草药治疗下腰痛
Appendices
Appendix 1. Current search strategy
MEDLINE
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
Randomized Controlled Trials/
-
Random Allocation/
-
Double‐Blind Method/
-
Single‐Blind Method/
-
or/1‐6
-
Animals/ not Human/
-
7 not 8
-
clinical trial.pt.
-
exp Clinical Trial/
-
(clin$ adj25 trial$).tw.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
-
Placebos/
-
placebo$.tw.
-
random$.tw.
-
Research Design/
-
(latin adj square).tw.
-
or/10‐18
-
19 not 18
-
20 not 9
-
Comparative Study/
-
exp Evaluation Studies/
-
Follow‐Up Studies/
-
Prospective Studies/
-
(control$ or prospective$ or Volunteer$).tw.
-
Cross‐Over Studies/
-
or/22‐27
-
28 not 8
-
29 not (9 or 21)
-
9 or 21 or 30
-
dorsalgia.ti,ab.
-
exp Back Pain/
-
backache.ti,ab.
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
sciatic neuropathy/
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
or/32‐41
-
31 and 42
-
Drugs, Chinese Herbal/
-
herbal.mp.
-
Plants, Medicinal/
-
phytomedicine.mp.
-
herb$.mp.
-
weed.mp.
-
algae.mp.
-
cryptophyta/ or haptophyta/ or exp glaucophyta/ or exp rhodophyta/ or exp viridiplantae/ or exp chlorophyta/ or exp streptophyta/ or exp stramenopiles/
-
exp Fungi/
-
exp Medicine, Traditional/
-
exp Phytotherapy/
-
exp Pharmacognosy/
-
(oriental adj traditional).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
(Camphora adj molmol).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
Capsicum/
-
exp Salicaceae/
-
(Maleluca adj alternifolia).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
Angelica sinensis/
-
Aloe/
-
(Thymus adj officinalis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
Menthe piperita.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
Arnica Montana.mp. or Arnica/
-
Curcuma longa.mp. or Curcuma/
-
Tanacetum parthenium.mp. or Tanacetum parthenium/
-
feverfew.mp.
-
Harpagophytum procumbens.mp. or exp Harpagophytum/
-
Zingiber officii.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
plant preparations/ or plant extracts/ or plant oils/
-
or/44‐71
-
43 and 72
-
31 and 42 and 72
-
limit 74 to yr=2013‐Current
-
limit 74 to ed=20130806‐20140911
-
75 or 76
EMBASE
From the 2013 strategy, line 31 was changed from 14 and 30 to 14 or 30
-
Clinical Article/
-
exp Clinical Study/
-
Clinical Trial/
-
Controlled Study/
-
Randomized Controlled Trial/
-
Major Clinical Study/
-
Double Blind Procedure/
-
Multicenter Study/
-
Single Blind Procedure/
-
Phase 3 Clinical Trial/
-
Phase 4 Clinical Trial/
-
crossover procedure/
-
placebo/
-
or/1‐13
-
allocat$.mp.
-
assign$.mp.
-
blind$.mp.
-
(clinic$ adj25 (study or trial)).mp.
-
compar$.mp.
-
control$.mp.
-
cross?over.mp.
-
factorial$.mp.
-
follow?up.mp.
-
placebo$.mp.
-
prospectiv$.mp.
-
random$.mp.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
-
trial.mp.
-
(versus or vs).mp.
-
or/15‐29
-
14 or 30
-
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
-
human/ or normal human/ or human cell/
-
32 and 33
-
32 not 34
-
31 not 35
-
dorsalgia.mp.
-
back pain.mp.
-
exp BACKACHE/
-
(lumbar adj pain).mp.
-
coccyx.mp.
-
coccydynia.mp.
-
sciatica.mp.
-
exp ISCHIALGIA/
-
spondylosis.mp.
-
lumbago.mp.
-
exp Low back pain/
-
or/37‐47
-
exp herbaceous agent/
-
herbal.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
exp medicinal plant/
-
exp phytotherapy/
-
exp herbal medicine/
-
phytomedicine.mp.
-
exp plant extract/
-
herb*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
weed.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
exp alga/
-
exp fungus/
-
exp traditional medicine/
-
exp pharmacognosy/
-
(oriental adj traditional).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
exp Chinese medicine/
-
exp pepper/
-
capsicum.mp.
