Estrógenos para la esquizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: tardive dyskinesia, 8/10 completers also had diagnosis of schizophrenia or schizoaffective disorder. | |
| Interventions | 1. Conjugated estrogen (Premarin): dose 1.25mg, oral. N=6. | |
| Outcomes | Adverse effects: AIMS. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised (in blocks of 2 and 4 by site). | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Estradiol (Estrace): dose 1 mg and medroxyprogesterone acetate (Provera): dose 2.5mg, oral. N=5. | |
| Outcomes | Mental state: PANSS. Unable to use ‐ | |
| Notes | * "Some patients may have been aware of status." ‐ Direct from Dr Good. No testing for double blindness was conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised (using a randomly generated list of numbers). | |
| Participants | Diagnosis: schizophrenia and related psychosis. | |
| Interventions | 1. Ethinylestradiol: dose 0.02mg, oral, and antipsychotic medication. N=11. | |
| Outcomes | Mental state: BPRS, SAPS and SANS. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised (allocated by giving each person identification number, this number was put into a computer that generated a "pseudo‐random" code determining group of allocation, only statistician and pharmacist could break code). | |
| Participants | Diagnosis: schizophrenia, schizophreniform or schizoaffective. | |
| Interventions | 1. Estradiol: dose 50mcg per 24h, transdermal. N=12. | |
| Outcomes | Mental State: PANSS. Unable to use ‐ | |
| Notes | * Conference abstract 2001b describes identical trial with N=44. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: active phase illness and met DSM IV criteria for schizophrenia. | |
| Interventions | 1. Conjugated estrogen: 0.625mg, oral. N=21. | |
| Outcomes | Mental state: BPRS and NSRS. Unable to use ‐ Simpson Angus Extrapyramidal Rating Scale and UKU side effects rating scale BPRS, Negative Symptoms Rating Scale, | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
AIMS ‐ Abnormal Involuntary Movements.
AVLT ‐ Rey Auditory Verbal Learning Test.
BPRS ‐ Brief Psychiatric Rating Scale.
BVRT ‐ Benton Visual Retention Test.
CGI ‐ Clinical Global Impression.
COWA ‐Controlled Oral Word Association.
GAF ‐ Global Assessment of Function.
PANSS ‐ Positive and Negative Symptom Scores.
SAPS ‐ Scale for Assessment of Positive Symptoms.
SANS ‐ Scale for Assessment of Negative Symptoms.
SAERS ‐ Simpson Angus Extrapyramidal Rating Scale
UKU ‐ side effects rating scale
UPSIT‐ University of Pennsylvania Smell Identification Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: placebo controlled, double blind, cross‐over design. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: unclear, "controlled trial" patients "divided" into treatment and non‐treatment groups. | |
| Allocation: unclear, "double blind crossover". | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised, crossover. | |
| Allocation: randomised. | |
| Allocation: people selected at random but unclear how treatments were allocated. | |
| Allocation: not randomised, survey. | |
| Allocation: unclear, unlikely randomised, stratified by age into groups and then divided into two treatment groups. Helpful contact with author. | |
| Allocation: randomised, crossover. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Unknown. |
| Methods | |
| Participants | Diagnosis: schizophrenia. |
| Interventions | Olanzapine plus: |
| Outcomes | Psychopathology. |
| Starting date | Unclear |
| Contact information | Prof Jayashri Kulkarni |
| Notes | Allocation: randomised. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||
| 1 Mental state: 1a. Average endpoint in general mental state scores (PANSS total, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 1 Mental state: 1a. Average endpoint in general mental state scores (PANSS total, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 1.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐15.44, 10.92] | ||||||||||||||||||||||||||||||||||||||||
| 1.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐14.60, 5.36] | ||||||||||||||||||||||||||||||||||||||||
| 2 Mental state: 1b. Average endpoint in general mental state scores (BPRS, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 2 Mental state: 1b. Average endpoint in general mental state scores (BPRS, skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 3 Mental state: 2a. Average endpoint in positive symptom scores (PANSS positive, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 3 Mental state: 2a. Average endpoint in positive symptom scores (PANSS positive, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 4 Mental state: 2b. Average endpoint in positive symptom scores ‐ 50mcg estrogen (PANSS positive, high=poor) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐5.36, 4.62] | ||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 4 Mental state: 2b. Average endpoint in positive symptom scores ‐ 50mcg estrogen (PANSS positive, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 5 Mental state: 2c. Average endpoint in positive symptom scores (SAPS, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 5 Mental state: 2c. Average endpoint in positive symptom scores (SAPS, skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 6 Mental state: 3a. Average endpoint in negative symptom scores (PANSS negative, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 6 Mental state: 3a. Average endpoint in negative symptom scores (PANSS negative, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 6.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐3.65, 2.63] | ||||||||||||||||||||||||||||||||||||||||
| 6.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐4.73, 0.31] | ||||||||||||||||||||||||||||||||||||||||
