Rutinsko struganje (skaliranje) i poliranje zubi za periodontalno zdravlje kod odraslih
Abstract
Background
Many dentists or hygienists provide scaling and polishing for patients at regular intervals, even if those patients are considered to be at low risk of developing periodontal disease. There is debate over the clinical effectiveness and cost effectiveness of 'routine scaling and polishing' and the 'optimal' frequency at which it should be provided for healthy adults.
A 'routine scale and polish' treatment is defined as scaling or polishing or both of the crown and root surfaces of teeth to remove local irritational factors (plaque, calculus, debris and staining), that does not involve periodontal surgery or any form of adjunctive periodontal therapy such as the use of chemotherapeutic agents or root planing.
Objectives
The objectives were: 1) to determine the beneficial and harmful effects of routine scaling and polishing for periodontal health; 2) to determine the beneficial and harmful effects of providing routine scaling and polishing at different time intervals on periodontal health; 3) to compare the effects of routine scaling and polishing with or without oral hygiene instruction (OHI) on periodontal health; and 4) to compare the effects of routine scaling and polishing provided by a dentist or dental care professional (dental therapist or dental hygienist) on periodontal health.
Search methods
We searched the following electronic databases: the Cochrane Oral Health Group's Trials Register (to 15 July 2013), CENTRAL (The Cochrane Library 2013, Issue 6), MEDLINE via OVID (1946 to 15 July 2013) and EMBASE via OVID (1980 to 15 July 2013). We searched the metaRegister of Controlled Trials and the US National Institutes of Health Clinical Trials Register (clinicaltrials.gov) for ongoing and completed studies to July 2013. There were no restrictions regarding language or date of publication.
Selection criteria
Randomised controlled trials of routine scale and polish treatments (excluding split‐mouth trials) with and without OHI in healthy dentate adults, without severe periodontitis.
Data collection and analysis
Two review authors screened the results of the searches against inclusion criteria, extracted data and assessed risk of bias independently and in duplicate. We calculated mean differences (MDs) (standardised mean differences (SMDs) when different scales were reported) and 95% confidence intervals (CIs) for continuous data and, where results were meta‐analysed, we used a fixed‐effect model as there were fewer than four studies. Study authors were contacted where possible and where deemed necessary for missing information.
Main results
Three studies were included in this review with 836 participants included in the analyses. All three studies are assessed as at unclear risk of bias. The numerical results are only presented here for the primary outcome gingivitis. There were no useable data presented in the studies for the outcomes of attachment change and tooth loss. No studies reported any adverse effects.
‐ Objective 1: Scale and polish versus no scale and polish
Only one trial provided data for the comparison between scale and polish versus no scale and polish. This study was conducted in general practice and compared both six‐monthly and 12‐monthly scale and polish treatments with no treatment. This study showed no evidence to claim or refute benefit for scale and polish treatments for the outcomes of gingivitis, calculus and plaque. The MD for six‐monthly scale and polish, for the percentage of index teeth with bleeding at 24 months was ‐2% (95% CI ‐10% to 6%; P value = 0.65), with 40% of the sites in the control group with bleeding. The MD for 12‐monthly scale and polish was ‐1% (95% CI ‐9% to 7%; P value = 0.82). The body of evidence was assessed as of low quality.
‐ Objective 2: Scale and polish at different time intervals
Two studies, both at unclear risk of bias, compared routine scale and polish provided at different time intervals. When comparing six with 12 months there was insufficient evidence to determine a difference for gingivitis at 24 months SMD ‐0.08 (95% CI ‐0.27 to 0.10). There were some statistically significant differences in favour of scaling and polishing provided at more frequent intervals, in particular between three and 12 months for the outcome of gingivitis at 24 months, with OHI, MD ‐0.14 (95% CI ‐0.23 to ‐0.05; P value = 0.003) and without OHI MD ‐0.21 (95% CI ‐0.30 to ‐0.12; P value < 0.001) (mean per patient measured on 0‐3 scale), based on one study. There was some evidence of a reduction in calculus. This body of evidence was assessed as of low quality.
‐ Objective 3: Scale and polish with and without OHI
One study provided data for the comparison of scale and polish treatment with and without OHI. There was a reduction in gingivitis for the 12‐month scale and polish treatment when assessed at 24 months MD ‐0.14 (95% CI ‐0.22 to ‐0.06) in favour of including OHI. There were also significant reductions in plaque for both three and 12‐month scale and polish treatments when OHI was included. The body of evidence was once again assessed as of low quality.
‐ Objective 4: Scale and polish provided by a dentist compared with a dental care professional
No studies were found which compared the effects of routine scaling and polishing provided by a dentist or dental care professional (dental therapist or dental hygienist) on periodontal health.
Authors' conclusions
There is insufficient evidence to determine the effects of routine scale and polish treatments. High quality trials conducted in general dental practice settings with sufficiently long follow‐up periods (five years or more) are required to address the objectives of this review.
PICO
Laički sažetak
Rutinsko struganje (skaliranje) i poliranje zubi za periodontalno zdravlje kod odraslih
Istraživačko pitanje
Struganje (skaliranje) i poliranje zubi može smanjiti naslage (zubni plak i kamenac), kao i krvarenje i upalu desni (gingivitis). Ublažavanje gingivitisa (blaža forma bolesti desni) će s vremenom smanjiti vjerojatnost napredovanja upale do periodontitisa (ozbiljna bolest desni).
Ovaj sustavni pregled ispituje dokaz učinaka tretmana rutinskog skaliranja i poliranja zubi. Sustavni pregled napravili su autori Cochrane uredničke skupine za oralno zdravlje (engl. Cohrane Oral Health Group) kako bi ocijenili prednosti inače rutinskog tretmana skaliranja i poliranja za zdravlje odraslih; kako bi ocijenili je li tretman učinkovitiji ako se ponudi zajedno s uputama kako najbolje održavati zdrave desni, i kako bi usporedili učinkovitost tretmana kada je proveden od strane liječnika dentalne medicine ili zubnog terapeuta ili tehničara.
Dosadašnje spoznaje
Mnogi liječnici dentalne medicine ili tehničari redovito provode skaliranje i poliranje zubi kod većine pacijenata u redovitim razmacima, čak i ako se smatra da imaju nizak rizik za razvoj bolesti desni. Još uvijek se raspravlja o kliničkoj učinkovitosti skaliranja i poliranja te koji je najbolji vremenski razmak između tretmana.
U svrhu ovoj pregleda „rutinsko skaliranje i poliranje“ je definirano kao skaliranje i poliranje obiju površina krune i korijena da bi se uklonili depoziti (uglavnom) bakterija zvani plak, i isto otvrdnuli plak poznat kao kamenac (tartar). Kamenac je toliko tvrd da ne može biti uklonjen samim četkanjem te se uz plak, druge naslage i obojenje na zubima uklanja skaliranjem i poliranjem. Skaliranje ili uklanjanje otvrdnulih depozita radi se posebno dizajniranim dentalnim instrumentima ili ultrazvučnim dlijetima, a poliranje mehanički s posebnim pastama.
U ovom pregledu uključeno je skaliranje iznad i ispod razine desni, međutim bilo kakvi kirurški postupak na desni, bilo kakvo kemijsko ispiranje prostora između desni i zuba (džepovi) i intenzivnije struganje (poliranje korijena) korijena od jednostavnog skaliranja je isključeno.
Značajke istraživanja
Dokazi na kojima se temelji ovaj sustavni pregled literature temelje se na studijama objavljenim do 15. srpnja 2013.
Uključena su tri pokusa sa 836 sudionika. U pojedinim studijama bilo je uključeno od 61 do 470 sudionika. Sudionici u dvama pokusima bili su odrasli u dobi od 18 do 73, u drugom pokusu mladi kadeti zračnih snaga.
Jedna studija je uključila pacijente koji su dolazili u tri ambulante opće dentalne prakse na rutinske preglede. Samo pacijenti s kamencem ili krvarenjem prilikom sondiranja i džepovima između zubi i desni manjim od 3,5 mm su uključeni. Jedna studija uključila je mlade muške kadete američkih zračnih snaga i ostale pacijente koji pohađaju školsku kliniku za dentalnu higijenu. Svi sudionici su imali različite stupnjeve gingivitisa, ali bez dokaza o gubitku kosti u kojoj su zubi ukotvljeni što uzrokuje periodontitis.
Ključni rezultati
Najbolji nađeni rezultat pripada jednoj studiji koja je temeljena na općoj praksi, što je najprimjerenije mjesto za izvođenje takvih terapija. Ta studija nije pokazala ni prednosti ni nedostatke za uobičajeni 6‐ do 12‐ mjesečni tretman skaliranja i poliranja u usporedbi s onim bez njih. Međutim, studija nad mladim kadetima zračnih snaga usporedila je tretman skaliranja i poliranja u različitom vremenskom periodu i pronašla razlike za gingivitis, plak i kamenac dok su tromjesečni tretmani uspoređeni s godišnjim tretmanima, pokazali bolji učinak kod tromjesečnih tretmana. Ta studija također je pogledala treba li tretman uključiti skaliranje i poliranje te upute o higijeni usne šupljine. Postojalo je povlačenje gingivitisa, plaka i kamenca. Nijedna studija nije usporedila doktore dentalne medicine s drugim stručnjacima iz područja oralnog zdravlja.
Skaliranje je invazivni postupak i povezano je s nizom nepovoljnih učinaka uključujući oštećenje zubnih površina i zubnu osjetljivost. Ova informacija nije zabilježena ili prijavljena od strane uključenih studija.
Nijedna od ovih studija uključenih u ovaj pregled nije opisala rezultate usmjerene na pacijenta kao što su kvaliteta života ili troškovi u zdravstvu.
Kvaliteta dokaza
S obzirom na visoku cijenu koja se troši na pružanje tih tretmana za odrasle u mnogim zemljama razočaravajuće je što postoji tako malo pouzdanih dokaza visoke kvalitete i dostupnih istraživanja koja mogu dati jasne naputke kliničku praksu. Kvaliteta dokaza je općenito niska, s jednom studijom koja je prikladnija od drugih.
Authors' conclusions
Summary of findings
| Routine scale and polish compared with no treatment for periodontal health | ||||
| Patient or population: Healthy dentate adults Settings: General dental practice Intervention: Routine scale and polish (either 6‐monthly or 12‐monthly) Comparison: No treatment | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis (proportion of index sites bleeding) at 24 months 6‐monthly scale and polish Mean proportion in control group is 0.40 sites | MD ‐0.02 (‐0.10 to 0.06) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| Calculus (mean depth in mm at index sites) at 24 months 6‐monthly scale and polish Mean in control group is 0.95 mm | MD ‐0.24 (‐0.51 to 0.03) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| Plaque (proportion of index sites with plaque) at 24 months 6‐monthly scale and polish Mean proportion in control group is 0.44 sites | MD ‐0.04 (‐0.13 to 0.05) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 1Single study at unclear risk of bias | ||||
| Routine scale and polish at different frequencies for periodontal health | ||||
| Patient or population: Healthy dentate young adults Settings: Air Force Academy Intervention: Routine scale and polish every 6 months with oral hygiene instruction Comparison: Routine scale and polish every 12 months with oral hygiene instruction | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis different indices used | SMD ‐0.08 (‐0.27 to 0.10) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are significant but based on only 1 study |
| Calculus different indices used | SMD ‐0.25 (‐0.44 to ‐0.06) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are significant but based on only 1 study |
| Plaque different indices used | SMD ‐0.16 (‐0.35 to 0.03) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are generally not significant |
| CI: confidence interval; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 12 studies at unclear risk of bias | ||||
| Routine scale and polish with and without oral hygiene instruction for periodontal health | ||||
| Patient or population: Healthy dentate young adults Settings: Air Force Academy Intervention: Routine scale and polish with oral hygiene instruction Comparison: Routine scale and polish without oral hygiene instruction | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean gingivitis score (0‐3 scale) in control group is 1.40 | MD ‐0.07 (‐0.18 to 0.04) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was significant |
| Calculus (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean calculus score (0‐3 scale) in control group is 0.29 mm | MD ‐0.02 (‐0.16 to 0.12) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was similar |
| Plaque (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean plaque score (0‐3 scale) in control group is 1.99 | MD ‐0.17 (‐0.31 to ‐0.03) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was not significant |
| CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 1Single study at unclear risk of bias | ||||
| Routine scale and polish undertaken by dentist or dental care professional for periodontal health | ||||
| Patient or population: Healthy dentate adults Settings: General dental practice Intervention: Routine scale and polish by dental professional Comparison: Routine scale and polish by dentist | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis | No studies | |||
| Calculus | No studies | |||
| Plaque | No studies | |||
| GRADE Working Group grades of evidence | ||||
Background
Description of the condition
'Periodontal (gum) disease' is a broad term that encompasses a cluster of diseases that result in inflammatory responses and chronic destruction of the tissues that surround and support the teeth, namely the gingiva, periodontal ligament, cementum and alveolar bone (collectively referred to as the 'periodontium'). Dental plaque is the principal aetiological factor in the pathogenesis of periodontal disease. Plaque is necessary but is not sufficient for periodontal disease to occur. The host response, the modifying effect of various risk factors and the bacterial attack from dental plaque can account for a variety of disease patterns, both between different individuals and between different sites in the mouth within the same individual. Calcified plaque (calculus) does not have a major role in the pathogenesis of periodontal disease, although it does act as a 'retention web' for bacteria (Ismail 1994) and reduces the effectiveness of personal oral hygiene control.