-
exp willow/
-
salix.mp.
-
exp Angelica sinensis/
-
exp Aloe/
-
exp Arnica montana/
-
exp Curcuma longa/
-
tanacetum/ or exp tanacetum parthenium/
-
exp harpagophytum/ or exp harpagophytum extract/ or exp harpagophytum procumbens extract/
-
or/49‐73
-
36 and 48 and 74
-
limit 75 to yr="2013‐Current"
-
limit 75 to em=201331‐201436
-
76 or 77
CENTRAL
#1 MeSH descriptor: [Low Back Pain] this term only
#2 MeSH descriptor: [Back Pain] this term only
#3 backache
#4 lumbago
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Drugs, Chinese Herbal] explode all trees
#7 MeSH descriptor: [Plants, Medicinal] explode all trees
#8 herbal
#9 phytomedicine
#10 herb*
#11 weed
#12 MeSH descriptor: [Algae] explode all trees
#13 MeSH descriptor: [Fungi] explode all trees
#14 MeSH descriptor: [Medicine, Traditional] explode all trees
#15 MeSH descriptor: [Phytotherapy] this term only
#16 MeSH descriptor: [Pharmacognosy] explode all trees
#17 Oriental next traditional
#18 MeSH descriptor: [Medicine, Chinese Traditional] this term only
#19 Camphora next molmo
#20 MeSH descriptor: [Capsicum] this term only
#21 MeSH descriptor: [Salix] this term only
#22 Maleluca next alternifolia
#23 MeSH descriptor: [Angelica sinensis] this term only
#24 MeSH descriptor: [Aloe] this term only
#25 Thymus next officinalis
#26 Menthe next piperita
#27 MeSH descriptor: [Arnica] this term only
#28 Arnica next Montana
#29 Curcuma next longa
#30 MeSH descriptor: [Curcuma] this term only
#31 MeSH descriptor: [Tanacetum parthenium] this term only
#32 MeSH descriptor: [Harpagophytum] this term only
#33 Harpagophytum next procumbens
#34 Zingiber next officii
#35 MeSH descriptor: [Plant Preparations] this term only
#36 MeSH descriptor: [Plant Oils] explode all trees
#37 MeSH descriptor: [Plant Extracts] explode all trees
#38 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37
#39 #5 and #38
#40 #39 Publication Year from 2013 to 2014, in Trials
CINAHL
S75 S73 OR S74
S74 S73 and EM 20130806‐20140911
S73 S49 and S71 Limiters ‐ Published Date: 20130801‐20140931
S72 S49 and S71
S71 S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70
S70 (MH "Devil's Claw")
S69 (MH "Feverfew")
S68 Curcuma longa
S67 (MH "Arnica")
S66 (MH "Aloe")
S65 (MH "Dong Quai")
S64 (MH "Willow Bark")
S63 ("Capsicum") or (MH "Cayenne Pepper")
S62 Camphora W1 molmol
S61 (MH "Medicine, Traditional")
S60 oriental W1 traditional
S59 Pharmacognosy
S58 ("Phytotherapy") or (MH "Medicine, Herbal")
S57 (MH "Medicine, Chinese Traditional")
S56 (MH "Fungi+")
S55 (MH "Algae+")
S54 "weed"
S53 herb*
S52 phytomedicine
S51 (MH "Plants, Medicinal+")
S50 (MH "Drugs, Chinese Herbal")
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
ClinicalTrials.gov
Condition: back pain
AND Intervention: herbal OR botanical
Received on or after 08/06/2013
WHO ICTRP
Condition: back pain
AND Intervention: herbal OR botanical
Date of registration between 06/08/2013‐(no date limit)
PubMed
((((herbal or botanical))) AND back pain) AND ((pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
Appendix 2. Previous search strategies
August 2013
Embase
The animal study filter was updated from 2010
-
Clinical Article/
-
exp Clinical Study/
-
Clinical Trial/
-
Controlled Study/
-
Randomized Controlled Trial/
-
Major Clinical Study/
-
Double Blind Procedure/
-
Multicenter Study/
-
Single Blind Procedure/
-
Phase 3 Clinical Trial/
-
Phase 4 Clinical Trial/
-
crossover procedure/
-
placebo/
-
or/1‐13
-
allocat$.mp.
