Administration orale de bêtamimétiques pour réduire les accouchements prématurés chez les femmes ayant une grossesse gémellaire
Appendices
Appendix 1. Search strategy
Authors wrote and ran:
MEDLINE (OvidSP) (January 1966 to 31 July 2015)
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp Pregnancy, Twin/
11. (twin adj6 pregnanc$).mp.
12. (twin$).mp.
13. 10 or 11 or 12
14. exp Adrenergic beta‐Agonists/
15. (adrenergic adj6 beta$).mp.
16. betamimetics.mp.
17. 14 or 15 or 16
18. 9 and 13 and 17
EMBASE (OvidSP) (EMBASE (January 1985 to 31 July 2015)
1. randomized controlled trial/
2. controlled clinical trial/
3. random*.tw.
4. placebo*.tw.
5. assign*.tw.
6. allocat*.tw.
7. (doubl* near/6 blind*).tw.
8. (singl* near/6 blind*).tw.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp Pregnancy, Twin/
11. (twin near/6 pregnanc*).tw.
12. (twin*).tw.
13. 10 or 11 or 12
14. exp Adrenergic beta‐Agonists/
15. (adrenergic near/6 beta*).tw.
16. betamimetics.tw.
17. 14 or 15 or 16
18. 9 and 13 and 17
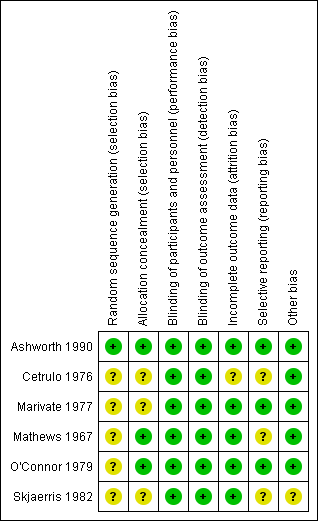
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
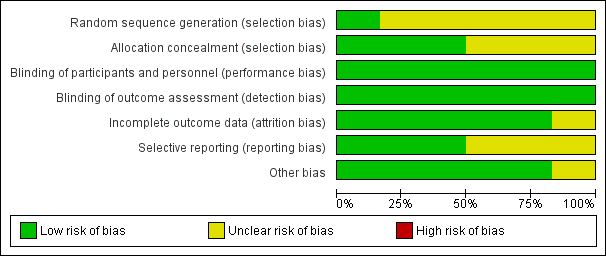
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
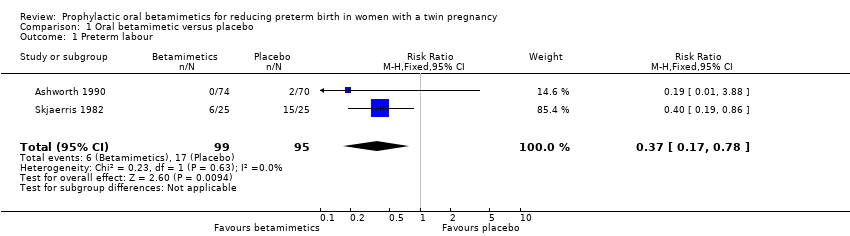
Comparison 1 Oral betamimetic versus placebo, Outcome 1 Preterm labour.
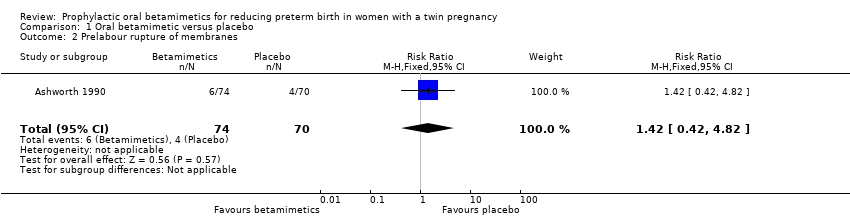
Comparison 1 Oral betamimetic versus placebo, Outcome 2 Prelabour rupture of membranes.

