抗生素治疗成人难辨梭菌(Clostridium difficile)相关性腹泻
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | CDAD | |
| Interventions | Teicoplanin dose study 100 mg twice daily (n = 49) versus 50 mg fours times a day (n = 43) | |
| Outcomes | Cure | |
| Notes | Also cited as Wistrom et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract reports randomised, but not how |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Abstract reports double blind |
| Incomplete outcome data (attrition bias) | High risk | 47% dropouts, 20/43 in 100 mg twice daily group and 25/49 in 50 mg four times daily group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Criteria for 'improved' outcome not described |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Rifamixin (n=10) versus vancomycin (n=10) | |
| Outcomes | Combined symptomatic and bacteriologic resolution | |
| Notes | Recurrence not assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | No recurrence data |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Unclear inclusion criteria and unclear cure criteria |
| Methods | RCT Multicenter USA and Europe | |
| Participants | Adults with diarrhoea and stool positive for C. difficile toxin A or B or both n = 535 | |
| Interventions | Fidaxomicin 200 mg (n = 270) every 12 hours or vancomycin 125 mg (n = 265) every 6 hours by mouth for 10 days | |
| Outcomes | < 3 bowel movements in 24 hours and toxin negative 20 other outcomes measured, the most important being symptomatic recurrence | |
| Notes | Non‐inferiority trial with intention‐to‐treat analysis not presented Identical design to (Louie 2011) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Participants and investigators were masked to treatment allocation and masking was maintained through database lock |
| Blinding (performance bias and detection bias) | Low risk | Double blind: study drugs were over‐encapsulated and identical in appearance |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts less than 10% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 24) versus teicoplanin (n = 27) | |
| Outcomes | Cure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | No mention of blinding of patients or outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 5/51 dropouts; 4/24 in vancomycin group and 1/27 in teicoplanin group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Not described |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 31) versus bacitracin (n = 31) | |
| Outcomes | Cure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear whether assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | 52% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Good randomisation technique, but did not test for C. difficile in stool until after randomisation |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin dose study 125 mg (n = 28) versus 500 mg (n = 28) both groups were dosed four times daily | |
| Outcomes | Cure | |
| Notes | High dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) | High risk | 18% dropouts, groups not specified |
| Selective reporting (reporting bias) | High risk | No recurrence data |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Wide range of treatment duration |
| Methods | RCT single centre | |
| Participants | Adults with > 3 unformed bowel movements in 24 hours and stool positive for C. difficile toxin (type unspecified) N = 79 | |
| Interventions | Following a standard course of either metronidazole or vancomycin for C. dif for 10‐14 days (physician choice), patients were randomised to placebo (n = 40) or rifaximin 400 mg (n = 39) by mouth for 20 days | |
| Outcomes | Recurrent diarrhoea and stool positive for C. dif after initial resolution of the illness | |
| Notes | Recurrence prophylaxis study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By study pharmacist |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by pharmacist |
