Modelo de assistência liderado por obstetrizes versus outros tipos de modelos de assistência obstétrica
Resumo
Introdução
Em muitas regiões do mundo, as obstetrizes são as principais responsáveis pelos cuidados oferecidos às mulheres grávidas. Contudo, existem poucos estudos avaliando diferenças entre os modelos de assistência conduzidos por obstetrizes versus outros tipos de modelos de assistência obstétrica quanto à morbidade e mortalidade materna ou aos desfechos psicossociais e de efetividade.
Objetivos
Comparar modelos de assistência liderados por obstetrizes versus outros tipos de modelo de assistência obstétrica para gestantes/parturientes e seus bebês.
Métodos de busca
A pesquisa foi realizada na base de dados eletrônica Cochrane Pregnancy and Childbirth Group’s Trials Register (em 28 de janeiro 2013). A busca também foi complementada pela análise das listas de referências dos artigos identificados.
Critério de seleção
Foram incluídos nesta revisão todos os ensaios clínicos randomizados publicados e não publicados, que compararam modelos de assistência obstétrica liderados por obstetrizes versus outros tipos de modelos de assistência existentes para mulheres durante a gestação e parto.
Coleta dos dados e análises
Todos os revisores avaliaram a qualidade metodológica dos estudos. Dois revisores verificaram a extração de dados.
Principais resultados
No total, foram incluídos 13 estudos, envolvendo 16.242 mulheres. As gestantes atendidas pelas obstetrizes tiveram menor probabilidade de receber analgesia regional (risco relativo [RR] médio 0,83, intervalo de confiança de 95% [IC95] 0,76 a 0,90), episiotomia (RR médio 0,84, IC95 0,76 a 0,92) e de serem submetidas a parto instrumental (RR médio 0,88, IC95 0,81 a 0,96). Elas apresentaram maior probabilidade de não receber nenhum tipo de analgesia/anestesia intraparto (RR médio 1,16, IC95 1,04 a 1,31), de terem um parto espontâneo vaginal (RR médio 1,05, IC95 1,03 a 1,08), de serem atendidas no parto por uma obstetriz conhecida (RR médio 7,83, IC95% 4,15 a 14,80) e de terem uma duração maior de trabalho de parto (diferença média em horas: 0,50, IC95 0,27 a 0,74). A proporção de cesarianas foi semelhante entre ambos modelos de assistência (RR médio 0,93, IC95 0,84 a 1,02).
As mulheres cuidadas pelas obstetrizes tiveram menor probabilidade de ter partos prematuros (RR médio 0,77, IC95 0,62 a 0,94) e óbito fetal antes da 24ª semana (RR médio 0,81, IC95 0,66 a 0,99). Entretanto, não houve diferença na taxa de óbito fetal/neonatal a partir da 24ª semana (RR médio 1,00, IC95 0,67 a 1,51) ou na taxa geral de óbito fetal/neonatal (RR médio 0,84, IC95 0,71 a 1,00).
A satisfação materna e os custos dos diversos tipos de modelos assistenciais foram relatados de forma descritiva nesta revisão porque não havia consistência na forma de apresentar esses dados nos estudos primários. Em grande parte dos estudos incluídos nesta revisão, a taxa de satisfação materna foi maior no grupo atendido pelas obstetrizes. Também foi identificada uma tendência de que o modelo de assistência liderado por obstetrizes teria um custo mais baixo que os outros modelos de assistência.
Conclusão dos autores
O modelo de assistência liderado por obstetrizes deve ser oferecido para a maioria das gestantes, e as mulheres devem ser encorajadas a solicitar esta opção. Porém, aconselha‐se prudência em fazer esta recomendação para mulheres com problemas clínicos ou obstétricos importantes.
PICO
Resumo para leigos
Modelo de assistência obstétrica liderado por obstetrizes versus outros tipos de modelos de assistência obstétrica
As obstetrizes são as principais responsáveis pela assistência oferecida às mulheres grávidas em muitas regiões do mundo. Em algumas regiões, os obstetras e os médicos generalistas podem ser os principais encarregados por esta assistência; alternativamente, esta responsabilidade pode ser compartilhada entre médicos e obstetrizes. Os princípios filosóficos que norteiam o modelo de assistência liderado por obstetrizes consideram a gestação um processo normal, no qual deve haver continuidade nos cuidados prestados à gestante, e que o parto deve ser assistido por uma obstetriz de confiança da parturiente. Este modelo enfatiza que a mulher é naturalmente capaz de passar pela experiência do parto com um mínimo de intervenções. Existem vários tipos de modelos de assistência liderados por obstetrizes. Em um deles uma “equipe” de obstetrizes é responsável por certo número de gestantes. Já em outro tipo de modelo, considerado “individual”, cada gestante é cuidada por uma única obstetriz ou seu par/substituto. A assistência liderada por obstetrizes é habitualmente oferecida dentro do contexto de uma rede multidisciplinar que inclui consultas e encaminhamentos para outros profissionais de saúde. Esse tipo de assistência difere do modelo de assistência liderado por médicos no qual o obstetra ou médico generalista é o principal responsável pelo atendimento à gestante. Nos modelos de assistência compartilhada, os cuidados são divididos entre os diversos profissionais de saúde.
Esta revisão incluiu modelos de assistência em que as obstetrizes cuidavam das gestantes ao longo da gravidez, no parto e pós‐parto. Foram identificados 13 estudos envolvendo um total de 16.242 mulheres de baixo/alto risco. O modelo liderado por obstetrizes, quando comparado com modelos de assistência médica ou compartilhados, apresentou diversos benefícios para as mães e os bebês, e não revelou efeitos adversos. Os principais benefícios foram a redução do uso de peridurais e as menores taxas de episiotomias ou de partos instrumentais. Esse tipo de modelo também aumentou a probabilidade de a gestante ser assistida na hora do parto por uma obstetriz conhecida e de ter um parto vaginal espontâneo. Não houve diferença na taxa de cesarianas. As mulheres no grupo atendido pelas obstetrizes tiveram menor probabilidade de passar por um parto prematuro ou de terem perdas fetais antes da 24ª semana de gestação. Todavia, não houve diferença no risco de perdas fetais depois da 24ª semana ou como um todo. Em todos os estudos incluídos nesta revisão, as obstetrizes eram profissionais diplomadas e não parteiras tradicionais ou curiosas. Nenhum estudo incluiu partos realizados fora do ambiente hospitalar.
A conclusão desta revisão é a de que o modelo de assistência obstétrica liderado por obstetrizes deve ser oferecido para a maioria das gestantes; porém deve‐se ter cuidado em recomendá‐lo a mulheres com problemas clínicos ou obstétricos sérios.
Authors' conclusions
Background
In many parts of the world, midwives are the primary providers of care for childbearing women (Koblinsky 2006). There are, however, considerable variations in the organisation of midwifery services and in the education and role of midwives (WHO 2006). Furthermore, in some countries, e.g. in North America, medical doctors are the primary care providers for the vast majority of childbearing women, while in other countries, e.g. Australia, New Zealand, the Netherlands, the United Kingdom and Ireland, various combinations of midwife‐led continuity, medical‐led, and shared models of care are available. Childbearing women are often faced with different opinions as to which option might be best for them (De Vries 2001). The midwife‐led continuity model of care is based on the premise that pregnancy and birth are normal life events. The midwife‐led continuity model of care includes: continuity of care; monitoring the physical, psychological, spiritual and social wellbeing of the woman and family throughout the childbearing cycle; providing the woman with individualised education, counselling and antenatal care; continuous attendance during labour, birth and the immediate postpartum period; ongoing support during the postnatal period; minimising technological interventions; and identifying and referring women who require obstetric or other specialist attention. Differences between midwife‐led continuity and other models of care often include variations in philosophy, relationship between the care provider and the pregnant woman, use of interventions during labour, care setting (home, home‐from‐home or acute setting) and in the goals and objectives of care (Rooks 1999). In addition, there is much debate about the clinical and cost effectiveness of the different models of maternity care (Ryan 2013) and hence continuing debate on the optimal model of care for routine ante, intra and postnatal care for healthy pregnant women (Sutcliffe 2012; Walsh 2012). There has been a lack of a single source of synthesised evidence on the effectiveness of midwife‐led continuity models of care when compared with other models of care. This review attempts to provide this evidence.
Midwife‐led continuity models of care have generally aimed to improve continuity of care over a period of time. However, the general literature on continuity notes that a lack of clarity in definition and measurement of different types of continuity has been one of the limitations in research in this field (Haggerty 2003). Continuity has been defined by Freeman 2007 as having three major types ‐ management, informational and relationship. Management continuity involves the communication of both facts and judgements across team, institutional and professional boundaries, and between professionals and patients. Informational continuity concerns the timely availability of relevant information. Relationship continuity means a therapeutic relationship of the service user with one or more health professionals over time. Relationship/personal continuity over time has been found to have a greater effect on user experience and outcome (Saultz 2004; Saultz 2005). Some models of midwife‐led care offer continuity with a group of midwives, and others offer personal or relational continuity, and thus the models of care that are the foci of this review are defined as follows.
(1) Midwife‐led continuity models of care
Whilst it is difficult to categorise maternity models of care exclusively due to the influence of generic policies and guidelines, it is assumed that the underpinning philosophy of a midwife‐led model of care is normality and the natural ability of women to experience birth without routine intervention. Midwife‐led continuity of care has been defined as care where "the midwife is the lead professional in the planning, organisation and delivery of care given to a woman from initial booking to the postnatal period" (RCOG 2001). Some antenatal and/or intrapartum and/or postpartum care may be provided in consultation with medical staff as appropriate. Within these models, midwives are, however, in partnership with the woman, the lead professional with responsibility for assessment of her needs, planning her care, referral to other professionals as appropriate, and for ensuring provision of maternity services. Thus, midwife‐led continuity models of care aim to provide care in either community or hospital settings, normally to healthy women with uncomplicated or 'low‐risk' pregnancies. In some models, midwives provide continuity of midwifery care to all women from a defined geographical location, acting as lead professional for women whose pregnancy and birth is uncomplicated, and continuing to provide midwifery care to women who experience medical and obstetric complications in partnership with other professionals.
Some models of midwife‐led continuity of care provide continuity of care to a defined group of women through a team of midwives sharing a caseload, often called 'team' midwifery. Thus, a woman will receive her care from a number of midwives in the team, the size of which can vary. Other models, often termed 'caseload midwifery', aim to offer greater relationship continuity, by ensuring that childbearing women receive their ante, intra and postnatal care from one midwife or her/his practice partner (McCourt 2006). There is continuing debate about the risks, benefits, and costs of team and caseload models of midwife‐led continuity of care (Ashcroft 2003; Benjamin 2001; Green 2000; Johnson 2005; Waldenstrom 1998).
