مداخلاتی برای پائین آوردن سطح هموسیستئین پلاسما در بیماران دیالیزی
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004683.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 mayo 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Riñón y trasplante
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Draft the protocol: AC, MG, MJ, AK, SK, SDN, SN, TN, VP
-
Study selection: MJ, AK, SK, SDN, SN, VP,
-
Extract data from studies: MJ, SK, SDN, SN, VP
-
Enter data into RevMan: MJ, SN
-
Carry out the analysis: MJ, SK, SDN, SN, TN, VP
-
Interpret the analysis: MJ, SK, SDN, SN, TN, VP, GS, SZ
-
Draft the final review: MJ, SK, SDN, SN, TN, VP
-
Disagreement resolution: AC, MG, MJ, AK, SK, SDN, SN, TN, VP, GS, SZ
-
Update the review: AC, MG, MJ, AK, SK, SDN, SN, TN, VP, GS, SZ
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Clinical Scientist in Nephrology award from the American Kidney Fund, USA.
Sagar U Nigwekar
Declarations of interest
-
Sagar U Nigwekar: none known
-
Amy Kang: none known
-
Sophia Zoungas: I have received speaker honoria from Servier, MSD, Novo Nordisk, Sanofi Aventis, Johnson and Johnson and Astra Zeneca/BMS. I have served on external advisory boards for MSD, Amgen, AbbVie, Novo Nordisk, Novartis, Takeda, Sanofi Aventis and Astra Zeneca.
-
Alan Cass: The Menzies School of Health Research has received unconditional research funding from AMGEN, Merck and Novartis for research in chronic kidney disease in Indigenous populations
-
Martin P Gallagher: Martin Gallagher has received competitive research funding from the Royal Australasian College of Physicians and the Australian National Health and Medical Research Council in the last 36 months.
-
Satyarth Kulshrestha: none known
-
Sankar D Navaneethan: none known
-
Vlado Perkovic: none known
-
Giovanni FM Strippoli: Institutional support from AIfa‐italian medicines agenda for Cedose trial funding (ESA dose); employment by Diaverum, renal service provider for dialysis
-
Meg J Jardine: is supported by a NHMRC Career Development Fellowship and National Heart Foundation Future Leader Fellowship.
Acknowledgements
We would like to thank the referees for their comments and feedback during the preparation of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 May 31 | Interventions for lowering plasma homocysteine levels in dialysis patients | Review | Sagar U Nigwekar, Amy Kang, Sophia Zoungas, Alan Cass, Martin P Gallagher, Satyarth Kulshrestha, Sankar D Navaneethan, Vlado Perkovic, Giovanni FM Strippoli, Meg J Jardine | |
| 2009 Apr 15 | Interventions for lowering plasma homocysteine levels in dialysis patients | Protocol | Sagar U Nigwekar, Alan Cass, Martin P Gallagher, Meg J Jardine, Amy Kang, Satyarth Kulshrestha, Sankar D Navaneethan, Vlado Perkovic, Giovanni FM Strippoli, Sophia Zoungas | |
| 2004 Jan 26 | Interventions for lowering plasma homocysteine levels in dialysis patients | Protocol | Kevan Polkinghorne, Pauline Branley, Peter G Kerr | |
Differences between protocol and review
In the protocol, we mentioned N‐acetyl cysteine as an intervention that lowers serum homocysteine levels to be considered for this review. Although it has this effect there are significant other effects including anti‐oxidant effects from this intervention and since this review is focused on interventions that primarily reduce homocysteine, we decided to not include studies that evaluated N‐acetyl cysteine in the ESKD setting in this review. Our search identified only four such studies (Ali 2003; Nascimento 2010; Scholze 2004; Tepel 2003; Thaha 2006).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Cardiovascular Diseases [etiology, *mortality];
- Cause of Death;
- Folic Acid [adverse effects, *therapeutic use];
- Homocysteine [blood];
- Hyperhomocysteinemia [*drug therapy];
- Kidney Failure, Chronic [*blood, therapy];
- Myocardial Infarction [epidemiology];
- *Renal Dialysis;
- Stroke [epidemiology];
- Venous Thrombosis [epidemiology];
- Vitamin B 12 [therapeutic use];
- Vitamin B 6 [therapeutic use];
- Vitamin B Complex [*therapeutic use];
Medical Subject Headings Check Words
Aged; Female; Humans; Male; Middle Aged;
PICO