-
assign$.mp.
-
blind$.mp.
-
(clinic$ adj25 (study or trial)).mp.
-
compar$.mp.
-
control$.mp.
-
cross?over.mp.
-
factorial$.mp.
-
follow?up.mp.
-
placebo$.mp.
-
prospectiv$.mp.
-
random$.mp.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
-
trial.mp.
-
(versus or vs).mp.
-
or/15‐29
-
14 and 30
-
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
-
human/ or normal human/ or human cell/
-
32 and 33
-
32 not 34
-
31 not 35
January 2011
Medline
Back terms and herbal medicine terms were updated from 2009
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
Randomized Controlled Trials/
-
Random Allocation/
-
Double‐Blind Method/
-
Single‐Blind Method/
-
or/1‐6
-
Animals/ not Human/
-
7 not 8
-
clinical trial.pt.
-
exp Clinical Trials/
-
(clin$ adj25 trial$).tw.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
-
Placebos/
-
placebo$.tw.
-
random$.tw.
-
Research Design/
-
(latin adj square).tw.
-
or/10‐18
-
19 not 18
-
20 not 9
-
Comparative Study/
-
exp Evaluation Studies/
-
Follow‐Up Studies/
-
Prospective Studies/
-
(control$ or prospective$ or Volunteer$).tw.
-
Cross‐Over Studies/
-
or/22‐27
-
28 not 8
-
29 not (9 or 21)
-
9 or 21 or 30
-
dorsalgia.ti,ab.
-
exp Back Pain/
-
backache.ti,ab.
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
sciatic neuropathy/
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
or/32‐41
-
31 and 42
-
Drugs, Chinese Herbal/
-
herbal.mp.
-
Plants, Medicinal/
-
phytomedicine.mp.
-
herb$.mp.
-
weed.mp.
-
algae.mp.
-
cryptophyta/ or haptophyta/ or exp glaucophyta/ or exp rhodophyta/ or exp viridiplantae/ or exp chlorophyta/ or exp streptophyta/ or exp stramenopiles/
-
exp Fungi/
-
exp Medicine, Traditional/
-
exp Phytotherapy/
-
exp Pharmacognosy/
-
(oriental adj traditional).mp.
-
(Camphora adj molmol).mp.
-
Capsicum/
-
exp Salicaceae/
-
(Maleluca adj alternifolia).mp.
-
Angelica sinensis/
-
Aloe/
-
(Thymus adj officinalis).mp.
-
Menthe piperita.mp.
-
Arnica Montana.mp. or Arnica/
-
Curcuma longa.mp. or Curcuma/
-
Tanacetum parthenium.mp. or Tanacetum parthenium/
-
feverfew.mp.
-
Harpagophytum procumbens.mp. or exp Harpagophytum/
-
Zingiber officii.mp.