(2) Other models of care
Other models of care include:
(a) Obstetrician‐provided care. This is common in North America, where obstetricians are the primary providers of antenatal care for most childbearing women. An obstetrician (not necessarily the one who provides antenatal care) is present for the birth, and nurses provide intrapartum and postnatal care.
(b) Family doctor‐provided care, with referral to specialist obstetric care as needed. Obstetric nurses or midwives provide intrapartum and immediate postnatal care but not at a decision‐making level, and a medical doctor is present for the birth.
(c) Shared models of care, where responsibility for the organisation and delivery of care, throughout initial booking to the postnatal period, is shared between different health professionals.
At various points during pregnancy, childbirth, and the postnatal period, responsibility for care can shift to a different provider or group of providers. Care is often shared by family doctors and midwives, by obstetricians and midwives, or by providers from all three groups. In some countries (e.g. Canada and the Netherlands), the midwifery scope of practice is limited to the care of women experiencing uncomplicated pregnancies, while in other countries (e.g. United Kingdom, France, Australia and New Zealand), midwives provide care to women who experience medical and obstetric complications in collaboration with medical colleagues. In addition, maternity care in some countries (e.g. Republic of Ireland, Iran and Lebanon), is predominantly provided by a midwife but is obstetrician‐led, in that the midwife might provide the actual care, but the obstetrician assumes overall responsibility for the care provided to the woman throughout her ante‐, intra‐ and postpartum periods.
This review complements other work on models of maternity care and attributes thereof, specifically, the work of Hodnett (Hodnett 2012) and Olsen (Olsen 2012) in which the relationships between the various birth settings and pregnancy outcomes were evaluated systematically. This review also subsumes the Cochrane review, 'Continuity of caregivers during pregnancy, childbirth, and the postpartum period' (Hodnett 2000).
Objectives
The primary objective of this review is to compare the effects of midwife‐led continuity models of care with other models of care for childbearing women and their infants.
We also explore whether the effects of midwife‐led continuity of care are influenced by: 1) models of midwife‐led care that provide differing levels of relationship continuity; 2) varying levels of obstetrical risk.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials including trials using individual or cluster randomisation methods. We also included trials where allocation may not have been truly random (e.g. where allocation was alternate or not clear).
Types of participants
Pregnant women.
Types of interventions
Models of care are classified as midwife‐led continuity of care, and other or shared care on the basis of the lead professional in the antepartum and intrapartum periods. In midwife‐led continuity models of care, the midwife is the woman's lead professional, but one or more consultations with medical staff are often part of routine practice. Other models of care include a) where the physician/obstetrician is the lead professional, and midwives and/or nurses provide intrapartum care and in‐hospital postpartum care under medical supervision; b) shared care, where the lead professional changes depending on whether the woman is pregnant, in labour or has given birth, and on whether the care is given in the hospital, birth centre (free standing or integrated) or in community setting(s); and c) where the majority of care is provided by physicians or obstetricians.
Types of outcome measures
Primary outcomes
Birth and immediate postpartum
-
Regional analgesia (epidural/spinal)
-
Caesarean birth
-
Instrumental vaginal birth (forceps/vacuum)
-
Spontaneous vaginal birth (as defined by trial authors)
-
Intact perineum
Neonatal
-
Preterm birth (less than 37 weeks)
-
Overall fetal loss and neonatal death (fetal loss was assessed by gestation using 24 weeks as the cut‐off for viability in many countries)
Secondary outcomes
-
Antenatal hospitalisation
-
Antepartum haemorrhage
-
Induction of labour
-
Amniotomy
-
Augmentation/artificial oxytocin during labour
-
No intrapartum analgesia/anaesthesia
-
Opiate analgesia
-
Attendance at birth by known midwife
-
Episiotomy
-
Perineal laceration requiring suturing
-
Mean labour length (hours)
-
Postpartum haemorrhage
-
Breastfeeding initiation
-
Duration of postnatal hospital stay (days)
-
Low birthweight (less than 2500 g)
-
Five‐minute Apgar score less than or equal to seven
-
Neonatal convulsions
-
Admission to special care nursery/neonatal intensive care unit
-
Mean length of neonatal hospital stay (days)
-
Fetal loss and neonatal death less than 24 weeks
-
Fetal loss and neonatal death equal to/after 24 weeks
-
Perceptions of control during labour and childbirth
-
Mean number of antenatal visits
-
Maternal death
-
Cord blood acidosis
-
Postpartum depression
-
Any breastfeeding at three months
-
Prolonged perineal pain
-
Pain during sexual intercourse
-
Urinary incontinence
-
Faecal incontinence
-
Prolonged backache
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For search methods used in the previous update of this review (Hatem 2008), seeAppendix 1.
Searching other resources
We searched for further studies in the reference list of the studies identified.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2. For this update we used the following methods.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. One review author entered data into Review Manager software (RevMan 2012) and this was independently checked by a second review author.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
One of the review authors (D Devane) is a co‐author of one of the included studies (Begley 2011) so was not involved in data extraction or in the 'Risk of bias' assessment for this study.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We viewed it unlikely that it would be possible to blind participants or personnel in these trials to the group to which women were randomised because the model of care that women are allocated to determines from whom and where they receive maternity care services. Nevertheless, we recognised that some authors may have attempted to blind control group participants or personnel. Therefore, we assessed the methods as:
-
low risk of bias;
-
high risk of bias;
-
unclear risk of bias.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a woman received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the risk of bias for blinding of outcome assessment as:
-
low risk;
-
high risk;
-
unclear risk.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed methods as:
-
low risk of bias (if attrition rate was less than 20% for all outcomes);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all outcomes stated in the methods section were adequately reported or explained in results);
-
high risk of bias (where not all the outcomes stated in the methods section were adequately reported or explained in result);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias such as considerable deviation from protocol, limitations in study design or significant imbalances in baseline characteristics.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
Measures of treatment effect
We conducted statistical analysis using the Review Manager software (RevMan 2012).
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. If necessary, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included one cluster‐randomised trial in the analyses along with individually‐randomised trials (North Stafford 2000). We adjusted the sample size using the methods described by Gates 2005 using an estimate of the intracluster correlation coefficient (ICC) derived from the trial. This trial estimated the ICC to be zero, so for the main analysis we used this estimate and did not adjust the sample sizes.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis based on the 'Risk of bias' assessment.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. Where asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). Due to the nature of this complex intervention it was agreed that there was sufficient clinical heterogeneity to expect that the underlying treatment effects differed between trials and, therefore, we used a random‐effect meta‐analysis for combining data to produce an overall summary of the average treatment effect across trials. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. Where the average treatment effect was not clinically meaningful, we did not combine trials.
The results are presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We carried out the following subgroup analyses:
-
variation in levels of personal continuity (caseload or team);
-
variation in levels of obstetric risk (low versus mixed).
Subgroup analyses were performed on primary outcomes. The following outcomes were used in subgroup analysis.
Delivery and immediate postpartum
-
Regional analgesia (epidural/spinal)
-
Caesarean birth
-
Instrumental vaginal birth (forceps/vacuum)
-
Spontaneous vaginal birth (as defined by trial authors)
-
Intact perineum
Neonatal
-
Overall fetal loss and neonatal death
-
Preterm birth (less than 37 weeks)
We assessed subgroup differences by interaction tests within RevMan (RevMan 2012). We reported the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analysis based on the quality of the included trials to identify the impact of the methodological quality on the overall results. For the purpose of this review, we defined "high quality" as a trial having adequate sequence generation, allocation concealment and an attrition rate of less than 20%.
Results
Description of studies
SeeCharacteristics of included studies table.
Our search strategy identified 55 citations relating to 33 studies for potential inclusion.
Of those, we included 13 trials involving 16,242 randomised women in total (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001) and excluded 20 studies (Berglund 1998; Berglund 2007; Bernitz 2011; Chambliss 1991; Chapman 1986; Giles 1992; Heins 1990; Hildingsson 2003; Hundley 1994; James 1988; Kelly 1986; Klein 1984; Law 1999; Marks 2003; Runnerstrom 1969; Slome 1976; Stevens 1988; Tucker 1996; Waldenstrom 1997; Walker 2012 (seeCharacteristics of excluded studies).
Included studies were conducted in the public health systems in Australia, Canada, Ireland, New Zealand and the United Kingdom with variations in model of care, risk status of participating women and practice settings. The Zelen method was used in three trials (Flint 1989; Homer 2001; MacVicar 1993) and one trial used cluster randomisation (North Stafford 2000).
Three studies offered a caseload team model of care (McLachlan 2012; North Stafford 2000; Turnbull 1996) and 10 studies provided a team model of care: (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; Rowley 1995; Waldenstrom 2001). The composition and modus operandi of the teams varied among trials. Levels of continuity (measured by the percentage of women who were attended during birth by a known carer varied between 63% to 98% for midwife‐led continuity models of care to 0.3% to 21% in other models of care).