Flow diagram showing study selection

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
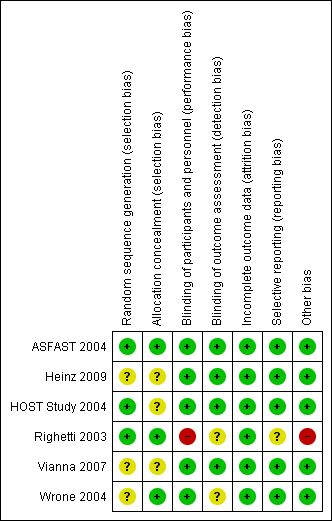
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
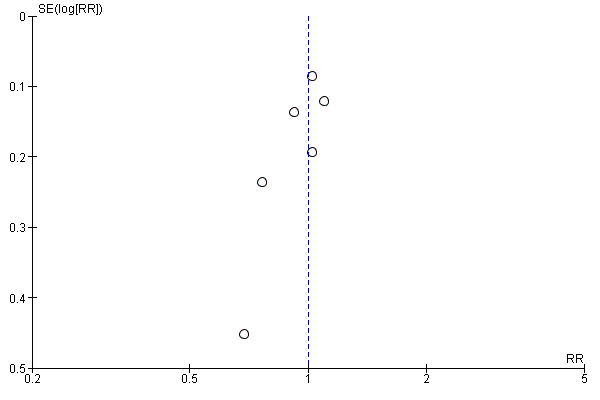
Funnel plot of comparison: 2 Secondary outcomes, outcome: 2.1 all‐cause mortality
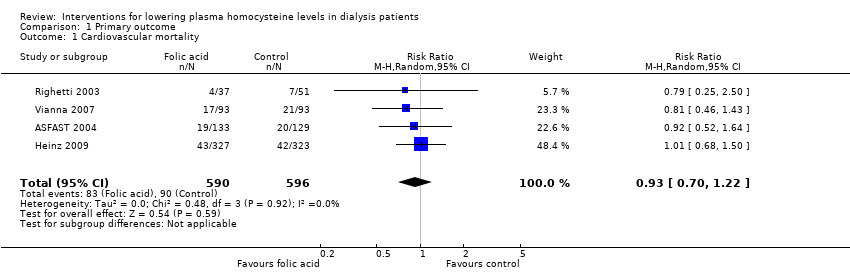
Comparison 1 Primary outcome, Outcome 1 Cardiovascular mortality.
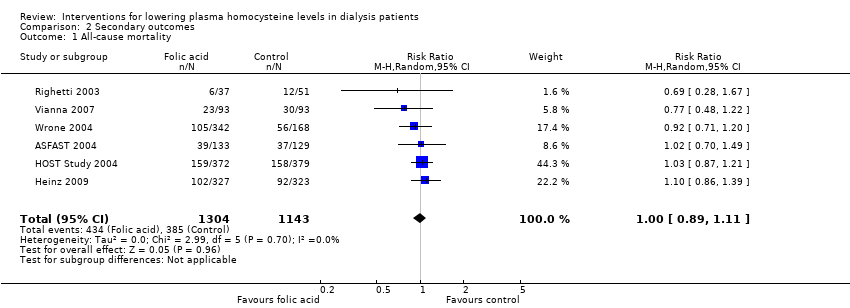
Comparison 2 Secondary outcomes, Outcome 1 All‐cause mortality.
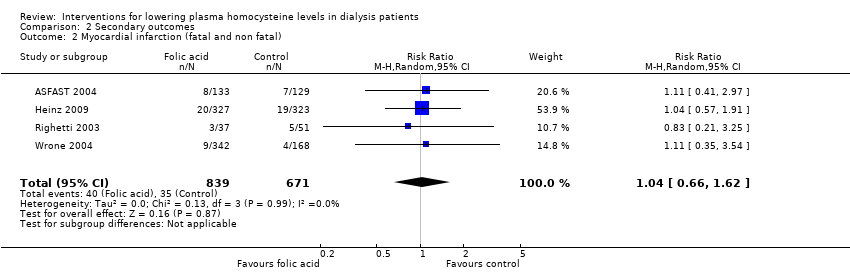
Comparison 2 Secondary outcomes, Outcome 2 Myocardial infarction (fatal and non fatal).
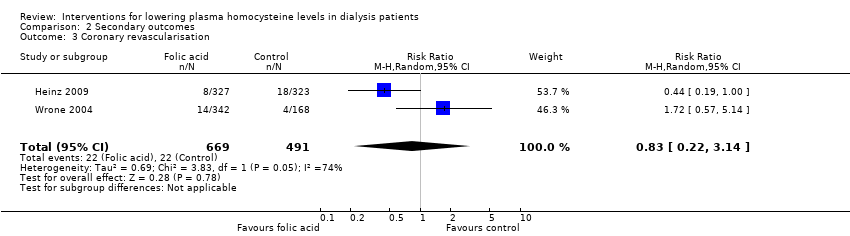
Comparison 2 Secondary outcomes, Outcome 3 Coronary revascularisation.

Comparison 2 Secondary outcomes, Outcome 4 Stroke.

Comparison 2 Secondary outcomes, Outcome 5 Deep venous thrombosis and pulmonary embolism.

Comparison 2 Secondary outcomes, Outcome 6 Thrombosis of dialysis access.

Comparison 2 Secondary outcomes, Outcome 7 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 4 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 6 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.11] |
| 2 Myocardial infarction (fatal and non fatal) Show forest plot | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.62] |
| 3 Coronary revascularisation Show forest plot | 2 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.14] |
| 4 Stroke Show forest plot | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.40] |
| 5 Deep venous thrombosis and pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Thrombosis of dialysis access Show forest plot | 2 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.14] |
| 7 Adverse events Show forest plot | 3 | 1248 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.51, 2.47] |

