Vitamina B12 oral versus vitamina B12 intramuscular para la deficiencia de vitamina B12
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004655.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos metabólicos y endocrinos
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors read and approved the final review draft.
Hai Yan Wang (HYW): trial selection, data analysis, data interpretation, quality of evidence assessment, and review writing.
Linyi Li (LYL): data extraction and 'Risk of bias' assessment.
Ling Ling Qin (LLQ): data extraction and 'Risk of bias' assessment.
Yanan Song (YNS): trial selection, 'Risk of bias' assessment, data analysis, data interpretation, and review update.
Josep Vidal‐Alaball (JVA): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft, and future review updates.
Tong Hua Liu (THL): trial selection, data analysis, quality of evidence assessment, and data interpretation.
Sources of support
Internal sources
-
Department of General Practice, Wales College of Medicine, Cardiff University, UK.
-
National Public Health Service for Wales, Wales, UK.
-
Department of General Practice and Elderly Care Medicine, VU University Medical Center, Amsterdam, Netherlands.
External sources
-
No sources of support supplied
Declarations of interest
HYW: none known
LYL: none known.
LLQ: none known.
YNS: none known.
JVA: none known.
THL: none known.
Acknowledgements
-
Christopher C Butler, Rebecca Cannings‐John, Andrew Goringe, Kerry Hood, Andrew McCaddon, Ian McDowell, Alexandra Papaioannou, Johannes C van der Wouden, and Rianne Langeveld (original Cochrane Review).
-
The search strategies for this review update were designed and run by the CMED Group's Information Specialist, Maria‐Inti Metzendorf.
-
We thank Ms Sai Zhao from Systematic Review Solution Ltd for being our methodology and reporting consultant.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 15 | Oral vitamin B<sub>12</sub> versus intramuscular vitamin B<sub>12</sub> for vitamin B<sub>12</sub> deficiency | Review | Haiyan Wang, Linyi Li, Ling Ling Qin, Yanan Song, Josep Vidal‐Alaball, Tong Hua Liu | |
| 2005 Jul 20 | Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency | Review | Josep Vidal‐Alaball, Christopher Butler, Rebecca Cannings‐John, Andrew Goringe, Kerry Hood, Andrew McCaddon, Ian McDowell, Alexandra Papaioannou | |
| 2004 Jan 26 | Oral vitamin B12 versus parenteral vitamin B12 for vitamin B12 deficiency. | Protocol | Josep Vidal‐Alaball, Chris Butler, Kerry Hood, Rebecca Cannings, Andrew McCaddon, Alexandra Papaioannou | |
Differences between protocol and review
The review authors' team changed for this update: Christopher C Butler, Rebecca Cannings‐John, Andrew Goringe, Kerry Hood, Andrew McCaddon, Ian McDowell, Alexandra Papaioannou, Johannes C van der Wouden, and Rianne Langeveld are no longer review authors.
Since the last publication of this review (Issue 3, 2005), multiple changes such as new methods and other standards were introduced, leading to a complete renewal of this Cochrane Review. We have revised the search strategies for this review update. The following sections have also been changed.
-
We used a cut‐off point below 200 pg/mL (below 148 pmol/L) as a threshold serum level for vitamin B12 deficiency to better differentiate vitamin B12 deficiency from borderline deficiency.
-
Types of outcomes: we added the section 'Method and timing of outcome measurement' to define each outcome and group the timing of outcome measurement.
-
Types of outcomes: we added a 'Summary of findings' table.
-
We updated the 'Assessment of risk of bias of included studies' section to provide further details about our judgements.
-
We revised the 'Data extraction and management' section by specifying data items we planned to extract; adding two sections: 'Unit of analysis issues' and ' Dealing with missing data'; and other minor amendments.
