Pemberian cecair dan makanan lebih awal berbanding dengan lewat untuk mengurangkan komplikasi selepas pembedahan abdominal ginekologikal major
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A prospective randomised controlled study in a single institution. Participants were randomised by using consecutively‐numbered, sealed, opaque envelopes according to the list generated from a random number table. Power calculation was performed a priori. | |
| Participants | Inclusion criteria: Women scheduled for major abdominal gynaecologic surgery; mean age: 40.8 years (early group), 41.1 years (delayed feeding group) | |
| Interventions | Early group: Participants were allowed to have sips of water within 8 hours after surgery. They were started on a soft diet in the morning of the 1st postoperative day and proceeded to a regular solid diet on the 2nd postoperative day Discharge criteria included the tolerance of a solid diet, passing flatus, and the discontinuance of intravenous fluids and medications. Participants were not required to have had a bowel movement. | |
| Outcomes | Hospital stay Gastrointestinal information | |
| Notes | Funding source: self‐funded Conflicts of interest: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by using consecutively numbered, sealed, opaque envelopes according to the list generated from a random number table." Comment: Random number table |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised by using consecutively numbered, sealed, opaque envelopes according to the list generated from a random number table." Comment: Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: The outcome assessors were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding to study outcomes was unclear. |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. | |
| Participants | Inclusion criteria: Gynecologic oncology patients aged 18 ‐ 75 years, undergoing laparotomy with associated intestinal resection; median age: 54 years (early group), 58 years (delayed feeding group). No significant difference in patient characteristics and surgical variables between the two groups except for a higher estimated blood loss in the delayed feeding group (median 800 ml vs. 300 ml). | |
| Interventions | Early group: Participants were offered liquids, mineral water (no gas), tea, chamomile infusion, or apple juice during the first 24 hours. If no nausea and vomiting, a regular diet of boiled or grilled beef, chicken, or fish was given starting on day 1 and continued for the entire hospital stay All participants underwent bowel preparation and preoperative antibiotics prophylaxis. In addition, a nasogastric tube was placed in all participants during surgery and was removed after the surgery finished. Postoperative analgesia was given via epidural catheter for 3 days (ropivacaine and fentanyl) or as intravascular continuous administration of ketorolac and tramadol in those without an epidural catheter. | |
| Outcomes | Hospital stay Recovery of bowel activity (time to first passage of gas and stool, time to tolerance of a solid diet) Other morbidities (wound infection, abdominal abscess, pneumonia, urinary tract infection, bacteraemia, wound dehiscence, marked postoperative bleeding, anastomotic leak, respiratory failure, cardiovascular instability, renal dysfunction, thromboembolic complications) Participants' satisfaction level and quality of life Analgesic and antiemetic drug requirements | |
| Notes | Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using the web‐based TENALEA randomization system (https://it.tenalea.net/ieo)." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized using the web‐based TENALEA randomization system (https://it.tenalea.net/ieo)." Comment: Central web‐based allocation |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay, subjective intestinal morbidities, participants' satisfaction level and quality of life) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Nursing staff, the primary outcome assessors, were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding on study outcomes was unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "11 were subsequently excluded (after randomisation) due to postoperative evidence of nongynecologic malignancy (n=3) and admission to ICU for more than 24 h (n=8)." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol was published in www.clinicaltrials.gov. The reported outcomes corresponded to those listed in the registered protocol. |
