 months (Sterling 1998). Lesions enlarge slowly and may reach a diameter of 5 to 10 mm in 6 to 12 weeks (Sterling 1998). After trauma (e.g. scratching) or spontaneously after several months, inflammatory changes result in the production of white fluid, crusting, and eventual destruction of the lesions. The duration of both the individual lesion and of the entire episode is highly variable. Crops of molluscum may appear to come and go for several months, and although most cases are self limiting and resolve within six to nine months, some may persist for more than three or four years.
A Japanese study described spontaneous resolution on average 6.5 months after infection in 205 out of 217 children (94.5%) affected by molluscum contagiosum (Takemura 1983). One month after the first consultation with the dermatologist, 23% of the children were cured. A recent community cohort study in the UK, Olsen 2015, followed 306 children with molluscum contagiosum aged 4 to 15 years. Only 19% of the children were reported to have received treatment. Mean time to resolution was 13.3 months; 30% had not resolved by 18 months, and 13% had not resolved by 24 months.
Secondary bacterial infection can occur, and when severe can result in scarring. This must be distinguished from the milder inflammatory reactions that molluscum lesions show after a scratching or when they are starting to resolve spontaneously, which may prompt parents to take their child to the general practitioner thinking they have become infected (Highet 1992).
Particularly in atopic people (who are prone to asthma, hay fever, or eczema), there is a tendency for a patch of eczema (which is often particularly itchy) to develop around one or more of the lesions a month or more after their arrival (Beaulieu 2000; De Oreo 1956). Erythema annulare centrifugum (a widespread rash of red inflammatory rings) has also been reported (Vasily 1978). Chronic conjunctivitis and superficial punctate keratitis may likewise complicate lesions on or near the eyelids (Haellmigk 1966; Redmond 2004). The eczema and conjunctivitis diminish naturally when the molluscum lesion is removed.
Molluscum contagiosum behaves differently in HIV‐infected individuals. As immunodeficiency progresses, molluscum contagiosum becomes more common and resistance to therapy increases. Frequently, multiple lesions in atypical areas such as the face and neck can be found (Husak 1997). Only limited data are available on the course of the disease in this group of people.
Description of the intervention
In people without an immune deficiency molluscum contagiosum is a self limiting disease (Chen 2013). Therapy is often not necessary for recovery, and awaiting spontaneous resolution is an important management strategy (Brown 2006; Chen 2013; Jones 2007; Olsen 2015; Takemura 1983). Most lesions resolve within months without scarring in otherwise healthy people (Ordoukhanian 1997). Treatment is intended to accelerate this process. Destruction of the lesions and the production of an inflammatory response are means by which resolution of the lesions could be hastened (Sterling 1998).
Reasons to treat molluscum contagiosum include the following:
-
alleviating discomfort, including itching;
-
cosmetic reasons;
-
social stigma associated with many visible lesions;
-
limiting its spread to other areas of the body and to other people;
-
preventing scarring and secondary infection; and
-
preventing trauma and bleeding of lesions.
There are a large number of treatment options for molluscum contagiosum (see Table 1 for an overview). These treatment options can be divided into three major categories:
| Treatment class | Treatment modality | Included studies | Other studies |
| 'Doing nothing' | Awaiting natural resolution | — | |
| Placebo | Antony 2001; Eichenfield 2005; Manchanda 1997b; Paller 2005a; Paller 2005b | — | |
| Surgical treatments | Cryotherapy | ||
| Curettage | |||
| Curettage with punch | — | ||
| Electric cauterisation | — | ||
| Physical expression (squeezing) | — | ||
| Pricking | — | ||
| Pulsed dye laser | — | ||
| Topical treatments | Acidified nitrite | ||
| Adapalene | — | ||
| Australian lemon myrtle oil | — | ||
| Benzoyl peroxide | — | ||
| Bromogeramine | — | ||
| Cantharidin | |||
| Cidofovir | — | ||
| Diphencyprone | — | ||
| Griseofulvin | — | ||
| Honey | — | ||
| Hydrogen peroxide cream | — | ||
| Hyperthermia | — | ||
| Imiquimod | Al‐Mutairi 2010; Eichenfield 2005; Hanna 2006; Paller 2005a; Paller 2005b; Seo 2010; Theos 2004 | Arican 2006; Barba 2001; Bayerl 2003; Hengge 2003; Lim 2003; Liota 2000; Metkar 2008; Skinner 2000; Skinner 2002; Syed 1998 | |
| Iodine | — | ||
| Iodine combined with tea tree oil | — | ||
| Milkweed | — | ||
| Povidone iodine plus salicylic acid | — | ||
| Phenol | |||
| Podophyllotoxin (HIV patients) | — | ||
| Potassium hydroxide | |||
| Retinoic acid | — | ||
| Salicylic acid | — | ||
| Salicylic acid combined with lactic acid | — | ||
| Salicylic acid combined with sodium nitrite | — | ||
| Silver nitrate | — | ||
| Tea tree oil | — | ||
| Tretinoin | — | ||
| Yellow oxide of mercury | — | ||
| Systemic treatments | Cimetidine | ||
| Calcarea carbonica (homeopathy) | |||
| Griseofulvin | — | ||
| Combinations of above | Potassium iodide followed by X‐rays | — |
-
physical destruction of the lesions;
-
topical agents (i.e. those applied directly to the lesions); and
-
systemic treatment (i.e. those affecting the whole body).
In the past, many authors have recommended physical destruction as the preferred method for treatment of molluscum contagiosum (Smith 2002; Stulberg 2003; Williams 1991). Dermatology textbooks mention removal of the lesion with a sharp curette (curettage) or the application of liquid nitrogen (cryotherapy) as being simple and usually effective treatments (Lowy 1999; Sterling 1998). Gentle squeezing or pricking with a sterile needle are alternatively recommended destructive therapies (Berger 1996). Most of these therapies need to be repeated at three to four weekly intervals. Treatment may be painful and may result in scarring (Friedman 1987). Squeezing of lesions may even lead to the formation of large abscesses due to the disruption of virus into the deeper layer of the skin (dermis) (Brandrup 1989).
Topical preparations such as podophyllotoxin, liquefied phenol, tretinoin, cantharidin, or potassium hydroxide are also used (Hughes 2013; Metkar 2008; Saryazdi 2004; Silverberg 2000; Syed 1994; Weller 1999). In children, prior application of local anaesthetic cream may reduce the pain of treatment involving physical destruction or local inflammation (de Waard 1990; Rosdahl 1988), although severe side effects have been reported in a case of excessive application of lidocaine‐prilocaine (Wieringa 2006). Other proposed topical treatments include immune response modifiers such as imiquimod and cidofovir.
Systemic treatment with cimetidine has been suggested as a possible treatment because of its systemic immunomodulatory effects; it increases lymphocyte proliferation and inhibits suppressor T‐cell function (Orlow 1993; Sterling 1998).
Little data are available with regard to prevailing practice. In a survey among paediatric dermatologists in the USA in 2008, respondents seemed to favour cantharidin, followed by imiquimod, watchful waiting, curettage, and cryotherapy (Coloe 2009). This differs from, for example, general practice in the Netherlands, where waiting for natural resolution is the most popular option (Van der Linden 2005). A more recent survey among physicians from various specialties in the USA showed that treatment preferences differed widely between specialties (Hughes 2013).
How the intervention might work
The working mechanism differs according to the type of treatment (Chen 2013). Curettage aims to remove the lesions entirely. Other techniques like pricking with a needle, cryotherapy, or pulsed‐dye laser aim at damaging the lesion, which may in itself induce an immune response. Topical preparations such as podophyllotoxin, tretinoin, cantharidin, or potassium hydroxide are supposed to evoke a local inflammatory response. Application of phenol or trichloroacetic acid also aims to destroy the lesions. Another topical preparation, imiquimod, supposedly induces an immune response. Cimetidine is a systemic immune modulator. Antiviral agents, especially cidofovir, have been used both systemically and locally (Chen 2013).
Why it is important to do this review
Molluscum contagiosum is a common reason for consultation in family practice and dermatology. Many treatment options are available, some of which are painful and some that may leave scars. A decision may be made in favour of active therapy to prevent further spread, relieve symptoms, prevent scarring, and for cosmetic and social reasons. Indeed, many parents are concerned about the stigma associated with the lesions. Children with molluscum may be excluded from attending nursery and from participating in physical activities such as swimming. However, the scientific basis for treatment is unclear. Consequently, many practitioners find themselves in a dilemma as to whether or not to promote active treatment and, if they do decide on an active treatment strategy, are unclear as to which is the best option. We carried out this systematic review to evaluate treatment options for molluscum contagiosum.
Objectives
To assess the effects of specific treatments and management strategies, including waiting for natural resolution, for cutaneous, non‐genital molluscum contagiosum in people without immune deficiency.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) for the treatment of molluscum contagiosum. We excluded trials on sexually transmitted molluscum contagiosum and in people with an immune deficiency (including those with HIV infection).
Types of participants
People with a diagnosis of molluscum contagiosum, except for those with immune deficiency or sexually transmitted molluscum contagiosum.
In general, treatment was based on a clinical diagnosis only, as molluscum contagiosum is an easy diagnosis to make and confusion is rare among clinicians. We therefore considered additional diagnostic criteria, such as histological examination and laboratory investigations, as unnecessary.
Types of interventions
All treatments aimed at eradicating molluscum contagiosum lesions, including:
-
physical interventions;
-
systemic treatments;
-
topical agents; and
-
awaiting natural resolution.
We excluded studies on other aspects of the treatment of molluscum contagiosum, for example on reducing pain in the studies that used analgesic EMLA (eutectic mixture of local anaesthetics) cream (de Waard 1990; Juhlin 1980).
Types of outcome measures
Primary outcomes
-
Short‐term clinical cure (up to three months after start of treatment).
We defined clinical cure as complete disappearance (clearance) of molluscum contagiosum skin lesions, as assessed by a physician.
Secondary outcomes
-
Medium‐ and long‐term clinical cure (after three months and up to six months after start of treatment, and beyond six months, respectively).
-
Short‐, medium‐, and long‐term improvement (including cure, intervals as above).
-
Time to cure.
-
Recurrences after 3, 6, and 12 months.
-
Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
-
Spread to other people.
-
Disease‐related quality of life.
Where included studies used the term 'complete clearance' or 'free of lesions' or 'cured or > 90% cleared', we classed these as our primary outcome 'short‐term clinical cure (up to three months after start of treatment)' or our secondary outcome 'medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)', and where they referred to 'partial clearance', we took this to mean our secondary outcome 'improvement'.
