Interventions for preventing occupational irritant hand dermatitis
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Individually randomised controlled trial Combination of parallel and split‐body design, 2x2 arms Duration: 12 months 3 follow‐ups at 3, 6, and 12 months | |
| Participants | Final number evaluable after 12 months: N = 96 healthy metal workers exposed to cutting fluids
Number of participants randomised: N = 100 (group 1: N = 50; group 2: N = 50) Lost to follow‐up: 4 in group 1 Mean age in years (participants evaluable after 12 months): group 1: 35.5 (range 17 to 59); group 2: 41.5 (range 17 to 59) Sex: male Inclusion criteria
Exclusion criteria
Setting: one medium‐sized German factory | |
| Interventions | Comparison of the effectiveness of skin protection, skin care, or both, versus no intervention (4 study arms, all relevant to the review). The metal workers' hands were randomised to:
No details on application described, but participants received a flyer and the product. One‐sided application using a glove for the other hand. Participants were randomised to use product on either left or right hand. | |
| Outcomes |
All outcomes were measured at baseline, after 3, 6, and 12 months. For the visits at 3 and 6 months, abnormal morphology was not reported while the other outcomes were reported in diagrams of the medians only. Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: the study investigators applied a score which consisted of 3 categories: 'unauffällige klinische Befundung' (inconspicuous findings), 'geringfügig auffällige klinische Befundung' (minor conspicuous findings) and 'auffällige klinische Befundung' (conspicuous findings). Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): the measurements were performed on four defined skin areas (back of the left and right hand, distal part of the volar site of the lower left and right arm) after a 20‐minute adjustment phase at a standardised temperature (20°C) and relative humidity (50%). The participants were asked not to take a shower, eat or drink anything and to refrain from smoking within the hour before the measurement. TEWL was measured according to international recommendations (Pinnagoda 1990, Rogiers 2001). For the corneometry, electrical measurements of conductibility (κ), impedance (Ω) and capacity (F) were performed. Strengths
Limitations
| |
| Notes | The left/right hand design may have led to failures when applying the products. Study funding sources: Vereinigung der Metall‐Berufsgenossenschaften (VMBG) (financial, content‐related and organisational support) Possible conflicts of interest not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. Quote: 'Während der Erstuntersuchung wurden die Probanden zufällig in zwei Gruppen aufgeteilt. Danach erfolgte in jeder Probandengruppe die zufällige Aufteilung der Probanden in jeweils zwei Interventionsarme (jeweils einer pro Hand).' (p. 7) [At baseline, probands were randomly assigned to two groups. Subsequently in each group of probands, the probands were randomly assigned to two intervention arms each (one for each hand).] |
| Allocation concealment (selection bias) | Unclear risk | Block randomisation was performed, no details given. Unclear selection bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of participants, participants could have influenced performance by intentionally applying products to the wrong hand. Possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) | Low risk | Physician was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | Low risk | Only 4 withdrawals, due to personal reasons, in group 1 (control/skin protection). |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes mentioned in the report were reported at least for first and last examination. |
| Other bias | Unclear risk | Design: more than one intervention arm. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. The split‐body design may have introduced contamination, especially concerning the control arm. (unclear risk) Baseline imbalances
Blocked randomisation in unblinded trials: block randomisation was performed, no details given. Unclear selection bias. Differential diagnostic activity: no different diagnostic activities across study arms. |
| Methods | Individually randomised controlled trial Duration: 1 year 3 follow‐ups at 4, 8, and 12 months | |
| Participants | Final number evaluable after 12 months: N = 497 initially healthy dye and print factory workers (intervention: N = 248; control: N = 249) Number of participants randomised: N = 868 (intervention: N = 428; control: N = 440) Lost to follow‐up: N = 211 (intervention: N = 102; control: N = 109) Excluded from review due to OIHD at beginning of study: N = 160 (intervention: N = 78; control: N = 82) Mean age in years (all included participants): intervention: 32.6; control group: 32.1 Sex: 91% male, balanced between groups Mean duration of employment (all 868 participants): barrier cream and control group, 7.9 years Inclusion criteria: dye and print industry workers Setting: field study in 13 dye and print factories, North Italy | |
| Interventions | Comparison of the effectiveness of:
The workers were randomised to receive either the barrier creams (silicone or hydrocarbon containing barrier creams), which they used twice a day for 1 year, or no intervention. | |
| Outcomes |
Assessments took place after 4, 8, and 12 months. Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): objective skin lesions assessed by study personnel (erythema, edema, exudation, vesicles, blisters, desquamation, hyperkeratosis, rhagades, dryness, atrophy, lichenisation) Strengths
Limitations
Broad outcome definition ‐> comparatively many cases expected Time frame: combined incidence at 4, 8, or 12 months Scoring system for the severity of OIHD: no score assessed | |
| Notes | Study funding sources: Not described. Possible conflicts of interest: Not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'La procedura di randomizzazione.....base di una sequenza di numeri casuali' (p. 233) [The randomisation procedure ... based on a sequence of random numbers] Matched pairs were used in order to achieve balanced groups. |