-
plant preparations/ or plant extracts/ or plant oils/
-
or/44‐71
-
43 and 72
-
limit 73 to yr="2003 ‐ 2011"
CINAHL
The strategy was updated from 2009
S73 S49 and S71 Limiters ‐ Published Date from: 20030101‐20111231
S72 S49 and S71
S71 S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70
S70 (MH "Devil's Claw")
S69 (MH "Feverfew")
S68 Curcuma longa
S67 (MH "Arnica")
S66 (MH "Aloe")
S65 (MH "Dong Quai")
S64 (MH "Willow Bark")
S63 ("Capsicum") or (MH "Cayenne Pepper")
S62 Camphora W1 molmol
S61 (MH "Medicine, Traditional")
S60 oriental W1 traditional
S59 Pharmacognosy
S58 ("Phytotherapy") or (MH "Medicine, Herbal")
S57 (MH "Medicine, Chinese Traditional")
S56 (MH "Fungi+")
S55 (MH "Algae+")
S54 "weed"
S53 herb*
S52 phytomedicine
S51 (MH "Plants, Medicinal+")
S50 (MH "Drugs, Chinese Herbal")
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
CENTRAL
New intervention terms were added from 2009
#1 MeSH descriptor Low Back Pain, this term only
#2 MeSH descriptor Back Pain, this term only
#3 backache
#4 lumbago
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Drugs, Chinese Herbal explode all trees
#7 MeSH descriptor Plants, Medicinal explode all trees
#8 herbal
#9 phytomedicine
#10 herb*
#11 weed
#12 MeSH descriptor Algae explode all trees
#13 MeSH descriptor Fungi explode all trees
#14 MeSH descriptor Medicine, Traditional explode all trees
#15 MeSH descriptor Phytotherapy, this term only
#16 MeSH descriptor Pharmacognosy explode all trees
#17 Oriental NEXT traditional
#18 MeSH descriptor Medicine, Chinese Traditional, this term only
#19 Camphora NEXT molmo
#20 MeSH descriptor Capsicum, this term only
#21 MeSH descriptor Salix, this term only
#22 Maleluca NEXT alternifolia
#23 MeSH descriptor Angelica sinensis, this term only
#24 MeSH descriptor Aloe, this term only
#25 Thymus NEXT officinalis
#26 Menthe NEXT piperita
#27 MeSH descriptor Arnica, this term only
#28 Arnica NEXT Montana
#29 Curcuma NEXT longa
#30 MeSH descriptor Curcuma, this term only
#31 MeSH descriptor Tanacetum parthenium, this term only
#32 MeSH descriptor Harpagophytum, this term only
#33 Harpagophytum NEXT procumbens
#34 Zingiber NEXT officii
#35 MeSH descriptor Plant Preparations, this term only
#36 MeSH descriptor Plant Oils explode all trees
#37 MeSH descriptor Plant Extracts explode all trees
#38 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37)
#39 (#5 AND #38), from 2003 to 2011
April 2010
EMBASE
The strategy was updated from 2009
-
Clinical Article/
-
exp Clinical Study/
-
Clinical Trial/
-
Controlled Study/
-
Randomized Controlled Trial/
-
Major Clinical Study/
-
Double Blind Procedure/
-
Multicenter Study/
-
Single Blind Procedure/
-
Phase 3 Clinical Trial/
-
Phase 4 Clinical Trial/
-
crossover procedure/
-
placebo/
-
or/1‐13
-
allocat$.mp.
-
assign$.mp.
-
blind$.mp.
-
(clinic$ adj25 (study or trial)).mp.
-
compar$.mp.
-
control$.mp.
-
cross?over.mp.
-
factorial$.mp.
-
follow?up.mp.
-
placebo$.mp.
-
prospectiv$.mp.
-
random$.mp.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
-
trial.mp.
-
(versus or vs).mp.
-
or/15‐29
-
14 and 30
-
human/
-
Nonhuman/
-
exp ANIMAL/
-
Animal Experiment/
-
33 or 34 or 35
-
32 not 36
-
31 not 36
-
37 and 38
-
38 or 39
-
dorsalgia.mp.
-
back pain.mp.
-
exp BACKACHE/
-
(lumbar adj pain).mp.
-
coccyx.mp.
-
coccydynia.mp.
-
sciatica.mp.
-
exp ISCHIALGIA/
-
spondylosis.mp.
-
lumbago.mp.
-
exp Low back pain/
-
or/41‐51
-
exp herbaceous agent/
-
herbal.mp.
-
exp medicinal plant/
-
exp phytotherapy/
-
exp herbal medicine/
-
phytomedicine.mp.
-
exp plant extract/
-
herb*.mp.
-
weed.mp.
-
exp alga/
-
exp fungus/
-
exp traditional medicine/
-
exp pharmacognosy/
-
(oriental adj traditional).mp.
-
exp Chinese medicine/
-
exp pepper/
-
capsicum.mp.
-
exp willow/
-
salix.mp.
-
exp Angelica sinensis/
-
exp Aloe/
-
exp Arnica montana/
-
exp Curcuma longa/
-
tanacetum/ or exp tanacetum parthenium/
-
exp harpagophytum/ or exp harpagophytum extract/ or exp harpagophytum procumbens extract/
-
or/53‐77
-
40 and 52 and 78
October‐November 2009
Medline
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
Randomized Controlled Trials/
-
Random Allocation/
-
Double‐Blind Method/
-
Single‐Blind Method/
-
or/1‐6
-
Animal/ not Human/
-
7 not 8
-
clinical trial.pt.