| 7 Mental state: 3b. Average endpoint in negative symptom scores (NSRS,skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 7 Mental state: 3b. Average endpoint in negative symptom scores (NSRS,skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 8 Mental state: 4a. Average endpoint in psychopathology scores (PANSS general symptoms subscale, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 8 Mental state: 4a. Average endpoint in psychopathology scores (PANSS general symptoms subscale, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 8.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐7.88, 6.22] | ||||||||||||||||||||||||||||||||||||||||
| 8.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.04 [‐7.01, 2.93] | ||||||||||||||||||||||||||||||||||||||||
| 9 Leaving the study early: up to 8 weeks Show forest plot | 4 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.15, 6.07] | ||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 9 Leaving the study early: up to 8 weeks. | ||||||||||||||||||||||||||||||||||||||||||||
| 10 Adverse effects: 1. Average endpoint movement disorder scores (skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.10
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 10 Adverse effects: 1. Average endpoint movement disorder scores (skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| 10.1 Abnormal Involuntary Movements Scale | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 10.2 Simpson & Angus Extrapyramidal Rating Scale | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| 11 Adverse effects: 2. Average endpoint adverse side‐effect scores (UKU, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.11
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 11 Adverse effects: 2. Average endpoint adverse side‐effect scores (UKU, skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Mental state: Average endpoint general mental state scores (PANSS, high= poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 1 Mental state: Average endpoint general mental state scores (PANSS, high= poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 PANSS postive symptom scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.52, 6.52] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 PANSS negative symptom scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐17.11, ‐0.89] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 PANSS psychopathology scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐14.30 [‐29.31, 0.71] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 PANSS total scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐25.30 [‐50.74, 0.14] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Leaving the study early ‐ up to 6 months Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 2 Leaving the study early ‐ up to 6 months. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Cognitive functioning: Average endpoint specific aspects of cognitive functioning Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 3 Cognitive functioning: Average endpoint specific aspects of cognitive functioning. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Visual Retention Test (BVRT total, high=good) | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐5.73, ‐1.27] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Visual Retention Test (BVRT errors, high=poor) | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐17.62, ‐6.38] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 Motor speed (Finger tapping dominant hand, high=good) | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐19.22, 9.02] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 Motor speed (Finger tapping non‐dominant hand, high=good) | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐23.72, 8.32] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Cognitive functioning: Average endpoint specific aspects of cognitive functioning Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 4 Cognitive functioning: Average endpoint specific aspects of cognitive functioning. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Motor dexterity (Grooved Pegboard, high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Design fluency (Design Fluency Test, high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Verbal Fluency (COWA,high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 Auditory‐Verbal Learning Test (AVLT trial, high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.5 Smell identification (UPSIT, high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.6 Smell sensitivity (Acuity, high=good) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 1 Mental state: 1a. Average endpoint in general mental state scores (PANSS total, high=poor).
| Study | Intervention | Mean | SD | N | Notes |
| Louza 2004 | Conjugated estrogens | 7.95 | 7.10 | 21 | |
| Louza 2004 | Placebo | 12.89 | 9.68 | 19 | |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 2 Mental state: 1b. Average endpoint in general mental state scores (BPRS, skewed data, high=poor).
| Study | Intervention | N | Mean | SD |
| Kulkarni 2001 | Estrogen 100mcg | 12 | 14.45 | 7.4 |
| Kulkarni 2001 | Placebo | 12 | 15.37 | 5.5 |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 3 Mental state: 2a. Average endpoint in positive symptom scores (PANSS positive, high=poor).

Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 4 Mental state: 2b. Average endpoint in positive symptom scores ‐ 50mcg estrogen (PANSS positive, high=poor).
| Study | Intervention | Mean | SD | N |
| Kulkarni 1996 | Estrogen | 22.20 | 23.91 | 11 |
| Kulkarni 1996 | Placebo | 25.90 | 16.24 | 7 |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 5 Mental state: 2c. Average endpoint in positive symptom scores (SAPS, skewed data, high=poor).
