早産児の退院後における栄養強化調合乳と標準的調合乳の比較
アブストラクト
背景
退院時における早産児の成長には、しばしば限界がある。退院後の早産児に標準的な調整粉乳ではなく栄養強化した調整粉乳を与えることは、成長の遅れを取り戻す助けとなり、発達を改善する可能性がある。
目的
退院後の早産児の成長と発達について、栄養強化した調整粉乳の効果を標準的な調整粉乳と比較すること。
検索戦略
標準的な検索方法で、Cochrane Neonatal Review Groupを検索した。コクラン・ライブラリのCochrane Central Register of Controlled Trials(2016年、第8号)、MEDLINE、Embase、Cumulative Index to Nursing and Allied Health Literature(CINAHL、2016年9月8日まで)、および会議の議事録や過去のレビューを検索した。
選択基準
退院後の早産児を対象として栄養強化した調整粉乳(退院後用調整粉乳または早産児用調整粉乳)の効果を、標準的な満期産用調整粉乳と比較したランダム化および準ランダム化比較試験。
データ収集と分析
2名のレビュー著者がそれぞれ試験の適格性とバイアスのリスクを評価し、データを抽出した。各試験に記載された治療効果を解析し、二値データについてはリスク比とリスク差を、連続データについては平均差(MD)を、95%信頼区間(CI)を付して報告した。メタアナリシスには固定効果モデルを用いた。また、感度解析を実施して考えられる異質性の原因を調べた。Grading of Recommendations Assessment, Development and Evaluation(GRADE)法を用いて、アウトカムレベルでエビデンスの質を評価した。
主な結果
計1251例の乳児を対象とした16件の適格な試験を選択した。試験の方法論的な質はさまざまで、割りつけの隠蔽化(コンシールメント)の欠如や追跡調査の未完了が、バイアスの主な原因として考えられた。退院後用調整粉乳群(エネルギー密度は約74kcal/100mL)と標準的な満期産用調整粉乳群(約67kcal/100mL)を比較した試験(N = 11)では、出産予定日後12~18カ月までの成長パラメーターについて一貫したエビデンスはみられなかった。GRADE評価では、エビデンスの質は中等度で、プールされた推定値の不一致が主な質の問題であることが示唆された。
早産児用調整粉乳群(約80kcal/100mL)は、満期産用調整粉乳群と比較した試験(N=5)で、幼年期を通じて成長率が高いことを示すエビデンスがみられた(出産予定日後12~18カ月時点の重み付け平均差:体重約500g、身長5~10mm、頭囲5mm)。GRADE評価では、エビデンスの質は中等度で、推定値の不一致が主な質の問題であることが示唆された。
神経発達アウトカムを評価した試験がわずかにあったが、これらの試験では出産予定日後18カ月の時点で発達指標に差は認められなかった。小児期を通じた成長や発達に関するデータはない。
著者の結論
退院後用調整粉乳を退院後の早産児に処方する現在の推奨は、入手可能なエビデンスでは支持されない。限定的なエビデンスでは、早産児用調整粉乳(通常は院内使用のみ)を退院後の早産児に与えると、出産予定日後18カ月までの成長率が増加する可能性を示唆している。
PICO
一般語訳
早産児の退院後における栄養強化調合乳と標準的調合乳の比較
レビューの論点:栄養を強化した早産児用調整粉乳は標準的な調整粉乳(満期産児用)と比較して、退院後の成長率を増加させ発達を改善するのか?
背景:早産児が誕生後に治療を受け、退院して帰宅する準備ができる頃、多くの早産児は、早産にならず満期まで子宮にいた場合よりも身長が低く体重が軽い。満期産児に用いられる標準的な調整粉乳よりも栄養を強化した早産児用調整粉乳は、早産児の成長を早めて満期産児に追いつくのを助け、発達を改善する可能性がある。
試験の特性:2016年9月8日に再検索し、計1251例の乳児を登録した16件の適格な試験を同定した。
主な知見:これらの試験では中等度の質のエビデンスにより、栄養強化した調整粉乳を無制限に与えても、標準的な調整粉乳と比較して、生後約18カ月までの成長と発達に重要な影響を与えないことが示されている。長期的な成長と発達についてはまだ評価されていない。
結論:退院後の早産児に栄養を強化した調整粉乳を処方する現在の推奨は、入手可能なエビデンスでは支持されない。
Authors' conclusions
Summary of findings
| Postdischarge formula compared with standard term formula for preterm infants after hospital discharge | ||||
| Patient or population: preterm infants after hospital discharge | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of participants | Quality of the evidence | Comments |
| Postdischarge formula vs standard term formula | ||||
| Weight (grams) 3‐4 months post term | MD 7.45 g lower | 523 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 62%) |
| Weight (grams) 6 months post term | MD 35.54 g higher | 576 | ⊕⊕⊕⊝ | Downgraded for inconsistency moderate (I² = 64%). |
| Crown‐heel length (mm) 3‐4 months post term | MD 2.45 mm higher | 523 | ⊕⊕⊕⊝ | Downgraded for high inconsistency (I² = 81%) |
| Crown‐heel length (mm) 6 months post term | MD 2.12 mm higher | 576 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 75%) |
| Head circumference (mm) 3‐4 months post term | MD 0.3 mm lower | 523 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 71%) |
| Head circumference (mm) 6 months post term | MD 2.28 mm higher | 576 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 69%) |
| Development ‐ Bayley Scales of Infant Development II: Mental Development Index | MD 0.9 higher | 184 | ⊕⊕⊕⊕ | |
| Preterm formula compared with standard term formula for preterm infants after hospital discharge | ||||
| Patient or population: preterm infants after hospital discharge | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of participants | Quality of the evidence | Comments |
| Preterm formula vs standard term formula | ||||
| Weight (grams) 3‐4 months post term | MD 74.41 g higher | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Weight (grams) 6 months post term | MD 74.6 g higher | 273 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Crown‐heel length (mm) 3‐4 months post term | MD 2.27 mm lower | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Crown‐heel length (mm) 6 months post term | MD 1.83 mm higher | 160 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Head circumference (mm) 3‐4 months post term | MD 3.61 mm higher | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Head circumference (mm) 6 months post term | MD 5.82 mm higher | 160 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Development ‐ Bayley Scales of Infant Development II: Mental Development Index | MD 1.44 lower | 143 | ⊕⊕⊕⊕ | |
Background
Compared with term infants, preterm infants have limited nutrient reserves at birth. Also, preterm infants, especially very preterm and very low birth weight (VLBW) infants, are subject to a variety of physiological and metabolic stresses that increase their nutrient needs. Recommended nutrient requirements for preterm infants are based on intrauterine growth studies and assume that the optimal rate of postnatal growth should be about the same as that of uncompromised fetuses of an equivalent postmenstrual age. Evidence indicates, however, that recommended target levels of nutrient input are rarely achieved in practice, and that most very preterm or VLBW infants accumulate significant energy, protein, mineral and other nutrient deficits during their initial hospital stay (Embleton 2001). By the time they are ready to go home, typically at around 36 to 40 weeks' postmenstrual age, many infants are substantially growth‐restricted relative to their term‐born peers (Clark 2003;Dusick 2003; Lucas 1984).
Description of the condition
After hospital discharge, responsively (demand) fed preterm infants often consume relatively more milk than term infants to attain 'catch‐up' growth (Lucas 1992a). Despite this, growth deficits can persist through childhood and adolescence (Bracewell 2008; Farooqi 2006; Ford 2000; Trebar 2007). Slow or incomplete catch‐up growth is associated with higher risk of neurodevelopmental impairment in later childhood, and with poorer cognitive and educational outcomes (Cooke 2003; Hack 1991; Leppanen 2014). Preterm infants who have accumulated mineral deficits have higher risks of metabolic bone disease and slower skeletal growth compared with infants born at term, although uncertainty remains about long‐term effects of such deficits on bone mass and health (Fewtrell 2011). Furthermore, nutritional deficiency and growth restriction in utero and during early infancy may have consequences for long‐term metabolic and cardiovascular health (Embleton 2013; Lapillonne 2013).
Description of the intervention
Because slow or incomplete catch‐up growth is associated with prolonged growth restriction and slower neurodevelopmental progression, attention has focused on nutritional interventions that might promote growth during the putative ‘critical window’ of early infancy in the post‐hospital discharge period. Two broad strategies for nutritional interventions are known (Dusick 2003; Fewtrell 2003; Klingenberg 2011).
-
Multi‐nutrient fortification of expressed milk for infants fed with human breast milk.
-
Nutrient‐enriched formula for formula‐fed infants.
Another Cochrane review addresses the question of whether multi‐nutrient fortification of human breast milk affects growth and development in preterm infants after hospital discharge (McCormick 2013). This review focuses on the comparison of nutrient‐enriched formula versus standard formula for formula‐fed preterm infants after hospital discharge.
