非甾体抗炎药治疗子宫内膜异位症妇女疼痛
Abstract
研究背景
子宫内膜异位症极大影响了女性生活质量,损害了工作、日常活动、性关系、非性关系、生育力。非甾体抗炎药是治疗子宫内膜异位症妇女疼痛的一线用药中最常用的。
研究目的
评估非甾体抗炎药对比安慰剂、其他非甾体抗炎药、其他止痛药或无治疗时,控制子宫内膜异位症妇女疼痛的效果。
检索策略
我们检索了Cochrane妇科和生殖组随机对照试验注册库(Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials,2016年10月),发表于Cochrane随机对照试验中心注册库(CENTRAL)的文献,MEDLINE(2008年1月‐2016年10月),Embase(日期限于2016年1月1日至2016年10月19日,因为所有更早的参考文献都作为Embase计划的结果被纳入到Cochrane随机对照试验中心数据库),正在进行试验的数据库以及所有相关文献的参考文献。除非将来有新的证据,我们不会再更新这个系统评价。
标准/纳入排除标准
我们纳入了所有描述使用非甾体抗炎药治疗任何年龄段女性子宫内膜异位症疼痛的随机对照试验。
数据收集与分析
2009年的更新版中,两个作者独立阅读纳入的试验,并且提取了数据。我们使用RevMan的逆方差法分析了交叉对照试验,计算了二分类结局的优势比。
主要结果
我们在这个2016年更新版中没有发现任何新的试验。这个系统评价纳入了2个试验,但是我们分析时只纳入了1个试验,共24个女性。
因为方法学细节缺失,总的偏倚风险不清楚。使用GRADE方法评估,我们认为证据质量非常低。因为偏倚风险和不精确,我们认为证据级别很低(置信区间很宽,证据是来自于一个单组小样本试验)。
非甾体抗炎药(萘普生)对比安慰剂的比较没有表明对子宫内膜异位症女性止痛的积极作用(优势比3.27,95%置信区间0.61‐17.69;一个试验,24个女性;证据质量很低)该证据指出,当对比安慰剂时,服用非甾体抗炎药(萘普生)是否会更难获得额外止痛效果(优势比0.12,95%置信区间0.01‐1.29;一个试验,24个女性,证据质量非常低)或者经历副作用(优势比0.46,95%置信区间0.09‐2.47;一个试验,24个女性;证据质量非常低)是不确定的。
研究没有提供任何关于生活质量、对日常活动的影响、缺席工作和学校情况、需要更加侵入性的治疗、治疗参与满意度的数据。
作者结论
因为缺乏高质量证据和重要结局的报道,我们不能对非甾体抗炎药(萘普生)是否对治疗子宫内膜异位症疼痛有效下结论。没有证据表明是否某一种非甾体抗炎药优于另一种。和其他Cochrane系统评价一样,女性使用非甾体抗炎药必须知道这些药物可能会产生非预期效果。
PICO
Plain language summary
非甾体抗炎药控制子宫内膜异位症妇女疼痛。
问题是什么?
子宫内膜异位症是一个常见的妇科疾病,通常患于育龄期女性。它可以导致疼痛症状包括痛经,性交疼痛或性交后疼痛,盆腔疼痛,下腹疼痛和不孕。它会极大影响女性的生活质量,损害工作、日常活动、性关系、非性关系、生育力。非甾体抗炎药因为副作用少,通常是治疗子宫内膜异位症的一线用药,而且许多是非处方药。
为什么这个问题很重要?
