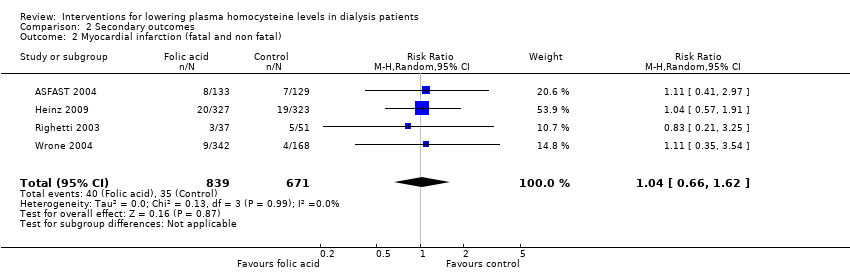
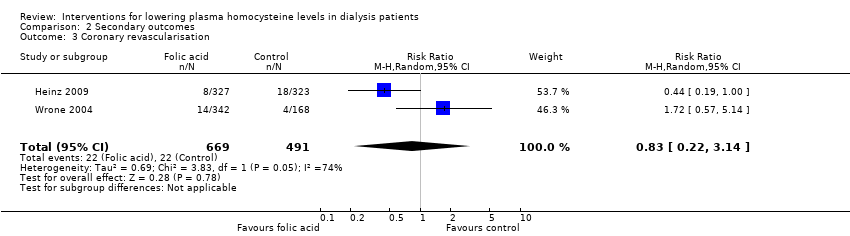
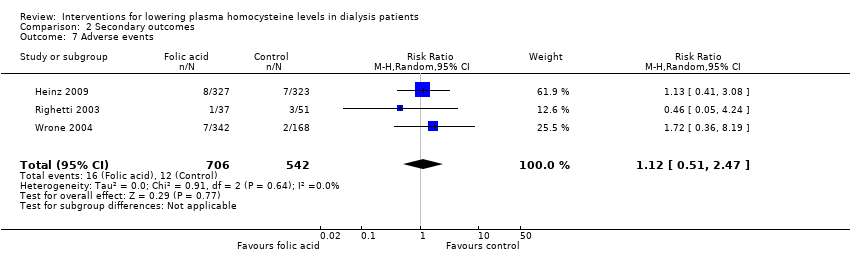
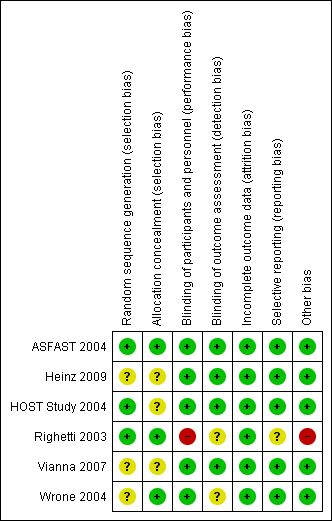
Ali 2003 {published data only}
Ali Z, Idham C, Effendi I.
The effect of n‐acetylcysteine on homocysteine level in chronic hemodialysis patients: randomized control trial [abstract].
Nephrology 2003;
8(Suppl 3):A62. [CENTRAL: CN‐00671778]
CENTRAL
Alvares Delfino 2007 {published data only}
Alvares Delfino V, de Andrade Vianna AC, Mocelin AJ, Barbosa DS, Mise RA, Matsuo T.
Folic acid therapy reduces plasma homocysteine levels and improves plasma antioxidant capacity in hemodialysis patients.
Nutrition 2007;
23(3):242‐7. [MEDLINE: 17321110]
CENTRAL
Anderson 2006 {published data only}
Anderson TJ, Sun YH, Hubacek J, Hyndman ME, Verma S, Shewchuk L, et al.
Effects of folinic acid on forearm blood flow in patients with end‐stage renal disease.
Nephrology Dialysis Transplantation 2006;
21(7):1927‐33. [MEDLINE: 16520350]
CENTRAL
Ardalan 2003 {published data only}
Ardalan MR, Etemadi J, Mortazavi M, Majidi J.
Hyperhomocysteinaemia therapy in haemodialysis patients with combination of Vitamin B6, B12 and folic acid [abstract no: W467].
Nephrology Dialysis Transplantation 2003;
18(4):696. [CENTRAL: CN‐00716091]
CENTRAL
Ardalan 2003a {published data only}
Ardalan MR, Mortazavi M, Etemadi J.
Hyperhomocysteinemia and its therapy in renal transplant recipients with combination of: Vit B6, Vit B12 and folic acid [abstract].
Nephrology Dialysis Transplantation 2003;
18(Suppl 4):509‐10. [CENTRAL: CN‐00444217]
CENTRAL
Armada 2003 {published data only}
Armada E, Perez C, Otero A, Esteban J, Camba M, Gayoso P, et al.
Neither folic nor folinic acid normalize homocysteine levels in hemodialysis patients.
Clinical Nephrology 2003;
60(3):168‐75. [MEDLINE: 14524579]
CENTRAL
Arnadottir 2003 {published data only}
Arnadottir M, Hultberg B.
The effect of vitamin B12 on total plasma homocysteine concentration in folate‐replete hemodialysis patients.
Clinical Nephrology 2003;
59(3):186‐9. [MEDLINE: 12653261]
CENTRAL
ATIC Study 2005 {published data only}
Nanayakkara PW, Kiefte‐De Jong JC, Stehouwer CD, van Ittersum FJ, Olthof MR, Kok RM, et al.
Association between global leukocyte DNA methylation, renal function, carotid intima‐media thickness and plasma homocysteine in patients with stage 2‐4 chronic kidney disease.
Nephrology Dialysis Transplantation 2008;
23(8):2586‐92. [MEDLINE: 18287179]
CENTRAL Nanayakkara PW, Kiefte‐de Jong JC, ter Wee PM, Stehouwer CD, van Ittersum FJ, Olthof MR, et al.
Randomized placebo‐controlled trial assessing a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on plasma asymmetric dimethylarginine concentration in mild to moderate CKD.
American Journal of Kidney Diseases 2009;
53(1):41‐50. [MEDLINE: 18786751]
CENTRAL Nanayakkara PW, Teerlink T, Stehouwer CD, Allajar D, Spijkerman A, Schalkwijk C, et al.
Plasma asymmetric dimethylarginine (ADMA) concentration is independently associated with carotid intima‐media thickness and plasma soluble vascular cell adhesion molecule‐1 (sVCAM‐1) concentration in patients with mild‐to‐moderate renal failure.
Kidney International 2005;
68(5):2230‐6. [MEDLINE: 16221223]
CENTRAL Nanayakkara PW, van Guldener C, ter Wee PM, Scheffer PG, van Ittersum FJ, Twisk JW, et al.
Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima‐media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti‐Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study.
Archives of Internal Medicine 2007;
167(12):1262‐70. [MEDLINE: 17592099]
CENTRAL Nanayakkara PW, van Guldener C, van Ittersum F, Stehouwer CD, ter Wee PM.
Effect of an oxidative‐stress‐reducing strategy on carotid intima‐media thickness, endothelial function and renal function in patients with mild‐to‐moderate chronic kidney disease [abstract no: F‐PO145].
Journal of the American Society of Nephrology 2006;
17(Abstracts):367A. [CENTRAL: CN‐00602094]
CENTRAL Nanayakkara PW, van Ittersum FJ, Fouque D, Charrie A, Stehouwer CD, ter Wee PM.
Plasma adiponectin is inversely associated with renal function in patients with Stage 3 and 4 chronic kidney disease [abstract no: F‐PO146].
Journal of the American Society of Nephrology 2006;
17(Abstracts):367A. [CENTRAL: CN‐00602093]
CENTRAL Thijs A, Nanayakkara PW, ter Wee PM, Huijgens PC, van Guldener C, Stehouwer CD.
Mild‐to‐moderate renal impairment is associated with platelet activation: a cross‐sectional study.
Clinical Nephrology 2008;
70(4):325‐31. [MEDLINE: 18826858]
CENTRAL Veringa SJ, Nanayakkara PW, van Ittersum FJ, Vegting IL, van Guldener C, Smulders YM, et al.
Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on arterial compliance and distensibility in patients with mild‐to‐moderate chronic kidney disease.
Clinical Nephrology 2012;
78(4):263‐72. [MEDLINE: 22981031]
CENTRAL
Azadibakhsh 2007 {published data only}
Azadibakhsh N, Hosseini RS, Atabak S, Nateghiyan N, Golestan B, Rad AH.
Efficacy of folate and vitamin B12 in lowering homocysteine concentrations in hemodialysis patients.
Saudi Journal of Kidney Diseases & Transplantation 2009;
20(5):779‐88. [MEDLINE: 19736473]
CENTRAL Azadibakhsh N, Hosseini RS, Atabak S, Nateghiyan N, Golestan B, Rad AH.
The effect of folate and vitamin B12 supplementation on homocysteine concentrations: a study in hemodialysis patients.
Tehran University Medical Journal 2007;
65(8):49‐56. [CENTRAL: CN‐00796293]
CENTRAL
Beavers 2008 {published data only}
Beavers KM, Beavers DP, Bowden RG, Wilson RL, Gentile M.
Effect of over‐the‐counter fish‐oil administration on plasma Lp(a) levels in an end‐stage renal disease population.
Journal of Renal Nutrition 2009;
19(6):443‐9. [MEDLINE: 19748798]
CENTRAL Beavers KM, Beavers DP, Bowden RG, Wilson RL, Gentile M.
Omega‐3 fatty acid supplementation and total homocysteine levels in end‐stage renal disease patients.
Nephrology 2008;
13(4):284‐8. [MEDLINE: 18331436]
CENTRAL
Bennett‐Richards 2002 {published data only}
Bennett‐Richards K, Kattenhorn M, Donald A, Oakley G, Varghese Z, Rees L, et al.
Does oral folic acid lower total homocysteine levels and improve endothelial function in children with chronic renal failure?.
Circulation 2002;
105(15):1810‐5. [MEDLINE: 11956124]
CENTRAL Bennett‐Richards K, Taylor M, Oakley G, Varghese Z, Deanfield J, Rees L.
Does oral folic acid lower total homocystine levels and improve endothelial function in children with CRF? [abstract].
Pediatric Nephrology 2001;
16(8):C167. [CENTRAL: CN‐00444396]
CENTRAL
Bostom 1996 {published data only}
Bostom AG, Shemin D, Lapane KL, Hume AL, Yoburn D, Nadeau MR, et al.
High dose‐B‐vitamin treatment of hyperhomocysteinemia in dialysis patients.
Kidney International 1996;
49(1):147‐52. [MEDLINE: 8770960]
CENTRAL
Bostom 2000 {published data only}
Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K, et al.
Controlled comparison of L‐5‐methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients.[Erratum appears in Circulation 2000 Aug 1;102(5):598].
Circulation 2000;
101(24):2829‐32. [MEDLINE: 10859289]
CENTRAL Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Spiegel P, et al.
Reduced folates are an expensive treatment for hyperhomocysteinemia in hemodialysis patients with no greater efficacy than folic acid: results of a controlled comparison study [abstract].
Journal of the American Society of Nephrology 2000;
11(Sept):257A. [CENTRAL: CN‐00550713]
CENTRAL Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Bagley P, Massy ZA, et al.
Treatment of hyperhomocysteinemia in hemodialysis patients and renal transplant recipients.
Kidney International ‐ Supplement 2001;
59(Suppl 78):S246‐52. [MEDLINE: 11169020]
CENTRAL Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Jacques PF, Dworkin L, et al.
Treatment of mild hyperhomocysteinemia in renal transplant recipients versus hemodialysis patients.
Transplantation 2000;
69(10):2128‐31. [MEDLINE: 10852611]
CENTRAL
Branley 2000 {published data only}
Branley P, D'Intini V, Thomson N, Fraenkel M, McNeil JJ.
Randomised placebo controlled clinical trial of supplementary folic acid in CRF [abstract].
Nephrology 2000;
5(3):A79. [CENTRAL: CN‐00400364]
CENTRAL
Brensing 2002 {published data only}
Brensing KA, Kaschell D, Hages M, Lutjohann D, Sigit J, Pietrizik K, et al.
Improved hyperhomocysteinemia in hemodialysed ESRD‐patients by intravenous folate or folinic acid: a randomized controlled trial [abstract no: O100].
Nephrology Dialysis Transplantation 2002;
17(Suppl 1):30. [CENTRAL: CN‐00509104]
CENTRAL
Brensing 2003 {published data only}
Brensing KA, Hages M, Raab P, Pietrzik K, von Bergmann K, Frotscher U, et al.
Vitamin B12 and folic‐acid as intravenous high‐dose vitamin therapy for hyperhomocysteinemia in hemodialysed ESRD‐patients: data of a prospective randomized controlled study [abstract no: SU‐PO1048].
Journal of the American Society of Nephrology 2003;
14(Nov):766A. [CENTRAL: CN‐00644257]
CENTRAL
Chang 2007 {published data only}
Chang TY, Chou KJ, Tseng CF, Chung HM, Fang HC, Hung YM, et al.
Effects of folic acid and vitamin B complex on serum C‐reactive protein and albumin levels in stable hemodialysis patients.
Current Medical Research & Opinion 2007;
23(8):1879‐86. [MEDLINE: 17605895]
CENTRAL
Chiu 2009 {published data only}
Chiu YW, Chang JM, Hwang SJ, Tsai JC, Chen HC.
Pharmacological dose of vitamin B12 is as effective as low‐dose folinic acid in correcting hyperhomocysteinemia of hemodialysis patients.
Renal Failure 2009;
31(4):278‐83. [MEDLINE: 19462276]
CENTRAL
Cianciolo 2008 {published data only}
Cianciolo G, La Manna G, Coli L, Donati G, D'Addio F, Persici E, et al.
5‐methyltetrahydrofolate administration is associated with prolonged survival and reduced inflammation in ESRD patients.
American Journal of Nephrology 2008;
28(6):941‐8. [MEDLINE: 18587236]
CENTRAL
Cutler 2009 {published data only}
Cutler MJ, Urquhart BL, Freeman DJ, Spence JD, House AA.
Mesna for the treatment of hyperhomocysteinemia in hemodialysis patients.
Blood Purification 2009;
27(3):306‐10. [MEDLINE: 19270449]
CENTRAL
De Angelis 2007 {published data only}
De Angelis S, Noce A, Dessi M, Pastore A, Scaccia F, Trombetta M, et al.
Hyperhomocysteinemia and alternate vitamin supplementation [Iperomocisteinemia nei pazienti uremici: effetti della supplementazione alternata di acido folico e vitamina B 12].
Giornale Italiano di Nefrologia 2007;
24(1):51‐5. [MEDLINE: 17342693]
CENTRAL Dessi M, Di Giovamberardino G, Pieri M, Noce A, Zenobi R, Di Daniele N, et al.
Influence of dialysis techniques and alternate vitamin supplementation on homocysteine levels in patients with known MTHFR genotypes.
Clinical & Experimental Nephrology 2015;
19(1):140‐5. [MEDLINE: 24652221]
CENTRAL
Del Pozo 2005 {published data only}
Del Pozo C, Alvarez L, Lopez‐Menchero R, Torregrosa I, Sanchez L, Albero MD.
Optimization of a therapy schedule with folinic acid to treat hyperhomocysteinemia in patients on hemodialysis [abstract no: SP261].
Nephrology Dialysis Transplantation 2005;
20(Suppl 5):v106. [CENTRAL: CN‐00671828]
CENTRAL
De Vecchi 2001 {published data only}
De Vecchi AF, Patrosso C, Novembrino C, Finazzi S, Colucci P, De Franceschi M, et al.
Folate supplementation in peritoneal dialysis patients with normal erythrocyte folate: effect on plasma homocysteine.
Nephron 2001;
89(3):297‐302. [MEDLINE: 11598393]
CENTRAL
De Vriese 2003 {published data only}
De Vriese AS, Langlois M, Bernard D, Geerolf I, Stevens L, Boelaert JR, et al.