A variety of standard and nutrient‐enriched formula preparations are available (Aggett 2006; Griffin 2007). These can be categorised broadly as:
-
standard term formula: designed for term infants, based on the composition of mature human breast milk. The typical energy content is 66 to 68 kcal/100 mL. The concentration of protein ‐ approximately 1.4 to 1.7 g/100 mL ‐ and calcium and phosphate content (about 50 mg/100 mL and 30 mg/100 mL, respectively) are not sufficient to satisfy recommended nutrient needs for stable and growing preterm infants;
-
postdischarge formula: specifically designed for preterm infants post discharge from the hospital. These are energy (about 72 to 74 kcal/100 mL) and protein (about 1.8 to 1.9 g/100 mL) enriched and are variably enriched with minerals, vitamins and trace elements compared with standard term formula. Expert bodies and authorities recommend these formulas for preterm infants for three to 12 months post discharge (Aggett 2006); and
-
preterm formula: energy‐enriched (about 80 kcal/100 mL), protein‐enriched (2.0 to 2.4 g/100 mL) and variably enriched with minerals, vitamins and trace elements to support intrauterine nutrient accretion rates. These formulas are commonly used for nutrition of preterm infants before hospital discharge and generally are not recommended for postdischarge feeding.
How the intervention might work
Feeding preterm infants after hospital discharge formula enriched with extra energy, protein, minerals and vitamins may be expected to promote rapid catch‐up growth. However, because preterm infants fed in response to hunger and satiation cues (demand or responsive feeding) adjust their volume of intake according to the energy density of formula, infants may consume less nutrient‐enriched milk than standard formula (Lucas 1992a). Consequently, infants fed responsively with preterm or postdischarge formula may not receive any more energy (or other nutrients, depending on the nutrient:energy ratio) than infants fed standard term formula.
Feeding of nutrient‐enriched formula may be associated with disordered gastric motility and emptying (Hancock 1984; Siegel 1984). Nutrient‐enriched formula may therefore be poorly tolerated, thereby reducing nutrient delivery and potentially removing any benefit for growth and development. Furthermore, catch‐up growth with accelerated weight gain and crossing of body mass index (BMI) percentiles might be associated with altered fat distribution and related ‘programmed’ metabolic consequences that may increase the risk of insulin resistance and cardiovascular disease (Doyle 2004; Euser 2005; Euser 2008; Hack 2003; Saigal 2006).
Why it is important to do this review
Given the potential for postdischarge nutrition strategies to affect growth and development in preterm infants, and the fact that uncertainty surrounds the balance between putative benefits and harms, this review was undertaken to detect, appraise and synthesise available evidence from randomised controlled trials to inform practice and research.
Objectives
To compare the effects of nutrient‐enriched formula versus standard formula on growth and development of preterm infants after hospital discharge.
Methods
Criteria for considering studies for this review
Types of studies
We included controlled trials using random or quasi‐random patient allocation, including cluster‐randomised controlled trials. We did not include cross‐over trials. Studies published as abstracts were eligible for inclusion only if assessment of study quality was possible, and if other criteria for inclusion were fulfilled.
Types of participants
We included preterm infants fed formula (exclusively or as a supplement to human breast milk) after discharge from hospital. The intervention may have commenced up to one week before planned discharge from hospital. We did not include in this review trials that randomly assigned infants to nutrient‐enriched formula versus standard term formula more than one week before hospital discharge (and then continued the intervention after hospital discharge).
Types of interventions
-
Standard term formula: energy content ≤ 72 kcal/100 mL and protein content ≤ 1.7 g/100 mL
versus
-
Postdischarge formula: energy content > 72 kcal/100 mL (but ≤ 75 kcal/100 mL) and protein content > 1.7 g /100 mL; or
-
Preterm formula: energy content > 75 kcal/100 mL and protein content > 2.0 g/100 mL.
The formula could be fed as the sole diet or as a supplement to human breast milk. Infants in trial groups should have received similar care other than the type of formula. Target levels prescribed for volume of intake and advice or support for demand feeding should have been no different among groups.
Types of outcome measures
Primary outcomes
-
Growth: weight, length, head growth, skinfold thickness, BMI and measures of body composition (lean/fat mass) and growth restriction (proportion of infants who remain < 10th percentile for distribution of weight, length or head circumference in the index population). Long‐term growth and growth restriction (proportion of infants who remain below the 10th percentile for distribution of weight, height or head circumference in the index population)
-
Development
-
Neurodevelopmental outcomes assessed by validated tools at ≥ 12 months' corrected age; classifications of disability, including non‐ambulant cerebral palsy, developmental delay and auditory and visual impairment
-
Cognitive and educational outcomes at ≥ 5 years: intelligence quotient and/or indices of educational achievement measured by a validated tool (including school examination results)
-
Secondary outcomes
-
Feed intolerance such as vomiting or diarrhoea that necessitates ceasing the study formula
-
Measures of bone mineralisation such as serum alkaline phosphatase level, or assessment of bone mineral content by dual‐energy X‐ray absorptiometry and clinical or radiological evidence of rickets on long‐term follow‐up
-
Blood pressure on long‐term follow‐up
-
Body mass index or other measures of overweight or obesity on long‐term follow‐up
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group.
Electronic searches
We updated the search on 8 September 2016 using a combination of the following terms: (infant nutrition OR infant formula OR milk OR formula OR nutrient OR fortif* OR supplemt*) AND (postdischarge OR post‐discharge OR discharge) plus the following database‐specific terms limited by relevant search filters for clinical trials, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.
-
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
-
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
-
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
We applied no language restrictions.
We searched ClinicalTrials.gov and Current Controlled Trials for completed and ongoing trials.
Searching other resources
We examined the references provided in studies identified as potentially relevant. We searched abstracts from annual meetings of the Pediatric Academic Societies (1993 to 2016), the European Society for Pediatric Research (1995 to 2016), the UK Royal College of Paediatrics and Child Health (2000 to 2016) and the Perinatal Society of Australia and New Zealand (2000 to 2016). We considered trials reported only as abstracts to be eligible if sufficient information was available from the report, or from contact with study authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
Two review authors screened the titles and abstracts of all studies identified by the above search strategy. We reassessed the full texts of potentially eligible reports and excluded studies that did not meet all of the inclusion criteria. We discussed disagreements until consensus was achieved.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted study data separately. We discussed disagreements until consensus was achieved and asked investigators for further information if data provided in the trial reports were insufficient.
Assessment of risk of bias in included studies
We used criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of included trials. Two review authors conducted assessment of risk of bias and resolved disagreements by discussion. We requested additional information from trial authors to clarify methods and results if necessary.
We made explicit judgements about whether studies were at high risk of bias across four domains, according to the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Random sequence generation: We categorised the method used to generate the allocation sequence as:
-
low risk ‐ any truly random process (e.g. random number table, computer random number generator);
-
high risk ‐ any non‐random process (e.g. odd or even date of birth, hospital or clinic record number); or
-
unclear risk ‐ no or unclear information provided.
-
-
Allocation concealment: We categorised the method used to conceal the allocation sequence as:
-
low risk (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
-
high risk ‐ open random allocation (e.g. unsealed or non‐opaque envelopes, alternation; date of birth); or
-
unclear ‐ no or unclear information provided.
-
-
Blinding: We assessed blinding of participants, clinicians and caregivers and outcome assessors separately for different outcomes and categorised the methods as:
-
low risk;
-
high risk; or
-
unclear risk.
-
-
Incomplete outcome data: We described completeness of data, including attrition and exclusions from analysis for each outcome and reasons for attrition or exclusion, when reported. We assessed whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or was supplied by trial authors, we planned to reinstate missing data to the analyses. We categorised completeness as:
-
-
low risk: ≤ 10% missing data;
-
high risk: > 10% missing data; or
-
unclear risk: no or unclear information provided.
-
We assessed the likely magnitude and direction of bias and whether we considered it likely for bias to impact study findings. We planned to explore the impact of the level of bias by performing sensitivity analyses.
Measures of treatment effect
We analysed treatment effects in individual trials by using Review Manager 5.3 and reported risk ratios (RRs) and risk differences (RDs) for dichotomous data and mean differences (MDs) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for analyses with statistically significant differences among RDs.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. For cluster‐randomised trials (had we identified any for inclusion), we planned to undertake analyses at the level of the individual while accounting for clustering of data by using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We requested additional data from trial investigators if data on important outcomes were missing or were reported unclearly. When data were still missing, we examined the impact of this on effect size estimates by performing sensitivity analyses.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high (I² > 50%) heterogeneity, we explored possible causes (e.g. differences in study design, participants or interventions; completeness of outcome assessments) by performing sensitivity analyses.
Assessment of reporting biases
If more than five trials were included in a meta‐analysis, we conducted a funnel plot analysis.
Data synthesis
We used the fixed‐effect model in RevMan 5.1 to perform meta‐analysis.
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a), which considers evidence from randomised controlled trials as of high quality that may be downgraded on the basis of consideration of any of five areas.