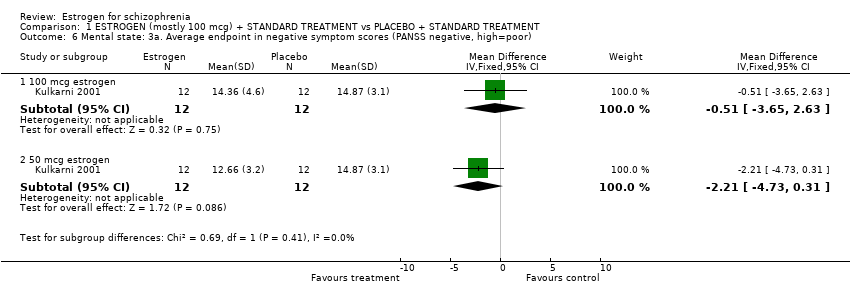
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 6 Mental state: 3a. Average endpoint in negative symptom scores (PANSS negative, high=poor).
| Study | Intervention | Mean | SD | N |
| Louza 2004 | Conjugated estrogen | 13.24 | 8048 | 21 |
| Louza 2004 | Placebo | 15.79 | 9.46 | 19 |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 7 Mental state: 3b. Average endpoint in negative symptom scores (NSRS,skewed data, high=poor).

Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 8 Mental state: 4a. Average endpoint in psychopathology scores (PANSS general symptoms subscale, high=poor).
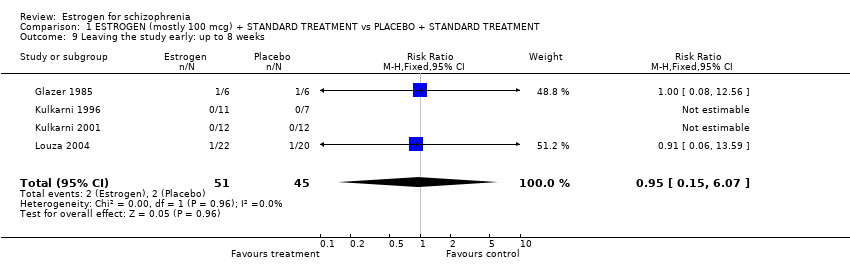
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 9 Leaving the study early: up to 8 weeks.
| Study | Intervention | Mean | SD | N |
| Abnormal Involuntary Movements Scale | ||||
| Glazer 1985 | Conjugated estrogens | 4.60 | 0.55 | 5 |
| Glazer 1985 | Placebo | 5.80 | 3.35 | 5 |
| Simpson & Angus Extrapyramidal Rating Scale | ||||
| Louza 2004 | Conjugated estrogens | 1.29 | 2.05 | 21 |
| Louza 2004 | Placebo | 1.89 | 3.31 | 19 |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 10 Adverse effects: 1. Average endpoint movement disorder scores (skewed data, high=poor).
| Study | Intervention | Mean | SD | N |
| Louza 2004 | Conjugated estrogens | 1.09 | 2.30 | 21 |
| Louza 2004 | Placebo | 3.05 | 4.08 | 19 |
Comparison 1 ESTROGEN (mostly 100 mcg) + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 11 Adverse effects: 2. Average endpoint adverse side‐effect scores (UKU, skewed data, high=poor).

Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 1 Mental state: Average endpoint general mental state scores (PANSS, high= poor).

Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 2 Leaving the study early ‐ up to 6 months.

Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 3 Cognitive functioning: Average endpoint specific aspects of cognitive functioning.