Eight studies compared a midwife‐led continuity model of care to a shared model of care (Begley 2011; Biro 2000; Flint 1989; Hicks 2003; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995), three studies compared a midwife‐led continuity model of care to medical‐led models of care (Harvey 1996; MacVicar 1993; Turnbull 1996) and two studies compared midwife‐led continuity of care with various options of standard care including midwife‐led (with varying levels of continuity), medical‐led and shared care (McLachlan 2012; Waldenstrom 2001).
Participating women received ante‐, intra‐ and postpartum care in 12 studies (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; McLachlan 2012; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001) and antenatal and intrapartum care in one study (MacVicar 1993).
Some midwife‐led continuity models included routine visits to the obstetrician or family physicians (GPs), or both. The frequency of such visits varied. Such visits were dependent on women's risk status during pregnancy (Biro 2000); routine for all women (one to three visits) (Flint 1989; Harvey 1996; Kenny 1994; MacVicar 1993; McLachlan 2012; Rowley 1995; Waldenstrom 2001) or determined based on the development of complications (Hicks 2003; Turnbull 1996) or antenatal care from midwives and, if desired by the woman, from the woman's general practitioner (Begley 2011).
Women were classified as being at low risk of complications in eight studies (Begley 2011; Flint 1989; Harvey 1996; Hicks 2003; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001) and as 'low and high' and 'high' in five studies (Biro 2000; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995).
The midwifery models of care were hospital‐based in four studies (Biro 2000; MacVicar 1993; Rowley 1995; Waldenstrom 2001) or offered (i) antenatal care in an outreach community‐based clinic and intra‐ and postpartum care in hospital (Homer 2001); (ii) ante‐ and postpartum community‐based care with intrapartum hospital‐based care (Hicks 2003; North Stafford 2000; Turnbull 1996) (iii) antenatal and postnatal care in the hospital and community settings with intrapartum hospital‐based care or (iv) postnatal care in the community with hospital‐based ante‐ and intrapartum care (Flint 1989; Harvey 1996; Kenny 1994; McLachlan 2012). Four studies offered intrapartum care in homelike settings, either to all women in the trial (Waldenstrom 2001), or to women receiving midwife‐led continuity of care only (Begley 2011; MacVicar 1993; Turnbull 1996).
Risk of bias in included studies
SeeFigure 1; Figure 2 for summary of 'Risk of bias' assessments.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies reported genuine random methods of generation of the randomisation sequence (Begley 2011; Biro 2000; Harvey 1996; Hicks 2003; Homer 2001; MacVicar 1993; McLachlan 2012; Rowley 1995; Turnbull 1996). Four gave no or insufficient information to form a clear judgement (Flint 1989; Kenny 1994; North Stafford 2000; Waldenstrom 2001). Allocation concealment was judged low risk of bias for 10 studies (Begley 2011; Biro 2000; Harvey 1996, Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001). Two studies were judged unclear risk of bias (Rowley 1995 gave no information about the process of random allocation, and Flint 1989 used sealed opaque envelopes but did not specify any numbering). The North Stafford 2000 trial was a cluster‐randomised trial, whereby allocation concealment was not possible and it was judged high risk of bias for allocation concealment.
Blinding
Five of the included studies were judged as high risk in blinding of participants and personnel (Begley 2011; Homer 2001; MacVicar 1993; North Stafford 2000; Rowley 1995) and eight studies were at unclear risk of bias (Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; McLachlan 2012; Turnbull 1996; Waldenstrom 2001).
One study was at low risk of bias for blinding of outcome assessment (McLachlan 2012), three were judged as high risk of bias (Begley 2011; Homer 2001; Rowley 1995) and nine studies were at unclear risk of bias (Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; North Stafford 2000; Turnbull 1996; Waldenstrom 2001).
Incomplete outcome data
Eleven of the included studies were judged at low risk of bias for incomplete outcome data on the basis that attrition rate was less than 20% for all outcomes (other than satisfaction) or missing outcome data were balanced across groups (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; McLachlan 2012; North Stafford 2000; Turnbull 1996; Waldenstrom 2001). Two of the studies (MacVicar 1993; Rowley 1995) did not provide sufficient information on loss to follow‐up and were judged as unclear.
Selective reporting
All outcomes stated in the methods section were adequately reported in results in all included studies (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001).
Other potential sources of bias
No other potential sources of bias were identified in any of the included studies.
Effects of interventions
We used random‐effects for all analyses ‐ where we identified statistical heterogeneity (I2 > 30%) we have reported the values of both Tau² and I².
Comparison 1 (main comparison): midwife‐led continuity models of care versus other models of care for childbearing women and their infants ‐ all trials
Primary outcomes
Women randomised to midwife‐led continuity models of care were, on average, less likely to experience:
-
regional analgesia (epidural/spinal) (average risk ratio (RR) 0.83, 95% confidence interval (CI) 0.76 to 0.90, 13 trials, n = 15,982, Tau² = 0.01, I² = 48%) (Analysis 1.1);
-
instrumental vaginal birth (forceps/vacuum) (average RR 0.88, 95% CI 0.81 to 0.96,12 trials, n = 15,809) (Analysis 1.3);
-
preterm birth (average RR 0.77, 95% CI 0.62 to 0.94, seven trials, n = 11,546, Tau² = 0.03, I² = 42%) (Analysis 1.6).
Women randomised to midwife‐led continuity models of care were on average more likely to experience:
-
a spontaneous vaginal birth (average RR 1.05, 95% CI 1.03 to 1.08, 11 trials, n = 14,995) (Analysis 1.4);
There were no statistically significant differences between groups for the following outcomes:
-
caesarean birth (average RR 0.93, 95% CI 0.84 to 1.02, 13 trials, n = 15,982) (Analysis 1.2);
-
intact perineum (average RR 1.03, 95% CI 0.94 to 1.13, nine trials, n = 11,494, Tau² = 0.01, I² = 59%) (Analysis 1.5);
-
overall fetal loss and neonatal death (average RR 0.84, 95% CI 0.71 to 1.00, 12 trials, n = 15,869) (Analysis 1.7)
Secondary outcomes
Women randomised to midwife‐led continuity models of care were, on average, less likely to experience:
-
amniotomy (average risk ratio (RR) 0.80, 95% CI 0.66 to 0.98, four trials, n = 3253, Tau² = 0.03, I² = 75%) (Analysis 1.11);
-
episiotomy (average RR 0.84, 95% CI 0.76 to 0.92,13 trials, n = 15,982, Tau² = 0.01, I² = 50%) (Analysis 1.16);
-
fetal loss/neonatal death before 24 weeks (average RR 0.81, 95% CI 0.66 to 0.99, 10 trials, n = 13,953) (Analysis 1.27).
Women randomised to midwife‐led continuity models of care were on average more likely to experience:
-
no intrapartum analgesia/anaesthesia (average RR 1.16, 95% CI 1.04 to 1.31, six trials, n = 8807) (Analysis 1.13);
-
alonger mean length of labour (hours) (mean difference (MD) 0.50, 95% CI 0.27 to 0.74, three trials, n = 3328) (Analysis 1.18); However, there was evidence of skewness in the data from one of the trials in the analyses of length of labour (Turnbull 1996);
-
women allocated to midwife‐led continuity models of care were more likely to be attended at birth by a known carer (average RR 7.83, 95% CI 4.15 to 14.80, six trials, n = 5225). However, the effect estimates for individual studies are highly variable (as reflected in substantial statistical heterogeneity, i.e., Tau² = 0.54; Chi² = 100.76, df = 5 (P < 0.00001), I² = 95%) (see Analysis 1.15)
There were no statistically significant differences between groups for the following outcomes:
-
antenatal hospitalisation (average RR 0.93, 95% CI 0.83 to 1.05, six trials, n = 6039, Tau² = 0.01, I² = 50%) (Analysis 1.8);
-
antepartum haemorrhage (average RR 0.89, 95% CI 0.57 to 1.40, four trials, n = 3654, Tau² = 0.07, I² = 31%) (Analysis 1.9);
-
induction of labour (average RR 0.95, 95% CI 0.86 to 1.03, 12 trials, n = 15,809, Tau² = 0.01, I² = 45%) (Analysis 1.10);
-
augmentation/artificial oxytocin during labour (average RR 0.89, 95% CI 0.79 to 1.01, 11 trials, n = 13,502, Tau² = 0.03, I² = 76% (Analysis 1.12);
-
opiate analgesia (average RR 0.90, 95% CI 0.80 to 1.01, 10 trials, n = 11,997, Tau² = 0.02, I² = 77% (Analysis 1.14);
-
perineal laceration requiring suturing (average RR 1.02, 95% CI 0.95 to 1.10, nine trials, n = 13,412, Tau² = 0.01, I² = 56%) (Analysis 1.17);
-
postpartum haemorrhage (average RR 0.97, 95% CI 0.84 to 1.11, nine trials, n = 12,522) (Analysis 1.19);
-
breastfeeding initiation (average RR 1.12, 95% CI 0.81 to 1.53, two trials, n = 2050, Tau² = 0.04, I² = 81%) (Analysis 1.20);
-
mean length of postnatal hospital stay (days) (MD ‐0.10, 95% CI ‐0.29 to 0.09, three trials, n = 3593, Tau² = 0.02, I² = 58%) (Analysis 1.21);
-
low birthweight infant (average RR 0.98, 95% CI 0.83 to 1.16, six trials, n = 9766) (Analysis 1.22);
-
five‐minute Apgar score less than or equal to seven (average RR 0.99, 95% CI 0.70 to 1.41, 10 trials, n = 10,854,Tau² = 0.11, I² = 38%) (Analysis 1.23);
-
neonatal convulsions (average RR 0.91, 95% CI 0.14 to 5.74, two trials, n = 2923) (Analysis 1.24);
-
admission of infant to special care or neonatal intensive care unit(s) (average RR 0.90, 95% CI 0.76 to 1.06, 12 trials, n = 15,869, Tau² = 0.04, I² = 48%) (Analysis 1.25);
-
mean length of neonatal hospital stay (days) (MD ‐3.63, 95% CI ‐7.57 to 0.30, two trials, n = 1979, Tau² = 6.69, I² = 80%) (Analysis 1.26);
-
fetal loss or neonatal death more than or equal to 24 weeks (average RR 1.00, 95% CI 0.67 to 1.51, 11 trials, n = 15,667) (Analysis 1.28).
The difference in the average treatment effect in overall fetal loss and neonatal death across included trials between women allocated to midwife‐led continuity models of care and women allocated to other models has a RR of 0.84 and a 95% CI of 0.71 to 1.00 (12 trials, n = 15,869, average RR 0.84, 95% CI 0.71 to 1.00) (Analysis 1.7). Given that (i) the 95% CI just reaches 1.00 and (ii) the absence of measurable heterogeneity in this outcome analysis, the probability is that midwife‐led continuity models of care are associated with a reduction in overall fetal loss and neonatal death by approximately 16%.
There was substantial statistical heterogeneity in many of the analyses. The I2 value was greater than 50% for 10 outcomes (antenatal hospitalisation, amniotomy, augmentation, opiate analgesia, attendance at birth by known carer, intact perineum, perineum requiring suturing, duration of postnatal hospital stay, duration of neonatal stay, breastfeeding initiation, and greater than 30% for a further six (antepartum haemorrhage, induction of labour, episiotomy, five‐minute Apgar score less than seven, preterm birth, admission to neonatal care).
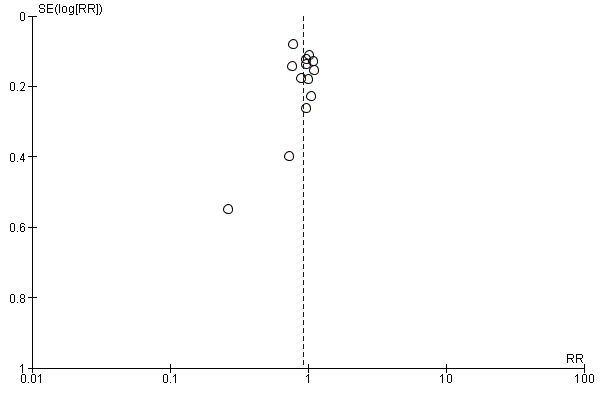
Visual inspection of funnel plots for analyses where there were 10 or more studies (Analysis 1.10, Analysis 1.12, Analysis 1.14, Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.16, Analysis 1.23, Analysis 1.25, Analysis 1.27, Analysis 1.28 and Analysis 1.7) suggested little evidence of asymmetry for most analyses. For three analyses (Analysis 1.1 regional analgesia, Analysis 1.2 caesarean delivery and Analysis 1.16 episiotomy) there was a some suggestion of asymmetry, though in all cases this was due to two small trials with large treatment effects in the same direction (Figure 3; Figure 4; Figure 5). There is therefore no strong evidence of reporting bias, though this is difficult to detect with the number of studies in this review, and whether it exists and the extent to which it affects the results may be clarified when more studies have been conducted.

Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.1 Regional analgesia (epidural/spinal).

Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.2 Caesarean birth.

Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.16 Episiotomy.
It was not possible to analyse the following outcomes, either because data were not reported by any studies or they were reported in a way that did not allow extraction of the necessary data for meta‐analysis, or losses and exclusions were more than 20% of the randomised participants. No maternal deaths were reported. Only one trial reported the following outcomes: mean number of antenatal visits, perceptions of control, and postpartum depression and so results were not included in a meta‐analysis. No trials reported on longer‐term outcomes: any breastfeeding at three months; prolonged perineal pain; pain during sexual intercourse; urinary incontinence; faecal incontinence; and prolonged backache.
Subgroup analyses
Comparison 2: variation in midwifery models of care (caseload or one‐to‐one versus team)
Three trials randomised 5118 women to compare a caseload model of care (defined as one midwife carrying responsibility for a defined caseload of women in partnership with a midwife partner) with other models of care (McLachlan 2012; North Stafford 2000; Turnbull 1996). Caseload size was reported to be 45 women per midwife per year (McLachlan 2012), 35 to 40 women (North Stafford 2000) and 32.4 women per midwife (Turnbull 1996). Ten trials randomised 11,124 women to compare team models of midwifery (defined as a group of midwives sharing responsibility for a caseload of women) with other models of care (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; Rowley 1995; Waldenstrom 2001).
There was no evidence of a difference between the caseload and team subgroups for any of the outcomes included in the subgroup analysis. Differences between the average treatment effects for the subgroups were generally small. The largest differences were for preterm birth: caseload (average RR 0.65, 95% CI 0.47 to 0.90); team (average RR 0.81, 95% CI 0.62 to 1.07) (Analysis 2.6); and overall fetal loss and neonatal death: caseload (RR 0.65, 95% CI 0.43 to 0.99); team (average RR 0.89 95% CI 0.73 to 1.07) (Analysis 2.7).
Comparison 3: variation in risk status (low risk versus mixed)
Eight trials randomised 11,195 women to compare midwife‐led continuity models of care versus other models of care in women defined to be at low risk by trial authors (Begley 2011; Flint 1989; Harvey 1996; Hicks 2003; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001). Five trials randomised 5047 women to compare midwife‐led continuity models of care with other models of care in women defined to be at mixed risk of complications by trial authors (Biro 2000; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995). Of these, two trials excluded women who booked late ‐ after 24 weeks' gestation (Biro 2000; Homer 2001) and 16 weeks' gestation (Kenny 1994). Two trials excluded women with a substance misuse problem (Kenny 1994; Rowley 1995) and two trials excluded women with significant medical disease/previous history of a classical or more than two caesareans (Homer 2001), or requiring admission to the maternal fetal medicine unit (Biro 2000).
There was no evidence of differences in treatment effect between the low risk and mixed risk subgroups for any of the outcomes included. Differences in treatment effect were very small, except for preterm birth: low risk (average RR 0.71, 95% CI 0.54 to 0.92); mixed risk (average RR 0.92, 95% CI 0.70 to 1.21) (Analysis 3.6); and overall fetal loss: low risk (average RR 0.94, 95% CI 0.73, to 1.20); mixed risk (average RR 0.76 95% CI 0.59 to 0.97) (Analysis 3.7).
Maternal satisfaction
Due to the lack of consistency in conceptualisation and measurement of women's experiences and satisfaction of care, a narrative synthesis of such data is presented. Nine studies reported maternal satisfaction with various components of the childbirth experiences (Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; Rowley 1995; Turnbull 1996; Waldenstrom 2001).
Given the ambiguity surrounding the concept of satisfaction, it was not surprising to find inconsistency in the instruments, scales, timing of administration and outcomes used to 'measure' satisfaction across studies. Because of such heterogeneity and as might be expected, response rates of lower than 80% for most of these studies, meta‐analysis for the outcome of satisfaction was considered inappropriate and was not conducted.
Satisfaction outcomes reported in the included studies included maternal satisfaction with information, advice, explanation, venue of delivery, preparation for labour and birth, as well as giving choice for pain relief and behaviour of the carer. One study assessed perceptions of control in labour (Flint 1989) using a three‐point scale. For convenience and ease of understanding, tabulated results of the overall satisfaction or indicators which directly relate to staff attitude, or both, are presented in Table 1. In brief, the majority of the included studies, showed a higher level of satisfaction in various aspects of care in the midwife‐led continuity compared to the other models of care.
| Satisfaction | Intervention (n/N) | Control (n/N) | Relative rate | 95% CI | Statistical test | P value |
| Staff in labour (very caring) | 252/275 (92%) | 208/256 (81%) | 1.1 | 1.0‐1.2 | ||
| Experience of labour (wonderful/enjoyable) | 104/246 (42%) | 72/223 (32%) | 1.3 | 1.0‐1.8 | ||
| Satisfaction with pain relief (very satisfied) | 121/209 (58%) | 104/205 (51%) | 1.1 | 0.9‐1.4 | ||
| Very well prepared for labour | 144/275 (52%) | 102/254 (40%) | 1.3 | 1.0‐1.7 | ||
| N = 1663 | N = 826 | Difference | ||||
| Very satisfied with antenatal care | 52% | 44% | 8.3% | 4.1‐12.5 | ||
| Very satisfied with care during labour | 73% | 60% | 12.9% | 9.1‐16.8 | ||
| N = 213 | N = 233 | |||||
| Carer skill, attitude and communication (antenatal care) | 57.1/60 | 47.7/60 | t = 12.4 | 0.0001 | ||
| Convenience and waiting (antenatal care) | 14.8/20 | 10.9/20 | t = 10.1 | 0.0001 | ||
| Expectation of labour/birth (antenatal care) | 9.8/18 | 9.3/18 | t = 1.4 | 0.16 | ||
| Asking questions (antenatal care) | 8.5/12 | 6.9/12 | t = 6.6 | 0.0001 | ||
| Information/communication (labour and birth) | 28.3/30 | 24.8/30 | t = 7.48 | 0.0001 | ||
| Coping with labour (labour and birth) | 20.9/30 | 19.3/30 | t = 2.83 | 0.005 | ||
| Midwife skill/caring (labour and birth) | 22.7/24 | 21.3/24 | t = 3.44 | 0.0007 | ||
| Help and advice (postnatal care) | 21.0/24 | 19.7/24 | t = 1.88 | 0.06 | ||
| Midwife skill and communication (postnatal care) | 16.6/18 | 15.4/18 | t = 4.48 | 0.0001 | ||
| Managing baby (postnatal care) | 8.7/12 | 8.5/12 | t = 0.77 | 0.77 | ||
| Self‐rated health (postnatal care) | 7.5/12 | 7.1/12 | t = 1.67 | 0.10 | ||
| OR | ||||||
| Encouraged to ask questions | N/A | 4.22 | 2.72‐6.55 | |||
| Given answers they could understand | N/A | 3.03 | 1.33‐7.04 | |||
| Able to discuss anxieties | N/A | 3.60 | 2.28‐5.69 | |||
| Always had choices explained to them | N/A | 4.17 | 1.93‐9.18 | |||
| Participation in decision making | N/A | 2.95 | 1.22‐7.27 | |||
| Midwives interested in woman as a person | N/A | 7.50 | 4.42‐12.80 | |||
| Midwives always friendly | N/A | 3.48 | 1.92 ‐ 6.35 | |||
| n/N | n/N | Mean difference ‐ satisfaction score | ||||
| Antenatal care | 534/648 | 487/651 | 0.48 | 0.55‐0.41 | ||
| Intrapartum care | 445/648 | 380/651 | 0.28 | 0.37‐0.18 | ||
| Hospital‐based postnatal care | 445/648 | 380/651 | 0.57 | 0.70‐0.45 | ||
| Home‐based postnatal care | 445/648 | 380/651 | 0.33 | 0.42‐0.25 | ||
| % | % | OR | ||||
| Overall antenatal care was very good (strongly agree) | 58.2% | 39.7% | 2.22 | 1.66‐2.95 | < 0.001 | |
| Happy with the physical aspect of intrapartum care (strongly agree) | 58.6% | 42.5% | 1.94 | 1.46‐2.59 | < 0.001 | |
| Happy with the emotional aspect of intrapartum care (strongly agree) | 58.8% | 44.0% | 1.78 | 1.34‐2.38 | < 0.001 | |
| Overall postnatal care was very good (strongly agree) | 37.6% | 33.2% | 1.27 | 0.97‐1.67 | 0.08 | |
| Care and sensitivity of staff (antenatal) | 1.32 | 1.77 | Mean difference? | 0.0000 | ||
| Care and sensitivity of staff (labour and delivery) | 1.26 | 1.58 | Mean difference? | 0.008 | ||
| Care and sensitivity of staff (postpartum at home) | 1.24 | 1.57 | Mean difference? | 0.0000 | ||
| Labour and Delivery Satisfaction Index + | 211 | 185 | 26 | 18.8‐33.1 | 0.001 | |
| Satisfaction with antenatal care (very good) | 195/344 (57%) | 100/287 (35%) | 1.24 | 1.13‐1.36 | 0.001 | |
| Satisfaction with intrapartum care (very good) | 215/241 (63%) | 134/282 (47%) | 1.11 | 1.03‐1.20 | 0.01 | |
| Satisfaction with postpartum care in hospital (very good) | 141/344 (41%) | 102/284 (31%) | 0.92 | 0.82‐1.04 | 0.22 |
*: 99% Confidence interval (CI) for Flint study was reported
N/A: not available
**:Mean satisfaction scores are reported: lower scale indicates higher satisfaction. Satisfaction scores were calculated on a 5‐point ordinal scale in which 1 = very satisfied and 5 = very dissatisfied.
Sensitivity analyses
We performed a sensitivity analysis excluding the cluster‐randomised North Staffordshire trial from all outcomes in the primary comparison (comparison 1) for which it had contributed data (North Stafford 2000). This did not alter the findings for any outcome, which remained consistent with overall findings with all trials included. Similarly, a sensitivity analysis for the primary outcomes including only the studies rated at low risk of bias (Begley 2011; Biro 2000; Harvey 1996; Hicks 2003; Homer 2001; McLachlan 2012; Turnbull 1996) found that there were only minor differences from the overall analyses. The main consequence was that confidence intervals were slightly wider, because of the smaller number of trials in the analysis. In no case were the conclusions of the analysis different. The primary outcome with the largest difference in this sensitivity analysis was preterm birth, where an analysis restricted to trials with lower risk of bias suggested a larger treatment effect: RR 0.64, (95% CI 0.51 to 0.81) compared with RR 0.77, (95% CI 0.62 to 0.94) in the overall analysis.
Economic analysis
Findings from economic analyses will vary depending on the structure of health care in a given country, and what factors are included in the modelling. Due to the lack of consistency in measurement of economic evaluations, a narrative synthesis of such data is presented. Five studies presented economic analysis in which various measures and items were included in the final cost estimation (Flint 1987; Homer 2001; Kenny 1994; Rowley 1995; Young 1997).
Flint 1989 examined the costs for a subgroup of women (n = 49) and estimated costs for antenatal admission and antenatal care, and found antenatal care was 20% to 25% cheaper for women in the midwife‐led continuity of care group due to differences in staff costs. Women in the midwife‐led continuity of care group had fewer epidurals (£19,360 versus £31,460).
