双胎妊娠女性の早産を減らすための経口β刺激薬による予防投与
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Blinding of intervention: yes. | |
| Participants | Country: Zimbabwe. | |
| Interventions | Salbutamol 4 mg 4 times a day vs placebo. | |
| Outcomes | Incidence of | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coding for salbutamol or placebo was computer‐randomised. |
| Allocation concealment (selection bias) | Low risk | The code was placed in a sealed envelope. |
| Blinding of participants and personnel (performance bias) | Low risk | Neither patients nor researchers knew the code. |
| Blinding of outcome assessment (detection bias) | Low risk | Neither patients nor researchers knew the code. |
| Incomplete outcome data (attrition bias) | Low risk | 6 from the salbutamol group and 10 from the control group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | Salbutamol was supplied by industry sponsorship, but none of the authors affiliated with the company. Negative results were reported. |
| Methods | Blinding of intervention: yes. | |
| Participants | Country: USA. | |
| Interventions | Ritodrine vs placebo. | |
| Outcomes | Incidence of | |
| Notes | No outcome data were shown in this preliminary report of 30 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) | Unclear risk | No report on complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The preliminary results were not significant, but no report on complete follow‐up. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
| Methods | Blinding of intervention: yes. | |
| Participants | Country: South Africa. | |
| Interventions | Fenoterol 5 mg once a day vs placebo. | |
| Outcomes | Incidence of | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Administration of the drug was "double blind". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double blind” and two independent observers assessed each infant. |
| Incomplete outcome data (attrition bias) | Low risk | 46 women completed the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
| Methods | Blinding of intervention: yes. | |
| Participants | Country: England. | |
| Interventions | Isoxuprine 30 mg 4 times a day vs placebo. | |
| Outcomes | Incidence of | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Low risk | Full treatments were made up in advance and dispensed when prescribed according to a random schedule so that neither patient nor doctor knew whether Isoxuprine or the placebo had been given. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) | Low risk | The primary outcome was reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | The outcome measurement was not predefined. |
