Interventions for cutaneous molluscum contagiosum
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind randomised placebo‐controlled trial. Method of generation of randomisation sequence is unclear, as is concealment of allocation. No intention‐to‐treat analysis. UK, Department (Dept) of Dermatology | |
| Participants | 38 patients (1 to 16 years, M/F 18/20) were enrolled, for 19 patients complete data were obtained, 8 of which had been randomised into the treatment arm. 19 patients withdrew from the study, no data on reasons for withdrawal | |
| Interventions | 35 mg/kg/day cimetidine, given once daily as oral suspension versus a matching placebo | |
| Outcomes | Complete clearance after 4 months treatment. Reduction of lesions. Adverse events: not mentioned | |
| Notes | 50% dropout rate. Published abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". No details in abstract |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described in the abstract |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Double‐blind placebo‐controlled"; "The dose of cimetidine was 35 mg/kg‐1/day ‐1"; "The placebo group received a manufactured placebo". Probably done, placebo‐controlled, both suspensions |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported in the abstract |
| Incomplete outcome data (attrition bias) | High risk | 4 months: 19/35 completed the treatment course. Quote: "The number of patients who received placebo or cimetidine was similar in the groups that did not attend or withdrew." > 30% withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The mean age and sex of the patients and incidence of atopic disease in each treatment group was similar." No compliance data |
| Methods | Randomised controlled trial. Body sides were randomised left‐right. UK, Dept of Dermatology | |
| Participants | 30 children (2 to 12 years of age, M/F 18/12) were recruited | |
| Interventions | Sterile normal 0.9% saline versus 5% potassium hydroxide | |
| Outcomes | Complete clearance of lesions; side‐effects | |
| Notes | Unpublished, year of study unclear. Unpublished paper obtained in 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomized to right or left side of body". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomized to right or left side of body". Insufficient information |
| Blinding (performance bias and detection bias) | Low risk | Quote: "30 patients were recruited in this double‐blind study". "All subjects were given seven bottles clearly labelled R and seven bottles labelled L, for use on the right and left side of the body respectively (patient and investigator did not know which is active site)" |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown when patients dropped out, no short‐term outcomes provided |
| Incomplete outcome data (attrition bias) | High risk | 12 weeks: 10/30 did not complete study, 2 withdrew due to severe stinging from KOH, and 8 children were lost to follow‐up. > 30% drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial, USA, outpatient clinic | |
| Participants | 31 children, mean age 4.6 years. Sex not reported | |
| Interventions | 10% lemon myrtle oil or vehicle (olive oil) | |
| Outcomes | Complete clearance or > 90% reduction in number of lesions | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomized to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100. Odd numbers were assigned to active treatment even numbers to vehicle" |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were randomized to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100." "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion." "A mild synthetic lemon fragrance not containing citral was added to scent the control olive oil preparation.This fragrance by itself had no therapeutic effect." Vehicle controlled |
| Incomplete outcome data (attrition bias) | Low risk | 21 days: 4/31 withdrew: 1/16 in lemon myrtle oil group lost to follow‐up; 3/15 missing in vehicle group, withdrew because of worsening of the molluscum. Withdrawn patients included in analysis as failures |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address medium and long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The mean number of lesions at enrolment did not differ between treatment groups. No sex or age comparison between groups. No compliance data |
| Methods | Randomised controlled trial, Canada, Montreal, Dermatology clinic | |
| Participants | 124 children, 1 to 16 years of age M/F 57/67 | |
| Interventions | Four arms: curettage; topical cantharidin 0.7%; topical salicylic acid 16.7% + lactic acid 16.7%; topical imiquimod cream 5% | |
| Outcomes | Number of visits required. Intervals between study visits not reported, so outcome data not suitable for inclusion | |
| Notes | Total number of patients unclear. Percentage of group 3 in table 1 does not correspond to number mentioned in text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)." Insufficient information |
| Blinding (performance bias and detection bias) | High risk | Quote: "This is not a double‐blind study." Physical versus topical treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial. UK, outpatient departments of teaching hospital and district general hospital | |
| Participants | 114 children, 1 to 15 years of age, sex not reported | |
| Interventions | Topical salicylic acid 12%, or phenol 10% with 70% alcohol, or 70% alcohol | |