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. | |
| Participants | Inclusion criteria: Gynecologic oncology patients aged 18 ‐ 75 years, undergoing laparotomy; mean age: 54 years (early group), 57 years (delayed feeding group). The majority of participants had ovarian malignancy, 59% in the early group and 57% in the delayed feeding group.Pelvic and aortic lymphadenectomy were performed in > 70% and in almost 50% of participants, respectively. No significant difference in participant characteristics between the two groups. | |
| Interventions | Early group: Participants were offered liquids, mineral water (no gas), tea, chamomile infusion, or apple juice during the first 24 hours. If no nausea and vomiting, a regular diet of boiled or grilled beef, chicken, or fish was given starting on day 1 and continued for the entire hospital stay. All participants underwent bowel preparation and preoperative antibiotics prophylaxis. In addition, a nasogastric tube was placed in all participants during surgery and was removed after the surgery finished. All participants received general anaesthesia. Postoperative analgesia was given via epidural catheter for 3 days (ropivacaine and fentanyl) or as intravascular continuous administration of ketorolac and tramadol in those without an epidural catheter. | |
| Outcomes | Hospital stay Recovery of bowel activity (time to first passage of gas and stool, time to tolerance for solid diet) Other morbidities (wound infection, abdominal abscess, pneumonia, urinary tract infection, bacteraemia, wound dehiscence, marked postoperative bleeding, anastomotic leak, respiratory failure, cardiovascular instability, renal dysfunction, thromboembolic complications) Participants' satisfaction level and quality of life Analgesic and antiemetic drug requirements | |
| Notes | Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized by means of the Web‐based Tenalea randomization system (https://it.tenalea.net/ieo)." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized by means of the Web‐based Tenalea randomization system (https://it.tenalea.net/ieo)." Comment: Central web‐based allocation |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay, subjective intestinal morbidities, participants' satisfaction level and quality of life) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Nursing staff, the primary outcome assessors, were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding on study outcomes was unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Twenty‐four patients (12 for each group) were subsequently excluded as a result of benign gynecologic pathology, nongynecologic pathology, and admission to the intensive care unit for > 24 h." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol was published in www.clinicaltrials.gov. The reported outcomes corresponded to those listed in the registered protocol. |
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. | |
| Participants | Inclusion criteria: All gynaecologic oncology patients undergoing non laparoscopic intra‐abdominal surgery | |
| Interventions | Early group: Participants began a clear liquid diet on the 1st postoperative day and then advanced to a regular diet as tolerated. | |
| Outcomes | Gastrointestinal information Other morbidities (febrile morbidity, pneumonia, wound complications, atelectasis) Hospital stay | |
| Notes | Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using a computer‐generated random number list." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation. It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: The outcome assessors were not blinded (information from the study's author). |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Five patients were nonevaluable, three in the early feeding group and two in the delayed feeding group. Of these, one patient in each group died of multiorgan system failure within 36 hours of surgery. The remaining patients had inoperable bowel obstructions, received gastrostomy tubes, and were placed on hospice care." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across groups, with similar reasons for missing data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