Effect of dialyser membrane pore size on plasma homocysteine levels in haemodialysis patients.
Nephrology Dialysis Transplantation 2003;
18(12):2596‐600. [MEDLINE: 14605283]
CENTRAL
Dierkes 1999 {published data only}
Dierkes J, Domrose U, Ambrosch A, Bosselmann HP, Neumann KH, Luley C.
Response of hyperhomocysteinemia to folic acid supplementation in patients with end‐stage renal disease.
Clinical Nephrology 1999;
51(2):108‐15. [MEDLINE: 10069646]
CENTRAL
Dierkes 2001 {published data only}
Dierkes J, Domrose U, Bosselmann KP, Neumann KH, Luley C.
Homocysteine lowering effect of different multivitamin preparations in patients with end‐stage renal disease.
Journal of Renal Nutrition 2001;
11(2):67‐72. [MEDLINE: 11295026]
CENTRAL
DIVINe Study 2010 {published data only}
House AA, Ehasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al.
Vitamin therapy for homocysteine accelerates loss of renal function in diabetic kidney disease [abstract no: SA‐FC343].
Journal of the American Society of Nephrology 2009;
20:80A. [CENTRAL: CN‐00740523]
CENTRAL House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al.
Effect of B‐vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial.
JAMA 2010;
303(16):1603‐9. [MEDLINE: 20424250]
CENTRAL
Dobronravov 2008 {published data only}
Dobronravov V, Ardebili M, Golubev R, Galkina O, Nabokow A, Kliem V, et al.
The effect of IV administration of sodium 2,3‐dimercaptopropane‐1‐sulfonate (DMPS) and 8‐week high‐dosed IV vitamin B therapy on total plasma homocysteine (tHcy) in patients undergoing chronic hemodialysis (HD) [abstract no: PUB584].
Journal of the American Society of Nephrology 2008;
19(Abstracts Issue):943A. [CENTRAL: CN‐00758243]
CENTRAL
Ducloux 2002 {published data only}
Ducloux D, Aboubakr A, Motte G, Toubin G, Fournier V, Chalopin JM, et al.
Hyperhomocysteinaemia therapy in haemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12.
Nephrology Dialysis Transplantation 2002;
17(5):865‐70. [MEDLINE: 11981075]
CENTRAL Ducloux D, Aboubakr A, Motte G, Toubin G, Fournier V, Chalopin JM, et al.
Hyperhomocysteinemia therapy in hemodialysis (HD) patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):354A‐5A. [CENTRAL: CN‐00445156]
CENTRAL
Elian 2002 {published data only}
Elian KM, Hoffer LJ.
Hydroxocobalamin reduces hyperhomocysteinemia in end‐stage renal disease.
Metabolism: Clinical & Experimental 2002;
51(7):881‐6. [MEDLINE: 12077735]
CENTRAL
Friedman 2003 {published data only}
Friedman AN, Bostom AG, Laliberty P, Selhub J, Shemin D.
The effect of N‐acetylcysteine on plasma total homocysteine levels in hemodialysis: a randomized, controlled study.
American Journal of Kidney Diseases 2003;
41(2):442‐6. [MEDLINE: 12552508]
CENTRAL
Galli 2003 {published data only}
Galli F, Benedetti S, Buoncristiani U, Piroddi M, Conte C, Canestrari F, et al.
The effect of PMMA‐based protein‐leaking dialyzers on plasma homocysteine levels.
Kidney International 2003;
64(2):748‐55. [MEDLINE: 12846775]
CENTRAL Galli F, Benedetti S, Floridi A, Canestrari F, Piroddi M, Buoncristiani E, et al.
Glycoxidation and inflammatory markers in patients on treatment with PMMA‐based protein‐leaking dialyzers.
Kidney International 2005;
67(2):750‐9. [MEDLINE: 15673326]
CENTRAL
Gonin 2003 {published data only}
Gonin JM, Nguyen H, Gonin R, Sarna A, Michels A, Masri‐Imad F, et al.
Controlled trials of very high dose folic acid, vitamins B12 and B6, intravenous folinic acid and serine for treatment of hyperhomocysteinemia in ESRD.
Journal of Nephrology 2003;
16(4):522‐34. [MEDLINE: 14696754]
CENTRAL Gonin JM, Sarna A, Loya A, Gonin R, Chassaing C, Wainer I, et al.
A double‐blind, controlled study of folate and vitamin therapy for hyperhomocysteinemia in hemodialysis [abstract].
Journal of the American Society of Nephrology 2000;
11(Sept):269A. [CENTRAL: CN‐00755224]
CENTRAL
Gonin 2003a {published data only}
Gonin J, Nguyen H, Michels A, Santos A, Gonin R, Cary D, et al.
Evaluation of folinic acid and serine in hyperhomocysteinemia in ESRD: a prospective placebo‐controlled randomized study [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):356A. [CENTRAL: CN‐00445513]
CENTRAL Gonin JM, Nguyen H, Gonin R, Sarna A, Michels A, Masri‐Imad F, et al.
Controlled trials of very high dose folic acid, vitamins B12 and B6, intravenous folinic acid and serine for treatment of hyperhomocysteinemia in ESRD.
Journal of Nephrology 2003;
16(4):522‐34. [MEDLINE: 14696754]
CENTRAL
Hauser 2001 {published data only}
Hagen W, Hauser A, Rehak PH, Buchmayer H, Fodinger M, Horl WH, et al.
Comparison of intravenous folinic acid with intravenous folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. A randomized, double blinded trial [abstract].
Journal of the American Society of Nephrology 2000;
11(Sept):148A. [CENTRAL: CN‐00583332]
CENTRAL Hagen W, Kletzmayr J, Fodinger M, Hauser AC, Horl WH, Sunder‐Plassmann G.
Effect of intravenous folic acid or folinic acid therapy on mean arterial pressure and pulse pressure in hemodialysis patients [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):483A. [CENTRAL: CN‐00583333]
CENTRAL Hauser AC, Hagen W, Rehak PH, Buchmayer H, Fodinger M, Papagiannopoulos M, et al.
Efficacy of folinic versus folic acid for the correction of hyperhomocysteinemia in hemodialysis patients.
American Journal of Kidney Diseases 2001;
37(4):758‐65. [MEDLINE: 11273876]
CENTRAL
Henning 2001 {published data only}
Henning BF, Zidek W, Riezler R, Graefe U, Tepel M.
Homocyst(e)ine metabolism in hemodialysis patients treated with vitamins B6, B12 and folate.
Research in Experimental Medicine 2001;
200(3):155‐68. [MEDLINE: 11426667]
CENTRAL
Hoffer 2005 {published data only}
Hoffer LJ, Saboohi F, Golden M, Barre PE.
Cobalamin dose regimen for maximum homocysteine reduction in end‐stage renal disease.
Metabolism: Clinical & Experimental 2005;
54(6):835‐40. [MEDLINE: 15931623]
CENTRAL
Hoffer 2005a {published data only}
Hoffer LJ, Djahangirian O, Bourgouin PE, Eid J, Saboohi F.
Comparative effects of hydroxocobalamin and cyanocobalamin on plasma homocysteine concentrations in end‐stage renal disease.
Metabolism: Clinical & Experimental 2005;
54(10):1362‐7. [MEDLINE: 16154437]
CENTRAL
HOPE‐2 Study 2006 {published data only}
Lonn E, Held C, Arnold JM, Probstfield J, McQueen M, Micks M, et al.
Rationale, design and baseline characteristics of a large, simple, randomized trial of combined folic acid and vitamins B6 and B12 in high‐risk patients: the Heart Outcomes Prevention Evaluation (HOPE)‐2 trial.
Canadian Journal of Cardiology 2006;
22(1):47‐53. [MEDLINE: 16450017]
CENTRAL Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al.
Homocysteine lowering with folic acid and B vitamins in vascular disease.[erratum appears in N Engl J Med. 2006 Aug 17;355(7):746].
