Single dose oral rofecoxib for acute postoperative pain in adults
Abstract
Background
Editor's note: The anti‐inflammatory drug rofecoxib (Vioxx) was withdrawn from the market at the end of September 2004 after it was shown that long‐term use (greater than 18 months) could increase the risk of heart attack and stroke in a study of secondary prevention of adenoma recurrence. Further information is available at www.vioxx.com.
Rofecoxib is a selective cyclooxygenase‐2 (COX‐2) inhibitor previously licensed for treating acute and chronic pain; it was associated with fewer gastrointestinal adverse events than conventional NSAIDs. An earlier Cochrane review (Barden 2005) showed that rofecoxib is at least as effective as conventional non‐steroidal anti‐inflammatory drugs (NSAIDs) for postoperative pain.
Objectives
To assess the analgesic efficacy and adverse effects of rofecoxib in single oral doses for moderate and severe postoperative pain.
Search methods
We searched Cochrane CENTRAL, MEDLINE, EMBASE and the Oxford Pain Relief Database for studies to June 2009.
Selection criteria
Randomised, double blind, placebo‐controlled trials of single dose orally administered rofecoxib in adults with moderate to severe acute postoperative pain.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Pain relief or pain intensity data were extracted and converted into the dichotomous outcome of number of participants with at least 50% pain relief over 4 to 6 hours, from which relative risk and number needed to treat to benefit (NNT) were calculated. Numbers of participants using rescue medication over specified time periods, and time to use of rescue medication, were sought as additional measures of efficacy. Information on adverse events and withdrawals was collected.
Main results
Twenty new studies and seven from the earlier review met the inclusion criteria. Twenty‐four studies were in dental surgery and three in other types of surgery. In total, 2636 participants were treated with rofecoxib 50 mg, 20 with rofecoxib 500 mg, and 1251 with placebo. The NNT for at least 50% pain relief over 4 to 6 hours with rofecoxib 50 mg was 2.2 (2.0 to 2.3) in all studies combined, 1.9 (1.8 to 2.0) in dental studies, and 6.8 (4.6 to 13) in other types of surgery. The median time to use of rescue medication was 14 hours for rofecoxib 50 mg and 2 hours for placebo. Significantly fewer participants used rescue medication following rofecoxib 50 mg than with placebo. Adverse events did not differ from placebo.
Authors' conclusions
Rofecoxib 50 mg (two to four times the standard daily dose for chronic pain) is an effective single dose oral analgesic for acute postoperative pain in adults, with a relatively long duration of action.
PICO
Plain language summary
Single dose oral rofecoxib for acute postoperative pain in adults
A high level of pain relief is experienced by about 60% of those with moderate to severe postoperative pain after a single dose of rofecoxib 50 mg, compared to about 10% with placebo. Based mainly on dental pain studies, one in every two participants treated with rofecoxib 50 mg had their pain levels halved, who would not have done so with placebo. Fewer people needed rescue medication with rofecoxib, and the time to use was relatively long, at 14 hours. Its efficacy and duration of action are better than that of many commonly used analgesics at standard doses. Efficacy was better in dental surgery than in other types of surgery. Adverse events did not differ from placebo in these single dose studies.
Editor's note: The anti‐inflammatory drug rofecoxib (Vioxx) was withdrawn from the market at the end of September 2004 after it was shown that long‐term use (greater than 18 months) could increase the risk of heart attack and stroke in a study of secondary prevention of adenoma recurrence. Further information is available at www.vioxx.com.
Authors' conclusions
Background
Editor's note: The anti‐inflammatory drug rofecoxib (Vioxx) was withdrawn from the market at the end of September 2004 after it was shown that long‐term use (greater than 18 months) could increase the risk of heart attack and stroke in a study of secondary prevention of adenoma recurrence. Further information is available at www.vioxx.com.
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Issue 1, 2005) on 'Single dose oral rofecoxib for postoperative pain' (Barden 2005). The title now states that the review is limited to acute postoperative pain in adults.
Rofecoxib was withdrawn by the original manufacturer in September 2004. It continues to be available in some parts of the world through different manufacturers. How much rofecoxib is used in not known. However, the original review demonstrated that it was a good analgesic, based on a large body of high quality trials. Other trials have subsequently been published, making an updated review necessary to ensure that previous results are robust.
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care. This review is one of a series of reviews whose aim is to increase awareness of the range of analgesics that are potentially available, and present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
Recent reviews include well established analgesics such as paracetamol (Toms 2008), naproxen (Derry C 2009a), diclofenac (Derry P 2009), and ibuprofen (Derry C 2009b), and newer cyclo‐oxygenase‐2 selective analgesics, such as lumiracoxib (Roy 2007), celecoxib (Derry 2008), etoricoxib (Clarke 2009), and parecoxib (Lloyd 2009).
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants is small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following 4 to 6 hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to 6 hours (Moore 2005a). Patients usually remain in the hospital or clinic for at least the first 6 hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
NSAIDs have pain‐relieving, antipyretic and anti‐inflammatory properties, are proven to be effective following day surgery and minor surgery, and have an opiate‐sparing effect after more major surgery (Grahame‐Smith 2002). However, a major concern regarding the use of conventional NSAIDs postoperatively is the possibility of bleeding from both the operative site (because of the inhibition of platelet aggregation) (Forrest 2002) and from the upper gastrointestinal tract, (especially in patients stressed by surgery, the elderly, frail, or dehydrated). Drug treatments that combine the pain‐relieving properties of NSAIDs without these adverse effects are likely to have a place in clinical practice.
Selective cyclo‐oxygenase‐2 inhibitors or 'coxibs' were developed to address the problem of upper gastrointestinal bleeding (Hawkey 2001). NSAIDs are thought to relieve pain by inhibiting cyclo‐oxygenases and thus the production of prostaglandins (Hawkey 1999). Prostaglandins occur throughout body tissues and fluids and act to stimulate pain nerve endings and promote/inhibit the aggregation of blood platelets. Cyclo‐oxygenase has at least two isoforms: COX‐1 and COX‐2. COX‐1 is constitutive while COX‐2 is induced at sites of inflammation and produces the prostaglandins involved in inflammatory responses and pain mediation (Grahame‐Smith 2002). Unlike traditional NSAIDs such as ibuprofen and ketoprofen, the 'coxibs' are selective inhibitors, blocking primarily the action of COX‐2 and causing fewer gastrointestinal effects (Moore 2005b). In common with other NSAIDS, COX‐2 inhibitors can give rise to fluid retention and renal damage (Garner 2002), so particular caution is needed in the elderly (Hawkey 2001). They have also been associated with increased cardiovascular problems, which led to the withdrawal of one coxib (Kearney 2006). Use of coxibs and non‐selective NSAIDs in patients with bowel problems such as ulcerative colitis and Crohn's Disease is complicated (Hawkey 2006).
COX‐2 inhibitors, like non‐selective NSAIDs, are also useful for the relief of acute pain, especially in patients with a high risk of upper gastrointestinal bleeding or those with a history of peptic ulcer. They should not precipitate bleeding events through inhibition of platelet aggregation (Straube 2005). In addition to the potential for a reduction in the number of adverse events, they may provide greater and more prolonged pain relief in the postoperative setting than traditional NSAIDs.
Rofecoxib (trade names Vioxx, Ceoxx, Ceeoxx) was the first COX‐2‐specific inhibitor approved for the treatment of acute pain in the USA. The recommended dose for acute pain treatment was 50 mg daily, which was two to four times the recommended daily dose for chronic arthritis pain.
The aim of this review is to evaluate the efficacy and safety of rofecoxib for the relief of acute postoperative pain.
Objectives
To evaluate the analgesic efficacy and safety of oral rofecoxib in the treatment of acute postoperative pain, using methods that permit comparison with other analgesics evaluated in the same way, using wider criteria of efficacy recommended by an in‐depth study at the individual patient level (Moore 2005a).
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were full publications of double blind trials of a single dose of oral rofecoxib against placebo for the treatment of moderate to severe postoperative pain in adults, with at least 10 participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose were available, and cross‐over studies were included provided that data from the first arm were presented separately.
Studies were excluded if they were:
-
posters or abstracts not followed up by full publication;
-
reports of trials concerned with pain other than postoperative pain (including experimental pain);
-
studies using healthy volunteers;
-
studies where pain relief was assessed by clinicians, nurses or carers (i.e., not patient‐reported);
-
studies of less than 4 hours' duration or which failed to present data over 4 to 6 hours post‐dose.
Types of participants
Studies of adult participants (15 years old or above) with established moderate to severe postoperative pain were included. For studies using a visual analogue scale (VAS), pain of at least moderate intensity was assumed when the VAS score was greater than 30 mm (Collins 1997). Studies of participants with postpartum pain were included provided the pain investigated resulted from episiotomy or Caesarean section (with or without uterine cramp). Studies investigating participants with pain due to uterine cramps alone were excluded.
Types of interventions
Orally administered rofecoxib or matched placebo for relief of postoperative pain.
Types of outcome measures
Data collected included the following:
-
characteristics of participants;
-
pain model;
-
patient‐reported pain at baseline (physician, nurse, or carer reported pain was not included in the analysis);
-
patient‐reported pain relief and/or pain intensity expressed hourly over 4 to 6 hours using validated pain scales (pain intensity and pain relief in the form of VAS or categorical scales, or both), or reported total pain relief (TOTPAR) or summed pain intensity difference (SPID) at 4 to 6 hours;
-
patient‐reported global assessment of treatment (PGE), using a standard five‐point scale
-
number of participants using rescue medication, and the time of assessment;
-
time to use of rescue medication;
-
withdrawals ‐ all cause, adverse event;
-
adverse events ‐ participants experiencing one or more, and any serious adverse event, and the time of assessment.
Search methods for identification of studies
The following databases were searched:
-
Cochrane CENTRAL (Issue 1, 2002 for the original review, and Issue 2, 2009 for the update).
-
MEDLINE via Ovid (1966 to March 2002 for the original review, and June 2009 for the update).
-
EMBASE via Ovid (1980 to June 2009).
-
Biological Abstracts (1985 to Dec 2001 for the original review).
-
CINAHL (1982 to Dec 2001 for the original review).