We did not initially specify secondary outcomes (2) and (7) in the protocol, but added them afterwards since we considered improvement at the end of the study important, as was disease‐related quality of life. For secondary outcome (2), we would combine 'improvement' and 'cure' (even though cure alone was a seperate outcome) because 'improvement' would be hard to interpret without also including those who were cured. For example: suppose in group A, 30% of participants were cured and another 20% improved. In group B, 40% of participants were cured and 10% improved. Comparing improvement rates between A and B (20% versus 10%) is misleading, whereas combining cure and improvement (50% versus 50%) is not.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised all of our search strategies in line with current Cochrane Skin practices. Details of the previous search strategies are available in Van der Wouden 2009.
We searched the following databases up to 21 July 2016:
-
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 6, in the Cochrane Library using the strategy in Appendix 2;
-
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
-
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
-
LILACS (Latin American and Caribbean Health Service Information database) (from 1982) using the strategy in Appendix 5.
Trials registers
For this update, we searched the following trials registers up to 4 August 2016, using 'molluscum' as the search term:
-
ISRCTN registry (www.isrctn.com);
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/);
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu); and
-
Netherlands Trial Register (www.trialregister.nl).
We searched Google combining the keyword 'molluscum' with author names of pertinent studies.
Searching other resources
Reference lists
We checked the bibliographies of included studies and review articles for further references to relevant studies.
Correspondence
We obtained further relevant published and unpublished trials via correspondence with selected pharmaceutical companies and authors of recent publications.
Three unpublished studies undertaken by 3M were brought to our attention by Dr Ken Katz, an American dermatologist.
Data collection and analysis
Selection of studies
Two review authors (of SK, RvdS, EK, JCvdW) independently read all abstracts or titles of identified trials. If one of the review authors considered the article to be potentially relevant, we obtained a full‐text copy of the article for further consideration. Two review authors (as before) independently examined all full‐text articles to determine whether or not they met our inclusion criteria. Disagreements were resolved by discussion between the review authors, with referral to a third review author (JCvdW or SK) when necessary.
We excluded trials on sexually transmitted molluscum contagiosum and in people with immune deficiency (including those with HIV infection), in order to increase homogeneity of studies. If the full text of a study was not available, we considered published abstracts for the review.
If an RCT included a variety of skin diseases, of which one was molluscum contagiosum, the number of molluscum participants needed to be at least five in the active treatment and placebo groups. We added this criterion after approval of the protocol when a we found a study that included 10 molluscum participants with a 9:1 distribution over the two treatment groups (Caballero 1996).
If the setting of the study was not explicitly mentioned in the text, we assumed it to be carried out at the affiliation of the first author.
Data extraction and management
Two review authors (of SK, RvdS, EK, JCvdW) independently carried out data extraction using specially developed and piloted data extraction forms. Discrepancies were resolved by a third review author (JCvdW or SK). We obtained missing data from study authors where possible. One review author (JCvdW) entered the data.
As planned in our protocol, the review authors were not blinded to the names of authors, journals, or institutions.
Assessment of risk of bias in included studies
Two review authors (of SK, RvdS, EK, JCvdW) independently assessed the included studies for risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011). The review authors were not blinded to the names of authors, journals, or institutions. A third review author (JCvdW or SK) acted as arbitrator when necessary. Our assessment included an evaluation of the following components.
-
Method of generation of the randomisation sequence: it was considered adequate when a computer program or a random number table was used, or a statistician was involved.
-
Method of allocation concealment: it was considered adequate if the assignment could not be foreseen.
-
Blinding of participants, clinicians: it was considered adequate when the study was called 'double‐blind' and precautions were taken to mask the nature of the interventions.
-
Blinding of outcome assessors: it was considered adequate when the study was called 'double‐blind' and it was unlikely that difference in treatment resulted in unmasking (e.g. in the case of staining due to one of the treatments).
-
Incomplete outcome data addressed (short, medium, and long term): it was scored 'unclear' if not reported and 'high risk' if > 20% of participants lost to follow‐up (short term) or > 30% lost to follow‐up (long term) (Back Review Group 2008).
-
Free of selective reporting: it was considered adequate if the reported outcomes were similar to those mentioned in the study protocol.
-
Free of other bias, such as baseline imbalance, compliance, and unit of analysis errors in the case of multiple lesions.
Items (5), (6), and (7) differed from the original protocol or were absent, and were initially adapted for the 2009 update. Items (3) and (4) were combined in one item in the previous versions of this review.
Assessment of quality of the evidence
We used GRADE to assess the overall quality of the evidence for each outcome of each comparison. Starting from 'high quality' (because we only included RCTs), we downgraded the quality for serious study limitations (high risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates (fewer than 300 events), or potential publication bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the results as risk ratios (RR) with 95% confidence intervals (CI) and as a number needed to treat (NNT) only for comparisons with two or more studies and only in case of statistically significant outcomes (the latter in order to avoid confusing confidence interval boundaries). For continuous outcomes, we expressed the results as weighted mean differences (WMD) with 95% CI.
Following the Cochrane Skin Group recommendations, we decided post hoc to re‐analyse results from individual studies with borderline significance and with low numbers of events (fewer than 10 in total) or a total sample size of less than 30, using Fisher’s exact test. The resulting P value was leading in interpreting the results.
Unit of analysis issues
We could expect unit of analysis issues regarding studies potentially using within‐participant comparisons (e.g. split‐body studies) and cross‐over trials. An additional problem of split‐body studies is when a locally applied treatment could induce a systemic response. Our protocol stated that data from these trials were to be analysed using techniques appropriate for paired designs, with the help of a statistician (Van der Wouden 2004).
Dealing with missing data
We attempted to obtain missing data from the trial authors. If this was not possible or not feasible, we used the data as reported.
Assessment of heterogeneity
We planned to explore heterogeneity between the studies using the I² statistic. If substantial heterogeneity (I² statistic > 50%) existed between studies for the primary outcome, we planned to explore the reasons for the heterogeneity, for example by using sensitivity analyses to examine the effects of excluding studies with high risk of bias.
Assessment of reporting biases
We planned to assess reporting bias by comparing the published trial publications with the study protocol.
Data synthesis
For studies with a similar type of intervention, we conducted meta‐analyses to calculate a weighted treatment effect across trials using a random‐effects model (DerSimonian and Laird model) (Higgins 2011).
When the same comparison between two interventions was made in more than one study, and studies appeared to have been executed in similar groups and settings, we used statistical tests for homogeneity between studies. In those studies where the available data were sufficiently homogenous and where a pooled estimate of the treatment effect made sense, we conducted a meta‐analysis.
Where a quantitative synthesis was not possible we provided a narrative synthesis of included trials, presenting the main characteristics of trials and their results.
For studies with a similar type of intervention, we conducted meta‐analyses to calculate a weighted treatment effect across
trials using a random‐effects model (DerSimonian and Laird model).
Sensitivity analysis
We planned to use sensitivity analyses to examine the effects of excluding studies with high risk of bias.
Summary of findings
We developed 'Summary of findings' tables subsequent to our protocol. For this update we produced a 'Summary of findings' table for what we believe is the most important comparison of this update: imiquimod versus vehicle.
Results
Description of studies
Results of the search
Our update of the database searches to July 2016 identified 64 records. We identified an additional 15 records from other sources including trial registers, resulting in a total of 79 records to be screened. We excluded 50 records based on titles and abstracts. Of the remaining 29 records, 5 were not available in full text and for 3 others were available in full text but insufficient information was provided to decide on inclusion or exclusion (see Characteristics of studies awaiting classification). We identified one ongoing study (see Characteristics of ongoing studies). We excluded a further nine studies (see Characteristics of excluded studies). We included 11 new studies (see Characteristics of included studies).
We combined these studies with those previously identified for this review, resulting in a total of 22 included studies. A flow diagram summarising the study selection process is shown in Figure 1.

Flow diagram of inclusion for this update.
Included studies
We identified 11 new studies for this update, resulting in a total of 22 included studies. The newly included studies are: Al‐Mutairi 2010; Chathra 2015; Coloe Dosal 2014; Eichenfield 2005; Handjani 2014; Machado 2010; Markum 2012; Paller 2005a; Paller 2005b; Seo 2010; Uçmak 2013.
Setting
Eight trials were performed in North America, five in the UK, eight in Asia, and one in South America (see Characteristics of included studies).
Most included studies were set in hospital outpatient or emergency departments. Only people without immune deficiency (non‐HIV participants) and non‐genital molluscum contagiosum participants were included in the studies. Participants therefore consisted primarily of children and young adults (adolescents).
Participants
The 22 included studies involved a total of 1650 randomised molluscum participants, more than three times as many as in the previous version of this review. The number of participants in each study ranged from 20, in Manchanda 1997b and Short 2006, to 379, in Paller 2005b.
Design
Most of the studies had two trial arms. Four studies had three arms (Leslie 2005; Machado 2010; Markum 2012; Ohkuma 1990), and one study had four arms (Hanna 2006). The number of participants per trial arm ranged from 5, in Ohkuma 1990, to 253, in Paller 2005b. Manchanda 1997b reported on two studies, both making the same comparison but one in a cross‐over design and one in a parallel design. We chose not to include the cross‐over study because fewer than five molluscum participants were assigned to one of the treatment arms. See below for more details on the study designs. Follow‐up duration ranged from 3 to 28 weeks after randomisation. Only five studies had longer than 3 months' follow‐up (Al‐Mutairi 2010; Antony 2001; Eichenfield 2005; Leslie 2005; Paller 2005b).
Publications
We obtained 17 studies as full‐text articles; in five cases these were unpublished manuscripts (Bazza 2007; Eichenfield 2005; Paller 2005a; Paller 2005b; Short 2006). The three unpublished studies by 3M, all addressing the same comparison, imiquimod versus placebo, were brought to our attention by Dr Ken Katz, an American dermatologist. These unpublished studies were mentioned in an FDA summary, Papadopoulos 2007, and subsequently in several journal articles (Katz 2013; Katz 2014; Katz 2015). Upon our request, the director of Meda Pharma BV (Amstelveen, the Netherlands) provided us with the full reports of these three trials.
Two studies were available only as published abstracts (Antony 2001; Saryazdi 2004). We requested and obtained additional information from the authors of several of the included studies (Manchanda 1997b; Ohkuma 1990; Short 2006).
Funding
Five studies reported obtaining commercial funding (Burke 2004; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b); three other studies obtained medication for free from pharmaceutical companies (Hanna 2006; Leslie 2005; Machado 2010); one study reported charity funding (Coloe Dosal 2014); and one study reported receiving no financial support (Chathra 2015). The other 12 studies did not report on funding.