Notes
We included a total of three trials in this update (one additional trial compared to the first published version in 2005).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Humans;
PICO
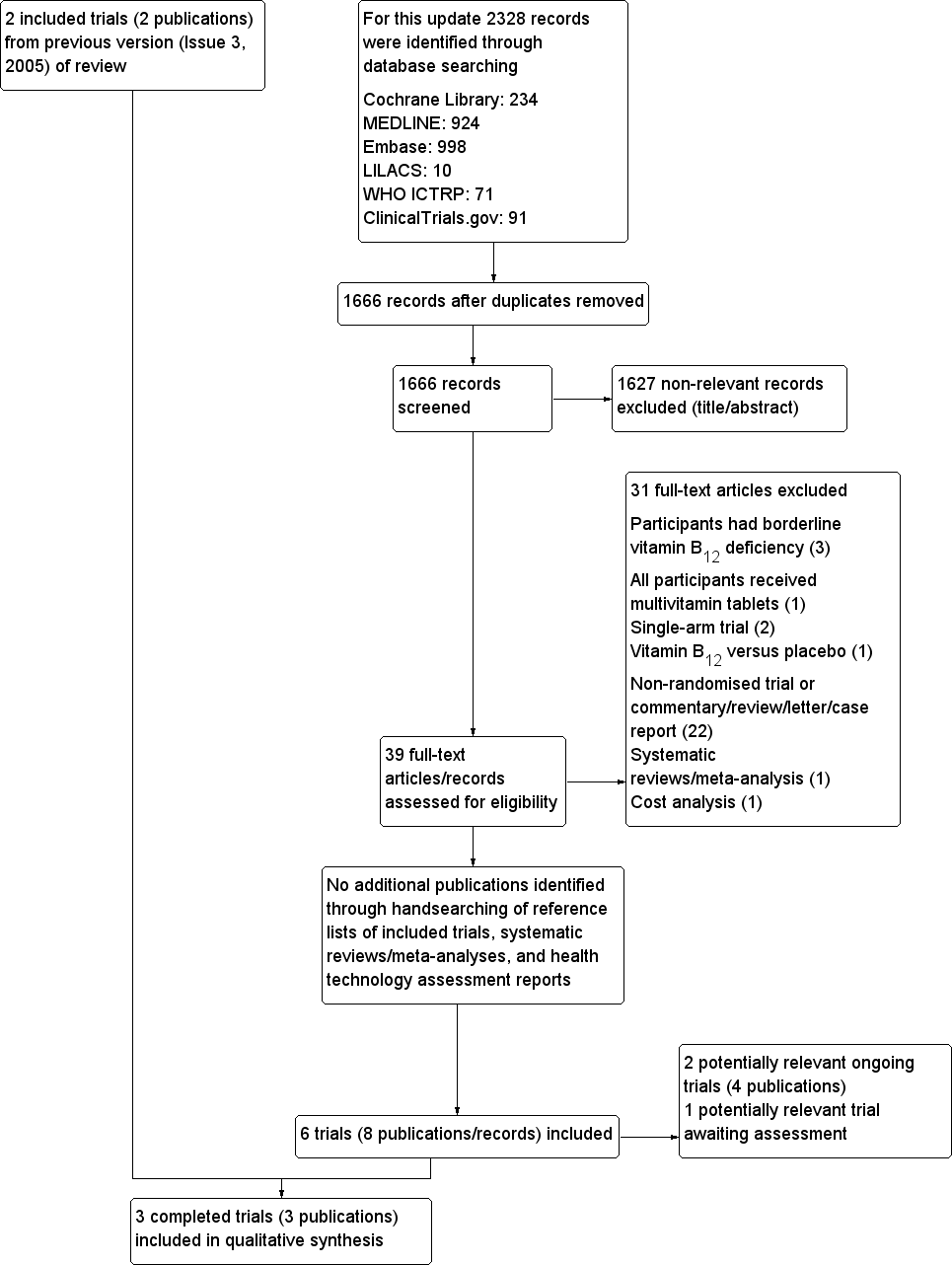
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not measured in some studies).