-
PsycINFO (1967 to Jan 2002 for the original review).
-
PubMed (March 2001 for the original review).
-
Oxford Pain Database (Jadad 1996a) (June 2009).
Reference lists of retrieved articles were searched.
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, Appendix 3 for the Cochrane CENTRAL search strategy.
Language
No language restriction was applied.
Additional sources
Abstracts, conference proceedings and other grey literature were not searched. The manufacturer of rofecoxib (Merck & Company) provided details of unpublished studies for an earlier individual patient meta‐analysis (Edwards 2004), but were not contacted for this update.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the updated review. Disagreements were resolved by consensus or referral to a third review author.
Quality assessment
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b).
The scale used is as follows:
-
Is the study randomised? If yes, add one point.
-
Is the randomisation procedure reported and is it appropriate? If yes, add one point, if no, deduct one point.
-
Is the study double blind? If yes, add one point.
-
Is the double blind method reported and is it appropriate? If yes, add one point, if no, deduct one point.
-
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The results are described in the 'Methodological quality of included studies' section below, and 'Characteristics of included studies' table.
Data management
Data were extracted by two review authors and recorded on a standard data extraction form. Data suitable for pooling were entered into RevMan 5.0.
Data analysis
QUOROM guidelines were followed (Moher 1999). For efficacy analyses we used the number of participants in each treatment group who were randomised, received medication, and provided at least one post‐baseline assessment. For safety analyses we used number of participants who received study medication in each treatment group. Analyses were planned for different doses. Sensitivity analyses were planned to investigate any effect of pain model (dental versus other postoperative pain), trial size (39 or fewer versus 40 or more per treatment arm), and quality score (two versus three or more) on the primary outcome. A minimum of two studies and 200 participants were required for any analysis (Moore 1998).
Primary outcome
Number of participants achieving at least 50% pain relief
For each study, mean TOTPAR (total pain relief) or SPID (summed pain intensity difference) for active and placebo groups were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR was calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR for active treatment and placebo was then used to calculate relative benefit (RB) and number‐needed‐to‐treat‐to‐benefit (NNT).
Pain measures accepted for the calculation of TOTPAR or SPID were:
-
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
-
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
-
VAS for pain relief;
-
VAS for pain intensity.
If none of these measures were available, numbers of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" were taken as those achieving at least 50% pain relief (Collins 2001).
Further details of the scales and derived outcomes are in the glossary (Appendix 4).
Secondary outcomes:
1. Use of rescue medication
Numbers of participants requiring rescue medication were used to calculate relative risk (RR) and numbers needed to treat to prevent (NNTp) use of rescue medication for treatment and placebo groups. Median (or mean) time to use of rescue medication was used to calculate the weighted mean of the median (or mean) for the outcome. Weighting was by number of participants.
2. Adverse events
Numbers of participants reporting adverse events for each treatment group were used to calculate RR and numbers needed to treat to harm (NNH) estimates for:
-
any adverse event;
-
any serious adverse event (as reported in the study);
-
withdrawal due to an adverse event.
3. Withdrawals
Withdrawals for reasons other than lack of efficacy (participants using rescue medication ‐ see above) and adverse events were noted, as were exclusions from analysis where data were presented.
Relative benefit or risk estimates were calculated with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT, NNTp and NNH with 95% CI were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the relative benefit did not include the number one.
Homogeneity of studies was assessed visually (L'Abbe 1987). The z test (Tramer 1997) was used to determine if there was a significant difference between NNTs for different groups in the sensitivity analyses.
Results
Description of studies
Searches identified 33 potentially relevant studies. Twenty one reports of 27 studies were included in the review: seven studies were included in the original review (Chang 2001; Chang 2002; Ehrich 1999; Fricke 2002; Malmstrom 1999; Morrison 1999; Reicin 2001) and 20 were new to this update (Chang 2004; Chang 2005; Chelly 2007; Christensen 2004; Daniels 2006; Desjardins 2004; Desjardins 2007; Edwards 2004; Haglund 2006; Jackson 2004; Kellstein 2004; Korn 2004; Malmstrom 2002; Michael‐Hill 2006). Two studies are described in Daniels 2006, and six unpublished studies are described along with eight published studies (all included) in Edwards 2004. Six studies were excluded (Lee 2006; Ong 2005; Reuben 2000; Reuben 2002; Riest 2006; Zacharias 2004).
Rofecoxib 50 mg was used in one treatment arm in each study, and 500 mg in a second treatment arm in one study (Ehrich 1999). In total, 2636 participants were treated with rofecoxib 50 mg, 20 with rofecoxib 500 mg, and 1251 with placebo.
One study was carried out in participants with pain following major orthopaedic surgery (Reicin 2001), one in participants with pain following knee arthroscopy (Chelly 2007), and one in participants with pain following first metatarsal bunionectomy (Desjardins 2004). The remaining 24 studies were carried out in participants with pain following surgical extraction of one or more impacted third molars.
Study duration was 6 hours in one study (Ehrich 1999), 8 hours in three studies (Haglund 2006; Michael‐Hill 2006; Malmstrom 1999), 12 hours in one study (Reicin 2001), and 24 hours in the remainder of the studies.
Details are in the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Risk of bias in included studies
Methodological quality of included studies
All included studies were both randomised and double blind. Four studies were awarded a quality score of three (Daniels 2006; Ehrich 1999; Fricke 2002), eight a quality score of four (Chang 2001; Chang 2005; Chelly 2007; Jackson 2004; Kellstein 2004; Korn 2004; Morrison 1999; Reicin 2001), and the remaining 15 a quality score of five. Points were mainly lost due to inadequate description of the methods of randomisation and double blinding. One study lost a point for failing to report withdrawals and drop‐outs (Ehrich 1999). Details are in the 'Characteristics of included studies' table.
Effects of interventions
Two studies (Jackson 2004; Michael‐Hill 2006) did not contribute data to the primary outcome. The former study reported TOTPAR at 8 hours and provided insufficient data to allow recalculation over 4 to 6 hours. The latter study reported the data in a form that could not be used to calculate number of participants with 50% pain relief, and a request for further data from the manufacturer was not answered.
Number of participants achieving at least 50% pain relief
Rofecoxib 50 mg versus placebo
Twenty‐five studies with 3687 participants provided data (Chang 2001; Chang 2002; Chang 2004; Chang 2005; Chelly 2007; Christensen 2004; Daniels 2006; Desjardins 2004; Desjardins 2007; Edwards 2004; Ehrich 1999; Fricke 2002; Haglund 2006; Kellstein 2004; Korn 2004; Malmstrom 1999; Malmstrom 2002; Morrison 1999; Reicin 2001).
-
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with rofecoxib 50 mg was 58% (1458/2519; range 28% to 74%).
-
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with placebo was 11% (134/1169; range 2% to 35%).
-
The relative benefit of treatment compared with placebo was 5.1 (4.3 to 6.1), giving an NNT for at least 50% pain relief over 4 to 6 hours of 2.2 (2.0 to 2.3) (Analysis 1.1).
Only one study used a different dose of rofecoxib; 20 participants received a 500 mg dose (Ehrich 1999). No sensible analysis could be carried out.
Sensitivity analysis of primary outcome
Methodological quality
All studies had scores of three or more, so no sensitivity analysis could be carried out for this criterion.
Pain model: dental versus other surgery
Twenty two studies (six in Edwards 2004 and two in Daniels 2006) with 3060 participants were in dental surgery. The proportion of participants with at least 50% pain relief over 4 to 6 hours was 61% (1332/2173) with rofecoxib 50 mg and 8% (73/887) with placebo, giving an NNT of 1.9 (1.8 to 2.0).
Three studies (Chelly 2007; Desjardins 2004; Reicin 2001) with 628 participants were in other types of surgery (orthopaedic and bunionectomy). The proportion of participants with at least 50% pain relief over 4 to 6 hours was 37% (127/346) with rofecoxib 50 mg and 22% (62/282) with placebo, giving an NNT of 6.8 (4.6 to 13).
There was a statistically significant difference between pain models (z = 9.95, P < 0.00001). (Summary of results A; Analysis 1.1; Figure 1; Figure 2).
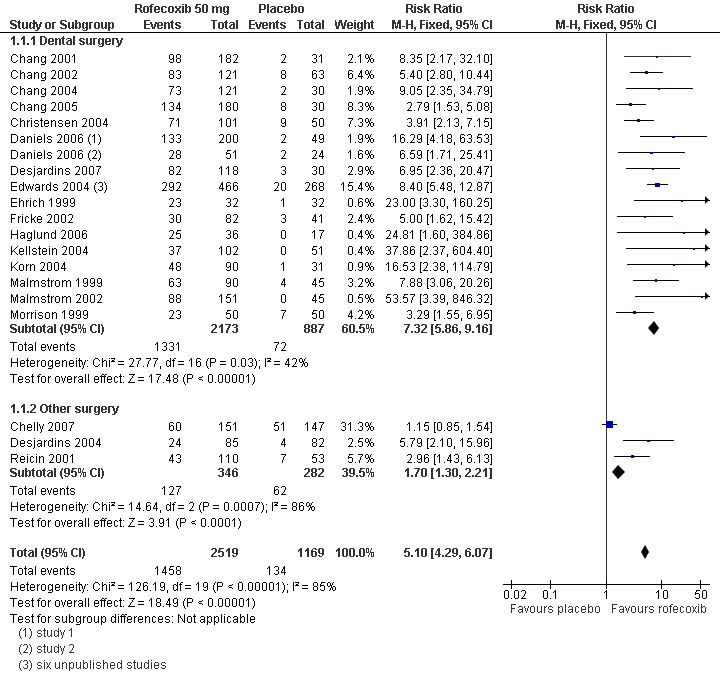
Forest plot of comparison: 1 Rofecoxib 50 mg v placebo, outcome: 1.5 Participants with at least 50% pain relief over 4 to 6 hours.
| Summary of results A: Number of participants with ≥50% pain relief over 4 to 6 hours | ||||||
| Dose (mg) | Surgery | Studies | Participants | Rofecoxib (%) | Placebo (%) | NNT (95%CI) |
| 50 | All | 25 | 3688 | 58 | 11 | 2.2 (2.0 to 2.3) |
| 50 | Dental | 22 | 3060 | 61 | 8 | 1.9 (1.8 to 2.0) |
| 50 | Other | 3 | 628 | 37 | 22 | 6.8 (4.6 to 13) |
Study size
There were insufficient data from studies with fewer than 40 participants in each treatment arm to carry out this analysis.