子宫内膜异位症很常见,但是很难诊断。2015年,全世界有180万女性(15‐49岁)被诊断为子宫内膜异位症。据估计大约60%痛经的女性患有子宫内膜异位症。子宫内膜异位症极大影响了女性生活质量,因为它会损害工作、日常活动、性关系、非性关系和生育力。英国一个由病人组织的机构进行了一个尚未发表的调查——子宫内膜异位症 英国(www.endometriosis‐uk.org/)——发现65%的子宫内膜异位症女性承认她们的疾病负面的影响了她们的工作。其中10%的女性不得不减少工作时间,30%的女性已经不能继续从事原来的工作。多达16%的女性不能继续进行任何工作,6%的女性需要领取政府补助金;因此,她们除了作为社会贡献者的失落感以外,她们也变得更依赖他人。这让她们更加感觉失去自尊和自信。子宫内膜异位症已经被视为一个重要的公共卫生话题,因为很多女性患此病,而且和此病相关的疾病也很严重。
如果用于止痛,非甾体抗炎药很容易买到,不需要处方。它们起效是通过阻止或减缓前列腺素的生成,这可以帮助缓解子宫内膜异位症伴随的疼痛痉挛。然而,一个关于使用非甾体抗炎药治疗痛经的Cochrane系统评价发现,非甾体抗炎药会增加胃部不适(例如恶心,腹泻)或其他副作用(例如头痛,昏睡,眩晕,口干)的风险。我们进行本篇系统评价,比较所有非甾体抗炎药治疗子宫内膜异位症引起的疼痛症状,对比安慰剂、其他止痛药、或者无治疗,评估非甾体抗炎药的疗效和安全性。
我们发现了什么证据?
我们检索了到2016年的最新证据,没有发现新的随机对照试验。
之前的更新版表明非甾体抗炎药(特别是萘普生)控制子宫内膜异位症疼痛的证据很有限。这个系统评价也很局限,因为只纳入了一项研究的数据用来分析,而且这项研究只有20个女性。可获得的证据质量非常低,主要是因为方法学报道缺乏、关于疼痛缓解的结果缺乏精确度、非预期的副作用、需要额外的疼痛缓解治疗。纳入的试验没有报道生活质量、对日常活动的影响、缺席工作和学校情况、治疗的参与满意度。
这意味着什么?
可获得的证据不允许我们下明确结论说明非甾体抗炎药是否对治疗子宫内膜异位症疼痛有效,或者某一种非甾体抗炎药优于另一种。和其他Cochrane系统评价一样,使用非甾体抗炎药的女性必须知道非甾体抗炎药可能产生不良反应,例如恶心,呕吐,头痛和昏睡。除非我们将来发现新证据,我们不能再次更新这个系统评价。
证据质量
证据质量非常低,因为偏倚风险和不精确(结果来自于一个单组小样本试验)。
Authors' conclusions
Summary of findings
| NSAID compared with placebo for pain in women with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with NSAID | |||||
| Pain relief assessed with: overall pain relief score follow‐up: median 2 months | 50 per 100 | 77 per 100 | OR 3.27 | 24 | ⊕⊝⊝⊝ | |
| Unintended effects from treatment follow‐up: median 2 months | 58 per 100 | 39 per 100 | OR 0.46 | 24 | ⊕⊝⊝⊝ | |
| Quality of life: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Effects on daily activities: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Absence from work or school: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Number of women requiring more invasive treatment: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Requirements for additional medication follow‐up: median 2 months | 83 per 100 | 38 per 100 | OR 0.12 | 24 | ⊕⊝⊝⊝ | |
| Participant satisfaction with treatment: not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to overall unclear risk of bias for included trial. bDowngraded two levels for imprecision because confidence interval is wide, consistent with benefit and harm and evidence based on a single small trial. | ||||||
Background
Description of the condition
Endometriosis is defined as the presence of endometrial tissue (stromal and glandular) outside the normal uterine cavity (Barbieri 1990). Endometriosis is a common gynaecological condition that can lead to painful symptoms and infertility. Symptoms may include dysmenorrhoea (painful periods), dyspareunia (pain during or after sexual intercourse) and pelvic or lower abdominal pain. A less common symptom is cyclical pain at other sites related to endometriosis (Prentice 2003). Endometriosis can be divided into four stages of severity (stage I: minimal disease; stage IV: severe disease), as defined by the classification system of the American Fertility Society (Canavan 2000). Staging does not correlate with degree or severity of symptoms but quantifies the extent of disease visible at laparoscopy. The link between the pain experienced by women and the extent of endometriosis observed is not well understood, and the severity of the pain experienced does not always directly correlate with the severity of endometriosis (Kauppila 1985). Even when endometriosis is diagnosed, it may not be the cause of a woman's symptoms, as the mechanism by which pain is caused is not fully understood.