Plaque‐induced periodontal disease has traditionally been divided into two general categories: gingivitis and periodontitis. Gingivitis is a reversible disease and can be defined as the presence of gingival inflammation (where the gum can appear reddened and swollen and may bleed easily) without loss of connective tissue attachment. Gingivitis is a precursor to periodontitis in some individuals ‐ that is, gingivitis does not inevitably progress to periodontitis. Periodontitis can be defined as the presence of gingival inflammation at sites where there has been a pathological loss of attachment (AAP 2003). This loss of attachment contributes to pocket formation and the denuded cementum may become contaminated by microorganisms and their products (Jenkins 2003).
The rate of progression of periodontitis is neither predictable nor steady. The disease is considered to progress in relatively short episodes of rapid tissue destruction, sometimes followed by some repair, and mostly by prolonged periods of quiescence (Pilot 1997). Some diseased sites may progress by as much as three mm per year (Haffajee 1991; Lindhe 1989).
Epidemiological studies of periodontal diseases are difficult to interpret due to the diversity of measures used to describe and quantify disease and the absence of uniform definition and classification. This is reflected in the World Health Organization Global Data Bank estimates (WHO 2004) which state that the prevalence of moderate severity disease ranges from 2% to 67% and that advanced disease occurs in 1% to 79% of the population. Gingivitis is highly prevalent in most populations and at most ages (Albandar 2002; Corbet 2002; Sheiham 1986) with global values ranging from 50% to 90%. In the UK, it was reported in the 1998 Adult Dental Health Survey (Kelly 2000) that 54% of dentate adults had some periodontal pocketing of 4 mm or more and 5% had deep pocketing (of 6 mm or more); 43% had some loss of attachment of 4 mm or more and 8% had loss of attachment of 6 mm or more. The prevalence of pocketing and loss of attachment increased with age. For example, the proportion of dentate adults with some loss of attachment increased from 14% among those aged 16 to 24 years to 85% of those aged 65 and over.
The goals of periodontal therapy have been defined in many different ways. Some authors have defined the ultimate aim of periodontal treatment as being to control disease progression or achieve a rate of progression which is compatible with a functional dentition for the lifetime of the individual (Pilot 1980; Sheiham 2002; Wennstrom 1990). Others have defined the key goals as improving periodontal health and thereby satisfying a patient's aesthetic and functional needs or demands. Currently accepted clinical signs of a healthy periodontium include the absence of inflammatory signs of disease such as redness, swelling, suppuration, and bleeding on probing; maintenance of a functional periodontal attachment level; minimal or no recession in the absence of interproximal bone loss; and, where present, functional dental implants (AAP 2001).
A fundamental component of the preventive management of periodontal disease is the control of dental plaque by the patient. Hence patient education and training in personal oral hygiene should form an integral part of any treatment plan for a patient with periodontal disease. Conventional periodontal therapy also includes non‐surgical treatment as well as a variety of surgical approaches (Needleman 2002). The precise choice of intervention may be influenced by the clinical severity of the disease, with surgery generally reserved for cases of advanced disease to allow for adequate access to, and full debridement of, areas with deep pocketing.
Description of the intervention
Scaling and polishing of the teeth by a dentist or a dental care professional (DCP) (dental therapist or dental hygienist) ) is a non‐surgical intervention that is intended to supplement (and is not a substitute for) the patient's home‐care plaque control. This is frequently provided as part of the dental recall appointment (Beirne 2005a). Scaling is the removal of plaque, mineralised plaque deposits (also referred to as calculus or tartar), debris and staining from the crown and root surfaces of the teeth. Specially designed sharp dental instruments ('hand scalers') or ultrasonic scalers can be used to perform the scaling procedure. Polishing is the mechanical removal of any residual extrinsic stains and deposits, typically undertaken by using a rubber cup or bristle brush loaded with a prophylaxis paste. Scaling and polishing can be used with or without a variety of adjuncts such as antimicrobial agents (either topical or systemic), gingival crevice irrigation and root planing. Root planing is a procedure for smoothening the root surface of a tooth that involves the "removal of cementum or surface dentin that is rough or impregnated with calculus, toxins or microorganisms" (Greenstein 1992). The rationale for root planing is to allow the gingival tissue to heal close to the root, shrinking the tissue and reducing the depth of the pocket that has formed (Bonito 2004).
Within the confines of this Cochrane review a 'routine scale and polish' is defined as scaling or polishing or both of the crown and root surfaces of teeth to remove local irritational factors (plaque, calculus, debris and staining), that does not involve periodontal surgery or any form of adjunctive periodontal therapy such as the use of chemotherapeutic agents or root planing. The definition includes both supragingival and subgingival scaling. The term 'routine' is simply used to indicate that the scale and polish is "a regular course or procedure" (Oxford Dict 1995) i.e. that the scale and polish is an intervention that is intended to be provided at 'regular intervals' to patients (without specifying any one particular frequency e.g. every month, every six months, every nine months, every 12 months, etc. at which patients may receive this intervention).
How the intervention might work
Scaling and polishing of the teeth may reduce plaque, calculus, bleeding and gingival inflammation over time, to reduce gingivitis and therefore progression to or progress of periodontitis.
Why it is important to do this review
Scaling and polishing of the teeth is a commonly provided intervention in general dental practice. In the United Kingdom approximately 50% of all adult courses of treatment provided under the National Health Service (NHS) (General Dental Services) regulations "consist of the patient having nothing more than an examination (and a) scale and polish" (DoH 2000). In 1999/2000, approximately 13 million scale and polishes were provided for NHS patients in England at a gross cost to the NHS of GBP 122 million (DoH 2000). In a survey of general dental practitioners preventive recommendations in western New York State, 86% of respondents stated that they would recommend scaling and polishing every six months for 'low risk' patients of all ages (a 'low risk' patient was defined as a patient having "adequate brushing and flossing habits" and "no history of periodontal disease") (Frame 2000). There has been debate over the clinical effectiveness and cost effectiveness associated with the routine scaling and polishing of teeth and the frequency with which it should be provided for patients. This debate is complicated by the fact that a 'routine scale and polish' is not a precisely defined intervention in periodontal disease management and there is no universally accepted definition of the term. In the United States the term 'oral prophylaxis' is most often used and has been defined as "the removal of plaque, calculus and stain from exposed and unexposed surfaces of the teeth by scaling and polishing as a preventive measure for the control of local irritational factors" (AAP 1992). The role and contribution of DCPs (dental hygienists and dental therapists) in maintaining periodontal health has increased in recent years. Any differences in treatment outcome following intervention by a dentist or DCP are not well understood and require investigation. This is an update of this review (Beirne 2005b; Beirne 2007).
Objectives
-
To determine the beneficial and harmful effects of routine scaling and polishing for periodontal health.
-
To determine the beneficial and harmful effects of providing routine scaling and polishing at different time intervals on periodontal health (which could be determined by tests).
-
To compare the beneficial and harmful effects of routine scaling and polishing with or without oral hygiene instruction.
-
To compare the beneficial and harmful effects of routine scaling and polishing provided by dentists or dental care professionals (dental therapist or dental hygienist) on periodontal health.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials with at least six months follow‐up. We excluded split‐mouth studies as this design does not reflect a routine scale and polish.
Types of participants
Healthy dentate adults. We included trials where participants had mild to moderate gingivitis at baseline. We excluded trials where participants were described as having severe periodontal disease (e.g. alveolar bone loss involving most teeth, or individuals requiring referral for specialist (surgical) periodontal treatment). We also included trials where participants had undergone specialist periodontal treatment in the six months prior to the study and were in the maintenance phase.
Types of interventions
We included trials where the intervention group(s) received scale and polish treatments with or without oral hygiene instruction delivered at planned regular intervals by a dentist, dental hygienist or dental therapist. We excluded trials where patients were given only a single scale and polish treatment.
We included trials where the comparison or control group received either:
-
no scale and polish (e.g. dental examination or oral health instruction only or both);
-
scale and polish in response to signs and symptoms of developing gingival or periodontal disease.
We also included trials directly comparing routine scale and polish treatments delivered at different time intervals (e.g. every six months versus every 12 months).
Types of outcome measures
We included trials reporting clinical status, patient‐centred and economic cost outcomes.
Primary outcomes
Periodontal disease, assessed by gingivitis indices (both inflammatory and bleeding).
Secondary outcomes
Clinical status factors
-
Calculus and plaque indices.
-
Changes in probing depth.
-
Changes in attachment level.
-
Periodontal indices.
-
Tooth loss.
-
Adverse events.
Patient‐centred factors
-
Halitosis.
-
Patient satisfaction with oral comfort.
-
Patient satisfaction with appearance (including gingival recession).
-
Patient satisfaction with actual care received.
-
Patient satisfaction with provider of care (i.e. dentist, therapist or hygienist).
Economic cost factors
-
Economic and resource cost of scale and polish.
Search methods for identification of studies
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (Appendix 1) but appropriately revised for each database to take account of differences in syntax rules and controlled vocabulary. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs (Appendix 2).
Electronic searches
The following databases were searched.
-
The Cochrane Oral Health Group's Trials Register (to 15 July 2013) (Appendix 3).
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6) (Appendix 4).
-
MEDLINE via OVID (1946 to 15 July 2013) (Appendix 1).
-
EMBASE via OVID (1980 to 15 July 2013) (Appendix 2).
Only handsearching carried out as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included in the search (see the Cochrane Masterlist of journals searched to date).
We searched for ongoing and completed trials using the following trial registries (Appendix 5):
-
The metaRegister of Controlled Trials (to July 2013) (www.controlled‐trials.com)
-
The US National Institutes of Health Clinical Trials Register (to July 2013) (www.clinicaltrials.gov).
Searching other resources
The reference lists of related review articles and all articles obtained were checked for further trials. The author(s) of some eligible studies published and any researchers involved in the ongoing debate on recall intervals were contacted, where possible and when considered necessary, to obtain the information on additional published or unpublished studies possibly eligible for inclusion.
Data collection and analysis
Selection of studies
Two review authors independently assessed titles, keywords and abstracts. The review authors remained unblinded regarding the author(s), their institutional affiliations and the site of publication of reports. The full report was obtained for all studies appearing to meet the inclusion criteria or in instances where there was insufficient information from the title, keywords and abstract to make a clear decision. All of the potentially relevant studies were assessed independently for eligibility by both review authors. Instances of disagreement in the study selection process were referred to the other members of the review team and ultimately resolved by mutual discussion among all review team members. Studies rejected at this or subsequent stages were recorded in a table of excluded studies, and reasons for exclusion noted. All of the studies meeting the inclusion criteria were subjected to risk of bias assessment and data extraction.
Data extraction and management
All randomised controlled trials which appeared to meet the inclusion criteria for this review were assessed by at least two review authors to confirm eligibility, assess risk of bias and extract data using a piloted data extraction form. The following data were recorded.
-
Study design, location, funding, number of centres.
-
Inclusion and exclusion criteria, number of patients recruited, number of patients randomised to each group, number of patients withdrawn, numbers evaluated.
-
Intervention(s), comparator, provider characteristics (dentist, hygienist, dental therapist or other), diagnostic criteria and diagnostic thresholds used.
-
Primary and secondary outcomes, times measured, numbers of patients included in the outcome evaluation, direct and indirect cost (where provided).
-
Whether a sample size calculation was performed.
Information was entered into the table of characteristics of included studies and additionally into an Excel spreadsheet from which a summary of the characteristics of the studies was made. Where the published paper was unclear concerning aspects of trial design, attempts were made to contact the study authors for clarification or more information or both.