New England Journal of Medicine 2006;
354(15):1567‐77. [MEDLINE: 16531613]
CENTRAL Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al.
Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease‐‐results of the renal Hope‐2 study.
Nephrology Dialysis Transplantation 2008;
23(2):645‐53. [MEDLINE: 18003666]
CENTRAL Ray JG, Kearon C, Yi Q, Sheridan P, Lonn E, Heart Outcomes Prevention Evaluation (HOPE‐2).
Homocysteine‐lowering therapy and risk for venous thromboembolism: a randomized trial.[Summary for patients in Ann Intern Med. 2007 Jun 5;146(11):I22; PMID: 17470823].
Annals of Internal Medicine 2007;
146(11):761‐7. [MEDLINE: 17470822]
CENTRAL Righetti M.
Hopeful for a second HOPE‐2 post hoc analysis.
Nephrology Dialysis Transplantation 2008;
23(7):2428‐9. [MEDLINE: 18403437]
CENTRAL Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E.
Homocysteine‐lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial.
Stroke 2009;
40(4):1365‐72. [MEDLINE: 19228852]
CENTRAL Sawka AM, Ray JG, Yi Q, Josse RG, Lonn E.
Randomized clinical trial of homocysteine level lowering therapy and fractures.
Archives of Internal Medicine 2007;
167(19):2136‐9. [MEDLINE: 17954810]
CENTRAL
House 1999 {published data only}
House A, Freeman D.
Larger intradialytic effect of high flux vs low flux polysulfone on homocysteine levels, but not on lipids or apo AI/CIII ratio [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):209A. [CENTRAL: CN‐00445778]
CENTRAL House AA, Donnelly JG.
Effect of multivitamins on plasma homocysteine and folate levels in patients on hemodialysis.
ASAIO Journal 1999;
45(1):94‐7. [MEDLINE: 9952016]
CENTRAL House AA, Donnelly JG.
Impact of methylenetetrahydrofolate reductase (MTHFR) genotype on folate status and homocysteine (hcy) in hemodialysis patients [abstract].
Journal of the American Society of Nephrology 1999;
10(Program & Abstracts):168A. [CENTRAL: CN‐00756935]
CENTRAL House AA, Wells GA, Donnelly JG, Nadler SP, Hebert PC.
Effect of high flux vs low flux hemodialysis on homocysteine, lipoprotein(a) and lipid profiles: a prospective randomized trial [abstract].
Journal of the American Society of Nephrology 1998;
9(Program & Abstracts):211A. [CENTRAL: CN‐00401331]
CENTRAL House AA, Wells GA, Donnelly JG, Nadler SP, Hebert PC.
Randomized trial of high‐flux vs low‐flux haemodialysis: effects on homocysteine and lipids.
Nephrology Dialysis Transplantation 2000;
15(7):1029‐34. [MEDLINE: 10862642]
CENTRAL
House 2004 {published data only}
House AA, Eliasziw M, Urquhart BL, Freeman DJ, Spence JD.
Dimercaptosuccinic acid for the treatment of hyperhomocysteinemia in hemodialysis patients: a placebo‐controlled, double‐blind, randomized trial.
American Journal of Kidney Diseases 2004;
44(4):689‐94. [MEDLINE: 15384020]
CENTRAL
Imani 2009 {published data only}
Imani H, Tabibi H, Atabak S, Rahmani L, Ahmadinejad M, Hedayati M.
Effects of soy consumption on oxidative stress, blood homocysteine, coagulation factors, and phosphorus in peritoneal dialysis patients.
Journal of Renal Nutrition 2009;
19(5):389‐95. [MEDLINE: 19577483]
CENTRAL Tabibi H, Imani H, Hedayati M, Atabak S, Rahmani L.
Effects of soy consumption on serum lipids and apoproteins in peritoneal dialysis patients: a randomized controlled trial.
Peritoneal Dialysis International 2010;
30(6):611‐8. [MEDLINE: 20378840]
CENTRAL
Isbel 2003 {published data only}
Isbel NM, Reiger K, Hayley CM, Campbell SB, Burke J, Bofinger A, et al.
Vitamin B12 is effective in the treatment of hyperhomocysteinaemia in patients with ESRD [abstract no: 58].
Nephrology 2003;
8(Suppl 3):A69. [CENTRAL: CN‐00758625]
CENTRAL
ISRCTN22151635 {published data only}
ISRCTN22151635.
Assessment of the lipid lowering agents on plasma homocysteine concentration and renal function. controlled‐trials.com/ISRCTN22151635 (accessed 4 February 2016).
CENTRAL
Jara 2001 {published data only}
Jara A, Morales M.
Intravenous folinic acid versus oral folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):357A. [CENTRAL: CN‐00445900]
CENTRAL
Kazory 2008 {published data only}
Kazory A, Kribs M, Fournier V, Motte G, Guy C, Devillard N, et al.
Homocysteine lowering effect of adsorptive dialysis membranes: a multicenter, randomized, open study [abstract no: 118].
American Journal of Kidney Diseases 2008;
51(4):A57. [CENTRAL: CN‐00756882]
CENTRAL
Klemm 2004 {published data only}
Klemm A, Franke C, Busch M, Muller A, Franke S, Lang D, et al.
Influence of hemodialysis membrane permeability on serum levels of advanced glycation end products (AGEs) and homocysteine metabolites.
Clinical Nephrology 2004;
61(3):191‐7. [MEDLINE: 15077870]
CENTRAL
Kooshki 2011 {published data only}
Kooshki A, Taleban FA, Tabibi H, Hedayati M.
Effects of marine omega‐3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients.
Annals of Nutrition & Metabolism 2011;
58(3):197‐202. [MEDLINE: 21757893]
CENTRAL Kooshki A, Taleban FA, Tabibi H, Hedayati M.
Effects of omega‐3 fatty acids on serum lipids, lipoprotein (a), and hematologic factors in hemodialysis patients.
Renal Failure 2011;
33(9):892‐8. [MEDLINE: 21859401]
CENTRAL
Koyama 2002 {published data only}
Koyama K, Usami T, Takeuchi O, Morozumi K, Kimura G.
Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high‐dose folic acid supplementation.
Nephrology Dialysis Transplantation 2002;
17(5):916‐22. [MEDLINE: 11981084]
CENTRAL
Koyama 2010 {published data only}
Koyama K, Ito A, Ichikawa T, Miura T, Ono M.
Short term regimen of homocysteine‐lowering therapy reduces serum concentration of asymmetric dimethylarginine in patients on chronic hemodialysis [abstract no: SaP317].
Nephrology Dialysis Transplantation 2007;
22(Suppl 6):vi339. [CENTRAL: CN‐00671829]
CENTRAL Koyama K, Ito A, Yamamoto J, Nishio T, Ichikawa T.
Co‐administration of methylcobalamin and folic acid normalizes hyperhomocysteinemia and reduces arterial stiffness in hemodialsyis patients [abstract no: PUB074].
Journal of the American Society of Nephrology 2005;
16:798A. [CENTRAL: CN‐00676039]
CENTRAL Koyama K, Ito A, Yamamoto J, Nishio T, Kajikuri J, Dohi Y, et al.
Randomized controlled trial of the effect of short‐term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long‐term hemodialysis.
American Journal of Kidney Diseases 2010;
55(6):1069‐78. [MEDLINE: 20430500]
CENTRAL
Kuhlmann 2004 {published data only}
Kuhlmann MK, Obeid R, Herrmann W.
Normalization of plasma homocystein (Hcy) levels in hyperhomocysteinemic hemodialysis patients by intravenous dosage of vitamins B6, B12 and folic acid. [abstract no SA‐PO345].
Journal of the American Society of Nephrology 2004;
15(Oct):377A. [CENTRAL: CN‐00757352]
CENTRAL
Kumar 2007b {published data only}
Kumar R, Vincent L, Perkins NJ, Tobe SW.
Impact of folic acid on homocysteine levels in haemodialysis patients, a randomized double blinded placebo controlled cross over trial [abstract no: SA‐PO788].