Interventions
Twenty studies evaluated topical therapies for molluscum contagiosum. Two studies included curettage as a treatment arm (Hanna 2006; Machado 2010). Two studies investigated systemic treatments (Antony 2001; Manchanda 1997b). Below we have provided some information regarding interventions, measurements, and loss to follow‐up for each study. For further details, see Characteristics of included studies.
Topical therapy
Three studies by 3M Pharmaceuticals all compared 5% imiquimod with its vehicle in children (Eichenfield 2005; Paller 2005a; Paller 2005b). Paller 2005a used a 1:1 randomisation scheme; the other two studies used a 2:1 scheme. In total, 532 children were randomised to the imiquimod arms and 295 children were randomised to the vehicle arms. Treatment frequency and duration varied from daily for 8 weeks, in Paller 2005a, to 3 times weekly for a maximum of 16 weeks (Eichenfield 2005; Paller 2005b). The total number of evaluable participants was 623, that is 76% of those randomised. Outcomes were lesion clearance, lesion counts, time to complete clearance, and side effects after 4, 8, 12, 16, 18, and 28 weeks after the start of treatment (Eichenfield 2005; Paller 2005b); and lesion clearance, lesion counts, time to complete clearance, and side effects 12 weeks after the start of treatment (Paller 2005a).
Al‐Mutairi 2010 assessed the effect of 5% imiquimod 5 times a week (n = 37) with that of cryospray once a week (n = 37) for a maximum of 16 weeks in children 2 to 12 years of age. No loss to follow‐up was reported. Outcomes were cure at 3, 6, 12, and 16 weeks; cosmetic outcome; and adverse effects.
Bazza 2007 assessed the effect of 5% potassium hydroxide compared to 0.9% saline twice daily. The design was a within‐participant comparison, with treatment randomised to the right or left side of the body. Treatment was continued for a maximum period of three weeks. The study included 30 participants, of whom 20 participants (2 to 12 years of age) completed the study. Outcomes were complete clearance of lesions and side effects after 12 weeks.
Burke 2004 assessed the effect of 10% Australian lemon myrtle tree oil once daily (n = 16). The control group (n = 15) received the vehicle, olive oil. Treatment was continued for 21 days. The mean age of the participants was 4.6 years. Four children were lost to follow‐up. Outcome was complete clearance or greater than 90% reduction in number of lesions after 3 weeks.
Chathra 2015 compared 5% imiquimod (n = 20) to 10% potassium hydroxide (n = 20) 3 times a week for up to 12 weeks. Age range was 1 to 18 years. There was no loss to follow‐up. Outcome was complete clearance after 4, 8, and 12 weeks.
Coloe Dosal 2014 compared 0.7% cantharidin (n = 16) with its vehicle (n = 16) for a maximum of 8 weeks, applied at 5 subsequent visits. Age of the participants was 5 to 10 years. No follow‐up data were available for three children in the 0.7% cantharidin group. Outcomes were complete clearance, lesion counts, and adverse effects at approximately 8 weeks after the start of treatment.
Handjani 2014 compared 10% potassium hydroxide 2 times daily (n = 15) with cryotherapy once weekly (n = 15) for up to 4 weeks. Age of the participants was 1 to 24 years, mean 6.4 years. There was no loss to follow‐up. Outcomes were lesion response and side effects 4 weeks after the start of treatment.
Hanna 2006 compared four treatments: 5% imiquimod (n = 29), 0.7% cantharidin (n = 30), 16.7% salicylic acid/16.7% lactic acid (n = 29), and curettage (physical destruction) (n = 31). Treatment frequency varied and neither treatment duration nor the time point for measuring whether participants were cured was reported. Age of the participants was 1 to 16 years. Loss to follow‐up was unclear. Outcome was the number of visits required. The intervals between study visits were not reported, therefore outcome data were not suitable for analysis.
Leslie 2005 compared the effect of 10% phenol/70% alcohol (n = 41) to 12% salicylic acid (n = 37) and to 70% alcohol (n = 36). Age of the participants was 1 to 15 years. Treatment frequency varied. Participants returned for additional treatment for up to six months. Thirty‐one participants (27%) were lost to follow‐up. Outcome was complete clearance of lesions after six months.
Machado 2010 reported on a study with three arms: 10% potassium hydroxide two times daily (n = 17); 14% salicylic acid plus 14% lactic acid in collodion once daily (n = 16); and curettage (n = 17). The first two groups were treated for 90 days; curettage was performed only once. Age of the participants was 3 to 15 years. No losses to follow‐up were reported. Outcomes were cure and adverse effects 90 days after the start of treatment.
Markum 2012 also reported on a study with three arms: iodine (n = 16); tea tree oil (Melaleuca alternifolia) (n = 18); and tea tree oil combined with iodine (n = 19) twice daily for a maximum of 30 days. The mean age of participants was 6.3 years. A total of five children were lost to follow‐up. Outcomes were cure or reduction in the number of lesions of greater than 90% and adverse effects 30 days after the start of treatment.
Ohkuma 1990 had three intervention arms: 10% povidone iodine solution combined with 50% salicylic acid plaster (n = 20), povidone iodine alone (n = 5), and salicylic plaster alone (n = 10), all once daily. Age of the participants was 2 to 9 years. Outcome was time to cure.
Ormerod 1999 assessed the effect of 5% sodium nitrite co‐applied daily with 5% salicylic acid, under occlusion (n = 16). The control group received an identical cream with 5% salicylic acid but without sodium nitrite (n = 14). Treatment was for three months or until participants were cured or dropped out if sooner. The median age of the participants was 6 years. Outcomes were time to complete resolution and adverse events.
Saryazdi 2004 compared the effect of 10% benzoyl peroxide cream with 0.05% tretinoin cream twice daily for 4 weeks. Participants were children; their age was not specified. The total number of participants was 30; we assumed these were equally divided between the 2 treatments. Outcomes were lesion count, lesion free, and side effects six weeks after the start of treatment.
Seo 2010 compared the effect of once daily 5% imiquimod cream (n = 15) with that of once daily 10% potassium hydroxide solution (n = 15) for a maximum of 12 weeks. Age of the participants was 1 to 36 years. Three participants were lost to follow‐up. Outcomes were cure and adverse effects after 2, 4, 8, and 12 weeks.
Short 2006 assessed the application of a 10% potassium hydroxide solution (n = 10) twice daily. The control group received saline (n = 10). Assessment of the therapeutic response took place up to 90 days after the start of treatment or 1 month after clearance, or both. Age of the participants was 2 to 9 years. One child was lost to follow‐up. Outcomes were time to resolution and adverse events three months after the start of treatment.
Theos 2004 assessed the effect of 5% imiquimod (n = 12) versus vehicle (n = 11) 3 times a week for up to 12 weeks. Participants were assessed every 2 weeks after treatment initiation, for up to 12 weeks. Age of the participants was 1 to 9 years. Two children were lost to follow‐up. Outcomes were complete and partial clearance and adverse events after 4, 8, and 12 weeks.
Uçmak 2013 compared 2.5% potassium hydroxide (n = 14) with 5% potassium hydroxide (n = 15) twice daily for 60 days. Age of the participants was 1 to 18 years. Three participants were lost to follow‐up. Outcomes were cure and adverse effects after 15, 30, 45, and 60 days.
Systemic therapy
Antony 2001 assessed the effect of 35 mg/kg/day of cimetidine given once daily as an oral suspension for three months. Thirty‐eight participants, aged 1 to 16 years, were enrolled in the trial, but assignment details were provided only for the 19 participants who completed the study. Eight of these participants were randomised to the treatment arm of the trial. The 11 participants in the control arm received a matched oral suspension. The follow‐up period was four months from the start of treatment. Outcomes were complete clearance after four months of treatment and reduction of lesions.
Manchanda 1997b evaluated different potencies of the homoeopathic drug calcarea carbonica given daily for 15 days (n = 14). Six participants were randomised to receive plain sugar globules as a placebo. Age of the participants was 0 to 30 years. Follow‐up duration was not reported. Outcome was improvement (not clear after what period).
Excluded studies
The most common reasons why studies were excluded was that they were case series rather than RCTs, or because the participant groups were outside the focus of the review. See the 'Characteristics of excluded studies' tables.
Studies awaiting classification
We have assigned eight studies to awaiting classification. Two studies classified as ongoing in the previous version of this review have likely been completed (NCT01348386; NCT02665260), however we are unaware of papers describing the results of these studies. For three other studies identified in the current search but rather old, we were unsuccessful in obtaining full‐text papers, and abstracts are missing as well (Elzawahry 1964; Tanissa 1951; Unknown Chinese author 1991). For the remaining three studies, full‐text papers were available, but insufficient information was provided to decide on inclusion or exclusion (Köse 2013; Muzaffar 2014; Rajouria 2011). All three studies compared topical treatments: a Turkish study compared a 10% potassium hydroxide solution with a combination of salicylic and lactic acid; a study from Pakistan compared two different potassium hydroxide solutions (5% versus 10%); and a study from Nepal compared 5% potassium hydroxide solution with 0.05% tretinoin cream. We have contacted the authors for additional information, but have been unsuccessful thus far.
Ongoing studies
We found one ongoing study comparing topical treatments: 10% sandalwood cream versus placebo cream. It is being conducted in the USA (NCT02024581).
Risk of bias in included studies
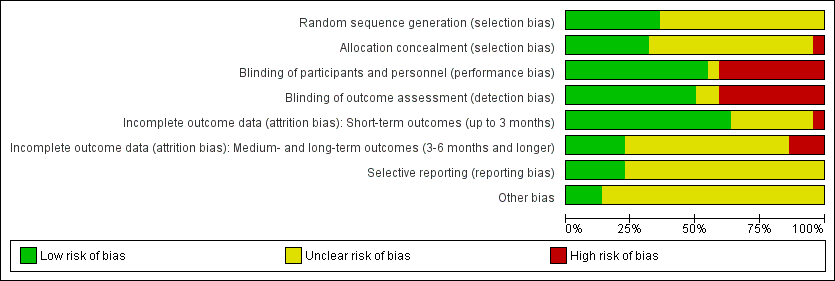
Figure 2 and Figure 3 show the results of the 'Risk of bias' assessment. Reasons for the choices that were made for each individual study can be found in the Characteristics of included studies. Overall, most study reports provided insufficient information to judge risk of bias, especially for allocation concealment and selective reporting. We considered only five studies to be at low risk of bias: Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b; all of these studies were identified during the 2016 update.

Risk of bias table: review authors' judgements about each methodological quality item for each included study.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twenty studies were described in the text as randomised trials. We obtained additional information from the authors of the other two papers, who confirmed in writing that the participants were randomised into the different treatment groups (Manchanda 1997b; Ohkuma 1990). Eight papers described the way the randomisation sequence was generated (Burke 2004; Coloe Dosal 2014; Eichenfield 2005; Hanna 2006; Leslie 2005; Markum 2012; Paller 2005a; Paller 2005b), which we judged as at low risk of bias (Figure 2). In a personal communication, Manchanda informed us that in his study this was "generated manually" (Manchanda 1997b).
