Penukar haba dan kelembapan berbanding alat pelembap yang dipanaskan untuk orang dewasa dan kanak‐kanak yang diventilasi secara mekanikal
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: men and women aged > 18 years admitted to an ICU and requiring mechanical ventilation for ≥ 72 hr. Mean (± SD) age: 62.66 ± 14.48 years. Respiratory diagnosis: HME 37.5%, HH 42.8%. Exclusion criteria: contraindication to HME such as a large haemoptysis. hypothermia or excessive tracheal secretions. Severity: mean APACHE II score: HME 27.50, HH 24.27. Setting: ICU, Brazil. | |
| Interventions | HME (hygroscopic): DAR filter Hygrobac S (Mallinckrodt Tyco Healthcare) changed every 24 hr. n = 8. HH: Misty 3 Intermed. n = 7. Time in study (median): HME 7 days, HH 7 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by 'simple lottery.' |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes measured by 2 different researchers. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, only VAP reported. |
| Other bias | High risk | Mean time in hospital: HME 9 days, HH 29 days ; median duration of ventilation: HME 201 hr, HH 161 hr; antibiotics used: HME 87.5%, HH 100%. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: infants weighing 5‐10 kg, ASA status I or II having peripheral surgery lasting 2‐3 hr. Mean age: HME 12 months, HH 11 months. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: paediatric hospital, Canada. | |
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 10. HH: MR450 (Fisher & Paykel) set at 37 ºC. n = 10. Time in study: 120 min. | |
| Outcomes |
| |
| Notes | Funding: National Institutes of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: infants and children weighing 5‐30 kg, ASA status I or II, having peripheral surgery lasting 1‐3 hr. Mean age: HME 4 years, HH 5 years. Exclusion criteria: without history of tympanic or middle ear problems. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: paediatric hospital, Canada. | |
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 8. HH: MR450 (Fisher & Paykel) set at 37 ºC. n = 10. Time in study: 120 min. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: all admissions to general ICU requiring mechanical ventilation for > 48 hr. Mean age: 51 years. Exclusion criteria: people with asthma, airway burns or pulmonary haemorrhage. Respiratory diagnosis: respiratory failure: HME 38/42, HH 37/41. Mean APACHE II score: HME 19, HH 18. Setting: adult ICU, Australia. | |
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck) changed every 24 hr. n = 42. HH (heated wire): MR730 (Fisher & Paykel) set at 37 ºC. n = 41. Change of circuit every 48 hr in both groups. Time in study (median): HME 6 days, HH 6 days. | |
| Outcomes |
| |
| Notes | Funding: Teleflex, Wayne, PA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all primary outcomes reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation for ≥ 48 hr. Mean age: HME 59 years, HH 60 years. Exclusion criteria: presenting history which suggested need for hot water humidification, e.g. airway haemorrhage, asthma or airway burns. Mean APACHE II score: HME 20, HH 20. Setting: ICU, Australia. | |
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck) changed every 24 hr or more frequently if required. n = 190. HH (heated wire): MR730 (Fisher & Paykel, single heated wire) set at 37 ºC or MR290 (Fisher & Paykel, double heated wire) set at 40 ºC. n = 191. Circuit unchanged for duration of ventilation. Time in study (median): HME 6 days, HH 8 days. | |
| Outcomes |
| |
| Notes | Funding: Teleflex, Wayne, PA. and Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all primary outcomes reported. |
| Other bias | High risk | Participants in HME group ventilated for a significantly shorter period (i.e. HME 6 days, HH 8 days). |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people in ICU requiring mechanical ventilation deemed suitable for HME. Mean age: HME 44 years, HH 41 years. Exclusion criteria: people deemed unsuitable for HME. Respiratory diagnosis: not stated. Mean SAPS score: HME 9, HH 8. Setting: surgical and medical ICUs, USA. | |
| Interventions | HME (hygroscopic): Baxter. n = 49. HH (heated wire): MR730 (Fisher & Paykel) set at 36 ºC. n = 54. Time in study (mean): HME 5 days, HH 4 days. | |
| Outcomes |
| |
| Notes | Funding: Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was accomplished using the last digit in the patient's medical record number ‐‐‐patients with an odd medical record number received an HCH [HME] and those with an even number received a heated humidifier." |
| Allocation concealment (selection bias) | High risk | "Randomization was accomplished at the bedside." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Low risk | No protocol available but the 2 primary outcomes of airway occlusion and pneumonia reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people following surgery, 15/26 breathing spontaneously and 11/26 ventilated. Mean age: 44 years. Exclusion criteria: not stated. Respiratory diagnosis: 58%. Severity: not stated. Setting: surgical ICU, USA. | |
| Interventions | HME (hygroscopic): Humid‐Vent 2 (Gibeck). n = 26. HME: Extended use (Mallinckrodt). n = 26. HH (heated wire): MR730 (Fischer & Paykel) set at 34 ºC. n = 26. Time in study: 1 hr for each type of humidification. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term trial. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: neonates requiring ventilation via ETT. Ventilated for > 8 hr before admission, obstruction in tracheobronchial tree, ventilated at a frequency > 60 cycles/min. Mean age: not stated. Exclusion criteria: not stated. Respiratory diagnosis: HME 55%, HH 44%. Severity: not stated. Setting: NICU, France. | |
| Interventions | HME (hygroscopic): Hygroflux (Vygon) changed daily. n = 29. HH: (Fisher & Paykel) set at 32‐34 ºC. n = 27. Time in study (mean): HME 4 days, HH 5 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people with ASA status I or II scheduled for gynaecological surgery. Mean age: 40 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: hospital, France. | |
| Interventions | HME (hydrophobic): Ultipor hydrophobic (Pall). n = 25. HH: Aquapor (Dräger) set at 42 ºC. n = 25. Time in study (mean): HME 155 min, HH 116 min. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Participants in HME group anaesthetized for significantly longer than those in HH group (HME 155 min, HH 116 min). |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving intubation. Median age: HME 61 years, HH 66 years. Exclusion criteria: previous pulmonary disease, hypothermia, pulmonary secretion or low expiratory volume. Respiratory diagnosis: not stated. Severity: not stated. Setting: ICU, Brazil. | |
| Interventions | HME (hygroscopic): not stated. n = 23. HH: not stated. n = 20. Time in study: not stated. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers list used to determine treatment sequence. |
