Hierbas medicinales para el dolor lumbar
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT with two groups. Patients were placed in groups by random number allocation. Period: Four weeks | |
| Participants | 118 participants were allocated to a H. procumbens (H) group (N = 59) and a placebo (P) group (N = 59)and 109 participants completed the trial (H; N = 54; P; N = 55). Inclusion criteria: participants were between 18 to 75 years of age, had at least six months of LBP not attributable to identifiable causes, were suffering from acute increases in pain, and were expected to require at least four weeks of symptomatic treatment. Exclusion criteria: participation in other clinical studies or had done so within the past 30 days, pregnancy, lactation, insufficient contraception, difficulties with language or cooperation, known allergy to proposed trial medication, history of drug or alcohol abuse, requirement of psychotherapeutic agents, or a serious organic illness affecting any of the organ systems. | |
| Interventions | Oral form of H. procumbens (devil's claw) standardized to a dosage of 50 mg harpagoside per day or 2400 mg of the crude extract. | |
| Outcomes | Primary: cumulative requirement for Tramadol (an oral opiate‐based analgesic) over the last three weeks of the study period. Secondary: number of pain free patients based on a five‐point visual rating scale and the Arhus LBP index. | |
| Notes | Total Quality Score: 7/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were placed in groups by random number allocation. |
| Allocation concealment (selection bias) | Unclear risk | Further description beyond randomized allocation is not included. |
| Blinding (performance bias and detection bias) | Low risk | Treatment group assignment blinded to participants. |
| Blinding (performance bias and detection bias) | Low risk | Treatment group assignment blinded to providers. |
| Blinding (performance bias and detection bias) | Low risk | Blinding done and unlikely the blinding was broken. |
| Incomplete outcome data (attrition bias) | Low risk | Only nine participants were lost to attrition, with the remaining 109 participants completing all outcome measures. Missing data not related to outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Treatment group had four participants not complete the final examination and one suffered tachycardia; the control group had four participants not complete the final examination, but participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | No significant difference within baseline characteristics between groups. |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes were reported. |
| Methods | RCT with three groups. Period: four weeks | |
| Participants | One hundred and ninety‐seven participants allocated to H. procumbens at 600 mg (N = 65), or 1200 mg (N = 66) or matched placebo (N = 66). Inclusion criteria: 18 to 75 years of age, six months of non‐specific LBP, a current exacerbation of their complaint that was effecting both rest and movement, which was giving rise to pain greater than five on a 1‐10 VAS and was expected to require at least four weeks of symptomatic treatment. Exclusion criteria: current or recent participation in any other clinical study, serious organic illness effecting any organ system, a history of drug or alcohol abuse or requirement for psychotherapeutic agents, pregnancy (actual or possible), or lactation, known allergy to any the proposed trial medications, difficulties with language or anticipated co‐operation. | |
| Interventions | H. procumbens extract WS 1531 600 mg (50 mg harpagoside), 1200 mg (100 mg harpagoside) | |
| Outcomes | Primary outcome: proportion of pain‐free participants without Tramadol for at least five days during the last week of treatment. Secondary outcomes: Arhus index, percentage requiring Tramadol, verbal pain ratings. | |
| Notes | Total Quality Score: 8/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted via stratified random allocation based on informed consent sequence. |
| Allocation concealment (selection bias) | Unclear risk | No additional information provided beyond randomization method. |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation blinded to participants. |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation blinded to providers. |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation blinded to both participants and providers and not likely broken. Treatment and placebo medications identical in appearance. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Groups were well matched for age, height, weight, and gender and of 120 matched indicators only four would have reached statistical significance in single isolated comparisons. |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes were reported. |
| Methods | RCT with three groups. No report of randomization method. Period: four weeks. | |
| Participants | Participants were recruited from the Haifa area in Israel between May and November. Two hundred and ten participants were randomized into three groups (N = 70 in each group) and 191 completed the trial (P; N = 59; 120; N = 67; 240; N = 65). | |
| Interventions | Extract of dry willow bark (S. alba): 120 mg salicin, 240 mg salicin. Matched placebo. | |
| Outcomes | Primary outcome: the proportion of participants who responded to treatment by being pain free without Tramadol for at least five days during the last week of treatment. Secondary outcome: The Arhus LBP Index scores | |
| Notes | Total quality score: 7/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Three group randomized double‐blind study with randomization conducted by "computerized list" but no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Participants were given identical coded tables. |