-
exp Clinical Trial/
-
(clinic$ adj trial$).tw.
-
((single$ or double$ or treble$ or triple$) adj (mask$ or blind$)).tw.
-
Placebos/
-
placebo$.tw.
-
random$.tw.
-
Research Design/
-
(Latin adj square).tw.
-
or/10‐18
-
19 not 8
-
20 not 9
-
Comparative Study/
-
exp Evaluation Studies/
-
Follow‐Up Studies/
-
Prospective Studies/
-
(control$ or prospective$ or volunteer$).tw.
-
Cross‐Over Studies/
-
or/22‐27
-
28 not 8
-
29 not (9 or 21)
-
9 or 21 or 30
-
low back pain/
-
low back pain.tw.
-
backache.mp.
-
lumbago.mp.
-
or/32‐35
-
exp Drugs, Chinese Herbal/
-
herbal.tw.
-
exp Plants, Medicinal/
-
phytomedicine.tw.
-
herb$.tw.
-
weed.tw.
-
exp Algae/
-
exp Fungi/
-
Medicine, Traditional/
-
exp Phytotherapy/
-
exp Pharmacognosy/
-
(oriental adj traditional).tw.
-
exp Medicine, Chinese Traditional/
-
(Camphora adj molmol).tw.
-
Capsicum/
-
Salix/
-
(Maleluca adj alternifolia).tw.
-
exp Angelica sinensis/
-
Aloe/
-
(Thymus adj officinalis).tw.
-
Menthe piperita.tw.
-
Arnica Montana.mp. or exp Arnica/
-
Curcuma longa.mp. or exp Curcuma/
-
exp Tanacetum parthenium/
-
Harpagophytum procumbens.mp. or exp Harpagophytum/
-
Zingiber officii.tw.
-
or/37‐62
-
31 and 36 and 63
-
limit 64 to yr="2005 ‐Current"
Embase
-
Randomized Controlled Trials/
-
Random Allocation/
-
Double‐Blind Method/
-
Single‐Blind Method/
-
4 or 1 or 3 or 2
-
Animal/ not Human/
-
5 not 6
-
exp clinical trial/
-
(clinic$ adj trial$).tw.
-
((single$ or double$ or treble$ or triple$) adj (mask$ or blind$)).tw.
-
Placebos/
-
placebo$.tw.
-
random$.tw.
-
Research Design/
-
(Latin adj square).tw.
-
or/8‐15
-
16 not 6
-
17 not 7
-
Comparative Study/
-
exp Evaluation Studies/
-
Follow‐Up Studies/
-
Prospective Studies/
-
(control$ or prospective$ or volunteer$).tw.
-
Cross‐Over Studies/
-
or/19‐24
-
25 not 6
-
26 not (7 or 18)
-
7 or 18 or 27
-
low back pain/
-
low back pain.tw.
-
backache.mp. or exp backache/
-
lumbago.mp.
-
/29‐32
-
exp Drugs, Chinese Herbal/
-
herbal.tw.
-
exp Plants, Medicinal/
-
phytomedicine.tw.
-
herb$.tw.
-
weed.tw.
-
exp Algae/
-
exp Fungi/
-
Medicine, Traditional/
-
exp Phytotherapy/
-
exp Pharmacognosy/
-
(oriental adj traditional).tw.
-
exp Medicine, Chinese Traditional/
-
(Camphora adj molmol).tw.
-
Capsicum/
-
Salix/
-
(Maleluca adj alternifolia).tw.
-
exp Angelica sinensis/
-
Aloe/
-
(Thymus adj officinalis).tw.
-
Menthe piperita.tw.
-
Arnica Montana.mp. or exp Arnica/
-
Curcuma longa.mp. or exp Curcuma/
-
exp Tanacetum parthenium/
-
Harpagophytum procumbens.mp. or exp Harpagophytum/
-
Zingiber officii.tw.