| Blinding (performance bias and detection bias) | Low risk | Double blind specified Study medication and matching placebo were dispensed with a specific study number to ensure blinding of investigators and patients |
| Incomplete outcome data (attrition bias) | High risk | 14% dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Non‐random distribution of primary antibiotic therapy |
| Methods | RCT | |
| Participants | 1118 patients from 200 centers in Europe (109) and the United States (91) with symptomatic primary or recurrent Clostridium difficile infection characterized by >3 bowel movements per day and a positive stool assay for CDI toxin | |
| Interventions | Tolevamer 3 g three times daily x 14 days (n = 563), oral metronidazole 375 mg per day x 10 days (n = 289) or oral vancomycin 125 mg per day x 10 days (n = 266) | |
| Outcomes | Symptomatic resolution of the diarrhoea to < 2 solid bowel movements per day Bacteriologic (toxin) confirmation was not sought unless symptoms failed to resolve or recurred | |
| Notes | Tolevamer is a resin that binds C. difficile toxin The results of the tolevamer arm of this study were not included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double blinding specified |
| Incomplete outcome data (attrition bias) | Low risk | 6 dropouts in the vancomycin group and 4 in the metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Vancomycin (n = 22) versus placebo (n = 22) | |
| Outcomes | Cure and bacteriologic resolution | |
| Notes | Recurrence not assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation by pharmacy |
| Blinding (performance bias and detection bias) | Unclear risk | Patients were blinded but it is unclear whether the outcome observer was blinded |
| Incomplete outcome data (attrition bias) | High risk | 52% dropouts; 12/22 in vancomycin group and 13/22 in placebo group and additional lost data |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Other pathogens not excluded Poor follow up procedures |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Metronidazole (n = 20) versus metronidazole plus rifampin (n = 19) | |
| Outcomes | Resolution of diarrhoea within 10 days and relapse within 40 days | |
| Notes | No dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer generated, and blinded study staff enrolled patients using numbered packages" |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence of randomization numbers was concealed until the end of the study" Comment: As study staff were blinded before enrolment, there does not seem to be a source of bias |
| Blinding (performance bias and detection bias) | High risk | Single‐blinded Quote: "A placebo was not used" Comment: This could have been placebo controlled although good justification was given" |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
| Methods | RCT ‐ multicenter | |
| Participants | Adult patients (> 18 years of age) with CDI | |
| Interventions | Surotomycin 125 mg once daily (n = 68) or 250 mg once daily (n = 71) or vancomycin 125 four times daily (n = 70) all by mouth for 10 days | |
| Outcomes | Cure (resolution of diarrhoea) and recurrence of diarrhoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) | Low risk | Pills identical Outcome assessors did not know allocation group |
| Incomplete outcome data (attrition bias) | Low risk | A bit problematic: Apparently 11/209 but PRISMA states 18/209 |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | There did not appear to be any other sources of bias |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | OPT 80 at 3 doses (100 mg, 200 mg and 400 mg) n = 16 in each group OPT‐80 became Fidaxomicin | |
| Outcomes | Resolution of diarrhoea and abdominal discomfort within the 10 day treatment period without requiring any additional therapy and relapse within 6 weeks of end of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used "interactive voice randomization system" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | "the study was a dose‐finding, randomized, open‐label study" |