| Allocation concealment (selection bias) | Unclear risk | Inadequate concealment of the allocation sequence may have resulted from pairwise assigning the first person of the pair to group A if the random number was even, to group B if odd. The second person of the pair was assigned to the alternative group. Efforts to conceal the allocation were not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants remains unclear. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'Controllo clinici periodici ....all`oscuro del gruppo' [Periodical clinical examination ... blinded to group] ‐ Outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | TEWL and corneometry were not measured. |
| Incomplete outcome data (attrition bias) | Unclear risk | 12 months follow‐up: 102 missing from the barrier cream group, 109 missing from the control group due to dropout and unavailability at the examination time points. The study investigators did mention ITT analysis but this merely meant that compliance was ignored. Missing values were not estimated. Dropout was due to the long duration of recruitment and due to workers leaving the factories, but there was no detailed analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | Design: no sources of bias were identified. Baseline imbalances: demographic data of participants were only available for the entire study population (N = 868). It remains unclear whether the demographic characteristics of the initially healthy workers from intervention and control group (N = 708) differed significantly in this study (low risk) Blocked randomisation in unblinded trials: not enough information was given to decide whether or not allocation concealment was broken by the pairwise allocation. (unclear allocation concealment bias) Differential diagnostic activity: no different diagnostic activities across study arms. |
| Methods | Cluster‐randomised controlled trial; 18 clusters (departments) Parallel design, 2 arms Duration: 1 year 1 follow‐up at 1 year | |
| Participants | Final number evaluable after 12 months: N = 212 initially healthy Danish gut cleaners (intervention: N = 59; control: N = 153) Number of participants randomised: N = 644 baseline responders (intervention: N = 205; control: N = 439) Lost to follow‐up: N = 228 were lost to follow‐up or stopped working as gut cleaners (intervention: N = 69; control: N = 159) Excluded from review due to OIHD at beginning of study: N = 204 (intervention: N = 77; control: N = 127) Mean age in years (all follow‐up respondents including those with OIHD at baseline and those not available at baseline): intervention: 36.1 (range 17‐62 years); control: 37.8 (range 17‐66) Sex: 66.3% male (intervention), 64.1% male (control) Inclusion criteria: not described Exclusion criteria: not described Setting: field study in 18 swine slaughterhouses in different Danish cities, all departments belonging to one company | |
| Interventions | Comparison of the effectiveness of a:
The prevention strategy consisted of a two part concept, with an evidence based prevention programme giving recommendations for prevention of work related skin problems in wet work occupations, and a documented method for implementation. The recommendations were aimed at the management and at the employees.
The local project group included 2‐5 gut cleaners who acted as role models. | |
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no score assessed | |
| Notes | 79 participants had stopped working as gut cleaners and were analysed as a separate group in the report regardless of the intervention. For this review, they were not considered and treated as dropouts. Demographic data were only available for participants who were not lost to follow‐up, including those with OIHD at baseline. There was a mistake in the paper concerning primary outcome 1, which MA Flyvholm corrected in an email (05/052015): 'For the intervention group (corrected: comparison group), 67% of those with eczema at baseline reported eczema at follow up and 33% no eczema; 37% with no eczema at baseline reported eczema at follow up.' (p. 646) Study funding sources: 'The project was financially supported by an appropriation for prevention of asthma and allergy, administered by the Danish Ministry of Health.' (p. 648) Possible conflicts of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described ('randomly allocated' p. 643). |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding, possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding likely to influence reporting of OIHD. |
| Blinding of outcome assessment (detection bias) | Unclear risk | TEWL and corneometry were not measured. |
| Incomplete outcome data (attrition bias) | Unclear risk | 149 participants (23,1%) lost to follow‐up, an unspecified number of which (max. 51) dropped out without stating reasons. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances
Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: no different diagnostic activities across study arms. |
| Methods | Individually randomised controlled trial Duration: 6 months (also reported as 30 weeks) for each participant, 3 years altogether 3‐weekly follow‐ups | |
| Participants | Final number evaluable after 6 months: N = 54 initially healthy, newly employed metal workers exposed to cutting fluids (barrier cream: N = 17; moisturiser: N = 14; control: N = 23) Number of participants randomised: N = 54 Lost to follow‐up: N = 0 Mean age in years: barrier cream group: 22 (range 16 to 37); moisturiser group: 22 (range 16 to 36); control: 22 (range 17 to 35) Sex: 50 out of 54 male Exclusion criteria: 'Only machinists who had not handled cutting fluids previously were included in the study. Machinists who had already worked for more than I week were excluded.' (p. 177) Setting: field study in the grinding and turning sections of one large ball‐bearing manufacturing factory, Singapore | |
| Interventions | Comparison of the effectiveness of:
The participants were randomised to receive either a barrier cream (60 g tube every 6 weeks used on the hands before work and after each meal break), or a moisturiser used daily as an after work emollient, or no intervention. Arretil is a water‐soluble, non‐oily, silicone‐free barrier cream. Keri lotion is a liquid paraffin lotion (16%) and contains lanolin oil. The participants were followed up every 3 weeks for 6 months. | |