Journal of the American Society of Nephrology 2007;
18(Abstracts Issue):516A. [CENTRAL: CN‐00716086]
CENTRAL
Kuo 2001a {published data only}
Kuo HT, Tsai JC, Chen LN, Chen HC, Guh JY, Hwang SJ, et al.
Intravenous methylcobalamin further improves hyperhomocysteinemia in hemodialysis patients under maintenance folic acid therapy: role of methyletetrahydrofolate reductase (mthfr) genotype [abstract].
Nephrology Dialysis Transplantation 2001;
16(6):A87. [CN‐00509297]
CENTRAL
LANDMARK Study 2006 {published data only}
Isbel N, Rakhit D, Armstrong K, Leano R, Hawley C, Campbell S, et al.
Rapid progression of myocardial ischaemia in patients with chronic kidney disease is not altered by risk factor modification [abstract no: PS107].
Nephrology 2005;
10(Suppl 3):A408. [CENTRAL: CN‐00583922]
CENTRAL Isbel NM, Haluska B, Johnson DW, Beller E, Hawley C, Marwick TH.
Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function.
American Heart Journal 2006;
151(3):745‐53. [MEDLINE: 16504645]
CENTRAL Isbel NM, Reiger K, Hawley CM, Campbell SB, Haluska B, Short L, et al.
Aggressive intervention in traditional cardiovascular risk factors in patients with chronic renal failure does not improve atheroma burden, cardiovascular function or outcome [abstract].
Nephrology 2004;
9(Suppl 1):A1‐2. [CENTRAL: CN‐00509247]
CENTRAL Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, et al.
Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification.
Nephrology 2007;
12(4):391‐8. [MEDLINE: 17635756]
CENTRAL Rakhit DJ, Marwick TH, Armstrong KA, Johnson DW, Leano R, Isbel NM.
Effect of aggressive risk factor modification on cardiac events and myocardial ischaemia in patients with chronic kidney disease.
Heart 2006;
92(10):1402‐8. [MEDLINE: 16606867]
CENTRAL
Libetta 2004 {published data only}
Libetta C, Corbetta R, Zucchi M, Gori E, Villa G, Bezoari G, et al.
Treatment of hyperhomocysteinemia in hemodialysis patients reduce progressive atherosclerosis: a long‐term controlled study [abstract no: SA‐PO343].
Journal of the American Society of Nephrology 2004;
15(Oct):376A‐7A. [CENTRAL: CN‐00626028]
CENTRAL Libetta C, Corbetta R, Zucchi M, Gori E, Villa G, Sepe V, et al.
Long‐term treatment of hyperhomocysteinemia prevents carotid artery atherosclerosis in haemodialysed patients [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal.
2004:4. [CENTRAL: CN‐00604353]
CENTRAL Libetta C, Villa G, Gori E, Zucchi M, Pisacco P, Bezoari G, et al.
Vitamins supplementation effectiveness on hyperhomocysteinemia in hemodialyzed patients: a two‐year single‐blind randomized study [abstract no: SA‐PO344].
Journal of the American Society of Nephrology 2004;
15(Oct):377A. [CENTRAL: CN‐00626027]
CENTRAL Libetta C, Villa G, Gori E, Zucchi M, Sepe V, Pisacco P, et al.
Effect of folate or folate plus vitamins B6 and B12 on hyperhomocysteinemia in hemodialyzed patients: a 2‐year controlled randomized study [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal.
2004:126. [CENTRAL: CN‐00636150]
CENTRAL
Madsen 2011 {published data only}
Madsen T, Christensen JH, Toft E, Aardestrup I, Lundbye‐Christensen S, Schmidt EB.
Effect of intravenous omega‐3 fatty acid infusion and hemodialysis on fatty acid composition of free fatty acids and phospholipids in patients with end‐stage renal disease.
Jpen: Journal of Parenteral & Enteral Nutrition 2011;
35(1):97‐106. [MEDLINE: 21224436]
CENTRAL
Manns 2001 {published data only}
Hyndman ME, Manns BJ, Snyder FF, Bridge PJ, Scott‐Douglas NW, Fung E, et al.
Vitamin B12 decreases, but does not normalize, homocysteine and methylmalonic acid in end‐stage renal disease: a link with glycine metabolism and possible explanation of hyperhomocysteinemia in end‐stage renal disease.
Metabolism: Clinical & Experimental 2003;
52(2):168‐72. [MEDLINE: 12601627]
CENTRAL Manns B, Hyndman E, Burgess E, Parsons H, Schaefer J, Snyder F, et al.
Oral vitamin B(12) and high‐dose folic acid in hemodialysis patients with hyper‐homocyst(e)inemia.
Kidney International 2001;
59(3):1103‐9. [MEDLINE: 11231366]
CENTRAL
Mazdeh 2005 {published data only}
Abolghasemi R, Mazdeh MM, Khatami M, Lessan‐Pezeshki M, Ahmadi F, Seifi S, et al.
Hyperhomocysteinemia therapy in haemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract no: MP318].
Nephrology Dialysis Transplantation 2005;
20(Suppl 5):v307.
CENTRAL Mazdeh MM, Abolghasemi R, Khatami M, Lessan‐Pezeshki M, Ahmadi F, Seifi S, et al.
Hyperhomocysteinemia therapy in hemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract no: W‐PO40014].
Nephrology 2005;
10(Suppl 1):A283. [CENTRAL: CN‐00756890]
CENTRAL
McGregor 2000a {published data only}
McGregor D, Shand B, Lynn K.
A controlled trial of the effect of folate supplements on homocysteine, lipids and hemorheology in end‐stage renal disease.
Nephron 2000;
85(3):215‐20. [MEDLINE: 10867536]
CENTRAL
Mudge 2005 {published data only}
Mudge D, Rogers R, Hollett P, Law B, Reiger K, Petrie JJP, et al.
Plasma homocysteine levels with standard high flux haemodialysis compared with FX class dialysers [abstract].
Nephrology 2004;
9(Suppl 1):A16. [CENTRAL: CN‐00509369]
CENTRAL Mudge DW, Rogers R, Hollett P, Law B, Reiger K, Petrie JJ, et al.
Randomized trial of FX high flux vs standard high flux dialysis for homocysteine clearance.
Nephrology Dialysis Transplantation 2005;
20(10):2178‐85. [MEDLINE: 16030045]
CENTRAL
Mueller 2001 {published data only}
Mueller T, Mueller N, Moser R, Stief T, Celle D, Pietrizk K.
Reduced folates have an anti‐inflammatory effect in hemodialysis patients [abstract].
Nephrology Dialysis Transplantation 2003;
18(Suppl 4):700. [CENTRAL: CN‐00446856]
CENTRAL Mueller TF, Mueller N, Moser R, Stief T, Nau H, Lange H, et al.
Effect of folate therapy on the inflammatory burden in hemodialysis patients [abstract no: SA‐FC185].
Journal of the American Society of Nephrology 2002;
13(September, Program & Abstracts):38A. [CENTRAL: CN‐00446855]
CENTRAL Mueller TF, Mueller N, Moser R, Stief T, Ziegler A, Nau H, et al.
Effect of oral substitution of reduced folates on homocysteine, neopterin, and lipid levels in patients treated with hemodialysis [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):360A‐1A. [CENTRAL: CN‐00756385]
CENTRAL
Muller 2001 {published data only}
Muller A, Busch M, Stein G, Hein GE, Funfstuck R.
L‐methionine loading test for the prediction of increased total homocysteine levels due to long term l‐methionine treatment [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):734A. [CENTRAL: CN‐00757145]
CENTRAL
Nakamura 2003 {published data only}
Nakamura T, Hirayama H, Tajiri M, Saionji K, Hata A.
Methylenetetrahydrofolate reductase (MTHFR) gene polymorphism influences the efficacy of folic acid (FA) and vitamin B12 (B12) on hyperhomocyteinemia in hemodialysis (HD) patients [abstract].