-
Design (risk of bias).
-
Consistency across studies.
-
Directness of the evidence.
-
Precision of estimates.
-
Presence of publication bias.
The GRADE approach is used to assess the quality of a body of evidence according to four grades (Schünemann 2013).
-
High: We are very confident that the true effect lies close to the estimate of effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Two review authors assessed independently the quality of the evidence for outcomes identified as critical or important for clinical decision making (growth, development).
In cases for which we considered risk of bias to arise from inadequate concealment of allocation, randomised assignment, complete follow‐up or blinded outcome assessment, to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency on the basis of similarity of point estimates, extent of overlap of confidence intervals and statistical criteria, including measurement of heterogeneity (I²). We downgraded the quality of evidence when inconsistency across study results was large and unexplained (i.e. some studies suggested important benefit, and others no effect or harm with no explanation) (Guyatt 2011c). We assessed precision accordingly with the 95% confidence interval (CI) around the pooled estimation (Guyatt 2011d). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e).
We entered data (pooled estimates of effects and 95% CIs) and explicit judgements for each of the above aspects assessed into the Guideline Development Tool, the software used to create 'Summary of findings (SoF)' tables (GRADEpro 2008). We explained our assessment of study characteristics in footnotes in the SoF table.
Subgroup analysis and investigation of heterogeneity
We prespecified the following subgroup analyses.
-
Very preterm (< 32 weeks' gestation) or VLBW (< 1500 g) infants (vs infants at 32 to 36 weeks' gestation or birth weight 1500 to 2499 g).
-
Infants who were small for gestational age (< 10th percentile for weight) at hospital discharge (vs infants ≥ 10th percentile).
-
Infants with chronic lung disease receiving supplemental oxygen therapy at hospital discharge (vs infants without chronic lung disease).
Results
Description of studies
We identified two potentially eligible study reports. We included one new trial (Roggero 2011). We have not yet been able to obtain the full article reporting a potentially eligible trial and have categorised this as a 'study awaiting classification' (Ekcharoen 2015). See Figure 1.
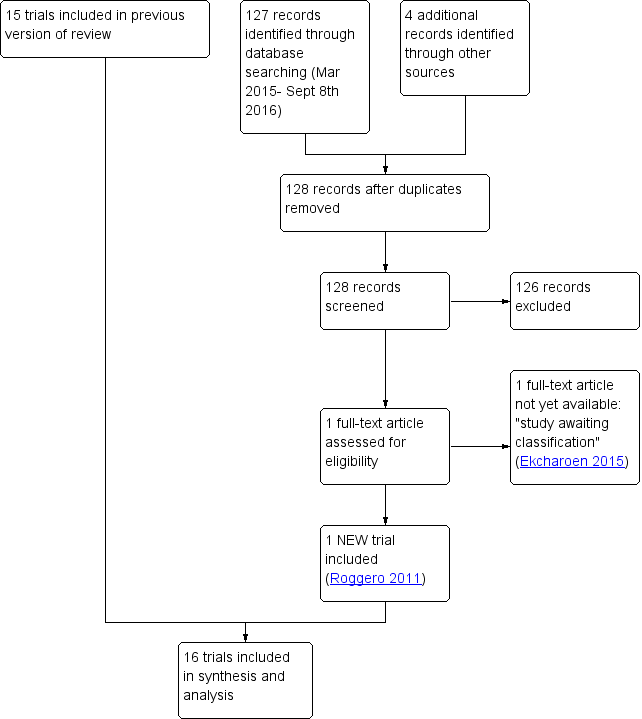
Study flow diagram: review update.
Included studies
In total, we identified 16 trials that fulfil review eligibility criteria (Agosti 2003; Atkinson 1999; Atkinson 2004; Carver 2001; Cooke 2001; De Curtis 2002; Jeon 2011; Koo 2006; Litmanovitz 2004; Lucas 1992; Lucas 2001; Peng 2004; Picaud 2005; Roggero 2011; Roggero 2012; Taroni 2009). See Characteristics of included studies.
Participants
Trials were undertaken within the past 20 years by investigators attached to perinatal centres in Europe, North America and the Middle East. In total, 1251 infants participated in these trials (range 20 to 229).
Most trials specified a maximum birth weight as the primary eligibility criterion.
-
1500 g: Agosti 2003; Litmanovitz 2004; Jeon 2011; Taroni 2009.
-
1750 g: Cooke 2001; De Curtis 2002; Lucas 2001; Picaud 2005.
-
1800 g: Atkinson 1999; Carver 2001.
-
1850 g: Lucas 1992; Peng 2004.
Four trials specified gestational age as an eligibility criterion.
-
< 35 weeks: Koo 2006.
-
< 37 weeks: Atkinson 2004; Roggero 2011; Roggero 2012.
Three trials specifically recruited participants who were small for gestational age.
-
Birth weight < 5th percentile: Atkinson 2004.
-
Birth weight < 10th percentile: Taroni 2009; Roggero 2012.
Although most reports of the other trials did not specify intrauterine or postnatal growth restriction as an exclusion criterion, it appears that very few participants in the trials were small for gestational age at birth or enrolment. Generally, infants with additional problems at discharge, particularly those with inadequate independent oral feeding or receipt of supplemental oxygen secondary to chronic lung disease, were not eligible to participate.
Interventions
Postdischarge formula versus standard term formula (Comparison 1)
Eleven trials (N = 885): Atkinson 1999; Atkinson 2004; Carver 2001; De Curtis 2002; Koo 2006; Litmanovitz 2004; Lucas 1992; Lucas 2001; Roggero 2011; Roggero 2012; Taroni 2009.
Preterm formula versus standard term formula (Comparison 2)
Five trials (N = 366): Agosti 2003; Cooke 2001; Jeon 2011; Peng 2004; Picaud 2005.
All participating infants were fed ad libitum. These feeds were intended to be the principal source of milk for a range of periods post term (or post hospital discharge).
-
One month: Taroni 2009.
-
Two months: De Curtis 2002; Picaud 2005.
-
Three months: Agosti 2003; Jeon 2011.
-
Six months: Cooke 2001; Litmanovitz 2004; Peng 2004; Roggero 2011; Roggero 2012.
-
Nine months: Lucas 1992; Lucas 2001.
-
Up to 12 months: Atkinson 1999; Atkinson 2004; Carver 2001; Koo 2006.
Outcomes
The main outcomes assessed were growth parameters (weight, length and occipitofrontal head circumference) assessed up to 12 to 18 months' corrected age. Three trials assessed neurodevelopmental outcomes at 18 months using Bayley Scales of Infant Development II (Cooke 2001; Jeon 2011; Lucas 2001). One trial used Griffiths' Developmental Scales at six, nine and 12 months' corrected age (Agosti 2003).
Excluded studies
We excluded nine studies (Amesz 2010; Bernbaum 1989; Bhatia 1991; Brunton 1998; Chan 1994; Cooper 1985; Friel 1993; Lapillonne 2004; Wheeler 1996) and listed reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
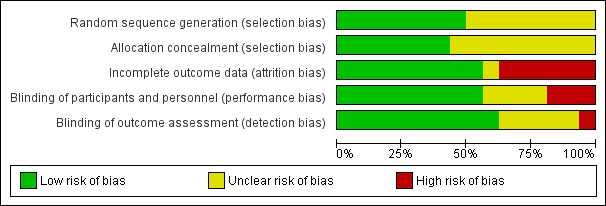
Trials were of variable methodological quality (Figure 2).
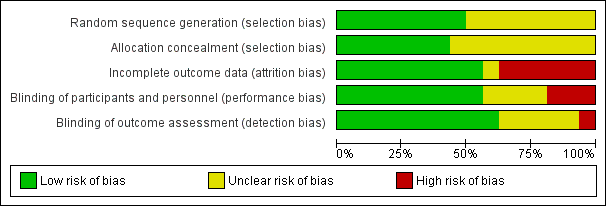
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In eight trials (Atkinson 1999; Atkinson 2004; Cooke 2001; Koo 2006; Lucas 2001; Picaud 2005; Roggero 2011; Roggero 2012), the method of randomisation described was likely to ensure blinding of allocation. In the other trials, it is not clear whether allocation concealment was adequate. In one of these trials, substantial between‐group differences in baseline demographic characteristics were evident, most likely due to allocation bias (Jeon 2011). This trial originally randomised participants to one of three intervention groups. We elected to discard data from the group in which infant characteristics were statistically significantly different from those of other groups at enrolment.
Blinding
Most trials blinded families to the type of milk that the infant received. In three trials (Agosti 2003; Jeon 2011; Peng 2004), it is likely that families were aware of which type of milk their infant had been allocated to receive. It is unclear whether blinding was satisfactory in another four trials (Litmanovitz 2004; Roggero 2011; Roggero 2012; Taroni 2009).