| Other bias | Low risk | Isoxuprine was supplied by industry sponsorship, but none of the authors affiliated with the company. Negative results were reported. |
| Methods | Blinding of intervention: yes. | |
| Participants | Country: Ireland. | |
| Interventions | Ritodrine 10 mg every 6 hours vs placebo. | |
| Outcomes | Incidence of | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Low risk | Patients were given a coded bottle of 100 tablets. The tablets contained either 10 mg of ritodrine hydrochloride or an inert placebo of identical appearance. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) | Low risk | There were 50 children in the intervention group and 48 children in the control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
| Methods | Blinding of intervention: yes. | |
| Participants | Country: Sweden. | |
| Interventions | Terbutaline 5 mg 3 times a day vs placebo. | |
| Outcomes | Incidence of | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) | Low risk | The study included 50 twin pregnancies. Two interrupted the treatment, one in the terbutaline group and one in the placebo group, both of them because of tachycardia and tremor. They were delivered in the 38th and 40th week, respectively. |
| Selective reporting (reporting bias) | Unclear risk | The outcome measurement was not predefined. |
| Other bias | Unclear risk | The conflict of interest was not declared. |
vs: versus
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Trial tested the addition of a betamimetic agent to cervical cerclage. | |
| Trial of maintenance tocolytic therapy. | |
| Women eligible for trial entry included triplet pregnancies. | |
| Trial of maintenance tocolytic therapy. | |
| Trial tested the effects of routine hospitalisation compared with selective hospitalisation in women receiving ritodrine. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm labour Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.78] |
| Analysis 1.1  Comparison 1 Oral betamimetic versus placebo, Outcome 1 Preterm labour. | ||||
| 2 Prelabour rupture of membranes Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.82] |
| Analysis 1.2 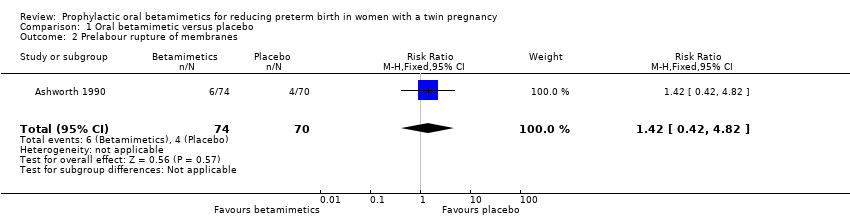 Comparison 1 Oral betamimetic versus placebo, Outcome 2 Prelabour rupture of membranes. | ||||
| 3 Preterm birth (less than 37 weeks' gestation) Show forest plot | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.10] |
| Analysis 1.3  Comparison 1 Oral betamimetic versus placebo, Outcome 3 Preterm birth (less than 37 weeks' gestation). | ||||
| 4 Preterm birth (less than 34 weeks' gestation) Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.50] |