Kenny 1994 examined the costs of care in detail. The average cost/client in the antenatal period was $158 midwife‐led continuity of care and $167 control. For high‐risk women the average cost/client was $390 midwife‐led continuity of care and $437 control, and for low‐risk women $119 midwife‐led continuity of care and $123 control. The average cost per woman for intrapartum care was $219 midwife‐led continuity of care and $220 control and for postnatal care was $745 midwife‐led continuity of care and $833 control. The total cost/woman was $1122 for midwife‐led continuity of care and $1220 control.
Rowley 1995 used the Australian national cost weights for diagnostic related groups (AN‐DRGs) to estimate maternity care in each study group. The average cost per delivery was higher in the standard care group ($3475) compared to the team‐midwifery group ($3324). This method was limited to the acute inpatient and did not include antenatal or postnatal care cost estimations. An assessment of midwife salaries from the first antenatal visit up to and including labour and delivery care resulted in a cost of $653 for each team care woman and $688 for each routine care woman. The amount of sick leave taken by team care midwives was half that taken by standard care midwives.
Young 1997 used the "individual patient‐based costing" approach, in which an assumption was made about the number of caseloads per midwife. When the assumption was based on a median caseload of 29 women per midwife, the cost of midwife managed care was not significantly different from the shared‐care group in the antenatal and intrapartum periods, but it was higher in the postpartum period. The authors also used an alternative assumption including a caseload of 39 women per midwife. A lower cost in the antenatal period for the midwife‐managed care was shown in comparison with the shared‐care group (mean: £346 versus £384, P = 0.05), but the postnatal care cost remained higher in the former group (£444 versus £397, respectively, P < 0.01). The authors did not recalculate the cost of intrapartum care for the second assumption, and used the same estimation as for the 29 caseload per midwife (since they indicated that the main effects were in the unit costs of clinic and home visits). They reported no significant differences between the midwifery and shared‐care group, in the cost of intrapartum care (£280 versus £276, P = 0.4).
Homer 2001 calculated the costs of all aspects of care from the healthcare provider's perspective, including salaries and wages; goods and services; and repair, maintenance and renewal (RMR). The associated costs for all stages of antenatal, intrapartum and postnatal care were calculated and presented as the mean cost per woman per group. The results showed a cost‐saving effect in the team midwifery group compared with the standard care arm of the study (mean cost per woman: $2579 versus $3483, respectively).
In summary, five studies presented cost data using different economic evaluation methods. All studies suggest a cost‐saving effect in intrapartum care. One study suggests a higher cost, and one study no differences in cost of postnatal care when midwife‐led continuity of care is compared with medical‐led maternity care. There is a lack of consistency in estimating maternity care cost among the available studies; however, there seems to be a trend towards the cost‐saving effect of midwife‐led continuity of care in comparison with medical‐led care.
Discussion
This review summarises 13 trials involving 16,242 women that took place in five countries in a wide variety of settings and health systems. All trials involved midwife‐led continuity models of care that included either team or caseload midwifery, and women classified as at low or mixed risk. All trials included licensed midwives, and none included lay or traditional midwives. The review includes trials that compared midwife‐led continuity of care given both during the antepartum and the intrapartum period with other models of care which included obstetricians or family physicians, or both, collaborating with nurses and midwives in a variety of organisational settings.No trial included models of care that offered out of hospital birth.
In the primary comparison, the results consistently show less use of some interventions for women who were randomised to receive midwife‐led continuity of care compared to women randomised to receive other models of care without detriment to outcomes. Specifically, women were on average less likely to experience amniotomy, the use of regional analgesia, episiotomy, and instrumental delivery. Women were on average more likely to experience spontaneous vaginal birth, a longer mean length of labour, and to be attended at birth by a known midwife, however, there were no differences in caesarean birth rates.
Stillbirth is not reported specifically due to differing gestational definitions, but is included within the outcome ‘Fetal loss/neonatal death equal to/after 24 weeks’. Women who were randomised to receive midwife‐led continuity of care compared to women randomised to receive other models of care were, on average, less likely to experience fetal loss before 24 weeks' gestation and preterm birth before 37 weeks. The difference in the average treatment effect in overall fetal loss and neonatal death across included trials between women allocated to midwife‐led continuity models of care and women allocated to other models has an average risk ratio (RR) of 0.84 and a 95% confidence interval (CI) of 0.71 to 1.00 (12 trials, n = 15,869, RR 0.84, 95% CI 0.71 to 1.00, random‐effects). Given that (i) the 95% CI just reaches 1.00 and (ii) the absence of measurable heterogeneity in this outcome analysis, the probability is that midwife‐led continuity models of care are associated with a reduction in overall fetal loss and neonatal death by approximately 16%. For a number of outcomes (induction of labour, augmentation, opiate analgesia, caesarean birth), the point estimate was less than 1 and the upper limit of the 95% confidence interval just exceeded 1. These outcomes therefore did not show a formally statistically significant effect using the conventional P = 0.05 cut‐off, but are suggestive that midwife‐led care may, on average, be beneficial. Further data may clarify the effects of the intervention on these outcomes.
The subgroup analyses of models of midwife‐led continuity of care and risk status did not find any significant subgroup interaction tests, indicating that there is no observable subgroup effect.
Overall, we did not find any increased likelihood for any adverse outcome for women or their infants associated with having been randomised to a midwife‐led continuity model of care. These results were moderate in magnitude and generally consistent across all the trials.
It is possible that practice settings such as midwife‐led units can be a confounding influence on outcomes of midwife‐led continuity of care Brocklehurst 2011, and home birth was not offered in any of the trials. Four trials offered care in midwife‐led units (Begley 2011; MacVicar 1993; Turnbull 1996; Waldenstrom 2001), which was available to women in both arms of one trial (Waldenstrom 2001) and only women in the midwife‐led group in three trials (Begley 2011; MacVicar 1993; Turnbull 1996). The increased likelihood of spontaneous vaginal birth in women randomised to midwife‐led continuity models of care may be a function of increased mobility due to less use of a range of analgesics, a much greater likelihood of attendance at birth by a known midwife, and the philosophy of care on offer. Midwife‐led continuity of care is a complex intervention, and it is impossible to unpick the relative importance of philosophy and continuity of care. However, in nine trials, care was provided on the labour ward, suggesting a separate effect to birth setting. To what extent the observed benefits can be attributed to the model of midwifery care or to the quality and degree of relationship between the care provider and women was outside the scope of this review and requires an in depth exploration.
The possible effects on fetal loss prior to 24 weeks and preterm birth are important. Aetiology of both these events are complex but potentially influenced by models of care. Medical interventions to prevent fetal loss prior to 24 weeks do exist, as this is mostly due to spontaneous miscarriage, (and are dependent on quick access to care potentially influenced by continuity), such as cerclage and progesterone. These interventions are targeted to 'at risk' women, and may explain why mixed risk populations (with the improved access to care and appropriate referral) have the effect. Low‐risk women may not be referred or when referred the interventions not used due to lack of evidence in low‐risk women. There is insufficient detail in the trials to elucidate reasons for loss (e.g. intrauterine death or spontaneous miscarriage) and this would be important in future research.
Government and hospital policies affect how midwives are 'allowed' to practise, and/or the institutional structure within which midwives practise, and would thus affect practices and outcomes by limiting the potential of midwife‐led continuity of care in some settings. This is in contrast to models of health care which offer relationship continuity over time, which have been found to prevent clients falling through 'gaps in care' (Cook 2000). Women's experiences of care reported in the original studies include maternal satisfaction with information, advice, explanation, venue of delivery and preparation for labour and birth, as well as perceptions of choice for pain relief and evaluations of carer's behaviour. In the majority of the included studies, satisfaction with various aspects of care appears to be higher in the midwife‐led continuity of care compared to the other models of care.
Although there were limitations in the way that satisfaction related outcomes were assessed and reported, the majority of the included studies showed a higher level of satisfaction with various aspects of care in the midwife‐led continuity of care compared to the other models of care. Estimates of cost and resource use employed different economic evaluation methods. Results generally suggest a cost‐saving effect in intrapartum care; one study suggests a higher cost of postnatal care when midwife‐led continuity of care is compared with medical‐led care. However, there is a lack of consistency in estimating maternity care cost among the available studies, and there seems to be a trend towards a cost‐saving effect of midwife‐led continuity of care in comparison with medical‐led care.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.1 Regional analgesia (epidural/spinal).
Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.2 Caesarean birth.
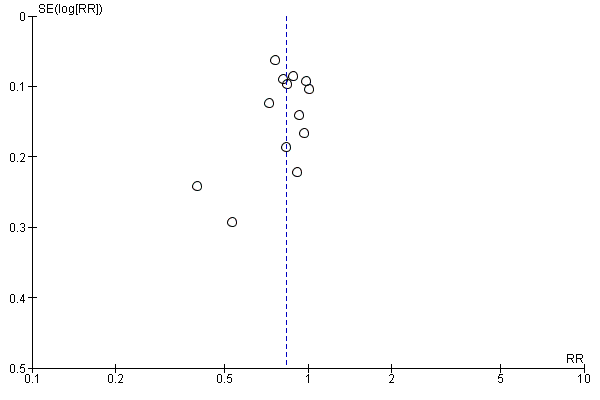
Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.16 Episiotomy.
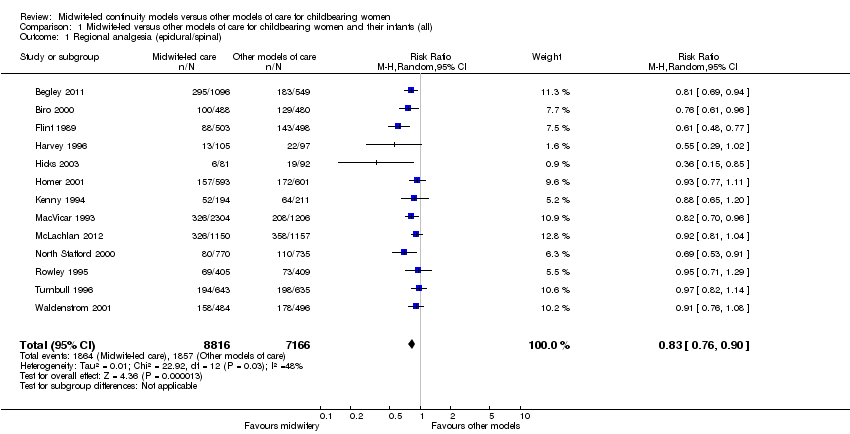
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 1 Regional analgesia (epidural/spinal).