Nephrology Dialysis Transplantation 2003;
18(Suppl 4):699‐700. [CENTRAL: CN‐00446896]
CENTRAL
Nakhoul 2004 {published data only}
Nakhoul F, Abassi Z, Plawner M, Khankin E, Ramadan R, Lanir N, et al.
Comparative study of response to treatment with supraphysiologic doses of B‐vitamins in hyperhomocysteinemic hemodialysis patients.
Israel Medical Association Journal ‐ Imaj 2004;
6(4):213‐7. [MEDLINE: 15115259]
CENTRAL Plawner M, Nakhoul F, Khankin E, Laneer N, Brenner B, Green J.
Folic acid and varying doses of B‐vitamines in the treatment of hyperhomocystinemia in hemodialysis patients [abstract].
Journal of the American Society of Nephrology 2000;
11(Sept):295A. [CENTRAL: CN‐00644331]
CENTRAL
Nascimento 2010 {published data only}
Nascimento MM, Suliman ME, Anderstam B, Silva MM, Hayashi S, Pecoits‐Filho R, et al.
Effect of N‐acetylcysteine supplementation on inflammatory and oxidative stress markers in peritoneal dialysis patients: a randomized placebo controlled study [abstract no: F‐PO769].
Journal of the American Society of Nephrology 2007;
18(Abstracts):270A. [CENTRAL: CN‐00756376]
CENTRAL Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, et al.
Effect of oral N‐acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo‐controlled study.
Peritoneal Dialysis International 2010;
30(3):336‐42. [MEDLINE: 20190028]
CENTRAL
OPACH Study 2006 {published data only}
Harving F, Svensson M, Flyvbjerg A, Schmidt EB, Jorgensen KA, Eriksen HH, et al.
n‐3 polyunsaturated fatty acids and adiponectin in patients with end‐stage renal disease.
Clinical Nephrology 2015;
83(5):279‐85. [MEDLINE: 25816805]
CENTRAL Kirkegaard E, Svensson M, Strandhave C, Schmidt EB, Jorgensen KA, Christensen JH.
Marine n‐3 fatty acids, atrial fibrillation and QT interval in haemodialysis patients.
British Journal of Nutrition 2012;
107(6):903‐9. [MEDLINE: 21791142]
CENTRAL Rasmussen LE, Svensson M, Jorgensen KA, Schmidt EB, Christensen JH.
The content of docosahexaenoic acid in serum phospholipid is inversely correlated with plasma homocysteine levels in patients with end‐stage renal disease.
Nutrition Research 2010;
30(8):535‐40. [MEDLINE: 20851307]
CENTRAL Svensson M, Christensen JH, Jorgensen KA, Schmidt EB.
Omacor as secondary prevention against cardiovascular disease in patients undergoing chronic hemodialysis: a randomised, placebo controlled intervention study [abstract no: F‐PO606].
Journal of the American Society of Nephrology 2005;
16:466A. [CENTRAL: CN‐00583663]
CENTRAL Svensson M, Frobert O, Schmidt EB, Jorgensen KA, Simonsen U, Christensen JH.
The effect of n‐3 fatty acids on levels of methylarginines in patients with end‐stage renal disease.
Journal of Nephrology 2010;
23(4):459‐64. [MEDLINE: 20349405]
CENTRAL Svensson M, Schmidt EB, Jorgensen KA, Christensen JH.
The effect of n‐3 fatty acids on heart rate variability in patients treated with chronic hemodialysis.
Journal of Renal Nutrition 2007;
17(4):243‐9. [MEDLINE: 17586422]
CENTRAL Svensson M, Schmidt EB, Jorgensen KA, Christensen JH.
The effect of n‐3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo‐controlled intervention study.
Nephrology Dialysis Transplantation 2008;
23(9):2918‐24. [MEDLINE: 18436564]
CENTRAL Svensson M, Schmidt EB, Jorgensen KA, Christensen JH, OPACH Study Group.
N‐3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo‐controlled intervention trial.
Clinical Journal of the American Society of Nephrology: CJASN 2006;
1(4):780‐6. [MEDLINE: 17699287]
CENTRAL
Ossareh 2009 {published data only}
Ossareh S, Shayan‐Moghaddam H, Salimi A, Asgari M, Farrokhi F.
Different doses of oral folic acid for homocysteine‐lowering therapy in patients on hemodialysis: a randomized controlled trial.[Erratum appears in Iran J Kidney Dis. 2010 Jan;4(1):88 Note: Salimi, Ashraf [added]; Asgari, Mojgan [added]].
Iranian Journal of Kidney Diseases 2009;
3(4):227‐33. [MEDLINE: 19841527]
CENTRAL
Pakfetrat 2013 {published data only}
Pakfetrat M, Shahroodi JR, Zolgadr AA, Larie HA, Nikoo MH, Malekmakan L.
Effects of zinc supplement on plasma homocysteine level in end‐stage renal disease patients: a double‐blind randomized clinical trial.
Biological Trace Element Research 2013;
153(1‐3):11‐5. [MEDLINE: 23475369]
CENTRAL
Pastore 2006 {published data only}
Pastore A, De Angelis S, Casciani S, Ruggia R, Di Giovamberardino G, Noce A, et al.
Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes.
Clinical Chemistry 2006;
52(1):145‐8. [MEDLINE: 16391331]
CENTRAL
Peng 2005 {published data only}
Peng L, Liu Q.
A prospective, randomized, placebo‐controlled study to evaluate the effect of combined treatment of folic acid and intravenous B‐complex vitamins on hyperhomocysteinemia in hemodialysis patients [abstract no: W‐PO40032].
Nephrology 2005;
10(Suppl 1):A288. [CENTRAL: CN‐00671827]
CENTRAL
Polkinghorne 2003 {published data only}
Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al.
A randomised placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract]. Kongres Nasional VIII Annual Meeting Perhimpunan Nefrologi Indonesia; 2002 Oct; Surabaya, Indonesia.
2002:2.5. [CENTRAL: CN‐00447252]
CENTRAL Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al.
A randomized placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract no: SU‐FC267].
Journal of the American Society of Nephrology 2002;
13(September, Program & Abstracts):55A. [CENTRAL: CN‐00447251]
CENTRAL Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al.
Randomized, placebo‐controlled trial of intramuscular vitamin B12 for the treatment of hyperhomocysteinaemia in dialysis patients.
Internal Medicine Journal 2003;
33(11):489‐94. [MEDLINE: 14656250]
CENTRAL Polkinghorne KR, Zoungas S, Branley P, Villnueva E, McNeil J, Atkins RC, et al.
A randomised placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract].
Nephrology 2002;
7(Suppl 3):A68.
CENTRAL
Poulia 2011 {published data only}
Poulia KA, Panagiotakos DB, Tourlede E, Rezou A, Stamatiadis D, Boletis J, et al.
Omega‐3 fatty acids supplementation does not affect serum lipids in chronic hemodialysis patients.
Journal of Renal Nutrition 2011;
21(6):479‐84. [MEDLINE: 21439849]
CENTRAL
Sanchez Alvarez 2005 {published data only}
Sanchez Alvarez JE, Perez Tamajon L, Hernandez D, Alvarez Gonzalez A, Delgado P, Lorenzo V.
Efficacy and safety of two vitamin supplement regimens on homocysteine levels in hemodialysis patients. Prospective, randomized clinical trial.
Nefrologia 2005;
25(3):288‐96. [MEDLINE: 16053010]
CENTRAL Sanchez JE, Molina E, De La Vega MJ, Hernandez D, Perez L, Garcia R, et al.
Sustained effect of two vitamin supplement regimens on hyperhomocysteinemia in maintenance hemodialysis (MHD) patients [abstract no: F‐PO773].
Journal of the American Society of Nephrology 2003;
14(Nov):231A. [CENTRAL: CN‐00583425]
CENTRAL
Scholze 2004 {published data only}
Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M.
Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end‐stage renal failure.
Circulation 2004;
109(3):369‐74. [MEDLINE: 14732754]
CENTRAL Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M.
Reduced plasma homocysteine concentration and improved pulse pressure in hemodialysis patients with acetylcysteine [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal.