| Blinding (performance bias and detection bias) | Low risk | Investigators were blinded from the medication coding scheme. |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation blinded to both participants and providers and not likely broken. Treatment and placebo medications identical in appearance. |
| Incomplete outcome data (attrition bias) | Low risk | 191 participants out of 210 completed baseline and final outcome measures. Analysis was conducted with and without drop‐out data. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | High risk | Baseline characteristics were similar across all three groups only differing on six reported factors out of 110. |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes data and analyses was available. |
| Methods | Open RCT with two groups comparing an herbal medicine (S. alba) to a synthetic anti‐rheumatic (rofecoxib). | |
| Participants | 228 participants divided equally in to two groups (N = 114 per group). | |
| Interventions | A proprietary extract of S. alba called Assalix at four capsules per day providing a total of 240 mg of salicin per day, or a single 12.5 mg tablet of rofecoxib per day. | |
| Outcomes | Pain on a VAS, modified Arhus index, its pain component and the total pain index, physician and patient‐rated success and the acceptability of the treatment on a verbal scale (very good, good, moderate, poor). | |
| Notes | Total quality score: 6/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization completed by pre‐determined computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Group allocation concealed prior to the start of the trial. |
| Blinding (performance bias and detection bias) | High risk | Participants only blinded to group allocation until after enrolment were non‐blinded at study start. |
| Blinding (performance bias and detection bias) | High risk | Providers not blinded to group allocation. |
| Blinding (performance bias and detection bias) | High risk | The only blinded provider was an independent reviewer for adverse outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Forty‐five participants were disenrolled prior to the trial conclusion, leaving 183 participants which allows adequate number of participants per group. The PAID group lost 21 participants (five due to non‐compliance, one for severe LBP, and 12 due to adverse events), the NSAID group lost 24 participants (six for non‐compliance, three for severe LBP, three for other pain reasons, and 14 to adverse events). |
| Incomplete outcome data (attrition bias) | High risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Similarity between groups at baseline was adequate. |
| Co‐interventions avoided or similar? | Low risk | Participants were allowed to continue with current medications, or current alternative treatments and therapies, or both. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes were reported. |
| Methods | RCT with two groups. Period: six weeks | |
| Participants | 88 participants allocated to H. procubens (N = 44) group or rofecoxib (N = 44). | |
| Interventions | H. procumbens in a proprietary aqueous extract called Doloteffin (standardized to contain 60 mg harpagoside) or 12.5 mg rofecoxib per day. | |
| Outcomes | Primary outcome: proportion of participants who recorded "no pain" without using Tramadol for at least five days in the final week or treatment. Secondary and other outcomes: proportion of patients in whom the averaged daily pain scores in the 6th week had decreased by 20 to 50% of the average in the first week; the percentage change from baseline of a modified Arhus LBP index; the percentage change from baseline on the Health Assessment Questionnaire; Tramadol requirement. | |
| Notes | Total Quality Score: 8/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospective, randomized, double‐blind, double‐dummy study with randomization via assigned random number. No further description of the randomization process. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information in the text. |
| Blinding (performance bias and detection bias) | Low risk | The trial is described as a double blind double‐dummy RCT. However, there is very little description of the blinding in the manuscript. |
| Blinding (performance bias and detection bias) | Low risk | The trial is described as a double blind double‐dummy RCT. However, there is very little description of the blinding in the manuscript. |
| Blinding (performance bias and detection bias) | Low risk | It is unclear if participants were blinded to the intervention medication or just the accompanying placebo. However if the study medication is unblinded it should not incur unacceptable bias into the outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 88 participants who enrolled, nine participants dropped out. This should not significantly impact the outcome data. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Baseline characteristics of participants between groups was similar with no significant differences noted. |
| Co‐interventions avoided or similar? | Low risk | Participants were allowed to supplement the trial medications with Tramadol liquid. However, this was used as an additional outcome measure. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes were reported. |
| Methods | RCT with two groups. Participants were placed in groups by computerized randomization list. Period: three weeks | |
| Participants | Two hundred and eighty‐two participants were allocated to Finalgon® CPD Warmecreme (a capsicum‐containing cream) (N = 140), or matched placebo (N = 141). Inclusion criteria: aged between 18 and 65 years, Caucasian, chronic pain of the soft tissues of the musculoskeletal apparatus, subjective pain at enrolment ≥ 5 (VAS 0–10; 0, no pain; 10, intolerable pain), ability and expressed willingness of the patient to follow the investigator’s instructions, i.e. meeting the prerequisites of the study, applying study medication according to the dosage regimen and filling in the questionnaires at the control visits, and granting of written informed consent. Exclusion criteria: severe co‐morbidity, addiction to alcohol or other drugs, pregnancy and lactation, insufficient contraceptive protection, participation in another clinical trial within the past four weeks, concomitant psychiatric disorders, a surgical procedure required in the immediate future, inability of the patient to understand the nature, importance and consequences of the study, muscle rupture, vertebral disk prolapse, spondylolisthesis, spinal canal stenosis, known or clinically proven instability of the spine, spinal fractures, tumours, infections, inflammatory joint conditions, seronegative spondyloarthropathies, osteoporosis as the cause of pain, chronic skin diseases, known hypersensitivity to capsaicin or other ingredients of the cream, anxiety or depressive conditions, ≥ 11 points of anxiety or depression scores (Hamilton Anxiety Depression Scale). | |
| Interventions | 'Finalgon® CPD Wärmecreme', of which 100 g contain 2.2 to 2.6 g soft extract of Capsici fructus acer corresponding to 53 mg capsaicin (0.05%), applied as a thin layer thrice daily over a three week period. | |
| Outcomes | Primary outcome: treatment response, defined as pain sum score reduction ≥ 30%. Secondary outcomes: median relative pain sum score improvement, average pain in the last 24 hours, worst pain in the past three days, average pain in the past three days, pain intensity at the moment of maximum pain relief, the delay between the application of cream and the onset of maximum effect, the duration of analgesia, efficacy as determined by the investigator (excellent, good, adequate, unsatisfactory) and patient (free of complaints, symptoms improved, unchanged, worsened). | |
| Notes | Total quality score: 7/12 Adverse effects: three patients in the treatment group experienced adverse effects and none in the placebo group. In the treatment group, these included unpleasant local heat sensation in two participants and pruritus in one participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization completed by computerized randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Participants were randomized to treatment groups with intervention and placebo medications identical in appearance. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessment unblinded but unlikely to influence outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | There were seven participants who withdrew during the course of the study, six from the treatment group (three due to symptom abatement, two for insufficient pain relief and one for refusal to continue) and one from the placebo group due to symptom abatement. |
| Incomplete outcome data (attrition bias) | High risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Baseline characteristics of participants between groups was similar with no significant differences noted. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions noted. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All prespecified outcomes were reported. |
| Methods | RCT with two groups. Period: 15 days | |
| Participants | Twenty participants allocated to Brazilian arnica gel (N = 10) or placebo gel (N = 10). Inclusion criteria: No specific criteria listed. Patient recruitment was based on spontaneous demand for treating lumbago within the academic community at UVV/ES and was accompanied by the physiotherapy clinic. All participants went through a screening process coordinated by the physiotherapist responsible for the orthopaedics, traumatology, and rheumatology sector of the clinic. After screening, participants were submitted to medical evaluations to diagnose the nature of their lumbago before being allowed to participate in the research program. Exclusion criteria: volunteers under 18 were not permitted to participate in the program, unless they had their parent's or legal guardian's permission, people who were not in otherwise good physical and/or mental condition, who did not pass the screening process, who were eliminated as a result of diagnoses made by the physiotherapy sector, or pregnant women. | |
| Interventions | 5% concentrated plant extract from aerial vegetative and reproductive parts of S. chilensis Meyen, diluted in propylene glycol and added at a proportion of 5% (w/v) in carbomer gel, corresponding to active substances in 5 g of dry raw material. 10 g of the placebo or arnica gels was manually and uniformly applied on the area of the lesion twice daily. | |
| Outcomes | Primary outcome: Change in perception of pain by VAS. Secondary outcome: lumbar flexibility, as determined by the modified Schober method | |
| Notes | Total quality score: 5/12 Adverse effects: nothing reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No method of randomization described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Participants were blinded to treatment group and were unaware of which compound was being applied. |
| Blinding (performance bias and detection bias) | Unclear risk | Providers were blinded to treatment group and were unaware of which compound was being applied. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up noted. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | There were no significant differences noted in baseline comparisons between the placebo and intervention group. |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text. |
| Compliance acceptable? | Low risk | No issued noted with compliance. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | Outcome measures were straightforward with pre‐specified outcomes being reported. |
| Methods | RCT with two groups. Period: three weeks | |
| Participants | Three hundred and twenty participants with chronic non‐specific LBP divided equally between capsicum plaster group and placebo group | |
| Interventions | Topical plaster containing an ethonolic extract of cayenne pepper standardized to 22 µg/cm2 of capsaicinoinds or placebo plaster | |
| Outcomes | Outcomes: Arhus LBP Rating Scale, global assessment of efficacy by patient and investigator, global assessment of safety by patient and investigator. | |
| Notes | Total Quality Score: 6/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was computer generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was done by external personnel not involved in the trial. |
| Blinding (performance bias and detection bias) | Low risk | Participants were blinded to study group, and study medication and placebo were identical in appearance. |
| Blinding (performance bias and detection bias) | Low risk | Providers were blinded to study group and study medication and placebo were identical in appearance. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Incomplete outcome data (attrition bias) | Low risk | There were seventy withdrawals in the course of the trial, which resulted in a reduction of 319 participants to 249 participants. Despite the withdrawals, the study groups remained balanced. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | With the exception of slightly more female participants in the placebo group, the groups were comparable. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions noted. |
| Compliance acceptable? | Low risk | No issues regarding compliance. |
| Timing outcome assessments similar? | Unclear risk | Unclear from text. |
| Selective Reporting | Low risk | All pre‐specified outcomes were reported. |
| Methods | RCT with two groups. Period: five days | |
| Participants | 120 patients allocated to Kytta‐Salbe (a cream containing Comfrey root extract) (N = 60) or a matched placebo cream (N = 60). Inclusion criteria: Age range 18 to 60 years, good general condition, written informed consent, acute back pain (either upper or lower back pain) not in combination, sensitivity to algometric pressure on the site contralateral to the painful trigger point at least 2.5 N/cm2, basic value of the pressure algometry on the trigger point shall not exceed 50% of the respective value of the site contralateral to the painful trigger point. Exclusion criteria: upper or lower back pain that is attributable to any identifiable cause, any recent trauma, any recent strains of the back muscles documented by clinical evaluation and anamnesis, chronic back pain, diabetes mellitus, risk factors for spinal infection, recent onset of bladder dysfunction or severe or progressive neurological deficit in the low extremity (as a possible indication of prolapsed disc), concomitant use of any anti‐inflammatory drugs, heparinoids or analgesics, including herbal preparations (glucocorticosteroids, NSAID, etc) for the same indication or other indications (e.g. rheumatoid arthritis), analgesics or NSAID applied by any route of administration within 10 days of study entry or corticoid drugs applied by any route of administration within 60 days of study entry, any other concomitant treatment or medication that interferes with the conduct of the trial, known intolerance or hypersensitivity (allergy) to the trial treatments, including known toxic reactions, local skin infections that do not allow the application of the test ointment, participation in a clinical trial within the previous 30 days before enrolment in the trial, participation in this study before or simultaneous participation in another clinical trial, pregnancy or lactation period, women with childbearing potential without an effective contraceptive method, abuse of alcohol, medicaments or illicit drugs, any patient in the investigator’s opinion not considered suitable for enrolment, legal incapacity or limited legal capacity to give informed consent. | |
| Interventions | Kytta‐Salbe f. 100 g contains 35 g 99% PA reduced Rad symphyti fluid extract. Four grams were applied topically, administered three times a day at intervals of approximately eight hours and continued for five days. | |
| Outcomes | Primary outcome: Area under the curve (AUC) of the VAS values on active standardized movement. Secondary outcomes: AUC of back pain at rest by VAS, AUC over five days of pressure algometry values, global assessment of efficacy by the patients, global assessment of efficacy by the investigators. | |
| Notes | Total quality score: 8/12 Adverse effects: four participants in the treatment group and three participants in the placebo group experienced adverse effects. In the treatment group, two participants experienced headaches and one participant experienced pruritus. In the control group, participants experienced eczema, cold, nausea, and rhinitis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear from text. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | The trial medication and placebo ointments were similar in appearance. |