Formulation
Studies described administration of an "oral dose" of rofecoxib, or active comparator or placebo. In some studies rofecoxib and placebo were encapsulated to facilitate blinding, but there was no suggestion of a different formulation in any study. No studies reported using the oral suspension.
Use of rescue medication
Proportion of participants using rescue medication
Six studies reported this outcome after 6 or 8 hours (Chang 2002; Chang 2004; Chelly 2007; Daniels 2006; Desjardins 2007; Haglund 2006). All these studies used rofecoxib 50 mg. The proportion of participants using rescue medication was 27% (217/798) with rofecoxib 50 mg and 74% (268/360) with placebo, giving a number needed to treat to prevent remedication (NNTp) of 2.1 (1.9 to 2.4). After exclusion of the one study in non‐dental pain (Chelly 2007) the weighted mean proportion was 20% (129/647) with rofecoxib 50 mg, and 79% (169/213) with placebo, giving a NNTp of 1.7 (1.5 to 1.9).
Two studies (Daniels 2006) reported this outcome after 12 hours. Both were in dental pain and used rofecoxib 50 mg. The proportion using rescue medication was 32% (81/251) with rofecoxib 50 mg, and 89% (65/73) with placebo, giving a NNTp of 1.8 (1.5 to 2.1).
Eight studies reported this outcome after 24 hours (Chang 2005; Christensen 2004; Fricke 2002; Jackson 2004; Korn 2004; Malmstrom 1999; Malmstrom 2002; Morrison 1999). All these studies were in dental pain and used rofecoxib 50 mg. The proportion using rescue medication was 52% (404/782) with rofecoxib 50 mg, and 87% (290/333) with placebo, giving a NNTp of 2.8 (2.5 to 3.3).
Significantly fewer participants required rescue medication with rofecoxib 50 mg than placebo at all time points up to 24 hours (Summary of results B; Analysis 1.2; Analysis 1.3; Analysis 1.4).
The only study to use rofecoxib 500 mg (Ehrich 1999) did not report this outcome.
| Summary of results B: Number of participants using rescue medication | ||||||
| Time (h) | Surgery | Studies | Participants | Rofecoxib 50 mg (%) | Placebo (%) | NNTp (95%CI) |
| 6 to 8 | All | 7 | 1158 | 27 | 74 | 2.1 (1.9 to 2.4) |
| 6 to 8 | Dental | 6 | 860 | 20 | 79 | 1.7 (1.9 to 1.5) |
| 12 | Dental | 2 | 324 | 32 | 89 | 1.8 (1.5 to 2.1) |
| 24 | Dental | 8 | 1115 | 52 | 87 | 2.8 (2.5 to 3.3) |
Time to use of rescue medication
Twenty‐five studies reported the median time to use of rescue medication (Chang 2001; Chang 2002; Chang 2004; Chang 2005; Chelly 2007; Christensen 2004; Daniels 2006; Desjardins 2004; Desjardins 2007; Edwards 2004; Ehrich 1999; Jackson 2004; Kellstein 2004; Korn 2004; Malmstrom 1999; Malmstrom 2002; Michael‐Hill 2006; Morrison 1999; Reicin 2001). The weighted mean of the median time to use of rescue medication was 13.8 hours for rofecoxib 50 mg, and 1.9 hours for placebo for all studies combined, and 16.2 hours for rofecoxib 50 mg and 1.7 hours for placebo in dental studies only.
Adverse events
Any adverse event
Four studies did not provide any data for this outcome (Chang 2004; Ehrich 1999; Haglund 2006; Reicin 2001). Another study (Christensen 2004) did not present single dose adverse events data in a usable format. It was not always clear whether studies continued to collect data for adverse events after participants withdrew, for example due to lack of efficacy (took rescue medication). Most studies, including Haglund 2006, reported that the majority of adverse effects were either mild or moderate in severity.
One study (Michael‐Hill 2006) collected data over 8 hours, twelve (Chang 2002; Chang 2005; Christensen 2004; Daniels 2006; Desjardins 2004; Desjardins 2007; Fricke 2002; Jackson 2004; Kellstein 2004; Malmstrom 2002; Morrison 1999) over 24 hours, one (Chang 2001) over 10 days, and two (Chelly 2007; Korn 2004) over 14 days. The duration over which adverse event data was collected in the six unpublished studies in Edwards 2004 was not reported. There was no obvious difference in rates of adverse events in studies conducted over the different time periods.
-
When all studies were considered together the adverse event rate was 34% (750/2236) for rofecoxib 50 mg and 35% (409/1168) for placebo, giving a relative risk of 0.96 (0.87 to 1.06)
-
For dental studies only the adverse event rate was 34% (673/2000) for rofecoxib 50 mg and 36% (339/939) for placebo, giving a relative risk of 0.94 (0.85 to 1.04).
-
For non‐dental studies only (Chelly 2007; Desjardins 2004) the adverse event rate was 33% (77/236) for rofecoxib 50 mg and 31% (70/229) for placebo, giving a relative risk of 1.07 (0.82 to 1.4).
There was no difference between rofecoxib 50 mg and placebo (Summary of results C; Analysis 1.5).
The single study using rofecoxib 500 mg (Ehrich 1999) provided no usable adverse events data.
| Summary of results C: Participants with at least one adverse event following single dose rofecoxib 50 mg | |||||
| Pain model | Studies | Participants | Rofecoxib 50 mg (%) | Placebo (%) | NNH (95% CI) |
| Dental surgery | 13 | 2939 | 34 | 36 | Not calculated |
| Other surgery | 2 | 465 | 33 | 31 | Not calculated |
| All | 15 | 3404 | 34 | 35 | Not calculated |
Serious adverse events
Three studies reported serious adverse events (Chang 2002; Chelly 2007; Malmstrom 2002). An asthma flare occurred in a patient randomised to placebo (Chang 2002). Deep vein thrombosis occurred in two participants randomised to rofecoxib (Chelly 2007). Neither was considered related to the study drug. One participant (Malmstrom 2002) experienced appendicitis five days after receiving rofecoxib. Seven studies (Edwards 2004; Korn 2004) that provided adverse events data did not explicitly state whether any serious adverse events had occurred.
Withdrawls
Participants who took rescue medication were classified as withdrawals due to lack of efficacy. Details are reported under 'Use of rescue medication' above.
A small number of participants were excluded from efficacy analyses, but these are unlikely to have affected results. Four participants were lost to follow‐up (Chang 2005; Chelly 2007; Jackson 2004), six withdrew consent (Chelly 2007; Daniels 2006), two were subject to protocol violations (Fricke 2002; Haglund 2006), and nine did not provide data by failing to complete questionnaires or attend post‐study visits (Haglund 2006; Malmstrom 1999; Malmstrom 2002).
Nine participants withdrew due to mild or moderate adverse events (Desjardins 2004; Jackson 2004; Malmstrom 1999), and one participant withdrew to a serious adverse event (Chang 2002). This adverse event was an asthma flare.
No details about withdrawals were available for the six unpublished studies reported in Edwards 2004, although the studies were all scored as reporting withdrawal data. Reicin 2001 did not provide any single dose data on withdrawals.