Most trials blinded outcome assessors and investigators to the type of milk that the infant received, but in five trials (Jeon 2011; Litmanovitz 2004; Roggero 2011; Roggero 2012; Taroni 2009), it is unclear whether blinding was satisfactory. In one trial (Peng 2004), physicians were unblinded.
Incomplete outcome data
Eleven trials (Atkinson 2004; Cooke 2001; De Curtis 2002; Jeon 2011; Litmanovitz 2004; Lucas 1992; Lucas 2001; Peng 2004; Roggero 2011; Roggero 2012; Taroni 2009) achieved complete or near‐complete (> 90%) assessment. In two other trials (Atkinson 1999; Koo 2006), 75% of infants underwent outcome assessments at latest follow‐up. In another two trials (Agosti 2003; Carver 2001), less than 50% of infants completed the planned 12‐month follow‐up assessment. In Picaud 2005, loss to follow‐up by 12 months in the control group was substantial (35%) and was greater than in the intervention group (9%).
Effects of interventions
See: Summary of findings for the main comparison Postdischarge formula compared with standard term formula for preterm infants after hospital discharge; Summary of findings 2 Preterm formula compared with standard term formula for preterm infants after hospital discharge
Postdischarge formula versus standard term formula (Comparison 1)
Growth (Outcomes 1.1 to 1.4)
Lucas 1992 detected no statistically significantly differences in weight, length or head circumference at the end of intervention and follow‐up periods (nine months' corrected age).
Atkinson 1999 reported that infants who received postdischarge formula were statistically significantly heavier at six, nine and 12 months' corrected age, and researchers noted no statistically significant differences in length or head circumference.
Carver 2001 detected no statistically significant differences in weight, length or head circumference at six and 12 months' corrected age. Loss to follow‐up during the trial was substantial, and as the published report does not state how many infants were assessed at various time points, we could not use data to calculate mean differences.
Lucas 2001 reported that at completion of the intervention period (nine months' corrected age), weight and length were statistically significantly greater among infants who received postdischarge formula but noted no statistically significant differences in head circumference. At 18 months, results showed no statistically significant differences in weight or head circumference. The group of infants who received postdischarge formula remained statistically significantly longer on average than infants in the control group (MD 9.0, 95% CI 0.3 to 17.7 mm).
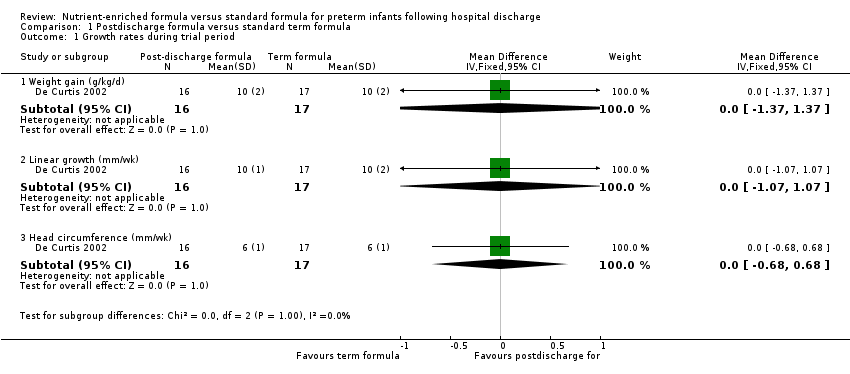
De Curtis 2002 found no statistically significant differences in rate of gain of weight, length or head circumference during the two‐month trial period.
Atkinson 2004 reported no statistically significant differences in rate of gain of weight, length or head circumference up to 12 months' corrected age (growth data reported as z scores).
Litmanovitz 2004 noted no statistically significant differences in weight, length or head circumference at six months' corrected age.
Koo 2006 reported that mean weight, head circumference and length were less in the nutrient‐enriched formula group at six, nine and 12 months after hospital discharge.
Taroni 2009 found no statistically significant differences in weight, length or head circumference at one month corrected age.
Roggero 2011 reported no statistically significant differences in weight, length or head circumference at three, six or 12 months' corrected age.
Roggero 2012 noted no statistically significant differences in weight, length or head circumference at three, six or 12 months' corrected age.
Meta‐analyses of growth data
-
Weight (Outcome 1.2; Figure 3): Meta‐analyses detected no statistically significant differences in weight at three to four and six months' corrected age. At nine months, meta‐analysis of data from four trials (Atkinson 1999; Koo 2006; Lucas 1992; Lucas 2001) indicated that infants in the postdischarge formula group were heavier (weighted mean difference (WMD) 244, 95% CI 17 to 471 g). At 12‐month follow‐up, results showed no statistically significant differences.
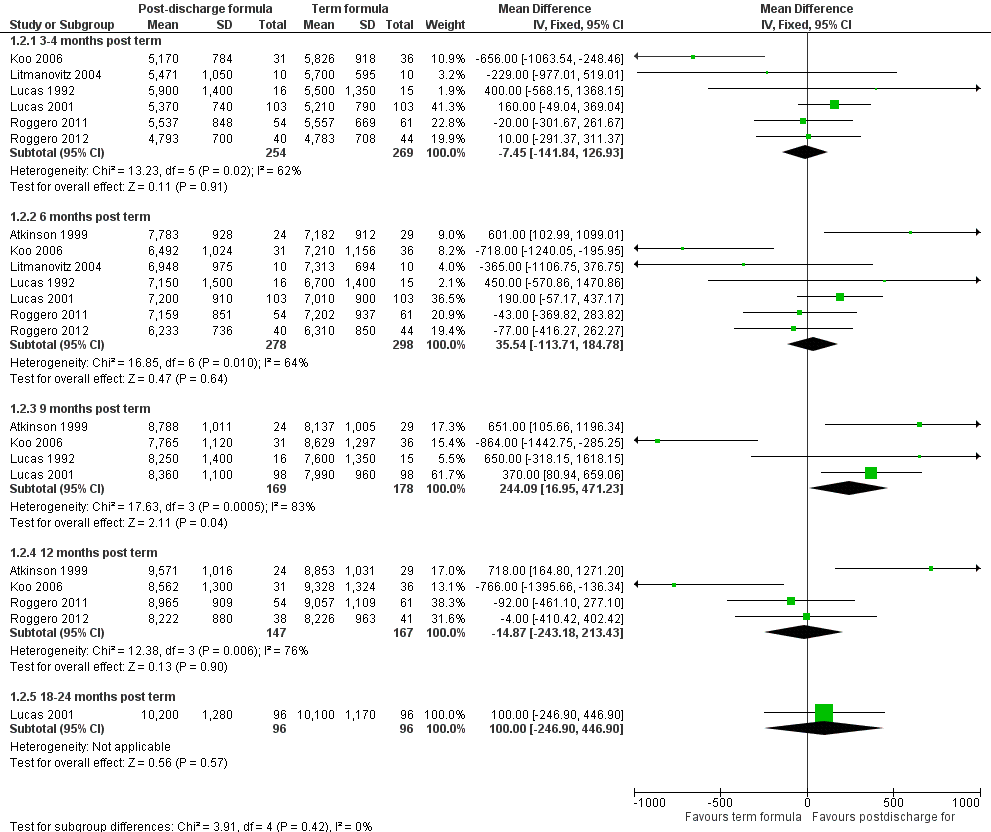
Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.2 Weight (grams).
-
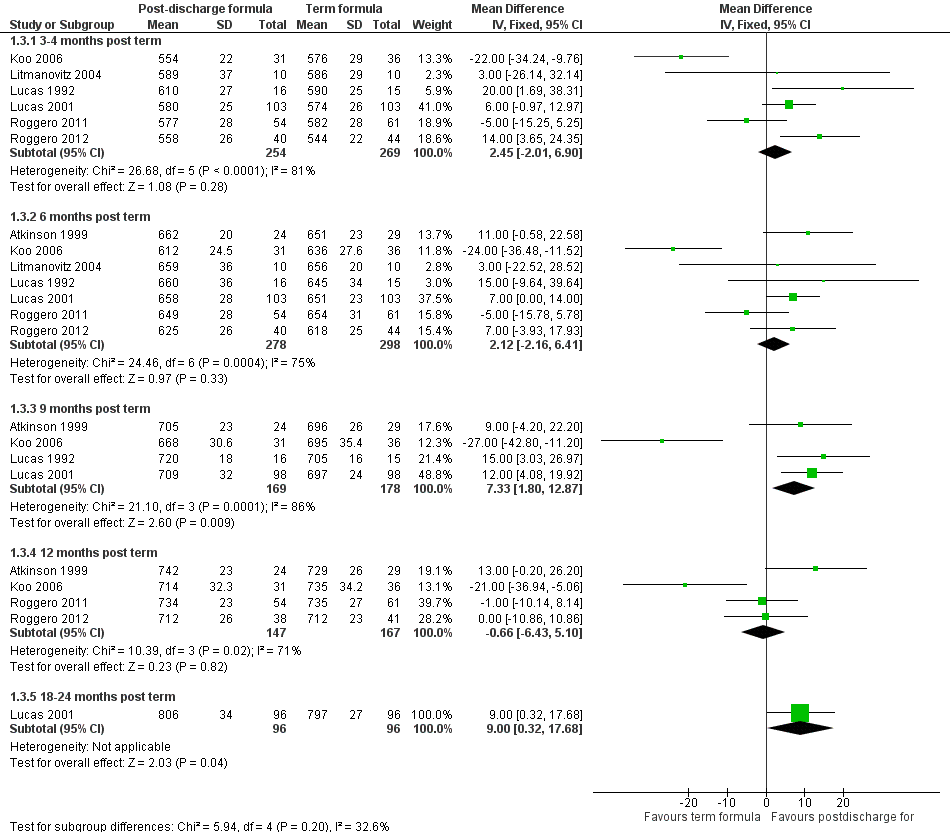
Length (Outcome 1.3; Figure 4): Meta‐analyses detected no statistically significant differences in weight at three to four and six months' corrected age. At nine months, meta‐analysis of data from four trials (Atkinson 1999; Koo 2006; Lucas 1992; Lucas 2001) indicated that infants in the postdischarge formula group were longer (WMD 7.3, 95% CI 1.8 to 12.9 mm). At 12‐month follow‐up, results showed no statistically significant differences.