Assessment of risk of bias in included studies
This was conducted using the recommended approach for assessing the risk of bias in studies included in Cochrane reviews (Higgins 2011). We used the two‐part tool, addressing six specific domains (namely sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias). Each domain included one specific entry in a 'Risk of bias' table. Within each study, the first part of the tool involved describing what was reported to have happened in the study. The second part of the tool involved assigning a judgement relating to the risk of bias for that entry. This was achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of 'low' indicated low risk of bias, 'high' indicated high risk of bias, and 'unclear' indicated unclear or unknown risk of bias.
All of the domains of sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting and other sources of bias were each addressed in the tool by a single entry for each study. It is not possible to blind patients to which intervention they are receiving. Scale and polish visits can be considered to be a 'complex intervention' as the delivery of the clinical care may have impact on oral hygiene behaviour in between scale and polish visits leading to different clinical outcomes. Blinding of participants was not therefore considered as a risk of bias domain, only blinding of outcome assessor. Where the patients self assessed the outcomes to the trial this was noted.
The risk of bias assessments were undertaken independently and in duplicate by two review authors as part of the data extraction process.
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories.
-
Low risk of bias (plausible bias unlikely to seriously alter the results) for all key domains.
-
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains were assessed as unclear.
-
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were assessed to be at high risk of bias.
A 'Risk of bias' table was completed for each included study. The results were also presented graphically.
Measures of treatment effect
For continuous outcomes, means and standard deviations were used to summarise the data for each group (standardised mean differences were used when different scales, measuring the same concept, were reported). For dichotomous outcomes, the estimates of effect were expressed as risk ratios together with 95% confidence intervals.
Dealing with missing data
Where data were missing from the published report of a trial we attempted to contact the author(s) to obtain the data and clarify any uncertainty. The analysis generally included only the available data (ignoring missing data) however, methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) were used if necessary. Otherwise we did not intend to undertake any imputations nor to use statistical methods to allow for missing data.
Assessment of heterogeneity
We planned to assess heterogeneity by inspection of the point estimates and confidence intervals on the forest plots. The variation in treatment effects was to be assessed by means of Cochran's test for heterogeneity and quantified by the I2 statistic. Heterogeneity was to be considered statistically significant if P value is < 0.1. A rough guide to the interpretation of I2 given in the Cochrane Handbook for Systematic Reviews of Interventions is: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, 75% to 100% considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). If asymmetry were identified we would have examined possible causes.
Data synthesis
A meta‐analysis was only to be conducted if there were studies of similar comparisons reporting the same outcome measures. A fixed‐effect model was used where there were fewer than four studies.
Subgroup analysis and investigation of heterogeneity
We planned to investigate clinical heterogeneity. Providing there were sufficient studies of each intervention and outcome, we planned a priori to conduct subgroup analyses for age, sex, smoking, oral cleanliness and degree of periodontal disease at baseline and different groups of systemically compromised adults.
Sensitivity analysis
Provided there were sufficient studies for each outcome and intervention, we planned to undertake sensitivity analysis based on the trials at low risk of bias.
Summary of results
The results for each objective of the review are presented in a 'Summary of findings' table, with the GRADE assessment of the quality of the body of evidence.
Results
Description of studies
Results of the search
All titles and abstracts retrieved through the search strategy were scanned for relevance and the full texts of 88 papers considered potentially relevant to the review were obtained. Seven of these papers were either partially or fully translated in order to determine their eligibility for the review (four German (Grimm 1986; Katay 1990; Sandig 1981 (partially translated); Schulz 1989 (fully translated)); one Finnish (Ketomaki 1993) (partially translated); one Norwegian (Lunder 1994) (fully translated) and one Japanese (Tsuboi 2003) (partially translated)).
Included studies
Following detailed assessment of all of the potentially relevant papers, five papers reporting three studies (Jones 2011; Lightner 1971; Listgarten 1985) were judged to have satisfied the eligibility criteria for the review.
Characteristics of the trial settings and investigators
SeeCharacteristics of included studies table for further details.
Of the three included studies, two were conducted in North America (Lightner 1971; Listgarten 1985), and one in the UK (Jones 2011).
The studies were conducted in different settings: dental school/hospital (Listgarten 1985), general dental practice (Jones 2011) and US Air Force Academy (Lightner 1971).
Treatment was provided by dental hygienists in Lightner 1971 and in Listgarten 1985, and using a pool of nine therapists and hygienists in Jones 2011.
Outcomes were assessed by dentists in two studies (Jones 2011; Lightner 1971); it was unclear who carried out the outcome assessment in Listgarten 1985.
One study was funded by the National Institute of Dental Research (NIDR) (Listgarten 1985), one study funded by a university grant (Jones 2011), the funding being unclear in the remaining study (Lightner 1971).
Characteristics of the participants
SeeCharacteristics of included studies table for further details.
The three trials included 836 participants in the analyses, ranging from 61 to 470 in each trial. Participants were adults aged 18 to 73 (Jones 2011), adults aged 20 to 73 (Listgarten 1985) whereas in the third study (Lightner 1971) participants were young air force cadets with an average age of 22 years.
One study was conducted on patients attending one of three general dental practices for check‐up appointments (Jones 2011). This study only included patients with calculus or bleeding on probing and no pockets greater than 3.5 mm. Another study (Lightner 1971) was conducted on young adult male US Air Force Academy cadets. The third study (Listgarten 1985) was conducted on patients attending a dental hygiene clinic at a dental school. All participants had varying degrees of gingivitis, but no evidence of alveolar bone loss. Some, but not all of the subjects had been receiving periodontal prophylaxes at intervals of three to six months (no further information on the latter point was provided in this paper).
Heterogeneity of participants
As can be seen from the descriptions provided above and from the Characteristics of included studies table there was considerable heterogeneity in the characteristics of the participants included in different studies.
Characteristics of the interventions
See the Characteristics of included studies table for further details.
Comparison 1: Scale and polish versus no scale and polish
In one study (Jones 2011) all participants received a scale and polish at baseline, and then were randomly allocated to a further scale and polish every six months, or every 12 months or to no further scale and polish during the study period (two years).
Comparison 2: Scale and polish at a fixed interval versus scale and polish in response to the signs or symptoms or both of periodontal disease
In the study by Listgarten 1985, the control group received a 'periodontal prophylaxis' every six months (no further details were given of the precise nature of the prophylaxis, however, it was assumed to fall within the definition of 'routine scale and polish' used in this review). In the experimental group, periodontal prophylaxes were administered according to a variable schedule, based on the outcome of differential dark‐field microscopy (DDFM) tests. For participants with negative DDFM tests, recall intervals were gradually extended from one to two, to three, to six, to 12, to 24 months (seeCharacteristics of included studies table for further details of the DDFM test and subsequent assignment of recall intervals on the basis of the test). Eleven subjects in the experimental group reached the end of the study without receiving a single prophylaxis over the three‐year duration of the trial.
Comparison 3: Scale and polish at a fixed interval versus scale and polish at a different fixed interval
There were two studies (Jones 2011; Lightner 1971) that made this comparison. One study (Lightner 1971) compared scale and polish treatments provided at three‐month, six‐month and 12‐month intervals (seeCharacteristics of included studies table for further details of the precise interventions provided). In Jones 2011 all the participants received a thorough scale and polish followed by a baseline examination. Patients were then allocated to groups having no scale and polish, one every six months and one every 12 months.
Comparison 4: Scale and polish with oral hygiene instruction (OHI) at a fixed interval versus scale and polish without OHI at the same fixed interval
Only one study (Lightner 1971) provided data for this comparison. The comparisons made were:
-
scale and polish treatment with OHI at three‐month intervals versus scale and polish treatment without OHI at three‐month intervals;
-
scale and polish treatment with OHI at 12‐month intervals versus scale and polish treatment without OHI at 12‐month intervals.
(SeeCharacteristics of included studies table for further details of the precise interventions provided.)
Comparison 5: Scale and polish by a dentist versus scale and polish by a dental care professional
No studies were found for this comparison.
Heterogeneity of interventions
As can be seen from the descriptions provided above and from the Characteristics of included studies table there was considerable heterogeneity in the characteristics of the interventions provided in different studies. The OHI components also varied, with some studies (Lightner 1971) giving toothbrushing instruction only, where the participants in those groups who received OHI were instructed to use the Bass technique for the lingual surface of the mandibular molars but the modified Roll technique for all other areas. In one study (Listgarten 1985) the intervention was described as a "periodontal prophylaxis" and there was no indication given regarding the delivery or otherwise of OHI to the participants. In Jones 2011 the participants were allocated a 15‐20 minute appointment for oral hygiene advice plus intervention, however additional time was permitted as required. In Lightner 1971 the duration of the scale and polish given to comparison groups varied from 30 to 50 minutes, with or without an additional 10 minutes of oral hygiene instruction. Only one study (Jones 2011) stated they used an ultrasonic scaler.
Characteristics of the outcome measures
Details of the different indices used in each individual trial to record the outcomes are presented in Additional Table 1 'Indices used in trials.'
| Study | Notes/references | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change | Periodontal indices |
| Jones 2011 | For references to index teeth used in trial seeRamfjord 1959 | Visual presence of any plaque on the 6 (Ramfjord) index teeth according to a dichotomous scale: plaque present/not present | Measurement of calculus in mm: 1 measurement, | Bleeding from the gingival margin of 6 (Ramfjord) index teeth. Bleeding was detected by running a blunt‐ended (PCP‐10) probe gently around the gingival margin of the tooth at a 60° | Not reported | Not reported | Not reported |
| Lightner 1971 | For references to indices used in trial seeO' Leary 1967. The examination system used was the Periodontal Screening Examination (O' Leary 1967). The mouth is divided into 6 segments. The highest score found for any tooth in a segment is recorded as the score for the segment | Plaque index (no description of the precise criteria used). Plaque scores range from 0 to 3. Data reported as mean plaque index scores | Hard deposit index. Hard deposit scores range from 0 to 3 (precise criteria not described). Data presented as mean hard deposit index scores | Gingival index (precise criteria not described). Gingival scores range from 0 to 3. Data reported as mean gingival index scores | Not reported | Loss of epithelial attachment. Data reported as mean attachment loss (mm). Reported data not used in this review as no standard deviations provided | Periodontal index. Possible scores of 0, 4, 5 or 6 (criteria described in full in the paper). To simplify the statistical handling of data, scores 4, 5 and 6 were transformed to 1, 2 and 3 respectively. Reported data were not used in this review as presented in an inappropriate format |
| Listgarten 1985 | For references to indices used in trial seeLoe 1967 | Modified plaque index. Index based on a 0‐3 score (Loe 1967). Each tooth was scored on the mid‐buccal and mid‐lingual surfaces as well as on the mesial buccal surface. The mean values for the whole mouth obtained by adding all mid‐buccal and mid‐lingual and the doubled value of the mesial buccal scores and dividing by the number of surfaces at risk. Data reported as mean plaque index scores for control and test groups | Not reported | Modified gingival index (Loe 1967). Index is based on a 0‐3 score. Data reported as mean gingival index scores for control and test groups for all surfaces measured at each examination | Probing depth. Data reported as mean probing depth (mm). Probing depth recorded to the nearest mm with uniform probes calibrated in mm, with a tip diameter of 0.35 mm | Recession recorded to the nearest mm as the distance from the gingival margin to the cemento‐enamel junction (only when a distinct cemento‐enamel junction was identifiable) | Not reported |
mm = millimetre
Details of the outcomes recorded in different studies, the time points when measured, and the frequency of provision of scale and polish for each of the three comparisons (scale and polish versus no scale and polish; scale and polish versus scale and polish in response to signs or symptoms or both of periodontal disease; scale and polish versus scale and polish at a different interval), are presented in Additional Table 2 , Table 3, and Table 4 respectively.
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change |
| 6 months | 24 | Jones 2011 | Jones 2011 | Jones 2011 | ||
| 12 months | 24 | Jones 2011 | Jones 2011 | Jones 2011 |
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change |
| 6 months | 6 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | ||
| 12 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 18 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 24 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 30 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 36 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 |
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change | Periodontal indices |
| 3 months versus 6 months | 12 | Lightner 1971 | Lightner 1971 | Lightner 1971 | |||
| 24 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 3 months versus 12 months | 24 | Lightner 1971 | Lightner 1971 | Lightner 1971 | |||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 6 months versus 12 months | 24 | Lightner 1971 Jones 2011 | Lightner 1971 Jones 2011 | Lightner 1971 Joners 2011 | |||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1973 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
Primary outcome measure
Gingivitis indices (inflammatory and bleeding)
Gingivitis/gingival bleeding was used as an outcome measure in all three studies and was measured at the following data points in each study:
-
12, 24, 36, 46 months (Lightner 1971)
-
6, 12, 18, 24, 30, 36 months (Listgarten 1985)
-
6, 12, 18, 24, 30, 36, 42, 48 months (Listgarten 1986). Due to a problem with study design only the six‐month data were used (for further details see the section below on 'Handling of data/data assumptions made in review')
-
24 months (Jones 2011).
Secondary outcome measures
There was little consistency in the other outcome measures reported.
Clinical status factors
-
Calculus and plaque indices
Calculus was used as an outcome measure in two studies (Jones 2011; Lightner 1971). It was measured at the following data points:
‐ 12, 24, 36, 46 months (Lightner 1971)
‐ 24 months (Jones 2011).
Plaque was used as an outcome measure in the three included studies and was measured at the following data points in each study:
‐ 12, 24, 36, 46 months (Lightner 1971)
‐ 6, 12, 18, 24, 30, 36 months (Listgarten 1985)
‐ 24 months (Jones 2011).
-
Changes in probing depth
Changes in probing depth were reported as an outcome measure in only one study (Listgarten 1985) and were measured at the following data points: 6, 12, 18, 24, 30, and 36 months.
-
Changes in attachment level
Changes in attachment level were reported as an outcome measure in only one study (Lightner 1971); however, the data could not be used as no standard deviations were provided.
-
Periodontal indices
Lightner 1971 reported periodontal index data which could not be used as they were in an inappropriate format for inclusion in this review.
-
Tooth loss
No studies reported this outcome.
-
Adverse events
No studies reported any adverse effects.
Patient‐centred factors and economic cost factors
One study (Jones 2011) reported some patient‐centred factors. No economic cost outcomes were reported in any of the included studies.
Handling of data/data assumption made in review
In one study (Lightner 1971) there were five comparison groups (groups 1, 2, 3, 4A and 4B) (seeCharacteristics of included studies table for full details of the interventions provided). For Comparison 3 we compared groups 2, 3 and 4A with each other (where oral hygiene instruction (OHI) was provided alongside the scale and polish), and compared groups 4B and 1 with each other (where no OHI was provided). We did not make comparisons between groups that did and did not receive OHI alongside the scale and polish in Comparison 3.
For Comparison 4 we compared group 4A (scale and polish with OHI at three‐month intervals) with group 4B (scale and polish without OHI at three‐month intervals) and group 2 (scale and polish with OHI at 12‐month intervals) with group 1 (scale and polish without OHI at 12‐month intervals). In this study, group 2 received one scale and polish treatment per year given in two 30‐minute appointments, five to 11 days apart. Similarly, group 3 received two scale and polish treatments per year, the first of which was provided over two visits, five to 11 days apart. In our comparisons and analyses, groups 2 and 3 have been described as '12‐month interval' and 'six‐month interval' groups respectively and no distinction has been drawn between the 'two‐visit' and 'one‐visit' scale and polish treatments.
In Listgarten 1985 no standard deviations for the data were given in the paper. In order to use the data we made the assumption that the standard deviations would be similar to those from the Listgarten 1986. To be conservative we assumed a common standard deviation for the test and control groups based on the control group standard deviation for the Listgarten 1986 study that was larger than that for the test. As no statistically significance differences were found, an analysis assuming larger standard deviations than this would not affect the results and conclusions for this study.
Excluded studies
Of the 88 potentially relevant papers considered, 83 study reports (of 75 studies) were excluded. Although many studies could be excluded for more than one reason, in general only the main reason for exclusion has been recorded in the Characteristics of excluded studies table.
-
Not routine scale and polish (27 studies: Addy 1988; Axelsson 1987; Bonner 2005; Brown 2002; Chapple 1995; Godin 1976; Greenwell 1985; Hill 1981; Hoffman 2005; Hugoson 2007; Kaldahl 1988; Kinane 2000; Knöfler 2007; Kwan‐Yat 2006; Loesche 2002; Lopez 2005; Mishkin 1986; Moëne 2010; Powell 1999; Rosling 1983; Schlagenhauf 1989; Serrano 2011; Smulow 1983; Van der Weijden 1994; Wennström 2011; Westfelt 1998; Zee 2006).
-
Unclear whether a randomised controlled trial (RCT) or judged not to be a randomised controlled trial (27 studies: Axelsson 1975; Axelsson 1981; Axelsson 2004; Badersten 1984; Budtz‐Jorgensen 2000; Chawla 1975; Cutress 1991; Feldman 1988; Grimm 1986; Gunay 1998; Hou 1989; Huber 1987; Ketomaki 1993; Klein 1985; Lee 2009; Lim 1996; Lunder 1994; Moimaz 2000; Mojon 1998; Rosen 2004; Saliba 1997; Sandig 1981; Schulz 1989; Suomi 1971; Suomi 1973; Tsuboi 2003), also including a cluster RCT with one cluster per intervention (Shaw 1991).
-
Length of follow‐up less than six months (four studies: Aldridge 1995; Tan 1978; White 1996; Zanatta 2011).
-
Children with mixed dentition at baseline (four studies: Ashley 1981; Axelsson 1977; Bellini 1981; Poulsen 1976).
-
All patients received scale and polish (two studies: Hellström 1996; Zimmerman 1993).
-
Interventions not relevant (three studies: Lembariti 1998; Rask 1988; Wang 1992).
-
No scale and polish (one study: Sato 2008).
-
None of the primary outcomes specified in our review were measured in this study (one study: Adachi 2002).
-
Patients had partial dentures (one study: Katay 1990).
-
Patients had severe periodontal disease, or had periodontal treatment and were in maintenance phase (five studies: Glavind 1977; Listgarten 1986; Nyman 1975; Rosling 1976; Westfelt 1983).
Ongoing studies
There is one ongoing study funded by the National Health Service in the UK, ISRCTN56465715, which will be included in a future version of this review (Characteristics of ongoing studies). The trial should be published in 2016/17.
Risk of bias in included studies
The review authors' judgements about each risk of bias item presented as percentages across all included studies is given in Figure 1, and the review authors' judgements about each risk of bias item for each included study is given in Figure 2.
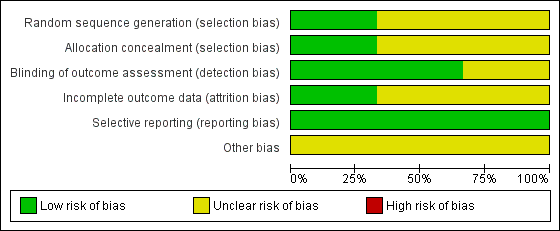
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
One study was judged to be at low risk of bias for random sequence generation, as a computer generated sequence was used (Jones 2011). The other two studies were judged to be at unclear risk of bias for this. Listgarten 1985 gave no more information than that they were "randomised" and Lightner 1971, that patients were "randomly assigned."
Allocation concealment
One study described the randomisation as centrally randomised and was judged as at low risk of bias (Jones 2011). Neither of the other studies described adequate methods of allocation concealment and were therefore classified as being at unclear risk of bias for this domain.
Blinding
Participant blinding was not possible in any of the studies and was not considered as part of the risk of bias assessment. We assessed blinding for the outcome assessors.
Two studies described adequate outcome assessor blinding and were therefore classified as being at low risk of bias for this domain (Jones 2011; Lightner 1971). The remaining study was classified as being at unclear risk of bias as the outcome assessor blinding was unclear in the report (Listgarten 1985).
Incomplete outcome data
Jones 2011 was considered to be at low risk of attrition bias due to approximately equal numbers withdrawing from each treatment group and full explanations provided of reasons for withdrawal. Risk of attrition bias was assessed as unclear in the other two trials due to either high attrition or lack of reporting of reasons.
Selective reporting
All three studies reported all the outcomes planned in the methods section in full and were all assessed as at low risk of reporting bias (Jones 2011; Lightner 1971; Listgarten 1985).
Other potential sources of bias
All three studies were assessed as being at unclear risk of bias for this domain for different reasons. There was possible bias resulting from the withdrawal of patients with Basic Periodontal Examination (BPE) > 3 in one study (Jones 2011). In the other two studies there was baseline imbalance in important prognostic factors (Lightner 1971; Listgarten 1985).
Overall risk of bias
We judged the risk of bias for all three studies as unclear (Jones 2011; Lightner 1971; Listgarten 1985).
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2 ; Summary of findings 3 ; Summary of findings 4
Comparison 1: Scale and polish versus no scale and polish (Objective 1)
Only one study at unclear risk of bias provided data for this comparison (Jones 2011), where both a six‐monthly scale and polish and a 12‐monthly scale and polish treatments are compared with no scale and polish. The clinical data are shown graphically as forest plots for the three outcomes of gingivitis, calculus and plaque at 24 months (Analysis 1.1; Analysis 1.2; Analysis 1.3).
Six‐monthly scale and polish versus no scale and polish
-
Mean difference (MD) = ‐0.02 (95% confidence interval (CI) ‐0.10 to 0.06) for gingivitis (mean proportion of bleeding sites per patient); P value = 0.65.
-
MD = ‐0.24 (95% CI ‐0.51 to 0.03) for calculus (mm); P value = 0.08.
-
MD = ‐0.04 (95% CI ‐0.13 to 0.05) for plaque (mean proportion of sites with plaque per patient); P value = 0.35.
12‐monthly scale and polish versus no scale and polish
-
MD = ‐0.01 (95% CI ‐0.09 to 0.07) for gingivitis (mean proportion of bleeding sites per patient); P value = 0.82.
-
MD= ‐0.06 (95% CI ‐0.33 to 0.21) for calculus (mm); P value = 0.67.
-
MD = ‐0.00 (95% CI ‐0.10 to 0.09) for plaque (mean proportion of sites with plaque per patient); P value = 0.97.
Patient‐centred outcomes
At 24 months, participants in the no scale and polish group were significantly less likely (P value < 0.001) to report a 'high' level of oral cleanliness (29%, odds ratio (OR) 0.36; 95% CI 0.20 to 0.65) compared to the six‐month group (52%), or the 12‐month group (47%, OR 0.45; 95% CI 0.25 to 0.81).
There is little evidence from this study that scale and polish at six‐monthly or 12‐monthly intervals was beneficial when compared with no scale and polish.
Comparison 2: Scale and polish at a fixed interval versus scale and polish in response to the signs or symptoms or both of periodontal disease (Objective 2)
One study assessed as at unclear risk of bias provided data for this comparison (Listgarten 1985). A six‐monthly scale and polish was compared with a variable scale and polish interval determined by the results of a differential dark‐field microscopy (DDFM) test. The data for the three outcomes reported (gingivitis, plaque and pocket depth) are shown for all time points measured (6, 12, 18, 24, 30, and 36 months) in Additional Table 5. There was no evidence that six‐monthly scale and polish was better than or worse than variable interval scale and polish for the outcomes of gingivitis, plaque or pocket depth. The mean differences for each outcome at 24 months are given below:
| Outcome measured | Variable interval | Fixed interval | ||||||
| Mean | SD | n | Mean | SD | n | MD (95% CI) | P value | |
| Gingivitis | ||||||||
| 6 months | 0.3 | 0.26 | 30 | 0.29 | 0.26 | 31 | 0.01 (‐0.12, 0.14) | 0.88 |
| 12 | 0.4 | 0.26 | 30 | 0.42 | 0.26 | 31 | ‐0.02 (‐0.15, 0.11) | 0.76 |
| 18 | 0.52 | 0.26 | 30 | 0.59 | 0.26 | 31 | ‐0.07 (‐0.20, 0.06) | 0.29 |
| 24 | 0.67 | 0.26 | 30 | 0.62 | 0.26 | 31 | 0.05 (‐0.08, 0.18) | 0.45 |
| 30 | 0.7 | 0.26 | 30 | 0.7 | 0.26 | 31 | 0.00 (‐0.13, 0.13) | 1.00 |
| 36 | 0.63 | 0.26 | 30 | 0.67 | 0.26 | 31 | ‐0.04 (‐0.17, 0.09) | 0.55 |
|
| ||||||||
| Plaque |
|
| ||||||
| 6 months | 0.43 | 0.24 | 30 | 0.53 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 12 | 0.55 | 0.24 | 30 | 0.62 | 0.24 | 31 | ‐0.07 (‐0.19, 0.05) | 0.25 |
| 18 | 0.6 | 0.24 | 30 | 0.7 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 24 | 0.59 | 0.24 | 30 | 0.69 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 30 | 0.68 | 0.24 | 30 | 0.74 | 0.24 | 31 | ‐0.06 (‐0.18, 0.06) | 0.33 |
| 36 | 0.6 | 0.24 | 30 | 0.68 | 0.24 | 31 | ‐0.08 (‐0.20, 0.04) | 0.19 |
|
| ||||||||
| Pocket depth |
|
|
|
|
|
|
|
|
| 6 months | 1.65 | 0.19 | 30 | 1.7 | 0.19 | 31 | ‐0.05 (‐0.15, 0.05) | 0.19 |
| 12 | 1.65 | 0.19 | 30 | 1.7 | 0.19 | 31 | ‐0.05 (‐0.15, 0.05) | 0.19 |
| 18 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
| 24 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
| 30 | 1.65 | 0.18 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.18 |
| 36 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
CI = confidence interval; MD = mean difference; SD = standard deviation
-
MD 0.05 (95% CI ‐0.08 to 0.18) gingivitis (mean per patient based on 0‐3 scale); P value = 0.45
-
MD ‐0.10 (95% CI ‐0.22 to 0.02) plaque (mean per patient based on 0‐3 scale); P value = 0.10
-
MD ‐0.05 (95% CI ‐0.14 to 0.04) pocket depth (mm); P value = 0.17.
Comparison 3: Scale and polish at a fixed interval versus scale and polish at a different fixed interval (Objective 2)
Two studies at unclear risk of bias provided data for this comparison comparing different fixed intervals of treatment (Jones 2011; Lightner 1971). The data are shown for gingivitis, calculus and plaque at all time points measured in Additional Table 6. As both studies provided data at the 24‐month follow‐up assessment, these data are also shown in forest plots (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4). The results for each individual comparison are summarised below.
| Comparison | Outcome measured | Variable interval | Fixed interval | MD (95% CI) | P value | ||||
| 3 versus 6 months | Gingivitis | Mean | SD | n | Mean | SD | n |
|
|
| Lightner 1971 | 12 | 1.58 | 0.31 | 64 | 1.63 | 0.31 | 110 | ‐0.05 (‐0.15, 0.05) | 0.30 |
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.43 | 0.31 | 110 | ‐0.10 (‐0.20, ‐0.00) | 0.04 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.34 | 0.31 | 110 | ‐0.07 (‐0.17, 0.03) | 0.15 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.34 | 0.31 | 110 | ‐0.09 (‐0.19, 0.01) | 0.06 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 0.26 | 0.41 | 64 | 0.3 | 0.41 | 110 | ‐0.04 (‐0.17, 0.09) | 0.53 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.27 | 0.41 | 110 | 0.00 (‐0.13, 0.13) | 1.00 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.23 | 0.41 | 110 | ‐0.01 (‐0.14, 0.12) | 0.88 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.15 | 0.41 | 110 | ‐0.02 (‐0.15, 0.11) | 0.76 |
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 1.85 | 0.41 | 64 | 1.84 | 0.41 | 110 | 0.01 (‐0.12, 0.14) | 0.88 |
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.77 | 0.41 | 110 | 0.05 (‐0.08, 0.18) | 0.44 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.58 | 0.41 | 110 | ‐0.05 (‐0.18, 0.08) | 0.44 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.47 | 0.41 | 110 | 0.01 (‐0.12, 0.14) | 0.88 |
|
| |||||||||
| 3 versus 12 months (with OHI) | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.47 | 0.31 | 121 | ‐0.14 (‐0.23, ‐0.05) | 0.003 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.39 | 0.31 | 121 | ‐0.12 (‐0.21, ‐0.03) | 0.01 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.4 | 0.31 | 121 | ‐0.15 (‐0.24, ‐0.06) | 0.002 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.4 | 0.41 | 121 | ‐0.13 (‐0.25, ‐0.01) | 0.04 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.32 | 0.41 | 121 | ‐0.10 (‐0.22, 0.02) | 0.11 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.26 | 0.41 | 121 | ‐0.13 (‐0.25, ‐0.01) | 0.04 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.84 | 0.41 | 121 | ‐0.02 (‐0.14, 0.10) | 0.75 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.68 | 0.41 | 121 | ‐0.15 (‐0.27, ‐0.03) | 0.02 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.53 | 0.41 | 121 | ‐0.05 (‐0.17, 0.07) | 0.43 |
|
|
|
|
|
|
|
|
|
|
|
| 3 versus 12 months (without OHI) | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.4 | 0.31 | 67 | 1.61 | 0.31 | 108 | ‐0.21 (‐0.30, ‐0.12) | < 0.001 |
| Lightner 1971 | 36 | 1.41 | 0.31 | 67 | 1.56 | 0.31 | 108 | ‐0.15 (‐0.24, ‐0.06) | 0.002 |
| Lightner 1971 | 48 | 1.34 | 0.31 | 67 | 1.55 | 0.31 | 108 | ‐0.21 (‐0.30, ‐0.12) | < 0.001 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.29 | 0.41 | 67 | 0.47 | 0.41 | 108 | ‐0.18 (‐0.30, ‐0.06) | 0.005 |
| Lightner 1971 | 36 | 0.29 | 0.41 | 67 | 0.45 | 0.41 | 108 | ‐0.16 (‐0.28, ‐0.04) | 0.01 |
| Lightner 1971 | 48 | 0.19 | 0.41 | 67 | 0.33 | 0.41 | 108 | ‐0.14 (‐0.26, ‐0.02) | 0.03 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.99 | 0.41 | 67 | 2.14 | 0.41 | 108 | ‐0.15 (‐0.27, ‐0.03) | 0.02 |
| Lightner 1971 | 36 | 1.9 | 0.41 | 67 | 2.04 | 0.41 | 108 | ‐0.14 (‐0.26, ‐0.02) | 0.03 |
| Lightner 1971 | 48 | 1.75 | 0.41 | 67 | 1.93 | 0.41 | 108 | ‐0.18 (‐0.30, ‐0.06) | 0.005 |
|
| |||||||||
| 6 versus 12 months | Gingivitis |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.379 | 0.303 | 107 | 0.388 | 0.307 | 100 | ‐0.01 (‐0.09, 0.07) | 0.03 |
| Lightner 1971 | 24 | 1.43 | 0.31 | 110 | 1.47 | 0.31 | 121 | ‐0.04 (‐0.12, 0.04) | 0.33 |
| Lightner 1971 | 36 | 1.34 | 0.31 | 110 | 1.39 | 0.31 | 121 | ‐0.05 (‐0.13, 0.03) | 0.22 |
| Lightner 1971 | 48 | 1.34 | 0.31 | 110 | 1.4 | 0.31 | 121 | ‐0.06 (‐0.14, 0.02) | 0.14 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.71 | 1.0 | 107 | 0.89 | 0.99 | 100 | ‐0.18 (‐0.45, 0.09) | 0.19 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 110 | 0.4 | 0.41 | 121 | ‐0.13 (‐0.24, ‐0.02) | 0.02 |
| Lightner 1971 | 36 | 0.23 | 0.41 | 110 | 0.32 | 0.41 | 121 | ‐0.09 (‐0.20, 0.02) | 0.10 |
| Lightner 1971 | 48 | 0.15 | 0.41 | 110 | 0.26 | 0.41 | 121 | ‐0.11 (‐0.22, ‐0.00) | 0.04 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.394 | 0.342 | 107 | 0.435 | 0.347 | 100 | ‐0.04 (‐0.13, 0.05) | 0.39 |
| Lightner 1971 | 24 | 1.77 | 0.41 | 110 | 1.84 | 0.41 | 121 | ‐0.07 (‐0.18, 0.04) | 0.19 |
| Lightner 1971 | 36 | 1.58 | 0.41 | 110 | 1.68 | 0.41 | 121 | ‐0.10 (‐0.21, 0.01) | 0.06 |
| Lightner 1971 | 48 | 1.47 | 0.41 | 110 | 1.53 | 0.41 | 121 | ‐0.06 (‐0.17, 0.05) | 0.27 |
CI = confidence interval; MD = mean difference; OHI = oral hygiene instruction; SD = standard deviation
Three months versus six months
Only one study at unclear risk of bias (Lightner 1971) provided data and there was no evidence of any differences in gingivitis, calculus or plaque comparing three and six‐monthly scale and polish treatments. The 24‐month outcome data are presented below:
-
MD ‐0.10 (95% CI ‐0.20 to 0.00) gingivitis (mean per patient based on 0‐3 scale); P value = 0.04
-
MD 0.00 (95% CI ‐0.13 to 0.13) calculus (mean per patient based on 0‐3 scale); P value = 1.00
-
MD 0.05 (95% CI ‐0.08 to 0.18) plaque (mean per patient based on 0‐3 scale); P value = 0.44.
Three months versus 12 months
Only one study at unclear risk of bias (Lightner 1971) provided data for this comparison. Levels of gingivitis, calculus and plaque were consistently lower in the three‐month scale and polish treatment group compared to the 12‐month group. The outcome data after 24 months of follow‐up are presented below:
-
MD ‐0.14 (95% CI ‐0.23 to ‐0.05) gingivitis (mean per patient based on 0‐3 scale); P value = 0.003
-
MD ‐0.13 (95% CI ‐0.25 to ‐0.01) calculus (mean per patient based on 0‐3 scale); P value = 0.04
-
MD ‐0.02 (95% CI ‐0.14 to 0.10) plaque (mean per patient based on 0‐3 scale); P value = 0.75.
For scale and polish alone, without oral hygiene instruction (OHI) in either group, there are consistent statistically significant differences favouring three‐monthly scale and polish compared to 12‐monthly scale and polish for the outcomes of gingivitis, calculus and plaque. The 24‐month outcome data are presented below:
-
MD ‐0.21 (95% CI ‐0.30 to ‐0.12) gingivitis (mean per patient based on 0‐3 scale); P value < 0.001
-
MD ‐0.18 (95% CI ‐0.30 to ‐0.06) calculus (mean per patient based on 0‐3 scale); P value = 0.005
-
MD ‐0.15 (95% CI ‐0.27 to ‐0.03) plaque (mean per patient based on 0‐3 scale); P value = 0.02.
Six months versus 12 months (with OHI)
Most of these results (Additional Table 6) were based on a single study (Lightner 1971), but outcome data for this comparison for 24 months of follow‐up were based on the meta‐analysis of two studies (Jones 2011; Lightner 1971) (Analysis 3.4), both assessed as at unclear risk of bias. A standardised mean difference (SMD) was used to combine the different scales used for all three outcome measurements:
-
SMD ‐0.08 (95% CI ‐0.27 to 0.10) gingivitis; P value = 0.38
-
SMD ‐0.25 (95% CI ‐0.44 to ‐0.06) calculus; P value = 0.009
-
SMD ‐0.16 (95% CI ‐0.35 to 0.03) plaque; P value = 0.10.
There is some weak evidence that calculus is reduced for the six‐monthly scale and polish treatment, but no difference between scale and polish at six‐month and 12‐month intervals for the outcomes of gingivitis and plaque.
Patient‐centred outcomes
Jones 2011 reported some patient‐centred outcome data. At 24 months there was no significant difference between the 12‐month group (47%, OR 0.95; 95% CI 0.53 to 1.70) and the six‐month group.