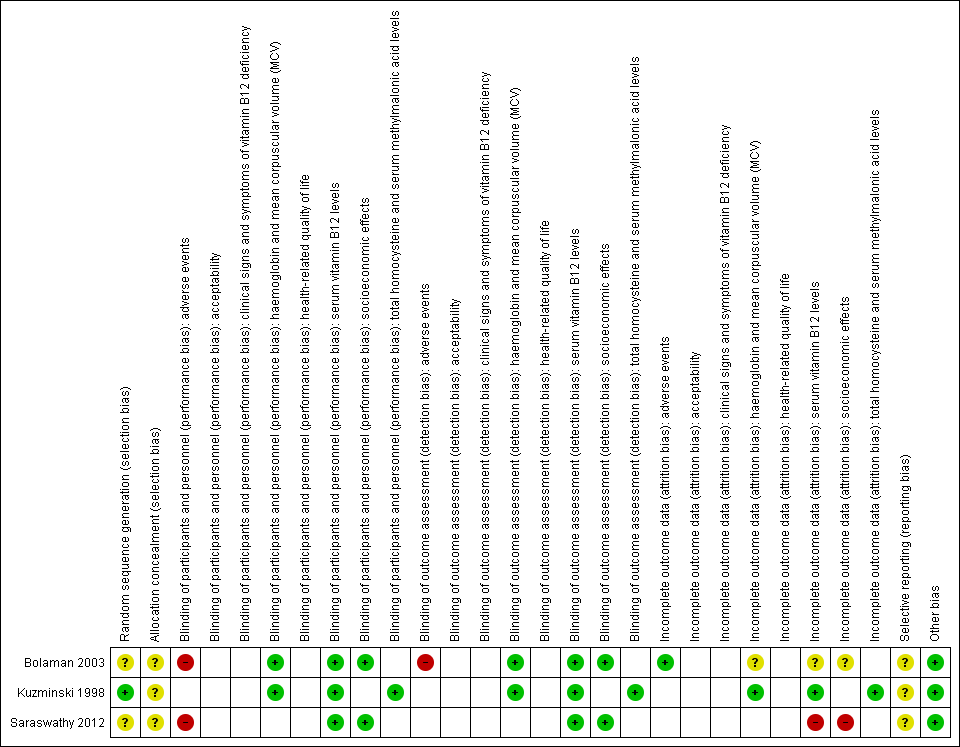
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not measure that particular outcome).

Comparison 1 Oral versus intramuscular vitamin B12, Outcome 1 Serum vitamin B12 levels.
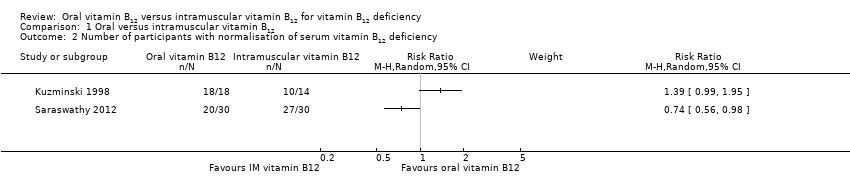
Comparison 1 Oral versus intramuscular vitamin B12, Outcome 2 Number of participants with normalisation of serum vitamin B12 deficiency.

Comparison 1 Oral versus intramuscular vitamin B12, Outcome 3 Adverse events.
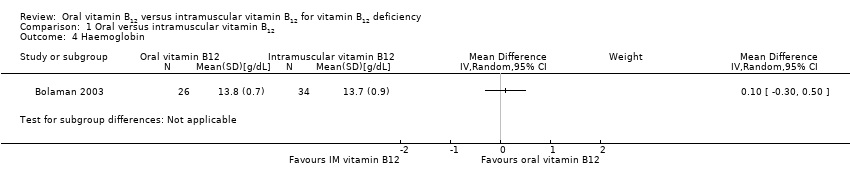
Comparison 1 Oral versus intramuscular vitamin B12, Outcome 4 Haemoglobin.
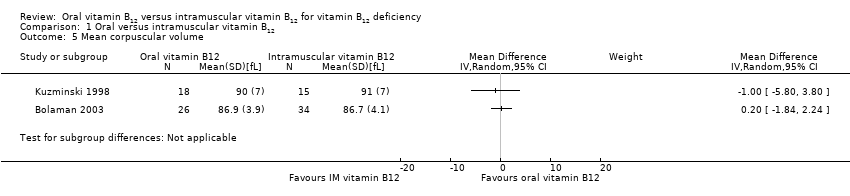
Comparison 1 Oral versus intramuscular vitamin B12, Outcome 5 Mean corpuscular volume.

Comparison 1 Oral versus intramuscular vitamin B12, Outcome 6 Total homocysteine.