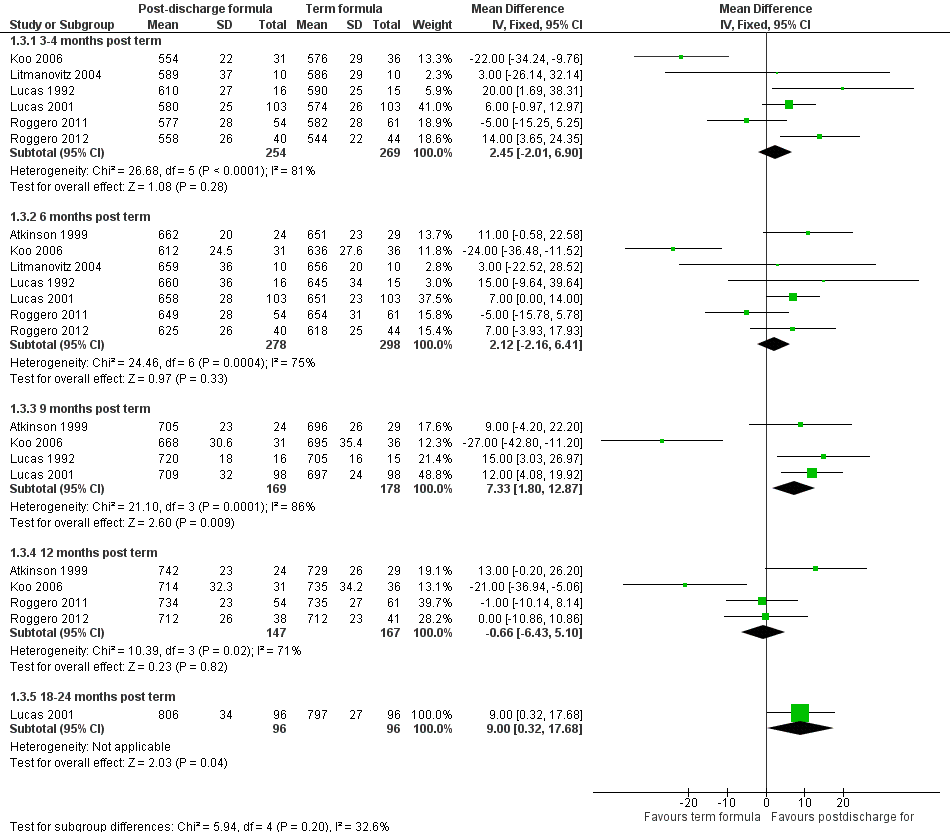
Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.3 Crown‐heel length (mm).
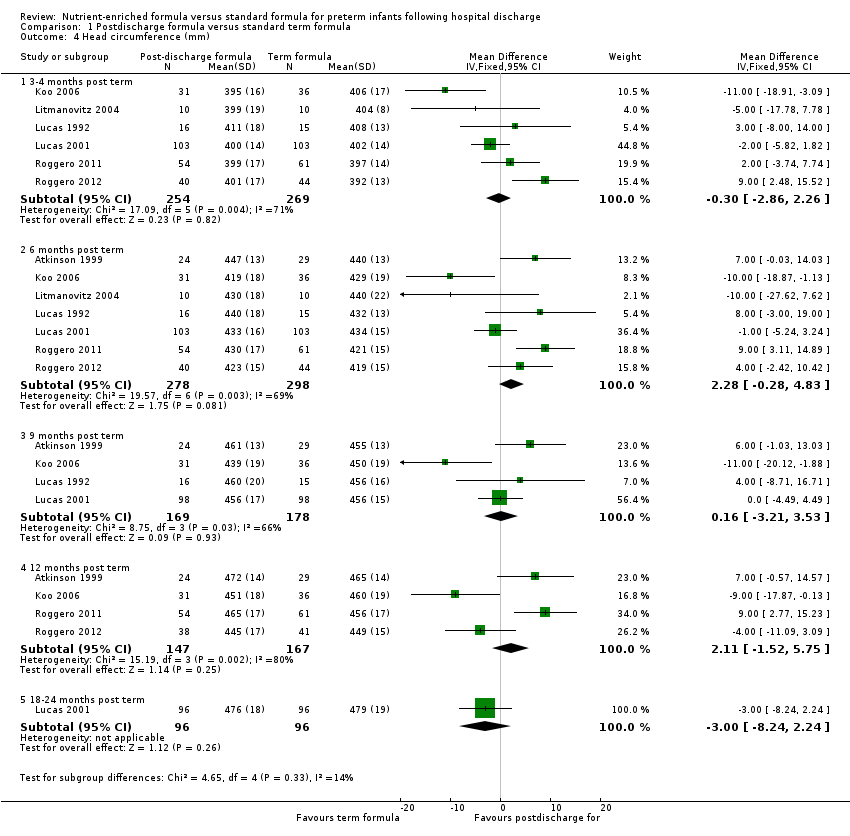
-
Head circumference (Outcome 1.4; Figure 5): Meta‐analyses detected no statistically significant differences at three to four, six, nine or 12 months.
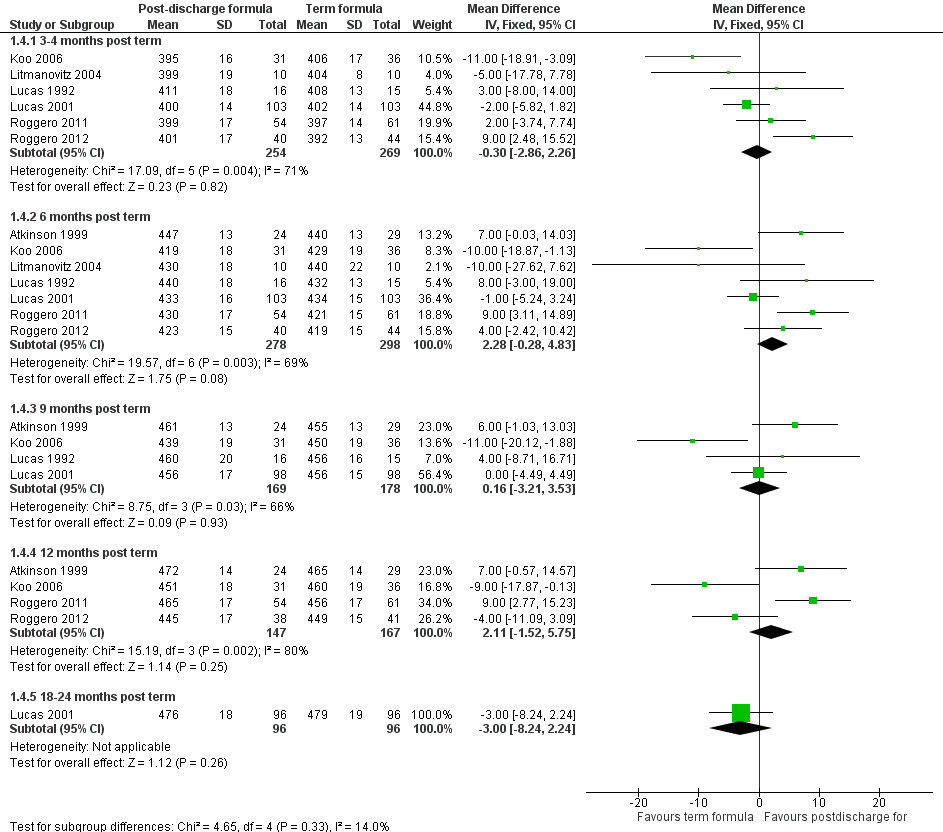
Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.4 Head circumference (mm).