| Study | Component tests | Interventions | Mean | SD | N |
| Motor dexterity (Grooved Pegboard, high=good) | |||||
| Good 1999 | Dominant hand | Estrogen plus progesterone | 149.6 | 167.2 | 5 |
| Good 1999 | Placebo | 189.0 | 96.8 | 3 | |
| Good 1999 | Non‐dominant hand | estrogen plus progesterone | 144.4 | 127.7 | 5 |
| Good 1999 | Placebo | 184.7 | 99.9 | 3 | |
| Good 1999 | |||||
| Good 1999 | |||||
| Design fluency (Design Fluency Test, high=good) | |||||
| Good 1999 | Design fluency free | Estrogen plus progesterone | 15.8 | 8.2 | 5 |
| Good 1999 | Placebo | 11.3 | 9.0 | 4 | |
| Good 1999 | Design fluency fixed | Estrogen plus placebo | 11.4 | 6.1 | 5 |
| Good 1999 | Placebo | 7.0 | 2.9 | 4 | |
| Good 1999 | |||||
| Good 1999 | |||||
| Verbal Fluency (COWA,high=good) | |||||
| Good 1999 | Estrogen plus progesterone | 33.6 | 6.9 | 5 | |
| Good 1999 | Placebo | 16.8 | 11.0 | 4 | |
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
| Auditory‐Verbal Learning Test (AVLT trial, high=good) | |||||
| Good 1999 | AVLT trial 1 | Estrogen plus progesterone | 7.2 | 5.0 | 5 |
| Good 1999 | Placebo | 2.8 | 2.2 | 4 | |
| Good 1999 | AVLT trial 1‐5 | Estrogen plus progesterone | 45.4 | 24.8 | 5 |
| Good 1999 | Placebo | 24.5 | 15.0 | 4 | |
| Good 1999 | AVLT delayed | Estrogen plus placebo | 8.6 | 5.9 | 5 |
| Good 1999 | Placebo | 3.8 | 2.9 | 4 | |
| Smell identification (UPSIT, high=good) | |||||
| Good 1999 | Estrogen plus progesterone | 31.2 | 7.2 | 5 | |
| Good 1999 | Placebo | 16.3 | 11.7 | 4 | |
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
| Smell sensitivity (Acuity, high=good) | |||||
| Good 1999 | Estrogen plus progesterone | 8.4 | 1.9 | 5 | |
| Good 1999 | Placebo | 5.8 | 3.8 | 4 | |
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
| Good 1999 | |||||
Comparison 2 ESTROGEN + PROGESTERONE + STANDARD TREATMENT vs PLACEBO + STANDARD TREATMENT, Outcome 4 Cognitive functioning: Average endpoint specific aspects of cognitive functioning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: 1a. Average endpoint in general mental state scores (PANSS total, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐15.44, 10.92] |
| 1.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐14.60, 5.36] |
| 2 Mental state: 1b. Average endpoint in general mental state scores (BPRS, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 3 Mental state: 2a. Average endpoint in positive symptom scores (PANSS positive, high=poor) Show forest plot | Other data | No numeric data | ||
| 4 Mental state: 2b. Average endpoint in positive symptom scores ‐ 50mcg estrogen (PANSS positive, high=poor) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐5.36, 4.62] |
| 5 Mental state: 2c. Average endpoint in positive symptom scores (SAPS, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 6 Mental state: 3a. Average endpoint in negative symptom scores (PANSS negative, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐3.65, 2.63] |
| 6.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐4.73, 0.31] |
| 7 Mental state: 3b. Average endpoint in negative symptom scores (NSRS,skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 8 Mental state: 4a. Average endpoint in psychopathology scores (PANSS general symptoms subscale, high=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 100 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐7.88, 6.22] |
| 8.2 50 mcg estrogen | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.04 [‐7.01, 2.93] |
| 9 Leaving the study early: up to 8 weeks Show forest plot | 4 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.15, 6.07] |
| 10 Adverse effects: 1. Average endpoint movement disorder scores (skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 10.1 Abnormal Involuntary Movements Scale | Other data | No numeric data | ||
| 10.2 Simpson & Angus Extrapyramidal Rating Scale | Other data | No numeric data | ||
| 11 Adverse effects: 2. Average endpoint adverse side‐effect scores (UKU, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: Average endpoint general mental state scores (PANSS, high= poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 PANSS postive symptom scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.52, 6.52] |
| 1.2 PANSS negative symptom scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐17.11, ‐0.89] |
| 1.3 PANSS psychopathology scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐14.30 [‐29.31, 0.71] |
| 1.4 PANSS total scores | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐25.30 [‐50.74, 0.14] |
| 2 Leaving the study early ‐ up to 6 months Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 3 Cognitive functioning: Average endpoint specific aspects of cognitive functioning Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Visual Retention Test (BVRT total, high=good) | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐5.73, ‐1.27] |
| 3.2 Visual Retention Test (BVRT errors, high=poor) | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐17.62, ‐6.38] |
| 3.3 Motor speed (Finger tapping dominant hand, high=good) | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐19.22, 9.02] |
| 3.4 Motor speed (Finger tapping non‐dominant hand, high=good) | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐23.72, 8.32] |
| 4 Cognitive functioning: Average endpoint specific aspects of cognitive functioning Show forest plot | Other data | No numeric data | ||
| 4.1 Motor dexterity (Grooved Pegboard, high=good) | Other data | No numeric data | ||
| 4.2 Design fluency (Design Fluency Test, high=good) | Other data | No numeric data | ||
| 4.3 Verbal Fluency (COWA,high=good) | Other data | No numeric data | ||
| 4.4 Auditory‐Verbal Learning Test (AVLT trial, high=good) | Other data | No numeric data | ||
| 4.5 Smell identification (UPSIT, high=good) | Other data | No numeric data | ||
| 4.6 Smell sensitivity (Acuity, high=good) | Other data | No numeric data | ||