| Blinding (performance bias and detection bias) | Low risk | The clinicians were blinded to treatment group. However, there is little description of care taken to disguise the intervention or placebo ointment. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed baseline to end of study measures. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Groups were well balanced at baseline, with slightly more female participants than males. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions noted. |
| Compliance acceptable? | Low risk | No issues regarding compliance. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All pre‐specified outcomes were reported. |
| Methods | RCT with 40 patients assigned to one of twp groups for period of 14 days. | |
| Participants | Forty participants with acute mechanical LBP were assigned to either the Rado‐Salil (a capsicum‐containing cream) group (N = 20) or a placebo group (N = 20). Each patient was also given 45 paracetamol 250 mg tablets. No other analgesic, anti‐inflammatory drug or physical treatment was allowed during the 12‐week period. | |
| Interventions | Rado‐Salil ointment (containing 17.64 mg ethysalicylate, 26.47 mg methylsalicylate, 8.82 mg glycosalicylate, 8.82 mg salicylic acid, 4.41 mg camphor, 55.14 mg menthol, and 15.44 mg Capsicum Oleoresin per 1 g) in the form of a 40 g stick applied as needed or a placebo (containing only the excipient with three times the amount of lavendula and bergamot essences) matched for appearance. | |
| Outcomes | Outcomes: pain evaluation on a 10 cm linear scale, duration of confinement to bed, muscular reflex contracture evaluation by the physician on a scale of 0 to 4, and spine mobility by determination of Schober's index, the finger to floor distance, the degree of lumbar extension, global appreciation of treatment by patient and physician. | |
| Notes | Total Quality Score: 5/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The exact method used for randomization was not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Participants were given either a treatment ointment or a placebo that are identical in appearance. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessments unblinded but unlikely to influence outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals noted in the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Unclear risk | Unclear from text. |
| Co‐interventions avoided or similar? | Low risk | Participants were given paracetamol tablets in addition to study medication or placebo. No other medication or physical treatment was allowed. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | High risk | No comparison of groups noted and certain baseline measures were not reported in the final results. |
| Methods | RCT with two groups. No report of randomization method. Period: one plaster per day at maximum pain site for four to 12 hours for three weeks. | |
| Participants | One hundred and fifty‐four participants were randomly allocated to a placebo plaster group (N = 77) and a capsicum plaster group (N = 77). A total of 132 participants completed the study, with data available for the intention to treat (ITT) analysis on 150 participants (P = 0.002). A total 22 participants were excluded due to premature discontinuation of the treatment (N = 19) failure to meet the inclusion criteria (N = 2) or unauthorized concurrent treatment (N = 1). Inclusion criteria: subjective back pain rating of five or more on an 11 grade VAS, as well as a duration of back pain for a minimum of three months at enrolment. Exclusion criteria: alcohol abuse, drug dependence, forms of specific back pain, concomitant systemic inflammatory rheumatic condition, no concurrent therapy for back pain. | |
| Interventions | Topical plaster type application of C. frutescens (cayenne) containing 12 mg of capsaicinoids per plaster. Matched placebo plaster. | |
| Outcomes | Primary outcome measure: Arhus Low Back Rating Scale. Secondary outcome measures: global assessment of efficacy and tolerance by physician and patient. | |
| Notes | Total quality score: 6/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No report of randomization method. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Study medication and placebo were identical in appearance. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text. |
| Incomplete outcome data (attrition bias) | Low risk | Out of 154 participants, 22 were excluded due to premature discontinuation of treatment (N = 19, failure to meet exclusion criteria (N = 2), and unauthorized outside treatment (N = 1). Of the remaining 132 participants the groups maintained adequately balanced. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Apart from a slightly higher BMI in the placebo group the groups were comparable. |
| Co‐interventions avoided or similar? | Low risk | No co‐intervention noted. |
| Compliance acceptable? | Low risk | No issues with compliance noted. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All pre‐specified outcomes were reported. |
| Methods | Thirty‐five participants randomized to two groups and a further 16 participants acted as controls. Period: four weeks | |
| Participants | Fifty‐one participants with 19 in the Salix alba group, 16 in a placebo group, and 16 in an acetylsalicylate group. | |
| Interventions | 786.48 mg twice per day of an ethanol extract of the bark of Salix daphnoides (240 mg salicin content per day), matched placebo, or 100 mg acetylsalicylate. | |
| Outcomes | Primary outcome: platelet aggregation | |
| Notes | Total quality score: 5/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not defined. |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text. |
| Blinding (performance bias and detection bias) | Low risk | Participants were blinded from treatment group allocation, and study medication and placebo were identical. |