Comparison 4: Scale and polish with OHI at a fixed interval versus scale and polish without OHI at the same fixed interval (Objective 3)
This comparison is to evaluate the effect of combining oral hygiene instruction with the scale and polish treatment. One study (Lightner 1971) provided data comparing a three‐monthly scale and polish treatment with or without OHI for the outcomes of gingivitis, calculus and plaque. This study also compared 12‐monthly scale and polish treatment with or without OHI. The data are shown for gingivitis, calculus and plaque at all time points measured in Additional Table 7. These data are also shown in forest plots (Analysis 4.1; Analysis 4.2). The results for each individual comparison are summarised below.
| Comparison | Outcome measured (months) | OHI | Without OHI | MD (95% CI) | P value | ||||
| Scale and polish every 3 months | Gingivitis | Mean | SD | n | Mean | SD | n |
|
|
| Lightner 1971 | 12 | 1.58 | 0.31 | 64 | 1.65 | 0.31 | 67 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.4 | 0.31 | 67 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.41 | 0.31 | 67 | ‐0.14 (‐0.25, ‐0.03) | 0.01 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.34 | 0.31 | 67 | ‐0.09 (‐0.20, 0.02) | 0.10 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 0.26 | 0.41 | 64 | 0.31 | 0.41 | 67 | ‐0.05 (‐0.19, 0.09) | 0.49 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.29 | 0.41 | 67 | ‐0.02 (‐0.16, 0.12) | 0.78 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.29 | 0.41 | 67 | ‐0.07 (‐0.21, 0.07) | 0.33 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.19 | 0.41 | 67 | ‐0.06 (‐0.20, 0.08) | 0.40 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 1.85 | 0.41 | 64 | 2.12 | 0.41 | 67 | ‐0.27 (‐0.41, ‐0.13) | < 0.001 |
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.99 | 0.41 | 67 | ‐0.17 (‐0.31, ‐0.03) | 0.02 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.9 | 0.41 | 67 | ‐0.37 (‐0.51, ‐0.23) | < 0.001 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.75 | 0.41 | 67 | ‐0.27 (‐0.41, ‐0.13) | < 0.001 |
|
| |||||||||
| Scale and polish every 12 months | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.47 | 0.31 | 121 | 1.61 | 0.31 | 108 | ‐0.14 (‐0.22, ‐0.06) | < 0.001 |
| Lightner 1971 | 36 | 1.39 | 0.31 | 121 | 1.56 | 0.31 | 108 | ‐0.17 (‐0.25, ‐0.09) | < 0.001 |
| Lightner 1971 | 48 | 1.4 | 0.31 | 121 | 1.55 | 0.31 | 108 | ‐0.15 (‐0.23, ‐0.07) | < 0.001 |
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.4 | 0.41 | 121 | 0.47 | 0.41 | 108 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 36 | 0.32 | 0.41 | 121 | 0.45 | 0.41 | 108 | ‐0.13 (‐0.24, ‐0.02) | 0.02 |
| Lightner 1971 | 48 | 0.26 | 0.41 | 121 | 0.33 | 0.41 | 108 | ‐0.07 (‐0.18, 0.04) | 0.20 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.84 | 0.41 | 121 | 2.14 | 0.41 | 108 | ‐0.30 (‐0.41, ‐0.19) | < 0.001 |
| Lightner 1971 | 36 | 1.68 | 0.41 | 121 | 2.04 | 0.41 | 108 | ‐0.36 (‐0.47, ‐0.25) | < 0.001 |
| Lightner 1971 | 48 | 1.53 | 0.41 | 121 | 1.93 | 0.41 | 108 | ‐0.40 (‐0.51, ‐0.29) | < 0.001 |
CI = confidence interval; MD = mean difference; OHI = oral hygiene instruction; SD = standard deviation
Three‐monthly scale and polish treatment
After 24 months there was evidence of a difference favouring three‐monthly scale and polish with OHI being associated with significantly lower plaque levels. There was no evidence of a difference in the outcomes of gingivitis or calculus for this comparison. The results for the 24‐month assessment are given below:
-
MD ‐0.07 (95% CI ‐0.18 to 0.04) gingivitis (mean per patient based on 0‐3 scale); P value = 0.20
-
MD ‐0.02 (95% CI ‐0.16 to 0.12) calculus (mean per patient based on 0‐3 scale); P value = 0.78
-
MD ‐0.17 (95% CI ‐0.31 to ‐0.03) plaque (mean per patient based on 0‐3 scale); P value = 0.02.
12‐monthly scale and polish treatment
There was evidence of a difference favouring 12‐monthly scale and polish with OHI being associated with lower gingivitis and plaque levels after 24 months of follow‐up.The results for the 24‐month assessment are given below:
-
MD ‐0.14 (95% CI ‐0.22 to ‐0.06) gingivitis (mean per patient based on 0‐3 scale); P value < 0.001
-
MD ‐0.07 (95% CI ‐0.18 to 0.04) calculus (mean per patient based on 0‐3 scale); P value = 0.20
-
MD ‐0.30 (95% CI ‐0.41 to ‐0.19) plaque (mean per patient based on 0‐3 scale); P value < 0.001.
Comparison 5: Scale and polish by a dentist versus scale and polish by a dental care professional (Objective 4)
No studies were found for this comparison.
Discussion
The three studies included in this review were clinically and methodologically heterogeneous, one being conducted in general practice, one in a dental school and the third on US Air Force cadets, however they did provide some limited data for three of the four objectives of the review. Only two studies compared the same outcomes for the same comparison and could be included in a meta‐analysis.
Summary of main results
Objective 1: Routine scale and polish versus no scale and polish
One study (Jones 2011) provided data for the comparison between scale and polish versus no scale and polish. The patient population and setting for Jones 2011 were most appropriate to answer the questions posed by this review, and this study was assessed as at unclear risk of bias. Jones 2011 provides a comparison between six‐monthly and 12‐monthly scale and polish treatments with no treatment, with a follow‐up of 24 months. This study was conducted in general dental practice and included patients attending for check‐up appointments who had no pockets greater than 3.5 mm. After 24 months of follow‐up there were no clinically important differences between scale and polish treatments and no treatment for the outcomes of gingivitis, calculus and plaque. This body of evidence (a single study, at unclear risk of bias), was considered to be of low quality and should be interpreted with caution.
Objective 2: Routine scale and polish at different time intervals on periodontal health (based on a test for periodontal disease)
One study (Listgarten 1985) provided data for the comparison between scale and polish at a fixed interval versus scale and polish in response to the signs or symptoms or both of periodontal disease. This study compared scale and polish provided at six‐monthly intervals with scale and polish provided in response to bacterial assessments. No statistically significant differences were observed between treatment (scale and polish in response to bacterial assessments) and control (six‐monthly scale and polish) groups at any time point over the course of this three‐year trial. Participants in the treatment group were initially recalled more frequently than those in the control group, however, by the end of the trial, recall intervals for scaling and polishing were considerably extended for many participants in the treatment group and 11 participants had not received a scale and polish by the end of the study. Despite this extension of recall interval, the periodontal health of participants in the test group (as measured using the outcomes gingivitis, plaque and pocket depth) was not adversely affected compared to the control group. However, neither treatment nor control regimen was successful in preventing gingivitis and mean gingival index scores in control and test groups generally increased at each time point over the course of the trial.
Two studies (Jones 2011; Lightner 1971) provided data for the comparison between scale and polish at a fixed interval versus scale and polish at a different fixed interval. The studies were conducted in two distinct populations ‐regular attenders in general practice (Jones 2011) and young adult males aged 17 to 22 years (Lightner 1971). As already mentioned the patient population and setting for Jones 2011 were most appropriate to answer some of the questions posed by this review. There were consistent results in Lightner 1971 towards improved outcomes (lower levels of plaque, calculus and gingivitis) in more frequent interval groups compared with less frequent interval groups. However, the differences generally only reached statistical significance when comparisons were made between the most frequent interval groups (scale and polish delivered at three or four‐monthly intervals) with the least frequent interval groups (scale and polish delivered at 12‐monthly intervals). We have reported the comparison between six‐monthly and 12‐monthly scale and polish intervals combined with oral hygiene instruction (Analysis 3.4) in the summary of findings table. The body of evidence was assessed as of low quality.
Objective 3: Scale and polish with and without oral hygiene instruction (OHI)
Only one study (Lightner 1971) provided data for the comparison between scale and polish with OHI at a fixed interval versus scale and polish without OHI at the same fixed interval. The provision of OHI in conjunction with three‐monthly and 12‐monthly scale and polish treatments was associated with significantly lower plaque levels and significantly lower plaque and gingivitis levels respectively after 24 months of follow‐up. However, the effect sizes were generally small. The body of evidence for comparing scale and polish treatments with and without OHI was assessed as of low quality.
Objective 4: Scale and polish provided by a dentist compared with a dental care professional
No included studies provided data for this objective.
Overall completeness and applicability of evidence
One of the included studies (Jones 2011) was more appropriate than the others for answering many of the questions posed by this review. This study was conducted on adults attending their dentist in general dental practice for check‐ups. More trials in a primary care setting including a more representative group of participants with higher levels of disease would enhance the evidence base for answering the questions posed by this review. The other two older studies were not conducted in primary care, and included patients who were either young, or who had had or were referred for treatment for periodontal disease. It is difficult to envisage how the experimental intervention in Listgarten 1985 could reasonably be used in general practice and its applicability in different patient populations with varying levels of risk for destructive periodontitis is uncertain.
Three of the four objectives were addressed by at least one included trial, however there were no trials addressing the fourth objective comparing scale and polish provided by a dentist with a professional complementary to dentistry.
Outcomes reported
The outcomes used in different studies were measured using different indices. The three included studies reported: three different gingivitis indices, two different calculus indices, three different plaque indices and probing depth was only reported in one study. Many primary and secondary outcomes were not reported in any of the three studies: tooth loss, attachment change, adverse events and economic cost factors. It is important that trials measure the harms as well as the benefits of interventions. Scaling is an invasive procedure and has been associated with a number of adverse effects, including damaged enamel surfaces by repeated scaling, tooth sensitivity and gingival recession (Brothwell 1998). It is also recognised that subgingival scaling of shallow pockets can cause permanent loss of periodontal attachment (Brothwell 1998). The latter would have been captured in 'attachment change' data but these were not reported in any of the trials included in this review. It would undoubtedly be advisable for future randomised controlled trials assessing the effects of scaling and polishing to report on adverse events to allow for a more balanced appraisal of benefits and harms.
It is increasingly recognised that the signs and symptoms of periodontal disease can impact on patients' oral health related quality of life (Needleman 2004). Therefore, interventions designed to ameliorate these signs and symptoms might be expected to have a positive impact on a patient's quality of life. However, studies of the effects of periodontal therapy have only rarely used patient‐centred outcome measures (Elley 2000; Needleman 2004) and none of the trials included in this review reported such outcomes. We are therefore unable to make any statements regarding the impact of routine scaling and polishing (as defined in this review) on patients' quality of life.
Similarly, although economic cost outcomes (such as time foregone to attend for appointments and provider costs) are relevant to the debate regarding the merits and demerits of routine scaling and polishing, such outcomes were not reported in any trials included in this review.
Again, it would be advisable for future trials to include quality of life and economic cost outcomes to allow for a full evaluation of the beneficial and harmful effects of this intervention.
Quality of the evidence
Given the considerable resources involved in providing routine scale and polish treatments for adults in many countries (DoH 2000; Frame 2000), it is disappointing that there is a paucity of good quality, reliable research evidence to inform clinical practice. Only three studies met the inclusion criteria for this review, all judged to be at unclear risk of bias. It is impossible to blind patients to the timing of scale and polish treatments, and as having a scale and polish treatment may influence personal oral hygiene behaviour, participant blinding was not included in the risk of bias assessment. The quality of the body of evidence available for each outcome (gingivitis, calculus and plaque) for three of the four objectives of this review was found to be low (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4). Therefore the results presented in this review should be interpreted cautiously.
Potential biases in the review process
A sensitive search strategy was used for this review. Every effort was made to identify all relevant studies. No studies were excluded due to language restrictions.
Data collection and analysis were done by two review authors independently, and any disagreement between review authors was resolved by discussion to minimise/exclude bias during the review process.
It is possible not all relevant studies have been identified for inclusion in this review due to grey literature bias and the publication of studies in non‐indexed journals. In addition, post‐hoc changes to a protocol may potentially bias the results of a review. For this updated review we made some important alternations to the selection criteria specified in the original protocol. Firstly, we excluded split‐mouth studies as this design does not reflect the manner in which routine scaling and polishing is provided in practice. Secondly, we excluded trials where participants were described as having severe periodontal disease (e.g. alveolar bone loss involving most teeth or individuals requiring referral for specialist periodontal treatment). We also excluded trials where participants had undergone specialist periodontal treatment in the six months preceding the trial and who were in the 'maintenance phase.' These exclusions were made because best clinical practice would suggest that these categories of patients should receive more advanced periodontal treatment than the 'routine scale and polish' as defined in this review. Finally, we also excluded trials where only a single scale and polish treatment was provided as, once again, this does not reflect the manner in which 'routine scaling and polishing' is provided in practice. As specified in the Background section of our review, a 'routine scale and polish' is an intervention that is intended to be provided at regular intervals to patients, and not as a 'stand alone' intervention provided at a single point in time.
We consider that these adjustments to the selection criteria have resulted in a review that will ultimately be more useful to practitioners who are providing 'routine scale and polish treatments' and patients who are receiving this intervention.