| Methods | A prospective randomised controlled study in a single institution. Participants were randomised by the clinic nurses according to a computer‐generated random number list. Power calculation was performed a priori. | |
| Participants | Inclusion criteria: Gynaecologic oncology and uro‐gynaecology patients undergoing laparotomy; mean age: 50 years (early group), 52 years (delayed feeding group) | |
| Interventions | Early group: Participants received clear fluids on the 1st postoperative day. After 500 ml of clear fluids was tolerated, a regular solid diet was given. | |
| Outcomes | Hospital stay Gastrointestinal information Other morbidities (wound infection, deep venous thrombosis, urinary tract infection, pneumonia, pulmonary oedema) | |
| Notes | Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were prospectively randomized with a computer‐generated random number list." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | High risk | Comment: Open random number list |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation. It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: The outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Seven women were excluded because of intraoperative injury of the gastrointestinal tract, and 4 patients were excluded because of self‐withdrawal." Comment: Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study compared early feeding with nasogastric decompression after major gynaecologic oncology surgery, not early versus delayed (traditional) feeding schedule. | |
| This study compared different doses of alvimopan and placebo after partial colectomy or simple/radical hysterectomy, not early versus delayed (traditional) feeding schedule. | |
| This is a non‐comparative study on effects of aggressive postradical hysterectomy bowel stimulation, which consisted of milk of magnesia and bis colic suppositories. | |
| This is a non‐comparative study that examined the effects of immediate postoperative feeding and bowel stimulation in 707 women who had major gynaecologic operations over a 5‐year period. | |
| This is a randomised controlled study that compared a semiliquid diet with clear feeds, both started at 6 hours after major abdominal gynaecological oncology surgery. The types of diet for early feeding were compared, not the timing. | |
| This study compared administration of water‐soluble, hyperosmolar, radio‐opaque contrast material and conventional management after gynaecologic surgery, not early versus delayed (traditional) feeding schedule. | |
| This study compared a high‐fibre diet plan or their usual diet after radical hysterectomy, not early versus delayed (traditional) feeding schedule. | |
| This is a non‐comparative study on effects of aggressive postradical hysterectomy bowel stimulation with oral 66% sodium phosphate solution. | |
| Participants had several different types of major gynaecologic surgeries, including abdominal and vaginal approaches, and were randomly allocated to feeding groups regardless of approach. | |
| This study compared a regular diet with clear liquid as the first meal after intra‐abdominal gynaecologic oncology surgery, not early versus delayed (traditional) feeding schedule. | |
| Although this prospective study compared early versus delayed (traditional) feeding after gynaecological surgery, the design was quasi‐randomisation. | |
| This study compared different doses of ADL 8‐2698, an investigational opioid antagonist and placebo after partial colectomy or simple hysterectomy, not early versus delayed (traditional) feeding schedule. | |
| This is a randomised controlled study that compared 8 different combinations of postoperative interventions including gum chewing, early oral hydration, and early mobilisation following abdominal gynaecologic surgery. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
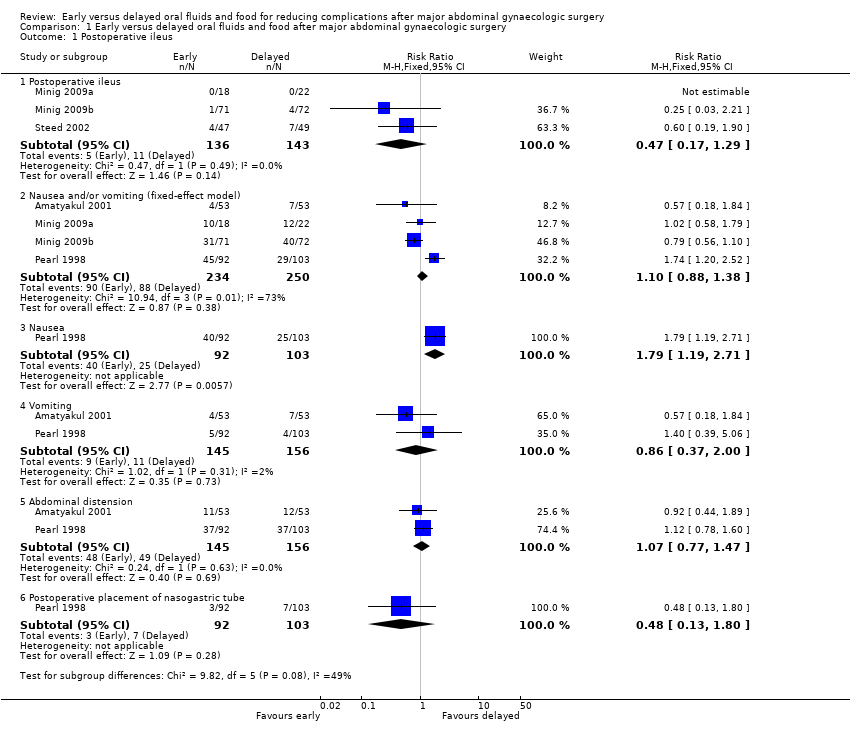
| 1 Postoperative ileus Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 1 Postoperative ileus. | ||||