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 2 Caesarean birth.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 3 Instrumental vaginal birth (forceps/vacuum).

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).
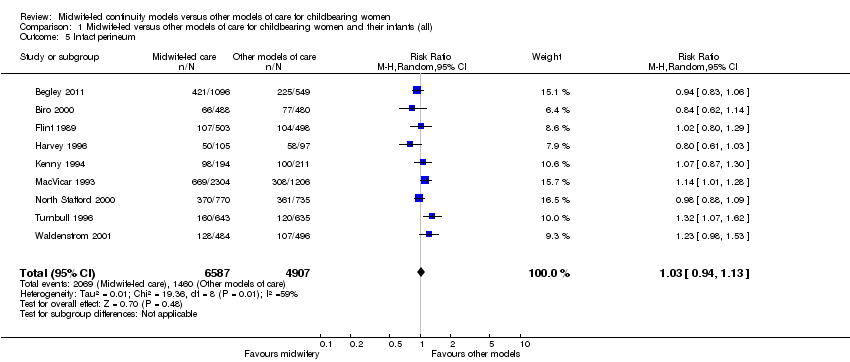
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 5 Intact perineum.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 6 Preterm birth (< 37 weeks).
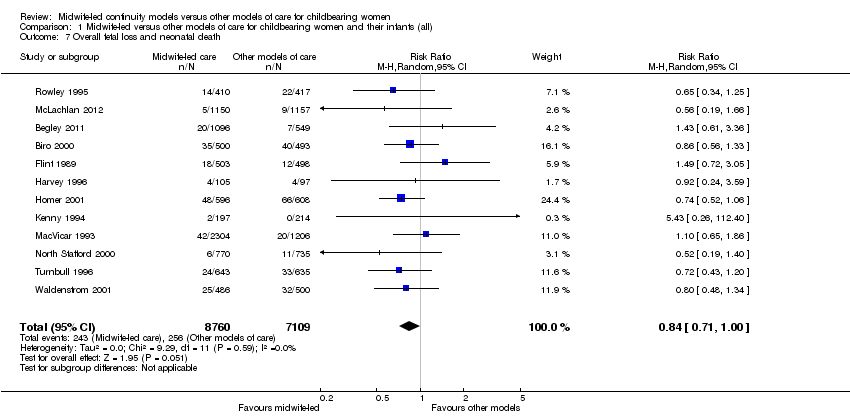
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 7 Overall fetal loss and neonatal death.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 8 Antenatal hospitalisation.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 9 Antepartum haemorrhage.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 10 Induction of labour.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 11 Amniotomy.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 12 Augmentation/artificial oxytocin during labour.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 13 No intrapartum analgesia/anaesthesia.
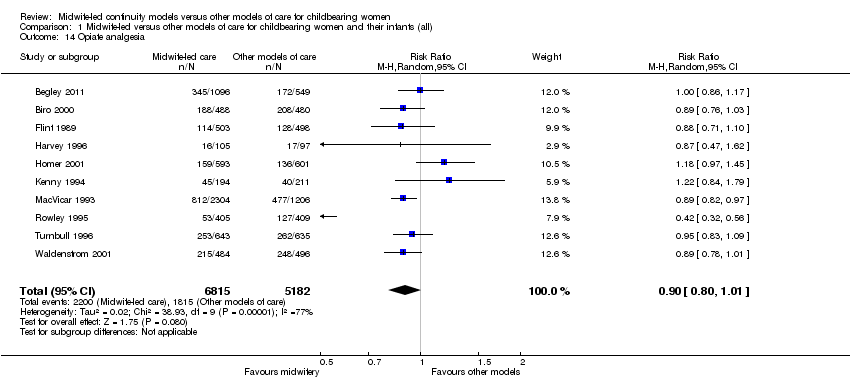
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 14 Opiate analgesia.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 15 Attendance at birth by known midwife.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 16 Episiotomy.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 17 Perineal laceration requiring suturing.
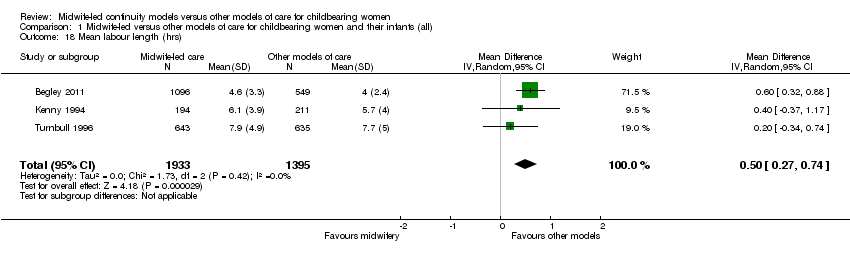
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 18 Mean labour length (hrs).