| Incomplete outcome data (attrition bias) | Low risk | 4% dropouts not included in analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Patients with severe disease were excluded and only patients with a primary episode or first relapse were included Patients were excluded if they had > 24 hrs antibiotic (metronidazole or vancomycin) prior to enrolment |
| Methods | RCT multicenter (USA and Canada) | |
| Participants | Adults with diarrhoea and stool positive for C. difficile toxin A and/or B (N = 629) | |
| Interventions | Fidaxomicin 200 mg (n = 302) every 12 hours or vancomycin 125 mg (n = 327) every 6 hours by mouth | |
| Outcomes | < 3 bowel movements in 24 hours and toxin negative 20 other outcomes measured, the most important being symptomatic recurrence | |
| Notes | Non‐inferiority trial with intention‐to‐treat analysis not presented Article written by part time Optimer Pharmaceuticals employee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) | Low risk | Double blind specified |
| Incomplete outcome data (attrition bias) | Low risk | < 10% dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Subgroup analyses by severity and other factors without prior stratification of the randomisation |
| Methods | RCT | |
| Participants | CDI (N = 84) | |
| Interventions | Cadazolid in 3 doses ‐ 250 mg twice daily (n = 20), 500 mg twice daily (n = 22) and 1000 mg twice daily (n = 20) verus vancomycin 125 mg four times daily (n = 22) | |
| Outcomes | Sustained clinical response | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double dummy |
| Incomplete outcome data (attrition bias) | Low risk | 6 dropouts ‐ 7% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | Not reported |
| Methods | RCT | |
| Participants | CDI (N = 72) | |
| Interventions | LFF571 (n = 46) versus vancomycin (n = 26) | |
| Outcomes | Sustained clinical resolution | |
| Notes | Initally 1:1 randomization, switched to 5:1 halfway through | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multicenter with randomization cards drawn at each center |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Evaluator blind" |
| Incomplete outcome data (attrition bias) | High risk | 17 dropouts ‐ 23.6% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | 8 (LFF) and 5 (V) serious adverse events |
| Methods | RCT | |
| Participants | CDI | |
| Interventions | Nitazoxanide in two doses (n = 44) versus metronidazole (n = 98) | |
| Outcomes | Resolution of diarrhoea at 7 and 31 days and time to resolution | |
| Notes | 32 dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised to 1 of 3 groups, in double‐blinded fashion" Comment: No mention is made of how randomisation was established |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double blind: tablets made to look identical |
| Incomplete outcome data (attrition bias) | High risk | 23% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
| Methods | RCT | |
| Participants | CDI (N = 49) | |
| Interventions | Vancomycin 125 mg 6 hourly (n = 27) versus nitazoxanide 500 mg 12 hourly (n = 22) | |
| Outcomes | Complete resolution of symptoms and signs attributable to C difficile within 3 days after completion of therapy Relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation (randomisation codes held by study sponsor) |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy: all patients had active and placebo tablets for the duration of the study |
| Incomplete outcome data (attrition bias) | Low risk | 2% dropout not included in analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported Unclear if recurrence separated from primary healing but follow‐up equal to most studies measuring recurrence |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | RCT | |
| Participants | CDI (N = 101) | |
| Interventions | Vancomycin (n = 56) versus metronidazole (n = 45) | |
| Outcomes | Cure | |
| Notes | No relapse data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 7% dropouts; 4 from vancomycin group and 3 from metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Also unclear as to which patients had original antibiotic removed |