Endometriosis greatly affects women's quality of life, impacting their careers, everyday activities, sexual and nonsexual relationships and fertility (Davies 2003; Jones 2002). In an unpublished survey conducted by a patient support organisation in the United Kingdom ‐ Endometriosis UK (www.endometriosis‐uk.org/) ‐ 65% of women with endometriosis reported that their condition had adversely affected their employment. Ten per cent of women had to reduce their hours of work, and 30% had not been able to continue in the same employment. As many as 16% of women were unable to continue in any employment, and 6% needed to claim state benefits; thus, in addition to their feelings of loss as contributors to society, they became dependent upon others, which increased their feelings of low self‐esteem.
Prevalence
The exact prevalence of endometriosis is unknown. However, endometriosis is a significant problem for a great number of women and incurs high socioeconomic costs (Prentice 2003). Endometriosis primarily affects women of reproductive age (i.e. women who are menstruating). In women who experience no symptoms, the prevalence of endometriosis has been estimated to range from 2% to 22%, depending on the diagnostic criteria used and the populations studied. In women with painful periods, the prevalence of endometriosis ranges from 40% to 60%, and in women with subfertility, from 20% to 30%. The severity of symptoms and the probability of diagnosis increase with age, peaking at about 40 years of age (Berube 1998; Vessy 1992). In the year 2000, 1.5 billion women aged 15 to 49 years were living in the world. Prevalence of 1% means, therefore, that 15 million women worldwide could have endometriosis, assuming that 20% prevalence would indicate that 300 million women would have endometriosis. The actual figure must lie somewhere between these but is unknown, as the epidemiology of endometriosis is difficult to understand (Kennedy 2003).
Diagnosis
Laparoscopy is considered the 'gold standard' for diagnosis of endometriosis (Canavan 2000; rAFS 1985). However, endometriosis may also be diagnosed (or presumed) on the basis of a description of symptoms provided by a woman (and that may be suspected by the practitioner on the basis of history, pelvic examination and other tests such as ultrasound, magnetic resonance imaging (MRI) and the CA‐125 blood test). However, examination results, test findings and the presence or absence of a classic history and symptoms cannot confirm or rule out endometriosis.
Description of the intervention
The practitioner can treat symptoms of endometriosis in many ways, but treating the underlying disease often requires repeated medical or surgical interventions. Management of endometriosis is varied. Medical treatments for endometriosis include oral contraceptives, progestogens, testosterone derivatives and gonadotrophin‐releasing hormone (GnRH) agonists (Rice 2002). Surgical treatments include ablative techniques (destroying the endometriosis with energy such as laser or electricity) and excision (using scissors, electricity or laser). The latter surgical approach aims to relieve symptoms whilst conserving reproductive function. Practitioners may also perform more radical surgery in the form of hysterectomy, removal of the ovaries (oophorectomy) or both. Conventional medical and surgical treatments for endometriosis aim to remove or decrease deposits of ectopic endometrium (tissue like that lining the uterus but found outside the uterus) (Barbieri 1990). Nonsteroidal anti‐inflammatory drugs (NSAIDs) are used most commonly as simple first‐line treatment for women with endometriosis because they have few side effects, and many are available over the counter. They do not remove or decrease deposits of ectopic endometrium. NSAIDs may act on local cytokines within actual endometriotic deposits and may act as analgesics.