Only seven studies described whether the investigators took any precautions to conceal the allocation schedule from those involved in entering participants into the study (Burke 2004; Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b; Short 2006).
Blinding
Eleven of the studies were described as double‐blind. However, only four of these studies provided information about the similarity in appearance and smell of treatments (Burke 2004; Coloe Dosal 2014; Manchanda 1997b; Markum 2012). In four so‐called vehicle‐controlled studies, visual similarity was not explicitly stated but can be assumed (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). None of the papers provided information on whether blinding was maintained throughout follow‐up. Ormerod reported brown staining on the skin in six participants with active treatment, but none of the controls, which may have unblinded outcome assessment (Ormerod 1999). In some studies blinding was impossible due to the comparison that was made (e.g. topical cream versus cryospray (Al‐Mutairi 2010)). Blinding was unclear in Saryazdi 2004. In Uçmak 2013, participants seemed to be blinded but for the outcome assessors this was unclear. We considered the following studies not to be (fully) blinded: Al‐Mutairi 2010; Chathra 2015; Handjani 2014; Hanna 2006; Leslie 2005; Ohkuma 1990; Ormerod 1999; Seo 2010. Further details are provided in Characteristics of included studies.
Incomplete outcome data
In Figure 2, we have reported this item separately for short‐term outcomes and longer‐term outcomes. For short‐term outcomes, only one study reported a loss to follow‐up of more than 20% (Bazza 2007). Six studies did not report any loss to follow‐up (Al‐Mutairi 2010; Chathra 2015; Handjani 2014; Machado 2010; Ohkuma 1990; Saryazdi 2004). For longer‐term outcomes, three studies reported a loss to follow‐up of over 30%.
Selective reporting
We could not evaluate selective reporting for most of the included studies because neither a study protocol was available, nor a trial register providing information on outcomes. We judged five studies to be free of reporting bias (Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b). Several studies reported that efficacy was assessed at several time points but results were provided only at the last time point. We did not qualify this as high risk of bias.
Other potential sources of bias
For most studies, it was unclear whether other sources of bias played a role, because no information was provided on baseline characteristics or adherence to the treatment protocol (Figure 2). One study reported that the treatment adherence differed between study arms (Machado 2010), and other studies reported considerable baseline imbalances for lesion count (Theos 2004), sex (Seo 2010), and skin dryness (Coloe Dosal 2014), but the differences were not statistically significant. We judged three studies to be at low risk of bias for this item because there was no relevant baseline imbalance and information was provided on adherence to the treatment protocol (Eichenfield 2005; Paller 2005a; Paller 2005b). For funding sources of studies, see above under Funding. We did not take the source of funding into account for the 'Risk of bias' assessment.
Effects of interventions
Our prespecified outcomes were as follows.
Primary outcomes
-
Short‐term clinical cure (up to three months after the start of treatment).
We defined clinical cure as complete disappearance (clearance) of molluscum contagiosum skin lesions, as assessed by a physician.
Secondary outcomes
-
Medium‐ and long‐term clinical cure (after three months and up to six months after start of treatment, and beyond six months, respectively).
-
Short‐, medium‐, and long‐term improvement (including cure, intervals as above).
-
Time to cure.
-
Recurrences after 3, 6, and 12 months.
-
Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
-
Spread to other people.
-
Disease‐related quality of life.
We describe here the prespecified outcomes found for all comparisons. Comparisons are sorted by type of treatment. Only two comparisons were addressed by more than one study and allowed pooling of study data: topical imiquimod versus vehicle cream (four studies), and imiquimod versus potassium hydroxide (two studies). For each comparison we have only reported the outcomes that were addressed in the included studies. If an outcome is not reported on, this is because the outcome was not assessed by the study/studies within that comparison.
Where included studies used the term 'complete clearance' or 'free of lesions' or 'cured or > 90% cleared', we classed these as our primary outcome 'short‐term clinical cure (up to three months after start of treatment)' or our secondary outcome 'medium‐ and long‐term cure (after three months and up to six months, and after six months, respectively)', and where they referred to 'partial clearance', we took this to mean our secondary outcome 'improvement'.
We could not include the results of the study by Hanna and colleagues in the analysis, as the outcome (cure rate) was reported only in number of visits, without stating at what time these visits took place (Hanna 2006). Four studies did not report adverse or side effects (Antony 2001; Leslie 2005; Manchanda 1997b; Ohkuma 1990). One study reported the rates but not the nature of the adverse effects (Hanna 2006).
See summary of findings Table for the main comparison for our grading of the evidence for the comparison 'imiquimod versus vehicle'.
Topical treatments
Two of our secondary outcomes were not reported in any of the studies: 6. Spread to other people and 7. Disease‐related quality of life. Only five studies reported on our secondary outcome 3. Time to cure (Eichenfield 2005; Paller 2005b; Ohkuma 1990; Ormerod 1999; Short 2006). With regard to our secondary outcome 5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes, three studies reported separately on: any side effect, application site reactions, and severe application site reactions (Eichenfield 2005; Paller 2005a; Paller 2005b).
Imiquimod versus vehicle
The quality of the evidence for this comparison was moderate to high, depending on the outcome; see summary of findings Table for the main comparison for details.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 5% imiquimod cream resulted in complete clearance in 79/544 participants versus 36/306 of the control group, who received vehicle cream (4 studies, 850 participants, risk ratio (RR) 1.33, 95% confidence interval (CI) 0.92 to 1.93, moderate‐quality evidence for little or no difference, Analysis 1.1) (time point: 12 weeks after start of treatment) (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). The pooled analysis showed no heterogeneity (I² = 0%).
Secondary outcomes
1.Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
Eighteen weeks after start of treatment, application of 5% imiquimod cream resulted in complete clearance in 112/470 participants versus 63/232 participants in the control (vehicle) group (2 studies, RR 0.88, 95% CI 0.67 to 1.14, I² = 0%, moderate‐quality evidence for no clear difference, Analysis 1.2) (Eichenfield 2005; Paller 2005b).
Twenty‐eight weeks after start of treatment, application of 5% imiquimod cream resulted in complete clearance in 180/470 participants versus 92/232 participants in the control (vehicle) group (2 studies, RR 0.97, 95% CI 0.79 to 1.17, I² = 0%, moderate‐quality evidence for no difference, Analysis 1.3) (Eichenfield 2005; Paller 2005b).
2. Improvement
Twelve weeks after start of treatment, application of 5% imiquimod cream resulted in partial or complete clearance in 273/544 participants versus 149/306 participants in the control (vehicle) group (4 studies, RR 1.14, 95% CI 0.89 to 1.47, high‐quality evidence for little or no difference, Analysis 1.4) (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). The I² value was 65%, showing substantial heterogeneity. Exploring heterogeneity, in the forest plot the small study Theos 2004 showed as a clear outlier. Excluding this study reduced I² to 10%, and resulted in a small change of the pooled outcome (RR 1.05, 95% CI 0.91 to 1.21).
Eighteen weeks after start of treatment, application of 5% imiquimod cream resulted in partial or complete clearance in 341/470 participants versus 174/232 participants in the control (vehicle) group (2 studies, RR 0.97, 95% CI 0.87 to 1.08, I² = 26%, high‐quality evidence for no difference, Analysis 1.5) (Eichenfield 2005; Paller 2005b).
3. Time to cure
In two unpublished studies, the median time to cure was the same in the group treated with 5% imiquimod and the group treated with its vehicle: 16 weeks in Eichenfield 2005 and 18 weeks in Paller 2005b (high‐quality evidence).
4. Recurrence
In two unpublished studies, recurrence was observed in 1/52 and 3/60 in the group treated with 5% imiquimod and 0/28 and 0/35 in the group treated with its vehicle after 28 weeks (Eichenfield 2005 and Paller 2005b, respectively) (RR 2.70, 95% CI 0.31 to 23.23, I² = 0%, moderate‐quality evidence, Analysis 1.6).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Three of the four related studies comparing topical 5% imiquimod with vehicle reported extensively on adverse effects (Eichenfield 2005; Paller 2005a; Paller 2005b); we had copies of the full trial reports of the 3M studies as submitted to the US Food and Drug Administration. In the imiquimod group of Theos 2004, pruritis was reported by 6/12 participants versus 5/11 in the vehicle group. Pain/tenderness was reported by one participant in each group. Other reported side effects were not interpretable because of unclear denominators.
In Eichenfield 2005, any side effect was reported in 149/217 versus 78/106 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 77/217 versus 21/106 participants; 7 versus 1 of these were qualified as severe. In Paller 2005a, any side effect was reported in 42/62 versus 41/63 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 32/62 versus 25/63 participants; 6 versus 1 of these were qualified as severe. In Paller 2005b, any side effect was reported in 166/253 versus 84/126 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 80/253 versus 31/126 participants; 3 versus 0 of these were qualified as severe. We pooled the results of the three 3M studies, with 827 evaluable participants. For any adverse effect, the pooled RR was 0.97 (95% CI 0.88 to 1.07, I² = 0%, high‐quality evidence for no difference, Analysis 1.7). For application site reactions, the pooled RR was 1.41 (95% CI 1.13 to 1.77, I² = 0%, moderate‐quality evidence for probably more harm for imiquimod, Analysis 1.8). The proportion of participants experiencing an application site reaction was 189/532 (36%) versus 77/295 (26%), giving a number needed to treat for an additional harmful outcome (NNTH) of 11. For severe application site reactions, the pooled RR was 4.33 (95% CI 1.16 to 16.19, I² = 0%, moderate‐quality evidence for probably more harm for imiquimod, Analysis 1.9). The proportion of participants experiencing a severe application site reaction was 16/532 (3%) versus 2/295 (0.7%), giving a NNTH of over 40.
Imiquimod versus cryospray
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 5% imiquimod cream resulted in complete clearance in 22/37 participants versus 37/37 participants who received cryospray after 6 weeks (RR 0.60, 95% CI 0.46 to 0.78, Analysis 2.1) (Al‐Mutairi 2010). (We selected the 6‐week time point for this analysis because the 12‐week time point was close to the 16‐week time point ‐ see below).
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
Application of 5% imiquimod cream resulted in complete clearance in 34/37 participants versus 37/37 participants who received cryospray after 16 weeks (RR 0.92, 95% CI 0.83 to 1.20, Analysis 2.2) (Al‐Mutairi 2010).