-
or/34‐59
-
28 and 33 and 60
-
limit 61 to yr="2005 ‐Current"
CENTRAL
#1 MeSH descriptor Low Back Pain, this term only
#2 MeSH descriptor Back Pain, this term only
#3 backache
#4 lumbago
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Drugs, Chinese Herbal explode all trees
#7 MeSH descriptor Plants, Medicinal explode all trees
#8 herbal
#9 phytomedicine
#10 herb*
#11 weed
#12 MeSH descriptor Algae explode all trees
#13 MeSH descriptor Fungi explode all trees
#14 MeSH descriptor Medicine, Traditional explode all trees
#15 MeSH descriptor Phytotherapy, this term only
#16 MeSH descriptor Pharmacognosy explode all trees
#17 Oriental NEXT traditional
#18 MeSH descriptor Medicine, Chinese Traditional, this term only
#19 Camphora NEXT molmo
#20 MeSH descriptor Capsicum, this term only
#21 MeSH descriptor Salix, this term only
#22 Maleluca NEXT alternifolia
#23 MeSH descriptor Angelica sinensis, this term only
#24 MeSH descriptor Aloe, this term only
#25 Thymus NEXT officinalis
#26 Menthe NEXT piperita
#27 MeSH descriptor Arnica, this term only
#28 Arnica NEXT Montana
#29 Curcuma NEXT longa
#30 MeSH descriptor Curcuma, this term only
#31 MeSH descriptor Tanacetum parthenium, this term only
#32 MeSH descriptor Harpagophytum, this term only
#33 Harpagophytum NEXT procumbens
#34 Zingiber NEXT officii
#35 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34)
#36 (#5 AND #35)
#37 (#36), from 2005 to 2009 (Searched with limiter Clinical Trials)
CINAHL
S55 S54 and S28 and S32
S54 S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53
S53 (MH "Devil's Claw")
S52 (MH "Feverfew")
S51 Curcuma longa
S50 (MH "Arnica")
S49 (MH "Aloe")
S48 (MH "Dong Quai")
S47 (MH "Willow Bark")
S46 ("Capsicum") or (MH "Cayenne Pepper")
S45 Camphora W1 molmol
S44 (MH "Medicine, Traditional")
S43 oriental W1 traditional
S42 Pharmacognosy
S41 ("Phytotherapy") or (MH "Medicine, Herbal")
S40 (MH "Medicine, Chinese Traditional")
S39 (MH "Fungi+")
S38 (MH "Algae+")
S37 "weed"
S36 herb*
S35 phytomedicine
S34 (MH "Plants, Medicinal+")
S33 (MH "Drugs, Chinese Herbal")
S32 S29 or S30 or S31
S31 "lumbago"
S30 "backache"
S29 ("low back pain") or (MH "Low Back Pain")
S28 S8 or S18 or S27
S27 S26 not (S18 or S8)
S26 S25 not S7
S25 S19 or S20 or S21 or S22 or S23 or S24
S24 (MH "Crossover Design")
S23 "Follow‐up Studies"
S22 control* or prospective* or volunteer*
S21 (MH "Prospective Studies")
S20 (MH "Evaluation Research+")
S19 (MH "Comparative Studies")
S18 S17 not S8
S17 S16 not S7
S16 S9 or S10 or S11 or S12 or S13 or S14 or S15
S15 Latin W1 Square
S14 (MH "Study Design")
S13 random*
S12 placebo*
S11 (MH "Placebos")
S10 clinic* W2 trial*
S9 "clinical trial"
S8 S6 not S7
S7 (MH "Animals") not ("Human")
S6 S1 or S2 or S3 or S4 or S5
S5 "Single‐Blind Method"
S4 "randomized controlled trial"
S3 "Double‐Blind Method"
S2 (MH "Random Assignment")
S1 (MH "Clinical Trials+")
Appendix 3. Criteria for assessing risk of bias for internal validity
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Blinding of personnel or care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel or care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Blinding of outcome assessors (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
-
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
-
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
-
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity or dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Intention‐to‐treat‐analysis
There is low risk of bias if all randomized patients were reported or analysed in the group to which they were allocated by randomization.
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Unclear' reflected the fact that there was insufficient information to determine whether this criterion was fulfilled or not.