| Allocation concealment (selection bias) | Low risk | Allocations made available in sealed and consecutively numbered envelopes. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. pneumonia and mortality, reported but airway occlusion not reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for > 48 hr. Mean age: HME 58 years, HH 62 years. Exclusion criteria: not stated. Respiratory diagnosis: HME 64%, HH 63%. Mean SAPS score: HME 16, HH 16. Setting: ICU, France. | |
| Interventions | HME (hygroscopic/hydrophobic): Hygrobac II (DAR) changed daily. n = 61. HH: Bennett cascade (Puritan‐Bennett) or MR460 (Fischer‐Paykel). n = 70. Time in study (median): HME 10 days, HH 12 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: people with acute or chronic respiratory failure difficult to wean from mechanical ventilation. Mean age: 69 years. Exclusion criteria: non‐co‐operative people, unable to have an oesophageal tube. Respiratory diagnosis: 100%. Mean SAPS score: 44. Setting: ICU, France. | |
| Interventions | HME (hygroscopic): Hygrobac (DAR). n = 11. HH (heated wire): MR730 (Fischer & Paykel). n = 11. Time in study: 2 × 20 min for HME (7 cm H2O/min and 18 cm H2O/min ventilation pressure), 2 × 20 min for HH (7 cm H2O/min and 18 cm H2O/min ventilation pressure). | |
| Outcomes |
* Data not used as this was unlikely to be a valid outcome in a cross‐over study. | |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | 3/15 participants withdrawn from study early because they could not "tolerate" HME and 1 participant refused to continue study. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults with ASA status I, II and III, scheduled lower abdominal surgery under endotracheal anaesthesia for 1‐4 hr. Mean age: HME: 43 years, HH: 45 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I, II and III. Setting: operating theatre, USA. | |
| Interventions | HME (hydrophobic): Ultipor (Pall). n = 21. HH: Fisher & Paykel set at 37 ºC. n = 14. Time in study (mean): HME 123 min, HH 141 min. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned using a computer‐generated random table." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Low risk | "Nursing personnel ‐‐‐ blinded to the patient group ‐‐‐ recorded temperatures." |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for ≥ 48 hr. Mean age: HME 53 years, HH 59 years. Exclusion criteria: hypothermic; intubated for 12 hr prior to ICU admission. Respiratory diagnosis: 40%. Mean SAPS score: HME 13, HH 13. Setting: ICU, Switzerland. | |
| Interventions | HME (hygroscopic): Hygroster (DAR). n = 59. HH: Fisher & Paykel set at 32 ºC. n = 56. Time in study (mean): HME 8 days, HH 8 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis used but there was a very high rate, i.e. 51%, that did not receive ventilation for ≥ 48 hr. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | High risk | Circuits changed every 48 hr in HH group and every 7 days in HME group. |
| Methods | Randomized cross‐over study comparing 2 types of HME to HH | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for acute respiratory failure. Mean age: 58 years. Exclusion criteria: COPD. Respiratory diagnosis: 100%. Severity: not stated. Setting: ICU, Italy. | |
| Interventions | HME (hygroscopic): Umid‐Vent 2S (Gibeck). n = 10. HME: Hygroster (DAR). n = 10. HH: MR 450 (Fisher & Paykel) set at 32‐34 ºC. n = 10. Time in study: 1‐2 hr. | |
| Outcomes |
| |
| Notes | Funding: Polytechnico S. Matteo and support from Hamilton Bonaduzz AG. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people undergoing neurosurgery. Median age: HME 52 years, HH 36 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: not stated. Setting: hospital, Denmark. | |
| Interventions | HME (hygroscopic): Engström Edith (Gambro). n = 15. HH: Hygrotherm. n = 15. Time in study: 72 hr. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | High risk | Participants in HME group were older. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation. Mean age: 47 years. Exclusion criteria: ventilated elsewhere. Respiratory diagnosis: not stated. Severity: not stated. Setting: trauma ICU, USA. | |
| Interventions | HME (hydrophobic): BB100‐F (Pall). n = 140. HH (heated wire). n = 140. Time in study: until removal of ETT, time not stated. | |
| Outcomes |
| |
| Notes | All 6 lost to follow‐up appeared to have been in HME group. Funding: University of Miami and Jackson Memorial Hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by a random number generated from a personal computer." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Low risk | "Laboratory and chest radiograph interpretation were blinded." |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether the 6 lost to follow‐up included in analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and pneumonia, reported but mortality not reported. |
| Other bias | Unclear risk | None identified but potential differences between groups not reported. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults requiring mechanical ventilation. Mean age: HME 58 years, HH 59 years. Exclusion criteria: ventilated elsewhere, previous heart or liver transplant, massive haemoptysis. Respiratory diagnosis: HME 16%, HH 18%. Mean APACHE II score: HME 17, HH 18. Setting: medical and surgical ICUs, USA. | |
| Interventions | HME (hygroscopic): duration extended use (Nellcor Puritan‐Bennett). n = 163. HH (heated wire): MR730 (Fisher & Paykel) set at 35‐36 ºC. = 147. Time in study (mean): both groups 4 days. | |
| Outcomes |
| |
| Notes | Funding: Nellcor Puritan‐Bennett. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | "Randomization was done using opaque, sealed envelopes;" opened when person enrolled in study. |
| Blinding of outcome assessment (detection bias) | Low risk | All participants suspected of having VAP were reviewed by an investigator blinded to treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people expected to require mechanical ventilation for > 48 hr. Mean age: HME 55 years, HH 55 years. Exclusion criteria: people already ventilated, contraindications to an HME or HH, admitted after cardiac arrest, enrolled in clinical trial or early decision to withdraw treatment. Respiratory diagnosis: HME 38%, HH 28%. Mean SAPS II score: HME 45.4, HH 49.3. Setting: 2 ICUs, France. | |
| Interventions | HME (hygroscopic): DAR Hygrobac (Tyco Healthcare/Nellcor) changed at 48‐hr intervals. n = 185. HH (heated wire): MR730 (Fisher & Paykel) and Aerodyne 2000 (Tyco Healthcare/Nellcor). n = 184. Time in study (mean): HME 14 days, HH 15 days. | |
| Outcomes |
| |
| Notes | Funding: Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised according to a computer‐generated randomization list, stratified by participating ICU." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | 5 times as many participants with HIV in HH group. Also higher PaO2/FiO2 in HH group. |
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving inspiratory pressure support during weaning trials from mechanical ventilation. Mean age: 63 years. Exclusion criteria: not stated. Respiratory diagnosis: 53%. Mean SAPS score: 16. Setting: hospital, France. | |