How the intervention might work
Nonsteroidal anti‐inflammatory drugs (NSAIDs) work by decreasing pain severity. NSAIDs (including cyclo‐oxygenase (COX)‐2 inhibitors) inhibit prostaglandin production. Prostaglandins are locally produced chemicals that are believed to be responsible for causing the pain of endometriosis. NSAIDs purchased over the counter may be taken in doses that are insufficient to relieve pain. However, NSAIDs taken in high doses have the potential to cause side effects. A Cochrane review of 31 studies that compared NSAIDs versus placebo for primary dysmenorrhoea found a statistically significantly increased risk of adverse effects in the gastrointestinal (e.g. nausea, diarrhoea) and nervous (e.g. headache, drowsiness, dizziness, dryness of the mouth) systems (Marjoribanks 2015). NSAIDs are analgesics that inhibit COX enzymes, thereby also inhibiting production of prostaglandins and alleviating cramps (Dawood 1986; Marjoribanks 2015). The first drug with this mode of action was aspirin (acetylsalicylic acid), which was introduced in 1899. However, the term 'NSAID' was not used until the 1950s, when phenylbutazone was developed. Since that time, NSAIDs have become more widely used (Hart 1984; Marjoribanks 2015).
Why it is important to do this review
Endometriosis is seen as a significant public health issue because a large number of women are affected and illnesses associated with this disease are significant (Murphy 2002). NSAIDs are widely used drugs that are readily available (both over the counter and by prescription). We conducted this review to compare all NSAIDs used to treat women with painful symptoms caused by endometriosis versus placebo, other pain management drugs or no treatment, to evaluate their effectiveness and safety.
Objectives
To assess effects of NSAIDs used for management of pain in women with endometriosis compared with placebo, other NSAIDs, other pain management drugs or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) describing use of NSAIDs to treat women of all ages with endometriosis. We included cross‐over trials, as the cross‐over is a valid design in this context.
Types of participants
We included women with endometriosis of any stage or severity. We diagnosed endometriosis by visualisation (e.g. laparoscopy, laparotomy), or we suspected the diagnosis on the basis of history and pelvic examination and other tests such as ultrasonography, MRI and the CA‐125 blood test. We excluded women with chronic pelvic pain that was known to be due to causes other than endometriosis.
Types of interventions
We included all RCTs involving NSAIDs as pain treatment for women with endometriosis versus placebo, other NSAIDs, other drug pain management approaches or no treatment. We considered RCTs describing NSAIDs of any type and administered at any dose, frequency or duration, or by any route of administration.
Types of outcome measures
We recorded data on each of the following outcomes as reported by included trials, when available.
Primary outcomes
-
Pain relief (measured by visual analogue scale (VAS) or another validated scale, or as a dichotomous outcome, e.g. improved, not improved)
-
Unintended effects of treatment (incidence and duration of total side effects and types of side effects)
Secondary outcomes
-
Quality of life (measured on a validated scale, e.g. Short Form (SF)‐36)
-
Effects on daily activities (measured as proportion of women who reported activity restriction)
-
Absence from work or school (measured as proportion of women reporting absences from work or school, and also as hours or days of absence as a more selective measure)
-
Number of women requiring more invasive treatment (e.g. laparoscopic surgery) and length of follow‐up
-
Requirements for additional medication (measured as proportion of women requiring analgesics (not NSAIDs) in addition to assigned treatment)
-
Participant satisfaction with treatment (measured as proportion of women who reported improvement and satisfaction with treatment)
Search methods for identification of studies
We searched for all published and unpublished RCTs on use of NSAIDs for management of pain in women with endometriosis, while applying no language restriction and working in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials (October 2016), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, and CINAHL to identify all publications which described, or might describe, randomised trials of any NSAID in the treatment of endometriosis. Search strategies are outlined in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6).
No new randomised controlled trials were identified in 2016.
Searching other resources
We also searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/network/en/index.html) and the US National Institutes of Health trial register (Clinicaltrials.gov; http://www.clinicaltrials.gov) for ongoing studies using the terms 'endometriosis' AND 'non steroidal anti inflammatory', OR 'NSAIDs'. We identified no ongoing trials that were relevant to this review. We checked references in relevant reports to identify additional studies.
Data collection and analysis
Selection of studies
In 2016, support staff from the Cochrane Gynaecology and Fertility Group screened the titles and abstracts of all retrieved records to identify trials for possible inclusion in the review. We obtained full copies of reports for each of the records not rejected and identified no new studies for inclusion.