2004:127. [CENTRAL: CN‐00509467]
CENTRAL
Seo 2003 {published data only}
Seo MD, Yoon S, Jeong JC, Choi SS, Kim SY, Son I, et al.
Effects of folic acid treatment on homocysteine levels and echocardiographic findings in ESRD [abstract no: SA‐PO900].
Journal of the American Society of Nephrology 2003;
14(Nov):497A. [CN‐00756462]
CENTRAL
Sepe 1999 {published data only}
Sepe V, Patrucco G, Santagostine A, Bolis V, Caminiti R, Cecere P, et al.
Hyperhomocysteinemia [HHcy] is not associated with elevated platelet annexin V [plt a‐v] expression in maintenance haemodialysis [HD] patients [abstract].
Nephrology Dialysis Transplantation 1999;
14(9):A192. [CENTRAL: CN‐00485810]
CENTRAL
Shemin 2001 {published data only}
Shemin D, Bostom AG, Selhub J.
Treatment of hyperhomocysteinemia in end‐stage renal disease.
American Journal of Kidney Diseases 2001;
38(4 Suppl 1):S91‐4. [MEDLINE: 11576930]
CENTRAL
Signorelli 2006 {published data only}
Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri CG, et al.
A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl L‐carnitine in patients with peripheral arterial disease requiring haemodialysis.
Drugs & Aging 2006;
23(3):263‐70. [MEDLINE: 16608381]
CENTRAL Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri CG, et al.
Propionyl‐L‐carnitine therapy: effects on endothelin‐1 and homocysteine levels in patients with peripheral arterial disease and end‐stage renal disease.
Kidney & Blood Pressure Research 2006;
29(2):100‐7. [MEDLINE: 16809937]
CENTRAL
Skoutakis 1975 {published data only}
Skoutakis VA, Acchiardo SR, Meyer MC, Hatch FE.
Folic acid dosage for chronic hemodialysis patients.
Clinical Pharmacology & Therapeutics 1975;
18(2):200‐4. [MEDLINE: 1098832]
CENTRAL
Stavrianaki 2002 {published data only}
Stavrianaki D, Bagiatoudi G, Salpigidis K, Sarris E, Siakotos M.
Treatment efficacy of folic or folinic acid in lowering total plasma homocysteine levels in patients on hemodialysis [abstract].
Nephrology Dialysis Transplantation 2002;
17(Suppl 12):256. [CENTRAL: CN‐00758491]
CENTRAL
Tamadon 2011 {published data only}
Tamadon MR, Jamshidi L, Soliemani A, Ghorbani R, Malek F, Malek M.
Effect of different doses of folic acid on serum homocysteine level in patients on hemodialysis.
Iranian Journal of Kidney Diseases 2011;
5(2):93‐6. [MEDLINE: 21368386]
CENTRAL
Tayyebi‐Khosroshahi 2010 {published data only}
Tayyebi‐Khosroshahi H, Houshyar J, Tabrizi A, Vatankhah AM, Razzagi ZN, Dehghan‐Hesari R.
Effect of omega‐3 fatty acid on oxidative stress in patients on hemodialysis.
Iranian Journal of Kidney Diseases 2010;
4(4):322‐6. [MEDLINE: 20852375]
CENTRAL
Tepel 2003 {published data only}
Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W.
The antioxidant acetylcysteine reduces cardiovascular events in patients with end‐stage renal failure: a randomized, controlled trial.
Circulation 2003;
107(7):992‐5. [MEDLINE: 12600912]
CENTRAL
Thaha 2006 {published data only}
Thaha M, Widodo, Yogiantoro M, Tomino Y.
Reduction of pulse pressure by intravenous N‐acetylcyseine administration in chronic hemodialysis patients [abstract no: 1620].
Nephrology 2006;
11(Suppl 2):A33. [CENTRAL: CN‐00653775]
CENTRAL Thaha M, Yogiantoro M, Tomino Y.
Intravenous N‐acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end‐stage renal disease.
Clinical Drug Investigation 2006;
26(4):195‐202. [MEDLINE: 17163251]
CENTRAL Thaha M, Yogiantoro M, Tomino Y.
Intravenous N‐acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end‐stage renal disease [abstract no: 1560].
Nephrology 2006;
11(Suppl 2):A18.
CENTRAL
Thaha 2008 {published data only}
Thaha M, Widodo B, Pranawa M, Yogiantoro HM, Tomino Y.
Intravenous N‐acetylcysteine during hemodialysis reduces homocysteine and asymmetric dimethylarginine in ESRD patients: a randomized double blind controlled trial [abstract no: 1159].
Nephrology 2007;
12(Suppl 2):A40. [CENTRAL: CN‐00740521]
CENTRAL Thaha M, Widodo, Pranawa W, Yogiantoro M, Tomino Y.
Intravenous N‐acetylcysteine during hemodialysis reduces asymmetric dimethylarginine level in end‐stage renal disease patients.
Clinical Nephrology 2008;
69(1):24‐32. [MEDLINE: 18226399]
CENTRAL
Thaha 2009 {published data only}
Thaha M, Mohani CI, Yogiantoro HM, Tomino Y.
Oral n‐acetyl cysteine to treat non‐diabetic chronic kidney disease stage 1‐4 with albuminuria [abstract no: SA‐PO2242].
Journal of the American Society of Nephrology 2009;
20:622A. [CENTRAL: CN‐00740560]
CENTRAL
Thambyrajah 2000 {published data only}
Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN.
Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure?.
Circulation 2000;
102(8):871‐5. [MEDLINE: 10952955]
CENTRAL Thambyrajah J, Landray MJ, Townend JN, Wheeler DC.
Folic acid decreases plasma homocysteine but does not improve endothelial function in chronic renal failure [abstract].
Journal of the American Society of Nephrology 2000;
11(Sept):372A. [CENTRAL: CN‐00583252]
CENTRAL
Tobe 1999 {published data only}
Tobe SW, Helmers K, Naimark DMJ, Perkins N, Baker B.
Impact on homocysteine levels of folic acid and adherance in haemodialysis patients. A randomized double blind placebo cross over trial [abstract no: A1332].
Journal of the American Society of Nephrology 2000;
11(Sept):253A. [CENTRAL: CN‐00583286]
CENTRAL Tobe SW, Naimark DN, Helmers KF, Perkins NJ, Baker B.
Plasma homocysteine (hcy) levels in patients on chronic haemodialysis are inversely correlated with folate and B12 levels [abstract].
Journal of the American Society of Nephrology 1999;
10(Program & Abstracts):306A. [CENTRAL: CN‐00583285]
CENTRAL
Tochihara 2008 {published data only}
Tochihara Y, Whiting MJ, Barbara JA, Mangoni AA.
Effects of pre‐ vs. intra‐dialysis folic acid on arterial wave reflections and endothelial function in patients with end‐stage renal disease.
British Journal of Clinical Pharmacology 2008;
66(5):717‐22. [MEDLINE: 18754845]
CENTRAL
Treleaven 2001 {published data only}
Treleaven D, Gough J, Lonn E, Strickland D, Kitching A, Churchill D, et al.
High dose folic acid in hemodialysis patients results in frequent side effects without improvement in plasma homocysteine levels [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):365A. [CENTRAL: CN‐00583283]
CENTRAL
Tremblay 2000 {published data only}
Tremblay R, Bonnardeaux A, Geadah D, Busque L, Lebrun M, Ouimet D, et al.
Hyperhomocysteinemia in hemodialysis patients: effects of 12‐month supplementation with hydrosoluble vitamins.
Kidney International 2000;
58(2):851‐8. [MEDLINE: 10916110]
CENTRAL
Trimarchi 2002 {published data only}
Trimarchi H, Schiel A, Freixas E, Diaz M.
Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients.
Nephron 2002;
91(1):58‐63. [MEDLINE: 12021520]
CENTRAL
Tungkasereerak 2006 {published data only}
Tungkasereerak P, Chaiyasoot W, Taruangsri P, Leowattana W, Vasuvattakul S, Vareesangthip K, et al.