| Methods | RCT | |
| Participants | CDI (N = 126) | |
| Interventions | Vancomycin (n = 31) versus metronidazole (n = 31) versus teicoplanin (n = 28) versus fusidic acid (n = 29) | |
| Outcomes | Cure | |
| Notes | Cost data presented N for each group a bit unclear as 126 randomised and 7 dropped out, leaving the specified numbers for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised according to a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | No mention of blinding of patients or outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 5.5% dropouts, groups not specified |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea Did exclude patients with no WBCs in stool sample "to insure inclusion of patients with significant disease due to C. difficile" Did however explicitly report that original offending antibiotic had been stopped |
| Methods | RCT | |
| Participants | CDI (N = 131) | |
| Interventions | Fusidic acid (n = 67) versus metronidazole (n = 64) | |
| Outcomes | Cessation of diarrhoea and conversion to toxin negative | |
| Notes | 17 dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician provided a computer‐generated list of random set numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "the investigator teams were unaware of the treatment allocation" |
| Blinding (performance bias and detection bias) | Low risk | All medication packs were coded and contained identical‐looking pills |
| Incomplete outcome data (attrition bias) | High risk | 26% dropouts; 20/67 in fusidic acid group and 14/64 from metronidazole group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | Did not exclude other pathogens in the stool as causes of diarrhoea |
| Methods | RCT | |
| Participants | CDI (N = 42) | |
| Interventions | Vancomycin versus bacitracin; 21 in each group | |
| Outcomes | Cure | |
| Notes | Cost data presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Patients blinded but unclear whether assessors were too ‐ although the abstract reports it was double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | Controls for diarrhoea resolution on removal of offending antibiotic |
| Methods | RCT | |
| Participants | CDI (N = 172) | |
| Interventions | Vancomycin (n = 82) versus metronidazole (n = 90) | |
| Outcomes | Cessation of diarrhoea Conversion to C. difficile toxin A negative stool Relapse at 21 days post cure | |
| Notes | 12.5% dropout, but achieved power | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a member of pharmacy staff randomised participants by selecting a card from a sealed envelope..." |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation by pharmacy using sealed envelope |
| Blinding (performance bias and detection bias) | Low risk | Quote: "patients received either vancomycin liquid and a placebo tablet that was similar in appearance to metronidazole or a metronidazole tablet and an unpleasantly‐flavoured placebo liquid" |
| Incomplete outcome data (attrition bias) | Unclear risk | 13% dropouts, missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | No patients with suspected or life‐threatening intraabdominal complications (perforation or obstruction) were included No patients in ITU or with pseudomembranous colitis were included Unclear whether prior antibiotics had been stopped The timing of randomisation for the stratification of severity of CDI was unclear No mention of stratification before randomisation was made in the text but the authors stated so by e‐mail correspondence |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Probiotic protocol | |
| Abstract only | |
| Non‐randomised study | |
| Fecal microbiota transplant | |
| C. difficile vaccine | |
| Non‐randomised study | |
| Duplicate data to (Louie 2015) | |
| Non‐randomised study | |
| Participants were asymptomatic carriers of Clostridium difficile without diarrhoea | |