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 19 Postpartum haemorrhage (as defined by trial authors).
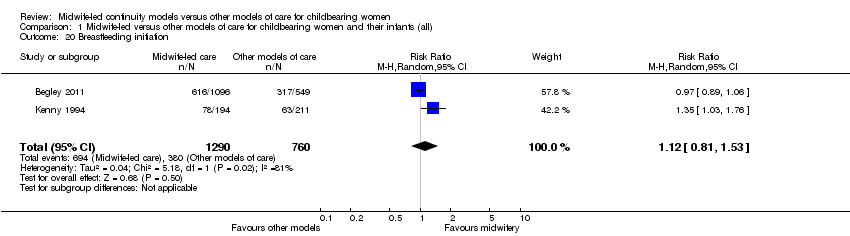
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 20 Breastfeeding initiation.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 21 Duration of postnatal hospital stay (days).
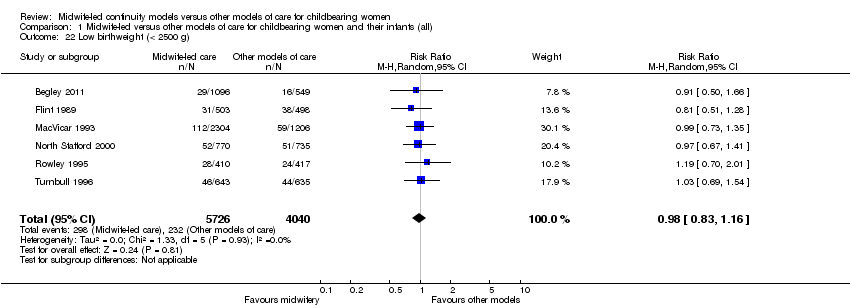
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 22 Low birthweight (< 2500 g).

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 23 5‐minute Apgar score below or equal to 7.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 24 Neonatal convulsions (as defined by trial authors).
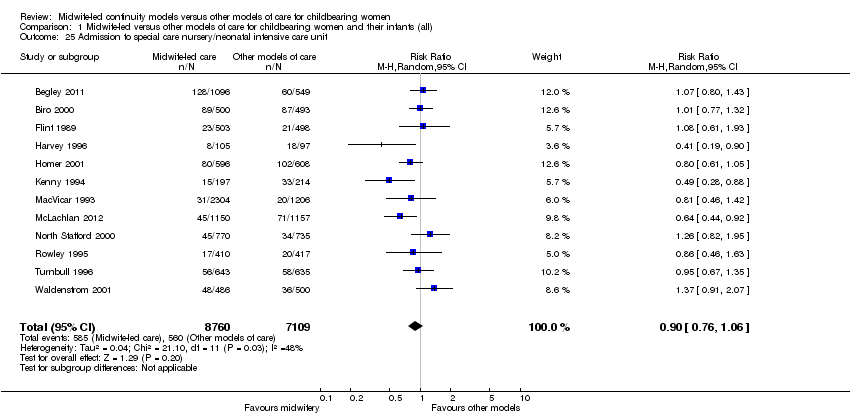
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 25 Admission to special care nursery/neonatal intensive care unit.

Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 26 Mean length of neonatal hospital stay (days).
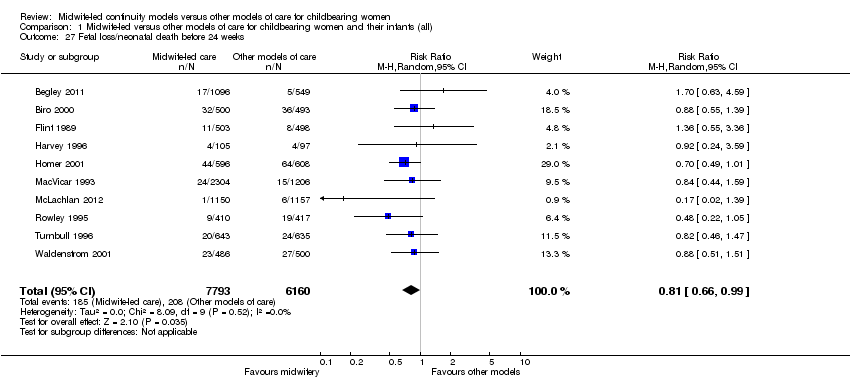
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 27 Fetal loss/neonatal death before 24 weeks.
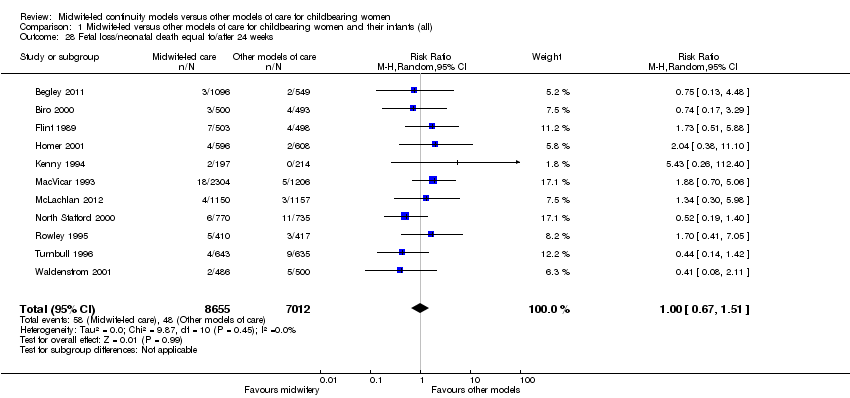
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 28 Fetal loss/neonatal death equal to/after 24 weeks.

Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 1 Regional analgesia (epidural/spinal).

Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 2 Caesarean birth.

Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 3 Instrumental vaginal birth (forceps/vacuum).
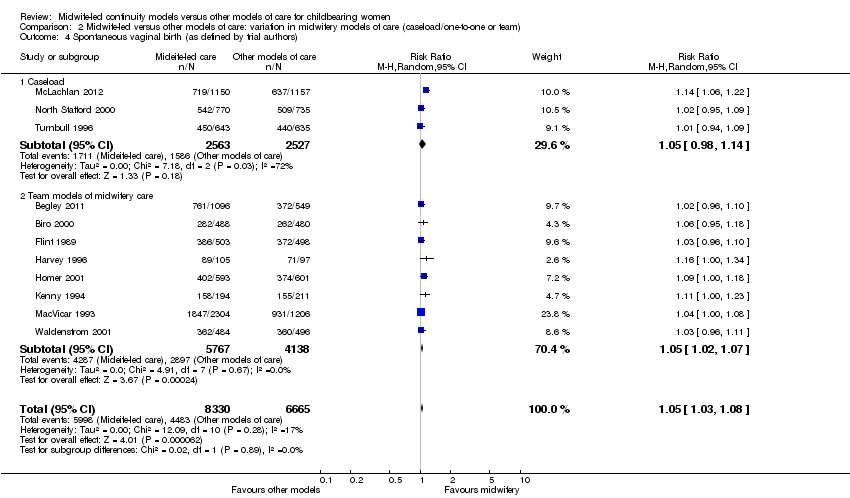
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).

Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 5 Intact perineum.
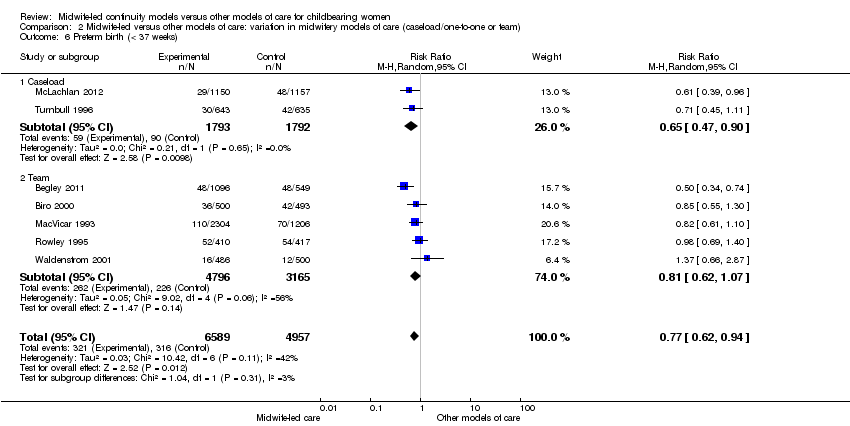
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 6 Preterm birth (< 37 weeks).

Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 7 Overall fetal loss and neonatal death.

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 1 Regional analgesia (epidural/spinal).

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 2 Caesarean birth.

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 3 Instrumental vaginal birth (forceps/vacuum).

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 5 Intact perineum.
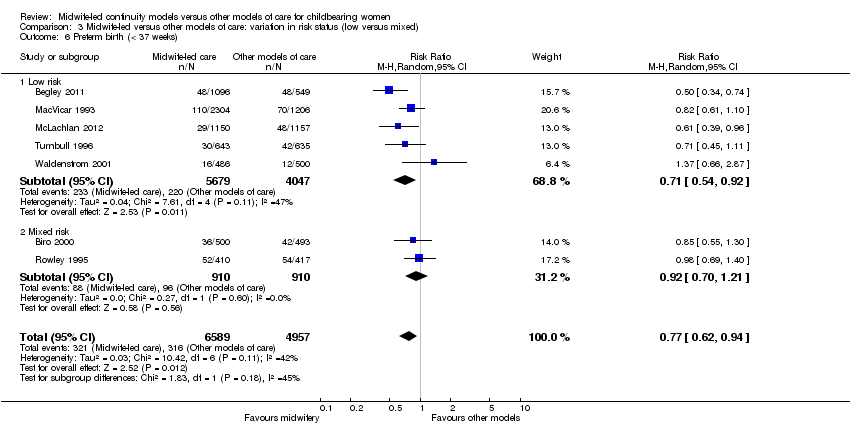
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 6 Preterm birth (< 37 weeks).

Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 7 Overall fetal loss and neonatal death.
| Satisfaction | Intervention (n/N) | Control (n/N) | Relative rate | 95% CI | Statistical test | P value |
| Staff in labour (very caring) | 252/275 (92%) | 208/256 (81%) | 1.1 | 1.0‐1.2 | ||
| Experience of labour (wonderful/enjoyable) | 104/246 (42%) | 72/223 (32%) | 1.3 | 1.0‐1.8 | ||
| Satisfaction with pain relief (very satisfied) | 121/209 (58%) | 104/205 (51%) | 1.1 | 0.9‐1.4 | ||
| Very well prepared for labour | 144/275 (52%) | 102/254 (40%) | 1.3 | 1.0‐1.7 | ||
| N = 1663 | N = 826 | Difference | ||||
| Very satisfied with antenatal care | 52% | 44% | 8.3% | 4.1‐12.5 | ||
| Very satisfied with care during labour | 73% | 60% | 12.9% | 9.1‐16.8 | ||
| N = 213 | N = 233 | |||||
| Carer skill, attitude and communication (antenatal care) | 57.1/60 | 47.7/60 | t = 12.4 | 0.0001 | ||
| Convenience and waiting (antenatal care) | 14.8/20 | 10.9/20 | t = 10.1 | 0.0001 | ||
| Expectation of labour/birth (antenatal care) | 9.8/18 | 9.3/18 | t = 1.4 | 0.16 | ||
| Asking questions (antenatal care) | 8.5/12 | 6.9/12 | t = 6.6 | 0.0001 | ||
| Information/communication (labour and birth) | 28.3/30 | 24.8/30 | t = 7.48 | 0.0001 | ||
| Coping with labour (labour and birth) | 20.9/30 | 19.3/30 | t = 2.83 | 0.005 | ||
| Midwife skill/caring (labour and birth) | 22.7/24 | 21.3/24 | t = 3.44 | 0.0007 | ||
| Help and advice (postnatal care) | 21.0/24 | 19.7/24 | t = 1.88 | 0.06 | ||
| Midwife skill and communication (postnatal care) | 16.6/18 | 15.4/18 | t = 4.48 | 0.0001 | ||
| Managing baby (postnatal care) | 8.7/12 | 8.5/12 | t = 0.77 | 0.77 | ||
| Self‐rated health (postnatal care) | 7.5/12 | 7.1/12 | t = 1.67 | 0.10 | ||
| OR | ||||||
| Encouraged to ask questions | N/A | 4.22 | 2.72‐6.55 | |||
| Given answers they could understand | N/A | 3.03 | 1.33‐7.04 | |||
| Able to discuss anxieties | N/A | 3.60 | 2.28‐5.69 | |||
| Always had choices explained to them | N/A | 4.17 | 1.93‐9.18 | |||
| Participation in decision making | N/A | 2.95 | 1.22‐7.27 | |||
| Midwives interested in woman as a person | N/A | 7.50 | 4.42‐12.80 | |||
| Midwives always friendly | N/A | 3.48 | 1.92 ‐ 6.35 | |||
| n/N | n/N | Mean difference ‐ satisfaction score | ||||
| Antenatal care | 534/648 | 487/651 | 0.48 | 0.55‐0.41 | ||
| Intrapartum care | 445/648 | 380/651 | 0.28 | 0.37‐0.18 | ||
| Hospital‐based postnatal care | 445/648 | 380/651 | 0.57 | 0.70‐0.45 | ||
| Home‐based postnatal care | 445/648 | 380/651 | 0.33 | 0.42‐0.25 | ||
| % | % | OR | ||||
| Overall antenatal care was very good (strongly agree) | 58.2% | 39.7% | 2.22 | 1.66‐2.95 | < 0.001 | |
| Happy with the physical aspect of intrapartum care (strongly agree) | 58.6% | 42.5% | 1.94 | 1.46‐2.59 | < 0.001 | |
| Happy with the emotional aspect of intrapartum care (strongly agree) | 58.8% | 44.0% | 1.78 | 1.34‐2.38 | < 0.001 | |
| Overall postnatal care was very good (strongly agree) | 37.6% | 33.2% | 1.27 | 0.97‐1.67 | 0.08 | |
| Care and sensitivity of staff (antenatal) | 1.32 | 1.77 | Mean difference? | 0.0000 | ||
| Care and sensitivity of staff (labour and delivery) | 1.26 | 1.58 | Mean difference? | 0.008 | ||
| Care and sensitivity of staff (postpartum at home) | 1.24 | 1.57 | Mean difference? | 0.0000 | ||
| Labour and Delivery Satisfaction Index + | 211 | 185 | 26 | 18.8‐33.1 | 0.001 | |
| Satisfaction with antenatal care (very good) | 195/344 (57%) | 100/287 (35%) | 1.24 | 1.13‐1.36 | 0.001 | |
| Satisfaction with intrapartum care (very good) | 215/241 (63%) | 134/282 (47%) | 1.11 | 1.03‐1.20 | 0.01 | |
| Satisfaction with postpartum care in hospital (very good) | 141/344 (41%) | 102/284 (31%) | 0.92 | 0.82‐1.04 | 0.22 | |
| *: 99% Confidence interval (CI) for Flint study was reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regional analgesia (epidural/spinal) Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.76, 0.90] |
| 2 Caesarean birth Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.02] |
| 3 Instrumental vaginal birth (forceps/vacuum) Show forest plot | 12 | 15809 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 4 Spontaneous vaginal birth (as defined by trial authors) Show forest plot | 11 | 14995 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.08] |
| 5 Intact perineum Show forest plot | 9 | 11494 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 6 Preterm birth (< 37 weeks) Show forest plot | 7 | 11546 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.94] |
| 7 Overall fetal loss and neonatal death Show forest plot | 12 | 15869 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 1.00] |
| 8 Antenatal hospitalisation Show forest plot | 6 | 6039 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.05] |
| 9 Antepartum haemorrhage Show forest plot | 4 | 3654 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.40] |
| 10 Induction of labour Show forest plot | 12 | 15809 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.03] |
| 11 Amniotomy Show forest plot | 4 | 3253 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.98] |
| 12 Augmentation/artificial oxytocin during labour Show forest plot | 11 | 13502 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 13 No intrapartum analgesia/anaesthesia Show forest plot | 6 | 8807 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.04, 1.31] |
| 14 Opiate analgesia Show forest plot | 10 | 11997 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 15 Attendance at birth by known midwife Show forest plot | 6 | 5225 | Risk Ratio (M‐H, Random, 95% CI) | 7.83 [4.15, 14.80] |
| 16 Episiotomy Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.92] |
| 17 Perineal laceration requiring suturing Show forest plot | 9 | 13412 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 18 Mean labour length (hrs) Show forest plot | 3 | 3328 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.27, 0.74] |
| 19 Postpartum haemorrhage (as defined by trial authors) Show forest plot | 9 | 12522 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 20 Breastfeeding initiation Show forest plot | 2 | 2050 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.53] |
| 21 Duration of postnatal hospital stay (days) Show forest plot | 3 | 3593 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.29, 0.09] |
| 22 Low birthweight (< 2500 g) Show forest plot | 6 | 9766 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.16] |
| 23 5‐minute Apgar score below or equal to 7 Show forest plot | 10 | 10854 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.70, 1.41] |
| 24 Neonatal convulsions (as defined by trial authors) Show forest plot | 2 | 2923 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.14, 5.74] |
| 25 Admission to special care nursery/neonatal intensive care unit Show forest plot | 12 | 15869 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.06] |
| 26 Mean length of neonatal hospital stay (days) Show forest plot | 2 | 1979 | Mean Difference (IV, Random, 95% CI) | ‐3.63 [‐7.57, 0.30] |
| 27 Fetal loss/neonatal death before 24 weeks Show forest plot | 10 | 13953 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 28 Fetal loss/neonatal death equal to/after 24 weeks Show forest plot | 11 | 15667 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regional analgesia (epidural/spinal) Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.76, 0.90] |
| 1.1 Caseload | 3 | 5090 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 1.2 Team models of midwifery care | 10 | 10892 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.89] |
| 2 Caesarean birth Show forest plot | 13 | 15966 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.02] |
| 2.1 Caseload | 3 | 5090 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.17] |
| 2.2 Team models of midwifery care | 10 | 10876 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 3 Instrumental vaginal birth (forceps/vacuum) Show forest plot | 12 | 16273 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.96] |
| 3.1 Caseload | 3 | 5090 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.04] |
| 3.2 Team models of midwifery care | 9 | 11183 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.97] |
| 4 Spontaneous vaginal birth (as defined by trial authors) Show forest plot | 11 | 14995 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.08] |
| 4.1 Caseload | 3 | 5090 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.14] |
| 4.2 Team models of midwifery care | 8 | 9905 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.02, 1.07] |
| 5 Intact perineum Show forest plot | 9 | 11494 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 5.1 Caseload | 2 | 2783 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.50] |
| 5.2 Team | 7 | 8711 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.13] |
| 6 Preterm birth (< 37 weeks) Show forest plot | 7 | 11546 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.94] |
| 6.1 Caseload | 2 | 3585 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 6.2 Team | 5 | 7961 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.07] |
| 7 Overall fetal loss and neonatal death Show forest plot | 12 | 15835 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 7.1 Caseload | 3 | 5090 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.99] |
| 7.2 Team | 9 | 10745 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regional analgesia (epidural/spinal) Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.76, 0.90] |
| 1.1 Low risk | 8 | 11096 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.2 Mixed risk | 5 | 4886 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 2 Caesarean birth Show forest plot | 13 | 15982 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.02] |
| 2.1 Low risk | 8 | 11096 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 2.2 Mixed risk | 5 | 4886 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 3 Instrumental vaginal birth (forceps/vacuum) Show forest plot | 12 | 15809 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 3.1 Low risk | 7 | 10923 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.99] |
| 3.2 Mixed risk | 5 | 4886 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
| 4 Spontaneous vaginal birth (as defined by trial authors) Show forest plot | 11 | 14995 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.08] |
| 4.1 Low risk | 7 | 10923 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.02, 1.08] |
| 4.2 Mixed risk | 4 | 4072 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.01, 1.10] |
| 5 Intact perineum Show forest plot | 9 | 11494 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 5.1 Low risk | 6 | 8616 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 5.2 Mixed risk | 3 | 2878 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 6 Preterm birth (< 37 weeks) Show forest plot | 7 | 11546 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.94] |
| 6.1 Low risk | 5 | 9726 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.92] |
| 6.2 Mixed risk | 2 | 1820 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.21] |
| 7 Overall fetal loss and neonatal death Show forest plot | 12 | 15835 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 7.1 Low risk | 7 | 10895 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 7.2 Mixed risk | 5 | 4940 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.97] |