Details of analgesia outcomes and use of rescue medication in individual studies are in Table 1, and of adverse events and withdrawals are in Table 2.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (hr) | % using |
| (1) Rofecoxib 50 mg, n = 182 (2) Paracetamol 600 mg plus codeine 60 mg, n = 180 (3) Placebo, n = 31 | TOTPAR 6: (1) 12.4 (2) 7.0 (3) 3.4 | (1) 98/182 (2) 23/180 (3) 2/31 | At 6 h: (1) 87/178 (2) 26/176 (3) 0/29 | (1) 9.6 (2) 2.3 (3) 1.6 | No data | |
| (1) Rofecoxib 50 mg, n = 121 (2) Diclofenac sodium 50 mg, n = 121 (3) Placebo, n = 63 | TOTPAR 6: (1) 14.5 (2) 6.9 (3) 4.5 | (1) 83/121 (2) 32/121 (3) 8/63 | At 8 h: (1) 79/121 (2) 21/121 (3) 6/63 | (1) >24:00 (2) 1.62 (3) 1.62 | At 8 h: (1) 19.8 (2) 66.9 (3) 76.2 | |
| (1) Rofecoxib 50 mg, n = 121 (2) Oxycodone/acetaminophen 10/650 mg, n = 120 (3) Placebo, n = 30 | TOTPAR 6: (1) 12.9 (2) 11.3 (3) 3.1 | (1) 73/121 (2) 61/120 (3) 2/30 | At 6 h: (1) 59/120 (2) 56/116 (3) 0/30 | (1) >6 (2) 5:05 (3) 1:37 | At 6 h: (1) 33.9 (2) 53.3 (3) 90.0 | |
| (1) Rofecoxib 50 mg, n = 180 (2) Codeine/acetaminophen 60/600 mg, n = 180 (3) Placebo, n = 30 | TOTPAR 6: (1) 15.5 (2) 10.7 (3) 6.7 | (1) 134/180 (2) 86/180 (3) 8/30 | At 6 h: (1) 124/180 (2) 68/180 (3) 3/30 | (1) >24 (2) 6.5 (3) 3.2 | At 24 h: (1) 43 (2) 86 (3) 73 | |
| (1) Rofecoxib 50 mg, n = 151 (2) Hydrocodone/acetaminophen 7.5/750 mg, n = 145 (3) Placebo, n = 147 | TOTPAR 6: (1) 9.3 (2) 10.7 (3) 8.4 | (1) 60/151 (2) 69/145 (3) 51/147 | At 6 h: (1) 91/151 (2) 99/147 (3) 76/147 | (1) 4.4 (2) 6.3 (3) 3.6 | At 6 h: (1) 58.3 (2) 47.9 (3) 67.1 | |
| (1) Rofecoxib 50 mg, n = 101 (2) Valdecoxib 40 mg, n = 99 (3) Placebo, n = 50 | TOTPAR 6: (1) 14.84 (2) 16.27 (3) 5.20 | (1) 71/101 (2) 78/99 (3) 9/50 | No usable data | (1) >24:00 (2) >24:00 (3) 2:10 | At 24 h: (1) 8.9 (2) 17.2 (3) 72.0 | |
| Daniels 2006 study 1 | (1) Rofecoxib 50 mg, n = 200 (2) Valdecoxib 20 mg, n = 201 (3) Placebo, n = 49 | TOTPAR 4: (1) 9.4 (2) 9.0 (3) 1.8 | (1) 133/200 (2) 127/201 (3) 2/49 | No usable data | (1) >24:00 (2) 23:58 (3) 2:04 | At 6 h: (1) 12.3 (2) 15.8 (3) 82.5 At 12 h: (1) 31.6 (2) 38.6 (3) 87.7 |
| Daniels 2006 study 2 | (1) Rofecoxib 50 mg, n = 51 (2) Valdecoxib 40 mg, n = 50 (3) Placebo, n = 24 | TOTPAR 4: (1) 8.0 (2) 8.7 (3) 2.4 | (1) 28/51 (2) 30/50 (3) 2/24 | No usable data | (1) >24:00 (2) >24:00 (3) 2:15 | At 6 h: (1) 17.5 (2) 21.1 (3) 84.2 At 12 h: (1) 35.1 (2) 29.8 (3) 93.0 |
| (1) Rofecoxib 50 mg, n = 85 (2) Diclofenac 100 mg, n = 85 (3) Placebo, n = 82 | TOTPAR 6: (1) 7.28 (2) 4.81 (3) 2.87 | (1) 24/85 (2) 13/85 (3) 4/82 | no usable data | (1) 4:02 (2) 2:03 (3) 1:41 | No data | |
| (1) Rofecoxib 50 mg, n = 118 (2) Oxycodone/acetaminophen 10/650 mg, n = 122 (3) Placebo, n = 30 | TOTPAR 6: (1) 14.6 (2) 12.8 (3) 3.9 | (1) 82/118 (2) 72/122 (3) 3/30 | No usable data | (1) >24:00 (2) >24:00 (3) 1:50 | At 6 h: (1) 20.3 (2) 37.7 (3) 73.3 | |
| Edwards 2004 trial 111 dental | (1) Rofecoxib 50 mg, n = 159 (2) Ibuprofen 400 mg, n = 53 (3) Placebo, n = 52 | No data | (1) 109/159 (2) 38/53 (3) 1/45 | No data | No data | No data |
| Edwards 2004 trial 154 dental | (1) Rofecoxib 50 mg, n = 91 (2) Acetaminophen/codeine 325/5 mg, n = 89 (3) Placebo, n = 30 | No data | (1) 60/91 (2) 17/89 (3) 4/30 | No data | No data | No data |
| Edwards 2004 trial 27 dental | (1) Rofecoxib 50 mg, n = 38 (2) Naproxen 550 mg, n = 39 (3) Placebo, n = 39 | No data | (1) 26/38 (2) 28/39 (3) 6/39 | No data | No data | No data |
| Edwards 2004 trial 51 dental | (1) Rofecoxib 50 mg, n = 72 (2) Naproxen 550 mg, n = 49 (3) Placebo, n = 48 | No data | (1) 40/72 (2) 24/49 (3) 3/48 | No data | No data | No data |
| Edwards 2004 trial 71 dental | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Placebo, n = 50 | No data | (1) 27/50 (2) 19/52 (3) 4/50 | No data | No data | No data |
| Edwards 2004 trial 84 dental | (1) Rofecoxib 50 mg, n = 56 (2) Ibuprofen 400 mg, n = 56 (3) Placebo, n = 56 | No data | (1) 30/56 (2) 30/56 (3) 2/56 | No data | No data | No data |
| Edwards 2004 all dental surgery | (1) Rofecoxib 50 mg (2) Placebo | (1) 15.5 (2) 1.6 | ||||
| Edwards 2004 all other surgery | (1) Rofecoxib 50 mg (2) Placebo | (1) 5.8 (2) 2.8 | ||||
| (1) Rofecoxib 50 mg, n = 32 (2) Rofecoxib 500 mg, n = 20 (3) Ibuprofen 400 mg, n = 20 (4) Placebo, n = 32 | TOTPAR 6: (1) 15.05 (2) 14.31 (3) 16.25 (4) 2.70 | (1) 23/32 (2) 14/20 (3) 16/20 (4) 1/32 | No usable data | (1) >6 (2) >6 (3) >6 (4) 1.6 | No usable data | |
| (1) Rofecoxib 50 mg, n = 82 (2) Valdecoxib 40 mg, n = 80 (3) Placebo, n = 41 | TOTPAR 6: (1) 8.6 (2) No usable data (3) 3.4 | (1) 30/82 (2) No usable data (3) 3/41 | No usable data | No data | At 24 h: (1) 58.5 (2) 40.0 (3) 85.4 | |
| (1) Rofecoxib 50 mg, n = 36 (2) Rofecoxib/acetaminophen 50 mg/1 g, n = 34 (3) Acetaminophen 1 g, n = 20 (4) Placebo, n = 17 | TOTPAR 6: (1) 14.5 (2) 16 (3) 11.5 (4) 0.25 | (1) 25/36 (2) 26/34 (3) 10/20 (4) 0/17 | No usable data | MEAN time to use of rescue medication (h) (1) 4.5 (2) 3.9 (3) 3.8 (4) 2.1 | At 8 h: (1) 16.7 (2) 8.8 (3) 40.0 (4) 70.6 | |
| (1) Rofecoxib 50 mg, n = 37 (2) Dexketoprofen trometamol 25 mg, n = 42 (3) Placebo, n = 41 | No usable data | No usable data | No usable data | (1) >24:00 (2) 6.6 (3) 2.5 | At 24 h: (1) 40.5 (2) 83.3 (3) 87.8 | |
| (1) Rofecoxib 50 mg, n = 102 (2) Lumiracoxib 400 mg, n = 101 (3) Celecoxib 200 mg, n = 101 (4) Placebo, n = 51 | TOTPAR 6: (1) 8.64 (2) 10.68 (3) 6.13 (4) 1.4 | (1) 37/102 (2) 48/101 (3) 23/101 (4) 0/51 | Rated excellent at 24 h: (1) 26/102 (2) 32/101 (3) 14/101 (4) 1/51 | (1) 3.8 (2) 7.2 (3) 2.0 (4) 1.3 | No usable data | |
| (1) Rofecoxib 50 mg, n = 90 (2) Oxycodone/acetaminophen 5/325 mg, n = 91 (3) Placebo, n = 31 | TOTPAR 4: (1) 7.2 (2) 4.0 (3) 1.2 TOTPAR 6: (1) 11.7 (2) 5.9 (3) 1.9 | (1) 48/90 (2) 13/91 (3) 1/31 | At 6 h: (1) 42/90 (2) 17/91 (3) 0/31 | (1) 8.3 (2) 3.3 (3) 1.7 | At 24 h: (1) 72.2 (2) 94.5 (3) 96.8 | |
| (1) Rofecoxib 50 mg, n = 90 (2) Celecoxib 200 mg, n = 91 (3) Ibuprofen 400 mg, n = 46 (4) Placebo, n = 45 | TOTPAR 6: (1) 13.17 (2) 9.55 (3) 12.85 (4) 3.07 | (1) 55/90 (2) 38/91 (3) 27/46 (4) 2/45 | At 8 h: (1) 50/90 (2) 26/91 (3) 23/46 (4) 3/45 | (1) >24 (2) 5.1 (3) 8.9 (4) 1.5 | At 24 h: (1) 78 (2) 49 (3) 76 (4) 91 | |
| (1) Rofecoxib 50 mg, n = 151 (2) Celecoxib 400 mg, n = 151 (3) Celecoxib 200 mg, n = 90 (4) Ibuprofen 400 mg, n = 45 (5) Placebo, n = 45 | TOTPAR 6: (1) 12.60 (2) 11.00 (3) 8.56 (4) 11.67 (5) 1.05 | (1) 88/151 (2) 75/151 (3) 32/90 (4) 24/45 (5) 0/45 | No usable data | (1) 15.9 (2) 10.6 (3) 6.8 (4) 10.0 (5) 1.6 | At 24 h: (1) 51.3 (2) 65.6 (3) 68.9 (4) 86.7 (5) 97.8 | |
| (1) Rofecoxib 50 mg, n = 80 (2) AZD3582 500 mg, n = 78 (3) AZD3582 750 mg, n = 83 (4) Placebo, n = 41 | No usable data | No usable data | No data | (1) 10.6 (2) 3.6 (3) 6.1 (4) 1.5 | No usable data | |
| (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 51 (3) Placebo, n = 50 | TOTPAR 6: (1) 10.12 (2) 9.26 (3) 4.15 | (1) 23/50 (2) 22/51 (3) 7/50 | No usable data | (1) 9.5 (2) 6.1 (3) 2.4 | At 24 h: (1) 84 (2) 56 (3) 92 | |
| (1) Rofecoxib 50 mg, n = 110 (2) Naproxen sodium 550 mg, n = 55 (3) Placebo, n = 53 | TOTPAR 6: (1) 10.7 (2) 9.8 (3) 5.4 | (1) 43/110 (2) 20/55 (3) 7/53 | No usable data | No data | No data | |
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other/Exclusions |
| (1) Rofecoxib 50 mg, n = 182 (2) Paracetamol 600 mg plus codeine 60 mg, n = 180 (3) Placebo, n = 31 | At 10 d: (1) 60/182 (2) 83/180 (3) 10/31 | None | (1) None (2) 1 vomited pill (3) None | None | |
| (1) Rofecoxib 50 mg, n = 121 (2) Diclofenac sodium 50 mg, n = 121 (3) Placebo, n = 63 | At 24 h: (1) 53/121 (2) 71/121 (3) 31/63 | (1) None (2) None (3) 1 asthma flare | (1) None (2) None (3) 1 asthma flare | None | |
| (1) Rofecoxib 50 mg, n = 121 (2) Oxycodone/acetaminophen 10/650 mg, n = 120 (3) Placebo, n = 30 | No single dose data | No single dose data | None | None | |