| Non‐randomised study | |
| Fecal microbiota transplant | |
| Pooled analysis of 2 trials | |
| RCT with a non antibiotic arm (tolevamer) | |
| Does not compare two antibiotics and focuses on the recurrence of Clostridium difficile rather than treatment of the existing infection | |
| RCT with a non antibiotic arm (Clostridium difficile immune whey) | |
| Non‐randomised study | |
| RCT ‐ prophylaxis of C. difficile | |
| Publication of antibiotic resistance development from a group previously reported by Wullt 2004. | |
| Assessment of immune whey efficacy | |
| Non‐randomised study | |
| Non‐randomised study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | CDAD (N = 232) |
| Interventions | Rifaximin (n = 117) versus vancomycin (n = 115) |
| Outcomes | Sustained clinical response |
| Notes | Abstract only published 2014 |
| Methods | RCT |
| Participants | CDAD n = 20 |
| Interventions | Oral vancomycin + intravenous metronidazole verus oral vancomycin alone |
| Outcomes | Sustained clinical response |
| Notes | Abstract only published 2014 |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Phase 3 study with cadazolid in CDAD (AC061A302) |
| Methods | RCT |
| Participants | CDI |
| Interventions | Cadazolid and vancomycin |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting |
| Trial name or title | Phase 3 study with cadazolid in CDAD |
| Methods | RCT |
| Participants | CDI |
| Interventions | Cadazolid and vancomycin |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sustained Symptomatic Cure with all exclusions treated as failures Show forest plot | 4 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| Analysis 1.1 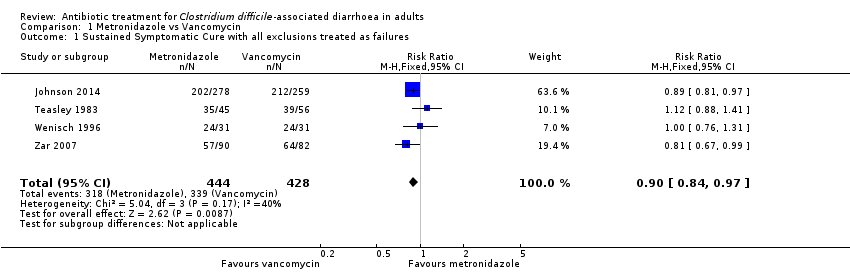 Comparison 1 Metronidazole vs Vancomycin, Outcome 1 Sustained Symptomatic Cure with all exclusions treated as failures. | ||||
| 2 Bacteriologic Cure Show forest plot | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| Analysis 1.2  Comparison 1 Metronidazole vs Vancomycin, Outcome 2 Bacteriologic Cure. | ||||
| 3 Sustained Cure (Combined symptomatic and bacteriologic cure) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.99] |
| Analysis 1.3 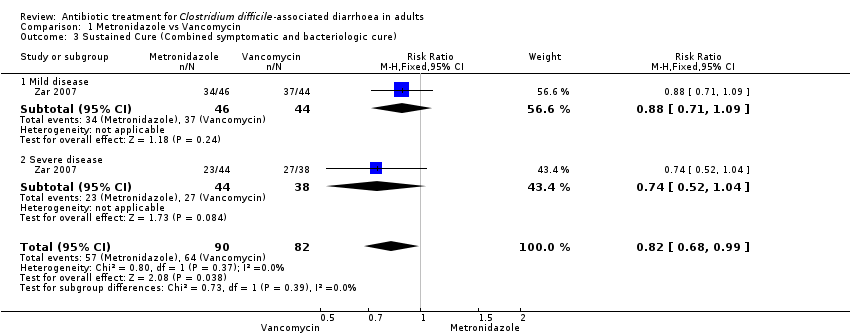 Comparison 1 Metronidazole vs Vancomycin, Outcome 3 Sustained Cure (Combined symptomatic and bacteriologic cure). | ||||
| 3.1 Mild disease | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 3.2 Severe disease | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
| Analysis 2.1  Comparison 2 Bacitracin vs Vancomycin, Outcome 1 Symptomatic Cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.00, 1.46] |
| Analysis 3.1  Comparison 3 Teicoplanin vs Vancomycin, Outcome 1 Symptomatic Cure. | ||||
| 2 Bacteriologic Cure Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.19, 2.78] |
| Analysis 3.2  Comparison 3 Teicoplanin vs Vancomycin, Outcome 2 Bacteriologic Cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomiatic Cure Show forest plot | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.88, 1.41] |
| Analysis 4.1  Comparison 4 Metronidazole vs Fusidic Acid, Outcome 1 Symptomiatic Cure. | ||||
| 2 Bacteriologic Cure Show forest plot | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
| Analysis 4.2  Comparison 4 Metronidazole vs Fusidic Acid, Outcome 2 Bacteriologic Cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.07, 1.27] |
| Analysis 5.1  Comparison 5 Fidaxomicin vs Vancomycin, Outcome 1 Symptomatic Cure. | ||||

Study flow diagram.
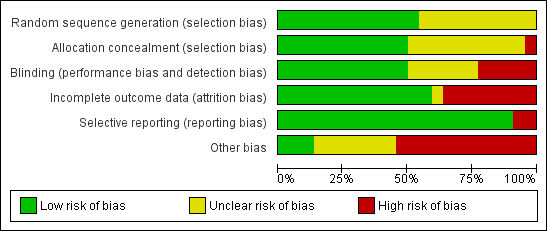
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
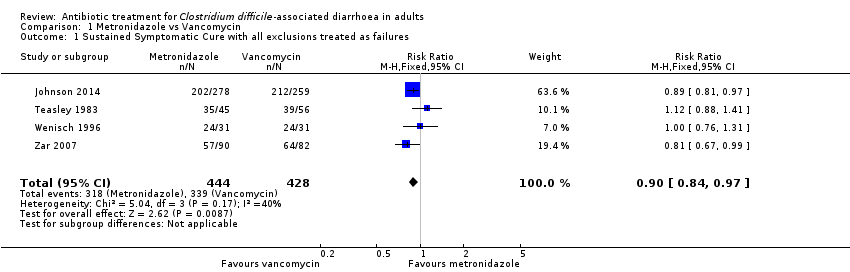
Comparison 1 Metronidazole vs Vancomycin, Outcome 1 Sustained Symptomatic Cure with all exclusions treated as failures.
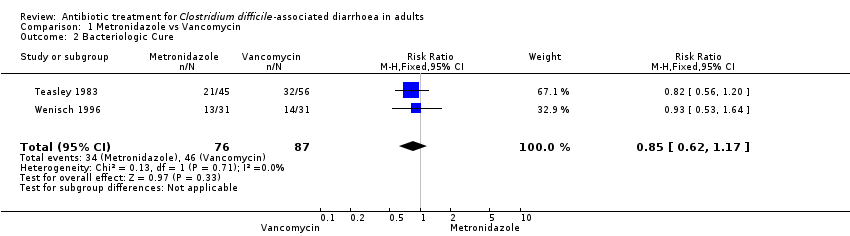
Comparison 1 Metronidazole vs Vancomycin, Outcome 2 Bacteriologic Cure.
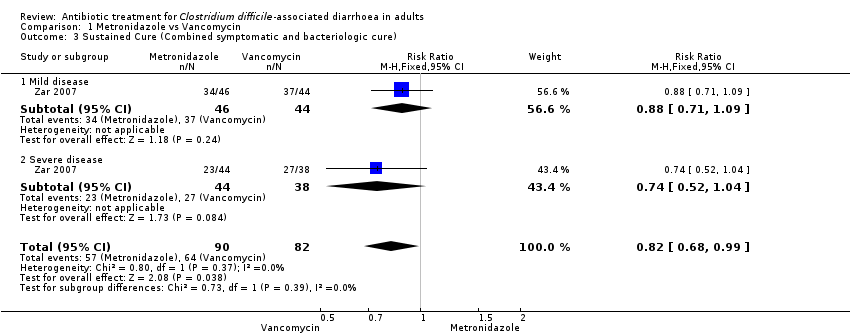
Comparison 1 Metronidazole vs Vancomycin, Outcome 3 Sustained Cure (Combined symptomatic and bacteriologic cure).