| Outcomes | Complete clearance of lesions | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomized according to a random number table" |
| Allocation concealment (selection bias) | High risk | Quote: "The investigators were not blinded to randomization" |
| Blinding (performance bias and detection bias) | High risk | Quote: "The patients in the salicylic acid groups were aware of their treatments. The other two groups treated with vehicle or phenol were single‐blinded, as the patients/parents were unaware of which treatment they received." "The vehicle and diluted phenol were prepared by the hospital pharmacy and labelled with a letter" |
| Incomplete outcome data (attrition bias) | Unclear risk | Up to 6 months: 31/114 lost to follow‐up: 13/37 in salicylic acid arm, 9/41 in dilute phenol arm, 9/36 in alcohol arm |
| Incomplete outcome data (attrition bias) | High risk | Up to 6 months: 31/114 lost to follow‐up: 13/37 in salicylic acid arm, 9/41 in dilute phenol arm, 9/36 in alcohol arm. > 30% drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The baseline characteristics of the three groups were similar." See also Table I, Baseline characteristics. No compliance data |
| Methods | Double‐blind randomised controlled trial, addressing various types of warts (n = 124), including molluscum contagiosum (n = 20). India, Homoeopathic Medical College & Hospital, New Delhi. Randomisation sequence was generated manually, identity of the drugs was kept secret in a sealed cover (personal communication with Dr Manchanda). No intention‐to‐treat analysis | |
| Participants | 14 molluscum patients (age and sex unknown) randomised into the treatment arm, 6 patients were randomised to receive plain sugar globules as a placebo (personal communication Dr Manchanda). 10 patients were aged below 10 years, 7 from 10 to 20 and 3 were from the age group 21 to 30 years (personal communication with Dr Manchanda) | |
| Interventions | Different potencies of a homeopathic drug called calcarea carbonica daily for 15 days (n = 14) versus sugar globules (placebo). Unclear which patients received what potency | |
| Outcomes | Improvement (not clear after what period) | |
| Notes | Paper reports on (1) cross‐over study (2) parallel study. The cross‐over study was excluded, because less than 5 patients in one of the arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." Randomisation not mentioned in paper, "sequence was generated manually" (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." "Therefore it was found that after decoding method of concealment is not described" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Two types of placebo controlled double‐blind clinical trials were undertaken." "The subjects were given both drug and placebo." Quote (personal communication): “The identity of the drugs was kept secret in a sealed cover which was opened only at the time un‐blinding the experiment." "The plain sugar globules looks like homoeopathic drug Calcerea carbonica was used as placebo." Probably done |
| Incomplete outcome data (attrition bias) | Low risk | 15 days: 20/124 dropouts, unclear what skin disease and group assignment |
| Incomplete outcome data (attrition bias) | High risk | Only 15 days |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
| Methods | Randomised controlled trial (written correspondence Dr Ohkuma), the method of generation of randomisation sequence remained unclear, as was the concealment of allocation. It was also unclear if participants were analysed according to the group to which they were randomised (intention‐to treat analysis) and how blinding was performed. Japan, Department of Dermatology | |
| Participants | 35 patients with molluscum contagiosum, aged between 2 and 9 years (M/F 21/14) | |
| Interventions | 3 interventions were compared: 10% povidone iodine solution combined with 50% salicylic acid plaster (n = 20), iodine alone (n = 5) and salicylic plaster alone (n = 10) | |
| Outcomes | Time to cure | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (personal communication, not in paper). Insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation |
| Blinding (performance bias and detection bias) | High risk | Probably not, iodine versus salicylic plaster: hard to mask |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss reported, all patients in outcome table. Follow‐up period unclear. Duration of treatment varied from 7 to 64 days |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss reported, all patients in outcome table. Follow‐up period unclear. Duration of treatment varied from 7 to 64 days |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "In the former, two girls and three boys between the age of 3 and 5 were included and 4 girls and 6 boys between 2 and 9 comprised the latter control group." No imbalance for sex. No compliance data |
| Methods | Group sequential double‐blind randomised trial. All participants were analysed according to group assignment (intention‐to‐treat). Two patients did not complete the trial. UK, Department of Dermatology | |
| Participants | 30 molluscum patients were enrolled, with 16 in the acidified nitrite group and 14 controls, with a median age of 6 years, 22 girls and 8 boys. Exclusion criteria were age below 1 year of age, pregnant or lactating women, and taking immunosuppressive drugs or known to have HIV infection | |