| (1) Rofecoxib 50 mg, n = 180 (2) Codeine/acetaminophen 60/600 mg, n = 180 (3) Placebo, n = 30 | At 24 h: (1) 40/180 (2) 63/180 (3) 14/30 | None | None | 1 participant lost to follow‐up | |
| (1) Rofecoxib 50 mg, n = 151 (2) Hydrocodone/acetaminophen 7.5/750 mg, n = 145 (3) Placebo, n = 147 | At 14 d: (1) 39/151 (2) 36/145 (3) 29/147 | Up to 14 d: (1) 2/151 both deep vein thrombosis (2) None (3) None | None | (1) 1 lost to follow‐up (2) 2 lost to follow‐up; 1 withdrew consent; 2 protocol deviations; 1 other unspecified (3) 4 withdrew consent | |
| (1) Rofecoxib 50 mg, n = 101 (2) Valdecoxib 40 mg, n = 99 (3) Placebo, n = 50 | At 24 h: 39‐44% of participants had ≥ 1 | None | None | None | |
| Daniels 2006 study 1 | (1) Rofecoxib 50 mg, n = 200 (2) Valdecoxib 20 mg, n = 201 (3) Placebo, n = 49 | At 24 h: (1) 79/200 (2) 74/201 (3) 24/49 | None | None | (1) 1 withdrew consent (2) None (3) None |
| Daniels 2006 study 2 | (1) Rofecoxib 50 mg, n = 51 (2) Valdecoxib 40 mg, n = 50 (3) Placebo, n = 24 | At 24 h: (1) 18/51 (2) 25/50 (3) 17/24 | None | None | (1) 1 withdrew consent (2) None (3) None |
| (1) Rofecoxib 50 mg, n = 85 (2) Diclofenac 100 mg, n = 85 (3) Placebo, n = 82 | No single dose data | None | (1) 3 (2) 1 (3) 4 | (1) None (2) 3 due to lack of efficacy (3) 3 due to lack of efficacy | |
| (1) Rofecoxib 50 mg, n = 118 (2) Oxycodone/acetaminophen 10/650 mg, n = 122 (3) Placebo, n = 30 | At 24 h: (1) 49/118 (2) No single dose data (3) 21/30 | None | (1) None (2) 1 due to syncope, headache, and vomiting (3) None | (1) None (2) 1 protocol deviation (3) None | |
| Edwards 2004 trial 111 dental | (1) Rofecoxib 50 mg, n = 159 (2) Ibuprofen 400 mg, n = 53 (3) Placebo, n = 52 | Time unknown (1) 52/159 (3) 28/52 | None | No data | No data |
| Edwards 2004 trial 154 dental | (1) Rofecoxib 50 mg, n = 91 (2) Acetaminophen/codeine 325/5 mg, n = 89 (3) Placebo, n = 30 | Time unknown (1) 36/91 (3) 14/30 | None | No data | No data |
| Edwards 2004 trial 27 dental | (1) Rofecoxib 50 mg, n = 38 (2) Naproxen 550 mg, n = 39 (3) Placebo, n = 39 | Time unknown (1) 17/38 (3) 12/39 | None | No data | No data |
| Edwards 2004 trial 51 dental | (1) Rofecoxib 50 mg, n = 72 (2) Naproxen 550 mg, n = 49 (3) Placebo, n = 48 | Time unknown (1) 34/72 (3) 13/48 | None | No data | No data |
| Edwards 2004 trial 71 dental | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Placebo, n = 50 | Time unknown (1) 21/50 (3) 25/50 | None | No data | No data |
| Edwards 2004 trial 84 dental | (1) Rofecoxib 50 mg, n = 56 (2) Ibuprofen 400 mg, n = 56 (3) Placebo, n = 56 | Time unknown (1) 19/56 (3) 22/56 | None | No data | No data |
| (1) Rofecoxib 50 mg, n = 32 (2) Rofecoxib 500 mg, n = 20 (3) Ibuprofen 400 mg, n = 20 (4) Placebo, n = 32 | No data | None reported | None | (1) 1 requested remedication before 90 min. (4) 1 requested remedication before 90 min. | |
| (1) Rofecoxib 50 mg, n = 82 (2) Valdecoxib 40 mg, n = 80 (3) Placebo, n = 41 | At 24 h: (1) 39/82 (2) 36/80 (3) 21/41 | None reported | None | (1) 1 protocol violation (2) None (3) None | |
| (1) Rofecoxib 50 mg, n = 36 (2) Rofecoxib/acetaminophen 50 mg/1 g, n = 34 (3) Acetaminophen 1 g, n = 20 (4) Placebo, n = 17 | No usable data | None | None | 1 protocol violation, 4 did not return questionnaire. Treatment groups not provided. | |
| (1) Rofecoxib 50 mg, n = 37 (2) Dexketoprofen trometamol 25 mg, n = 42 (3) Placebo, n= 41 | At 24 h: (1) 6/37 (2) 5/42 (3) 4/41 | None | None | (1) 1 not dosed due to nausea. (2) 2 lost to follow‐up. (3) None | |
| (1) Rofecoxib 50 mg, n = 102 (2) Lumiracoxib 400 mg, n = 101 (3) Celecoxib 200 mg, n = 101 (4) Placebo, n = 51 | At 24 h: (1) 12/102 (2) 21/101 (3) 20/101 (4) 9/51 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 90 (2) Oxycodone/acetaminophen 5/325 mg, n = 91 (3) Placebo, n = 31 | At 14 d: (1) 46/90 (2) 59/91 (3) 15/31 | No data | None reported | None | |
| (1) Rofecoxib 50 mg, n = 90 (2) Celecoxib 200 mg, n = 91 (3) Ibuprofen 400 mg, n = 46 (4) Placebo, n = 45 | No data | No data | (1) None (2) None (3) None (4) 1 bleeding | (1) None (2) None (3) None (4) 4 did not return for post‐study visit | |
| (1) Rofecoxib 50 mg, n = 151 (2) Celecoxib 400 mg, n = 151 (3) Celecoxib 200 mg, n = 90 (4) Ibuprofen 400 mg, n = 45 (5) Placebo, n = 45 | At 24 h: (1) 42/151 (2) 38/151 (3) 22/90 (4) 8/45 (5) 12/45 | (1) 1 appendicitis 5 days after dosing (2) None (3) 1 pregnancy 6 days after dosing. Spontaneous abortion 45 days later (4) None (5) None | None | (1) 1 did not provide any efficacy data (2) None (3) None (4) None (5) None | |
| (1) Rofecoxib 50 mg, n = 80 (2) AZD3582 500 mg, n = 78 (3) AZD3582 750 mg, n = 83 (4) Placebo, n = 41 | At 8 h: (1) 11/80 (2) 25/78 (3) 21/83 (4) 12/41 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 51 (3) Placebo, n = 50 | At 24 h: (1) 6/50 (2) 13/51 (3) 17/50 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 110 (2) Naproxen sodium 550 mg, n = 55 (3) Placebo, n = 53 | No single dose data | No single dose data | No single dose data | No single dose data | |
See Analysis 1.1 for analysis of primary outcome and Figure 2 for heterogeneity between studies in dental and other types of surgery. See Analysis 1.2, Analysis 1.3 and Analysis 1.4 for numbers of participants using rescue medication, and Analysis 1.5 for participants with any adverse event.

L'Abbé plot showing heterogeneity between studies in dental pain (yellow) and in other types of surgery (pink). Study size is proportional to size of circle (inset scale).
Discussion
This updated review includes 20 additional studies (Chang 2004; Chang 2005; Chelly 2007; Christensen 2004; Daniels 2006; Desjardins 2004; Desjardins 2007; Edwards 2004; Haglund 2006; Jackson 2004; Kellstein 2004; Korn 2004; Malmstrom 2002; Michael‐Hill 2006) and increases the amount of information available in comparisons with placebo from 982 to 3907 participants. All new studies used rofecoxib 50 mg. Studies were all of sufficiently high reporting quality to minimise reporting bias, and the amount of information such as to minimise any possible effect of publication bias. This updated review provides a more robust estimate of the primary outcome of at least 50% pain relief over 4 to 6 hours, considers differences in efficacy for different types of surgery, and analyses in greater detail the use of rescue analgesia, in terms of both numbers of participants using it and time to use.
The new information does not change the result for the primary outcome; the NNT for at least 50% pain relief over 4 to 6 hours with rofecoxib 50 mg compared to placebo was 2.2 (2.0 to 2.3). That is, one of every two participants treated with rofecoxib 50 mg will achieve at least 50% pain relief who would not have done so had they received placebo.
For studies in dental surgery only, the NNT for rofecoxib 50 mg compared to placebo was 1.9 (1.8 to 2.0), and for studies in other types of surgery the NNT was 6.8 (4.6 to 13). This difference is statistically significant (P < 0.00001). It has previously been difficult to demonstrate a difference in efficacy between dental and other types of surgery (Barden 2004), although a recent review of ibuprofen 400 mg with a very large data set did demonstrate superiority of analgesia in dental surgery compared with other types of surgery (Derry C 2009b), as did a recent review of diclofenac (Derry P 2009). It may be that there is indeed a difference, but previously other data sets have been too small to show statistical significance. In this review non‐dental surgery comprised major orthopaedic surgery, knee arthroscopy, and first metatarsal bunionectomy. Both the extent of the surgery and the context may influence the perception of pain and make this a highly heterogeneous group. Efficacy in bunionectomy in particular appears to be poor. There has never been sufficient data for any one type of non‐dental surgery to be compared with dental surgery, or indeed with other types of non‐dental surgery.
