کنسانتره فاکتور VIIa نوترکیب در برابر کنسانتره مشتق از پلاسما، برای درمان اپیزودهای خونریزی حاد در افراد مبتلا به هموفیلی و مهارکنندهها
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open‐label cross‐over multicentre RCT. | |
| Participants | Individuals with severe haemophilia A with inhibitors not undergoing ITI. A total of 66 individuals were enrolled, but 14 withdrew prior to treatment or were treated only once. Diaries for a further 4 participants were not adequately completed. Age: mean 27.5 years (range 8 ‐ 55 years). Mean inhibitor titre 8.6 BU/ml (range 0 ‐ 1800). 96 episodes in 48 participants. | |
| Interventions | aPCC (FEIBA®) 75 ‐ 100 IU/kg (target 85 IU/kg) as a single IV bolus. Activated rFVII (NovoSeven®) 90 ‐ 120 mcg/kg (target 105 mcg/kg) as IV bolus repeated after 2 hours. Both treatments were administered a mean of 2 hours after bleeding onset. | |
| Outcomes | Subjective evaluation of treatment efficacy based on a four level scale (effective, partially effective, poorly effective, not effective); efficacy was defined as effective or partially effective by participant rating at 6 hours (primary) and at various times from 2 ‐ 48 hours (secondary). Subjective evaluation of stop of bleeding (binary outcome). Additonal treatments and the occurrence of re‐bleeding were recorded. | |
| Notes | Use of analgesics was allowed and its distribution in the treatment group was evaluated. A significantly different number of knee (higher in Novoseven®‐treated participants) and elbow (higher in aPCC‐treated participants) bleeding events were recorded. The analysis technique used to balance for the uneven distribution of knee bleeds is unclear and not sufficiently detailed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in association with the first bleeding event was performed in a block of participants equally divided into two. |
| Allocation concealment (selection bias) | High risk | Open‐label trial. A randomisation list specifying the order of treatment for enrolled participants was provided to each participating centre. |
| Blinding (performance bias and detection bias) | High risk | Blinding was not possible for physicians and participants because of difference between the 2 products (physical appearance and required volume for injection). Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | High risk | The trial was analysed on a per protocol basis. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and the results sections correspond. |
| Other bias | High risk | Use of analgesics allowed during the trial. A significantly different number of knee (higher in participants treated with Novoseven®) and elbow (higher in participants treated with aPCC) bleeding events were recorded. The analysis technique used to balance for the uneven distribution of knee bleeds is unclear and not sufficiently detailed. |
| Methods | Open‐label cross‐over multicentre 3‐tier RCT. The comparison between rFVIIa and aPCC was open label, while the comparison between the two different rFVIIa regimens was concealed. Outcome assessor was blinded. | |
| Participants | Individuals with severe haemophilia A and B with inhibitor (the number of participants with A and B was not separately specified). A total of 42 were randomised, with 21 completing all 3 arms of treatment. Age: mean 19.5 years (range 1 ‐ 54 years). | |
| Interventions | Activated rrF VII (NovoSeven®) 90 mcg/kg as IV bolus administered at 0, 3 and 6 hours. Activated recombinant factor VII (NovoSeven®) 270 mcg/kg as single IV bolus (followed by 2 placebo infusions). aPCC (FEIBA®) 75 IU/kg as a single IV bolus. | |
| Outcomes | Primary outcomes Number of participants requiring additional treatment. Secondary outcomes Subjective pain and mobility scale rating evaluated as global treatment response (composite end‐point) and separately. Rate of adverse events. | |
| Notes | The trial states that the analysis was performed on an intention‐to‐treat basis, but the data seems to have been analysed on‐treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 6 treatment sequences were generated by the permutation of the 3 dosing regimens. The sequences were randomly assigned to the participants. |
| Allocation concealment (selection bias) | Unclear risk | The comparison between rFVIIa and aPCC was open label, while the comparison between the 2 different rFVIIa regimens was described as blinded without details about randomisation code concealment. |
| Blinding (performance bias and detection bias) | High risk | Participants and clinicians could not be blinded to comparison between both NovoSeven® treatments and the FEIBA® treatment due to differences in physical appearance and required volume for injection, but comparison of 2 NovoSeven® treatments was blinded (3 active versus 1 active and 2 placebo doses). Outcome assessment was blinded for the treatments. |
| Incomplete outcome data (attrition bias) | High risk | The trial states that the analysis was performed on an intention‐to‐treat basis, but the data seems to have been analysed on‐treatment. |
| Selective reporting (reporting bias) | Low risk | Outcome data reported in the methods and results sections correspond. |
| Other bias | Unclear risk | Use of analgesics allowed during the trial. Distribution of analgesics use among group was evaluated. The trial was interrupted by the sponsor for unspecified reasons. |
aPCC: activated prothrombin complex concentrates
BU: Bethesda units
ITI: immune tolerance induction
IV: intravenous
RCT: randomised controlled trial
rFVIIa: recombinant factor VIIa
vs: versus
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised or quasi‐randomised controlled trial. | |
| Dosage‐finding trial: comparator is not an alternative therapy. | |
| Double‐blind cross‐over RCT comparing two different regimens of rFVIIa. | |
| RCT comparing two different regimens of rFVIIa. | |
| Dosage‐finding trial: comparator is not an alternative therapy. | |
| Dosage‐finding trial: comparator is not an alternative therapy. | |
| RCT comparing two different regimens of rFVIIa. | |
| RCT comparing two different regimens of rFVIIa. | |
| Open label randomised RCT comparing two different regimens of rFVIIa (90 mcg/kg boluses versus continuous infusion) in people with haemophilia undergoing major surgery. | |
| Open label cross‐over RCT comparing two different regimens of rFVIIa. | |
| Not a randomised or quasi‐randomised controlled trial. | |
| Dosage‐finding trial: comparator is not an alternative therapy. | |
| Dosage‐finding trial: comparator is not an alternative therapy. |
RCT: randomised controlled trial
rFVIIa: recombinant factor VIIa
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
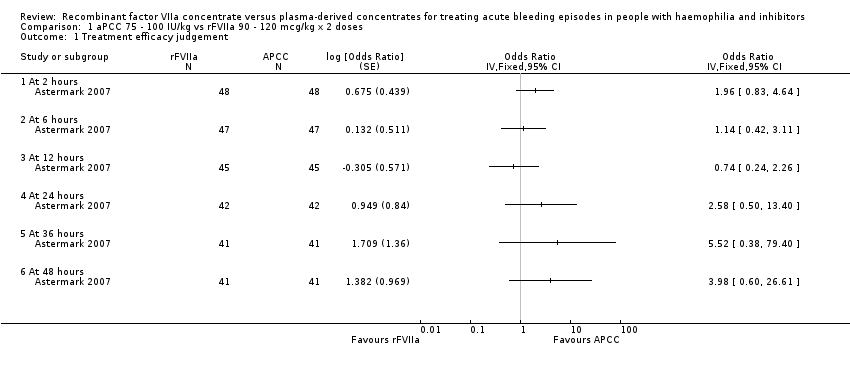
| 1 Treatment efficacy judgement Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 aPCC 75 ‐ 100 IU/kg vs rFVIIa 90 ‐ 120 mcg/kg x 2 doses, Outcome 1 Treatment efficacy judgement. | ||||
| 1.1 At 2 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 12 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 24 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 At 36 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 At 48 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
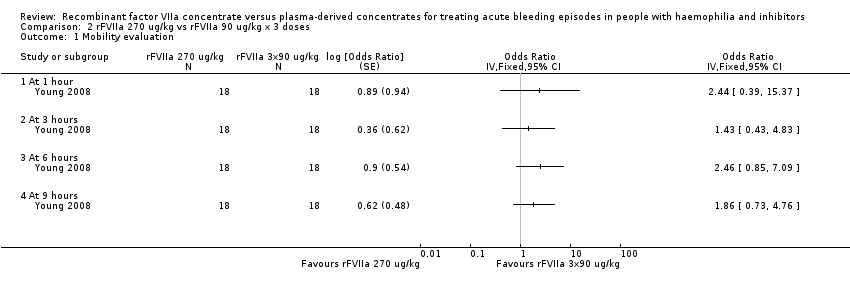
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 1 Mobility evaluation. | ||||
| 1.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 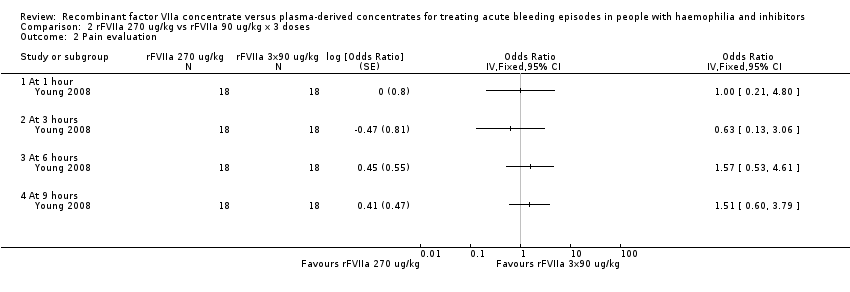 Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 2 Pain evaluation. | ||||
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 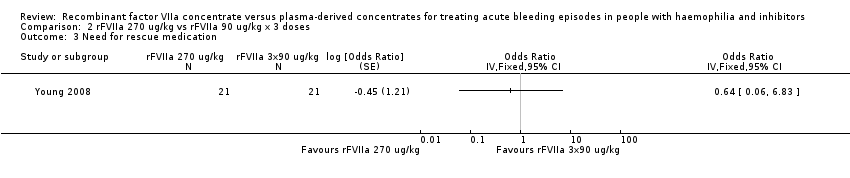 Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 3 Need for rescue medication. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 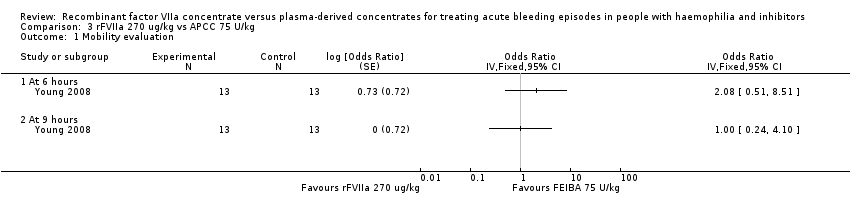 Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 1 Mobility evaluation. | ||||
| 1.1 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 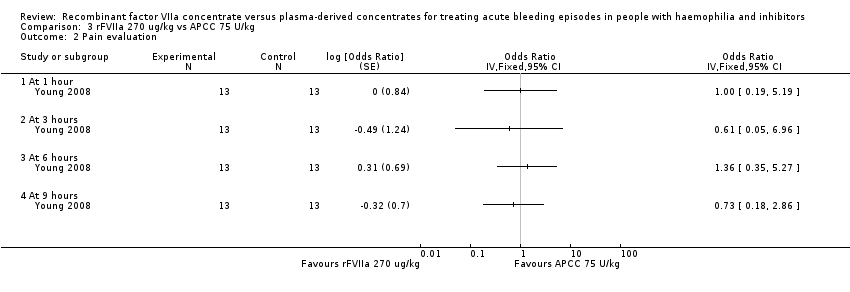 Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 2 Pain evaluation. | ||||
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 3 Need for rescue medication. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 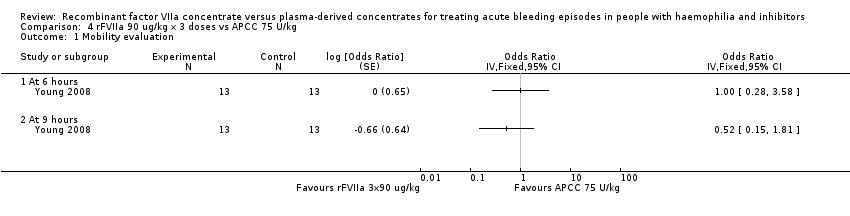 Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 1 Mobility evaluation. | ||||
| 1.1 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 2 Pain evaluation. | ||||
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 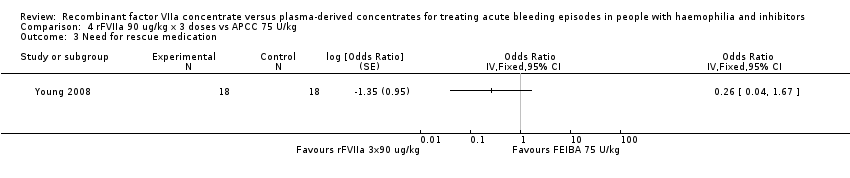 Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 3 Need for rescue medication. | ||||
Comparison 1 aPCC 75 ‐ 100 IU/kg vs rFVIIa 90 ‐ 120 mcg/kg x 2 doses, Outcome 1 Treatment efficacy judgement.
Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 1 Mobility evaluation.
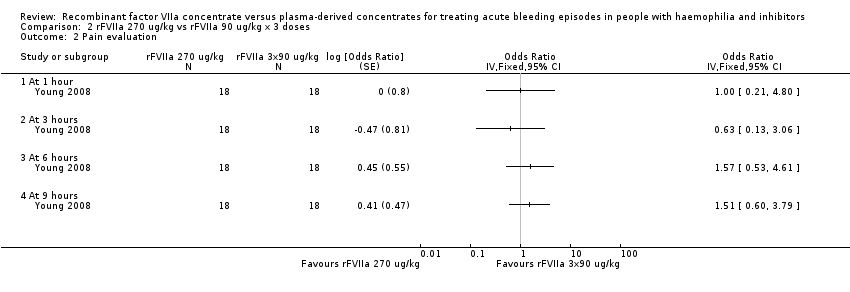
Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 2 Pain evaluation.
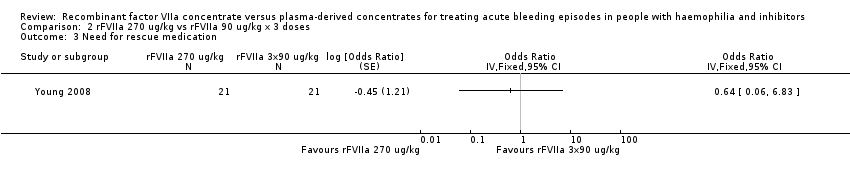
Comparison 2 rFVIIa 270 ug/kg vs rFVIIa 90 ug/kg x 3 doses, Outcome 3 Need for rescue medication.

Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 1 Mobility evaluation.
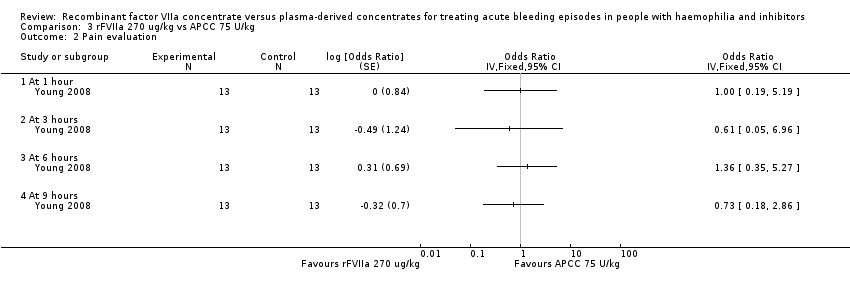
Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 2 Pain evaluation.

Comparison 3 rFVIIa 270 ug/kg vs APCC 75 U/kg, Outcome 3 Need for rescue medication.

Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 1 Mobility evaluation.

Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 2 Pain evaluation.