| 1.1 Postoperative ileus | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.29] |
| 1.2 Nausea and/or vomiting (fixed‐effect model) | 4 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 1.3 Nausea | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.19, 2.71] |
| 1.4 Vomiting | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.00] |
| 1.5 Abdominal distension | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.47] |
| 1.6 Postoperative placement of nasogastric tube | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.80] |
| 2 Time intervals (fixed‐effect model) [days] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 ![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 2 Time intervals (fixed‐effect model) [days].](/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-02.png) Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 2 Time intervals (fixed‐effect model) [days]. | ||||
| 2.1 Time to the presence of bowel sound (fixed‐effect model) [days] | 2 | 338 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.11] |
| 2.2 Time to the passage of flatus [days] | 3 | 444 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.01] |
| 2.3 Time to the first solid diet (fixed‐effect model) [days] | 2 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.34, ‐1.05] |
| 2.4 Time to the first passage of stool [days] | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.09] |
| 2.5 Hospital stay (fixed‐effect model) [days] | 4 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.83, ‐0.35] |
| 3 Other major postoperative complications Show forest plot | 4 | 1286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| Analysis 1.3  Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 3 Other major postoperative complications. | ||||
| 3.1 Febrile morbidity | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 3.2 Infectious complications | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.73] |
| 3.3 Wound complications | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 3.4 Pneumonia | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.73] |
| 4 Satisfaction visual analog scale [mm] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 ![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 4 Satisfaction visual analog scale [mm].](/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-04.png) Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 4 Satisfaction visual analog scale [mm]. | ||||
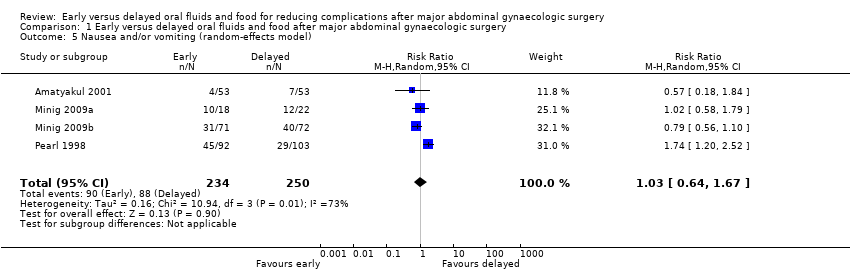
| 5 Nausea and/or vomiting (random‐effects model) Show forest plot | 4 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.67] |
| Analysis 1.5 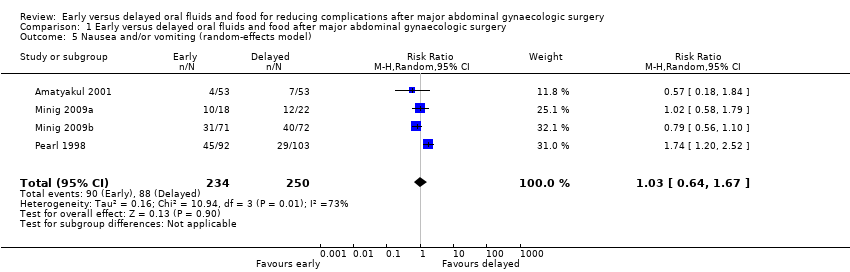 Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 5 Nausea and/or vomiting (random‐effects model). | ||||
| 6 Time to the presence of bowel sound (random‐effects model) [days] Show forest plot | 2 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.61, ‐0.03] |
| Analysis 1.6 ![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 6 Time to the presence of bowel sound (random‐effects model) [days].](/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-06.png) Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 6 Time to the presence of bowel sound (random‐effects model) [days]. | ||||
| 7 Time to the first solid diet (random‐effects model) [days] Show forest plot | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.26, ‐0.68] |
| Analysis 1.7 ![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 7 Time to the first solid diet (random‐effects model) [days].](/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-07.png) Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 7 Time to the first solid diet (random‐effects model) [days]. | ||||
| 8 Hospital stay (random‐effects model) [days] Show forest plot | 4 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.53, ‐0.31] |
| Analysis 1.8 ![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 8 Hospital stay (random‐effects model) [days].](/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-08.png) Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 8 Hospital stay (random‐effects model) [days]. | ||||
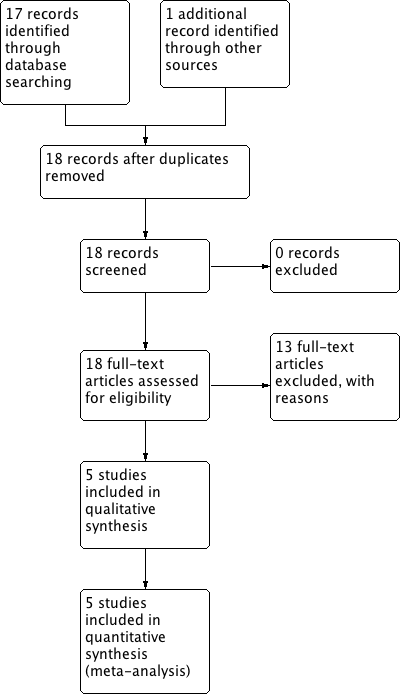
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 1 Postoperative ileus.