Data extraction and management
In previous updates, two review authors (CA and SH) independently extracted data using a prespecified data extraction form and resolved disagreements by discussion. Information extracted from each included trial consisted of the following.
-
Characteristics of trial participants (including age, stage and severity of disease and method of diagnosis) and inclusion and exclusion criteria of the trial.
-
Type of intervention (including type, dose, duration and frequency of NSAID vs placebo; type, dose, duration and frequency of another NSAID; another pain management drug; or no treatment).
-
Type of outcome measure (including level of pain reduction, improvement in quality of life score (based on a validated scale), effects on daily activities, absence from work or school, length of follow‐up, unintended effects of treatment, number of women requiring more invasive treatment and length of follow‐up).
When data for a trial were insufficient or missing, we sought information from the named contact author of the trial. We attempted to contact Dr Kauppila but have been unable to elicit a response.
Assessment of risk of bias in included studies
For previous updates, two review authors used the Cochrane 'Risk of bias' tool (Higgins 2011) to independently assess included studies for the following risks of bias.
-
Selection (random sequence generation and allocation concealment).
-
Perfomance (blinding of participants and personnel).
-
Detection (blinding of outcome assessors).
-
Attrition (incomplete outcome data).
-
Reporting (selective reporting).
-
Other bias.
We assigned judgements as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011), and resolved disagreements by discussion. We described all judgements and presented all conclusions in the 'Risk of bias' table.
Measures of treatment effect
Both of the trials identified for inclusion in this review were cross‐over trials. Only one trial (Kauppila 1985) provided sufficient information for inclusion in a meta‐analysis. We analysed this trial using the method described by Elbourne and colleagues (Elbourne 2002), that is, by analysing information from both parts of the two‐period, two‐treatment cross‐over trial. For each binary outcome, we calculated the log odds ratio as a measure of different effects of the two treatments, along with its corresponding standard error. We then applied this information in the meta‐analysis using the inverse variance method available in RevMan.
Unit of analysis issues
We utilised the method described above to facilitate appropriate inclusion of cross‐over data in the meta‐analysis.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. by including all randomised participants in the analysis, in the groups to which they were randomised). We attempted to obtain missing data from the original trialists but could not obtain them.
Assessment of heterogeneity
We intended to consider whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We intended to assess statistical heterogeneity by using the measure of I2, with I2 greater than 50% taken to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we had included 10 or more studies in the analysis, we would have used a funnel plot to explore the possibility of small study effects (i.e. tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We carried out statistical analyses using Review Manager software (RevMan 2014) and a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses by analysing women with endometriosis and type of NSAID or type of diagnosis (by direct visualisation or just presumed), as data were insufficient. The decision about whether to combine the results of individual trials was dependent on assessment of heterogeneity. In the first instance, we assessed trials for clinical and methodological homogeneity. We had decided that when we judged trials to be sufficiently homogeneous, we would carry out a meta‐analysis of these trials and would investigate statistical heterogeneity in the event that we did not carry out the meta‐analysis.
Sensitivity analysis
We intended to conduct sensitivity analyses for the primary outcomes to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis, with regard to statistical model (fixed‐effect vs random‐effects) and choice of effect estimate (odds ratio vs risk ratio). However, data were insufficient for performance of such sensitivity analyses.
Results
Description of studies
Results of the search
In earlier updates of this review, searches identified 53 citations. Of these, we obtained full papers for eight possibly relevant trials. We identified only two trials that met the inclusion criteria for this review. We found one trial that was potentially relevant but decided to exclude it from the review. The other five possibly relevant trials investigated use of naproxen specifically for dysmenorrhoea, not for endometriosis. We identified no ongoing trials.
Included studies
The first trial (Kauppila 1979) was a two‐period, four‐treatment cross‐over trial that compared indomethacin (25 mg, three times per day), acetylsalicylic acid (500 mg, three times per day), tolfenamic acid (200 mg, three times per day) and placebo (three times per day) in 24 women with symptomatic endometriosis (stage and severity not described). Each woman received each of the four drugs for two menstrual cycles, but how the women were randomised was unclear. Diagnosis of endometriosis was based on laparoscopic results or pelvic examination findings.