4. Recurrence
Al‐Mutairi 2010 reported 0/37 in the imiquimod group and 3/37 in the cryospray group with recurrence after 5 months (RR 0.14, 95% CI 0.01 to 2.67, Analysis 2.3).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the 5% imiquimod group of Al‐Mutairi 2010, 27/37 participants reported pain during application; 28/37, erythema; 9/37, itching; 5/37, a burning sensation; and 2/37, pigmentary changes. In the cryospray group, 22/37 participants reported pain during application; 34/37, a burning sensation; 18/37, erosions; 17/37, erythema; 11/37, itching; 9/37, vesicles/bullae; 15/37, pigmentary changes; and 8/37, scarring/atrophy.
Imiquimod versus potassium hydroxide
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Two small studies compared 5% imiquimod cream with 10% potassium hydroxide (Chathra 2015; Seo 2010), resulting in complete clearance 12 weeks after start of treatment in 18/34 (imiquimod) versus 27/33 (potassium hydroxide) (RR 0.65, 95% CI 0.46 to 0.93), favouring 10% potassium hydroxide (I² = 0%) and resulting in a number needed to treat for an additional beneficial outcome of 3 (Analysis 3.1).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Two small studies compared 5% imiquimod cream with 10% potassium hydroxide (Chathra 2015; Seo 2010). In the 5% imiquimod group 10/33 participants reported adverse effects (erythema, burning, itching, ulceration, scaling, hypo‐ and hyperpigmentation), versus 16/34 in the 10% potassium hydroxide group (RR 0.68, 95% CI 0.25 to 1.81, Analysis 3.2). The I² value was 57%, showing substantial heterogeneity; however, as only two studies were included in the analysis we were unable to investigate heterogeneity.
Lemon myrtle oil versus olive oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% lemon myrtle oil resulted in complete disappearance (or reduction of > 90% of lesions) after three weeks in 9/16 participants versus 0/15 of the control group, who received only the vehicle (olive oil) (RR 17.88, 95% CI 1.13 to 282.72, Analysis 4.1) (Burke 2004). The Fisher exact test resulted in a P value of 0.001.
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Application of 10% lemon myrtle oil resulted in local redness in 2/16 participants versus 1/15 participants in the control (vehicle) group (Burke 2004).
Benzoyl peroxide versus tretinoin
We considered the evidence for the outcome for this comparison to be of low quality due to small study size, unknown risk of bias, and imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% benzoyl peroxide resulted in complete disappearance of lesions after six weeks in 11/15 participants compared to 5/15 participants who received 0.05% tretinoin (RR 2.20, 95% CI 1.01 to 4.79, Analysis 5.1) (Saryazdi 2004).
Potassium hydroxide versus saline
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
The Bazza 2007 study randomised treatments to the right or left side of the body, comparing application of 5% potassium hydroxide to 0.9% saline. On both sides of the body, 17/20 participants showed complete clearance. Due to the absence of paired data in the study report, it was impossible to take the split‐body design into account in the analysis, therefore we did not pool the study results with those of Short 2006.
Short 2006 made a similar comparison, showing 10% potassium hydroxide solution to be successful in 7/10 participants (70%) compared with 2/10 (20%) in the saline group, which was not statistically significant (RR 3.50, 95% CI 0.95 to 12.90, Analysis 6.1). The Fisher exact test resulted in a P value of 0.07.
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In Short 2006 most participants treated with 10% potassium hydroxide reported mild stinging, and two participants reported severe stinging, one of whom withdrew. Also, two participants developed post‐inflammatory pigmentary changes at the treatment site (unclear in which group). No participants in the saline group withdrew due to side effects.
In Bazza 2007 12/20 participants reported mild to moderate stinging; 2/20, severe stinging; and 2/20, hypopigmentation in the group treated with 5% potassium hydroxide. In the group receiving topical 0.9% saline, 8/20 participants reported mild to moderate stinging and 2/20 reported hypopigmentation.
Potassium hydroxide versus potassium hydroxide
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found complete clearance in 3/13 versus 8/12 participants after 60 days (RR 0.35, 95% CI 0.12 to 1.01, Analysis 7.1), in favour of the 5% solution. Fisher's exact test resulted in a P value of 0.047.
Secondary outcomes
2. Improvement
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found clinical cure or improvement in 8/13 versus 10/12 participants after 60 days (RR 0.74, 95% CI 0.45 to 1.22, Analysis 7.2), favouring the 5% solution.
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found at least one adverse effect at 15 days in 11/13 versus 11/12 participants; at 60 days: 6/13 versus 5/12, respectively. The most common adverse effect was a burning sensation.
Potassium hydroxide versus salicylic acid plus lactic acid
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Machado 2010 compared 10% potassium hydroxide to a combination of 14% salicylic acid plus 14% lactic acid, and found 14/17 versus 15/16 participants were cured after a maximum of 90 days treatment (RR 0.88, 95% CI 0.68 to 1.13, Analysis 8.1).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
In the 10% potassium hydroxide group of Machado 2010, 10/17 participants reported moderate pain; in the group treated with 14% salicylic acid plus 14% lactic acid, 8/16 reported mild pain.
Potassium hydroxide versus curettage
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Machado 2010 compared 10% potassium hydroxide to curettage, and found 14/17 versus 15/17 participants were cured after a maximum of 90 days treatment (RR 0.93, 95% CI 0.71 to 1.24, Analysis 9.1).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the 10% potassium hydroxide group of Machado 2010, 10/17 participants reported moderate pain; in the curettage group, 12/17 reported mild pain.
Potassium hydroxide versus cryotherapy
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Handjani 2014 compared 10% potassium hydroxide to cryotherapy, and found 13/15 versus 14/15 participants were cured after a maximum of 4 weeks treatment (RR 0.93, 95% CI 0.73 to 1.18, Analysis 10.1).
Secondary outcomes
2. Improvement
Handjani 2014 compared 10% potassium hydroxide to cryotherapy, and found 14/15 versus 15/15 participants were cured or improved after a maximum of 4 weeks treatment (RR 0.94, 95% CI 0.78 to 1.12, Analysis 10.2).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Handjani 2014 found hyperpigmentation in 4/15 participants (10% potassium hydroxide) and 7/15 (cryotherapy), and hypopigmentation in 6/15 (10% potassium hydroxide) and 5/15 (cryotherapy).
Povidone iodine versus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine was effective in 3/5 participants (60%) compared to 7/10 (70%) who received salicylic acid plaster alone (RR 0.86, 95% CI 0.38 to 1.95, not statistically significant) (Ohkuma 1990) (Analysis 11.1). (Please note that this study did not clearly state when cure was measured but stated "treatment continued as long as necessary, range was 7‐64 days, mean 26 days".)
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine resulted in a mean time to cure of 86 days versus 47 days for the group receiving salicylic acid plaster.
Povidone iodine versus povidone iodine plus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine solution was effective in 3/5 participants (60%) compared with 20/20 (100%) who received 10% povidone iodine plus salicylic acid plaster (RR 0.60, 95% CI 0.30 to 1.18, Analysis 12.1) (Ohkuma 1990). See note above after results of Analysis 11.1.
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine resulted in a mean time to cure of 86 days versus 26 days for the group receiving 10% povidone iodine plus salicylic acid plaster.
Povidone iodine plus salicylic acid plaster versus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine solution plus 50% salicylic acid plaster was effective in curing 20/20 participants (100%) compared with 7/10 (70%) who received salicylic acid plaster alone (RR 1.43, 95% CI 0.95 to 2.16, Analysis 13.1) (Ohkuma 1990). Fisher's exact test resulted in a P value of 0.03. See note above after results of Analysis 11.1.
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine plus salicylic acid plaster resulted in a mean time to cure of 26 days versus 47 days for the group receiving salicylic acid plaster (Ohkuma 1990).
Cantharidin versus vehicle
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Coloe Dosal 2014 compared 0.7% cantharidin cream to its vehicle, and found 2/13 versus 1/16 participants free of lesions at the end of 5 treatments over 2 months (RR 2.46, 95% CI 0.25 to 24.21, Analysis 14.1).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In Coloe Dosal 2014, 3/13 participants reported pain; 12/13, blistering; and 6/13, hypo‐ or hyperpigmentation in the group treated with 0.7% cantharidin. In the vehicle group, the same effects were found in 1/16, 8/16, and none of the participants, respectively.
Salicylic acid versus sodium nitrate in salicylic acid
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Treatment with 5% sodium nitrite co‐applied daily with 5% salicylic acid under occlusion resulted in significantly more participants with complete resolution of lesions three months after start of treatment: 12/16 (75%) compared with 3/14 (21%) participants in the control group, which was treated with an identical cream but omitting sodium nitrite (RR 3.50, 95% CI 1.23 to 9.92, Analysis 15.1) (Ormerod 1999).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the group treated with 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, brown staining was reported in 6 of the 16 participants (Ormerod 1999). Four out of 16 participants (25%) in this group stopped the treatment because of irritation and lack of efficacy. Two additional participants, who were cured, complained of significant irritation. In the group treated with 5% salicylic acid only, 4/14 participants complained of irritation.
Salicylic acid versus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 12% salicylic acid with 70% alcohol, and assessed cure after a maximum of 6 months. In the 12% salicylic acid group, 21/37 participants were cured versus 16/36 in the 70% alcohol group (RR 1.28, 95% CI 0.81 to 2.02, Analysis 16.1).
Salicylic acid versus phenol plus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 12% salicylic acid with 10% phenol plus 70% alcohol, and assessed cure after a maximum of 6 months. In the 12% salicylic acid group, 21/37 participants were cured versus 17/41 in the 10% phenol plus 70% alcohol group (RR 1.37, 95% CI 0.86 to 2.17, not statistically significant, Analysis 17.1).
Salicylic acid plus lactic acid versus curettage
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
The third comparison of Machado 2010 was a combination of 14% salicylic acid plus 14% lactic acid versus curettage, resulting in 14/17 versus 15/17 participants showing complete clearance (RR 1.06, 95% CI 0.86 to 1.32, Analysis 18.1).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the group treated with 14% salicylic acid plus 14% lactic acid, 8/16 participants reported mild pain, whereas in the curettage group 12/17 reported mild pain.
Alcohol versus phenol plus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 70% alcohol plus 10% phenol with 70% alcohol, and assessed cure after a maximum of 6 months. In the 70% alcohol group, 16/36 participants were cured versus 17/41 in the 70% alcohol plus 10% phenol group (RR 1.07, 95% CI 0.64 to 1.79, Analysis 19.1).
Iodine versus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
In a three‐armed study, Markum 2012 compared iodine, tea tree oil, and the two combined. Application of iodine compared to tea tree oil resulted in clinical cure (> 90% reduction of lesions) in 1/16 versus 3/18 participants (RR 0.38, 95% CI 0.04 to 3.25, Analysis 20.1).