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): classification of dermatitis severity: mild = less than 25% of the total surface area of either hand involved (up to wrist line), moderate = more than 25% of the total surface area of either hand involved (up to wrist line). Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no score assessed Quality of bioengineering methods (Pinnagoda 1990): 'Baseline TEWL measurement was made on the skin overlying the dorsal 3rd metarcarpophalangeal (MCP) joint of both hands with an Evaporimeter (Servomed, Vallingby, Sweden). We chose the 3rd MCP joint because of the ease of identifying the exact spot for repeat measurements. We conducted a pilot measurement on mid‐dorsal hand, 1st, 3rd, 5th dorsal MCP joints, mid‐ventral forearms and mid‐dorsal forearms on the 1st 30 volunteers. All showed fairly consistent recordings. We found the 3rd dorsal MCP joints to have the highest TEWL recordings and that these were fairly consistently reproducible. The method of TEWL measurement was as follows. The machinist rested in the examination room for 10 to 15 min. The TEWL on the dorsal 3rd MCP joint of each hand was then measured. TEWL values on the left hand followed by those on the right hand were recorded. The procedure was repeated once. 2 recordings of TEWL values for each hand were thus obtained; the average was recorded.' (p. 177) Strengths
| |
| Notes | Groups were comparable at baseline. No dropouts Study funding sources not described. ('A research grant was obtained.' p. 176) Possible conflicts of interest not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Machinists were randomly assigned.' (p. 177). No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded to the intervention because original samples of the products were supplied. Possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | No influence of blinding is expected for the objective measurement of TEWL. Corneometry was not measured. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported in either group. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. No protocol available. |
| Other bias | Unclear risk | Design: participants were recruited consecutively (when they started work) over a period of 3 years, in which the work conditions and exposures might have been changed ‐> unclear bias More than one intervention group. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. Baseline imbalances: baseline imbalance regarding turning vs grinding section: controls ‐ 14 vs 9; barrier cream ‐ 8 vs 9; emollient cream ‐ 4 vs 10 ‐> unclear bias Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
| Methods | Individually randomised controlled trial Duration: 2x2 weeks 2 follow‐ups (at 2 and 4 weeks or at time of dropout) | |
| Participants | Final number completing the 2x2 weeks: N = 70 initially healthy cleaners and kitchen workers Number of participants randomised: N = 111 (N = 56 started with moisturiser; N = 55 started with no treatment) Lost to follow‐up: N = 41 Mean age in years (groups I, II, III): 41 (range 19 to 67) Mean duration of employment (groups I, II, III): 10 (range 1 to 35) years Sex: 110 out of 111 included participants were female. Setting: field study, Denmark | |
| Interventions | Comparison of the effectiveness of:
in a 2x2 weeks cross‐over design. Application requirements were not described. Locobase, manufactured by Ferndale Laboratories, USA, is a wound and skin emulsion formulation intended for topical application. No details were given about the composition of the cream. | |
| Outcomes | The study investigators reported the results after grouping all participants in 4 groups according to their ability to complete the 4 week study. Finally, only results of groups I and II were reported. Comparisons of intervention vs no treatment were only reported for corneometry, TEWL, and sum score in groups I and II
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): the degree of certainty could not be assessed because no diagnostic criteria for OIHD were described. Scoring system for the severity of OIHD
Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): 'Skin surface temperature (digital thermometer, Ellab type TRD, probe diameter 12 mm), transepidermal water loss (TEWL) (Evaporimeter EPI, Stockholm, Servomed, Sweden), and electrical capacitance (Corneometer CM420, Schwarzhaupt, W. Germany) were measured on both the right and left side and on the volar and dorsal aspect of the distal part of the 3rd finger, middle of the hands, and on the forearms (12 measurement points). The results are presented as overall mean values (the mean values for all measurements on all sites). All probes were hand‐held. The measurements were performed in a separate room with minor draught and protection shields. The mean room temperature was 23°C (20‐26°C) and the mean relative humidity (RH) 34% (25‐42%).' (p. 267). Strengths
| |
| Notes | The study investigators did not report the results according to the groups randomised, but regrouped all participants in 4 groups according their ability to complete the 4 week study period. Therefore not included in meta‐analysis. Study funding sources: 'The study was supported by the Danish Insurance Association, Copenhagen, L. P. Hansen's fund, Possible conflicts of interest not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'They were randomised into 2 groups' (p. 267). No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded due to study design. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was probably not done. Quote: 'Clinical examination and skin physiological measurements were performed on entry...' (p. 267). |
| Blinding of outcome assessment (detection bias) | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | High risk | N = 23 dropped out in period C after developing severe dryness of the skin, N = 12 violated the protocol or declined to continue the study because they developed or feared to develop hand dermatitis, N = 6 turned up only once, did not find Locobase acceptable, or went on vacation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout analysis did not address this outcome conclusively. |
| Selective reporting (reporting bias) | High risk | Results were not reported according to the original randomisation, but according to the ability of the participants to complete the study period. This was an attempt to deal with the high risk of attrition bias. Consequently, the results were not reported in a way appropriate for an RCT. |
| Other bias | High risk | In‐study use of moisturiser was twice as high in participants who dropped out in the no‐treatment period (N = 23) compared to the participants who completed both parts of the study (2.3 g/person/day versus 1.2 g/person/day). Design: cross‐over ‐> unclear bias Baseline imbalances: pre‐study use of moisturiser differed significantly between the groups despite randomisation (96% versus 82%, P < 0.01). ‐> high risk Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: not applicable because study arms were not evaluated separately. |