The second trial (Kauppila 1985) was a two‐period, two‐treatment cross‐over trial that compared naproxen sodium (275 mg, four times per day) with placebo (four times per day) in 24 women with endometriosis as classified by the American Fertility Society (mild endometriosis, n = 7; moderate endometriosis, n = 8; severe endometriosis, n = 6). Diagnosis was based on pelvic examination, history of menstrual distress or direct visualisation of pelvic regions at laparoscopy or laparotomy. Each woman received naproxen sodium for two menstrual cycles followed by placebo for two menstrual cycles, or placebo for two menstrual cycles followed by naproxen sodium for two menstrual cycles.
Excluded studies
Review authors excluded a trial (Cobellis 2004) that assessed use of a COX‐2‐specific inhibitor (rofecoxib) for management of pain related to endometriosis. However, this drug was withdrawn from the marketplace in November 2004 on the grounds of safety; therefore, it is inappropriate to assess the efficacy of the product in this review. If the drug is re‐launched, we will review this decision at the time the review would be updated.
Risk of bias in included studies
We summarised information on potential risk of bias for each of the included studies in a 'Risk of bias' table and in an overall summary presented in Figure 1 and Figure 2.
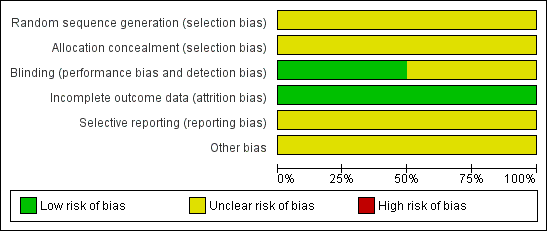
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Neither of the included trials (Kauppila 1979; Kauppila 1985) provided information on the method of randomisation or concealment of allocation sequence. One trial (Kauppila 1985) described how each woman received naproxen sodium for two menstrual cycles followed by placebo for two menstrual cycles, or placebo for two menstrual cycles followed by naproxen sodium for two menstrual cycles. In Kauppila 1979, each woman received each of four drugs (indomethacin, acetylsalicylic acid, tolfenamic acid and placebo) for two menstrual cycles, but how the women were randomised remains unclear. We rated both studies as having unclear risk of selection bias.
Blinding
Investigators described both of the trials (Kauppila 1979; Kauppila 1985) as double‐blind and specifically mentioned that drugs were dispensed in identical capsules in one trial (Kauppila 1985). Researchers in both trials provided no information on blinding of outcome assessors or data analysers. We considered Kauppila 1985 to be at low risk for this bias, and Kauppila 1979 to be at unclear risk.
Incomplete outcome data
In Kauppila 1979, researchers randomised 24 women but included only 18 in the analysis. Trialists did not provide reasons for loss to follow‐up. In Kauppila 1985, investigators randomised 24 women and included only 20 in the analysis. Reasons given for loss to follow‐up included pregnancy (n = 1), psychiatric problems (n = 1) and unknown reasons (n = 2).
Kauppila 1979 presented data graphically for each menstrual cycle (144 cycles), not per woman included in the trial. Therefore, it was not possible to link each of the menstrual cycles from one treatment with the corresponding menstrual cycle from an alternative treatment. It also was not possible to link the two menstrual cycles for each woman with each treatment. It appears as though the study authors, in carrying out their analysis, did not account for pairing within each treatment group. For these reasons, we did not include this trial in the overall analysis.
We rated both studies as having low risk of attrition bias.
Selective reporting
We judged both included trials (Kauppila 1979; Kauppila 1985) as having unclear risk of bias because neither trial clearly prespecified primary and secondary outcomes.
Other potential sources of bias
We judged both included trials as having unclear risk of bias owing to insufficient information to enable a judgement of low risk of bias.