Secondary outcomes
2. Improvement
Application of iodine compared to tea tree oil resulted in clinical improvement in 5/16 versus 8/18 participants (RR 0.70, 95% CI 0.29 to 1.71, Analysis 20.2) (Markum 2012).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 2/16 participants treated with iodine and 1/18 participants treated with tea tree oil. None of the iodine‐treated participants and 4/18 participants treated with tea tree oil noted a sensation of warmth during application (Markum 2012).
Iodine versus iodine plus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
In a three‐armed study, Markum 2012 compared iodine, tea tree oil, and the two combined. Application of iodine compared to tea tree oil combined with iodine resulted in > 90% reduction of lesions in 1/16 versus 16/19 participants (RR 0.07, 95% CI 0.01 to 0.50, Analysis 21.1).
Secondary outcomes
2. Improvement
Application of iodine compared to tea tree oil combined with iodine resulted in improvement in 5/16 versus 18/19 participants (RR 0.29, 95% CI 0.14 to 0.62, Analysis 21.2).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 2/16 participants treated with iodine and 1/19 participants treated with iodine combined with tea tree oil. None of the iodine‐treated participants and 3/19 participants treated with the combination noted a sensation of warmth during application (Markum 2012).
Tea tree oil versus iodine plus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
Application of tea tree oil alone compared to iodine combined with tea tree oil resulted in > 90% reduction of lesions in 3/18 versus 16/19 participants (RR 0.20, 95% CI 0.07 to 0.57, Analysis 22.1) (Markum 2012), favouring the combined treatment.
Secondary outcomes
2. Improvement
Application of tea tree oil alone compared to iodine combined with tea tree oil resulted in improvement in 8/18 versus 18/19 participants (RR 0.47, 95% CI 0.28 to 0.79, Analysis 22.2) (Markum 2012), favouring the combined treatment.
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 1/18 participants treated with tea tree oil and 1/19 participants treated with the combination. The number of participants noting a sensation of warmth during application was 4/18 and 3/19 treated with tea tree oil or the combination (Markum 2012).
Systemic treatments
Of the two studies addressing systemic treatments, only one study reported on our primary outcome but did not include any of our secondary outcomes (Manchanda 1997b). The other study reported on two of our secondary outcomes (Antony 2001).
Cimetidine versus placebo
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
Treatment with systemic cimetidine 35 mg/kg/day cleared lesions completely in 4/8 participants (50%) after 4 months of treatment, compared with 5/11 participants (46%) given placebo in the same period (Analysis 23.1) (Antony 2001). The difference was not statistically significant (RR 1.10, 95% CI 0.43 to 2.84). No data were reported for the 50% (19/38) of participants who withdrew from the study.
2. Improvement
Treatment with systemic cimetidine 35 mg/kg/day resulted in completely cleared lesions or self reported improvement in 7/8 participants after 4 months of treatment, compared with 9/11 participants given placebo in the same period (Analysis 23.2) (Antony 2001). The difference was not statistically significant (RR 1.07, 95% CI 0.73 to 1.57).
Calcarea carbonica versus placebo
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
Treatment with calcarea carbonica resulted in 100% improvement in 13/14 participants in the treatment arm and 1/6 in the placebo arm of the trial (RR 5.57, 95% CI 0.93 to 33.54, Analysis 24.1) (Manchanda 1997b). Fisher's exact test resulted in a P value of 0.002. However, study duration, time to resolution, and adverse events were not reported, and the study was not analysed by the intention‐to‐treat principle. The number of dropouts (20/104 for the whole trial, including other skin conditions) was unclear for the molluscum participants.
Discussion
Summary of main results
Twenty‐two studies, with a total of 1650 participants, examined the effects of topical (20 studies) and systemic (2 studies) interventions. Altogether, we could extract evaluable data for 24 comparisons.
We found limited evidence of low quality from 11 comparisons for the short‐term cure efficacy of the following topical treatments: cryospray when compared to 5% imiquimod; 10% potassium hydroxide compared to 5% imiquimod; 5% sodium nitrite co‐applied with 5% salicylic acid compared to 5% salicylic acid alone; tea tree oil combined with iodine compared to tea tree oil or iodine alone; 10% Australian lemon myrtle oil compared to olive oil; and 10% benzoyl peroxide compared to 0.05% tretinoin. We found some evidence to suggest that 10% potassium hydroxide is more effective than saline; 5% solution of potassium hydroxide is favoured compared to 2.5% solution of potassium hydroxide; 10% povidone iodine solution plus 50% salicylic acid plaster is favoured compared to salicylic acid plaster alone; and homeopathic calcarea carbonica is favoured compared to placebo. We found no statistically significant differences for the other comparisons not including imiquimod.
The addition of three unpublished trial reports, with a total of over 800 participants, resulted in high‐ to moderate‐quality evidence for the comparison of topical 5% imiquimod versus vehicle. We conclude that compared to vehicle, topical 5% imiquimod is probably no more effective in terms of clinical cure, makes little or no difference in terms of short‐term improvement or local side effects, but appears to induce more application site reactions.
Overall, study limitations included lack of blinding, many dropouts, and no intention‐to‐treat analysis. Small study sizes resulted in broad confidence intervals and may have led to important differences being missed. None of the evaluated treatment options were associated with serious adverse effects, except for 5% imiquimod (severe application site reactions).
Although this update of our original review identified 11 new studies for inclusion, the overall conclusions have hardly changed, due to the small size of most of the studies and methodological shortcomings. We found no strong evidence either for or against the most commonly used treatment options for molluscum contagiosum. The evidence identified by this systematic review is therefore insufficient to propose any one intervention for molluscum contagiosum.
Overall completeness and applicability of evidence
Of the 11 trials added during the 2016 update, only three contributed to comparisons for which trials had been found in previous versions of this review. The other eight addressed new comparisons, thus increasing the fragmentation of the evidence. There remains an evidence gap regarding many promoted and used treatment options for molluscum contagiosum, with many interventions assessed by single, low‐quality studies. For example, of the two studies that included a curettage arm (Hanna 2006; Machado 2010), the outcomes of Hanna 2006 were not suitable for inclusion in this review, and Machado 2010 was a very small study, with 50 participants divided over three study arms. We could include only two studies on cryotherapy (Al‐Mutairi 2010; Handjani 2014).
Due to the small sample sizes of the low‐quality, single‐study comparisons that were included and for which no differences were found, it is possible that clinically relevant differences could be found if treatments would be evaluated in larger samples.
About half of the studies did not compare an active treatment to some sort of placebo, but rather compared two active treatments. This implies that for these interventions, the magnitude of benefit compared to placebo or doing nothing is unclear.
Several issues remain unclear due to lack of details in the published papers. For example, it is unclear whether duration of treatment, as used in Ormerod 1999, can be taken as a valid indicator for time to cure given dropouts and other possible reasons for stopping treatment. Although Antony 2001 did not report on adverse events, the 50% loss to follow‐up rate in the trial might have been caused by adverse effects of the treatment. It is unclear which dosing regimen was used in Manchanda 1997b when evaluating calcarea carbonica.
Several of the outcomes important to participants and clinicians were not measured in most of the studies we found, or not at all; these included recurrences, and, especially, spread to other people and quality of life.
We initially chose our primary outcome measure to be clinical cure after one month, calculated from the last day of treatment. However, this may not be the most appropriate outcome measure to cover the variety of treatments for molluscum. For example, when comparing a method of physical destruction (e.g. curettage) with a topical treatment that is applied during several days or weeks, our primary outcome measure might favour the first type of treatment. For this update we have adapted our primary outcome to make it more manageable: short‐term clinical cure (up to three months after start of treatment). A time point beyond one month after start of treatment for assessing cure was also suggested by Mc Cuaig 2011, commenting on the 2009 update of this review. An example of when time to cure since the last day of treatment is not appropriate is when treatment is continued until resolution of all lesions (e.g. Ohkuma 1990). Although no clear‐cut solution seems available, and so far few trials have studied physical destruction (e.g. Hanna 2006; Machado 2010), it is advisable to always consider multiple outcome measures and also to take the burden of treatment into account.
We could perform a meta‐analysis for only two comparisons: 5% imiquimod versus its vehicle and 5% imiquimod versus 10% potassium hydroxide.
We excluded studies on genital molluscum contagiosum and in participants with immune deficiency, so our conclusions do not apply to these participant groups, as the need for treatment is probably higher and may require different treatments.
Quality of the evidence
We included 22 studies with a total of 1650 participants in this update. Most of the included studies had small sample sizes, with a median study size of just over 30 molluscum participants, and only five studies with more than 100 participants. This impacted on our GRADE assessments. The small studies may have limited power, which was reflected in the wide confidence intervals around the risk ratios. In addition, many of the studies had large losses to follow‐up, up to 50% (Antony 2001).
Furthermore, most of the included studies used a control treatment that was not a placebo. Examples of comparator treatments in these studies were olive oil, saline, and alcohol, which may have had potential treatment effects. It was therefore difficult to compare the net effect of interventions due to the absence of a placebo group.
We could assign a low risk of bias for only a small proportion of items in the 'Risk of bias' table (Figure 2). The lack of reported details on several methodological issues and follow‐up periods, together with the small number of participants, gives rise to doubts about the validity of the results of some of the studies. We were not able to assess publication bias in this review, for example by constructing a funnel plot, due to the lack of directly comparable studies.
Notably, we judged five of the nine studies included in the 2016 update to be at low risk of bias (Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b), suggesting a trend toward better study design or reporting, or both. However, with the exception of the three 'imiquimod versus vehicle' studies, most of the studies added during this update were small. The imiquimod studies overall had a low risk of bias (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004), therefore we did not downgrade the outcomes in summary of findings Table for the main comparison for this domain.
As most of the evidence included in this review was based on low‐quality studies, we were unable to reach a robust conclusion regarding the objective of the review.
Potential biases in the review process
Despite conducting a thorough search, we cannot be certain that we did not miss relevant randomised trials. One study is ongoing, and several others are awaiting classification and have not yet been incorporated into the review. Given that 19 of the included studies were performed or published, or both after 2000, we expect more studies comparing treatments for molluscum contagiosum to be published in the coming years.
We made several amendments to the original protocol, some of which were 'data‐driven' in the sense that we encountered situations that we had not expected when we developed the protocol. This may have introduced bias.