Indirect comparisons of NNTs for at least 50% pain relief over 4 to 6 hours in reviews of other analgesics using identical methods indicate that rofecoxib 50 mg has equivalent efficacy to acetaminophen and codeine 1000/60 mg (2.2 (1.8 to 2.9)) (Toms 2009). Rofecoxib 50 mg has superior efficacy to acetaminophen 1000 mg alone (3.6 (3.2 to 4.1)) (Toms 2008), ibuprofen 400 mg (2.5 (2.4 to 2.6)) (Derry C 2009b), naproxen 200 mg (3.4 (2.4 to 5.8)) (Derry C 2009a), and celecoxib 200 mg (3.2 (2.7 to 3.9)) (Derry 2008). Rofecoxib 50 mg appears inferior to etoricoxib 120 mg (1.9 (1.7 to 2.1)) (Clarke 2009) although the confidence intervals (CIs) overlap and the difference is very small. A current listing of reviews of analgesics in the single dose postoperative pain model can be found at www.medicine.ox.ac.uk/bandolier/index.html.
When prescribed to treat chronic osteoarthritis pain, rofecoxib was given at a dose of 12.5 mg daily, and increased if necessary to a maximum of 25 mg (BNF 2001). All of the studies included in this review of postoperative pain relief administered rofecoxib as a single dose of 50 mg, which is between two and four times the standard daily dose for regular chronic pain treatment. One study (Ehrich 1999) also included 20 participants who received rofecoxib 500 mg: this gave an NNT of 1.5 (1.1 to 2.2), based on extremely limited data. However, rofecoxib 500 mg is an experimental dose being 20 to 40 times the standard daily dose, and was never used in a clinical setting. As this was the only study to use a dose other than 50 mg, a dose‐response analysis could not be performed.
It has been suggested that data on use of rescue medication, whether as a proportion of participants requiring it, or the median time to use of it, might be helpful in assessing the usefulness of an analgesic (Moore 2005a). Data on remedication was inconsistently reported in the trials reviewed, but such analysis as was possible demonstrated that the weighted mean of the median time to use of rescue medication was 13.7 hours and 1.9 hours for rofecoxib 50 mg and placebo respectively for all studies combined, and 16.2 hours and 1.7 hours for dental studies only. This is the same as was found in the earlier review, which included only dental studies. For dental studies only, the weighted mean proportion of participants taking rescue medication within 6 to 8 hours was 20% with rofecoxib 50 mg, and 79% with placebo, giving a NNTp of 1.7 (1.5 to 1.9). This outcome measured at 24 hours was 52% with rofecoxib 50 mg and 87% with placebo, giving a NNTp of 2.8 (2.5 to 3.3).
Rofecoxib compares favourably with commonly used analgesics, such as paracetamol 1000 mg (Toms 2008; median time to rescue medication 3.8 hours, NNTp at 4 to 6 hours 5.2), ibuprofen 400 mg (Derry C 2009b; 5.6 hours, NNTp at 6 hours 2.7) and naproxen 500 mg (Derry C 2009a; 8.9 hours, NNTp at 12 hours 6.9), but is shorter than with etoricoxib 120 mg (Clarke 2009; > 20 hours, NNTp at 24 hours 2.4). In a postoperative setting, where patients may feel nauseated, a longer time before remedication is required, may be of benefit to the patient, and may also reduce time demands on nursing staff. Whether duration of action is a useful outcome by which to compare different analgesics awaits the completion of other reviews to allow examination of a more substantial body of evidence. It has also been suggested that rescue medication data might be useful in distinguishing between different doses of an analgesic (Moore 2005a), and indeed such differences have been found with ibuprofen (Derry C 2009b). Only one study included in this review used a dose of rofecoxib other than 50 mg (Ehrich 1999), however, and provided no data on the use of rescue medication.
Reporting of adverse events was more complete in these studies than for many older single dose analgesic studies. Adverse events were collected using various methods such as spontaneous reports, patient diaries or questionnaires, and monitoring of vital signs over different periods of time. This may have included periods after the use of rescue medication, which may have caused its own adverse events. Problems with analysis of reports of adverse events in acute pain trials have been noted before (Edwards 1999). The usefulness of single dose studies for assessing adverse events is questionable, but it is non‐the‐less reassuring that no increase in adverse events was demonstrated with rofecoxib 50 mg relative to placebo. Serious adverse events and adverse event withdrawals were rare, and generally not thought to be related to the test drug. The asthma flare leading to withdrawal reported by Chang 2002 occurred in a patient randomised to placebo. Long term multiple dose studies should be used for meaningful analysis of adverse events since, even in acute pain settings, analgesics are likely to be used in multiple doses. The difficulty in the postoperative setting is that there are many sequelae of surgery and anaesthesia that manifest as adverse events, such as nausea, vomiting, or abdominal discomfort, while others, like headache, can be caused by events such as acute caffeine withdrawal over the postoperative period. The main issue is that of rare but serious adverse events, and these are more likely to be found in large observational studies.
Loss of information from withdrawals or exclusions was small, and is unlikely to have led to an overestimate of efficacy because it is as likely to be related to poor reporting as poor methods. In single dose studies most exclusions occur for protocol violations such as failing to meet baseline pain requirements, or failing to return for post‐treatment visits after the acute pain results are concluded (McQuay 1982). For missing data it has been shown that over the 4 to 6 hour period, there is no difference between the baseline observation carried forward, which gives the more conservative estimate, and last observation carried forward (Moore 2005a).

Forest plot of comparison: 1 Rofecoxib 50 mg v placebo, outcome: 1.5 Participants with at least 50% pain relief over 4 to 6 hours.

L'Abbé plot showing heterogeneity between studies in dental pain (yellow) and in other types of surgery (pink). Study size is proportional to size of circle (inset scale).
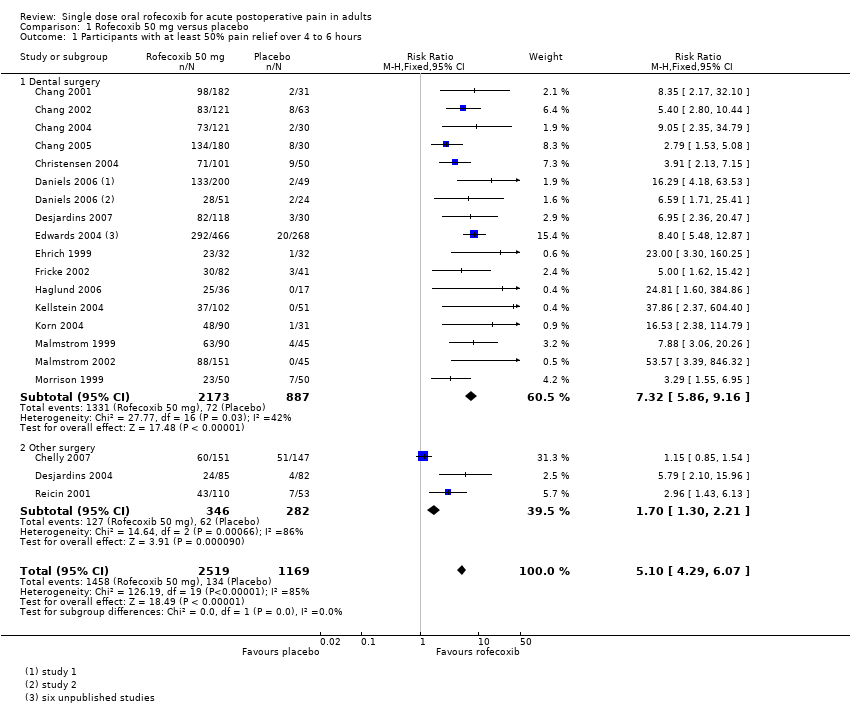
Comparison 1 Rofecoxib 50 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.

Comparison 1 Rofecoxib 50 mg versus placebo, Outcome 2 Participants using rescue medication at 6 to 8 hours.

Comparison 1 Rofecoxib 50 mg versus placebo, Outcome 3 Participants using rescue medication at 12 hours.

Comparison 1 Rofecoxib 50 mg versus placebo, Outcome 4 Participants using rescue medication at 24 hours.
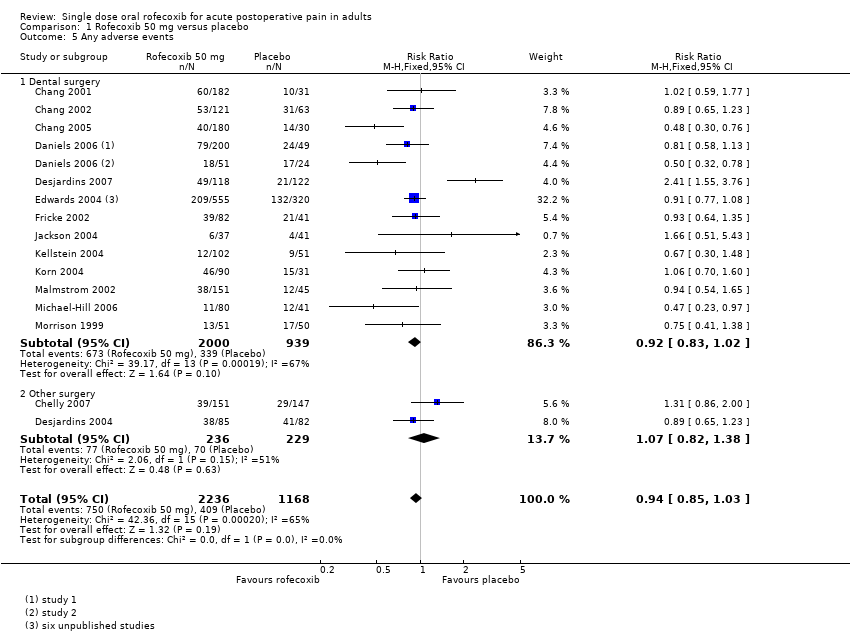
Comparison 1 Rofecoxib 50 mg versus placebo, Outcome 5 Any adverse events.