| Methods | Cluster‐randomised controlled trial; 18 clusters (enterprises) Parallel design, 4 arms Duration: 12 months 2 follow‐ups at 6 and 12 months | |
| Participants | Final number evaluable after 12 months: N = 800 initially healthy metal workers exposed to cutting fluids (group 1: N = 217 in 6 enterprises; group 2: N = 209 in 5 enterprises; group 3: N = 213 in 4 enterprises; control: N = 161 in 3 enterprises) Number of participants randomised: N = 1020 (group 1: N = 263; group 2: N = 253; group 3: N = 258; control: N = 246) Lost to follow‐up: N = 220 (21.6%) withdrawal/exclusion at 2nd follow‐up (group 1: N = 46; group 2: N = 44; group 3: N = 45; control: N = 85) Median age (all included participants): group 1: 40; group 2: 40; group 3: 44,5; control: 42 Sex: male Inclusion criteria
Exclusion criteria:
Setting: German factories mainly of small or medium‐size | |
| Interventions | Comparison of the effectiveness of skin protection and/or skin care creams vs no recommendation in a 4‐armed trial
Pragmatic approach: 'All participants used the skin care and protection products provided by the employer.' (p. 363). | |
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: percentage change of skin score as primary outcome and relative change from baseline as secondary outcome: 'A quantitative skin score was used. In brief, the score comprised all morphological criteria and physiological abnormalities (e.g. dryness) characteristic of hand eczema. Extent, intensity and anatomical site of each type of skin lesion were also recorded.' (pp. 363‐4). 'All three physicians underwent standardized training before using the score. Moreover, during the study period digital photographs of the hands of randomly chosen participants were regularly evaluated and results discussed in order to maintain similarity of visual assessments between observers.' (p. 366). Strengths
Quality of bioengineering methods (Pinnagoda 1990): not relevant | |
| Notes | Primary intervention: no inclusion of workers with manifest hand eczema (despite high baseline values on their very sensitive score). This was confirmed through correspondence with H Drexler. Study funding sources: 'The study was funded by the German Statutory Accident Insurance (DGUV) and the Franz‐Koelsch‐Stiftung e.V.' (p. 369). Possible conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Randomization was performed on the basis of the 19 included enterprises, assigning each enterprise randomly to one of four study arms.' (p. 363) Apparently, an undescribed method was applied to ensure approximately equal size of the groups despite varying numbers of workers per enterprise. |
| Allocation concealment (selection bias) | Unclear risk | It cannot be excluded that allocation was biased by the results of the baseline screening. Potential confounders differed significantly at baseline. Even though the study investigators do not report significant associations with their outcomes, they did not test the association with review outcome 'proportion of OIHD'. Baseline skin condition was better in the control group, thus allowing less improvement after 1 year compared to other groups. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding reported; possible to influence proportion of OIHD. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of participants (= outcome assessors) was probably not done. This would be likely to influence reporting of OIHD, but clear information on blinding is lacking. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | Unclear risk | 78.4% of recruited participants were available at last follow‐up (1 year), no analysis of dropouts. Attrition in control was 2x as high as in other groups. 'High risk' judgement was therefore considered but discarded because dropout reasons are unknown. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Skin score was presented in various ways: percentage change of skin score as primary outcome, categorized percentage change as secondary outcome; further outcomes including absolute change of skin score were reported. This is questionable, but at least the study investigators did not omit their less significant calculations. No protocol available |
| Other bias | High risk | Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. More than one intervention group. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. Baseline imbalances: significant differences of potential confounders and dyshidrotic eczema, but not of other dermatological disorders. It remains unclear whether this was due to lack of allocation concealment or happened by chance and was worsened by the cluster design ‐> high risk of bias Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: no different diagnostic activities across study arms. |
| Methods | Cluster‐randomised controlled trial; 14 clusters (nursing schools) Parallel groups, 2 arms Duration: 3 years 2 follow‐ups at 1.5 and 3 years | |
| Participants | Final number evaluable after 3 years: N = 250 initially healthy 1st‐year nurse apprentices (intervention: N = 121; control N = 129) Number of participants randomised: N = 521 (numbers of participants allocated to groups were not reported) Lost to follow‐up: N = 196 (37.6%; dropout per group was not reported) Excluded from review due to OIHD at beginning of study: only the number of healthy participants who were evaluable were provided (N = 250). This included participants who had OIHD at least at 1 follow‐up before they dropped out. Mean age in years (all included participants): intervention: 20.9 (SD 4.9); control: 23.6 (SD 7.7) Sex: 13% male (intervention), 12% male (control) Mean duration of employment: 0 years, baseline examination was before beginning of the nurse training Inclusion criteria: not described Setting: field study in 14 nursing schools (general nurses, paediatric and geriatric nurses, midwifes), Central Germany | |
| Interventions | Comparison of the effectiveness of a:
The intervention group received regular training (educational lecture with practical parts), and skin care cream (Asche Basis Creme). The lectures took place 3 times in the first year, 2 times in the second and third year. The nurse apprentices were encouraged to use alcoholic hand disinfection instead of hand washing or scrubbing. The effect of applying skin care cream was assessed by the use of a fluorescence technique with the Dermalux‐system as part of the training. Participants in the control group also received the skin care cream. | |