Comparison 1 Oral betamimetic versus placebo, Outcome 3 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 4 Preterm birth (less than 34 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 5 Neonatal mortality.
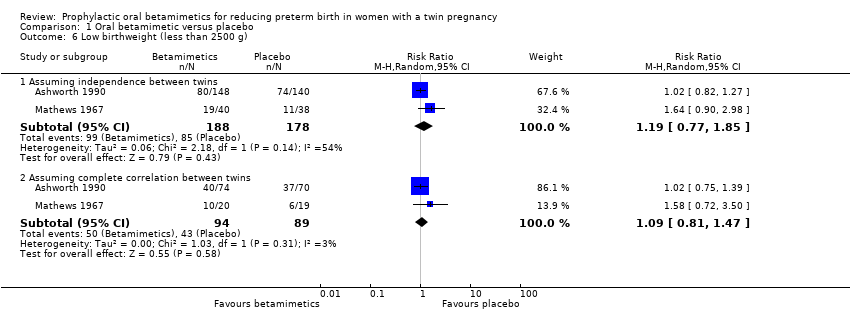
Comparison 1 Oral betamimetic versus placebo, Outcome 6 Low birthweight (less than 2500 g).

Comparison 1 Oral betamimetic versus placebo, Outcome 7 Small‐for‐gestational age (birthweight less than 10th centile).

Comparison 1 Oral betamimetic versus placebo, Outcome 8 Birthweight (not prespecified).

Comparison 1 Oral betamimetic versus placebo, Outcome 9 Respiratory distress syndrome.
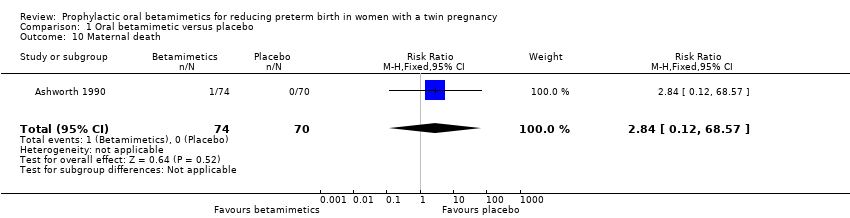
Comparison 1 Oral betamimetic versus placebo, Outcome 10 Maternal death.
| Oral betamimetic versus placebo for pregnant women with a twin pregnancy to prevent preterm birth | ||||||
| Patient or population: pregnant women with a twin pregnancy Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral betamimetic versus placebo | |||||
| Preterm labour | Study population | RR 0.37 | 194 | ⊕⊕⊝⊝ | ||
| 179 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 116 per 1000 | |||||
| Prelabour rupture of membranes | Study population | RR 1.42 | 144 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 81 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.85 | 276 | ⊕⊕⊝⊝ | ||
| 478 per 1000 | 406 per 1000 | |||||
| Moderate | ||||||
| 427 per 1000 | 363 per 1000 | |||||
| Very preterm birth (less than 34 weeks' gestation) | Study population | RR 0.47 | 144 | ⊕⊕⊝⊝ | ||
| 114 per 1000 | 54 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm labour Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.78] |
| 2 Prelabour rupture of membranes Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.82] |
| 3 Preterm birth (less than 37 weeks' gestation) Show forest plot | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.10] |
| 4 Preterm birth (less than 34 weeks' gestation) Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.50] |
| 5 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Assuming independence between twins | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.15, 5.37] |
| 5.2 Assuming complete correlation between twins | 3 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.38] |
| 6 Low birthweight (less than 2500 g) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Assuming independence between twins | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.85] |
| 6.2 Assuming complete correlation between twins | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 7 Small‐for‐gestational age (birthweight less than 10th centile) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Assuming independence between twins | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.99] |
| 7.2 Assuming complete correlation between twins | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.42, 2.13] |
| 8 Birthweight (not prespecified) Show forest plot | 3 | 478 | Mean Difference (IV, Fixed, 95% CI) | 111.22 [22.21, 200.24] |
| 9 Respiratory distress syndrome Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Assuming independence between twins | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.77] |
| 9.2 Assuming complete correlation between twins | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.11, 1.16] |
| 10 Maternal death Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 68.57] |