All meta‐analyses showed substantial statistical heterogeneity (I2 > 50%).
Development (Outcome 1.5)
Lucas 2001 did not detect a statistically significant difference in the Bayley Scales Mental or Psychomotor Development Index at 18 months' corrected age. None of the included trials assessed later cognitive and educational outcomes.
Feed intolerance
Only one trial assessed this outcome (Lucas 1992) and reported no statistically significant difference in the mean number of vomits or possets per day. None of the participating infants ceased taking a study formula because of feed intolerance.
Bone mineralisation (Outcome 1.6)
Atkinson 2004 found no statistically significant differences in bone mineral content assessed at 12 months' corrected age (numerical data not available).
De Curtis 2002 reported no statistically significant differences in bone mineral content nor in bone area at the end of the two‐month study period.
Koo 2006 reported that at the end of the 12‐month study period, infants who received nutrient‐enriched formula had statistically significantly lower bone mass (measured by dual‐energy X‐ray absorptiometry). Investigators presented data in graphs only, and data could not be extracted or obtained for calculation of mean differences.
Lucas 1992 assessed bone width and bone mineral content of the radius at nine months' corrected age. Bone width was not statistically significantly different between groups. Bone mineral content was statistically significantly greater in the group of infants who received the postdischarge formula: mean difference 20.6, 95% CI 7.8 to 33.4 mg/cm.
Litmanovitz 2004 found no statistically significant differences in bone strength assessed as 'bone speed of sound' on measurement with ultrasonography, nor in serum levels of bone‐specific alkaline phosphatase, at six months' corrected age.
No trials assessed the effect of the intervention on clinical or radiological evidence of rickets.
Blood pressure on long‐term follow‐up
No included trials performed this assessment.
Body mass index on long‐term follow‐up
No included trials performed this assessment.
Subgroup analyses
-
VLBW or very preterm infants: Two trials (Litmanovitz 2004; Taroni 2009) recruited exclusively VLBW infants. As described above, investigators found no statistically significant differences in weight, length or head circumference, nor in measures of bone mineralisation, up to six months' corrected age.
-
Infants who remain small for gestational age at hospital discharge: Three trials (Atkinson 2004; Roggero 2012; Taroni 2009) recruited infants who were growth‐restricted at birth. These trials detected no statistically significant effects on weight up to 12 months' corrected age, but meta‐analyses of data from the two trials that undertook follow‐up at six months (Atkinson 2004; Roggero 2012) revealed statistically significant effects on crown‐heel length (WMD 8.88, 95% CI 0.94 to 16.83 mm) and on head circumference (WMD 5.36, 95% CI 0.62 to 10.11 mm).
-
Infants with chronic lung disease requiring home supplemental oxygen therapy: No trials exclusively recruited infants with chronic lung disease. Subgroup data were not available.
Preterm formula versus standard term formula (Comparison 2)
Growth (Outcomes 2.1‐2.4)
Cooke 2001 found no statistically significant differences in rate of weight gain during the trial period. Researchers presented data in graphs only, and data could not be extracted or obtained for calculation of the mean difference. At 18 months' corrected age, the nutrient‐enriched formula group was statistically significantly heavier than the control group (MD 500, 95% CI 26 to 974 g), but investigators noted no statistically significant differences in length or head circumference.
Agosti 2003 reported no statistically significant differences in mean weight, length or head circumference at four, six and 12 months after hospital discharge.
Peng 2004 noted no statistically significant differences in mean weight, length or head circumference at monthly intervals up to six months' corrected age.
Picaud 2005 observed no statistically significant differences in rate of gain of weight, length or head circumference during the first four months of the trial period and no statistically significant differences in weight, length or head circumference between groups at four months. At 12 months post discharge, infants in the preterm formula group were heavier (MD 1007, 95% CI 211 to 1803 g) (Outcome 2.2), were longer (MD 27, 95% CI 2 to 52 mm) (Outcome 2.3) and had larger head circumference (MD 12, 95% CI 0.2 to 24 mm) (Outcome 2.4) than control infants. However, loss to follow‐up by 12 months was substantial in the control group (35%) and was greater than that reported for the intervention group (9%).
Jeon 2011 found no statistically significant differences in mean weight, length or head circumference at three, 12 and 18 months after hospital discharge.
Meta‐analyses of growth data
-
Weight (Outcome 2.2; Figure 6): Meta‐analysis of data from four trials (Agosti 2003; Cooke 2001; Jeon 2011; Picaud 2005) showed statistically significantly higher weight in the preterm formula group at 12 months' corrected age (WMD 540, 95% CI 255 to 824 g). Meta‐analysis of data from two trials (Cooke 2001; Jeon 2011) revealed statistically significantly higher weight in the preterm formula group at 18 months (WMD 491, 95% CI 142 to 839 g) (Outcome 2.2).
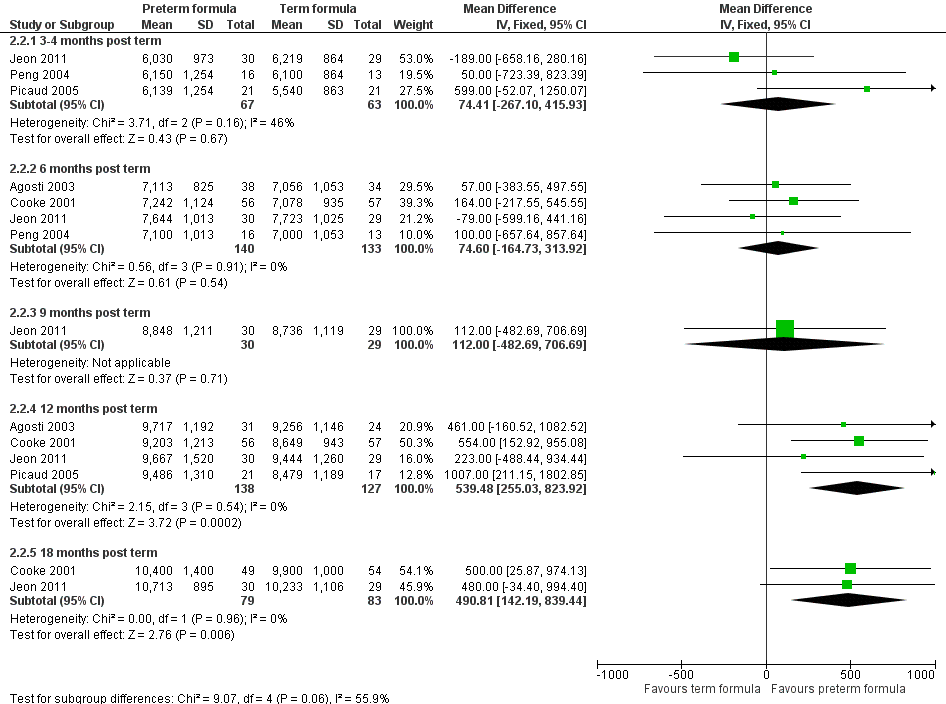
Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.2 Weight (grams).
-
Length (Outcome 2.3; Figure 7): Meta‐analysis of data from three trials (Agosti 2003; Jeon 2011; Picaud 2005) showed no statistically significant difference at 12 months' corrected age (WMD 5.1, 95% CI ‐4.2 to 14.5 mm). Meta‐analysis of data from two trials (Cooke 2001; Jeon 2011) revealed statistically significantly longer crown‐heel length in the preterm formula group at 18 months (WMD 11, 95% CI 2 to 20 mm).
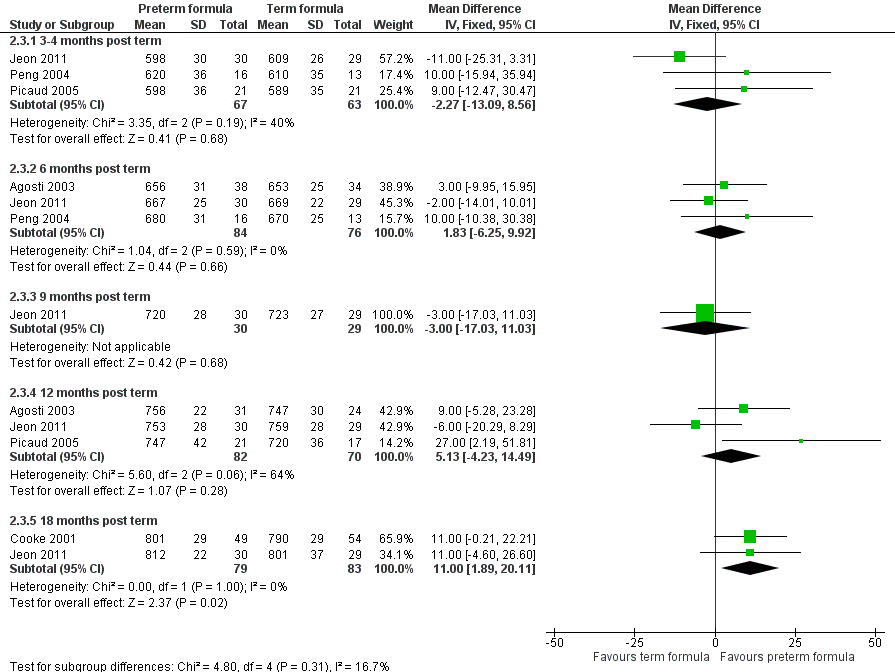
Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.3 Crown‐heel length (mm).