Comparison 2 Rofecoxib 500 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (hr) | % using |
| (1) Rofecoxib 50 mg, n = 182 (2) Paracetamol 600 mg plus codeine 60 mg, n = 180 (3) Placebo, n = 31 | TOTPAR 6: (1) 12.4 (2) 7.0 (3) 3.4 | (1) 98/182 (2) 23/180 (3) 2/31 | At 6 h: (1) 87/178 (2) 26/176 (3) 0/29 | (1) 9.6 (2) 2.3 (3) 1.6 | No data | |
| (1) Rofecoxib 50 mg, n = 121 (2) Diclofenac sodium 50 mg, n = 121 (3) Placebo, n = 63 | TOTPAR 6: (1) 14.5 (2) 6.9 (3) 4.5 | (1) 83/121 (2) 32/121 (3) 8/63 | At 8 h: (1) 79/121 (2) 21/121 (3) 6/63 | (1) >24:00 (2) 1.62 (3) 1.62 | At 8 h: (1) 19.8 (2) 66.9 (3) 76.2 | |
| (1) Rofecoxib 50 mg, n = 121 (2) Oxycodone/acetaminophen 10/650 mg, n = 120 (3) Placebo, n = 30 | TOTPAR 6: (1) 12.9 (2) 11.3 (3) 3.1 | (1) 73/121 (2) 61/120 (3) 2/30 | At 6 h: (1) 59/120 (2) 56/116 (3) 0/30 | (1) >6 (2) 5:05 (3) 1:37 | At 6 h: (1) 33.9 (2) 53.3 (3) 90.0 | |
| (1) Rofecoxib 50 mg, n = 180 (2) Codeine/acetaminophen 60/600 mg, n = 180 (3) Placebo, n = 30 | TOTPAR 6: (1) 15.5 (2) 10.7 (3) 6.7 | (1) 134/180 (2) 86/180 (3) 8/30 | At 6 h: (1) 124/180 (2) 68/180 (3) 3/30 | (1) >24 (2) 6.5 (3) 3.2 | At 24 h: (1) 43 (2) 86 (3) 73 | |
| (1) Rofecoxib 50 mg, n = 151 (2) Hydrocodone/acetaminophen 7.5/750 mg, n = 145 (3) Placebo, n = 147 | TOTPAR 6: (1) 9.3 (2) 10.7 (3) 8.4 | (1) 60/151 (2) 69/145 (3) 51/147 | At 6 h: (1) 91/151 (2) 99/147 (3) 76/147 | (1) 4.4 (2) 6.3 (3) 3.6 | At 6 h: (1) 58.3 (2) 47.9 (3) 67.1 | |
| (1) Rofecoxib 50 mg, n = 101 (2) Valdecoxib 40 mg, n = 99 (3) Placebo, n = 50 | TOTPAR 6: (1) 14.84 (2) 16.27 (3) 5.20 | (1) 71/101 (2) 78/99 (3) 9/50 | No usable data | (1) >24:00 (2) >24:00 (3) 2:10 | At 24 h: (1) 8.9 (2) 17.2 (3) 72.0 | |
| Daniels 2006 study 1 | (1) Rofecoxib 50 mg, n = 200 (2) Valdecoxib 20 mg, n = 201 (3) Placebo, n = 49 | TOTPAR 4: (1) 9.4 (2) 9.0 (3) 1.8 | (1) 133/200 (2) 127/201 (3) 2/49 | No usable data | (1) >24:00 (2) 23:58 (3) 2:04 | At 6 h: (1) 12.3 (2) 15.8 (3) 82.5 At 12 h: (1) 31.6 (2) 38.6 (3) 87.7 |
| Daniels 2006 study 2 | (1) Rofecoxib 50 mg, n = 51 (2) Valdecoxib 40 mg, n = 50 (3) Placebo, n = 24 | TOTPAR 4: (1) 8.0 (2) 8.7 (3) 2.4 | (1) 28/51 (2) 30/50 (3) 2/24 | No usable data | (1) >24:00 (2) >24:00 (3) 2:15 | At 6 h: (1) 17.5 (2) 21.1 (3) 84.2 At 12 h: (1) 35.1 (2) 29.8 (3) 93.0 |
| (1) Rofecoxib 50 mg, n = 85 (2) Diclofenac 100 mg, n = 85 (3) Placebo, n = 82 | TOTPAR 6: (1) 7.28 (2) 4.81 (3) 2.87 | (1) 24/85 (2) 13/85 (3) 4/82 | no usable data | (1) 4:02 (2) 2:03 (3) 1:41 | No data | |
| (1) Rofecoxib 50 mg, n = 118 (2) Oxycodone/acetaminophen 10/650 mg, n = 122 (3) Placebo, n = 30 | TOTPAR 6: (1) 14.6 (2) 12.8 (3) 3.9 | (1) 82/118 (2) 72/122 (3) 3/30 | No usable data | (1) >24:00 (2) >24:00 (3) 1:50 | At 6 h: (1) 20.3 (2) 37.7 (3) 73.3 | |
| Edwards 2004 trial 111 dental | (1) Rofecoxib 50 mg, n = 159 (2) Ibuprofen 400 mg, n = 53 (3) Placebo, n = 52 | No data | (1) 109/159 (2) 38/53 (3) 1/45 | No data | No data | No data |
| Edwards 2004 trial 154 dental | (1) Rofecoxib 50 mg, n = 91 (2) Acetaminophen/codeine 325/5 mg, n = 89 (3) Placebo, n = 30 | No data | (1) 60/91 (2) 17/89 (3) 4/30 | No data | No data | No data |
| Edwards 2004 trial 27 dental | (1) Rofecoxib 50 mg, n = 38 (2) Naproxen 550 mg, n = 39 (3) Placebo, n = 39 | No data | (1) 26/38 (2) 28/39 (3) 6/39 | No data | No data | No data |
| Edwards 2004 trial 51 dental | (1) Rofecoxib 50 mg, n = 72 (2) Naproxen 550 mg, n = 49 (3) Placebo, n = 48 | No data | (1) 40/72 (2) 24/49 (3) 3/48 | No data | No data | No data |
| Edwards 2004 trial 71 dental | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Placebo, n = 50 | No data | (1) 27/50 (2) 19/52 (3) 4/50 | No data | No data | No data |
| Edwards 2004 trial 84 dental | (1) Rofecoxib 50 mg, n = 56 (2) Ibuprofen 400 mg, n = 56 (3) Placebo, n = 56 | No data | (1) 30/56 (2) 30/56 (3) 2/56 | No data | No data | No data |
| Edwards 2004 all dental surgery | (1) Rofecoxib 50 mg (2) Placebo | (1) 15.5 (2) 1.6 | ||||
| Edwards 2004 all other surgery | (1) Rofecoxib 50 mg (2) Placebo | (1) 5.8 (2) 2.8 | ||||
| (1) Rofecoxib 50 mg, n = 32 (2) Rofecoxib 500 mg, n = 20 (3) Ibuprofen 400 mg, n = 20 (4) Placebo, n = 32 | TOTPAR 6: (1) 15.05 (2) 14.31 (3) 16.25 (4) 2.70 | (1) 23/32 (2) 14/20 (3) 16/20 (4) 1/32 | No usable data | (1) >6 (2) >6 (3) >6 (4) 1.6 | No usable data | |
| (1) Rofecoxib 50 mg, n = 82 (2) Valdecoxib 40 mg, n = 80 (3) Placebo, n = 41 | TOTPAR 6: (1) 8.6 (2) No usable data (3) 3.4 | (1) 30/82 (2) No usable data (3) 3/41 | No usable data | No data | At 24 h: (1) 58.5 (2) 40.0 (3) 85.4 | |
| (1) Rofecoxib 50 mg, n = 36 (2) Rofecoxib/acetaminophen 50 mg/1 g, n = 34 (3) Acetaminophen 1 g, n = 20 (4) Placebo, n = 17 | TOTPAR 6: (1) 14.5 (2) 16 (3) 11.5 (4) 0.25 | (1) 25/36 (2) 26/34 (3) 10/20 (4) 0/17 | No usable data | MEAN time to use of rescue medication (h) (1) 4.5 (2) 3.9 (3) 3.8 (4) 2.1 | At 8 h: (1) 16.7 (2) 8.8 (3) 40.0 (4) 70.6 | |
| (1) Rofecoxib 50 mg, n = 37 (2) Dexketoprofen trometamol 25 mg, n = 42 (3) Placebo, n = 41 | No usable data | No usable data | No usable data | (1) >24:00 (2) 6.6 (3) 2.5 | At 24 h: (1) 40.5 (2) 83.3 (3) 87.8 | |
| (1) Rofecoxib 50 mg, n = 102 (2) Lumiracoxib 400 mg, n = 101 (3) Celecoxib 200 mg, n = 101 (4) Placebo, n = 51 | TOTPAR 6: (1) 8.64 (2) 10.68 (3) 6.13 (4) 1.4 | (1) 37/102 (2) 48/101 (3) 23/101 (4) 0/51 | Rated excellent at 24 h: (1) 26/102 (2) 32/101 (3) 14/101 (4) 1/51 | (1) 3.8 (2) 7.2 (3) 2.0 (4) 1.3 | No usable data | |
| (1) Rofecoxib 50 mg, n = 90 (2) Oxycodone/acetaminophen 5/325 mg, n = 91 (3) Placebo, n = 31 | TOTPAR 4: (1) 7.2 (2) 4.0 (3) 1.2 TOTPAR 6: (1) 11.7 (2) 5.9 (3) 1.9 | (1) 48/90 (2) 13/91 (3) 1/31 | At 6 h: (1) 42/90 (2) 17/91 (3) 0/31 | (1) 8.3 (2) 3.3 (3) 1.7 | At 24 h: (1) 72.2 (2) 94.5 (3) 96.8 | |
| (1) Rofecoxib 50 mg, n = 90 (2) Celecoxib 200 mg, n = 91 (3) Ibuprofen 400 mg, n = 46 (4) Placebo, n = 45 | TOTPAR 6: (1) 13.17 (2) 9.55 (3) 12.85 (4) 3.07 | (1) 55/90 (2) 38/91 (3) 27/46 (4) 2/45 | At 8 h: (1) 50/90 (2) 26/91 (3) 23/46 (4) 3/45 | (1) >24 (2) 5.1 (3) 8.9 (4) 1.5 | At 24 h: (1) 78 (2) 49 (3) 76 (4) 91 | |
| (1) Rofecoxib 50 mg, n = 151 (2) Celecoxib 400 mg, n = 151 (3) Celecoxib 200 mg, n = 90 (4) Ibuprofen 400 mg, n = 45 (5) Placebo, n = 45 | TOTPAR 6: (1) 12.60 (2) 11.00 (3) 8.56 (4) 11.67 (5) 1.05 | (1) 88/151 (2) 75/151 (3) 32/90 (4) 24/45 (5) 0/45 | No usable data | (1) 15.9 (2) 10.6 (3) 6.8 (4) 10.0 (5) 1.6 | At 24 h: (1) 51.3 (2) 65.6 (3) 68.9 (4) 86.7 (5) 97.8 | |
| (1) Rofecoxib 50 mg, n = 80 (2) AZD3582 500 mg, n = 78 (3) AZD3582 750 mg, n = 83 (4) Placebo, n = 41 | No usable data | No usable data | No data | (1) 10.6 (2) 3.6 (3) 6.1 (4) 1.5 | No usable data | |
| (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 51 (3) Placebo, n = 50 | TOTPAR 6: (1) 10.12 (2) 9.26 (3) 4.15 | (1) 23/50 (2) 22/51 (3) 7/50 | No usable data | (1) 9.5 (2) 6.1 (3) 2.4 | At 24 h: (1) 84 (2) 56 (3) 92 | |
| (1) Rofecoxib 50 mg, n = 110 (2) Naproxen sodium 550 mg, n = 55 (3) Placebo, n = 53 | TOTPAR 6: (1) 10.7 (2) 9.8 (3) 5.4 | (1) 43/110 (2) 20/55 (3) 7/53 | No usable data | No data | No data | |
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other/Exclusions |