Effects of interventions
Comparison of naproxen sodium versus placebo
Primary outcomes
Overall pain relief
One trial reported data on overall pain relief (Kauppila 1985). Whether results showed a difference between naproxen sodium (275 mg, four times a day) and placebo in producing excellent to moderate relief of pain caused by endometriosis was not clear (odds ratio (OR) 3.27, 95% CI 0.61 to 17.69; one RCT, 24 women; very low‐quality evidence).
Unintended effects of treatment
One trial reported data on unintended effects of treatment (Kauppila 1985). Whether results showed a difference between women taking naproxen sodium and experiencing unintended effects of treatment and those taking placebo and experiencing unintended effects of treatment is unclear (OR 0.46, 95% CI 0.09 to 2.47; one RCT, 24 women; very low‐quality evidence). Unintended effects of treatment reported with naproxen sodium (n = 4) included fatigue, light‐headedness, eye lid swelling and chest pain. Unintended effects of treatment reported with placebo (n = 7) included hypomenorrhoea (loss of a small amount of menstrual blood with still regular menstrual cycles), diarrhoea, increased diuresis, headache, epigastric pain, nausea and vomiting, tremor and dizziness.
Secondary outcomes
Requirement for additional medication
One trial (Kauppila 1985) provided data on the need for supplementary analgesia. Whether results showed a difference between women taking naproxen sodium and requiring supplementary analgesia and those taking placebo and requiring supplementary analgesia (OR 0.12, 95% CI 0.01 to 1.29; one RCT, 24 women) for pain caused by endometriosis is unclear.
The included trial did not report on the other secondary outcomes of this review (quality of life, effects on daily activities, absence from work or school, number of women requiring more invasive treatment, participant satisfaction with treatment).
Discussion
Summary of main results
Despite rigorous searches, we identified only two randomised controlled trials (RCTs) comparing nonsteroidal anti‐inflammatory drugs (NSAIDs) versus placebo for treatment of women with pain associated with endometriosis. Whether results showed a difference between NSAIDs and placebo for overall pain relief, unintended effects of treatment or requirement for additional medication remains unclear (summary of findings Table for the main comparison). Investigators provided no data on the other secondary outcomes of this review (quality of life, effects on daily activities, absence from work or school, number of women requiring more invasive treatment, participant satisfaction with treatment). The evidence is surprising, given that NSAIDs are widely prescribed and are bought over the counter for management of pain caused by endometriosis. In comparison, much of the literature suggests that NSAIDs can be used as treatment for women with primary dysmenorrhoea. It is likely that prostaglandins are involved in causing pain in both groups of patients. A recent Cochrane review (Marjoribanks 2015) presented evidence indicating that NSAIDs provide effective treatment for women with pain caused by primary dysmenorrhoea, although women taking NSAIDs must be aware of the risk of unintended effects of treatment.
Overall completeness and applicability of evidence
Owing to the lack of RCTs undertaken to explore the use of NSAIDs as treatment for women with pain associated with endometriosis, we are unable to comment on many of the outcomes that are important to women who have endometriosis, such as quality of life, effects on daily activities and absence from school or work. We assessed only four NSAIDs (naproxen sodium, indomethacin, acetylsalicylic acid and tolfenamic acid) for the management of pain associated with endometriosis (Kauppila 1985; Kauppila 1979). One trial (Kauppila 1979) analysed three of these NSAIDs (indomethacin, acetylsalicylic acid and tolfenamic acid), but we did not analyse study findings in this review, as the study had serious methodological flaws. However, many other prescribed and over‐the‐counter NSAIDs are available. We found no evidence to support use of these to control pain caused by endometriosis.
The only remaining trial (Kauppila 1985) included and analysed in this review provided no evidence of an effect when comparing NSAIDs (naproxen sodium) versus placebo for the management of pain caused by endometriosis. Evidence was inconclusive to show whether women taking NSAIDs were less likely to take supplementary analgesia than those taking placebo. We believe that this most likely occurred because the trial was very small (randomising only 24 women); therefore, we would recommend caution when these results are applied to a larger population.
