Ventilação mecânica para pacientes com esclerose lateral amiotrófica/doença do neurônio motor
Resumo
Introdução
A esclerose lateral amiotrófica (ELA), também conhecida como doença do neurônio motor, é uma doença neurodegenerativa fatal. A insuficiência respiratória de origem neuromuscular é a causa mais comum de morte, que geralmente ocorre dentro de dois a cinco anos após o início da doença. O uso de ventilação mecânica para ajudar a respiração pode aumentar a sobrevida e a qualidade de vida dos pacientes com essa doença. Esta é a segunda atualização de uma revisão originalmente publicada em 2009.
Objetivos
Avaliar os efeitos da ventilação mecânica (ventilação assistida pela traqueostomia e ventilação não‐invasiva (VNI)) na sobrevida, medidas funcionais de progressão da doença e qualidade de vida de pacientes com ELA, e avaliar os eventos adversos relacionados à intervenção.
Métodos de busca
Em 30 de janeiro de 2017, fizemos buscas nas seguintes bases de dados: The Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL Plus e AMED. Também fizemos buscas por estudos em andamento em duas plataformas de registros de ensaios clínicos.
Critério de seleção
Incluímos ensaios clínicos randomizados (ECRs) e quasi‐randomizados que avaliaram o uso da VNI ou da ventilação assistida pela traqueostomia em participantes com diagnóstico clínico de ELA, independente dos desfechos relatados. Incluímos comparações com nenhuma intervenção ou com o melhor tratamento padrão.
Coleta dos dados e análises
Na revisão original, quatro autores, trabalhando de forma independente, selecionaram os estudos. Nesta atualização, dois autores avaliaram os resultados da mais nova busca. Os autores ,trabalhando de forma independente, extraíram os dados dos estudos selecionados para leitura na íntegra e avaliaram o risco de viés dos estudos que preencheram os critérios de inclusão. Tentamos obter os dados faltantes sempre que possível. Havíamos planejado coletar dados sobre eventos adversos dos estudos incluídos.
Principais resultados
Na revisão original, os autores identificaram e incluíram dois ECRs envolvendo 54 participantes com ELA submetidos à VNI. Não identificamos novos ECRs ou quasi‐randomizados na primeira atualização. Nesta segunda atualização, Identificamos um novo ECR mas ele foi excluído pelas razões descritas abaixo.
Um dos estudos publicados, comparando início precoce versus tardio da VNI em 13 participantes, apresentava dados incompletos no artigo. Entramos em contato com os autores desse ECR, mas eles não foram capazes de nos fornecer os dados que faltavam. Portanto, os resultados da revisão são baseados em um único estudo (com 41 participantes), que comparou VNI versus tratamento padrão. De uma forma geral, esse estudo foi bem conduzido e tinha um baixo risco de viés. Porém, a falta (ou incerteza) de cegamento pode ter introduzido viés para os desfechos relatados pelos pacientes ou pelos profissionais de saúde (como qualidade de vida).
Existe evidência de qualidade moderada (proveniente desse estudo) de um aumento significante da sobrevida mediana no grupo VNI comparado ao grupo que recebeu cuidados habituais. A sobrevida mediana no grupo VNI foi 48 dias mais longa do que no grupo que recebeu o tratamento padrão: 219 dias versus 171 dias, IC 95% 12 a 91 dias, p = 0,0062. Esse aumento da sobrevida foi acompanhado de melhora na qualidade de vida. Na análise por subgrupos, a sobrevida e a qualidade de vida foram maiores no subgrupo de pacientes com função bulbar normal ou com acometimento moderado (20 participantes): sobrevida mediana 205 dias maior (216 dias no grupo VNI versus 11 dias no grupo de tratamento padrão), P=0,0059, evidência de baixa qualidade. A VNI não prolongou a vida nem melhorou a qualidade de vida dos pacientes com pouca função bulbar (21 participantes), porém melhorou significantemente os escores médios dos sintomas no questionário Sleep Apnea Quality of Life Index. Nenhum dos estudos apresentou dados clínicos sobre eventos adversos associados à intervenção.
Conclusão dos autores
Existe evidência de moderada qualidade, proveniente de um único ECR sobre VNI (com 41 participantes), sugerindo que esta intervenção prolonga significativamente a sobrevida de pacientes com ELA. Há também evidência de baixa qualidade de que a VNI melhora ou mantém a qualidade de vida desses pacientes. A sobrevida e a qualidade de vida melhoraram significativamente no subgrupo de pacientes com função bulbar melhor, mas não naqueles com comprometimento bulbar mais grave. Os efeitos adversos relacionados ao uso da VNI devem ser sistematicamente relatados, uma vez que no momento existe pouca informação a este respeito. Será difícil gerar mais evidência proveniente de ECRs para apoiar o uso da VNI em pacientes com ELA, uma vez que não seria mais ético deixar de oferecer VNI ao grupo controle. Estudos futuros devem avaliar os benefícios da intervenção precoce com VNI e estabelecer o momento mais apropriado para iniciá‐la, visando obter o seu maior benefício. Também é necessário fazer um ECR para avaliar o efeito de adicionar técnicas que promovam a tosse à VNI. Futuros estudos devem também avaliar aspectos econômicos relacionados à VNI. O acesso à VNI ainda é restrito em muitos lugares do mundo, incluindo na Europa e na América do Norte. Precisamos entender melhor os fatores pessoais e econômicos que influenciam o acesso à VNI.
PICOs
Resumo para leigos
Ventilação mecânica para pessoas com esclerose lateral amiotrófica/doença do neurônio motor
Pergunta da revisão
A ventilação mecânica melhora a sobrevida de pessoas com esclerose lateral amiotrófica (ELA)? Como a ventilação mecânica afeta a progressão da doença e a qualidade de vida desses pacientes? Ela tem algum efeito indesejado?
Introdução
A esclerose lateral amiotrófica (ELA), também conhecida como doença do neurônio motor, é uma condição em que os nervos que controlam os movimentos param de funcionar. Nos últimos dez anos houve muitos avanços nos cuidados para pacientes com ELA, Embora a ELA ainda seja uma doença sem cura, alguns tratamentos podem ajudar no manejo dos sintomas. A ELA causa fraqueza muscular progressiva, inclusive fraqueza dos músculos responsáveis pela respiração. A insuficiência ventilatória (capacidade de fazer o ar entrar e sair dos pulmões) é uma causa importante de morte dos pacientes com ELA. A ventilação mecânica consiste no uso de máquinas que ajudam as pessoas a respirar. A ventilação mecânica pode ser invasiva ou não‐invasiva. A ventilação invasiva envolve a colocação de um tubo na garganta (traqueostomia). A ventilação não‐invasiva (VNI) é um método para ajudar as pessoas a respirar que não requer uma traqueostomia. A VNI consiste no uso de uma máscara (colocada no rosto ou no nariz do paciente) conectada por um tubo a um pequeno ventilador portátil.
Características do estudo
Nesta atualização da revisão, avaliamos a evidência proveniente de dois ensaios clínicos randomizados (ECRs) que testaram o uso da VNI em um total de 54 pacientes com ELA. Um dos estudos, que avaliou quando iniciar a VNI, não forneceu dados que pudessem ser usados na revisão. Um terceiro estudo, identificado em uma base de registros de ensaios clínicos, ainda não tem resultados publicados até o momento.
Resultados principais e qualidade da evidência
Apenas um estudo com 41 participantes tinha dados completos que puderam ser usados. Os resultados deste estudo forneceram evidência de qualidade moderada indicando que a VNI prolonga significativamente a sobrevida dos pacientes com ELA. Este estudo também forneceu evidência de baixa qualidade que a VNI melhora ou mantém a qualidade de vida destes pacientes em comparação com o tratamento padrão. A sobrevida mediana do grupo que recebeu a VNI aumentou em cerca de 48 dias. Ou seja, a sobrevida, que era de 171 dias (no grupo que recebeu o tratamento padrão), foi para 219 dias (no grupo que recebeu a VNI). O aumento da sobrevida com a VNI foi bem maior nos pacientes com ELA que tinham pouco ou nenhum envolvimento dos músculos usados para falar, mastigar e engolir (músculos bulbares). Entre os 20 participantes com essas características, o VNI aumentou a sobrevida mediana em aproximadamente 205 dias. Ou seja, a sobrevida no grupo com a VNI foi de 216 dias, em comparação com 11 dias no grupo que recebeu o tratamento padrão. Nos participantes com fraqueza leve ou moderada dos músculos bulbares, a qualidade de vida também se manteve. Nos 21 pacientes com fraqueza importante dos músculos bulbares, a VNI não prolongou a vida nem manteve os escores de qualidade de vida, porém melhorou uma avaliação de sintomas relacionados ao sono. Nenhum dos estudos apresentou dados sobre efeitos adversos relacionados ao uso da VNI. Tanto os participantes como os profissionais de saúde sabiam a qual grupo os participantes pertenciam. Isso pode ter influenciado as avaliações de qualidade de vida.
É pouco provável que mais estudos comparando VNI versus tratamento padrão em pacientes com ELA sejam feitos, uma vez que não seria ético deixar de utilizar a VNI. Estudos futuros devem avaliar a introdução precoce da VNI e descobrir qual seria o melhor momento de iniciá‐la.
A evidência está atualizada até janeiro de 2017.
Authors' conclusions
Summary of findings
| Non‐invasive ventilation compared with standard care for amyotrophic lateral sclerosis (ALS) | ||||||
| Patient or population: people with ALS Settings: people with ALS attending a single regional care centre Intervention: non‐invasive ventilation Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Non‐invasive ventilation (NIV) | |||||
| Survival | All participants Median survival was 171 days. | All participants Median survival was 48 days longer (12 to 91 days1 longer). | ‐ | 41 (1 study) | ⊕⊕⊕⊝ Moderate2 | 21 of the 41 participants had poor bulbar function. P = 0.0059 better bulbar function, P = 0.92 poor bulbar function |
| Participants with better (good or moderately impaired) bulbar function Median survival was 11 days. | Participants with better (good or moderately impaired) bulbar function Median survival was 205 days longer (CI not given). | |||||
| Participants with poor bulbar function Median survival was 261 days. | Participants with poor bulbar function Median survival was 39 days shorter (CI not given). | |||||
| Quality of life (SF‐36 MCS) | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 99 days. | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 69 days longer (45 to 667 days longer). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 195 days longer (P = 0.001, CI not given). | |||||
| Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 164 days. | Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 37 days shorter (P = 0.64, CI not given). | |||||
| Quality of life (SF‐36 PCS) | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 81 days. | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 69 days longer (P = 0.004). | ‐ | ‐ | ‐ | CI not given |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 175 days longer (P < 0.001). | |||||
| Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 18 days longer (P = 0.88). | |||||
| Quality of life (SAQLI) | All participants Median duration that SAQLI remained above 75% of baseline was 99 days. | All participants Median duration that SAQLI remained above 75% of baseline was 74 days longer (P = 0.031). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | CI not given |
| Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of baseline was 4 days. | Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of the baseline was 195 days longer (P = < 0.001). | |||||
| Participants with poor bulbar function Median duration that SAQLI remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SAQLI remained above 75% of the baseline was 29 days shorter (P = 0.77). | |||||
| Adverse events (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Calculated CIs are approximate. | ||||||
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a fatal neurodegenerative disease characterised by loss of upper and lower motor neurons in the brain and spinal cord (Brooks 1994; Brooks 2000). The incidence of ALS is 1 to 2 per 100,000 of the population, and the age‐specific incidence and mortality rates peak at 55 to 75 years (Worms 2001). The average life expectancy is two to three years from the onset of symptoms, although 10% of people with ALS may survive for 10 years or more (Haverkamp 1995; Turner 2003). Death usually results from respiratory failure due to denervation weakness in respiratory muscles. Respiratory muscle function at any time point during the disease trajectory is the most important predictor of survival and an important predictor of quality of life (Bach 1995; Haverkamp 1995; Vitacca 1997; Stambler 1998; Fitting 1999; Chaudri 2000; Bourke 2001; Lyall 2001a; Varrato 2001; Lechtzin 2002). Measures of respiratory muscle strength (e.g. forced vital capacity and sniff nasal inspiratory pressure) are useful in monitoring the progression of respiratory muscle weakness, but no single test of respiratory function can reliably predict the onset of respiratory failure. Furthermore, respiratory function tests have limitations in people with bulbar weakness, who cannot blow effectively (Lyall 2001a).
Assisted ventilation has long been used to support ventilation in respiratory failure (Annane 2014). Assisted ventilation can be provided by invasive (tracheostomy ventilation (TV)) and non‐invasive (NIV) means. Tracheostomy ventilation can prolong survival for many years (Bach 1993; Cazzolli 1996), but it is resource‐intensive and risks ventilator entrapment, which exacts a significant emotional toll on people with ALS and their carers (Moss 1993; Cazzolli 1996; Moss 1996). Tracheostomy ventilation may prolong life in the face of increasing disability and dependency, and hence quality of life may not be sustained. Nevertheless, people affected by ALS are increasingly aware of this option. Tracheostomy ventilation in ALS is not encouraged in Europe and North America (Hayashi 1997; Borasio 1998; Yamaguchi 2001). In Japan, however, TV is the predominant form of ventilation offered to people with ALS, and the cost is fully covered by the government and medical insurance (Kawata 2008).
Non‐invasive ventilation is another option for treating respiratory failure in people with ALS. Non‐invasive ventilation utilises a face or nasal mask and a volume‐cycled or bilevel pressure‐limited ventilator to provide an intermittent positive pressure to support ventilation. Until the turn of the century, the use of NIV varied greatly across North America and Europe (Melo 1999; Borasio 2001; Bradley 2001; Cedarbaum 2001; Chio 2001; Bourke 2002). Evidence from several retrospective and some prospective studies indicated that NIV may be associated with gain in survival (Pinto 1995; Aboussouan 1997; Kleopa 1999; Bach 2002), improved quality of life (Hein 1997; Hein 1999; Aboussouan 2001; Bourke 2001; Jackson 2001; Lyall 2001b), and improved cognitive function (Newsom‐Davis 2001). People with ALS who have little or no bulbar muscle weakness may tolerate NIV better than those with significant bulbar involvement (Cazzolli 1996; Aboussouan 1997). In the absence of a randomised controlled trial (RCT), uncertainties remained over the benefits and unwanted effects of TV and NIV.
Annane 2014, a Cochrane Review of nocturnal mechanical ventilation for a mixed group of people with chronic hypoventilation, identified eight randomised trials, three of which involved people with ALS. This review concluded that nocturnal ventilation may relieve chronic hypoventilation‐related symptoms and prolong survival, but that the quality of the studies was poor and the benefit of long‐term mechanical ventilation should be confirmed in further trials. The evidence was thought to be strongest for NIV in people with ALS.
Over the past few years, the use of NIV in ALS has greatly increased (O'Neill 2012). An RCT evaluated the effects of NIV on survival and quality of life in people with ALS (Bourke 2006). The National Institute for Health and Care Excellence UK (NICE) has published guidelines on the use of NIV in people with ALS (NICE 2010). The aim of this review was to assimilate the evidence for mechanical ventilation in ALS and to assess the benefits and unwanted effects of TV and NIV. The conclusions of Radunovic 2009, the original version of this review, were based on the results of this single study of 41 participants (Bourke 2006), as the other available study provided no usable data (Jackson 2001). For this 2017 update, we identified no new RCTs or quasi‐RCTs.
Objectives
To assess the effects of mechanical ventilation (tracheostomy‐assisted ventilation and non‐invasive ventilation) on survival, functional measures of disease progression, and quality of life in ALS, and to evaluate adverse events related to the intervention.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs involving NIV or TV. Quasi‐RCTs are trials where treatment allocation was intended to be random but may have been biased (e.g. alternate allocation or allocation according to the day of the week). We selected studies independently of reported outcomes.
Types of participants
People with a clinical diagnosis of ALS/MND (pure mixed upper motor neuron and lower motor neuron degeneration with supportive electromyogram) according to the El Escorial criteria (Brooks 1994; Brooks 2000), at any stage of disease and with any clinical pattern of the condition (e.g. bulbar and limb onset). Subgroups of interest were participants with or without significant bulbar symptoms as categorised by the authors of the papers reviewed.
We did not consider trials of mixed neuromuscular or chest wall conditions, which are included in a separate review (Annane 2014).
Types of interventions
All forms of NIV (irrespective of pressure settings and timings) and TV, compared to no intervention or the best standard care.
Types of outcome measures
Our outcomes were not selection criteria, but rather a list of outcomes of interest within included studies.
Primary outcomes
The primary outcome was overall survival after initiation of assisted ventilation, as assessed by a pooled hazard ratio using life table/Cox regression methods to combine disparate periods of observation from all studies. This would have been supplemented where possible by pooled estimates of the 75%, 50% (median) survival times and confidence intervals (CIs). This is to allow for the situation where the proportional hazards assumption, necessary for Cox regression, has not been met.
Secondary outcomes
-
Survival at one month and six months or longer.
-
Quality of life assessed using validated health status questionnaires, e.g. 36‐Item Short Form Health Survey (SF‐36) at one month and six months or longer (Lyall 2001b).
-
Any validated functional rating scale, such as the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) or the ALSFRS‐Revised (ALSFRS 1996; Cedarbaum 1999), Norris (Norris 1974), or Appel scales at one month and six months or longer (Haverkamp 1995).
-
The proportion of people experiencing adverse events related to mechanical ventilation. We would have considered adverse events in two categories. The first category would have included the proportion of participants experiencing any adverse event attributed to ventilation (e.g. fistulae, pneumothorax, bleeding, local infection, hospitalisation, or death), and the second category would have included participants experiencing severe complications of mechanical ventilation, including life‐threatening episodes, prolonged hospitalisation, and death.
Search methods for identification of studies
We searched the following databases:
-
the Cochrane Neuromuscular Specialised Register (30 January 2017) (Appendix 1);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (30 January 2017, in the Cochrane Register of Studies Online (CRSO)) (Appendix 2);
-
MEDLINE (1966 to 30 January 2017) (Appendix 3);
-
Embase (1980 to 30 January 2017) (Appendix 4);
-
CINAHL Plus (1937 to 30 January 2017) (Appendix 5);
-
AMED (Allied and Complementary Medicine Database) (1985 to 30 January 2017) (Appendix 6).
We also searched for ongoing or unpublished trials on 1 February 2017 in:
-
the US National Institutes of Health trials registry ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 7);
-
the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) (Appendix 8).
Data collection and analysis
Selection of studies
For the original review, all four review authors (AR, DA, KJ, NM) checked titles and abstracts identified by the searches for RCTs or quasi‐RCTs, and two review authors (MKR and DA) reviewed the searches for the update. We obtained the full texts of all potentially relevant studies, which the review authors independently assessed.
Data extraction and management
All review authors independently extracted data onto a specially designed form. We tried to obtain missing or additional data from the study authors wherever possible. For our primary outcome, overall survival after initiation of assisted ventilation, we planned to extract hazard ratios with standard errors or confidence intervals (CIs), or median survival times with 95% CIs, or the numbers required to construct a life table, for example the numbers surviving or failing to survive after initiation of assisted ventilation for a sequence of specified time intervals.
In any studies requiring translation, the translator would extract data directly onto a data extraction form. If possible, the review authors would have checked any numerical data extraction against the study report.
Assessment of risk of bias in included studies
All review authors decided which trials met the inclusion criteria for the review and assessed the risk of bias for the included studies. Any disagreements about inclusion were resolved by discussion between review authors. We completed a 'Risk of bias' assessment for all included studies according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We evaluated random sequence generation, allocation concealment, blinding (participants and personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. We then made a judgement on each of these criteria of 'high risk', 'low risk', or 'unclear risk', where 'unclear risk' indicates an unclear or unknown risk of bias.
Measures of treatment effect
In Appendix 9 we have reported in full methods that the review authors will follow if more trials become available in the future and meta‐analysis becomes possible.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes.
-
Length of survival following initiation of mechanical ventilation.
-
Quality of life.
-
Adverse events.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence (studies that contribute data for the prespecified outcomes). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used footnotes to justify all decisions to down‐ or upgrade the quality of the evidence and made comments to aid readers' understanding of the review where necessary.
We reported summary scores for quality of life outcomes. We reported the median days at which quality of life remained above 75% of baseline in the 'Summary of findings' table, as raw scores were not available, and of the two quality of life analyses reported in Bourke 2006, this is more easily interpreted.
Dealing with missing data
We contacted investigators in order to verify key study characteristics and to obtain missing numerical outcome data.
Assessment of reporting biases
Our search strategies were comprehensive. For this update, we searched trials registries to identify any completed but unpublished trials.
We considered non‐randomised evidence related to adverse events, cost, and cost‐effectiveness of different forms of mechanical ventilation in the Discussion.
Results
Description of studies
Results of the search
The updated database searches for this update produced the following results: MEDLINE 224 (78 new references), Embase 190 (92 new), AMED 6 (1 new), CINAHL 11 (2 new), Cochrane Neuromuscular Specialised Register 66 (33 new), and CENTRAL 63 (37 new). We reviewed 242 new references from database searches for this update, with 217 after deduplication, but none were eligible for inclusion. We also reviewed 77 records in ClinicalTrials.gov and 2 records in WHO ICTRP. See Figure 1 for a flow chart of the study selection process.
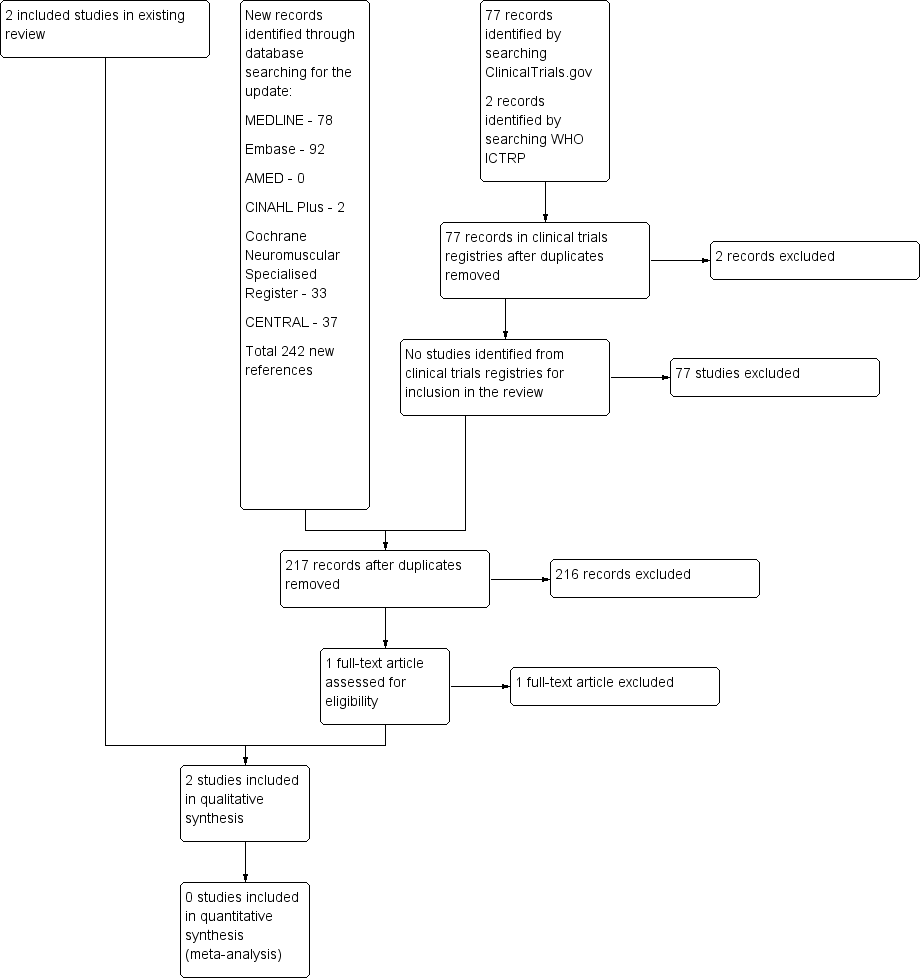
A flow diagram illustrating the study selection process.
We also searched the Database of Abstracts of Reviews of Effects (4 references), Health Technology Assessment database (4 references), and NHS Economic Evaluation Database (3 references) for information for inclusion in the Discussion.
Included studies
All review authors agreed on the inclusion of two studies (Jackson 2001; Bourke 2006).
The first study was a prospective randomised three‐month study in three ALS centres in the USA (Jackson 2001). The study included 20 people with ALS (no age or sex provided), of whom 13 were randomised when overnight oximetry studies documented oxygen saturation below 90% for at least one cumulative minute throughout the duration of the study (a minimum of six hours) and the individual had at least two significant symptoms of nocturnal hypoventilation. There were two groups: an early group where the participants were started immediately on NIV (seven participants) and a late group (six participants) in whom NIV was initiated when forced vital capacity (FVC) was less than 50% predicted. The report provided no demographic characteristics for the participants. The effects of NIV on ALSFRS respiratory version, Pulmonary Symptom Scale, and SF‐36 were estimated. No survival data were available from this study.
The second study was an RCT performed in a single ALS centre in the UK. It included 92 participants, of whom 41 met the criteria for randomisation (orthopnoea or maximum inspiratory pressures less than 60% or symptomatic hypercapnia, or both) and were followed up for at least 12 months or until death (Bourke 2006). Random allocation was computer generated by minimisation, a process that allows all significant prognostic factors to be included in the model. Twenty‐two participants were assigned to the NIV group and 19 participants to the standard care group. Demographic and functional characteristics of the participants in the two groups were similar at randomisation (Characteristics of included studies). The effect of NIV on survival and quality of life outcome domains (e.g. SF‐36 and the Sleep Apnea Quality of Life Index (SAQLI)) was estimated in the whole cohort but also in the subgroups of participants with and without severe impairment of bulbar function. Trialists defined severe impairment as a score of three or less on a simple six‐point assessment scale.
Excluded studies
We excluded one study that was terminated early due to problems recruiting participants (Perez 2003). We excluded Pinto 2003 as it used historical controls. One study was a controlled study of exercise in people with ALS with respiratory insufficiency (Pinto 1999). One study was an anecdotal study (Sivak 1982). Twelve studies were retrospective (Goulon 1989; Kamimoto 1989; Bach 1993; Cazzolli 1996; David 1997; Buhr‐Schinner 1999; Kleopa 1999; Saito 1999; Cedarbaum 2001; Winterholler 2001; Lo Coco 2007; Shoesmith 2007), of which only Kleopa 1999 had a control group. Six studies were non‐randomised prospective observational studies (Pinto 1995; Aboussouan 1997; Lyall 2001b; Newsom‐Davis 2001; Lo Coco 2006; Mustfa 2006). Berlowitz 2016 was a cohort study examining the effects of NIV on pulmonary function decline and survival across MND/ALS phenotypes.
Pinto 1995 was a single‐centre study in Portugal that included 20 consecutive participants with bulbar features and probable or definite ALS according to El Escorial criteria. The first 10 participants were treated with oxygen, bronchodilators, and physiotherapy. The following 10 participants were submitted to bilevel positive airway pressure. Two participants were excluded, one in the first group because the participant had tracheostomy, and the other in the second group for refusing the treatment. Despite the limitation of small sample size, the study demonstrated a significant improvement in total survival time (P < 0.004) and in the survival from the onset of diurnal gas exchange disorder (P < 0.006). Although it was a controlled trial, participants were not randomised, and hence selection bias cannot be excluded.
A 2016 study looked at the early initiation of NIV within a sham and an interventional arm (Jacobs 2016). It essentially compared 4 cm continuous positive airway pressure against bilevel ventilation at 8/4 cmH2O at a stage at which NIV would not normally be used. The two groups used NIV (or sham) for only 2.0 hours/day and 3.3 hours/day, respectively. The reported outcome was decline in percentage predicted FVC during the follow‐up period. Once both groups reached the time when they met the standard criteria for starting NIV, both groups were offered NIV. There was no difference in the survival of the two groups. The review authors considered that the main objective of this study was to assess the benefits of early initiation of NIV, and the trial did not evaluate NIV against no intervention, therefore we could not consider this data as complementary to the objectives of this review.
We excluded four apparently unpublished trials in clinical trials registries that compared different settings, modes, or timings of NIV (NCT00537446; NCT00560287; NCT01363882; NCT01641965). We excluded an ongoing trial in which different modes of ventilation were to be compared (NCT01746381). We enquired about two further registry entries from investigators. The contact person for NCT00958048 informed us that because participants were not willing to be randomised, investigators converted the trial to an observational study, which was not eligible for this review. An investigator of NCT00386464 reported that the trial had recruitment problems and was neither completed nor published.
Risk of bias in included studies
For a 'Risk of bias' summary see Figure 2.
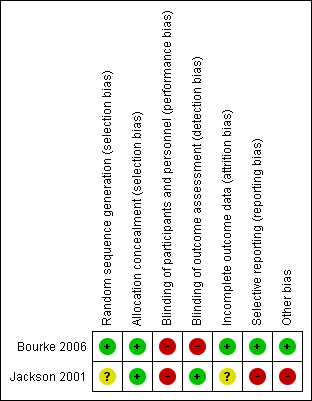
Methodological quality summary: review authors' judgements about each methodological quality item for each included study. A green plus sign indicates low risk of bias; a red minus sign indicates high risk of bias; and a yellow question mark indicates unclear risk of bias.
Allocation
Allocation was adequately concealed in both included studies. Bourke 2006 performed centralised randomisation; random allocation was computer generated by the process of minimisation. Although the Jackson 2001 paper gives no data for allocation concealment, the study authors informed us that an independent statistician performed randomisation by preparing two sets of random assignment in blocks of four, which were then allocated to each centre.
Blinding
Blinding of participants was not possible in the included studies, as it was not possible to blind delivery of NIV. Outcome assessors were blinded in Jackson 2001; no information was given in Bourke 2006 as to whether outcome assessors were blinded to knowledge of allocation intervention.
Incomplete outcome data
Jackson 2001 provided data on only six participants from the early‐intervention group. The outcome of one randomised participant was not clear, and the paper provided no data for the late‐intervention group. We noted no obvious attrition bias in Bourke 2006. Thirteen participants withdrew during surveillance, but none withdrew after randomisation, and all participants were followed up until the end of the study or death.
Selective reporting
Bourke 2006 was free of the suggestion of selective outcome reporting, as the study protocol was available, and the trial authors reported all primary and secondary outcomes in the prespecified way. No study protocol was available for Jackson 2001. The results of Jackson 2001 were intended to be used as preliminary data, but unfortunately the investigators did not secure funding for a subsequent study.
Other potential sources of bias
In Jackson 2001, trial authors did not define nocturnal hypoventilation according to the universally accepted criteria; they accepted oxygen desaturation below 90% for at least one cumulative minute as evidence of nocturnal hypoventilation. Nocturnal oximetry showing oxygen desaturation below 88% for at least five consecutive minutes or oxygen saturation below 90% for more than 5% of sleep time are considered sufficient to initiate NIV (Mehta 2001).
Effects of interventions
Both included studies used NIV.
Jackson 2001 provided insufficient data to be included in meta‐analyses, and this prevented pooled analysis of either primary or secondary outcome measures. Our review was therefore based on the results of one study, Bourke 2006. Neither were we able to obtain individual participant data or life tables from the Bourke 2006 trialists.
Primary outcome
Bourke 2006 showed that the overall median survival after initiation of assisted ventilation was significantly different between the NIV and standard care groups (P = 0.0062). The median survival for the NIV group participants was 48 days longer than the standard care group participants (219 days compared to 171 days). The published information did not provide a 95% confidence interval (CI) for the median survival difference (48 days) or for our secondary outcomes. We were able to derive approximate CIs from P values and median survival estimates under an assumption that median survival follows a lognormal distribution. A statistical method based on a lognormal survival model gave the following estimates for the 95% CI: 12 to 91 days for the estimated 48‐day survival difference (private communication from statistical referee Dan Moore).
The overall median survival of the subgroup with better (good or moderately impaired) bulbar function was also significantly different in the NIV group (P = 0.0059), with NIV group participants surviving 205 days longer than the standard care group participants (median 216 days in NIV group versus 11 days in the standard care group).
In participants with poor bulbar function, NIV did not confer survival advantage (P = 0.92), with an overall median survival for NIV group participants of 39 days less than the standard care group (222 versus 261 days). We considered the quality of the survival evidence as moderate, as it was based on a single RCT (see summary of findings Table for the main comparison).
Secondary outcomes
1. Survival at one or six months or longer
No data were available for survival at one and six months.
2. Quality of life assessed using validated health status questionnaires at one and six months or longer
No data were available at one and six months for either study.
Jackson 2001 did not systematically report quality of life data.
Bourke 2006 reported the time SAQLI and SF‐36 scores were maintained above 75% of pre‐randomisation assessment, and time‐weighted mean improvements in SF‐36 and sleep‐related SAQLI (the median (range) values of AUC (area under the curve) above baseline divided by time from randomisation to death). We have reported here the summary scores but also provide the SAQLI symptoms score, which the Bourke 2006 trialists designated as primary.
Summary scores
We were unable to calculate mean difference and 95% CI from the available data. Based on interpretation of P values from two‐sided tests of significance (0.05 significance level) on intention‐to‐treat data, the median time after initiation of assisted ventilation that quality of life was maintained above 75% of the baseline, based on the SF‐36 mental component summary (MCS), Physical Component Summary (PCS), and the SAQLI score, was significantly longer with NIV than standard care in the group as a whole and in the subgroup with normal and moderately impaired bulbar function. In participants with poor bulbar function, NIV conferred no significant benefit using these measures. For numerical data, see Table 1.
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV (n = 22) | Standard care | P | NIV | Standard care | P | NIV (n = 11) | Standard care | P value | |
| SF‐36 MCS | 168 (45 to 1357) | 99 (0 to 690) | 0.0017 | 199 (48 to 552) | 4 (0 to 196) | 0.001 | 127 (45 to 1357) | 164 (2 to 690) | 0.64 |
| SF‐36 PCS | 150 (27 to 908) | 81 (0 to 273) | 0.0014 | 179 (36 to 548) | 4 (0 to 94) | < 0.001 | 150 (27 to 908) | 132 (2 to 273) | 0.88 |
| SAQLI symptoms | 192 (48 to 1357) | 46 (0 to 703) | 0.0013 | 205 (69 to 629) | 4 (0 to 143) | < 0.001 | 143 (48 to 1357) | 100 (2 to 703) | 0.26 |
| SAQLI score | 173 (25 to 1357) | 99 (0 to 645) | 0.031 | 199 (61 to 595) | 4 (0 to 193) | < 0.001 | 103 (25 to 1357) | 132 (2 to 645) | 0.77 |
Data are median (range). Data from Bourke 2006.
Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary
The time‐weighted mean improvement in the SF‐36 MCS score and the sleep‐related SAQLI were found to be significantly greater in the NIV group than the standard care group in the whole cohort (n = 41) and in the participants with better bulbar function (n = 20), but not in those with poor bulbar function (n = 21). The time‐weighted mean improvement in the SF‐36 PCS was greater in the participants with better bulbar function, but not in the whole group or in the subgroup with poor bulbar function. For numerical data, see Table 2.
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV | Standard care (n = 19) | P | NIV (n = 11) | Standard care (n = 9) | P | NIV | Standard care (n = 10) | P value | |
| SF‐36 MCS | 2.31 (0 to 11.54) | 0 (0 to 5.23) | 0.0082 | 2.18 (0 to 11.54) | 0 (0 to 1.39) | 0.0052 | 4.47 (0 to 7.75) | 0.88 (0 to 5.23) | 0.24 |
| SF‐36 PCS | 0.18 (0 to 10.62) | 0 (0 to 6.73) | 0.51 | 0.14 (0 to 10.62) | 0 (0 to 0.39) | 0.031 | 0.21 (0 to 5.41) | 0.48 (0 to 6.73) | 0.37 |
| SAQLI symptoms | 1.07 (0 to 3.20) | 0 (0 to 1.14) | < 0.001 | 1.73 (0.52 to 2.95) | 0 (0 to 0) | < 0.001 | 0.90 (0 to 3.20) | 0.04 (0 to 1.14) | 0.018 |
| SAQLI score | 0.44 (0 to 1.59) | 0 (0 to 0.42) | < 0.001 | 0.50 (0 to 0.88) | 0 (0 to 0.07) | < 0.001 | 0.28 (0 to 1.59) | 0.04 (0 to 0.42) | 0.066 |
Data are median (range) values of area under the curve above baseline divided by time from randomisation to death. Data from Bourke 2006.
Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary
Other primary quality of life outcomes in the included study
Bourke 2006 chose the SF‐36 MCS (reported above) and the sleep‐related SAQLI symptoms domain as primary quality of life outcome measures, based on the results of a pilot study. The study report and the trial protocol do not indicate whether these outcomes were prespecified as primary.
The study showed that the median time the SAQLI symptoms domain score was maintained above 75% of the baseline after initiation of NIV was significantly longer with NIV than with standard care in the group as a whole and in the subgroup with normal and moderately impaired bulbar function. In participants with poor bulbar function, NIV conferred no significant benefit in maintaining the SAQLI symptoms score above 75% of baseline. See Table 1.
A significant difference in time‐weighted mean improvement of SAQLI between the NIV and standard care group was present in the group as a whole and in both subgroups. See Table 2.
2a. Quality of life median values at one and six months
No data were available.
3. Functional rating scale at one or six months or longer
No data were available for the ALSFRS to be analysed.
3a. Functional rating scales median values
No data were available.
4. Proportion experiencing adverse events related to mechanical ventilation
Neither Jackson 2001 nor Bourke 2006 reported adverse events related to mechanical ventilation.
Discussion
Two reports of RCTs of nocturnal mechanical ventilation in ALS were available for this review. Both trials employed NIV. Since only one of the reports provided data suitable for analysis (Bourke 2006), we were not able to perform a meta‐analysis.
Bourke 2006 was designed to assess the effect of NIV on survival and quality of life in people with ALS. The study provided moderate‐quality evidence that NIV prolongs median survival, and low‐quality evidence that it maintains quality of life in people with ALS overall. The benefit of NIV was striking in the subgroup of participants with better (normal or moderately impaired) bulbar function, but it is, however, important to note that six of nine participants in the standard group died within two weeks of randomisation, thus probably overestimating the effect of NIV in this subgroup of people with ALS. Non‐invasive ventilation did not prolong survival in people with ALS who had severe bulbar dysfunction, but did improve sleep‐related symptoms in this subgroup.
Despite the above shortcomings, Bourke 2006 has shown that NIV significantly improves quality of life over standard care, prolonging life for longer than is reported with riluzole (Miller 2012). The study has confirmed previous observations from non‐randomised trials of survival advantage and improved quality of life in people with ALS who start and can tolerate NIV at the onset of respiratory impairment (Bach 1993; Pinto 1995; Aboussouan 1997; Kleopa 1999; Lyall 2001b). It is unlikely that there will be further RCTs of NIV in unselected cohorts of people with ALS. In the view of the review authors, evidence from Bourke 2006 supports the belief that NIV is a major advance in the management of ALS, and it would be unethical in future trials not to offer NIV to people with ALS who have symptoms of nocturnal hypoventilation. The evidence supports benefit from NIV in people with ALS. The National Institute for Health and Care Excellence (NICE) carried out a cost‐effectiveness analysis using the Markov model and concluded that the use of NIV in the management of people with ALS represents a cost‐effective use of resources (NICE 2010).
A UK‐wide survey demonstrated that the provision of NIV to people with ALS is increasing (O'Neill 2012). A study has also reported on the obstacles people with ALS experience in using NIV and how to address them (Baxter 2013a). Some people with ALS are unable to tolerate NIV and decide to discontinue its use. Careful attention to secretion management, humidification, and comfortable interfaces may improve compliance with NIV. Detailed explanation to the patient along with emphasis of benefits may pre‐empt difficulties. A qualitative study has addressed concerns about the use of NIV in the terminal phase of the disease (Baxter 2013b). This study did not find the use of NIV in the last days of life to be associated with any adverse effects. The authors advised rapid withdrawal rather than gradual weaning when patients express a wish to discontinue NIV. The study concluded that patients' wishes should guide the use of NIV at the end of life. Another study investigated the impact of NIV on the family carer and concluded that NIV had no significant impact on carer burden (Baxter 2013c).
Conclusions from Bourke 2006 cannot be extended to people with ALS who do not have respiratory symptoms. It has been suggested that early treatment with NIV may offer a survival benefit above that demonstrated in Bourke 2006. There is some evidence from non‐controlled studies that early NIV improves survival and reduces decline of FVC in ALS (Carratu 2009). NCT01641965 is an ongoing RCT evaluating the impact of early NIV in people with ALS who have mild respiratory involvement. Jacobs 2016 is a pilot study that aimed to determine the feasibility of conducting a randomised, double‐blind, placebo‐controlled trial of early NIV versus sham NIV in people with ALS. The study was not powered to assess clinical outcomes.
Non‐invasive ventilation settings may require titration with disease progression. A randomised trial is evaluating the use of polysomnography in guiding the initiation and further titration of NIV therapy during the course of the disease (NCT01363882). Different ventilator modes and settings are to be assessed in two NIV trials in people with ALS. The Italian multicentre randomised NIV study was designed to evaluate clinical efficacy, the participants' tolerance and quality of life and the frequency of changing settings in people with ALS who are undergoing NIV with pressure support ventilation or volume‐assisted ventilation (NCT00560287). However, this trial is compromised due to incomplete outcome data. The Columbia University study is designed to measure difference in pulmonary function and respiratory muscle pressure testing, gas exchange, and subjective dyspnoea between baseline and two different ventilator modes (high‐ and low‐level non‐invasive positive pressure ventilation) (NCT00537446). Another study is evaluating the use of an Intelligent Volume‐Assured Pressure Support (iVAPS) ventilator in ALS (NCT01746381). Most of these studies are not eligible for this review, which focuses on NIV compared to no NIV or standard care. They may, however, inform the best pressure settings of NIV and the ideal time of its initiation to obtain the maximum benefit.
Another Cochrane Review of nocturnal ventilation for daytime hypoventilation in neuromuscular and chest wall disorders concluded, while noting several methodological limitations, that mechanical ventilation is associated with survival benefit in MND (Annane 2014). This conclusion was based on three trials (Pinto 1995; Jackson 2001; Bourke 2006). Jackson 2001 is included in this review, but we consider it to be at high risk of bias due to selective reporting. We excluded Pinto 1995 as we considered it a non‐randomised controlled study. Another review on the management of respiratory problems in neurodegenerative disease concluded that the evidence to support the role of NIV was strongest for MND, although the evidence itself was weak (Jones 2012).
A limitation of the evidence in this review is that it was based on a single RCT with 41 participants, and hence we assessed the quality of the evidence as moderate for improvement in survival. However, findings of this trial are consistent with the findings of several other studies, which together offer strong support for the benefit of NIV for people with ALS in respiratory failure. We contacted investigators of registered but unreported trials to determine whether they had been abandoned or were completed but unpublished. We did not identify any study that addressed the adverse effects of NIV.
The review protocol did not define the quality of life outcome in detail. The protocol and study report of Bourke 2006 do not state whether the primary quality of life domains or analyses were prespecified (Bourke 2006; ISRCTN76330611). To reduce the risk of selective reporting in the review, we reported summary scores for both scales and both types of analysis, including results for the SAQLI symptoms score, which was a primary quality of life outcome in the trial.
The review authors clarified eligible comparisons in the methods, explicitly excluding trials assessing the timing of initiation and other aspects of NIV delivery or specification. This decision excludes some studies of NIV, limiting its scope to the central question of the effects of NIV versus 'standard care' or versus no NIV as originally intended. New, ongoing studies are addressing further issues about the timing of NIV initiation and further titration with the disease progression. Future reviews will assess different modes, schedules, and initiation of ventilation.

A flow diagram illustrating the study selection process.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. A green plus sign indicates low risk of bias; a red minus sign indicates high risk of bias; and a yellow question mark indicates unclear risk of bias.
| Non‐invasive ventilation compared with standard care for amyotrophic lateral sclerosis (ALS) | ||||||
| Patient or population: people with ALS Settings: people with ALS attending a single regional care centre Intervention: non‐invasive ventilation Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Non‐invasive ventilation (NIV) | |||||
| Survival | All participants Median survival was 171 days. | All participants Median survival was 48 days longer (12 to 91 days1 longer). | ‐ | 41 (1 study) | ⊕⊕⊕⊝ Moderate2 | 21 of the 41 participants had poor bulbar function. P = 0.0059 better bulbar function, P = 0.92 poor bulbar function |
| Participants with better (good or moderately impaired) bulbar function Median survival was 11 days. | Participants with better (good or moderately impaired) bulbar function Median survival was 205 days longer (CI not given). | |||||
| Participants with poor bulbar function Median survival was 261 days. | Participants with poor bulbar function Median survival was 39 days shorter (CI not given). | |||||
| Quality of life (SF‐36 MCS) | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 99 days. | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 69 days longer (45 to 667 days longer). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 195 days longer (P = 0.001, CI not given). | |||||
| Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 164 days. | Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 37 days shorter (P = 0.64, CI not given). | |||||
| Quality of life (SF‐36 PCS) | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 81 days. | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 69 days longer (P = 0.004). | ‐ | ‐ | ‐ | CI not given |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 175 days longer (P < 0.001). | |||||
| Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 18 days longer (P = 0.88). | |||||
| Quality of life (SAQLI) | All participants Median duration that SAQLI remained above 75% of baseline was 99 days. | All participants Median duration that SAQLI remained above 75% of baseline was 74 days longer (P = 0.031). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | CI not given |
| Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of baseline was 4 days. | Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of the baseline was 195 days longer (P = < 0.001). | |||||
| Participants with poor bulbar function Median duration that SAQLI remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SAQLI remained above 75% of the baseline was 29 days shorter (P = 0.77). | |||||
| Adverse events (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Calculated CIs are approximate. | ||||||
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV (n = 22) | Standard care | P | NIV | Standard care | P | NIV (n = 11) | Standard care | P value | |
| SF‐36 MCS | 168 (45 to 1357) | 99 (0 to 690) | 0.0017 | 199 (48 to 552) | 4 (0 to 196) | 0.001 | 127 (45 to 1357) | 164 (2 to 690) | 0.64 |
| SF‐36 PCS | 150 (27 to 908) | 81 (0 to 273) | 0.0014 | 179 (36 to 548) | 4 (0 to 94) | < 0.001 | 150 (27 to 908) | 132 (2 to 273) | 0.88 |
| SAQLI symptoms | 192 (48 to 1357) | 46 (0 to 703) | 0.0013 | 205 (69 to 629) | 4 (0 to 143) | < 0.001 | 143 (48 to 1357) | 100 (2 to 703) | 0.26 |
| SAQLI score | 173 (25 to 1357) | 99 (0 to 645) | 0.031 | 199 (61 to 595) | 4 (0 to 193) | < 0.001 | 103 (25 to 1357) | 132 (2 to 645) | 0.77 |
| Data are median (range). Data from Bourke 2006. Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary | |||||||||
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV | Standard care (n = 19) | P | NIV (n = 11) | Standard care (n = 9) | P | NIV | Standard care (n = 10) | P value | |
| SF‐36 MCS | 2.31 (0 to 11.54) | 0 (0 to 5.23) | 0.0082 | 2.18 (0 to 11.54) | 0 (0 to 1.39) | 0.0052 | 4.47 (0 to 7.75) | 0.88 (0 to 5.23) | 0.24 |
| SF‐36 PCS | 0.18 (0 to 10.62) | 0 (0 to 6.73) | 0.51 | 0.14 (0 to 10.62) | 0 (0 to 0.39) | 0.031 | 0.21 (0 to 5.41) | 0.48 (0 to 6.73) | 0.37 |
| SAQLI symptoms | 1.07 (0 to 3.20) | 0 (0 to 1.14) | < 0.001 | 1.73 (0.52 to 2.95) | 0 (0 to 0) | < 0.001 | 0.90 (0 to 3.20) | 0.04 (0 to 1.14) | 0.018 |
| SAQLI score | 0.44 (0 to 1.59) | 0 (0 to 0.42) | < 0.001 | 0.50 (0 to 0.88) | 0 (0 to 0.07) | < 0.001 | 0.28 (0 to 1.59) | 0.04 (0 to 0.42) | 0.066 |
| Data are median (range) values of area under the curve above baseline divided by time from randomisation to death. Data from Bourke 2006. Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary | |||||||||