| (1) Rofecoxib 50 mg, n = 182 (2) Paracetamol 600 mg plus codeine 60 mg, n = 180 (3) Placebo, n = 31 | At 10 d: (1) 60/182 (2) 83/180 (3) 10/31 | None | (1) None (2) 1 vomited pill (3) None | None | |
| (1) Rofecoxib 50 mg, n = 121 (2) Diclofenac sodium 50 mg, n = 121 (3) Placebo, n = 63 | At 24 h: (1) 53/121 (2) 71/121 (3) 31/63 | (1) None (2) None (3) 1 asthma flare | (1) None (2) None (3) 1 asthma flare | None | |
| (1) Rofecoxib 50 mg, n = 121 (2) Oxycodone/acetaminophen 10/650 mg, n = 120 (3) Placebo, n = 30 | No single dose data | No single dose data | None | None | |
| (1) Rofecoxib 50 mg, n = 180 (2) Codeine/acetaminophen 60/600 mg, n = 180 (3) Placebo, n = 30 | At 24 h: (1) 40/180 (2) 63/180 (3) 14/30 | None | None | 1 participant lost to follow‐up | |
| (1) Rofecoxib 50 mg, n = 151 (2) Hydrocodone/acetaminophen 7.5/750 mg, n = 145 (3) Placebo, n = 147 | At 14 d: (1) 39/151 (2) 36/145 (3) 29/147 | Up to 14 d: (1) 2/151 both deep vein thrombosis (2) None (3) None | None | (1) 1 lost to follow‐up (2) 2 lost to follow‐up; 1 withdrew consent; 2 protocol deviations; 1 other unspecified (3) 4 withdrew consent | |
| (1) Rofecoxib 50 mg, n = 101 (2) Valdecoxib 40 mg, n = 99 (3) Placebo, n = 50 | At 24 h: 39‐44% of participants had ≥ 1 | None | None | None | |
| Daniels 2006 study 1 | (1) Rofecoxib 50 mg, n = 200 (2) Valdecoxib 20 mg, n = 201 (3) Placebo, n = 49 | At 24 h: (1) 79/200 (2) 74/201 (3) 24/49 | None | None | (1) 1 withdrew consent (2) None (3) None |
| Daniels 2006 study 2 | (1) Rofecoxib 50 mg, n = 51 (2) Valdecoxib 40 mg, n = 50 (3) Placebo, n = 24 | At 24 h: (1) 18/51 (2) 25/50 (3) 17/24 | None | None | (1) 1 withdrew consent (2) None (3) None |
| (1) Rofecoxib 50 mg, n = 85 (2) Diclofenac 100 mg, n = 85 (3) Placebo, n = 82 | No single dose data | None | (1) 3 (2) 1 (3) 4 | (1) None (2) 3 due to lack of efficacy (3) 3 due to lack of efficacy | |
| (1) Rofecoxib 50 mg, n = 118 (2) Oxycodone/acetaminophen 10/650 mg, n = 122 (3) Placebo, n = 30 | At 24 h: (1) 49/118 (2) No single dose data (3) 21/30 | None | (1) None (2) 1 due to syncope, headache, and vomiting (3) None | (1) None (2) 1 protocol deviation (3) None | |
| Edwards 2004 trial 111 dental | (1) Rofecoxib 50 mg, n = 159 (2) Ibuprofen 400 mg, n = 53 (3) Placebo, n = 52 | Time unknown (1) 52/159 (3) 28/52 | None | No data | No data |
| Edwards 2004 trial 154 dental | (1) Rofecoxib 50 mg, n = 91 (2) Acetaminophen/codeine 325/5 mg, n = 89 (3) Placebo, n = 30 | Time unknown (1) 36/91 (3) 14/30 | None | No data | No data |
| Edwards 2004 trial 27 dental | (1) Rofecoxib 50 mg, n = 38 (2) Naproxen 550 mg, n = 39 (3) Placebo, n = 39 | Time unknown (1) 17/38 (3) 12/39 | None | No data | No data |
| Edwards 2004 trial 51 dental | (1) Rofecoxib 50 mg, n = 72 (2) Naproxen 550 mg, n = 49 (3) Placebo, n = 48 | Time unknown (1) 34/72 (3) 13/48 | None | No data | No data |
| Edwards 2004 trial 71 dental | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Placebo, n = 50 | Time unknown (1) 21/50 (3) 25/50 | None | No data | No data |
| Edwards 2004 trial 84 dental | (1) Rofecoxib 50 mg, n = 56 (2) Ibuprofen 400 mg, n = 56 (3) Placebo, n = 56 | Time unknown (1) 19/56 (3) 22/56 | None | No data | No data |
| (1) Rofecoxib 50 mg, n = 32 (2) Rofecoxib 500 mg, n = 20 (3) Ibuprofen 400 mg, n = 20 (4) Placebo, n = 32 | No data | None reported | None | (1) 1 requested remedication before 90 min. (4) 1 requested remedication before 90 min. | |
| (1) Rofecoxib 50 mg, n = 82 (2) Valdecoxib 40 mg, n = 80 (3) Placebo, n = 41 | At 24 h: (1) 39/82 (2) 36/80 (3) 21/41 | None reported | None | (1) 1 protocol violation (2) None (3) None | |
| (1) Rofecoxib 50 mg, n = 36 (2) Rofecoxib/acetaminophen 50 mg/1 g, n = 34 (3) Acetaminophen 1 g, n = 20 (4) Placebo, n = 17 | No usable data | None | None | 1 protocol violation, 4 did not return questionnaire. Treatment groups not provided. | |
| (1) Rofecoxib 50 mg, n = 37 (2) Dexketoprofen trometamol 25 mg, n = 42 (3) Placebo, n= 41 | At 24 h: (1) 6/37 (2) 5/42 (3) 4/41 | None | None | (1) 1 not dosed due to nausea. (2) 2 lost to follow‐up. (3) None | |
| (1) Rofecoxib 50 mg, n = 102 (2) Lumiracoxib 400 mg, n = 101 (3) Celecoxib 200 mg, n = 101 (4) Placebo, n = 51 | At 24 h: (1) 12/102 (2) 21/101 (3) 20/101 (4) 9/51 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 90 (2) Oxycodone/acetaminophen 5/325 mg, n = 91 (3) Placebo, n = 31 | At 14 d: (1) 46/90 (2) 59/91 (3) 15/31 | No data | None reported | None | |
| (1) Rofecoxib 50 mg, n = 90 (2) Celecoxib 200 mg, n = 91 (3) Ibuprofen 400 mg, n = 46 (4) Placebo, n = 45 | No data | No data | (1) None (2) None (3) None (4) 1 bleeding | (1) None (2) None (3) None (4) 4 did not return for post‐study visit | |
| (1) Rofecoxib 50 mg, n = 151 (2) Celecoxib 400 mg, n = 151 (3) Celecoxib 200 mg, n = 90 (4) Ibuprofen 400 mg, n = 45 (5) Placebo, n = 45 | At 24 h: (1) 42/151 (2) 38/151 (3) 22/90 (4) 8/45 (5) 12/45 | (1) 1 appendicitis 5 days after dosing (2) None (3) 1 pregnancy 6 days after dosing. Spontaneous abortion 45 days later (4) None (5) None | None | (1) 1 did not provide any efficacy data (2) None (3) None (4) None (5) None | |
| (1) Rofecoxib 50 mg, n = 80 (2) AZD3582 500 mg, n = 78 (3) AZD3582 750 mg, n = 83 (4) Placebo, n = 41 | At 8 h: (1) 11/80 (2) 25/78 (3) 21/83 (4) 12/41 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 51 (3) Placebo, n = 50 | At 24 h: (1) 6/50 (2) 13/51 (3) 17/50 | None | None | None | |
| (1) Rofecoxib 50 mg, n = 110 (2) Naproxen sodium 550 mg, n = 55 (3) Placebo, n = 53 | No single dose data | No single dose data | No single dose data | No single dose data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 19 | 3688 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.10 [4.29, 6.07] |
| 1.1 Dental surgery | 16 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.32 [5.86, 9.16] |
| 1.2 Other surgery | 3 | 628 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.30, 2.21] |
| 2 Participants using rescue medication at 6 to 8 hours Show forest plot | 6 | 1158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.38, 0.48] |
| 2.1 Dental surgery | 5 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.21, 0.30] |
| 2.2 Other surgery | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.03] |
| 3 Participants using rescue medication at 12 hours Show forest plot | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.45] |
| 3.1 Dental surgery | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.45] |
| 4 Participants using rescue medication at 24 hours Show forest plot | 8 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.57, 0.66] |
| 4.1 Dental surgery | 8 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.57, 0.66] |
| 5 Any adverse events Show forest plot | 15 | 3404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 5.1 Dental surgery | 13 | 2939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 5.2 Other surgery | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with at least 50% pain relief over 4 to 6 hours Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.0 [3.06, 47.01] |