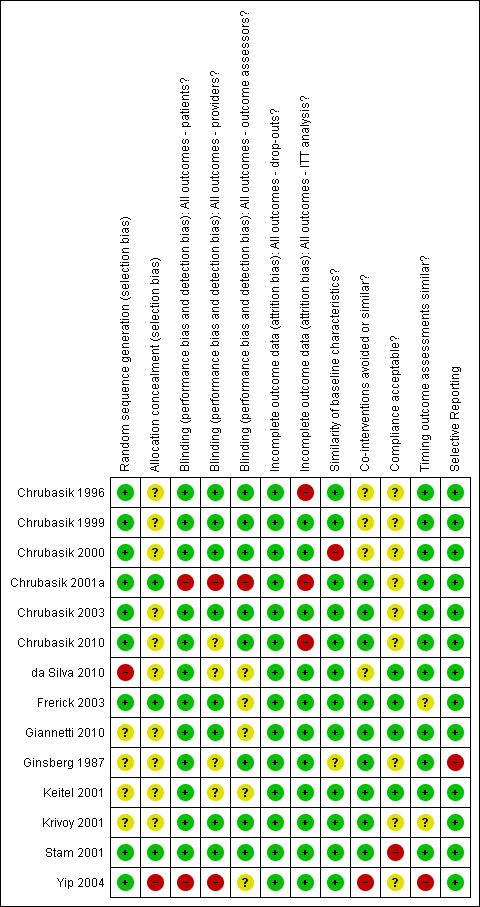
Summary of risk of bias for each of the included trials.
| Brazilian arnica extract compared to placebo for patients with non‐specific chronic back pain or soft tissue pain | |||
| Patient or population: patients with back pain Settings: outpatient clinic Intervention: extract of Brazilian arnica Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain reduction based on Pain VAS instrument 0‐100 scale | 20 | ⊕⊝⊝⊝ | Very small sample size only N = 10 in the treatment group. This trial found that topical application of Brazilian arnica reduced the perception of pain and increased flexibility in the treated group compared to baseline values in that group. Unknown if acute or chronic LBP. |
| GRADE Working Group grades of evidence | |||
| 1Selection bias was high to unclear, performance bias was low risk to unclear risk, with other attributes being low risk. | |||
| Topical capsaicin cream or plaster compared to placebo for patients with non‐specific chronic back pain or soft tissue pain | |||
| Patient or population: patients with chronic LBP or soft tissue pain Settings: Outpatient clinic Intervention: topical capsicum cream or plaster Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain perception according to the Pain VAS scale 0‐10 | 755 | ⊕⊕⊕⊝ | All three trials found a statistically significant difference between the capsaicin intervention vs. placebo. In three trials minor adverse effects were noted in the treatment groups requiring no specific follow‐up treatments. |
| GRADE Working Group grades of evidence | |||
| 1All three trials exhibited low to unclear risk in selection bias, performance bias and attrition bias. One trial was at high risk for selective reporting. | |||
| Topical capsaicin cream compared with placebo for patients with acute non‐specific LBP | |||
| Patient or population: patients with acute mechanical LBP Settings: outpatient clinic Intervention: Rado‐Salil ointment Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain evaluation on a 10 cm linear scale | 40 (one trial) | ⊕⊝⊝⊝ | Pain improvements were significantly greater in the capsicum cream group up to day 14. Adverse events: Pruritis, one in placebo, one in Rado‐Salil group. Local erythema and burning, three in the Rado‐Salil group. |
| GRADE Working Group grades of evidence | |||
| 1Exhibited unclear risk for selection bias as well unclear baseline similarities. Performance bias was low risk as was attrition bias but it was high risk for incomplete outcome data. 2As under 400 participants were included, evidence was downgraded to very low from low. | |||
| H. procumbens compared to placebo for non‐specific chronic back pain | |||
| Patient or population: patients with chronic back pain Settings: outpatient clinic Intervention:H. procumbens extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Arhus pain index scale 0‐130 | 315 | ⊕⊕⊝⊝ | In one trial a 50mg dose of H. procumbens was used, and in the second trial a 50 mg and 100 mg dose was used with both trialss showing a significantly improved pain score over placebo. |
| GRADE Working Group grades of evidence | |||
| 1Both included trials exhibited low risk of bias regarding selection bias with one trial at unclear risk of bias. Performance bias was at low risk of bias, as was attrition bias with one trial at high risk of bias for incomplete outcome data. 2Two trials included under 400 participants and we downgraded the evidence to low from moderate. | |||
| H. procumbens extract compared to Vioxx®for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic Intervention:H. procumbens extract Comparison: Vioxx® | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Modified Arhus Index Scale 0‐120 | 88 | ⊕⊝⊝⊝ | H. procumbens was compared to Vioxx® and while both groups showed similar pain reduction scores there were no demonstrable difference among groups. There were adverse effects noted in both groups. |
| GRADE Working Group grades of evidence | |||