Comparison 1 Oral versus intramuscular vitamin B12, Outcome 7 Serum methylmalonic acid.
| Oral versus intramuscular vitamin B12 for vitamin B12 deficiency | ||||||
| Patient: people with vitamin B12 deficiency Intervention: oral versus IM vitamin B12 | ||||||
| Outcomes | IM vitamin B12 | Oral vitamin B12 | Relative effect | No. of participants | Quality of the evidence | Comments |
| Serum vitamin B12 levels Normal value: > 300 pg/mL (> 221 pmol/L) Follow‐up: 90 days and 4 months | See comment | See comment | Not estimable | 153 (3) | ⊕⊕⊝⊝ | 1 trial (60 participants) used 1000 μg/day oral or IM vitamin B12 (total dose 15 mg): MD was ‐11.7 pg/mL (95% CI ‐29.5 to 6.1) (Bolaman 2003). 1 trial (33 participants) used 2000 μg/day vitamin B12 (total dose 240 mg) or 1000 μg/day IM vitamin B12 (total dose 9 mg): MD was 680 pg/mL (95% CI 392.7 to 967.3) in favour of oral vitamin B12 (Kuzminski 1998). 1 trial (60 participants) (using 1000 μg/day oral or IM vitamin B12 (total dose 90 mg and 15 mg, oral and IM respectively) reported that 27/30 in the IM vitamin B12 group (90%) and 20/30 in the oral vitamin B12 group (66.7%) achieved normalisation of serum vitamin B12, defined as ≥ 200 pg/mL (Saraswathy 2012). |
| Clinical signs and symptoms | Not reported | |||||
| Adverse events | See comment | See comment | Not estimable | 120 (2) | ⊕⊝⊝⊝ | Bolaman 2003 reported no treatment‐related adverse events in both the oral and IM vitamin B12 groups. Saraswathy 2012 reported that 2/30 participants (6.7%) in the oral vitamin B12 group left the trial early due to adverse events. |
| Health‐related quality of life | Not reported | |||||
| Acceptability | Not reported | |||||
| Socioeconomic effects Follow‐up: 90 days | See comment | See comment | Not estimable | 60 (1) | ⊕⊕⊝⊝ | Only 1 trial reported data for this outcome (Bolaman 2003). The costs per treatment were USD 80 per person in the oral vitamin B12 group compared with USD 220 per person in the IM group. |
| CI: confidence interval; IM: intramuscular; MD: mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels due to serious imprecision (low numbers of trials and participants); see Appendix 12. | ||||||
| Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible | Randomised | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| Bolaman 2003 (parallel RCT) | I: 1000 µg oral vitamin B12 | — | — | 26 | 26 | 26 | 100 | 90 days (none) |
| C: 1000 µg IM vitamin B12 | 34 | 34 | 34 | 100 | ||||
| Total: | 60b | 60 | 60 | 100 | ||||
| Kuzminski 1998 (parallel RCT) | I: 2000 µg oral vitamin B12 | — | 138 | 18 | 18 | 18 | 100 | 4 months (none) |
| C: 1000 µg IM vitamin B12 | 15 | 15 | 15 | 100 | ||||
| Total: | 33c | 33 | 33 | 100 | ||||
| Saraswathy 2012 (parallel RCT) | I: 1000 μg oral vitamin B12 | "Sample size was estimated to be 23 in each study arm, assuming equal response of 90% and non inferiority margin of 25% /alpha = 0.25, 1‐beta = 80%)" | — | 30 | 22 | 22 | 73 | 3 months (none) |
| C: 1000 μg IM vitamin B12 | 30 | 27 | 27 | 90 | ||||
| Total: | 60 | 49 | 49 | 82 | ||||
| Grand total | All interventions | 74 | 66 | |||||
| All comparators | 79 | 76 | ||||||
| All interventions and comparators | 153 | 142 | ||||||
| — denotes not reported C: comparator; I: intervention; IM: intramuscular; RCT: randomised controlled trial aFollow‐up under randomised conditions until end of trial (= duration of intervention + follow‐up postintervention or identical to duration of intervention); extended follow‐up refers to follow‐up of participants once the original trial was terminated as specified in the power calculation. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum vitamin B12 levels Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Number of participants with normalisation of serum vitamin B12 deficiency Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Haemoglobin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Mean corpuscular volume Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Total homocysteine Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Serum methylmalonic acid Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |