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): Irritant Skin changes were recorded using the operational definitions of Uter 1998b. Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no scores assessed | |
| Notes | Demographic data and dropout rates were only available for the entire study population of 521 nurse trainees. Study funding sources: 'This work was generously supported by a grant from Asche Chiesi GmbH, Hamburg, Germany.' (p. 207) Possible conflicts of interest: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Randomly divided' (p. 203); details not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants unclear, possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluation of skin changes was performed by 2 blinded physicians. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: 'The dropout rate was 37.6% which was not due to skin problems, but due to their absence because of illness at the date of evaluation.' (p. 204) Quote: 'For follow up of dropouts, we performed a telephone interview with the trainee or (if he could not be reached) with his teacher. The aim of this interview was to evaluate, whether or not skin changes were responsible for the dropout.' (p. 204) Low bias because the study investigators convincingly explain that they ensured no dropout was related to hand eczema |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances: no imbalances Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
| Methods | Cluster‐randomised controlled trial; 48 clusters (hospital departments) Parallel design with different recruitment dates, 2 arms Duration: 12 months 4 follow‐ups at 3, 6, 9, and 12 months | |
| Participants | Final number evaluable after 12 months: N = 981 initially healthy hospital employees handling irritants during work (intervention: N = 559; control: N = 422) After correction for ICC = 0.005: N = 893 initially healthy hospital employees handling irritants during work (intervention: N = 509; control: N = 384) Number of participants randomised: N = 1649 (intervention: N = 876; control: N = 773) Excluded from review due to OIHD at beginning of study: N = 144 (intervention: N = 64; control: N = 80) Lost to follow‐up: N = 524 (intervention: N = 253; control: N = 271) out of the 1505 initially healthy participants Mean age in years (all included participants): intervention: 40.07 years, SD 11.5; controls: 40.8 years, SD 11.3 Sex: 78.4% female (intervention), 78.3% female (control) Inclusion criteria
Exclusion criteria: not handling irritants during work Setting: field study in 48 hospital departments (different cities in the Netherlands) | |
| Interventions | Comparison of the effectiveness of:
The multifaceted implementation strategy included participatory working groups, role modes, educational programme including reminders, and a leaflet. The main recommendations were: '1. When there is no visible contamination of the hands, use an alcohol‐based hand disinfectant instead of water and soap to * This means that the use of disinfectant should be increased and the use of water and soap should be decreased.' (p. 3) All workers who were present at the educational session received a bag with one moisturiser, a pair of cotton undergloves, and two disinfectants (no product names or details reported). The intervention went on for 4 months. The comparison group received only the leaflet. | |
| Outcomes |
Study investigators did not evaluate healthy skin score separately for participants in incident cases (as defined as review outcome). Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: 'Workers assessed the health of the skin on their hands by means of the following question: ‘How would you judge the health of your hands/forearms at the moment on a scale from 0 to 10?’ (0, unhealthy skin; 10, healthy skin). This question was based on question D12 of the NOSQ‐2002.' (p. 4) Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): not relevant | |
| Notes | Demographic data and only reported for the study population including those with hand eczema at baseline. Contingency table and dropouts were calculated based on the raw data received from the study investigators. Study funding sources: 'This study was supported by a grant from the Netherlands Organization for Health Research and Development (ZONMW).' Possible conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Based on the sequence of inclusion, randomisation was performed in strata of two by an independent researcher.' (Meer 2011, p. 3) |
| Allocation concealment (selection bias) | Unclear risk | Not described. Randomisation took place before baseline measurements by an independent researcher and was stratified by cluster‐based criteria. The study investigators excluded 17 out of 1666 baseline responders after randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: 'Workers were not informed about the design of the study and outcome of randomisation.' (pp. 2‐3) No blinding of personnel and department managers is expected to introduce only a low risk. |
| Blinding of outcome assessment (detection bias) | Low risk | OIHD was self‐reported and participants were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only superficial analysis of dropouts, reasons for dropout unknown. Quote: ‘Another limitation was that there was a non‐response rate of >30% at the final follow‐up measurement. However, the differences between the baseline values of the non‐responders and the baseline values of the total population were minimal.’ (p. 11) |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Primary study outcome predefined in strategy and reported; secondary study outcomes predefined in strategy. |
| Other bias | Unclear risk | Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances (whole study population including participants with HE at baseline)
Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: study investigators speculate that participants of the intervention group are more aware of HE and were therefore more likely to report them: 'The educational session given during the intervention period might have increased awareness among the participants in the intervention group concerning their hand eczema symptoms. They may have evaluated their symptoms — which might have already been present at baseline — differently after the educational session. This might have led to an increase in hand eczema reports at follow‐up as compared with the control group that may be independent of a change in clinical status.' (p. 10) |