| Interventions | HME (hygroscopic): Hygrobac (DAR). HH: MR450 (Fisher & Paykel). n = 15. Time in study: 20 min. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults ASA status I or II undergoing laparotomies > 3 hr. Mean age: HME 43 years, HH 40 years. Exclusion criteria: serious circulatory, pulmonary or metabolic disease, heavy intraoperative bleeding or shock. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: hospital, Finland. | |
| Interventions | HME: Servo 150 (Siemens‐Elema AB). n = 10. HH: MR418 (Fisher & Paykel) set at 37‐40 ºC. n = 10. Time in study: 4 hr. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults expected to require ventilation for ≥ 5 days. Mean age: HME 56 years, HH 55 years. Exclusion criteria: aged < 18 years, HIV, blood leukocytes < 1000/mm3, solid or haematological tumour, immunosuppressive therapy. Respiratory diagnosis: HME 24%, HH 31%. Mean APACHE II score: HME 18.11, HH 18.72. Setting: ICU, Spain. | |
| Interventions | HME: Edith Flex (Datex‐Ohmeda) changed at 48‐hr intervals. n = 53. HH (heated wire): MR 850 (Fisher & Paykel) and Aerodyne 2000 (Tyco Healthcare/Nellcor) set at 37 ºC. n = 51. Time in study (mean): HME 20 days, HH 21 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned ‐‐‐ by a random number list generated using Excel software." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing an HME to 2 types of HH. | |
| Participants | Inclusion criteria: critically ill people undergoing mechanical ventilation. Age range: 18‐34 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: not stated. Setting: ICU, Italy. | |
| Interventions | HME (hygroscopic): Hygrobac (DAR) changed every 24 hr. n = 15. HH: Cascade II set at 8 (Puritan‐Bennett). n = 15. HH: MR600 (Fisher & Paykel) set at 37 ºC. n = 15. Time in study: 3‐7 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: clinically stable people requiring mechanical ventilation. Mean age: 60 years. Exclusion criteria: not stated. Respiratory diagnosis: 35%. Severity: not stated. Setting: hospital, USA. | |
| Interventions | HME (hygroscopic): Servo humidifier. n = 26. HH: Puritan Bennett. n = 26. Time in study: 24 hr for each type of humidification. | |
| Outcomes |
* Not considered valid because of the cross‐over design and, therefore, not used. | |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for > 24 hr. Mean age: HME 61 years, HH 54 years. Exclusion criteria: not stated. Respiratory diagnosis: HME 48%, HH 51%. Severity: not stated. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): Ultipor (Pall) replaced at least daily. n = 31. HH: set at 31 ºC. n = 42. Time in study (mean): HME 10 days, HH 14 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | Mean number of days: HH 14, HME 10. |
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: sedated, paralysed people requiring mechanical ventilation for > 3 days. Mean age: 64 years. Exclusion criteria: not stated. Respiratory diagnosis: 100%. Severity: not stated. Setting: ICU, France. | |
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 11. HH: Cascade II (Bennett). n = 11. Time in study: 24 hr for each method of humidification. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | High risk | "This study was ‐ unblinded." |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: intubated people requiring mechanical ventilation with evidence of respiratory or systemic infection. Mean age: HME 48 years, HH 46 years. Exclusion criteria: ventilated < 48 hr. Respiratory diagnosis: 36%. Mean APACHE score: HME 32, HH 31. Setting: medical/surgical ICU, Saudi Arabia. | |
| Interventions | HME (hygroscopic): Hudson RCI. n = 120. HH: not stated. n = 123. Time in study (mean): HME 9 days, HH 9 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group balance was maintained within each block of 20." |
| Allocation concealment (selection bias) | Low risk | "The randomization record was kept with the hospital biostatistician." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. mortality and pneumonia, reported but airway occlusion not reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people expected to be mechanically ventilated for > 5 days. Mean age: HME 53 years, HH 49 years. Exclusion criteria: ventilated < 5 days. Respiratory diagnosis: not stated. Mean SAPS score: HME 14, HH 13. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): Ultipor BB2215 (Pall) changed daily. n = 30. HH: Cascade II (Puritan‐Bennett) or MR450 (Fisher & Paykel) set at 32‐34 ºC. n = 26. Time in study (mean): HME 12 days, HH 11 days. | |
| Outcomes |
| |
| Notes | Variance reported as SEMs but based on P values appear to have been SDs. Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome, airway occlusion, was reported for this long‐term study. |
| Other bias | Unclear risk | None identified. |
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people with moderate acute respiratory failure, receiving pressure‐support ventilation. Mean age: 54 years. Exclusion criteria: COPD. Respiratory diagnosis: 100%. Mean SAPS score: 12. Setting: ICU, Italy. | |
| Interventions | HME (hygroscopic): Hygroster. n = 7. HME: Hygrobac‐S (DAR). n = 7. HH: MR450 (Fisher & Paykel). n = 14. Time in study: 90 min. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but 3 respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel/cross‐over* study comparing 4 types of HME to HH. * Participants randomized to 2/5 interventions. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation. Mean age: 54 years. Exclusion criteria: not stated. Respiratory diagnosis: 58%. Mean SAPS score: 14. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): Ultipor Filter BB2215 (Pall). n = 20. HME (hydrophobic): Biomedical BB50 (Pall). n = 20. HME (hydrophobic/hygroscopic): Ultipor Filter BB100 (Pall). n = 20. HME (hydrophobic/hygroscopic): Hygrobac (DAR). n = 10. HH: Fischer‐Paykel. n = 15. Time in study: 3 hr. | |
| Outcomes |
| |
| Notes | Funding: authors declared they did not have any funding support and had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available but 2 respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation through an ETT. Mean age: HME 53 years, HH 49 years. Exclusion criteria: requiring high‐frequency jet ventilation. Respiratory diagnosis: HME 53%, HH 38%. Severity: HME 12, HH 12. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): BB2215 (Pall), changed daily. n = 55. HH: Aquapor (Dräger) set at 31 ºC. n = 61. Time in study (mean): HME 11 days, HH 8 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | HME group in study for mean of 11 days compared to 8 days in HH group. |