![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 2 Time intervals (fixed‐effect model) [days].](/es/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-02.png)
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 2 Time intervals (fixed‐effect model) [days].

Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 3 Other major postoperative complications.
![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 4 Satisfaction visual analog scale [mm].](/es/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-04.png)
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 4 Satisfaction visual analog scale [mm].
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 5 Nausea and/or vomiting (random‐effects model).
![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 6 Time to the presence of bowel sound (random‐effects model) [days].](/es/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-06.png)
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 6 Time to the presence of bowel sound (random‐effects model) [days].
![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 7 Time to the first solid diet (random‐effects model) [days].](/es/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-07.png)
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 7 Time to the first solid diet (random‐effects model) [days].
![Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 8 Hospital stay (random‐effects model) [days].](/es/cdsr/doi/10.1002/14651858.CD004508.pub4/media/CDSR/CD004508/image_n/nCD004508-CMP-001-08.png)
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 8 Hospital stay (random‐effects model) [days].
| Early oral feeding compared to delayed oral feeding for women who had major abdominal gynaecologic surgery | ||||||
| Patient or population: Women who had major abdominal gynaecologic surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed oral feeding | Early oral feeding | |||||
| Postoperative ileus | 77 per 1000 | 36 per 1000 | RR 0.47 | 279 | ⊕⊕⊕⊝ | |
| Nausea or vomiting or both | 352 per 1000 | 363 per 1000 | RR 1.03 | 484 | ⊕⊕⊕⊝ | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) |
| Time to bowel sounds [days] | The mean time to the presence of bowel sound [days] in the intervention groups was | 338 | ⊕⊕⊕⊝ | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| Time to the passage of flatus [days] | The mean time to the passage of flatus [days] in the intervention groups was | 444 | ⊕⊕⊕⊕ | |||
| Time to the first solid diet [days] | The mean time to the first solid diet [days] in the intervention groups was | 301 | ⊕⊕⊕⊝ | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| Time to first passage of stool [days] | The mean time to first passage of stool [days] in the intervention groups was | 249 | ⊕⊕⊕⊝ | |||
| Hospital stay [days] | The mean hospital stay [days] in the intervention groups was | 484 | ⊕⊕⊕⊝ | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 For the three studies contributing data, all were at high risk of performance bias, two were at unclear risk of detection bias, and one was at high risk of selection bias (no allocation concealment). 4 This outcome may be influenced by the high risk of performance bias in all studies that provided data. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative ileus Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Postoperative ileus | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.29] |
| 1.2 Nausea and/or vomiting (fixed‐effect model) | 4 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 1.3 Nausea | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.19, 2.71] |
| 1.4 Vomiting | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.00] |
| 1.5 Abdominal distension | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.47] |
| 1.6 Postoperative placement of nasogastric tube | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.80] |
| 2 Time intervals (fixed‐effect model) [days] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Time to the presence of bowel sound (fixed‐effect model) [days] | 2 | 338 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.11] |
| 2.2 Time to the passage of flatus [days] | 3 | 444 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.01] |
| 2.3 Time to the first solid diet (fixed‐effect model) [days] | 2 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.34, ‐1.05] |
| 2.4 Time to the first passage of stool [days] | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.09] |
| 2.5 Hospital stay (fixed‐effect model) [days] | 4 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.83, ‐0.35] |
| 3 Other major postoperative complications Show forest plot | 4 | 1286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 3.1 Febrile morbidity | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 3.2 Infectious complications | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.73] |
| 3.3 Wound complications | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 3.4 Pneumonia | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.73] |
| 4 Satisfaction visual analog scale [mm] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Nausea and/or vomiting (random‐effects model) Show forest plot | 4 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.67] |
| 6 Time to the presence of bowel sound (random‐effects model) [days] Show forest plot | 2 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.61, ‐0.03] |
| 7 Time to the first solid diet (random‐effects model) [days] Show forest plot | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.26, ‐0.68] |
| 8 Hospital stay (random‐effects model) [days] Show forest plot | 4 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.53, ‐0.31] |