| Methods | Individually randomised controlled trial Duration: 2 x 2 weeks Follow‐ups: day 12, day 15, day 26, day 29 | |
| Participants | Final number evaluable after 2x2 weeks: N = 16 initially healthy 2nd year apprentice hairdressers (numbers allocated to groups not reported) Number of participants randomised: N = 21 (numbers allocated to groups not reported) Lost to follow‐up: N = 5 (numbers allocated to groups not reported) Sex: 20 female out of 21 included participants Median age (all included participants): 18 (range 16 to 30) Atopy: 1 had a probable and 3 had a possible atopic disposition Inclusion criteria: at least 5 times shampooing per day without gloves Setting: field study with apprentices from the main regional occupational training centre, Geneva, Switzerland | |
| Interventions | Comparison of the effectiveness of:
The participants were randomised to start either with barrier cream (Excipial protect) or with its vehicle. Excipial protect contains aluminium hydroxychloride and glycerine. Glycerine promotes water retention in the skin and the aluminium salt reduces excess sweating. The vehicle was designed specifically for skin care for occupational users. The first cream was applied 5 days a week for 2 weeks with a washout period of 2 days followed by another 2‐week treatment period with the second cream. | |
| Outcomes |
Participants were assessed at the beginning at day 12, day 15, day 26, and day 29. The primary outcome measures were the proportion of irritant skin changes (score defined by study investigators). The secondary outcome measures were the amount of skin barrier impairment (TEWL), skin hydration (corneometry), and skin colour (chromometry) in the groups. A further secondary outcome measure was the subjective opinion of the participants concerning different features of the creams (ease of use, consistency, oiliness, protective effect, tolerance, general aspects). Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): not relevant Scoring system for the severity of OIHD: three scores were assessed: Dryness, redness, breaks in the skin. Scale of 0 to 3 (0 = none, 1 = mild, 2 = strong, 3 = maximum). Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990) 'The biometric measurements were taken on the back of the dominant hand. The following instruments
Strengths
Limitations
| |
| Notes | Not included in meta‐analysis because no quantitative data reported. Study funding sources: not described Possible conflicts of interest: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'The subjects were randomly assigned.' No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | The participants were blinded: They received 3x 50 g tubes with identical markings at the beginning of the study and another 3 tubes after 2 weeks. Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'Double‐blind' (p. 135). Participants, and outcome assessors were blinded. Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) | Unclear risk | 5 out of 21 apprentices dropped out during only 4 weeks. The study investigators stated that the dropouts were 'for reasons not related to the study. Their withdrawal did not effect the balance between the 2 groups to which they had been assigned' (p. 134). No further details provided. Dropout reasons unknown although it is doubtful they were truly unrelated to the study or outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Results concerning primary outcome measure was only summarised briefly. Quote: 'Clinical scores were generally very low... under either cream' (p. 136), but not reported separately for verum and vehicle. |
| Other bias | Unclear risk | Application frequency of barrier cream and vehicle unclear. Design: cross‐over ‐> no impact because no quantitative data for proportion of HE provided Baseline imbalances: comparability of groups at baseline unclear, no details given. Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
g: gram
HE: hand eczema
ITT: intention‐to‐treat
N: number
OIHD: occupational irritant hand dermatitis
RCT: randomised controlled trial
SD: standard deviation
TEWL: transepidermal water loss
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Double‐blind CCT: inclusion of workers with hand dermatitis (intervention group: 21.0%, control group 21.4%), no separate data for healthy workers available. No inclusion of dropouts and withdrawals in the analysis. | |
| RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. | |
| CCT: quality criteria for quasi‐RCT not fulfilled. No blinding of participants, clinicians and outcome assessors. | |
| RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. | |
| Not randomised, but groups were similar. Housewives. | |
| Not randomised. No primary intervention (8 out of 397 apprentices showed HD at inclusion). | |
| Qualitative study, intervention implementation research, no quantitative data available. | |
| RCT: no field study, laboratory experiment. | |
| RCT: effect of industrial hand cleansers on TEWL was evaluated. This type of intervention was not considered in this review. No data on OIHD, only outcome is TEWL. | |
| RCT: no separate data for healthy dental technicians available. Only 5 laboratories were randomised to 4 products; only partial cross‐over‐design (2 out of 4 products were tested in the same laboratory). | |
| Probably not randomised. Under field conditions it was only investigated whether or not the protective ointment had a negative influence on the handling of dental instruments. The protective effects were only measured in a laboratory experiment. | |
| CCT: inclusion of apprentices with hand dermatitis (intervention group 25%, control group 20%), no separate data for healthy apprentices available. No blinding of participants and clinicians, blinding of outcome assessors unclear. | |
| RCT: inclusion of workers with hand dermatitis (intervention group 25%, control group 30% with two or more of the following symptoms: redness, vesicles, papules, itching, scaling, dryness, fissuring, rough and thickened, or suppurate skin changes), no separate data for healthy workers available. | |
| RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. | |
| RCT: the objectives were to investigate the impact of an alcohol‐based hand rub on knowledge, compliance, and hand colonisation of healthcare workers. The prevention of OIHD was not addressed. | |
| RCT in an intensive care unit: unclear if only healthy participants were included, only preliminary data available. No quantitative data on OIHD available. | |