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation through an ETT for acute respiratory failure. Mean age: 45 years. Exclusion criteria: not stated. Respiratory diagnosis: 100%. Mean SAPS score: 15. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): BB100 (Pall). n = 10. HME: BactHME (Pharma system AB). n = 10. HH: Cascade II (Puritan‐Bennett). n = 10. Time in study: 24 hr. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | High risk | "A prospective controlled unblinded study." |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people in ICU requiring mechanical ventilation. Mean age: HME 63 years, HH 60 years. Exclusion criteria: haemorrhagic disorders, intubated for > 24 hr before admission. Respiratory diagnosis: not stated. Mean SAPS score: HME 17, HH 17. Setting: ICU, France. | |
| Interventions | HME (hydrophobic): BB2215 (Pall). n = 8. HME: Hygrobac (DAR) changed daily. n = 8. HH: MR310 (Fisher & Paykel) set at 32 ºC. n = 7. Time in study (mean): HME 6 days, HH 6 days. | |
| Outcomes |
| |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: elderly people having total hip arthroplasty for osteoarthritis. Mean age: HME 70 years, HH 68 years. Exclusion: grossly obese, endocrine diseases, people with pyrexia. Respiratory diagnosis: not stated. Severity: not stated. Setting: operating theatre, UK. | |
| Interventions | HME: Thermovent 1200 (Portex) n = 20. HH: Cascade (Puritan Bennett) n = 20. Time in study (mean): HME 128 min, HH 133 min. | |
| Outcomes |
| |
| Notes | Funding: HME supplied by Portex. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
APACHE: Acute Physiology & Chronic Health Score; ASA: American Society of Anesthesiologists; COPD: chronic obstructive pulmonary disease; ETT: endotracheal tube; HH: heated humidifier; HME: heat and moisture exchanger; hr: hour; ICU: intensive care unit; ITT: intention‐to‐treat analysis; LOS: length of stay; min: minute; n: number of participants; NICU: neonatal intensive care unit; PaCO2: arterial pressure of carbon dioxide; PaO2: arterial pressure of oxygen; SaO2: arterial oxygen saturation; SAPS: Simplified Acute Physiologic Score; SD: standard deviation; SEM: standard error; VAP: ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Data unavailable. | |
| Not randomized. Allocation based on clinical algorithm. | |
| HME connected to HH. | |
| Did not measure any relevant clinical outcomes ‐ measured inspired humidity. | |
| Studied HME. Only switched to HH if there were complications. | |
| Only studied HMEs. | |
| Not a randomized controlled trial. | |
| Not a randomized controlled trial. | |
| Not a randomized controlled trial. | |
| Not randomized ‐ all participants received HH first. | |
| HME in ventilator circuit with HH. | |
| Not randomized. | |
| Did not measure any relevant clinical outcomes ‐ measured core temperature and inspired and expired humidity. | |
| Non‐randomized study of HMEs use in children during anaesthesia. | |
| Not a trial. Commentary on trial by Lacherade 2005. | |
| All participants received HH first. | |
| HME used in conjunction with HH. | |
| Participants were cold to start with ‐ study compared no heat via HME with HH. | |
| Invasive ventilation was not used. | |
| Data unavailable. Results presented only in graphical form. | |
| Used non‐invasive ventilation. | |
| Not randomized. | |
| Not randomized. HME removed when person was hypercapnic and HH used. | |
| Did not measure any relevant clinical outcomes ‐ measured inspired humidity. | |
| Did not measure any relevant clinical outcomes ‐ measured water vapour pressure. | |
| Not randomized. | |
| Home‐made humidifier compared to no humidification. | |
| Participants spontaneously breathing via tracheostomy. | |
| Not randomized ‐ alternate allocation. |
HH: heated humidifier; HME: heat and moisture exchanger.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized trial of HME‐Booster and HH. |
| Participants | 41 mechanically ventilated people in an ICU in Turkey. |
| Interventions | 21 participants randomized to HME‐Booster and 20 participants to HH with conventional microbiological filter. |
| Outcomes | Endotracheal tube occlusion due to secretions, VAP, PaCO2. |
| Notes | Study awaiting classification, attempted to contact authors for additional information including outcome data. |
| Methods | Randomized trial of HME and HH. |
| Participants | 35 mechanically ventilated participants from the first day of intubation, did not have preintubation pneumonia, no infections or antibiotics for pulmonary infections or evidence of infiltration with chest radiography in a private hospital ICU in Turkey. |
| Interventions | 18 participants randomized to HME and 17 participants to HH. |
| Outcomes | Pneumonia ‐ presence of infiltrate over 7 days. |
| Notes | Infiltrate identified in 6 participants in HME group and 5 participants in HH group. |
HH: heated humidifier; HME: heat and moisture exchange; ICU: intensive care unit; PaCO2: arterial pressure of carbon dioxide; VAP: ventilator‐associated pneumonia.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| Analysis 1.1  Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 1 Artificial airway occlusion. | ||||
| 2 Mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 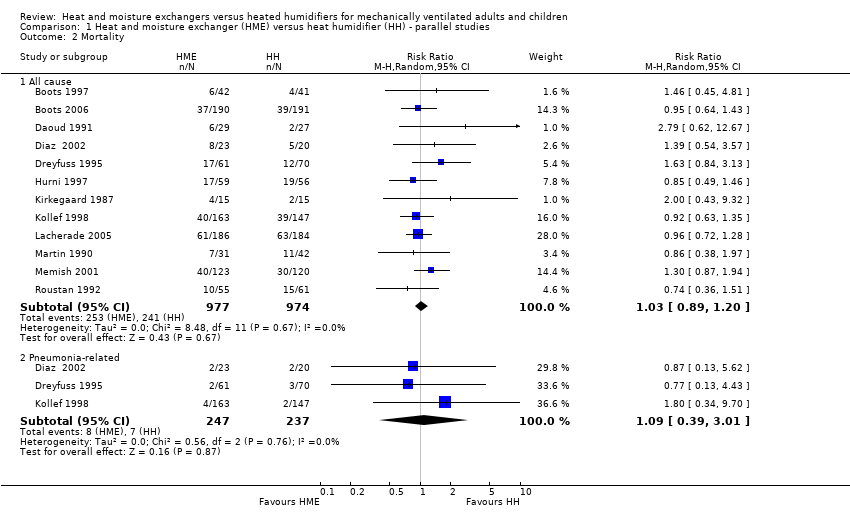 Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 2 Mortality. | ||||
| 2.1 All cause | 12 | 1951 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.20] |
| 2.2 Pneumonia‐related | 3 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.01] |
| 3 Pneumonia Show forest plot | 13 | 2251 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| Analysis 1.3  Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 3 Pneumonia. | ||||
| 3.1 Throughout ventilation | 7 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.2 ≥ 48 hours after ventilation | 6 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.64, 1.46] |
| 4 Atelectasis Show forest plot | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.40] |
| Analysis 1.4 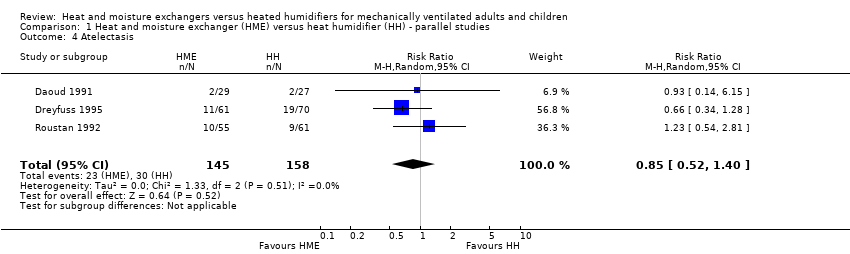 Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 4 Atelectasis. | ||||
| 5 Tracheal aspirations (per day) Show forest plot | 3 | 290 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.41, 0.47] |
| Analysis 1.5  Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 5 Tracheal aspirations (per day). | ||||
| 6 Saline instillations (number per day) Show forest plot | 3 | 276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.17] |
| Analysis 1.6  Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 6 Saline instillations (number per day). | ||||
| 7 Change in body temperature (absolute data) (ºC) Show forest plot | 6 | 321 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.96, ‐0.02] |
| Analysis 1.7 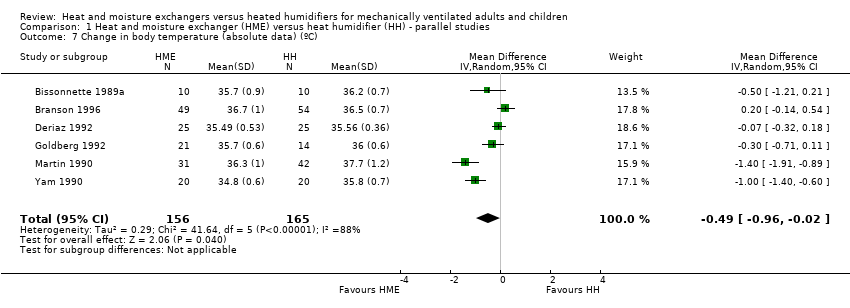 Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 7 Change in body temperature (absolute data) (ºC). | ||||
| 8 Change in body temperature mean data) (ºC) Show forest plot | 3 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.82, ‐0.36] |
| Analysis 1.8 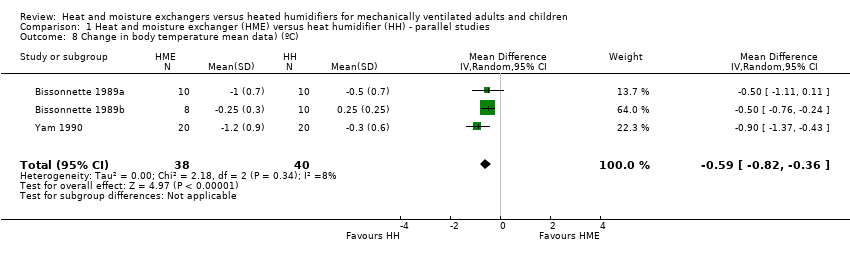 Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 8 Change in body temperature mean data) (ºC). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PaO2 (mmHg) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 1 PaO2 (mmHg). | ||||