| Analysis 1.4 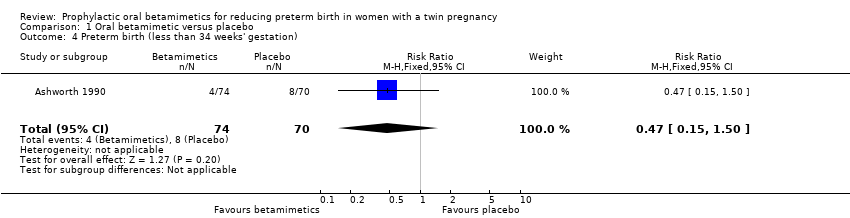 Comparison 1 Oral betamimetic versus placebo, Outcome 4 Preterm birth (less than 34 weeks' gestation). | ||||
| 5 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Oral betamimetic versus placebo, Outcome 5 Neonatal mortality. | ||||
| 5.1 Assuming independence between twins | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.15, 5.37] |
| 5.2 Assuming complete correlation between twins | 3 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.38] |
| 6 Low birthweight (less than 2500 g) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6 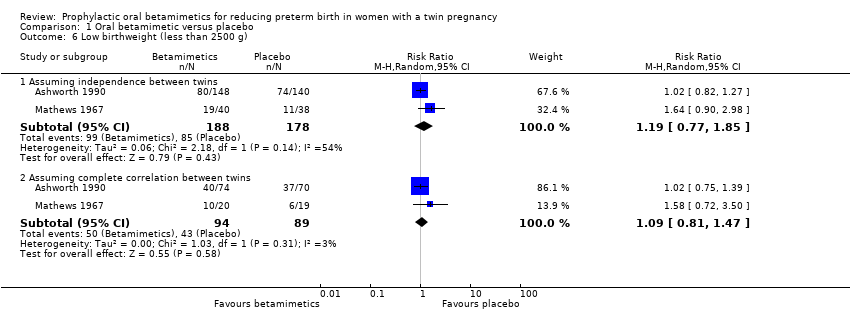 Comparison 1 Oral betamimetic versus placebo, Outcome 6 Low birthweight (less than 2500 g). | ||||
| 6.1 Assuming independence between twins | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.85] |
| 6.2 Assuming complete correlation between twins | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 7 Small‐for‐gestational age (birthweight less than 10th centile) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 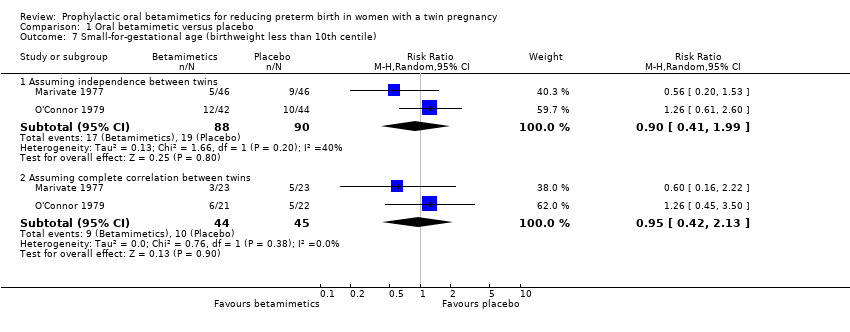 Comparison 1 Oral betamimetic versus placebo, Outcome 7 Small‐for‐gestational age (birthweight less than 10th centile). | ||||
| 7.1 Assuming independence between twins | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.99] |
| 7.2 Assuming complete correlation between twins | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.42, 2.13] |
| 8 Birthweight (not prespecified) Show forest plot | 3 | 478 | Mean Difference (IV, Fixed, 95% CI) | 111.22 [22.21, 200.24] |
| Analysis 1.8 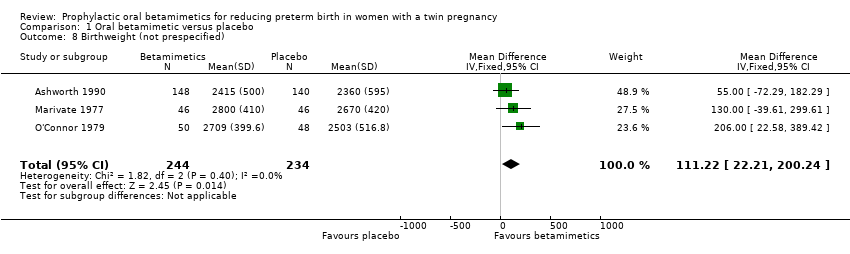 Comparison 1 Oral betamimetic versus placebo, Outcome 8 Birthweight (not prespecified). | ||||
| 9 Respiratory distress syndrome Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 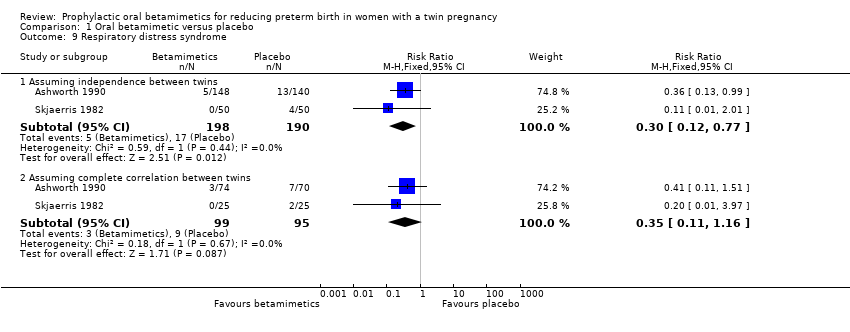 Comparison 1 Oral betamimetic versus placebo, Outcome 9 Respiratory distress syndrome. | ||||
| 9.1 Assuming independence between twins | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.77] |
| 9.2 Assuming complete correlation between twins | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.11, 1.16] |
| 10 Maternal death Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 68.57] |
| Analysis 1.10 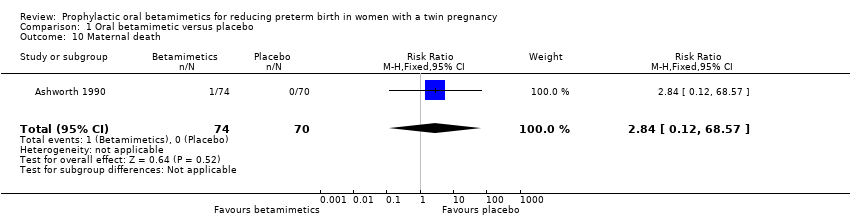 Comparison 1 Oral betamimetic versus placebo, Outcome 10 Maternal death. | ||||
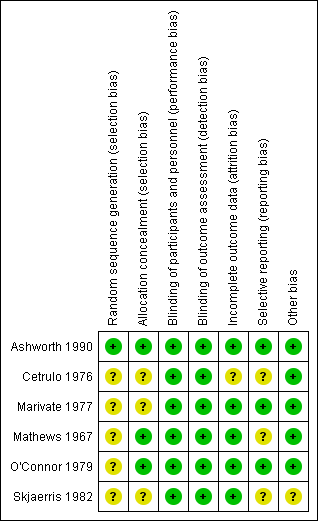
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
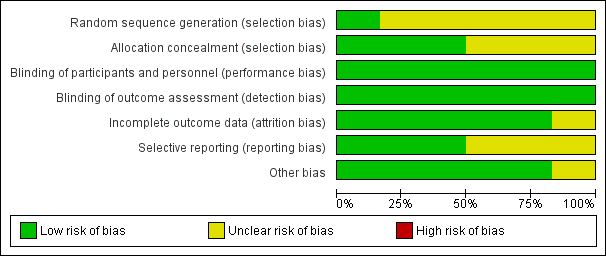
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
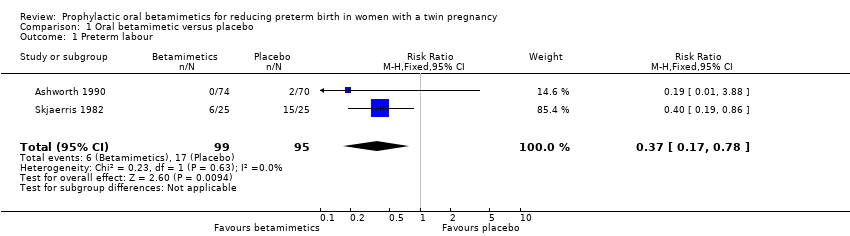
Comparison 1 Oral betamimetic versus placebo, Outcome 1 Preterm labour.