| Interventions | 5% sodium nitrite co‐applied daily with 5% salicylic acid under occlusion versus identical cream with 5% salicylic acid omitting sodium nitrite | |
| Outcomes | Time to complete resolution | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Group sequential design in which subjects were randomized to receive either". Insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding (performance bias and detection bias) | High risk | Quote: "Double‐blind, group sequential design in which subjects were randomized to receive either 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, or identical cream with 5% salicylic acid but omitting sodium nitrite, as a control." Not done, active intervention was associated with brown staining |
| Incomplete outcome data (attrition bias) | Unclear risk | Only long‐term data |
| Incomplete outcome data (attrition bias) | High risk | 21/30 dropouts after 3 months |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No compliance data. Duration and number of lesions were very similar (communication with author) |
| Methods | Randomised trial, Iran, hospital dermatology clinic Outcomes given for 23 patients of 30 randomised, original distribution unknown, assumed 15:15 | |
| Participants | 30 children, age and sex unknown | |
| Interventions | Topical benzoyl peroxide 10% cream versus tretinoin 0.05% cream, 2 times daily (TD) for 4 weeks | |
| Outcomes | Count of lesions, lesion free after 6 weeks Side‐effects limited to mild dermatitis in both groups | |
| Notes | Information based on abstract, proportions cured used for estimating absolute numbers. Abstract published in 2004 ‐ unclear when study was carried out | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | "Investigator masked" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline characteristics nor compliance data |
| Methods | Double‐blind randomised placebo controlled trial. UK, Department of Dermatology, London. The method of generation of the randomisation sequence is unclear as is concealment of allocation. All participants were analysed according to group assignment (intention‐to‐treat analysis). 19/20 completed the study. | |
| Participants | 20 children from a paediatric dermatology clinic, age range 2 to 12 years, M/F 6/14. Exclusion criteria were known immunodeficiency and facial lesions | |
| Interventions | Application of 10% potassium hydroxide solution twice daily applied with a cotton swab, continued until the lesions showed signs of inflammation (n = 10). The control group received saline (n = 10) | |
| Outcomes | Time to resolution | |
| Notes | Number of patients who completed the study differs between unpublished paper (18/20) and published paper (19/20). Latter number included in corrected version of 2009 update (December 2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments". Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments." Central allocation: Pharmacy‐controlled |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both the patients and the observer were blinded". "Both solutions were dispensed in identical, unlabeled bottles. The sequence was not revealed until the end of the study." Staining and stinging reported in the KOH group. Patient, care provider, and outcome assessor probably blinded |
| Incomplete outcome data (attrition bias) | Low risk | 2 weeks: 1/20 not completed the study; 1/10 in the KOH group withdrew after 2 weeks because of discomfort of the skin localised to the application site |
| Incomplete outcome data (attrition bias) | Low risk | 90 days: 1/20 not completed the study; 1/10 in the KOH group withdrew after 2 weeks because of discomfort of the skin localised to the application site |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance for sex, lesion site, and numbers. No compliance data |
| Methods | Randomised controlled trial. USA, Alabama, Illinois, New York | |
| Participants | 23 children, 1 to 9 years of age, M/F 12/11 | |
| Interventions | Imiquimod cream 5% or vehicle | |
| Outcomes | Complete or partial clearance (> 30% decrease from baseline lesion count) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomized to either imiquimod or vehicle". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomized to either imiquimod or vehicle". Insufficient information |
| Blinding (performance bias and detection bias) | Low risk | Quote: "In a Double Blind, Randomized Pilot Trial"; "imiquimod vs vehicle". Only patients and physicians involved |
| Incomplete outcome data (attrition bias) | Low risk | 2 weeks: 2/23 not completed the study (discontinued treatment) |
| Incomplete outcome data (attrition bias) | Low risk | 2 weeks: 2/23 not completed the study (discontinued treatment) |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Baseline imbalance for mean lesion count, imiquimod: 27.0 versus vehicle: 19.4 (not statistically significant). No compliance data |
KOH = Potassium Hydroxide
NS = Normal Saline
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| HIV‐infected patients (n = 40) | |
| RCT comparing 2 types of cryotherapy for cutaneous skin lesions: 124 patients, among which 10 molluscum patients, distributed 9:1 over 2 arms | |
| Not randomised (personal communication) | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 83) | |
| Large parallel controlled study (n = 1656), with 4 arms, no randomisation (personal communication with Dr He through Taixiang Wu) | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 24) | |