Evidence was also inconclusive to show whether women taking NSAIDs were more likely to experience unintended effects of treatment compared with women taking placebo. Four women experienced unintended effects of treatment while taking naproxen sodium compared with seven women who experienced these effects while taking placebo. Unintended effects reported when women were randomised to placebo may have been related to the pain caused by endometriosis, rather than to the placebo itself. However, drawing firm conclusions is difficult because this trial included a small number of women.
Quality of the evidence
We judged the risk of bias to be unclear owing to lack of methodological detail. We used the GRADE method and judged that the overall quality of the evidence was very low owing to unclear risk of bias, imprecision with wide confidence intervals and evidence derived from a single small study (summary of findings Table for the main comparison).
Potential biases in the review process
The review authors believe that we have minimised potential biases in the review process by searching published and unpublished literature with no restrictions on date of publication or language. We were unable to judge the potential effect of publication bias, as we identified fewer than 10 trials.
Agreements and disagreements with other studies or reviews
Despite the findings reported in this review, we believe it is important to highlight the findings of another Cochrane review on NSAIDs for management of pain caused by primary dysmenorrhoea (Marjoribanks 2015), which showed that NSAIDs appear to provide very effective treatment for women with dysmenorrhoea, but evidence is insufficient to show which (if any) individual NSAID is most safe and effective.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
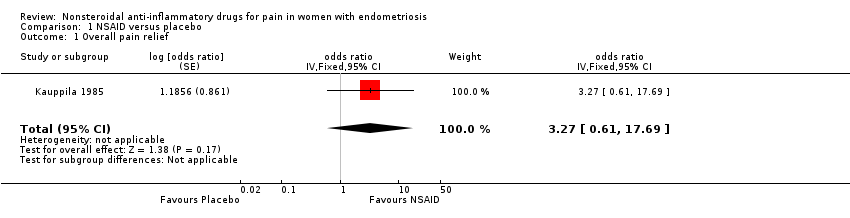
Comparison 1 NSAID versus placebo, Outcome 1 Overall pain relief.
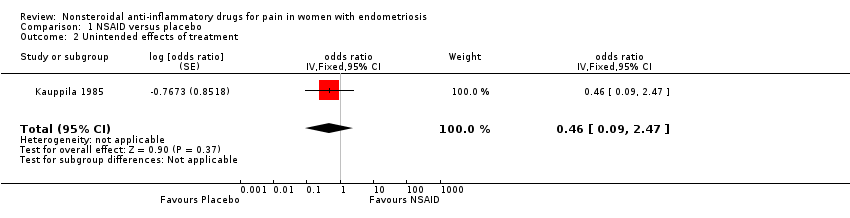
Comparison 1 NSAID versus placebo, Outcome 2 Unintended effects of treatment.

Comparison 1 NSAID versus placebo, Outcome 3 Requirements for additional medication.
| NSAID compared with placebo for pain in women with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with NSAID | |||||
| Pain relief assessed with: overall pain relief score follow‐up: median 2 months | 50 per 100 | 77 per 100 | OR 3.27 | 24 | ⊕⊝⊝⊝ | |
| Unintended effects from treatment follow‐up: median 2 months | 58 per 100 | 39 per 100 | OR 0.46 | 24 | ⊕⊝⊝⊝ | |
| Quality of life: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Effects on daily activities: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Absence from work or school: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Number of women requiring more invasive treatment: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Requirements for additional medication follow‐up: median 2 months | 83 per 100 | 38 per 100 | OR 0.12 | 24 | ⊕⊝⊝⊝ | |
| Participant satisfaction with treatment: not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to overall unclear risk of bias for included trial. bDowngraded two levels for imprecision because confidence interval is wide, consistent with benefit and harm and evidence based on a single small trial. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall pain relief Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 3.27 [0.61, 17.69] | |
| 2 Unintended effects of treatment Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.46 [0.09, 2.47] | |
| 3 Requirements for additional medication Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.12 [0.01, 1.29] | |