-
Head circumference (Outcome 2.4; Figure 8): Meta‐analysis of data from three trials (Agosti 2003; Jeon 2011; Picaud 2005) showed a statistically significantly larger head circumference in the preterm formula group at 12 months' corrected age (WMD 6.1, 95% CI 1.1 to 11.1 mm). Meta‐analysis of data from two trials (Cooke 2001; Jeon 2011) revealed a statistically significantly larger head circumference in the preterm formula group at 18 months (WMD 5.4, 95% CI 0.7 to 10.1 mm).
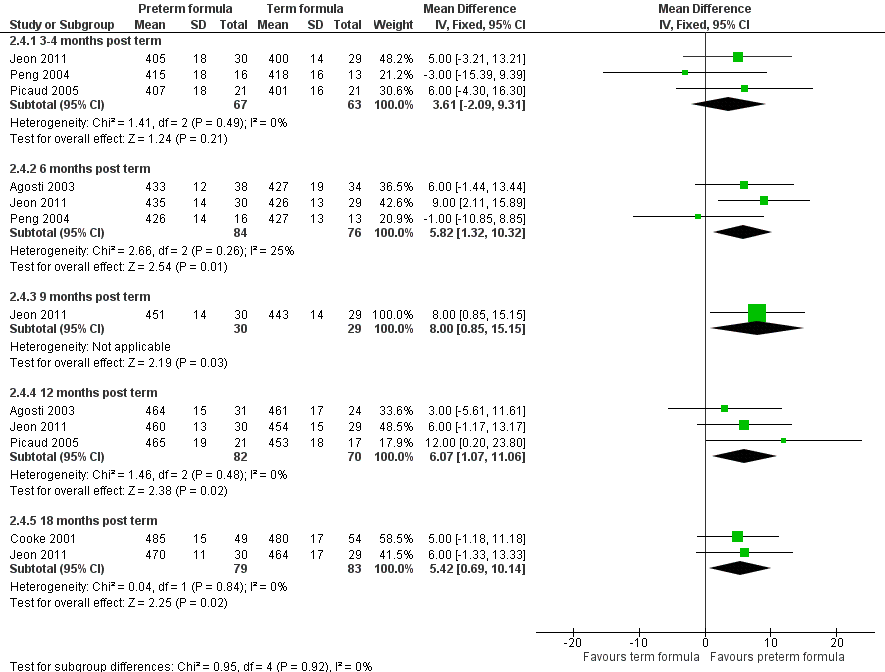
Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.4 Head circumference (mm).
Development (Outcome 2.5)
Neither Cooke 2001 nor Jeon 2011 nor a meta‐analysis of data from both trials detected a statistically significant difference in the Bayley Scales Mental Development Index (WMD ‐1.4, 95% CI ‐6.2 to 3.4) or in the Psychomotor Development Index (WMD ‐1.1, 95% CI ‐4.2 to 1.93). Agosti 2003 noted no statistically significant differences in Griffiths' Developmental Scale evaluations at six, nine and 12 months' corrected age (numerical data not available from report nor from trialists).
Feed intolerance
No included trials performed this assessment.
Bone mineralisation
Cooke 2001 assessed body composition with dual‐energy X‐ray absorptiometry at six and 12 months' corrected age and noted no statistically significant differences in bone area, bone mineral mass or bone mineral density measurements between groups. In the published report, all data were presented in graphs and could not be extracted for estimation of mean differences. Investigators also reported that they found no statistically significant differences in serum phosphorus, calcium and alkaline phosphatase levels measured at intervals during the study period (up to six months post term). These data were presented mainly in graphs and could not be extracted for estimation of mean differences.
Blood pressure on long‐term follow‐up
No included trials performed this assessment.
Body mass index on long‐term follow‐up
No included trials performed this assessment.
Subgroup analyses
-
VLBW or very preterm infants: Two trials (Agosti 2003; Jeon 2011) exclusively recruited VLBW infants. See details of findings described above. Subgroup data from the other trials were not available.
-
Infants who remain small for gestational age at hospital discharge: Subgroup data were not available.
-
Infants with chronic lung disease requiring home supplemental oxygen therapy: Subgroup data were not available.
Discussion
Summary of main results
Data from 11 randomised controlled trials with a total of 885 participants provided no evidence that feeding postdischarge formula (˜74 kcal/100 mL) versus standard term formula (˜67 kcal/100 mL) to preterm infants after hospital discharge affects growth parameters up to 12 to 18 months post term.
The five trials that examined the effect of feeding preterm formula (˜80 kcal/100 mL) versus standard term formula provided some evidence of an effect on growth parameters. Meta‐analyses showed a weighted mean difference of about 500 g for weight, 11 mm for length and 5 to 6 mm for head circumference at 12 to 18 months post term. It is not yet known whether any of these differences persist through later childhood.
Evidence of the effects of nutrient‐enriched formula on long‐term development is unclear. The only trial of postdischarge versus term formula conducted to assess developmental outcomes showed no difference in the Bayley Scales Mental or Psychomotor Development Index assessed at 18 months' corrected age (Lucas 2001). Similarly, meta‐analyses of data from two trials provided no evidence that feeding preterm versus term formula affects neurodevelopmental outcomes at 18 months' corrected age. Data on longer‐term cognitive and educational outcomes are not yet available.
Overall completeness and applicability of evidence
We identified 11 eligible trials that compared feeding with postdischarge formula versus term formula, but studies generally were small and of variable methodological quality. Quantitative synthesis was limited, as only seven of these trials (Atkinson 1999; Koo 2006; Litmanovitz 2004; Lucas 1992; Lucas 2001; Roggero 2011; Roggero 2012) presented data that could be included in meta‐analyses of growth outcomes. Interpretation of meta‐analyses was further limited by heterogeneity. The source of heterogeneity is not clear, as these trials were of similar design (intervention given for six to 12 months) and methodological quality (satisfactory processes to ensure allocation concealment and achievement of about 70% to 80% follow‐up at longer than six months post term). Meta‐analyses of data from the five trials that compared preterm formula versus term formula were more complete and revealed no statistical heterogeneity.
Differences in measured effects on growth parameters between postdischarge formula and preterm formula may simply be related to total nutrient content and intake. An additional factor is that whereas postdischarge formula contains about 10% more calories and 20% to 25% more protein and bone minerals than term formula, preterm formula is about 20% energy‐enriched and contains 40% to 60% more protein and minerals than term formula. Demand (responsively) fed infants regulate their volume of milk intake relative to its calorie density; therefore, infants in comparison groups may have had similar total energy intake. However, infants fed postdischarge formula would still have received about 10% more protein and minerals than those fed term formula, but infants fed preterm formula would have received up to about 25% more protein and minerals than those given term formula. It is possible that protein and mineral intake (per unit of energy) is the key factor in determining catch‐up growth rates and, specifically, lean and skeletal growth, in this population of infants.
The applicability of currently available data is limited by the short duration of follow‐up reported in clinical trials. No trials planned to undertake or undertook assessment of growth or development beyond 12 to 18 months' corrected age, and some trials reported growth outcomes only up to six months. Similarly, no trials have reported data related to possible adverse metabolic consequences of nutrient supplementation in early infancy, nor to any long‐term measures of obesity (such as body mass index (BMI), fat mass) or risk factors for cardiovascular disease (such as elevated blood pressure).
Quality of the evidence
Interpretation of review findings is limited by methodological weaknesses associated with potential for bias in some trials (Figure 2). Methods used to preserve allocation concealment are uncertain for some trials. Only one trial (Jeon 2011) reported substantial between‐group differences in baseline demographics that are likely due to allocation bias. We elected to exclude one arm of this three‐arm trial because of substantial differences in mean birth weight, gestational age and proportion of growth‐restricted infants. The other methodological limitation apparent in six trials was incomplete outcome assessment (loss to follow‐up > 20%). In most of these trials (Atkinson 1999; Koo 2006; Peng 2004; Picaud 2005), loss to follow‐up was less than 30% and was distributed evenly between intervention and control groups. In two trials (Agosti 2003; Carver 2001), loss to follow‐up at 12‐month assessment was greater than 50%. However, these trials did not contribute substantially to any meta‐analyses.
The GRADE assessment revealed that evidence for key growth outcomes was of moderate quality because of inconsistency (moderate or high heterogeneity in meta‐analyses of trials of postdischarge formula vs standard formula; summary of findings Table for the main comparison) and imprecision (few trials with low numbers of participants included in meta‐analyses of preterm formula vs standard formula; summary of findings Table 2).