| Cross‐over study with different types of warts (n = 43), 10 molluscum patients. 1 of the treatment arms (placebo first?) had less than 2 patients | |
| Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 55) | |
| Not randomised but alternate assignment (personal communication, Alireza Firooz) | |
| RCT, n = 150, mainly genital lesions, which is not a focus of this review | |
| RCT, n = 100, mainly genital lesions, which is not a focus of this review | |
| Controlled trial (n = 16), comparing phenol ablation and physical expression. Lesions were unit of treatment and analysis. No randomisation | |
| Controlled trial, N=34, aged 2 to 12 years. 10% potassium hydroxide versus placebo. Not randomised, but alternate assignment |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy of cantharidin in molluscum contagiosum: a randomised, blinded, placebo‐controlled prospective study |
| Methods | Randomized, double‐blind (subject, caregiver, investigator, outcomes assessor) |
| Participants | Molluscum patients |
| Interventions | Topical cantharidin 0.7% Vehicle |
| Outcomes | Complete and partial clearance after 8 weeks or 5 visits |
| Starting date | January 2008 |
| Contact information | |
| Notes | ‐ |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance or > 90% reduction after 3 weeks Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.88 [1.13, 282.72] |
| Analysis 1.1  Comparison 1 Topical: 10% Australian lemon myrtle oil vs. vehicle (olive oil), Outcome 1 Complete clearance or > 90% reduction after 3 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance after 4 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.25, 86.72] |
| Analysis 2.1  Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 1 Complete clearance after 4 weeks. | ||||
| 2 Partial clearance after 4 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.85 [0.88, 217.26] |
| Analysis 2.2  Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 2 Partial clearance after 4 weeks. | ||||
| 3 Complete clearance after 12 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.48, 28.00] |
| Analysis 2.3  Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 3 Complete clearance after 12 weeks. | ||||
| 4 Partial clearance after 12 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.98, 13.67] |
| Analysis 2.4  Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 4 Partial clearance after 12 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Free of lesions after 6 weeks Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [1.01, 4.79] |
| Analysis 3.1  Comparison 3 Topical: 10% benzoyl peroxide cream vs. 0.05% tretinoin cream (ITT), Outcome 1 Free of lesions after 6 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (3 months) Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.36, 7.75] |
| Analysis 4.1  Comparison 4 Topical: 10% KOH vs. saline, Outcome 1 Clinical cure at medium‐term follow‐up (3 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.85, 3.30] |
| Analysis 5.1  Comparison 5 Topical: 10% povidone iodine and 50% salicylic plaster vs. 10% povidone iodine alone, Outcome 1 Clinical cure at end of study (duration unknown). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.95, 2.16] |
| Analysis 6.1  Comparison 6 Topical: 10% povidone iodine and 50% salicylic acid plaster vs. 50% salicylic plaster alone, Outcome 1 Clinical cure at end of study (duration unknown). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.38, 1.95] |
| Analysis 7.1  Comparison 7 Topical: 10% povidone iodine vs. 50% salicylic acid plaster, Outcome 1 Clinical cure at end of study (duration unknown). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (3 months) Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [1.23, 9.92] |
| Analysis 8.1  Comparison 8 Topical: 5% sodium nitrite in 5% salicylic acid vs. 5% salicylic acid alone, Outcome 1 Clinical cure at medium‐term follow‐up (3 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (max 6 months) Show forest plot | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.56] |
| Analysis 9.1  Comparison 9 Topical: 10% phenol/70% alcohol vs. 70% alcohol (ITT), Outcome 1 Complete clearance at end of study (max 6 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (6 months max) Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| Analysis 10.1  Comparison 10 Topical: 12% salicylic acid vs. 70% alcohol (ITT), Outcome 1 Complete clearance at end of study (6 months max). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (max 6 months) Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.86, 2.17] |
| Analysis 11.1  Comparison 11 Topical: 12% salicylic acid vs. 10% phenol/70% alcohol (ITT), Outcome 1 Complete clearance at end of study (max 6 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (4 months) Show forest plot | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.43, 2.84] |
| Analysis 12.1  Comparison 12 Systemic: cimetidine vs. placebo, Outcome 1 Clinical cure at medium‐term follow‐up (4 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement at end of study (duration unknown) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 5.57 [0.93, 33.54] |
| Analysis 13.1  Comparison 13 Systemic: calcarea carbonica vs. placebo, Outcome 1 Improvement at end of study (duration unknown). | ||||
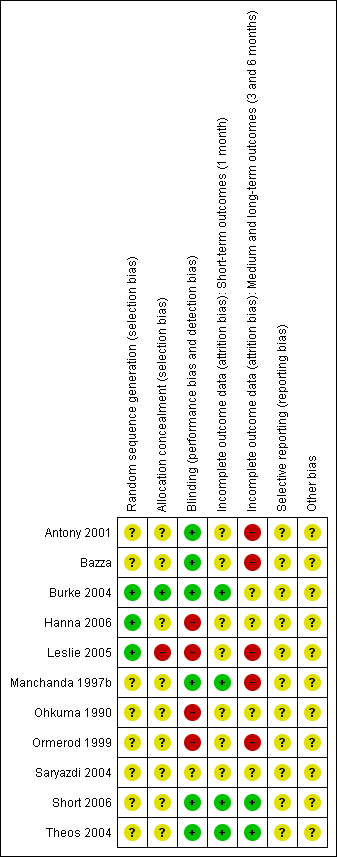
Risk of bias table: review authors' judgements about each methodological quality item for each included study.