| Blinding (performance bias and detection bias) | Low risk | Providers were blinded from treatment group allocation. |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessors were unblinded. However knowing the outcome of interest, platelet aggregation, is unlikely to effect outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals noted. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | The groups were similar in baseline measures except gender; there were more female participants in the placebo group (P = 0.01). |
| Co‐interventions avoided or similar? | Low risk | Participants were disallowed the use of anti‐inflammatory drugs within the trial period. Tramadol was allowed as an emergency medication. |
| Compliance acceptable? | Unclear risk | Unclear from text. |
| Timing outcome assessments similar? | Unclear risk | Unclear from text. |
| Selective Reporting | Low risk | All pre‐specified measures were reported. |
| Methods | RCT with two groups (no placebo). Randomization was performed using RCODE software (Version 3.4) in blocks of four. Period: seven days. | |
| Participants | One hundred and sixty‐one participants were randomly allocated to either group. A total of six participants were lost to follow‐up (SLR = 2; CCC = 4). Twenty‐one participants met all per protocol criteria. Inclusion criteria: between the ages of 18 and 65, acute attack of LBP within previous 72 hours, free from back pain during the previous three months, at least moderately painful limitation of movement on physical examination. Exclusion criteria: radicular symptoms, pain above T12, rheumatoid arthritis, ankylosing spondylitis, known hypersensitivity to treatment compounds, use of analgesics other than paracetamol during the treatment period, use of NSAIDs during the treatment period, receiving other treatment for acute LBP, pregnancy, over 96 hours elapsed since onset of pain, including washout for analgesic or NSAIDs or both. | |
| Interventions | Spiroflor SLR homeopathic gel (SLR) group (N = 83) or CCC group (N = 78). Each gel was applied at 3 g per day. | |
| Outcomes | Primary outcome: reduction in VAS scores for pain (100 mm scale) and the proportion of treatment success (a VAS reduction of at least 80%). Secondary outcome measures: proportion of participants using paracetamol, number of nights with disturbed sleep, duration of absence from work and an overall assessments of treatment efficacy or usefulness by the general practitioners (GP) and the patients | |
| Notes | Total quality score: 9/12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using RCODE software (version 3.4) in blocks of four. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was adequate, providers were not influential in group selection. |
| Blinding (performance bias and detection bias) | Low risk | Participants were blinded to treatment group and although trial medications were not identical, they were packed in identical containers. |
| Blinding (performance bias and detection bias) | Low risk | Providers were blinded to group allocation and were only able to access treatment group allocation if there was an adverse event which required unblinding. The trial medications were not available to the providers in the trial country at the time and all stakeholders assumed both medications held active ingredients. |
| Blinding (performance bias and detection bias) | Low risk | This was a double‐blinded trial. While there were reservations with the blinding due to non‐similar medications, there was no evidence that unblinding occurred. |
| Incomplete outcome data (attrition bias) | Low risk | One hundred and fifty‐four out of 161 participants were able to provide evaluable results from baseline to seven days. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | Group comparison was similar with no significant differences noted between groups at baseline. |
| Co‐interventions avoided or similar? | Low risk | Anti‐inflammatory drugs were disallowed during the trial phase with paracetamol used as an emergency medication. |
| Compliance acceptable? | High risk | Poor compliance was noted among participants. Rapid improvement and three applications per day may have influenced non‐compliance. Compliance was equal across groups. |
| Timing outcome assessments similar? | Low risk | No differences noted in timing. |
| Selective Reporting | Low risk | All pre‐specified outcomes reported. |
| Methods | Unblinded RCT with two groups. Period: three weeks | |
| Participants | Sixty‐one patients were allocated to acupressure with lavender oil (N = 32) or conventional treatment (N = 29). Inclusion criteria: aged 18 or above with non‐specific sub‐acute LBP for most days in the past four weeks; who had not received acupuncture, physiotherapy, or manipulative therapy in the past week; who could understand the explanation of the study, complete the interview and comprehend the instructions. Non‐specific sub‐acute LBP was defined as pain on most days in the past four weeks, in the area between the lower coastal margins and the gluteal folds without known specific cause, such as a spinal deformity. Exclusion criteria: LBP caused by specific entities, such as infection, metastases, neoplasm osteoporosis, fractures, spine deformity, or prolapsed intervertebral disc; had undergone surgery or had dislocation, fracture, neurological deficits, spinal disease, varicose vein, blood dyscrasia, cancer or systemic disorders; were pregnant; were allergic to natural lavender aromatic oil; had a wound at any of the acupoints at the back or on the lower limb; or had had a surgical intervention within the last three months. | |