| 1This trial was at low risk of bias for all risk of bias factors, with the exception of allocation concealment and compliance which were at unclear risk of bias. 2Downgraded to very low versus low as under 400 participants were included. | |||
| Willow bark extract compared to placebo for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic and public advertisement Intervention: willow bark extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS Scale 0‐10 | 261 | ⊕⊕⊕⊝ | The high dose (240 mg) treatment group showed a significant reduction in pain scores versus the low dose (120 mg) group and the placebo group. There was one severe allergic reaction related to the extract noted. One trial (N = 51) also examined the effect of the extract on platelet aggregation. |
| GRADE Working Group grades of evidence | |||
| 1Both trials were at low to unclear risk for selection bias, low risk for performance bias with one trial exhibiting high risk in baseline characteristics similarity. Both trials were rated as an overall low risk of bias since they met our predetermined cut‐point of 50% of the criteria on which the trial methods were assessed. 2Downgraded from high to moderate as under 400 participants were included between both trials. | |||
| Willow bark extract compared to rofecoxib for non‐specific chronic LBP | |||
| Patient or population: patients with chronic LBP Settings: outpatient clinic Intervention: willow bark extract Comparison: rofecoxib | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Arhus Index Scale 0‐130 Pain VAS Scale 0‐10 | 228 | ⊕⊝⊝⊝ | There was no significant difference in the effectiveness and adverse events between the extract and rofecoxib. |
| GRADE Working Group grades of evidence | |||
| 1Low risk for selection bias, high risk for performance bias, and high and low risk for attrition bias. 2Downgraded from low to very low due as under 400 participants were included. | |||
| Comfrey root extract compared to placebo for acute lower and upper back non‐specific pain | |||
| Patient or population: patients with acute lower and upper back pain Settings: outpatient setting Intervention: comfrey root extract Comparison: placebo | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS sum (decrease) on active standardized movement (mm) | 120 | ⊕⊕⊝⊝ | The root extract showed a statistically and clinically relevant reduction in acute back pain versus placebo. |
| GRADE Working Group grades of evidence | |||
| 1Unclear risk for selection bias, low risk for both performance and attrition bias. 2Downgraded from moderate to low as under 400 participants were included. | |||
| Lavender oil acupressure massage and acupoint stimulation compared to usual treatment for acute non‐specific LBP | |||
| Patient or population: patients with acute LBP Settings: old aged home and community centre Intervention: lavender oil massage Comparison: usual therapy | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS 0‐10 scale | 61 | ⊕⊝⊝⊝ | One week post‐study the treatment group showed a significant (P = 0.0001) reduction in VAS pain as well as improved walking time and lateral spine flexion range. |
| GRADE Working Group grades of evidence | |||
| 1Sequence generation was at low risk of bias but allocation concealment was at high risk. Performance bias was at high and unclear risk. Co‐interventions and timing outcome assessment factors were at high risk of bias. | |||
| Spiroflor SRL compared to CCC for chronic non‐specific LBP | |||
| Patient or population: patients with acute and chronic LBP Settings: outpatient clinic Intervention: Spiroflor SRL Comparison: CCC | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| Pain VAS 0‐100 scale | 161 | ⊕⊝⊝⊝ | Spiroflor SRL and CCC were equally effective in treating acute LBP but the CCC group experienced greater adverse events and adverse drug reactions. |
| GRADE Working Group grades of evidence | |||
| CCC = Cremor Capsici Compositus FNA; SRL = Homeopathic combination of Symphytum officinale, Rhus toxicodendron and Ledum palustre 1All risk of bias factors were at low risk of bias, except patient compliance which was at high risk. 2Downgraded from low to very low as under 400 participants were included. | |||