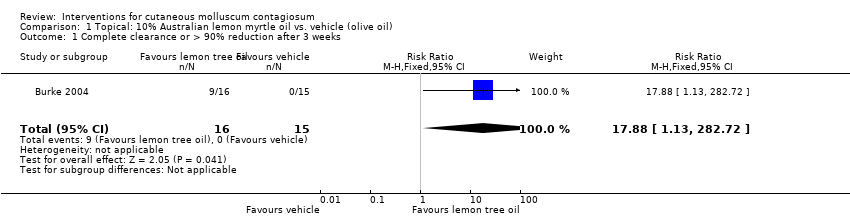
Comparison 1 Topical: 10% Australian lemon myrtle oil vs. vehicle (olive oil), Outcome 1 Complete clearance or > 90% reduction after 3 weeks.

Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 1 Complete clearance after 4 weeks.

Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 2 Partial clearance after 4 weeks.

Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 3 Complete clearance after 12 weeks.
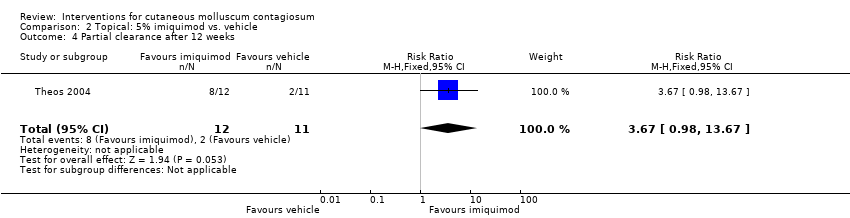
Comparison 2 Topical: 5% imiquimod vs. vehicle, Outcome 4 Partial clearance after 12 weeks.
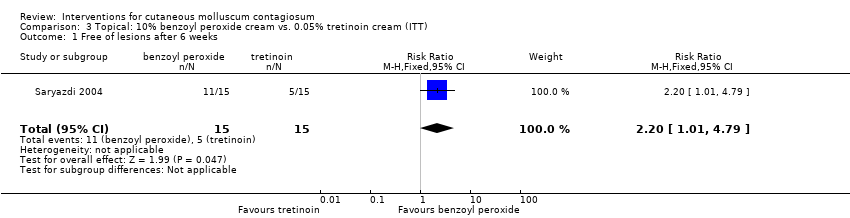
Comparison 3 Topical: 10% benzoyl peroxide cream vs. 0.05% tretinoin cream (ITT), Outcome 1 Free of lesions after 6 weeks.

Comparison 4 Topical: 10% KOH vs. saline, Outcome 1 Clinical cure at medium‐term follow‐up (3 months).

Comparison 5 Topical: 10% povidone iodine and 50% salicylic plaster vs. 10% povidone iodine alone, Outcome 1 Clinical cure at end of study (duration unknown).
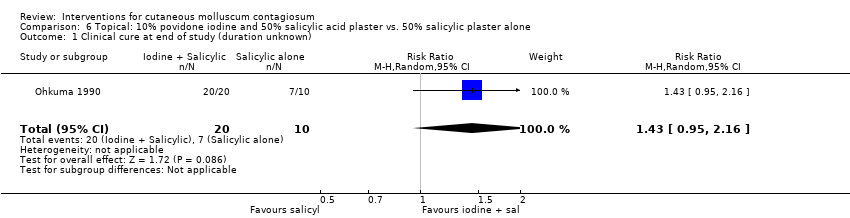
Comparison 6 Topical: 10% povidone iodine and 50% salicylic acid plaster vs. 50% salicylic plaster alone, Outcome 1 Clinical cure at end of study (duration unknown).