Agreements and disagreements with other studies or reviews
At the time of the 2009 update of this review, we had found one other systematic review (Schmitt 2008). This review included six randomised trials, all of which were also included in our review. The conclusions of this review were similar to ours. During the 2016 update, we identified a new partly systematic (they described their databases, search strategy, and search date, but did not select studies or extract data in couples, did not state clear inclusion criteria, and did not assess risk of bias) review (Chen 2013), which addressed not only epidemiology, virology, and immunology of molluscum contagiosum, but also clinical management. They summarised the findings of the previous version of this review (Van der Wouden 2009), and mentioned three new trials (Al‐Mutairi 2010; Köse 2013; Seo 2010), the first two of which have been included in this update, and the last which is awaiting assessment. They concluded that no evidence‐based consensus has been reached on which is the best treatment for molluscum contagiosum in people without immune deficiency.
The cure rates found in the non‐randomised study by Weller 1999 for physical expression and phenol ablation (75% and 77% of lesions, respectively, after one month) compare favourably to the 23% of children found cured in the Japanese cohort study on the natural history of the disease (Takemura 1983).
In the vehicle group of the 3M studies, clearance rates were 12% within 3 months; 27% after 3 to 6 months; and 40% beyond 6 months. Another, more recent cohort study from the UK (Olsen 2015), where only a small proportion of children were reported to have received treatment, found somewhat lower cure rates: around 10% after 6 months; 40% after 12 months; 75% after 18 months; and 80% after 24 months. Comparing these figures, 'vehicle' seems to work better than no treatment at all.

Flow diagram of inclusion for this update.

Risk of bias table: review authors' judgements about each methodological quality item for each included study.
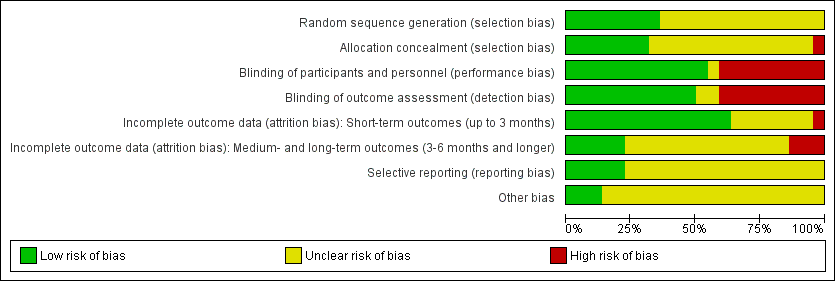
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
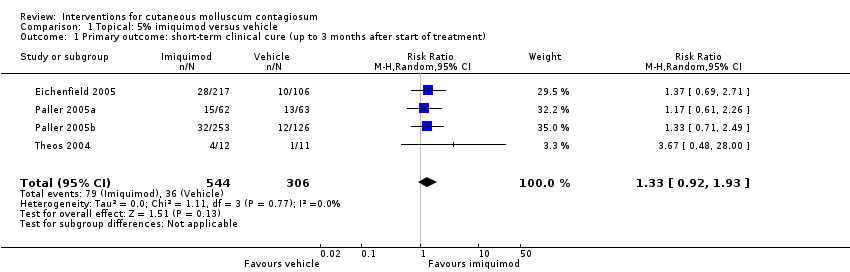
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
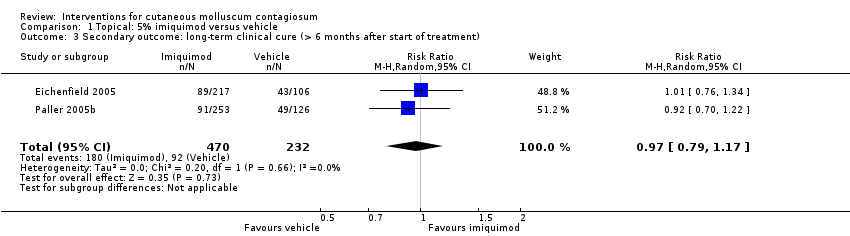
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment).
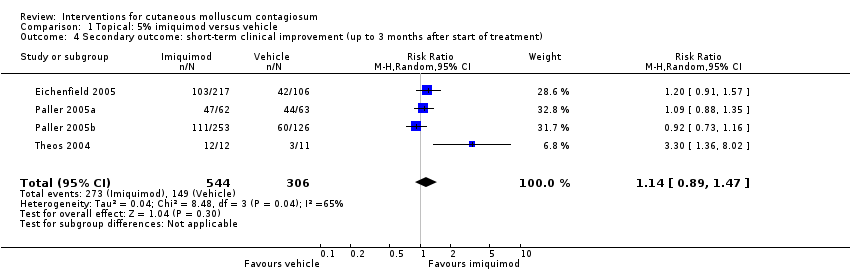
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
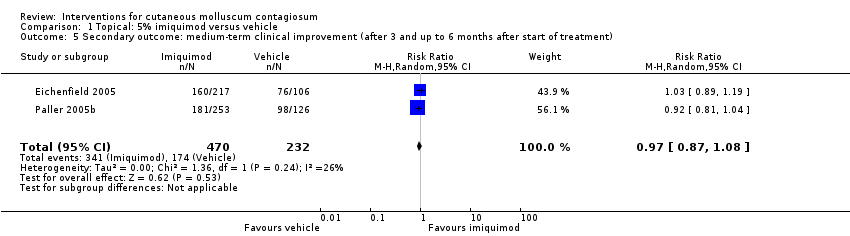
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment).
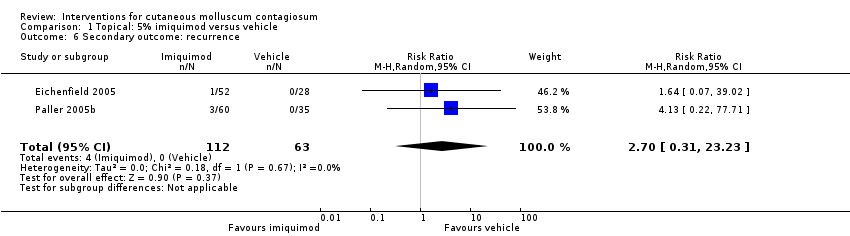
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 6 Secondary outcome: recurrence.
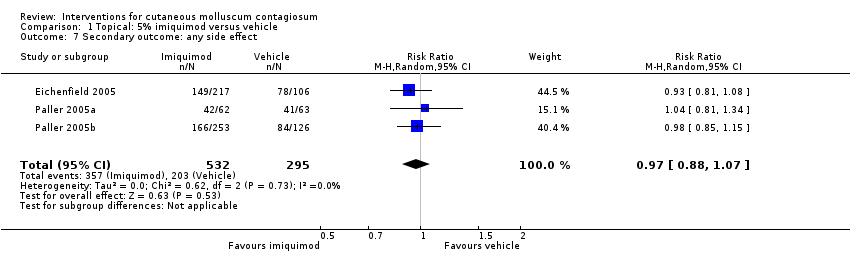
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 7 Secondary outcome: any side effect.
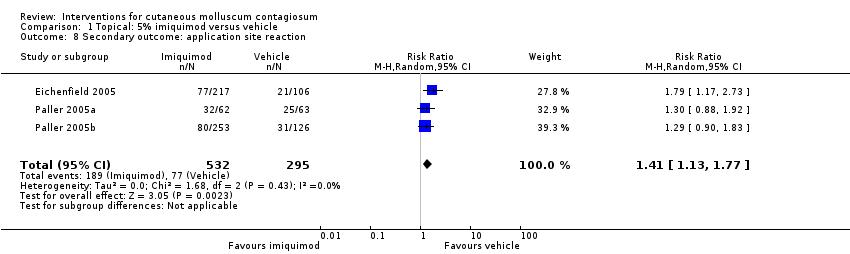
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 8 Secondary outcome: application site reaction.

Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 9 Secondary outcome: severe application site reaction.
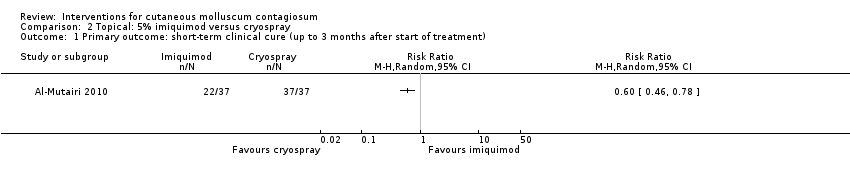
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
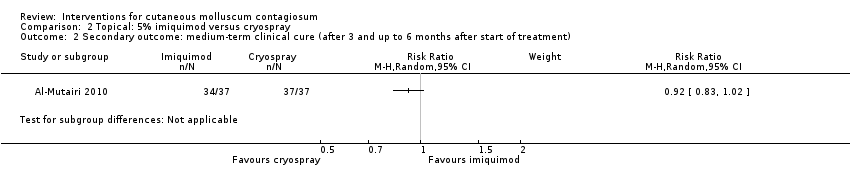
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
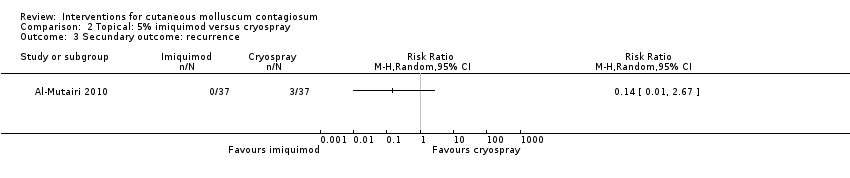
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 3 Secundary outcome: recurrence.
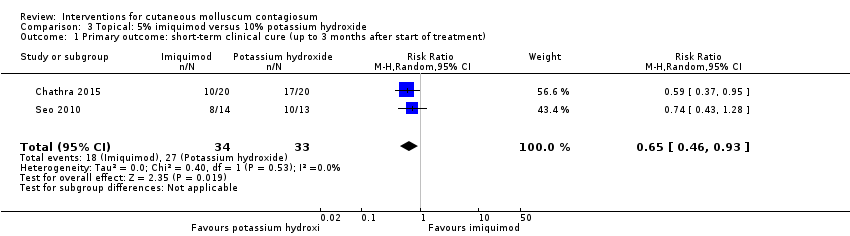
Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 2 Secondary outcome: any side effect.