| CCT: inclusion of apprentices with hand dermatitis (intervention group 13.7%, control group 27.8%), no separate data for healthy apprentices available. No blinding of participants, clinicians, and outcome assessors. No information on loss to follow‐up. | |
| CCT: inclusion of workers with hand dermatitis (12%), data on healthy workers were provided, but study could not be included because of violation of quality criteria (no blinding of participants, clinicians, and outcome assessors). | |
| RCT: Primary and secondary intervention mixed. Winker answered to our emails, but did not provide the requested data for initially healthy participants (primary prevention). He stated that the number of cases (HE) would be too small to detect significant differences between groups. This is probably true, but may introduce reporting bias to our review. Their study included 456 participants without and 27 with HE at baseline. At last follow‐up, 24 participants had eczema. | |
| RCT: Comparison of a non‐medicated soap vs an alcohol‐based hand rinse: This type of intervention was not considered in this review. Primary outcome proportion of OIHD was not assessed. Only 8 days of duration. |
CCT: controlled clinical trial
HE: hand eczema
RCT: randomised controlled trial
OIHD: occupational irritant hand dermatitis
TEWL: transepidermal water loss
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial Parallel design, 2 arms Duration: 4 weeks 3 follow‐ups at 1, 2, and 4 weeks |
| Participants | Number of participants randomised: N = 63 intensive care HCWs (over 60% with knuckle dryness and erythema scores of 2 at baseline) Setting: hospital |
| Interventions | 'The objective was to determine the effects on hand skin condition of
'Application was 33 daily.' |
| Outcomes | 'The primary outcome was skin condition measured as
after 1, 2, and 4 weeks.' |
| Notes | Conference publication, only abstract available. 'There was significant irritant dermatitis at baseline as over 60% of HCWs had knuckle dryness and erythema scores of 2.' This trial will only be eligible if the data on OIHD is available in a dichotomised form and separately provided for HCWs without OIHD at baseline. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A behavioural change package to prevent hand dermatitis in nurses working in the national health service (the SCIN trial) |
| Methods | Cluster‐randomised controlled trial (35 sites) Parallel design, 4 arms Duration: 12 months |
| Participants | Nurses working in the National Health Service (NHS) who are particularly at risk Focus on two groups of staff:
Sample size: 'Field workers will be encouraged to recruit as many eligible student nurses and ICU nurses as possible, with the aim of recruiting at least 40 student nurses and 40 nurses from the ICUs at each site.' Setting: '12 NHS acute hospital trusts/health boards which provide OH care to both student and ICU nurses, 18 NHS trusts which provide OH care to ICU nurses and 5 university OH departments which provide OH care to student nurses' |
| Interventions | Comparison of the effectiveness of:
|
| Outcomes |
|
| Starting date | 'At the time of submission, the main trial has been underway since September 2014, with final recruitment planned for March 2016.' |
| Contact information | |
| Notes | Inclusion and exclusion criteria might be refined. It is unclear whether or not nurses with hand dermatitis present at baseline will be included. Study funding sources: The SCIN Trial is funded by the National Institute of Health Research, Health Technology Assessment (HTA) programme grant: NIHR grant number 11/94/01. The trial is sponsored by the National Institute for Health Research (NIHR) Biomedical Research Centre based as Guy's and St Thomas' NHS Foundation Trust and the UKCRC‐registered King's Clinical Trials Unit at King's College London. Possible conflicts of interest: 'There are no competing interests to declare by any of the authors.' |
| Trial name or title | The healthy hands project: Effectiveness of a skincare programme for the prevention of contact dermatitis in healthcare workers |
| Methods | Cluster‐randomised controlled trial Parallel design, 2 arms Duration: 18 months Questionnaire every 6 months |
| Participants | Number of participants randomised: healthcare workers (N unknown, 34 wards) Setting: University Medical Center |
| Interventions | 'The experimental intervention will comprise provision of hand creams in dispensers at the wards, with regular training and feedback, including reports of the electronically monitored consumption. Both the experimental and control groups will receive basic education on skin protection (as care as usual).' |
| Outcomes | 'The primary outcome is the change in Hand Eczema Severity Index from baseline to 12 months. The secondary outcomes are the natural moisturizing factor levels in the stratum corneum as a biomarker of skin barrier damage, and the total consumption of creams per ward.' |
| Starting date | Probably 2016 |
| Contact information | Not known |
| Notes | Conference publication, only abstract available Unknown whether purely primary prevention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 4 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| Analysis 1.1  Comparison 1 Barrier creams versus no treatment, Outcome 1 Proportion of OIHD. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.09] |
| Analysis 2.1  Comparison 2 Moisturisers versus no treatment, Outcome 1 Proportion of OIHD. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.42] |
| Analysis 3.1  Comparison 3 Barrier creams and moisturisers vs no treatment, Outcome 1 Proportion of OIHD. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 3 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.08] |
| Analysis 4.1  Comparison 4 Skin protection education versus no or minimal intervention, Outcome 1 Proportion of OIHD. | ||||
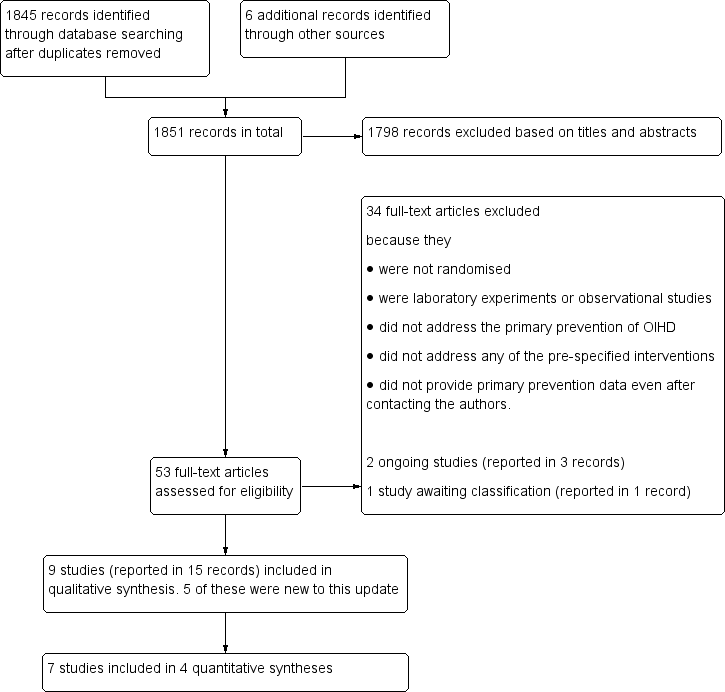
Study flow diagram including all previous searches.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

Comparison 1 Barrier creams versus no treatment, Outcome 1 Proportion of OIHD.
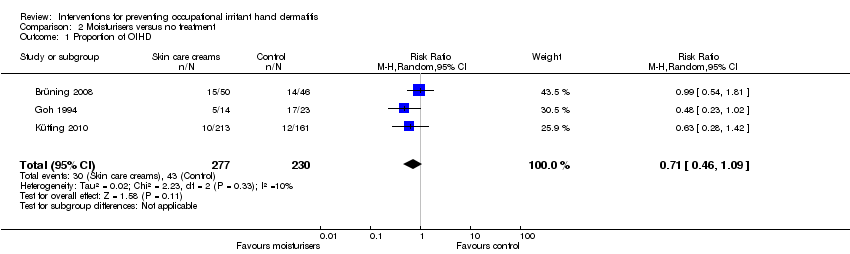
Comparison 2 Moisturisers versus no treatment, Outcome 1 Proportion of OIHD.
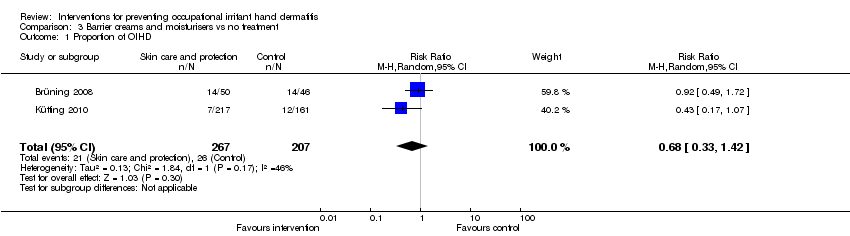
Comparison 3 Barrier creams and moisturisers vs no treatment, Outcome 1 Proportion of OIHD.

Comparison 4 Skin protection education versus no or minimal intervention, Outcome 1 Proportion of OIHD.