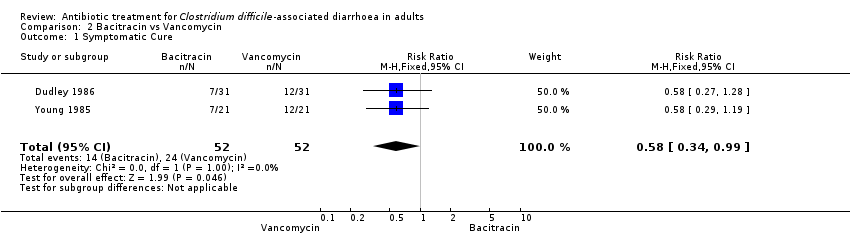
Comparison 2 Bacitracin vs Vancomycin, Outcome 1 Symptomatic Cure.
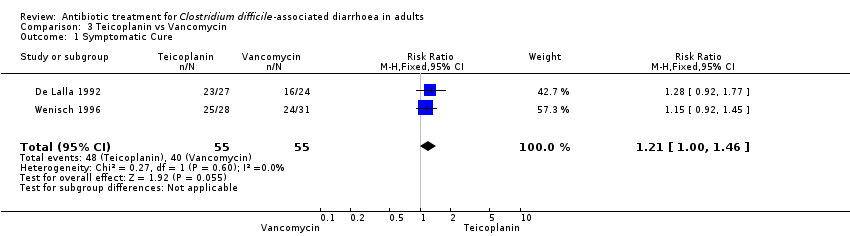
Comparison 3 Teicoplanin vs Vancomycin, Outcome 1 Symptomatic Cure.
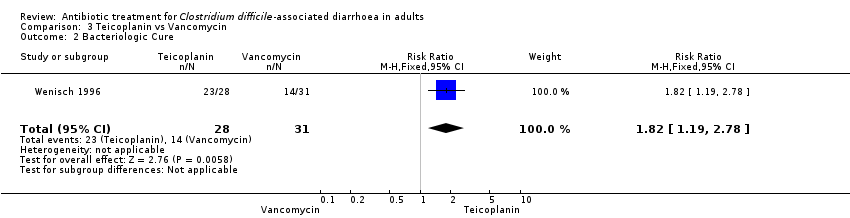
Comparison 3 Teicoplanin vs Vancomycin, Outcome 2 Bacteriologic Cure.
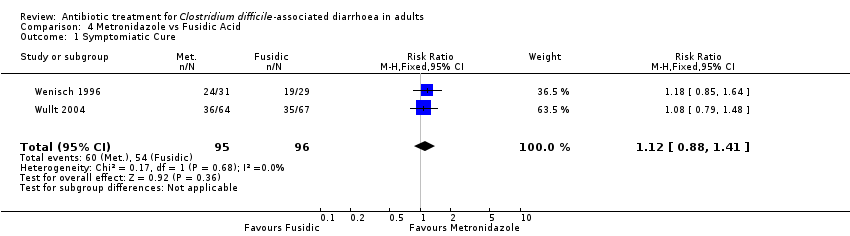
Comparison 4 Metronidazole vs Fusidic Acid, Outcome 1 Symptomiatic Cure.

Comparison 4 Metronidazole vs Fusidic Acid, Outcome 2 Bacteriologic Cure.