| 1.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | ‐3.24 [‐16.08, 9.60] | |
| 1.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | ‐3.87 [‐16.73, 9.00] | |
| 1.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | ‐4.41 [‐17.09, 8.27] | |
| 2 PaCO2 (mmHg) Show forest plot | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.2 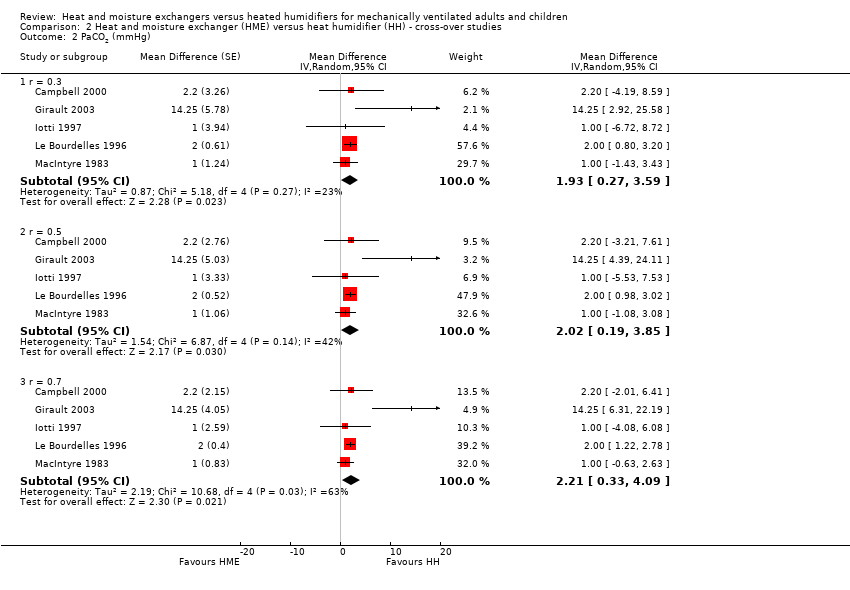 Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 2 PaCO2 (mmHg). | ||||
| 2.1 r = 0.3 | 5 | Mean Difference (Random, 95% CI) | 1.93 [0.27, 3.59] | |
| 2.2 r = 0.5 | 5 | Mean Difference (Random, 95% CI) | 2.02 [0.19, 3.85] | |
| 2.3 r = 0.7 | 5 | Mean Difference (Random, 95% CI) | 2.21 [0.33, 4.09] | |
| 3 Breathing rate (breaths/minute) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.3 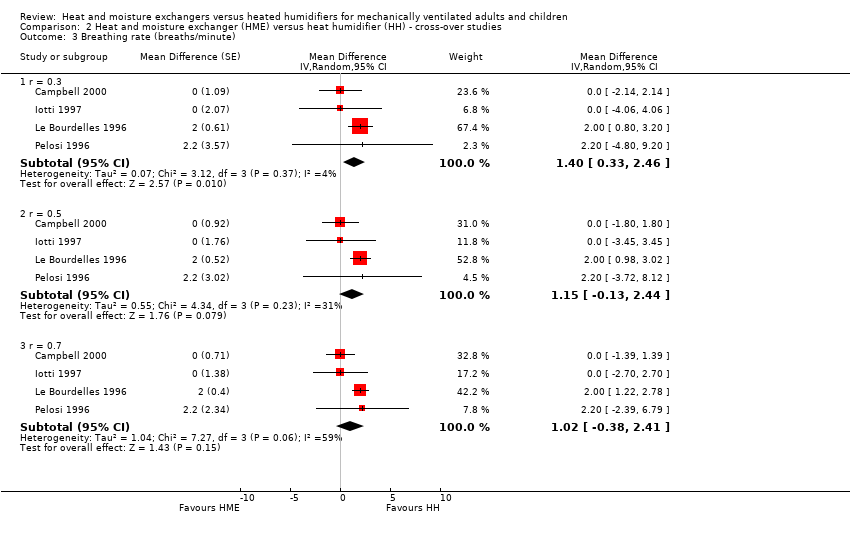 Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 3 Breathing rate (breaths/minute). | ||||
| 3.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | 1.40 [0.33, 2.46] | |
| 3.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | 1.15 [‐0.13, 2.44] | |
| 3.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | 1.02 [‐0.38, 2.41] | |
| 4 Tidal volume (L) Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.4 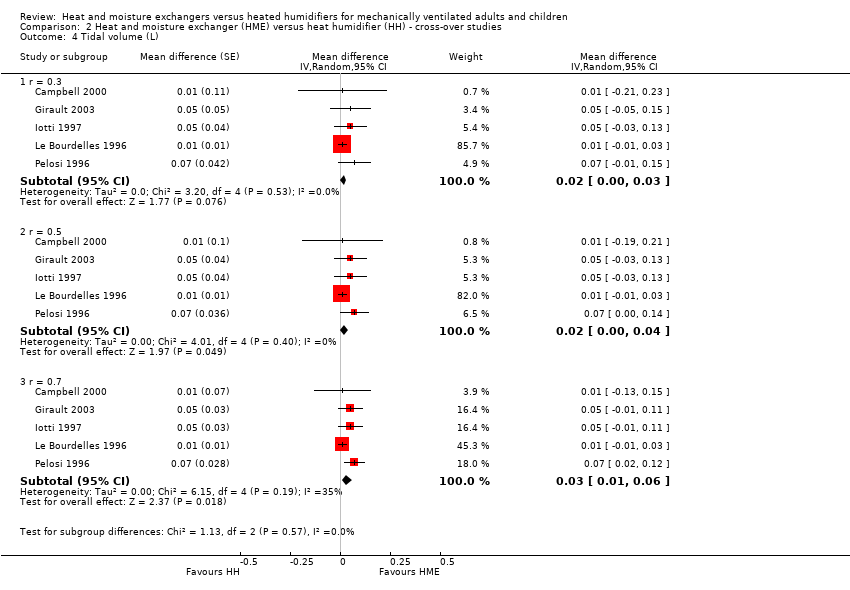 Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 4 Tidal volume (L). | ||||
| 4.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 0.02 [‐0.00, 0.03] | |
| 4.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 0.02 [0.00, 0.04] | |
| 4.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 0.03 [0.01, 0.06] | |
| 5 Minute ventilation (L/minute) Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 5 Minute ventilation (L/minute). | ||||
| 5.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 1.20 [0.78, 1.61] | |
| 5.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 1.19 [0.63, 1.75] | |
| 5.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 1.18 [0.55, 1.80] | |
| 6 Body temperature (ºC) Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.6 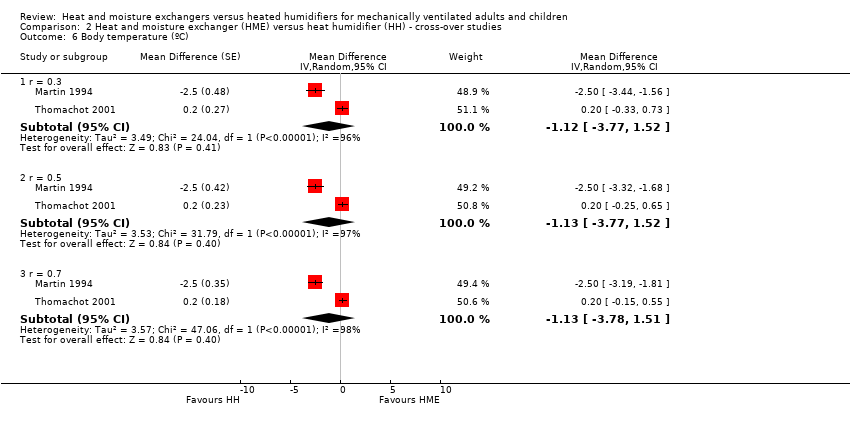 Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 6 Body temperature (ºC). | ||||
| 6.1 r = 0.3 | 2 | Mean Difference (Random, 95% CI) | ‐1.12 [‐3.77, 1.52] | |
| 6.2 r = 0.5 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.77, 1.52] | |
| 6.3 r = 0.7 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.78, 1.51] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 1 Artificial airway occlusion. | ||||
| 1.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.33, 1.26] |
| 1.2 Adults | 14 | 2115 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.65, 5.76] |
| 2 All‐cause mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 2 All‐cause mortality. | ||||
| 2.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.62, 12.67] |
| 2.2 Adults | 11 | 1895 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 1 Artificial airway occlusion. | ||||
| 1.1 Medium‐term | 8 | 1031 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.41, 7.30] |
| 1.2 Long‐term | 7 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.34, 6.36] |
| 2 All‐cause mortality Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 2 All‐cause mortality. | ||||
| 2.1 Medium‐term | 5 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.31] |
| 2.2 Long‐term | 6 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 3 Pneumonia Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 3 Pneumonia. | ||||
| 3.1 Medium‐term | 5 | 892 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |
| 3.2 Long‐term | 6 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1 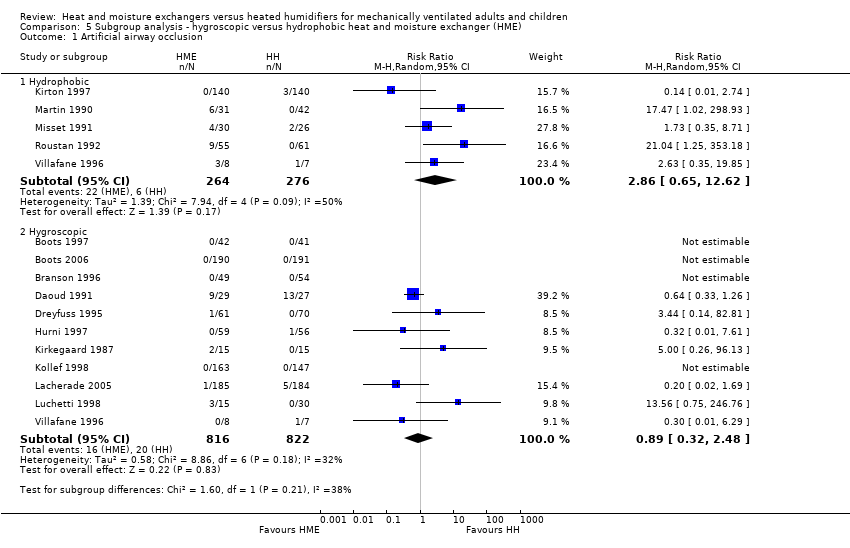 Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 1 Artificial airway occlusion. | ||||
| 1.1 Hydrophobic | 5 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.65, 12.62] |
| 1.2 Hygroscopic | 11 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.48] |
| 2 All‐cause mortality Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.2 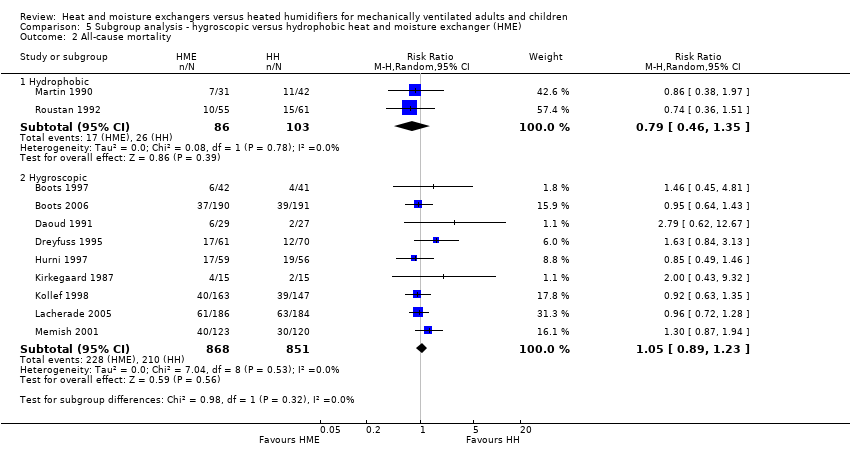 Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 2 All‐cause mortality. | ||||
| 2.1 Hydrophobic | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.35] |
| 2.2 Hygroscopic | 9 | 1719 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 3 Pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 3 Pneumonia. | ||||
| 3.1 Hydrophobic | 3 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.82] |
| 3.2 Hygroscopic | 9 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 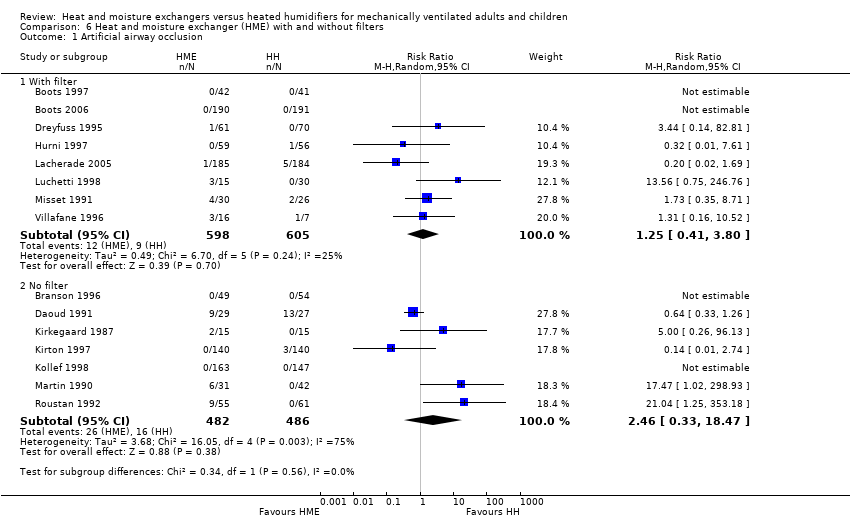 Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 1 Artificial airway occlusion. | ||||
| 1.1 With filter | 8 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.80] |
| 1.2 No filter | 7 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.33, 18.47] |
| 2 All‐cause mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 2 All‐cause mortality. | ||||
| 2.1 With filter | 5 | 1080 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
| 2.2 No filter | 7 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.85, 1.36] |
| 3 Pneumonia Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3 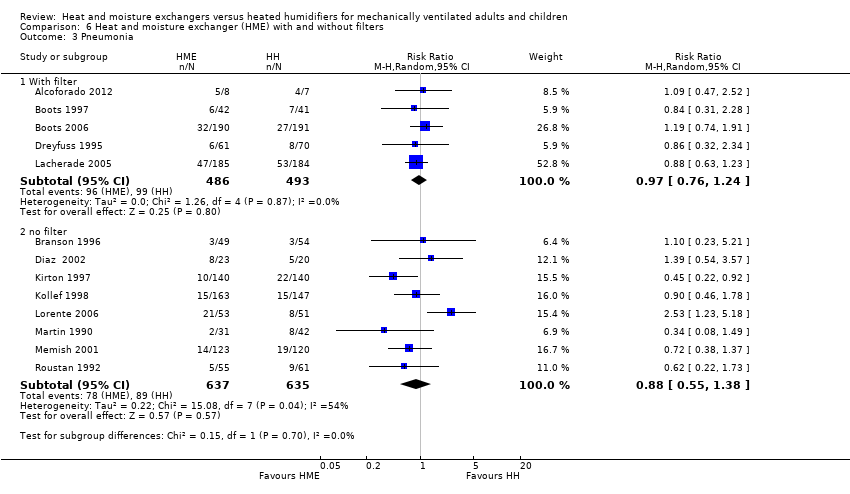 Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 3 Pneumonia. | ||||
| 3.1 With filter | 5 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 3.2 no filter | 8 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 1 Mortality. | ||||
| 1.1 Low risk of bias | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.90] |
| 1.2 Unknown risk of bias | 10 | 1665 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.16] |
| 2 Pneumonia Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 2 Pneumonia. | ||||
| 2.1 Low risk of bias | 3 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| 2.2 Unknown risk of bias | 9 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 2.3 High risk of bias | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.23, 5.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| Analysis 8.1  Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 1 Artificial airway occlusion. | ||||
| 1.1 Low risk of bias | 2 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.74] |
| 1.2 Unclear risk of bias | 13 | 1581 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.69, 5.34] |
| 2 Pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.2  Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 2 Pneumonia. | ||||
| 2.1 Low risk of bias | 4 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.36] |
| 2.2 Unknown risk of bias | 8 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.33] |
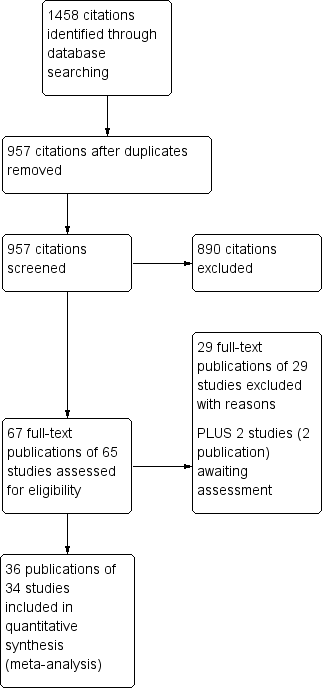
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
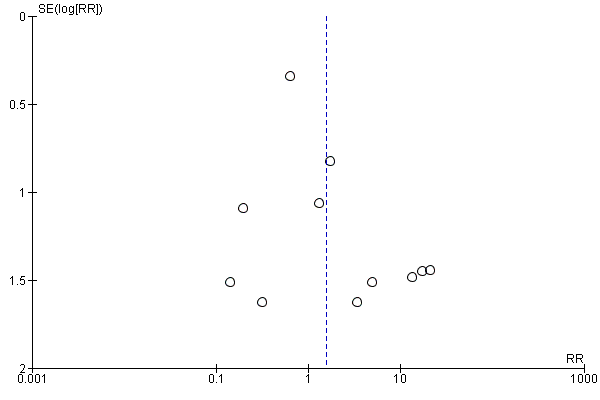
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.1 Artificial airway occlusion.
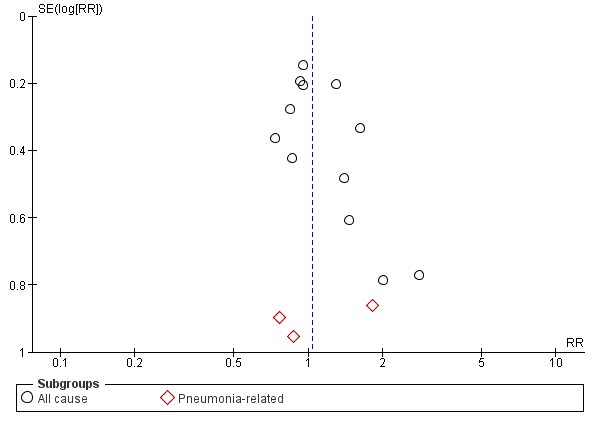
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.2 Mortality.
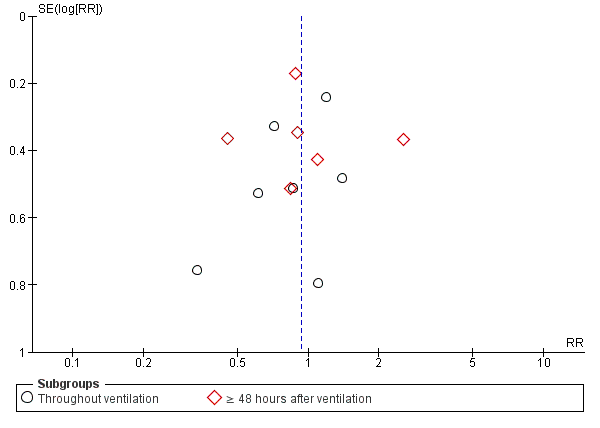
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.3 Pneumonia.

Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 1 Artificial airway occlusion.

Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 2 Mortality.

Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 3 Pneumonia.
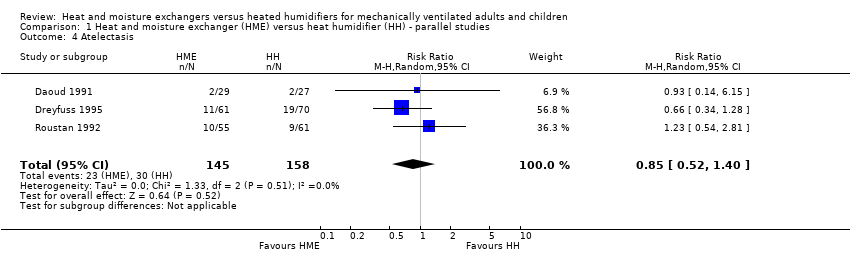
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 4 Atelectasis.
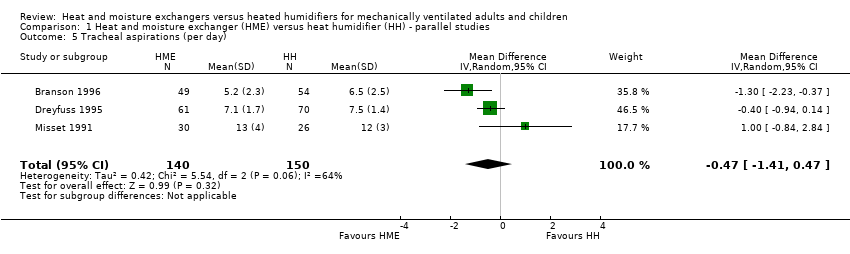
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 5 Tracheal aspirations (per day).
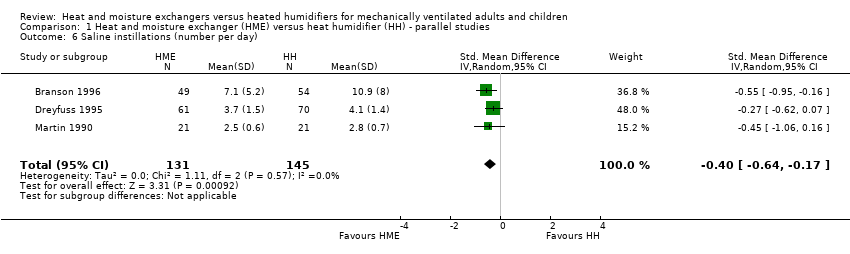
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 6 Saline instillations (number per day).
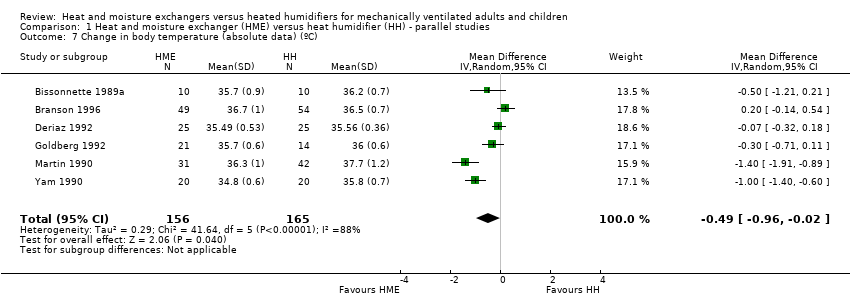
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 7 Change in body temperature (absolute data) (ºC).
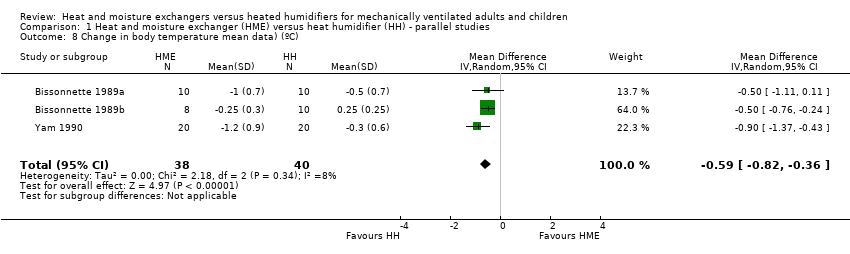
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 8 Change in body temperature mean data) (ºC).
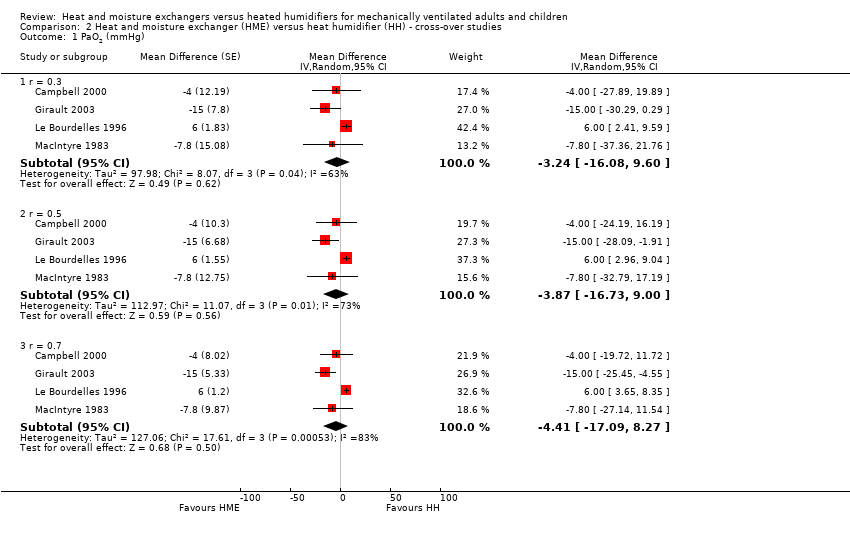
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 1 PaO2 (mmHg).

Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 2 PaCO2 (mmHg).

Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 3 Breathing rate (breaths/minute).

Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 4 Tidal volume (L).
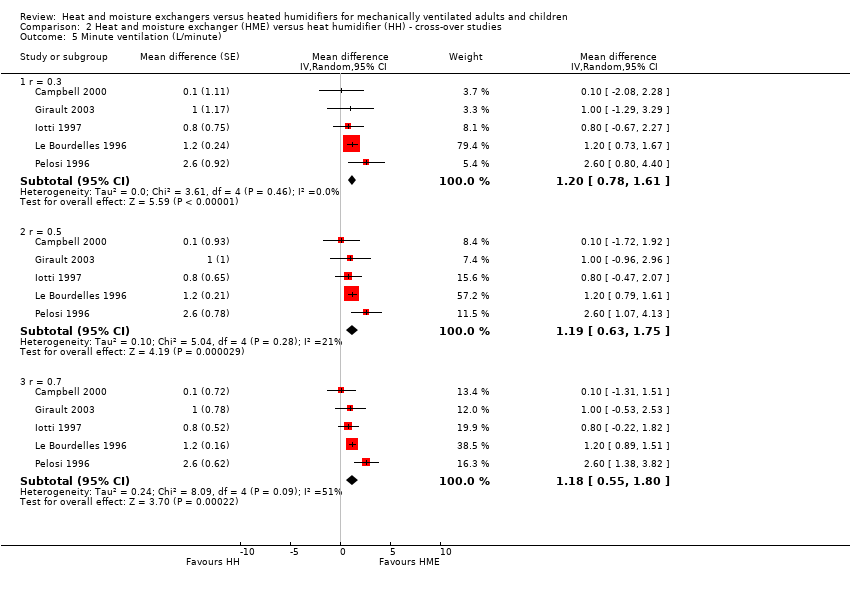
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 5 Minute ventilation (L/minute).

Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 6 Body temperature (ºC).

Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 1 Artificial airway occlusion.

Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 2 All‐cause mortality.

Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 1 Artificial airway occlusion.

Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 2 All‐cause mortality.
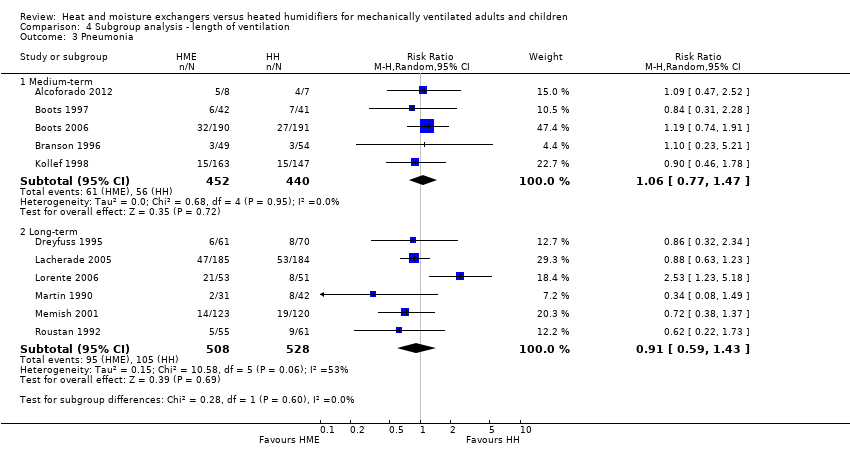
Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 3 Pneumonia.
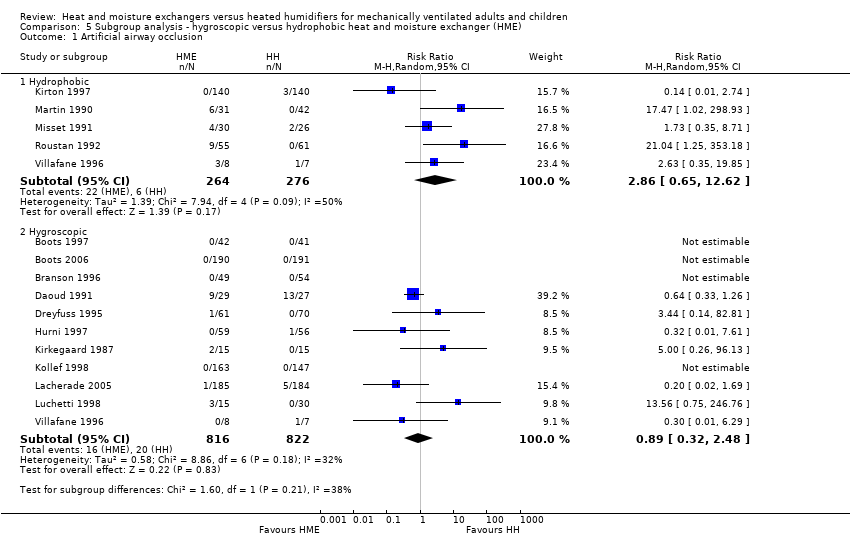
Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 1 Artificial airway occlusion.

Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 2 All‐cause mortality.
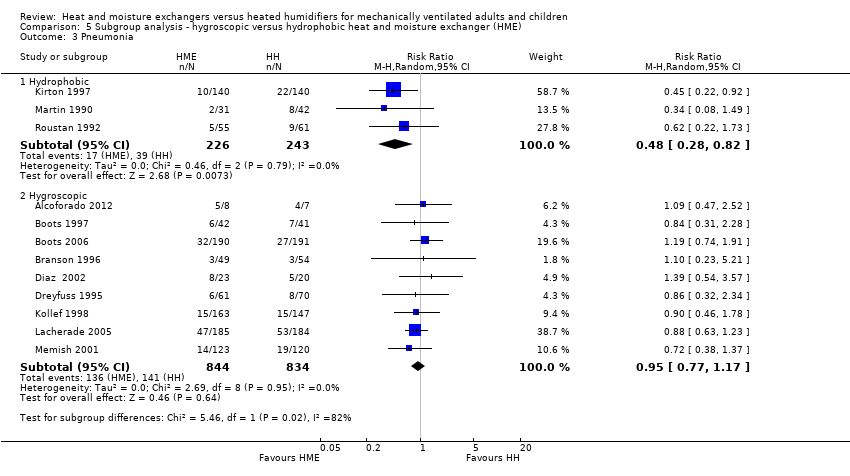
Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 3 Pneumonia.
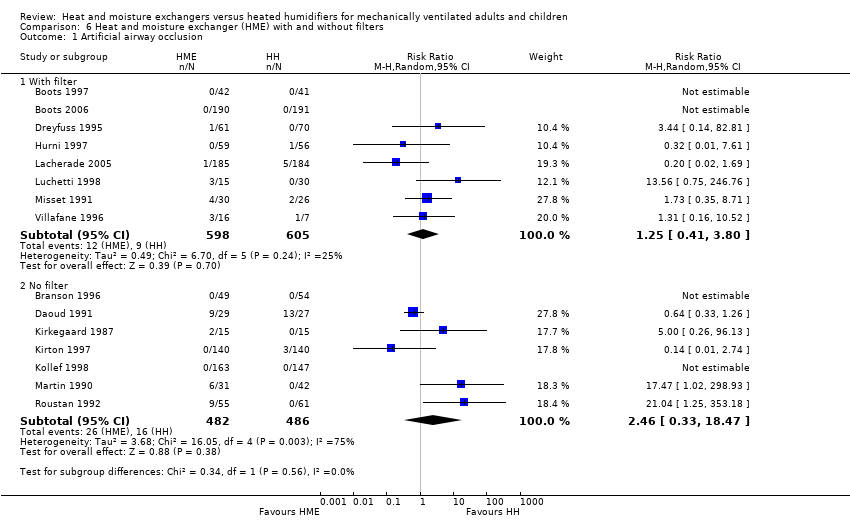
Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 1 Artificial airway occlusion.

Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 2 All‐cause mortality.

Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 3 Pneumonia.

Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 1 Mortality.
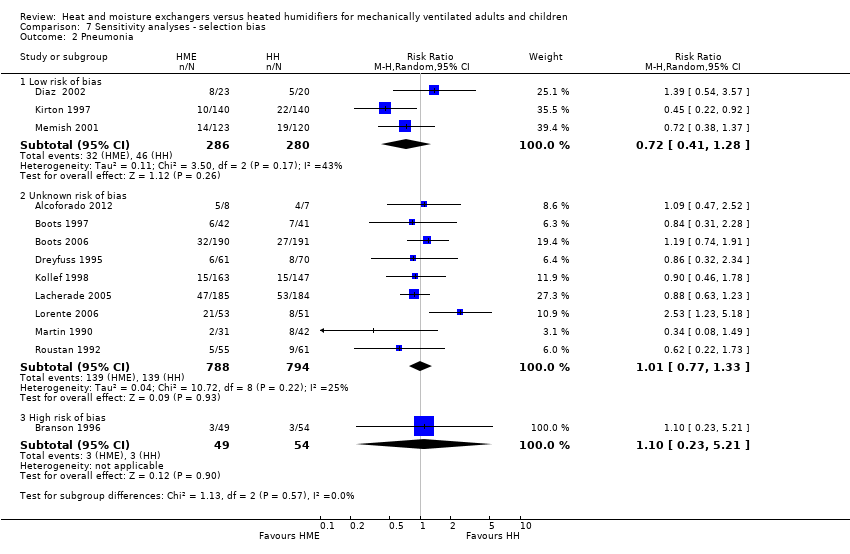
Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 2 Pneumonia.

Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 1 Artificial airway occlusion.
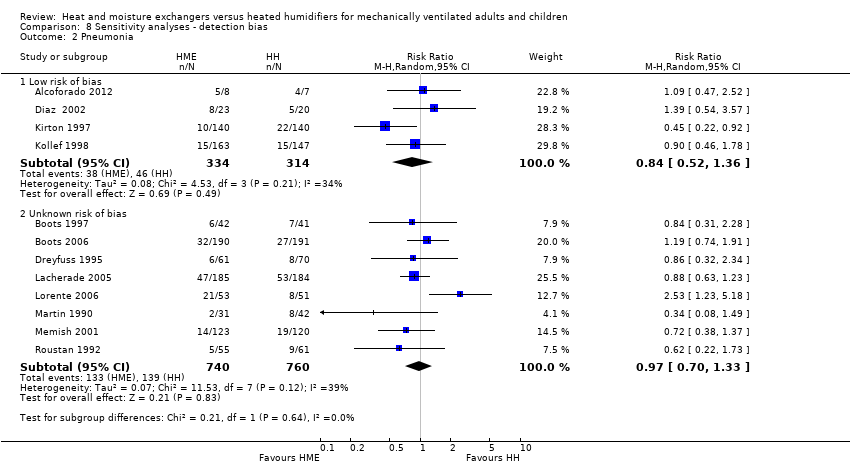
Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 2 Pneumonia.
| Heat and moisture exchangers (HME) compared to heated humidifiers (HH) for ventilated adults and children | ||||||
| Patient or population: ventilated adults (18 trials) and children (1 trial) Settings: ICUs (17), NICU (1), and hospitals (1) in France (7), USA (3), Australia (2), Brazil (2), Denmark (1), Italy (1), Saudi Arabia (1), Spain (1), Switzerland (1) Intervention: HME Comparison: HH | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HH | HME | |||||
| Artificial airway occlusion (measured over 3‐15 days (median 4 days)) | 23 per 1000 | 37 per 1000 | RR 1.59 | 2171 | ⊕⊕⊝⊝ 1 L ow | Allocation and blinding unclear in 13 studies; moderate heterogeneity. |
| Mortality ‐ all cause (Measured over 3‐15 days (median 8 days)) | 247 per 1000 | 257 per 1000 | RR 1.03 | 1951 | ⊕⊕⊝⊝ 2 L ow | Allocation and blinding unclear in 9 and 11 studies; low heterogeneity. |
| Pneumonia (Measured over 4‐21 days (median 4 days)) | 32 per 1000 | 30 per 1000 | RR 0.93 | 2251 | ⊕⊕⊝⊝ 3 L ow | Allocation and blinding unclear in more than half of studies; moderate heterogeneity though this was due to only 1 study. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed and corresponding risks were calculated from data in included trials. 1 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in three studies and could be taken out of the HME group in three studies. 2 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in two studies and could be taken out of the HME group in three studies. 3 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in two studies and could be taken out of the HME group in three studies. | ||||||
| Study | HH | HME | No of participants |
| 9 | 7 | 381 | |
| 4 | 4 | 43 | |
| 13.80 | 11.10 | 104 | |
| 5.30 | 5.70 | 310 | |
| 25.3 | 21.4 | 369 | |
| 9.30 | 13.90 | 116 | |
| HH: heated humidification; HME: heat and moisture exchangers. | |||
| Study | HH | HME | No of participants |
| 11 | 10 | 43 | |
| 16.50 | 16.50 | 310 | |
| HH: heated humidification; HME: heat and moisture exchangers. | |||
| Study | HME | HH | Units | Participants |
| 6.72 | 8.20 | AUD/day | 83 | |
| 8.62 | 9.27 | AUD/day | 381 | |
| 4.70 | 8.97 | USD/day | 99 | |
| 5.00 | 11.00 | USD/day (France) | 131 | |
| 17.46 | 27.80 | USD/participant | 280 | |
| 15.98 | 38.26 | USD/participant | 310 | |
| HH: heated humidification; HME: heat and moisture exchangers. | ||||
| Study | HH | HME | Participants |
| 9.86 | 16.50 | 11 | |
| 13.6 | 20.8 | 10 | |
| HH: heated humidification; HME: heat and moisture exchangers. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| 2 Mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 All cause | 12 | 1951 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.20] |
| 2.2 Pneumonia‐related | 3 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.01] |
| 3 Pneumonia Show forest plot | 13 | 2251 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 3.1 Throughout ventilation | 7 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.2 ≥ 48 hours after ventilation | 6 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.64, 1.46] |
| 4 Atelectasis Show forest plot | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.40] |
| 5 Tracheal aspirations (per day) Show forest plot | 3 | 290 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.41, 0.47] |
| 6 Saline instillations (number per day) Show forest plot | 3 | 276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.17] |
| 7 Change in body temperature (absolute data) (ºC) Show forest plot | 6 | 321 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.96, ‐0.02] |
| 8 Change in body temperature mean data) (ºC) Show forest plot | 3 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.82, ‐0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PaO2 (mmHg) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | ‐3.24 [‐16.08, 9.60] | |
| 1.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | ‐3.87 [‐16.73, 9.00] | |
| 1.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | ‐4.41 [‐17.09, 8.27] | |
| 2 PaCO2 (mmHg) Show forest plot | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 r = 0.3 | 5 | Mean Difference (Random, 95% CI) | 1.93 [0.27, 3.59] | |
| 2.2 r = 0.5 | 5 | Mean Difference (Random, 95% CI) | 2.02 [0.19, 3.85] | |
| 2.3 r = 0.7 | 5 | Mean Difference (Random, 95% CI) | 2.21 [0.33, 4.09] | |
| 3 Breathing rate (breaths/minute) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | 1.40 [0.33, 2.46] | |
| 3.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | 1.15 [‐0.13, 2.44] | |
| 3.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | 1.02 [‐0.38, 2.41] | |
| 4 Tidal volume (L) Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 4.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 0.02 [‐0.00, 0.03] | |
| 4.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 0.02 [0.00, 0.04] | |
| 4.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 0.03 [0.01, 0.06] | |
| 5 Minute ventilation (L/minute) Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 5.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 1.20 [0.78, 1.61] | |
| 5.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 1.19 [0.63, 1.75] | |
| 5.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 1.18 [0.55, 1.80] | |
| 6 Body temperature (ºC) Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 r = 0.3 | 2 | Mean Difference (Random, 95% CI) | ‐1.12 [‐3.77, 1.52] | |
| 6.2 r = 0.5 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.77, 1.52] | |
| 6.3 r = 0.7 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.78, 1.51] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.33, 1.26] |
| 1.2 Adults | 14 | 2115 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.65, 5.76] |
| 2 All‐cause mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.62, 12.67] |
| 2.2 Adults | 11 | 1895 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Medium‐term | 8 | 1031 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.41, 7.30] |
| 1.2 Long‐term | 7 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.34, 6.36] |
| 2 All‐cause mortality Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Medium‐term | 5 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.31] |
| 2.2 Long‐term | 6 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 3 Pneumonia Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Medium‐term | 5 | 892 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |
| 3.2 Long‐term | 6 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Hydrophobic | 5 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.65, 12.62] |
| 1.2 Hygroscopic | 11 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.48] |
| 2 All‐cause mortality Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hydrophobic | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.35] |
| 2.2 Hygroscopic | 9 | 1719 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 3 Pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Hydrophobic | 3 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.82] |
| 3.2 Hygroscopic | 9 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 With filter | 8 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.80] |
| 1.2 No filter | 7 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.33, 18.47] |
| 2 All‐cause mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 With filter | 5 | 1080 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
| 2.2 No filter | 7 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.85, 1.36] |
| 3 Pneumonia Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 With filter | 5 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 3.2 no filter | 8 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.90] |
| 1.2 Unknown risk of bias | 10 | 1665 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.16] |
| 2 Pneumonia Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias | 3 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| 2.2 Unknown risk of bias | 9 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 2.3 High risk of bias | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.23, 5.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Artificial airway occlusion Show forest plot | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| 1.1 Low risk of bias | 2 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.74] |
| 1.2 Unclear risk of bias | 13 | 1581 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.69, 5.34] |
| 2 Pneumonia Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias | 4 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.36] |
| 2.2 Unknown risk of bias | 8 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.33] |