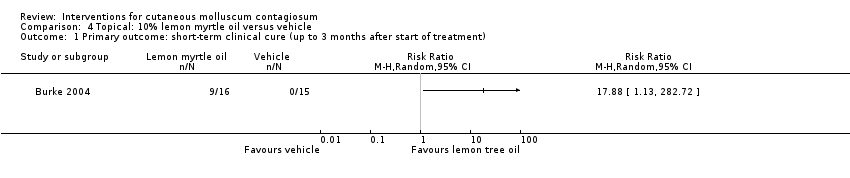
Comparison 4 Topical: 10% lemon myrtle oil versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
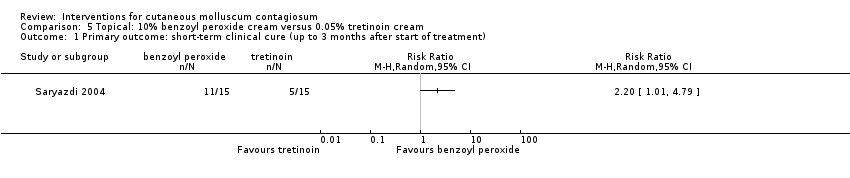
Comparison 5 Topical: 10% benzoyl peroxide cream versus 0.05% tretinoin cream, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
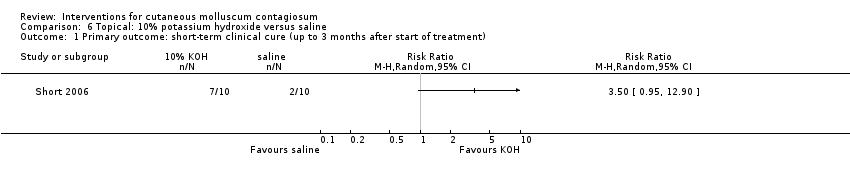
Comparison 6 Topical: 10% potassium hydroxide versus saline, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
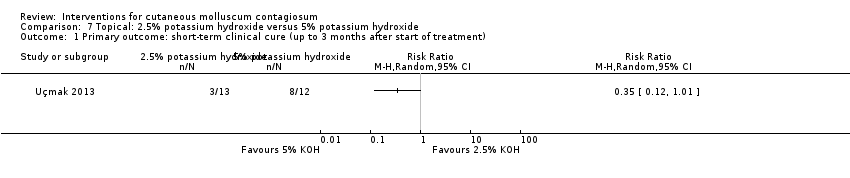
Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment).

Comparison 8 Topical: 10% potassium hydroxide versus 14% salicylic acid + 14% lactic acid, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 9 Topical: 10% potassium hydroxide versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
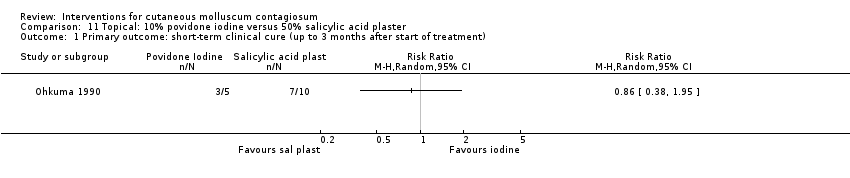
Comparison 11 Topical: 10% povidone iodine versus 50% salicylic acid plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 12 Topical: 10% povidone iodine alone versus 10% povidone iodine and 50% salicylic plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 13 Topical: 10% povidone iodine and 50% salicylic acid plaster versus 50% salicylic plaster alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 14 Topical: 0.7% cantharidin versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment)..

Comparison 15 Topical: 5% sodium nitrite in 5% salicylic acid versus 5% salicylic acid alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
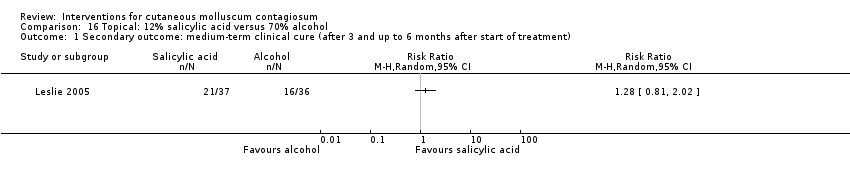
Comparison 16 Topical: 12% salicylic acid versus 70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
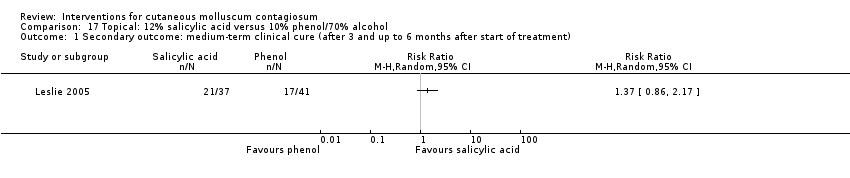
Comparison 17 Topical: 12% salicylic acid versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).

Comparison 18 Topical: 14% salicylic acid + 14% lactic acid versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 19 Topical: 70% alcohol versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).

Comparison 20 Topical: iodine versus tea tree oil, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
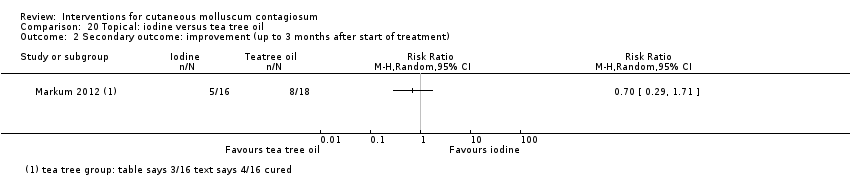
Comparison 20 Topical: iodine versus tea tree oil, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).

Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).

Comparison 23 Systemic: cimetidine versus placebo, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment).
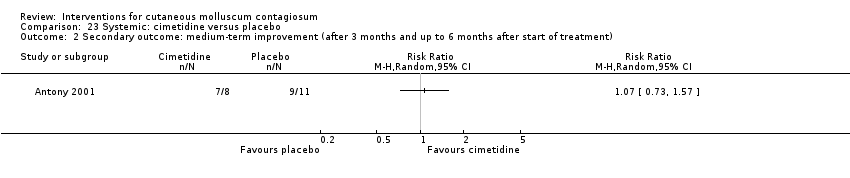
Comparison 23 Systemic: cimetidine versus placebo, Outcome 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment).
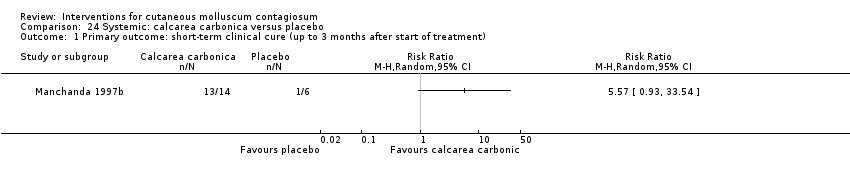
Comparison 24 Systemic: calcarea carbonica versus placebo, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
| Imiquimod versus vehicle for cutaneous molluscum contagiosum | ||||||
| Patient or population: molluscum contagiosum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical vehicle | Risk with topical imiquimod | |||||
| Short‐term clinical cure (up to 3 months after start of treatment) (completely cleared short term) | Study population | RR 1.33 | 850 | ⊕⊕⊕○ | Analysis 1.1 | |
| 118 per 1000 | 156 per 1000 | |||||
| Medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) (completely cleared medium term) | Study population | RR 0.88 | 702 | ⊕⊕⊕○ | Analysis 1.2 | |
| 272 per 1000 | 239 per 1000 | |||||
| Long‐term clinical cure (beyond 6 months after start of treatment) (completely cleared long term) | Study population | RR 0.97 | 702 | ⊕⊕⊕○ | Analysis 1.3 | |
| 401 per 1000 | 389 per 1000 | |||||
| Short‐term clinical improvement (up to 3 months after start of treatment) | Study population | RR 1.14 | 850 | ⊕⊕⊕⊕ | Analysis 1.4 | |
| 487 per 1000 | 555 per 1000 | |||||
| Any adverse effect | Study population | RR 0.97 | 827 | ⊕⊕⊕⊕ | Analysis 1.7 | |
| 688 per 1000 | 667 per 1000 | |||||
| Application site reactions | Study population | RR 1.41 | 827 | ⊕⊕⊕○ | Analysis 1.8. This outcome was not prespecified in our protocol. | |
| 261 per 1000 | 368 per 1000 | |||||
| Severe application site reactions | Study population | RR 4.33 | 827 | ⊕⊕⊕○ | Analysis 1.9. This outcome was not prespecified in our protocol. | |
| 7 per 1000 | 29 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. 2Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as both studies were judged to be at low risk of bias. 3We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. We also decided not to downgrade for inconsistency as removing one outlier eliminated inconsistency but hardly affected pooled estimate. 4We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. 5Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias. | ||||||
| Treatment class | Treatment modality | Included studies | Other studies |
| 'Doing nothing' | Awaiting natural resolution | — | |
| Placebo | Antony 2001; Eichenfield 2005; Manchanda 1997b; Paller 2005a; Paller 2005b | — | |
| Surgical treatments | Cryotherapy | ||
| Curettage | |||
| Curettage with punch | — | ||
| Electric cauterisation | — | ||
| Physical expression (squeezing) | — | ||
| Pricking | — | ||
| Pulsed dye laser | — | ||
| Topical treatments | Acidified nitrite | ||
| Adapalene | — | ||
| Australian lemon myrtle oil | — | ||
| Benzoyl peroxide | — | ||
| Bromogeramine | — | ||
| Cantharidin | |||
| Cidofovir | — | ||
| Diphencyprone | — | ||
| Griseofulvin | — | ||
| Honey | — | ||
| Hydrogen peroxide cream | — | ||
| Hyperthermia | — | ||
| Imiquimod | Al‐Mutairi 2010; Eichenfield 2005; Hanna 2006; Paller 2005a; Paller 2005b; Seo 2010; Theos 2004 | Arican 2006; Barba 2001; Bayerl 2003; Hengge 2003; Lim 2003; Liota 2000; Metkar 2008; Skinner 2000; Skinner 2002; Syed 1998 | |
| Iodine | — | ||
| Iodine combined with tea tree oil | — | ||
| Milkweed | — | ||
| Povidone iodine plus salicylic acid | — | ||
| Phenol | |||
| Podophyllotoxin (HIV patients) | — | ||
| Potassium hydroxide | |||
| Retinoic acid | — | ||
| Salicylic acid | — | ||
| Salicylic acid combined with lactic acid | — | ||
| Salicylic acid combined with sodium nitrite | — | ||
| Silver nitrate | — | ||
| Tea tree oil | — | ||
| Tretinoin | — | ||
| Yellow oxide of mercury | — | ||
| Systemic treatments | Cimetidine | ||
| Calcarea carbonica (homeopathy) | |||
| Griseofulvin | — | ||
| Combinations of above | Potassium iodide followed by X‐rays | — |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.92, 1.93] |
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.14] |
| 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.17] |
| 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 4 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.89, 1.47] |
| 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment) Show forest plot | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| 6 Secondary outcome: recurrence Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.31, 23.23] |
| 7 Secondary outcome: any side effect Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 8 Secondary outcome: application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.13, 1.77] |
| 9 Secondary outcome: severe application site reaction Show forest plot | 3 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [1.16, 16.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Secundary outcome: recurrence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.93] |
| 2 Secondary outcome: any side effect Show forest plot | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