| Interventions | Acupoint stimulation with a digital Electronic Muscle Stimulator for 10 minutes, followed by acupressure massage, consisting of the application of a light to medium finger press with 3% aromatic natural lavender oil with grape seed oil as the base on eight fixed acupoints for two minutes each. Treatment lasted 35 to 40 minutes and occurred eight times over a three‐week period. | |
| Outcomes | Primary outcome: Pain intensity rating on VAS. Secondary outcomes: range of motion of lateral spine flexion, quantified by lateral fingertip‐to‐ground distance, walking time for 15 meters, interference with daily activities measured by the modified Aberdeen LBP scale. | |
| Notes | Total quality score: 4/12 Adverse effects: Reported no adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated by the research team consulting a random numbers table. |
| Allocation concealment (selection bias) | High risk | Patients and clinicians were aware of group allocation. |
| Blinding (performance bias and detection bias) | High risk | Intervention treatment and control treatment were dissimilar with no blinding. |
| Blinding (performance bias and detection bias) | High risk | Providers were aware and involved in the treatment allocation process. |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) | Low risk | Of the 61 original participants, 10 participants dropped out but there was no difference in dropout rate between groups. Reasons for withdrawal by participants were not related to study procedures and should have little effect on outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Participants appeared to be analysed in the groups to which they were allocated. |
| Similarity of baseline characteristics? | Low risk | No significant differences between study groups noted. |
| Co‐interventions avoided or similar? | High risk | No discussion or controlling for medication or additional treatment modalities noted. |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | High risk | There was no description of the control group's therapy beyond being a conventional therapy. |
| Selective Reporting | Low risk | All pre‐specified outcomes were reported. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT. | |
| Did not study non‐specific LBP. | |
| Note on another article. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Comment on another study. | |
| Not a RCT. | |
| Abstract from a symposium. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT | |
| Not a RCT. | |
| Not a RCT. | |
| Did not study LBP. | |
| Not a RCT. | |
| Not a herbal medicine. | |
| Not a RCT, not a herbal medicine. | |
| Not a herbal medicine. | |
| Not a herbal medicine. | |
| Did not study LBP. | |
| Not a RCT. | |
| Conference abstract only, unknown participants type, unknown if a herbal medicine. | |
| Abstract or full text not available. | |
| Not a RCT. | |
| Comment on another article. | |
| Mixed low back and upper back pain with no subgroup analyses. | |
| Herbal medicine given by injection | |
| Not a herbal medicine. | |
| Not a RCT. | |
| Not a RCT, not a herbal medicine. | |
| Not a RCT, not a herbal medicine. | |
| Not LBP. | |
| Did not use herbal medicine. | |
| Not a RCT. | |
| Not an oral or topical route of administration. | |
| Not LBP, not a herbal medicine. | |
| Not a RCT. | |
| Not LBP. |
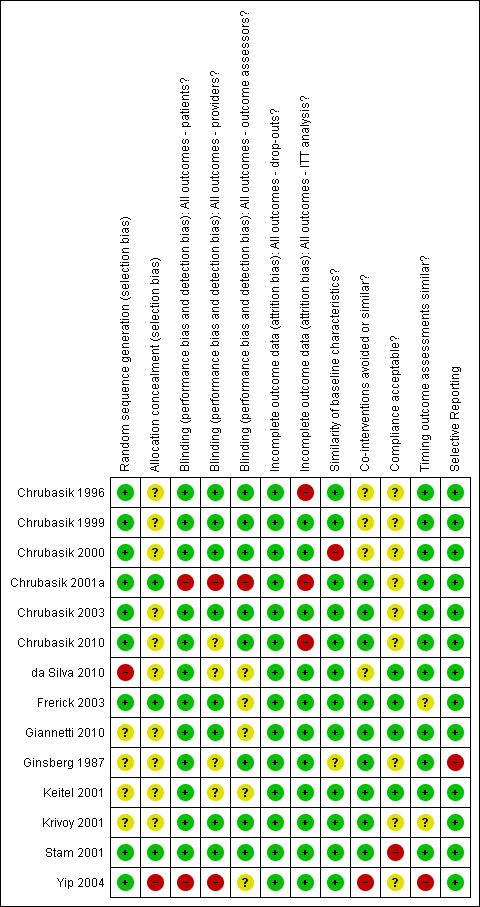
Summary of risk of bias for each of the included trials.
| Brazilian arnica extract compared to placebo for patients with non‐specific chronic back pain or soft tissue pain | |||
| Patient or population: patients with back pain Settings: outpatient clinic Intervention: extract of Brazilian arnica Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain reduction based on Pain VAS instrument 0‐100 scale | 20 | ⊕⊝⊝⊝ | Very small sample size only N = 10 in the treatment group. This trial found that topical application of Brazilian arnica reduced the perception of pain and increased flexibility in the treated group compared to baseline values in that group. Unknown if acute or chronic LBP. |
| GRADE Working Group grades of evidence | |||
| 1Selection bias was high to unclear, performance bias was low risk to unclear risk, with other attributes being low risk. | |||
| Topical capsaicin cream or plaster compared to placebo for patients with non‐specific chronic back pain or soft tissue pain | |||
| Patient or population: patients with chronic LBP or soft tissue pain Settings: Outpatient clinic Intervention: topical capsicum cream or plaster Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain perception according to the Pain VAS scale 0‐10 | 755 | ⊕⊕⊕⊝ | All three trials found a statistically significant difference between the capsaicin intervention vs. placebo. In three trials minor adverse effects were noted in the treatment groups requiring no specific follow‐up treatments. |
| GRADE Working Group grades of evidence | |||
| 1All three trials exhibited low to unclear risk in selection bias, performance bias and attrition bias. One trial was at high risk for selective reporting. | |||
| Topical capsaicin cream compared with placebo for patients with acute non‐specific LBP | |||
| Patient or population: patients with acute mechanical LBP Settings: outpatient clinic Intervention: Rado‐Salil ointment Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain evaluation on a 10 cm linear scale | 40 (one trial) | ⊕⊝⊝⊝ | Pain improvements were significantly greater in the capsicum cream group up to day 14. Adverse events: Pruritis, one in placebo, one in Rado‐Salil group. Local erythema and burning, three in the Rado‐Salil group. |
| GRADE Working Group grades of evidence | |||