Potential biases in the review process
Our main concern with the review process is that findings may be subject to publication and other reporting biases, including greater availability of numerical data for inclusion in meta‐analyses from trials that reported statistically significant or clinically important effects (Hopewell 2009). We attempted to minimise this threat by searching the proceedings of major international perinatal conferences to identify trial reports that were not (or were not yet) published in full form in academic journals. However, we cannot be sure whether other trials have been undertaken but not reported, and we remain concerned that such trials are less likely than published trials to have detected statistically significant or clinically important effects. The meta‐analyses that we performed did not include sufficient trials to explore the symmetry of funnel plots as a means of identifying possible publication or reporting bias.

Study flow diagram: review update.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.2 Weight (grams).
Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.3 Crown‐heel length (mm).

Forest plot of comparison: 1 Postdischarge formula versus standard term formula, outcome: 1.4 Head circumference (mm).

Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.2 Weight (grams).

Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.3 Crown‐heel length (mm).

Forest plot of comparison: 2 Preterm formula versus standard term formula, outcome: 2.4 Head circumference (mm).
Comparison 1 Postdischarge formula versus standard term formula, Outcome 1 Growth rates during trial period.

Comparison 1 Postdischarge formula versus standard term formula, Outcome 2 Weight (grams).

Comparison 1 Postdischarge formula versus standard term formula, Outcome 3 Crown‐heel length (mm).
Comparison 1 Postdischarge formula versus standard term formula, Outcome 4 Head circumference (mm).

Comparison 1 Postdischarge formula versus standard term formula, Outcome 5 Development.

Comparison 1 Postdischarge formula versus standard term formula, Outcome 6 Bone mineralisation.

Comparison 2 Preterm formula versus standard term formula, Outcome 1 Growth rates during trial period.

Comparison 2 Preterm formula versus standard term formula, Outcome 2 Weight (grams).

Comparison 2 Preterm formula versus standard term formula, Outcome 3 Crown‐heel length (mm).

Comparison 2 Preterm formula versus standard term formula, Outcome 4 Head circumference (mm).

Comparison 2 Preterm formula versus standard term formula, Outcome 5 Development.
| Postdischarge formula compared with standard term formula for preterm infants after hospital discharge | ||||
| Patient or population: preterm infants after hospital discharge | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of participants | Quality of the evidence | Comments |
| Postdischarge formula vs standard term formula | ||||
| Weight (grams) 3‐4 months post term | MD 7.45 g lower | 523 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 62%) |
| Weight (grams) 6 months post term | MD 35.54 g higher | 576 | ⊕⊕⊕⊝ | Downgraded for inconsistency moderate (I² = 64%). |
| Crown‐heel length (mm) 3‐4 months post term | MD 2.45 mm higher | 523 | ⊕⊕⊕⊝ | Downgraded for high inconsistency (I² = 81%) |
| Crown‐heel length (mm) 6 months post term | MD 2.12 mm higher | 576 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 75%) |
| Head circumference (mm) 3‐4 months post term | MD 0.3 mm lower | 523 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 71%) |
| Head circumference (mm) 6 months post term | MD 2.28 mm higher | 576 | ⊕⊕⊕⊝ | Downgraded for moderate inconsistency (I² = 69%) |
| Development ‐ Bayley Scales of Infant Development II: Mental Development Index | MD 0.9 higher | 184 | ⊕⊕⊕⊕ | |
| Preterm formula compared with standard term formula for preterm infants after hospital discharge | ||||
| Patient or population: preterm infants after hospital discharge | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of participants | Quality of the evidence | Comments |
| Preterm formula vs standard term formula | ||||
| Weight (grams) 3‐4 months post term | MD 74.41 g higher | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Weight (grams) 6 months post term | MD 74.6 g higher | 273 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Crown‐heel length (mm) 3‐4 months post term | MD 2.27 mm lower | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Crown‐heel length (mm) 6 months post term | MD 1.83 mm higher | 160 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Head circumference (mm) 3‐4 months post term | MD 3.61 mm higher | 130 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Head circumference (mm) 6 months post term | MD 5.82 mm higher | 160 | ⊕⊕⊕⊝ | Downgraded for imprecision |
| Development ‐ Bayley Scales of Infant Development II: Mental Development Index | MD 1.44 lower | 143 | ⊕⊕⊕⊕ | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Growth rates during trial period Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Weight gain (g/kg/d) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.37, 1.37] |
| 1.2 Linear growth (mm/wk) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
| 1.3 Head circumference (mm/wk) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.68, 0.68] |
| 2 Weight (grams) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3‐4 months post term | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | ‐7.45 [‐141.84, 126.93] |
| 2.2 6 months post term | 7 | 576 | Mean Difference (IV, Fixed, 95% CI) | 35.54 [‐113.71, 184.78] |
| 2.3 9 months post term | 4 | 347 | Mean Difference (IV, Fixed, 95% CI) | 244.09 [16.95, 471.23] |
| 2.4 12 months post term | 4 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐14.87 [‐243.18, 213.43] |
| 2.5 18‐24 months post term | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | 100.0 [‐246.90, 446.90] |
| 3 Crown‐heel length (mm) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 3‐4 months post term | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | 2.45 [‐2.01, 6.90] |
| 3.2 6 months post term | 7 | 576 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [‐2.16, 6.41] |
| 3.3 9 months post term | 4 | 347 | Mean Difference (IV, Fixed, 95% CI) | 7.33 [1.80, 12.87] |
| 3.4 12 months post term | 4 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐6.43, 5.10] |
| 3.5 18‐24 months post term | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [0.32, 17.68] |
| 4 Head circumference (mm) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 3‐4 months post term | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.86, 2.26] |
| 4.2 6 months post term | 7 | 576 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [‐0.28, 4.83] |
| 4.3 9 months post term | 4 | 347 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐3.21, 3.53] |
| 4.4 12 months post term | 4 | 314 | Mean Difference (IV, Fixed, 95% CI) | 2.11 [‐1.52, 5.75] |
| 4.5 18‐24 months post term | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐8.24, 2.24] |
| 5 Development Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Bayley Scales of Infant Development II: Mental Development Index | 1 | 184 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.24, 5.04] |
| 5.2 Bayley Scales of Infant Development II: Psychomotor Development Index | 1 | 184 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐1.28, 6.68] |
| 6 Bone mineralisation Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Bone area at 2 months post term (cm2) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐15.46, 29.46] |
| 6.2 Bone mineral content at 2 months post term (grams) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐4.73, 11.13] |
| 6.3 Bone 'speed of sound' assessed on ultrasonography at 6 months post term (mm/s) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 45.0 [‐18.48, 108.48] |
| 6.4 Bone specific serum alkaline phosphatase at 6 months post term (units/L) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐42.01, 24.01] |
| 6.5 Bone width at 9 months post term (cm) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 6.6 Bone mineral content at 9 months post term (mg/cm) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 20.60 [7.78, 33.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Growth rates during trial period Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Weight gain (g/d) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐0.16, 7.56] |
| 1.2 Linear growth (mm/wk) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.09, 1.91] |
| 1.3 Head circumference (mm/wk) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.04, 1.04] |
| 2 Weight (grams) Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3‐4 months post term | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | 74.41 [‐267.10, 415.93] |
| 2.2 6 months post term | 4 | 273 | Mean Difference (IV, Fixed, 95% CI) | 74.60 [‐164.73, 313.92] |
| 2.3 9 months post term | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 112.0 [‐482.69, 706.69] |
| 2.4 12 months post term | 4 | 265 | Mean Difference (IV, Fixed, 95% CI) | 539.48 [255.03, 823.92] |
| 2.5 18 months post term | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 490.81 [142.19, 839.44] |
| 3 Crown‐heel length (mm) Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 3‐4 months post term | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐13.09, 8.56] |
| 3.2 6 months post term | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐6.25, 9.92] |
| 3.3 9 months post term | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐17.03, 11.03] |
| 3.4 12 months post term | 3 | 152 | Mean Difference (IV, Fixed, 95% CI) | 5.13 [‐4.23, 14.49] |
| 3.5 18 months post term | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 11.00 [1.89, 20.11] |
| 4 Head circumference (mm) Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 3‐4 months post term | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | 3.61 [‐2.09, 9.31] |
| 4.2 6 months post term | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | 5.82 [1.32, 10.32] |
| 4.3 9 months post term | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [0.85, 15.15] |
| 4.4 12 months post term | 3 | 152 | Mean Difference (IV, Fixed, 95% CI) | 6.07 [1.07, 11.06] |
| 4.5 18 months post term | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 5.42 [0.69, 10.14] |
| 5 Development Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Bayley Scales of Infant Development II: Mental Development Index | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐1.44 [‐6.22, 3.35] |
| 5.2 Bayley Scales of Infant Development II: Psychomotor Development Index | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐4.19, 1.93] |