Agreements and disagreements with other studies or reviews
We did not find any other reviews that addressed the specific objectives of this Cochrane review. Reviews of oral health promotion and dental health education have demonstrated that, in general, oral hygiene instruction can induce limited behaviour change in patients and reduce plaque levels in the short term, but there is no lasting effect (Brown 1994; Kay 1996; Kay 1998; Sprod 1996). In our review only one trial (Lightner 1971) evaluated the effects of providing oral hygiene instruction in conjunction with scale and polish treatments on plaque levels. The provision of oral hygiene instruction with three‐monthly and 12‐monthly scale and polish treatments was associated with small but statistically significant reductions in plaque at 24 and 48 months when compared with three‐monthly and 12‐monthly scale and polish treatments without oral hygiene instruction.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Scale and polish versus no scale and polish (control), Outcome 1 Gingivitis at 24 months.

Comparison 1 Scale and polish versus no scale and polish (control), Outcome 2 Calculus at 24 months.

Comparison 1 Scale and polish versus no scale and polish (control), Outcome 3 Plaque at 24 months.
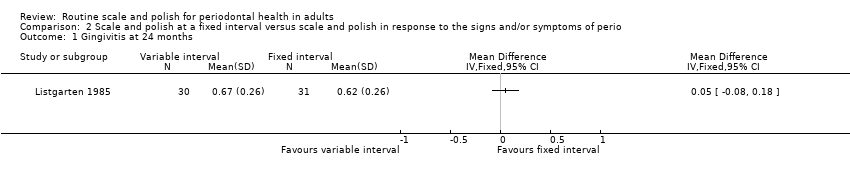
Comparison 2 Scale and polish at a fixed interval versus scale and polish in response to the signs and/or symptoms of perio, Outcome 1 Gingivitis at 24 months.

Comparison 2 Scale and polish at a fixed interval versus scale and polish in response to the signs and/or symptoms of perio, Outcome 2 Plaque at 24 months.

Comparison 2 Scale and polish at a fixed interval versus scale and polish in response to the signs and/or symptoms of perio, Outcome 3 Pocket depth at 24 months.
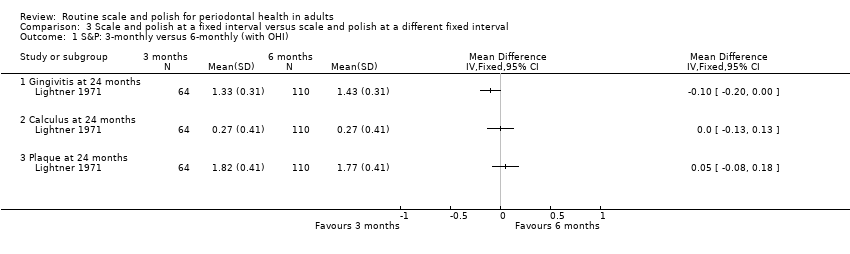
Comparison 3 Scale and polish at a fixed interval versus scale and polish at a different fixed interval, Outcome 1 S&P: 3‐monthly versus 6‐monthly (with OHI).

Comparison 3 Scale and polish at a fixed interval versus scale and polish at a different fixed interval, Outcome 2 S&P: 3‐monthly versus 12‐monthly (with OHI).
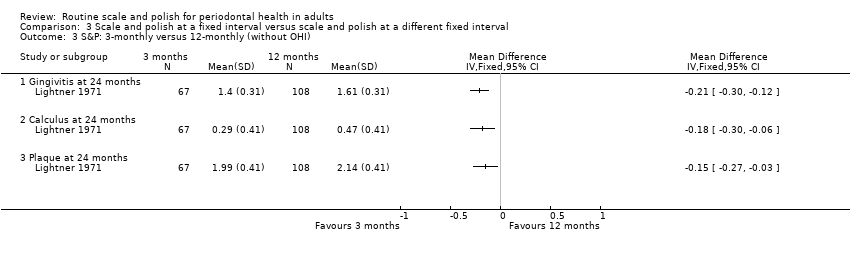
Comparison 3 Scale and polish at a fixed interval versus scale and polish at a different fixed interval, Outcome 3 S&P: 3‐monthly versus 12‐monthly (without OHI).

Comparison 3 Scale and polish at a fixed interval versus scale and polish at a different fixed interval, Outcome 4 S&P: 6‐monthly versus 12‐monthly (with OHI).

Comparison 4 Scale and polish at a fixed interval with OHI versus scale and polish without OHI at the same fixed interval, Outcome 1 S&P every 3 months with OHI versus without OHI.

Comparison 4 Scale and polish at a fixed interval with OHI versus scale and polish without OHI at the same fixed interval, Outcome 2 S&P every 12 months with OHI versus without OHI.
| Routine scale and polish compared with no treatment for periodontal health | ||||
| Patient or population: Healthy dentate adults Settings: General dental practice Intervention: Routine scale and polish (either 6‐monthly or 12‐monthly) Comparison: No treatment | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis (proportion of index sites bleeding) at 24 months 6‐monthly scale and polish Mean proportion in control group is 0.40 sites | MD ‐0.02 (‐0.10 to 0.06) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| Calculus (mean depth in mm at index sites) at 24 months 6‐monthly scale and polish Mean in control group is 0.95 mm | MD ‐0.24 (‐0.51 to 0.03) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| Plaque (proportion of index sites with plaque) at 24 months 6‐monthly scale and polish Mean proportion in control group is 0.44 sites | MD ‐0.04 (‐0.13 to 0.05) | 1 study1 (207 participants) | ⊕⊕⊝⊝ | The results for 12‐monthly scale and polish were similar and also not significant |
| CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 1Single study at unclear risk of bias | ||||
| Routine scale and polish at different frequencies for periodontal health | ||||
| Patient or population: Healthy dentate young adults Settings: Air Force Academy Intervention: Routine scale and polish every 6 months with oral hygiene instruction Comparison: Routine scale and polish every 12 months with oral hygiene instruction | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis different indices used | SMD ‐0.08 (‐0.27 to 0.10) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are significant but based on only 1 study |
| Calculus different indices used | SMD ‐0.25 (‐0.44 to ‐0.06) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are significant but based on only 1 study |
| Plaque different indices used | SMD ‐0.16 (‐0.35 to 0.03) | 2 studies1 (438 participants) | ⊕⊕⊝⊝ | The results for comparing 3 versus 12 months are generally not significant |
| CI: confidence interval; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 12 studies at unclear risk of bias | ||||
| Routine scale and polish with and without oral hygiene instruction for periodontal health | ||||
| Patient or population: Healthy dentate young adults Settings: Air Force Academy Intervention: Routine scale and polish with oral hygiene instruction Comparison: Routine scale and polish without oral hygiene instruction | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean gingivitis score (0‐3 scale) in control group is 1.40 | MD ‐0.07 (‐0.18 to 0.04) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was significant |
| Calculus (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean calculus score (0‐3 scale) in control group is 0.29 mm | MD ‐0.02 (‐0.16 to 0.12) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was similar |
| Plaque (mean on 0‐3 scale) at 24 months 3‐monthly scale and polish Mean plaque score (0‐3 scale) in control group is 1.99 | MD ‐0.17 (‐0.31 to ‐0.03) | 1 study1 (131 participants) | ⊕⊕⊝⊝ | Results for 12‐monthly scale and polish was not significant |
| CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| 1Single study at unclear risk of bias | ||||
| Routine scale and polish undertaken by dentist or dental care professional for periodontal health | ||||
| Patient or population: Healthy dentate adults Settings: General dental practice Intervention: Routine scale and polish by dental professional Comparison: Routine scale and polish by dentist | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Gingivitis | No studies | |||
| Calculus | No studies | |||
| Plaque | No studies | |||
| GRADE Working Group grades of evidence | ||||
| Study | Notes/references | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change | Periodontal indices |
| Jones 2011 | For references to index teeth used in trial seeRamfjord 1959 | Visual presence of any plaque on the 6 (Ramfjord) index teeth according to a dichotomous scale: plaque present/not present | Measurement of calculus in mm: 1 measurement, | Bleeding from the gingival margin of 6 (Ramfjord) index teeth. Bleeding was detected by running a blunt‐ended (PCP‐10) probe gently around the gingival margin of the tooth at a 60° | Not reported | Not reported | Not reported |
| Lightner 1971 | For references to indices used in trial seeO' Leary 1967. The examination system used was the Periodontal Screening Examination (O' Leary 1967). The mouth is divided into 6 segments. The highest score found for any tooth in a segment is recorded as the score for the segment | Plaque index (no description of the precise criteria used). Plaque scores range from 0 to 3. Data reported as mean plaque index scores | Hard deposit index. Hard deposit scores range from 0 to 3 (precise criteria not described). Data presented as mean hard deposit index scores | Gingival index (precise criteria not described). Gingival scores range from 0 to 3. Data reported as mean gingival index scores | Not reported | Loss of epithelial attachment. Data reported as mean attachment loss (mm). Reported data not used in this review as no standard deviations provided | Periodontal index. Possible scores of 0, 4, 5 or 6 (criteria described in full in the paper). To simplify the statistical handling of data, scores 4, 5 and 6 were transformed to 1, 2 and 3 respectively. Reported data were not used in this review as presented in an inappropriate format |
| Listgarten 1985 | For references to indices used in trial seeLoe 1967 | Modified plaque index. Index based on a 0‐3 score (Loe 1967). Each tooth was scored on the mid‐buccal and mid‐lingual surfaces as well as on the mesial buccal surface. The mean values for the whole mouth obtained by adding all mid‐buccal and mid‐lingual and the doubled value of the mesial buccal scores and dividing by the number of surfaces at risk. Data reported as mean plaque index scores for control and test groups | Not reported | Modified gingival index (Loe 1967). Index is based on a 0‐3 score. Data reported as mean gingival index scores for control and test groups for all surfaces measured at each examination | Probing depth. Data reported as mean probing depth (mm). Probing depth recorded to the nearest mm with uniform probes calibrated in mm, with a tip diameter of 0.35 mm | Recession recorded to the nearest mm as the distance from the gingival margin to the cemento‐enamel junction (only when a distinct cemento‐enamel junction was identifiable) | Not reported |
| mm = millimetre | |||||||
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change |
| 6 months | 24 | Jones 2011 | Jones 2011 | Jones 2011 | ||
| 12 months | 24 | Jones 2011 | Jones 2011 | Jones 2011 |
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change |
| 6 months | 6 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | ||
| 12 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 18 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 24 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 30 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 | |||
| 36 | Listgarten 1985 | Listgarten 1985 | Listgarten 1985 |
| Frequency of scale and polish | Data points | Plaque | Calculus | Gingivitis/bleeding | Pocket depth | Attachment change | Periodontal indices |
| 3 months versus 6 months | 12 | Lightner 1971 | Lightner 1971 | Lightner 1971 | |||
| 24 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 3 months versus 12 months | 24 | Lightner 1971 | Lightner 1971 | Lightner 1971 | |||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| 6 months versus 12 months | 24 | Lightner 1971 Jones 2011 | Lightner 1971 Jones 2011 | Lightner 1971 Joners 2011 | |||
| 36 | Lightner 1971 | Lightner 1971 | Lightner 1973 | ||||
| 46 | Lightner 1971 | Lightner 1971 | Lightner 1971 | ||||
| Outcome measured | Variable interval | Fixed interval | ||||||
| Mean | SD | n | Mean | SD | n | MD (95% CI) | P value | |
| Gingivitis | ||||||||
| 6 months | 0.3 | 0.26 | 30 | 0.29 | 0.26 | 31 | 0.01 (‐0.12, 0.14) | 0.88 |
| 12 | 0.4 | 0.26 | 30 | 0.42 | 0.26 | 31 | ‐0.02 (‐0.15, 0.11) | 0.76 |
| 18 | 0.52 | 0.26 | 30 | 0.59 | 0.26 | 31 | ‐0.07 (‐0.20, 0.06) | 0.29 |