Comparison 5 Fidaxomicin vs Vancomycin, Outcome 1 Symptomatic Cure.
| Metronidazole versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Metronidazole versus Vancomycin | |||||
| Symptomatic cure with all exclusions treated as failures | 792 per 10001 | 713 per 1000 | RR 0.9 | 872 | ⊕⊕⊕⊝ | |
| Bacteriologic cure | 529 per 10001 | 449 per 1000 | RR 0.85 | 163 | ⊕⊝⊝⊝ | |
| Cure (combined symptomatic and bacteriologic cure) ‐ mild disease | 841 per 10001 | 740 per 1000 | RR 0.88 | 90 | ⊕⊝⊝⊝ | |
| Cure (combined symptomatic and bacteriologic cure) ‐ severe disease | 711 per 10001 | 526 per 1000 | RR 0.74 | 82 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Teicoplanin versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Teicoplanin versus Vancomycin | |||||
| Symptomatic Cure | 727 per 10001 | 880 per 1000 | RR 1.21 | 110 | ⊕⊝⊝⊝ | |
| Bacteriologic Cure | 452 per 10001 | 822 per 1000 | RR 1.82 | 59 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Fidaxomicin compared to Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vancomycin | Fidaxomicin | |||||
| Symptomatic Cure | 610 per 10001 | 713 per 1000 | RR 1.17 | 1164 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Bacitracin versus Vancomycin for Clostridium difficile‐associated diarrhoea in adults | ||||||
| Patient or population: patients with Clostridium difficile‐associated diarrhoea in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bacitracin versus Vancomycin | |||||
| Symptomatic Cure | 462 per 10001 | 268 per 1000 | RR 0.58 | 104 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Study | n=Total | Deaths | Harms due to Intervention | Attrition% | Stratified by Severity |
| 49 | 1 | joint pain | <10% | ||
| 20 | 0 | 0 | 0 | ||
| 535 | 20 Fid 17 Van | none | 4.9% | Yes post randomisation | |
| 51 | 2 | NR | 10% | ||
| 62 | 0 | NR | 52% | ||
| 46 | 0 | NR | 18% | ||
| 79 | skin rash | 14% | |||
| 1118 | 23 Met 16 Van | none | 1.8% | Yes post randonisation | |
| 39 | 2 | 0 | |||
| 209 | 4 | 5 | 7% | ||
| 49 | 0 | 4% | |||
| 629 | 16 Fid 21 Van | elevated liver enzymes | 5.2% | Yes post randomisation | |
| 82 | 2 | 7.3% | |||
| 72 | 0 | 44% | |||
| 142 | 4 | 23% | |||
| 50 | 0 | 2% | Yes post randomisation | ||
| 101 | 2 | NR | 7% | ||
| 126 | 3 | 5.5% | |||
| 131 | 0 | 26% | |||
| 42 | 0 | NR | 0 | ||
| 172 | 7 | 13% | Yes but uncertain when | ||
| TOTAL | 140 | ||||
| NR: None reported | |||||
| HPA1 | ESCMID2 | SHEA & IDSA3 | |
| MILD CDAD | Stop inciting antibiotic and observe, or oral metronidazole 500 mg three times daily Alternate dosing also recommended and change to vancomycin if no better in 4 days | Stop inciting antibiotic and observe, or oral metronidazole 500 mg three times daily | Stop inciting antibiotic, or oral metronidazole 500 mg three times daily for 10 days |
| SEVERE CDAD | Oral vancomycin 500 mg four times daily with tapering | Oral vancomycin 125 mg four times daily for 10 days | Oral vancomycin 125 mg four times daily for 10 days |
| SEVERE AND CANNOT TOLERATE ORAL MEDS | Intravenous metronidazole and vancomycin via nasogastric tube or enemas four times daily | Same | Same |
| SURGERY | For toxic megacolon or lactate > 5 | For perforation, toxic megacolon, Ileus, lactate > 5 | |
| RECURRENCE | First as Primary 2nd: oral vancomycin with taper | Same | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sustained Symptomatic Cure with all exclusions treated as failures Show forest plot | 4 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 2 Bacteriologic Cure Show forest plot | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| 3 Sustained Cure (Combined symptomatic and bacteriologic cure) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.99] |
| 3.1 Mild disease | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 3.2 Severe disease | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.00, 1.46] |
| 2 Bacteriologic Cure Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.19, 2.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomiatic Cure Show forest plot | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.88, 1.41] |
| 2 Bacteriologic Cure Show forest plot | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic Cure Show forest plot | 2 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.07, 1.27] |