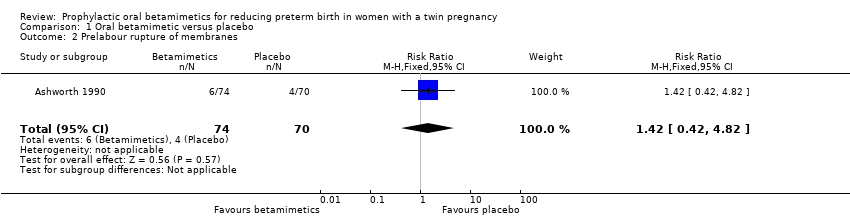
Comparison 1 Oral betamimetic versus placebo, Outcome 2 Prelabour rupture of membranes.

Comparison 1 Oral betamimetic versus placebo, Outcome 3 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 4 Preterm birth (less than 34 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 5 Neonatal mortality.
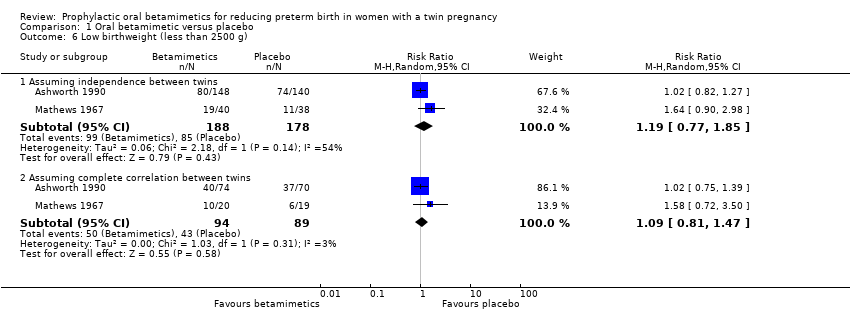
Comparison 1 Oral betamimetic versus placebo, Outcome 6 Low birthweight (less than 2500 g).

Comparison 1 Oral betamimetic versus placebo, Outcome 7 Small‐for‐gestational age (birthweight less than 10th centile).

Comparison 1 Oral betamimetic versus placebo, Outcome 8 Birthweight (not prespecified).

Comparison 1 Oral betamimetic versus placebo, Outcome 9 Respiratory distress syndrome.
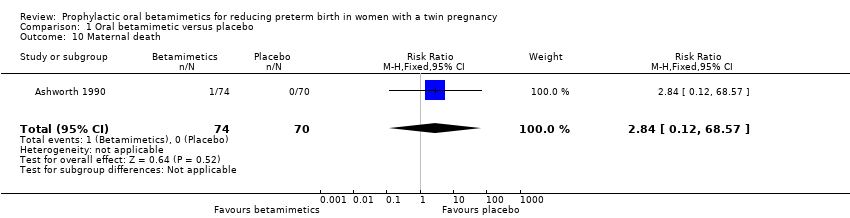
Comparison 1 Oral betamimetic versus placebo, Outcome 10 Maternal death.
| Oral betamimetic versus placebo for pregnant women with a twin pregnancy to prevent preterm birth | ||||||
| Patient or population: pregnant women with a twin pregnancy Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral betamimetic versus placebo | |||||
| Preterm labour | Study population | RR 0.37 | 194 | ⊕⊕⊝⊝ | ||
| 179 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 116 per 1000 | |||||
| Prelabour rupture of membranes | Study population | RR 1.42 | 144 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 81 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.85 | 276 | ⊕⊕⊝⊝ | ||
| 478 per 1000 | 406 per 1000 | |||||
| Moderate | ||||||
| 427 per 1000 | 363 per 1000 | |||||
| Very preterm birth (less than 34 weeks' gestation) | Study population | RR 0.47 | 144 | ⊕⊕⊝⊝ | ||
| 114 per 1000 | 54 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm labour Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.78] |
| 2 Prelabour rupture of membranes Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.82] |
| 3 Preterm birth (less than 37 weeks' gestation) Show forest plot | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.10] |
| 4 Preterm birth (less than 34 weeks' gestation) Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.50] |
| 5 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Assuming independence between twins | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.15, 5.37] |
| 5.2 Assuming complete correlation between twins | 3 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.38] |
| 6 Low birthweight (less than 2500 g) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Assuming independence between twins | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.85] |
| 6.2 Assuming complete correlation between twins | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 7 Small‐for‐gestational age (birthweight less than 10th centile) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Assuming independence between twins | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.99] |
| 7.2 Assuming complete correlation between twins | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.42, 2.13] |
| 8 Birthweight (not prespecified) Show forest plot | 3 | 478 | Mean Difference (IV, Fixed, 95% CI) | 111.22 [22.21, 200.24] |
| 9 Respiratory distress syndrome Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Assuming independence between twins | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.77] |
| 9.2 Assuming complete correlation between twins | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.11, 1.16] |
| 10 Maternal death Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 68.57] |