| 24 | 0.67 | 0.26 | 30 | 0.62 | 0.26 | 31 | 0.05 (‐0.08, 0.18) | 0.45 |
| 30 | 0.7 | 0.26 | 30 | 0.7 | 0.26 | 31 | 0.00 (‐0.13, 0.13) | 1.00 |
| 36 | 0.63 | 0.26 | 30 | 0.67 | 0.26 | 31 | ‐0.04 (‐0.17, 0.09) | 0.55 |
|
| ||||||||
| Plaque |
|
| ||||||
| 6 months | 0.43 | 0.24 | 30 | 0.53 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 12 | 0.55 | 0.24 | 30 | 0.62 | 0.24 | 31 | ‐0.07 (‐0.19, 0.05) | 0.25 |
| 18 | 0.6 | 0.24 | 30 | 0.7 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 24 | 0.59 | 0.24 | 30 | 0.69 | 0.24 | 31 | ‐0.10 (‐0.22, 0.02) | 0.10 |
| 30 | 0.68 | 0.24 | 30 | 0.74 | 0.24 | 31 | ‐0.06 (‐0.18, 0.06) | 0.33 |
| 36 | 0.6 | 0.24 | 30 | 0.68 | 0.24 | 31 | ‐0.08 (‐0.20, 0.04) | 0.19 |
|
| ||||||||
| Pocket depth |
|
|
|
|
|
|
|
|
| 6 months | 1.65 | 0.19 | 30 | 1.7 | 0.19 | 31 | ‐0.05 (‐0.15, 0.05) | 0.19 |
| 12 | 1.65 | 0.19 | 30 | 1.7 | 0.19 | 31 | ‐0.05 (‐0.15, 0.05) | 0.19 |
| 18 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
| 24 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
| 30 | 1.65 | 0.18 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.18 |
| 36 | 1.65 | 0.17 | 30 | 1.7 | 0.17 | 31 | ‐0.05 (‐0.14, 0.04) | 0.17 |
| CI = confidence interval; MD = mean difference; SD = standard deviation | ||||||||
| Comparison | Outcome measured | Variable interval | Fixed interval | MD (95% CI) | P value | ||||
| 3 versus 6 months | Gingivitis | Mean | SD | n | Mean | SD | n |
|
|
| Lightner 1971 | 12 | 1.58 | 0.31 | 64 | 1.63 | 0.31 | 110 | ‐0.05 (‐0.15, 0.05) | 0.30 |
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.43 | 0.31 | 110 | ‐0.10 (‐0.20, ‐0.00) | 0.04 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.34 | 0.31 | 110 | ‐0.07 (‐0.17, 0.03) | 0.15 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.34 | 0.31 | 110 | ‐0.09 (‐0.19, 0.01) | 0.06 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 0.26 | 0.41 | 64 | 0.3 | 0.41 | 110 | ‐0.04 (‐0.17, 0.09) | 0.53 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.27 | 0.41 | 110 | 0.00 (‐0.13, 0.13) | 1.00 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.23 | 0.41 | 110 | ‐0.01 (‐0.14, 0.12) | 0.88 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.15 | 0.41 | 110 | ‐0.02 (‐0.15, 0.11) | 0.76 |
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 1.85 | 0.41 | 64 | 1.84 | 0.41 | 110 | 0.01 (‐0.12, 0.14) | 0.88 |
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.77 | 0.41 | 110 | 0.05 (‐0.08, 0.18) | 0.44 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.58 | 0.41 | 110 | ‐0.05 (‐0.18, 0.08) | 0.44 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.47 | 0.41 | 110 | 0.01 (‐0.12, 0.14) | 0.88 |
|
| |||||||||
| 3 versus 12 months (with OHI) | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.47 | 0.31 | 121 | ‐0.14 (‐0.23, ‐0.05) | 0.003 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.39 | 0.31 | 121 | ‐0.12 (‐0.21, ‐0.03) | 0.01 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.4 | 0.31 | 121 | ‐0.15 (‐0.24, ‐0.06) | 0.002 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.4 | 0.41 | 121 | ‐0.13 (‐0.25, ‐0.01) | 0.04 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.32 | 0.41 | 121 | ‐0.10 (‐0.22, 0.02) | 0.11 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.26 | 0.41 | 121 | ‐0.13 (‐0.25, ‐0.01) | 0.04 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.84 | 0.41 | 121 | ‐0.02 (‐0.14, 0.10) | 0.75 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.68 | 0.41 | 121 | ‐0.15 (‐0.27, ‐0.03) | 0.02 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.53 | 0.41 | 121 | ‐0.05 (‐0.17, 0.07) | 0.43 |
|
|
|
|
|
|
|
|
|
|
|
| 3 versus 12 months (without OHI) | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.4 | 0.31 | 67 | 1.61 | 0.31 | 108 | ‐0.21 (‐0.30, ‐0.12) | < 0.001 |
| Lightner 1971 | 36 | 1.41 | 0.31 | 67 | 1.56 | 0.31 | 108 | ‐0.15 (‐0.24, ‐0.06) | 0.002 |
| Lightner 1971 | 48 | 1.34 | 0.31 | 67 | 1.55 | 0.31 | 108 | ‐0.21 (‐0.30, ‐0.12) | < 0.001 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.29 | 0.41 | 67 | 0.47 | 0.41 | 108 | ‐0.18 (‐0.30, ‐0.06) | 0.005 |
| Lightner 1971 | 36 | 0.29 | 0.41 | 67 | 0.45 | 0.41 | 108 | ‐0.16 (‐0.28, ‐0.04) | 0.01 |
| Lightner 1971 | 48 | 0.19 | 0.41 | 67 | 0.33 | 0.41 | 108 | ‐0.14 (‐0.26, ‐0.02) | 0.03 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.99 | 0.41 | 67 | 2.14 | 0.41 | 108 | ‐0.15 (‐0.27, ‐0.03) | 0.02 |
| Lightner 1971 | 36 | 1.9 | 0.41 | 67 | 2.04 | 0.41 | 108 | ‐0.14 (‐0.26, ‐0.02) | 0.03 |
| Lightner 1971 | 48 | 1.75 | 0.41 | 67 | 1.93 | 0.41 | 108 | ‐0.18 (‐0.30, ‐0.06) | 0.005 |
|
| |||||||||
| 6 versus 12 months | Gingivitis |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.379 | 0.303 | 107 | 0.388 | 0.307 | 100 | ‐0.01 (‐0.09, 0.07) | 0.03 |
| Lightner 1971 | 24 | 1.43 | 0.31 | 110 | 1.47 | 0.31 | 121 | ‐0.04 (‐0.12, 0.04) | 0.33 |
| Lightner 1971 | 36 | 1.34 | 0.31 | 110 | 1.39 | 0.31 | 121 | ‐0.05 (‐0.13, 0.03) | 0.22 |
| Lightner 1971 | 48 | 1.34 | 0.31 | 110 | 1.4 | 0.31 | 121 | ‐0.06 (‐0.14, 0.02) | 0.14 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.71 | 1.0 | 107 | 0.89 | 0.99 | 100 | ‐0.18 (‐0.45, 0.09) | 0.19 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 110 | 0.4 | 0.41 | 121 | ‐0.13 (‐0.24, ‐0.02) | 0.02 |
| Lightner 1971 | 36 | 0.23 | 0.41 | 110 | 0.32 | 0.41 | 121 | ‐0.09 (‐0.20, 0.02) | 0.10 |
| Lightner 1971 | 48 | 0.15 | 0.41 | 110 | 0.26 | 0.41 | 121 | ‐0.11 (‐0.22, ‐0.00) | 0.04 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Jones 2011 | 24 | 0.394 | 0.342 | 107 | 0.435 | 0.347 | 100 | ‐0.04 (‐0.13, 0.05) | 0.39 |
| Lightner 1971 | 24 | 1.77 | 0.41 | 110 | 1.84 | 0.41 | 121 | ‐0.07 (‐0.18, 0.04) | 0.19 |
| Lightner 1971 | 36 | 1.58 | 0.41 | 110 | 1.68 | 0.41 | 121 | ‐0.10 (‐0.21, 0.01) | 0.06 |
| Lightner 1971 | 48 | 1.47 | 0.41 | 110 | 1.53 | 0.41 | 121 | ‐0.06 (‐0.17, 0.05) | 0.27 |
| CI = confidence interval; MD = mean difference; OHI = oral hygiene instruction; SD = standard deviation | |||||||||
| Comparison | Outcome measured (months) | OHI | Without OHI | MD (95% CI) | P value | ||||
| Scale and polish every 3 months | Gingivitis | Mean | SD | n | Mean | SD | n |
|
|
| Lightner 1971 | 12 | 1.58 | 0.31 | 64 | 1.65 | 0.31 | 67 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 24 | 1.33 | 0.31 | 64 | 1.4 | 0.31 | 67 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 36 | 1.27 | 0.31 | 64 | 1.41 | 0.31 | 67 | ‐0.14 (‐0.25, ‐0.03) | 0.01 |
| Lightner 1971 | 48 | 1.25 | 0.31 | 64 | 1.34 | 0.31 | 67 | ‐0.09 (‐0.20, 0.02) | 0.10 |
|
| |||||||||
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 0.26 | 0.41 | 64 | 0.31 | 0.41 | 67 | ‐0.05 (‐0.19, 0.09) | 0.49 |
| Lightner 1971 | 24 | 0.27 | 0.41 | 64 | 0.29 | 0.41 | 67 | ‐0.02 (‐0.16, 0.12) | 0.78 |
| Lightner 1971 | 36 | 0.22 | 0.41 | 64 | 0.29 | 0.41 | 67 | ‐0.07 (‐0.21, 0.07) | 0.33 |
| Lightner 1971 | 48 | 0.13 | 0.41 | 64 | 0.19 | 0.41 | 67 | ‐0.06 (‐0.20, 0.08) | 0.40 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 12 | 1.85 | 0.41 | 64 | 2.12 | 0.41 | 67 | ‐0.27 (‐0.41, ‐0.13) | < 0.001 |
| Lightner 1971 | 24 | 1.82 | 0.41 | 64 | 1.99 | 0.41 | 67 | ‐0.17 (‐0.31, ‐0.03) | 0.02 |
| Lightner 1971 | 36 | 1.53 | 0.41 | 64 | 1.9 | 0.41 | 67 | ‐0.37 (‐0.51, ‐0.23) | < 0.001 |
| Lightner 1971 | 48 | 1.48 | 0.41 | 64 | 1.75 | 0.41 | 67 | ‐0.27 (‐0.41, ‐0.13) | < 0.001 |
|
| |||||||||
| Scale and polish every 12 months | Gingivitis |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.47 | 0.31 | 121 | 1.61 | 0.31 | 108 | ‐0.14 (‐0.22, ‐0.06) | < 0.001 |
| Lightner 1971 | 36 | 1.39 | 0.31 | 121 | 1.56 | 0.31 | 108 | ‐0.17 (‐0.25, ‐0.09) | < 0.001 |
| Lightner 1971 | 48 | 1.4 | 0.31 | 121 | 1.55 | 0.31 | 108 | ‐0.15 (‐0.23, ‐0.07) | < 0.001 |
|
| Calculus |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 0.4 | 0.41 | 121 | 0.47 | 0.41 | 108 | ‐0.07 (‐0.18, 0.04) | 0.20 |
| Lightner 1971 | 36 | 0.32 | 0.41 | 121 | 0.45 | 0.41 | 108 | ‐0.13 (‐0.24, ‐0.02) | 0.02 |
| Lightner 1971 | 48 | 0.26 | 0.41 | 121 | 0.33 | 0.41 | 108 | ‐0.07 (‐0.18, 0.04) | 0.20 |
|
| |||||||||
|
| Plaque |
|
|
|
|
|
|
|
|
| Lightner 1971 | 24 | 1.84 | 0.41 | 121 | 2.14 | 0.41 | 108 | ‐0.30 (‐0.41, ‐0.19) | < 0.001 |
| Lightner 1971 | 36 | 1.68 | 0.41 | 121 | 2.04 | 0.41 | 108 | ‐0.36 (‐0.47, ‐0.25) | < 0.001 |
| Lightner 1971 | 48 | 1.53 | 0.41 | 121 | 1.93 | 0.41 | 108 | ‐0.40 (‐0.51, ‐0.29) | < 0.001 |
| CI = confidence interval; MD = mean difference; OHI = oral hygiene instruction; SD = standard deviation | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gingivitis at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Calculus at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 6‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 12‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Plaque at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 6‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12‐monthly S&P | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gingivitis at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Plaque at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pocket depth at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 S&P: 3‐monthly versus 6‐monthly (with OHI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Gingivitis at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Calculus at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Plaque at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 S&P: 3‐monthly versus 12‐monthly (with OHI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Gingivitis at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calculus at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Plaque at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 S&P: 3‐monthly versus 12‐monthly (without OHI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Gingivitis at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calculus at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Plaque at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 S&P: 6‐monthly versus 12‐monthly (with OHI) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Gingivitis at 24 months | 2 | 438 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.27, 0.10] |
| 4.2 Calculus at 24 months | 2 | 438 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.44, ‐0.06] |
| 4.3 Plaque at 24 months | 2 | 438 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.35, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 S&P every 3 months with OHI versus without OHI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Gingivitis at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Calculus at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Plaque at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 S&P every 12 months with OHI versus without OHI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Gingivitis at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calculus at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Plaque at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