Comparison 4 rFVIIa 90 ug/kg x 3 doses vs APCC 75 U/kg, Outcome 3 Need for rescue medication.
| Study ID | Hours (pts number) | aPCC n (%) | rFVIIa n (%) | 90% CI of the difference (%) | P value |
| 2 (48) | 36 (75.0) | 29 (60.4) | ‐0.73 to 29.9 | 0.482 | |
| 6 (47) | 38 (80.9) | 37 (78.7) | ‐11.42 to 15.67 | 0.059 | |
| 12 (45) | 38 (80.0) | 38 (84.4) | ‐18.08 to 9.19 | 0.101 | |
| 24 (42) | 40 (95.2) | 36 (85.7) | ‐1.29 to 20.33 | 0.202 | |
| 36 (41) | 41 (100) | 37 (90.2) | 2.13 to 17.38 | 0.129 | |
| 48 (41) | 40 (97.6) | 35 (85.4) | 2.05 to 22.34 | 0.325 | |
| The table reports the number and % of participants who judged the treatment efficacious for any treatment and any time point. The 90% CIs of the difference test the hypothesis of equivalence between the treatments. When considering the difference at 2 hours, it has to be taken into account that this time point is before the administration of the second rFVIIa bolus. aPCC: activated prothrombin complex concentrates | |||||
| Study ID | Hours (number of participants) | aPCC (%) | rFVIIa (%) | 90% CI of the difference (%) | P value |
| 2 (47) | 53.2 | 38.3 | 0.06 to 29.72 | 0.495 | |
| 6 (46) | 76.1 | 65.2 | ‐2.73 to 24.47 | 0.309 | |
| 12 (45) | 77.8 | 75.6 | ‐11.92 to 16.37 | 0.069 | |
| 24 (42) | 90.5 | 85.7 | ‐4.75 to 14.28 | 0.038 | |
| 36 (41) | 95.1 | 87.8 | ‐1.45 to 16.09 | 0.075 | |
| 48 (41) | 95.1 | 92.7 | 4.48 to 9.36 | 0.001 | |
| The table reports the number and % of participants who judged the treatment efficacious for any treatment and any time point. The 90% CIs of the difference test the hypothesis of equivalence between the treatments. When considering the difference at 2 hours, it has to be taken into account that this time point is before the administration of the second rFVIIa bolus. aPCC: activated prothrombin complex concentrates | |||||
| Study ID | Outcome | rFVIIa 270 mcg/kg (N = 24) | rFVIIa 90 mcg/kg x 3 (n = 22) | aPCC 75 IU/kg (n = 22) |
| Positive treatment response (%) | 45.8 | 54.5 | 27.3 | |
| The response was globally evaluated 9 hours after treatment. The positive response were defined as at least 3 positive assessments at 1, 3, 6 and 9 hours. The positive assessment was defined on the base of a 3‐level scale (more pain, no difference, less pain). There were no statistically significant differences between treatments (P = 0.219). aPCC: activated prothrombin complex concentrates | ||||
| Study ID | Outcome | rFVIIa 270 mcg/kg (N = 24) | rFVIIa 90 mcg/kg x 3 (n = 22) | aPCC 75 IU/kg (n = 22) |
| Positive treatment response (%) | 25.0 | 45.5 | 22.7 | |
| The response was globally evaluated 9 hours after treatment. The positive response were defined as at least 3 positive assessments at 1, 3, 6 and 9 hours. The positive assessment was defined on the base of a 3‐level scale (more mobility, no difference, less mobility). There were no statistically significant differences between treatments (P = 0.903). aPCC: activated prothrombin complex concentrates | ||||
| Study ID | Outcome | rFVIIa 270 mcg/kg (n = 24) | rFVIIa 90 mcg/kg x 3 (n = 22) | aPCC 75 IU/kg (n = 22) |
| Participants requiring rescue medication n (%) | 2 (8.3) | 2 (9.1) | 8 (36.4) | |
| Participants with an insufficient treatment response within 6 hours of the first treatment administration were evaluated in the clinic or by phone to consider the use of rescue medication. Rescue medication was defined as additional haemostatic treatment within 9 hours post first administration of trial product. The difference between rFVIIa 270 mcg/kg vs aPCC was statistically significant (P = 0.032). The efficacy difference between the aPCC treatment group and the rFVIIa 90 x 3 mcg/kg did not reach statistical difference (P = 0.069). aPCC: activated prothrombin complex concentrates | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment efficacy judgement Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 2 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 12 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 24 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 At 36 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 At 48 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mobility evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain evaluation Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 hour | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 9 hours | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Need for rescue medication Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |

