Técnicas y materiales para el cierre de la pared abdominal en una cesárea
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | 76 women undergoing caesarean section with more than 2 cm of subcutaneous fat. Excluded if no time for adequate consent. | |
| Interventions | Treatment group: closure of the subcutaneous tissue. Three randomised groups: closure of subcutaneous tissue; subcutaneous drain used; no closure of subcutaneous tissue or use of subcutaneous drain. | |
| Outcomes | Wound infection and wound complication incidence available on all randomised women. Wounds assessed prior to discharge and at staple removal (7 to 10 days postpartum). Further complications identified by retrospective chart review (timing not stated). | |
| Notes | USA. 1995 to 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial. | |
| Participants | 164 women undergoing caesarean section. Excluded if prescribed antibiotics in the two weeks prior to caesarean, or if given antibiotics for cardiac prophylaxis. | |
| Interventions | Treatment group: closure of the subcutaneous fat. | |
| Outcomes | Wound infection and wound complication incidence available on 82 women in the closure group and 77 women in the non‐closure group. Wounds assessed during hospital admission. Women were asked to return to the hospital "if they developed any problems". | |
| Notes | Turkey. 1995 to 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial. | |
| Participants | 327 women undergoing caesarean section. Excluded if delayed primary closure or drain insertion was planned preoperatively. | |
| Interventions | Treatment group: closure of the subcutaneous fat. | |
| Outcomes | Wound infection and wound complication incidence available on 135 in the closure group and 143 in the non‐closure group. Outcomes assessed at a postpartum visit (4 to 8 weeks postpartum). | |
| Notes | USA. 1995 to 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial. | |
| Participants | 451 women undergoing caesarean section. No stated exclusion criteria. No information on whether the women were obese or 'non‐obese'. Mean body mass index in both groups was greater than 30 kg/m2. | |
| Interventions | Treatment group: closure of the camper fascia. | |
| Outcomes | Superficial wound disruption incidence (including wound infection, haematoma or seroma) available on 222 women in the closure group and 216 women in the non‐closure group. Wounds assessed during hospital admission. Women were asked to return to the hospital "if they developed any problems". A chart review was also carried out at six weeks postpartum. | |
| Notes | USA. 1991 to 1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial. | |
| Participants | 964 women undergoing caesarean section were randomised preoperatively. Excluded if: no time for adequate consent; less than 2 cm of subcutaneous fat (when measured intraoperatively). | |
| Interventions | Treatment group: closure of the subcutaneous tissue. Three randomised groups: closure of subcutaneous tissue; subcutaneous drain used; no closure of subcutaneous tissue or use of subcutaneous drain. | |
| Outcomes | Wound infection, wound complication and endometritis incidence available on all randomised women at staple removal (7 to 10 days postpartum). Unclear at which point in the follow up the recorded outcomes were diagnosed. | |
| Notes | USA. 1998 to 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial. | |
| Participants | 245 women undergoing caesarean section with more than 2 cm of subcutaneous fat. No stated exclusion criteria. | |
| Interventions | Treatment group: closure of the subcutaneous fat. | |
| Outcomes | Wound infection and wound complication incidence available on all randomised women. Outcomes assessed at hospital discharge, and at staple removal (7 to 10 days postnatally). There were significantly fewer wound complications in the closure group than in the non‐closure group. | |
| Notes | USA. 1991 to 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial. | |
| Participants | 204 women undergoing caesarean section. No stated exclusion criteria. | |
| Interventions | Treatment group: closure of the uterus, peritoneum and rectus sheath using dexon with blunt needles (fat closure optional). One woman excluded because unable to use dexon throughout the operation. | |
| Outcomes | Wound infection incidence available on 97 women in the blunt needle group and on 106 women in the sharp needle group at discharge (four days postnatally). No significant difference in complications between the blunt needle and the sharp needle groups. Wounds assessed at discharge. | |
| Notes | UK. 1994 to 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 5 | 1348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.50] |
| Analysis 1.1  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 1 Wound infection. | ||||
| 2 Haematoma +/‐ seroma Show forest plot | 5 | 1348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| Analysis 1.2  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 2 Haematoma +/‐ seroma. | ||||
| 3 Aggregate wound complications (infection, wound separation, haematoma or seroma) Show forest plot | 6 | 1786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
| Analysis 1.3  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 3 Aggregate wound complications (infection, wound separation, haematoma or seroma). | ||||
| 4 Endometritis Show forest plot | 1 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.46, 1.28] |
| Analysis 1.4  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 4 Endometritis. | ||||
| 5 Duration of surgery (minutes) Show forest plot | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.29, 3.49] |
| Analysis 1.5  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 5 Duration of surgery (minutes). | ||||
| 6 Mean blood loss (ml) Show forest plot | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐24.29, 42.29] |
| Analysis 1.6  Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 6 Mean blood loss (ml). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.54, 13.76] |
| Analysis 2.1  Comparison 2 Blunt needles versus sharp needles for closure at caesarean section, Outcome 1 Wound infection. | ||||
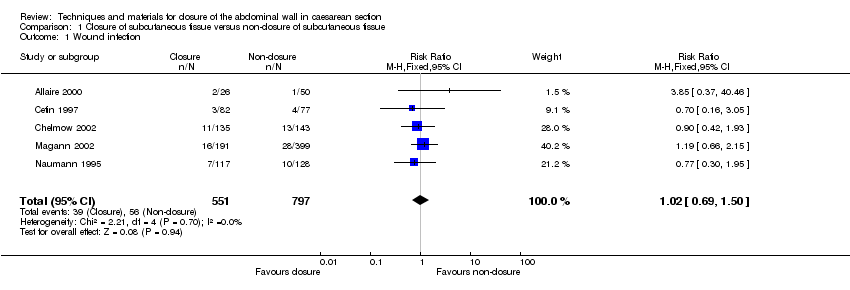
Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 1 Wound infection.
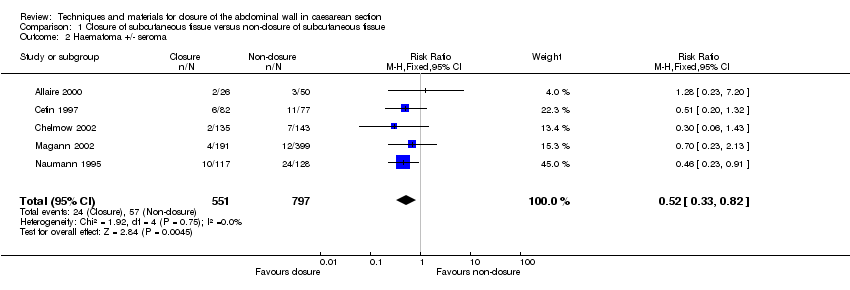
Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 2 Haematoma +/‐ seroma.

Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 3 Aggregate wound complications (infection, wound separation, haematoma or seroma).

Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 4 Endometritis.

Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 5 Duration of surgery (minutes).

Comparison 1 Closure of subcutaneous tissue versus non‐closure of subcutaneous tissue, Outcome 6 Mean blood loss (ml).

Comparison 2 Blunt needles versus sharp needles for closure at caesarean section, Outcome 1 Wound infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 5 | 1348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.50] |
| 2 Haematoma +/‐ seroma Show forest plot | 5 | 1348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 3 Aggregate wound complications (infection, wound separation, haematoma or seroma) Show forest plot | 6 | 1786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
| 4 Endometritis Show forest plot | 1 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.46, 1.28] |
| 5 Duration of surgery (minutes) Show forest plot | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.29, 3.49] |
| 6 Mean blood loss (ml) Show forest plot | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐24.29, 42.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.54, 13.76] |