| Barrier creams compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment | Risk with barrier creams | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 6 months to 12 months | Study population | RR 0.87 | 999 | ⊕⊕⊝⊝ | ||
| 334 per 1000 | 291 per 1000 | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months | All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the individual trials ranged from 0% to 24%. | 111 (3 RCTs) | ⊕⊕⊕⊝ | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; OIHD: occupational irritant hand dermatitis | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, by study personnel, or by the participants. 2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison the dropout analyses were not detailed enough to extract whether or not adverse effects were among the reasons. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge. | ||||||
| Moisturisers compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment | Risk with moisturisers | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 6 months to 12 months | Study population | RR 0.71 | 507 | ⊕⊕⊝⊝ | ||
| 187 per 1000 | 133 per 1000 | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months | All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the individual trials ranged from 0% to 37% | 133 (2 RCTs) | ⊕⊕⊕⊝ | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, by study personnel, or by the participants. 2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison the dropout analyses were not detailed enough to extract how often adverse effects were a reason. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge. | ||||||
| Barrier creams and moisturisers compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment | Risk with barrier creams and moisturisers | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: median 12 months | Study population | RR 0.68 | 474 | ⊕⊕⊝⊝ | ||
| 126 per 1000 | 85 per 1000 | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months | All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the trial ranged from 8% to 24% | 100 (1 RCT) | ⊕⊕⊕⊝ | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, or by the participants. 2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining study in this comparison no dropout analysis was performed. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge. | ||||||
| Skin protection education compared to no or minimal intervention for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no or minimal intervention | Risk with skin protection education | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 1 years to 3 years | Study population | RR 0.76 | 13551 | ⊕⊝⊝⊝ | ||
| 275 per 1000 | 209 per 1000 | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months | All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the trial ranged from 35% to 38% | 250 (1 RCT) | ⊕⊕⊕⊝ | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 'effective sample size' after correcting for cluster design in one study; number of natural participants: N = 1443 2 Downgraded by three levels. Downgraded one level for risk of bias as the risk ratios may have been overestimated (due to detection bias and baseline imbalance) or underestimated (due to differential diagnostic criteria). Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by a physician, or by the participants. 3 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison no dropout reasons were reported. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge. | ||||||
| Excluded trials | Rationale for exclusion | Number of included trials | Number of included participants | RR (95% CI) | P | I2 |
| ‐ | ‐ | 4 | 999 | 0.87 (0.72 ‐ 1.06) | 0.18 | 9% |
| cluster design without correction | 3 | 629 | 0.89 (0.75 ‐ 1.06) | 0.18 | 0% | |
| split‐body design without paired analysis | 3 | 907 | 0.84 (0.70 ‐ 1.00) | 0.05 | 0% | |
| PO1 was not manifest hand dermatitis | 2 | 410 | 0.75 (0.43 ‐ 1.29) | 0.30 | 35% |
| Study arm | N | Skin hydration1 baseline | Skin hydration1 last follow‐up | TEWL2 baseline | TEWL2 last follow‐up |
| Barrier cream + moisturiser | 50 | 24.73 | 27.18 | 17.65 | 14.9 |
| Moisturiser | 50 | 24.42 | 26.43 | 16.93 | 14.4 |
| Barrier cream | 46 | 25.15 | 28.5 | 16 | 13.68 |
| Control3 | 46 | 25.73 | 27.35 | 16 | 14.5 |
| 1 Skin hydration measured by corneometry 2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h) 3 Differences of interventions compared to the control were not significant at baseline or last follow‐up (no P values reported) | |||||
| Study arm | Skin hydration1 after 11 days control | Skin hydration1 after 11 days barrier cream | TEWL2 after 11 days control | TEWL2 after 11 days barrier cream |
| Control before barrier cream | 67 | 60 | 10 | 14 |
| Barrier cream before control | 74 | 64 | 13 | 12 |
| Mean of both study arms3 | 70.5 | 62 | 11.5 | 13 |
| 1 Skin hydration measured by corneometry (units), figures extracted from diagram 2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h), figures extracted from diagram 3 Differences of barrier cream compared to control group were significant for skin hydration (P < 0.01) and not significant for TEWL (no P values reported) | ||||
| Excluded trials | Rationale for exclusion | Number of included trials | Number of included participants | RR (95% CI) | P | I2 |
| ‐ | ‐ | 3 | 507 | 0.71 (0.46 ‐ 1.09) | 0.11 | 10% |
| cluster design without correction | 2 | 133 | 0.71 (0.36 ‐ 1.43) | 0.34 | 53% | |
| PO1 was not manifest hand dermatitis; split‐body design | 2 | 411 | 0.55 (0.31 ‐ 0.94) | 0.03 | 0% |
| Study arm | N | Skin hydration1 after 2 weeks control | Skin hydration1 after 2 weeks moisturiser | TEWL2 after 2 weeks control | TEWL2 after 2 weeks moisturiser |
| Control before moisturiser group I3 | 70 | 72 | 80 | 39 | 42 |
| Moisturiser before control group I | 70 | 72 | 83 | 40 | 41 |
| Control before moisturiser group II4 | 0 | ‐ | ‐ | ‐ | ‐ |
| Moisturiser before control group II | 23 | 66 | 86 | 41 | 37 |
| Mean of both study arms and both groups5 | ‐ | 71.2 | 82.1 | 39.7 | 40.9 |
| 1 Skin hydration measured by corneometry (capacitance), figures extracted from diagram 2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h), figures extracted from diagram 3 Group 1: participants who completed both periods 4 Group 2: participants who completed the moisturiser period but dropped out of period C after an average of 6 days (1‐10 days) because they developed severe dryness of the skin 5 Differences of moisturiser compared to control were not significant for skin hydration or TEWL (no P values reported) | |||||
| Excluded trials | Rationale for exclusion | Number of included trials | Number of included participants | RR (95% CI) | P | I2 |
| ‐ | ‐ | 2 | 474 | 0.68 (0.33 ‐ 1.42) | 0.30 | 46% |
| cluster design without correction | 1 | 96 | 0.92 (0.49 ‐ 1.72) | 0.79 | ‐ | |
| PO1 was not manifest hand dermatitis; split‐body design | 1 | 378 | 0.43 (0.17 ‐ 1.07) | 0.07 | ‐ |
| Excluded trials | Rationale for exclusion | Number of included trials | Number of included participants | RR (95% CI) | P | I2 |
| ‐ | ‐ | 3 | 1355 | 0.76 (0.54 ‐ 1.08) | 0.12 | 55% |
| cluster design without correction | 2 | 1143 | 0.86 (0.43 ‐ 1.70) | 0.66 | 78% | |
| cluster design without correction; PO1 was not manifest hand dermatitis | 2 | 1105 | 0.90 (0.51 ‐ 1.61) | 0.73 | 54% | |
| cluster design without correction | 1 | 893 | 1.26 (0.67 ‐ 2.35) | 0.47 | ‐ |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 4 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of OIHD Show forest plot | 3 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.08] |