Comparison 7 Topical: 10% povidone iodine vs. 50% salicylic acid plaster, Outcome 1 Clinical cure at end of study (duration unknown).
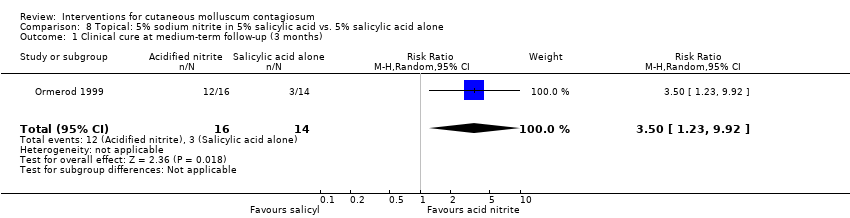
Comparison 8 Topical: 5% sodium nitrite in 5% salicylic acid vs. 5% salicylic acid alone, Outcome 1 Clinical cure at medium‐term follow‐up (3 months).

Comparison 9 Topical: 10% phenol/70% alcohol vs. 70% alcohol (ITT), Outcome 1 Complete clearance at end of study (max 6 months).

Comparison 10 Topical: 12% salicylic acid vs. 70% alcohol (ITT), Outcome 1 Complete clearance at end of study (6 months max).

Comparison 11 Topical: 12% salicylic acid vs. 10% phenol/70% alcohol (ITT), Outcome 1 Complete clearance at end of study (max 6 months).

Comparison 12 Systemic: cimetidine vs. placebo, Outcome 1 Clinical cure at medium‐term follow‐up (4 months).
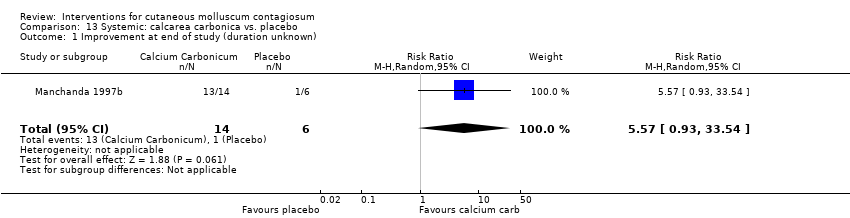
Comparison 13 Systemic: calcarea carbonica vs. placebo, Outcome 1 Improvement at end of study (duration unknown).
| Treatment class | Treatment modality | Included studies | Other studies |
| Surgical treatments | Cryotherapy | ||
| Curettage | |||
| Curettage with punch | |||
| Electric cauterisation | |||
| Physical expression (squeezing) | |||
| Pricking | |||
| Pulsed dye laser | |||
| Topical treatments | Acidified nitrite | ||
| Australian lemon myrtle oil | |||
| Benzoyl peroxide | |||
| Bromogeramine | |||
| Cantharidin | |||
| Cidofovir | |||
| Diphencyprone | |||
| Griseofulvin | |||
| Imiquimod | Syed 1998; Liota 2000; Barba 2001; Skinner 2002; Bayerl 2003; Hengge 2003 | ||
| Milkweed | |||
| Povidone iodine + salicylic acid | |||
| Phenol | |||
| Podophyllotoxin (HIV patients) | |||
| Potassium hydroxide | |||
| Retinoic acid | |||
| Salicylic acid | |||
| Salicylic acid combined with sodium nitrite | |||
| Silver nitrate | |||
| Tretinoin | |||
| Yellow oxide of mercury | |||
| Systemic treatments | Cimetidine | ||
| Calcarea carbonica (homeopathy) | |||
| Griseofulvin | |||
| Combinations of above | Potassium iodide followed by X‐rays |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance or > 90% reduction after 3 weeks Show forest plot | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.88 [1.13, 282.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance after 4 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.25, 86.72] |
| 2 Partial clearance after 4 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.85 [0.88, 217.26] |
| 3 Complete clearance after 12 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.48, 28.00] |
| 4 Partial clearance after 12 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.98, 13.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Free of lesions after 6 weeks Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [1.01, 4.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (3 months) Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.36, 7.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.85, 3.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.95, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at end of study (duration unknown) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.38, 1.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (3 months) Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [1.23, 9.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (max 6 months) Show forest plot | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (6 months max) Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete clearance at end of study (max 6 months) Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.86, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure at medium‐term follow‐up (4 months) Show forest plot | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.43, 2.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement at end of study (duration unknown) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 5.57 [0.93, 33.54] |