Can folate and vitamin B supplementation reduce total homocysteine levels and atherosclerosis in hemodialysis patients? [abstract no: SA‐PO472].
Journal of the American Society of Nephrology 2004;
15(Oct):406A. [CENTRAL: CN‐00583721]
CENTRAL Tungkasereerak P, Ong‐ajyooth L, Chaiyasoot W, Ong‐ajyooth S, Leowattana W, Vasuvattakul S, et al.
Effect of short‐term folate and vitamin B supplementation on blood homocysteine level and carotid artery wall thickness in chronic hemodialysis patients.
Journal of the Medical Association of Thailand 2006;
89(8):1187‐93. [MEDLINE: 17048428]
CENTRAL
Urquhart 2008 {published data only}
Urquhart BL, Freeman DJ, Cutler MJ, Mainra R, Spence JD, House AA.
Mesna for treatment of hyperhomocysteinemia in hemodialysis patients: a placebo‐controlled, double‐blind, randomized trial.
Clinical Journal of the American Society of Nephrology: CJASN 2008;
3(4):1041‐7. [MEDLINE: 18337551]
CENTRAL
van Guldener 1998 {published data only}
van Guldener C, Janssen M, Lambert J, ter Wee P, Donker A, Stehonwer C.
Treatment of hyperhomocysteinaemia with folic acid does not ameliorate endothelial function in chronic haemodialysis patients [abstract].
Nephrology Dialysis Transplantation 1997;
12(9):A114. [CENTRAL: CN‐00261358]
CENTRAL van Guldener C, Janssen MJ, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD.
Folic acid treatment of hyperhomocysteinemia in peritoneal dialysis patients does not ameliorate endothelial function [abstract].
Journal of the American Society of Nephrology 1997;
8(Program & Abstracts):274A. [CENTRAL: CN‐00448137]
CENTRAL van Guldener C, Janssen MJ, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD.
Folic acid treatment of hyperhomocysteinemia in peritoneal dialysis patients: no change in endothelial function after long‐term therapy.
Peritoneal Dialysis International 1998;
18(3):282‐9. [MEDLINE: 9663892]
CENTRAL van Guldener C, Janssen MJ, Lambert J, ter Wee PM, Jakobs C, Donker AJ, et al.
No change in impaired endothelial function after long‐term folic acid therapy of hyperhomocysteinaemia in haemodialysis patients.
Nephrology Dialysis Transplantation 1998;
13(1):106‐12. [MEDLINE: 9481724]
CENTRAL van Guldener C, Janssen MJ, Stehouwer CD, Ter Wee PM, Donker AJ.
Treatment of hyperhomocysteinemia in chronic dialysis patients with folic acid and betaine [abstract no: A1266].
Journal of the American Society of Nephrology 1996;
7(9):1502. [CENTRAL: CN‐00758506]
CENTRAL van Guldener C, Janssen MJ, Stehouwer CD, ter Wee PM, Donker AJ.
Folic acid and betaine in the treatment of hyperhomocysteinaemia in haemodialysis patients [abstract].
Netherlands Journal of Medicine 1996;
48(5):A67. [CENTRAL: CN‐00416837]
CENTRAL van Guldener C, Janssen MJ, de Meer K, Donker AJ, Stehouwer CD.
Effect of folic acid and betaine on fasting and postmethionine‐loading plasma homocysteine and methionine levels in chronic haemodialysis patients.
Journal of Internal Medicine 1999;
245(2):175‐83. [MEDLINE: 10081520]
CENTRAL van Guldener C, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD.
Carotid artery stiffness in patients with end‐stage renal disease: no effect of long‐term homocysteine‐lowering therapy.
Clinical Nephrology 2000;
53(1):33‐41. [MEDLINE: 10661480]
CENTRAL
Van Tellingen 2001 {published data only}
Van Tellingen A, Grooteman MP, Bartels PC, Van Limbeek J, Van Guldener C, Wee PM, et al.
Long‐term reduction of plasma homocysteine levels by super‐flux dialyzers in hemodialysis patients.
Kidney International 2001;
59(1):342‐7. [MEDLINE: 11135089]
CENTRAL
VIENNA Study 2000 {published data only}
Sunder‐Plassmann G, Fodinger M, Buchmayer H, Papagiannopoulos M, Wojcik J, Kletzmayr J, et al.
Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study.
Journal of the American Society of Nephrology 2000;
11(6):1106‐16. [MEDLINE: 10820175]
CENTRAL Sunder‐Plassmann G, Fodinger M, Buchmayer H, Wolfl G, Papagiannoupoulos M, Wojzik J, et al.
High dose folic acid therapy in hemodialysis (HD) patients. The Vienna multicenter study [abstract].
Journal of the American Society of Nephrology 1999;
10(Program & Abstracts):269A. [CENTRAL: CN‐00756862]
CENTRAL
Vrentzos 2001 {published data only}
Vrentzos G, Ganotakis E, Vardakis K, Maliaraki N, Stratigis S, Stilianou K, et al.
Effect of vitamin B12 supplementation in serum total homocysteine and folate in patients with end‐stage renal failure [abstract].
Atherosclerosis Supplements 2001;
2(2):107. [CENTRAL: CN‐00671820]
CENTRAL
Vychytil 2003 {published data only}
Vychytil A, Fodinger M, Pleiner J, Mullner M, Konner P, Skoupy S, et al.
Acute effect of amino acid peritoneal dialysis solution on vascular function.
American Journal of Clinical Nutrition 2003;
78(5):1039‐45. [MEDLINE: 14594793]
CENTRAL Vychytil A, Fodinger M, Pleiner J, Mullner M, Wolzt M, Sunder‐Plassmann G.
Acute effect of amino acid peritoneal dialysis (PD) solution on vascular function ‐ no association with HCY [abstract].
Journal of Inherited Metabolic Disease 2003;
26(Suppl 1):94. [CENTRAL: CN‐00677763]
CENTRAL Vychytil A, Foedinger M, Pleiner J, Muellner M, Konner P, Skoupy S, et al.
Acute effect of amino acid peritoneal dialysis solution on vascular function [abstract].
Nephrology Dialysis Transplantation 2003;
18(Suppl 4):215.
CENTRAL Vychytil A, Foedinger M, Pleiner J, Muellner M, Konner P, Skoupy S, et al.
Acute effect of amino acid peritoneal dialysis solution on vascular function. [abstract no: SU‐PO935].
Journal of the American Society of Nephrology 2003;
14(Nov):741A.
CENTRAL
Westphal 2001 {published data only}
Westphal S, Dierkes J, Luley C.
Effects of fenofibrate and gemfibrozil on plasma homocysteine.
Lancet 2001;
358(9275):39‐40. [MEDLINE: 11454380]
CENTRAL
Yango 2001 {published data only}
Ghandour H, Bagley PJ, Shemin D, Hsu N, Jacques PF, Dworkin L, et al.
Distribution of plasma folate forms in hemodialysis patients receiving high daily doses of L‐folinic or folic acid.
Kidney International 2002;
62(6):2246‐9. [MEDLINE: 12427152]
CENTRAL Yango A, Shemin D, Hsu N, Jacques PF, Dworkin L, Selhub J, et al.
Rapid communication: L‐folinic acid versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients.
Kidney International 2001;
59(1):324‐7. [MEDLINE: 11135086]
CENTRAL
Zeman 2006 {published data only}
Zeman M, Zak A, Vecka M, Tvrzicka E, Pisarikova A, Stankova B.
N‐3 fatty acid supplementation decreases plasma homocysteine in diabetic dyslipidemia treated with statin‐fibrate combination.
Journal of Nutritional Biochemistry 2006;
17(6):379‐84. [MEDLINE: 16214329]
CENTRAL
Zuo 2001 {published data only}
Zuo L, Wang M, Feng L, Wei H, Wang H.
Can high dose of folic acid normalize fast total plama homocysteine (thcy) level in maintenance hemodialysis patients? [abstract].
Journal of the American Society of Nephrology 2001;
12(Program & Abstracts):421A. [CENTRAL: CN‐00689429]
CENTRAL