| 1Exhibited unclear risk for selection bias as well unclear baseline similarities. Performance bias was low risk as was attrition bias but it was high risk for incomplete outcome data. 2As under 400 participants were included, evidence was downgraded to very low from low. | |||
| H. procumbens compared to placebo for non‐specific chronic back pain | |||
| Patient or population: patients with chronic back pain Settings: outpatient clinic Intervention:H. procumbens extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Arhus pain index scale 0‐130 | 315 | ⊕⊕⊝⊝ | In one trial a 50mg dose of H. procumbens was used, and in the second trial a 50 mg and 100 mg dose was used with both trialss showing a significantly improved pain score over placebo. |
| GRADE Working Group grades of evidence | |||
| 1Both included trials exhibited low risk of bias regarding selection bias with one trial at unclear risk of bias. Performance bias was at low risk of bias, as was attrition bias with one trial at high risk of bias for incomplete outcome data. 2Two trials included under 400 participants and we downgraded the evidence to low from moderate. | |||
| H. procumbens extract compared to Vioxx®for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic Intervention:H. procumbens extract Comparison: Vioxx® | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Modified Arhus Index Scale 0‐120 | 88 | ⊕⊝⊝⊝ | H. procumbens was compared to Vioxx® and while both groups showed similar pain reduction scores there were no demonstrable difference among groups. There were adverse effects noted in both groups. |
| GRADE Working Group grades of evidence | |||
| 1This trial was at low risk of bias for all risk of bias factors, with the exception of allocation concealment and compliance which were at unclear risk of bias. 2Downgraded to very low versus low as under 400 participants were included. | |||
| Willow bark extract compared to placebo for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic and public advertisement Intervention: willow bark extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS Scale 0‐10 | 261 | ⊕⊕⊕⊝ | The high dose (240 mg) treatment group showed a significant reduction in pain scores versus the low dose (120 mg) group and the placebo group. There was one severe allergic reaction related to the extract noted. One trial (N = 51) also examined the effect of the extract on platelet aggregation. |
| GRADE Working Group grades of evidence | |||
| 1Both trials were at low to unclear risk for selection bias, low risk for performance bias with one trial exhibiting high risk in baseline characteristics similarity. Both trials were rated as an overall low risk of bias since they met our predetermined cut‐point of 50% of the criteria on which the trial methods were assessed. 2Downgraded from high to moderate as under 400 participants were included between both trials. | |||
| Willow bark extract compared to rofecoxib for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic Intervention: willow bark extract Comparison: rofecoxib | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Arhus Index Scale 0‐130 Pain VAS Scale 0‐10 | 228 | ⊕⊝⊝⊝ | There was no significant difference in the effectiveness and adverse events between the extract and rofecoxib. |
| GRADE Working Group grades of evidence | |||
| 1Low risk for selection bias, high risk for performance bias, and high and low risk for attrition bias. 2Downgraded from low to very low due as under 400 participants were included. | |||
| Comfrey root extract compared to placebo for acute lower and upper back non‐specific pain | |||
| Patient or population: patients with acute lower and upper back pain Settings: outpatient setting Intervention: comfrey root extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS sum (decrease) on active standardized movement (mm) | 120 | ⊕⊕⊝⊝ | The root extract showed a statistically and clinically relevant reduction in acute back pain versus placebo. |
| GRADE Working Group grades of evidence | |||
| 1Unclear risk for selection bias, low risk for both performance and attrition bias. 2Downgraded from moderate to low as under 400 participants were included. | |||
| Lavender oil acupressure massage and acupoint stimulation compared to usual treatment for acute non‐specific LBP | |||
| Patient or population: patients with acute LBP Settings: old aged home and community centre Intervention: lavender oil massage Comparison: usual therapy | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS 0‐10 scale | 61 | ⊕⊝⊝⊝ | One week post‐study the treatment group showed a significant (P = 0.0001) reduction in VAS pain as well as improved walking time and lateral spine flexion range. |
| GRADE Working Group grades of evidence | |||
| 1Sequence generation was at low risk of bias but allocation concealment was at high risk. Performance bias was at high and unclear risk. Co‐interventions and timing outcome assessment factors were at high risk of bias. | |||
| Spiroflor SRL compared to CCC for chronic non‐specific LBP | |||
| Patient or population: patients with acute and chronic LBP Settings: outpatient clinic Intervention: Spiroflor SRL Comparison: CCC | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS 0‐100 scale | 161 | ⊕⊝⊝⊝ | Spiroflor SRL and CCC were equally effective in treating acute LBP but the CCC group experienced greater adverse events and adverse drug reactions. |
| GRADE Working Group grades of evidence | |||
| CCC = Cremor Capsici Compositus FNA; SRL = Homeopathic combination of Symphytum officinale, Rhus toxicodendron and Ledum palustre 1All risk of bias factors were at low risk of bias, except patient compliance which was at high risk. 2Downgraded from low to very low as under 400 participants were included. | |||

