முழங்கால் கீல்வாதத்திற்கான உடற்பயிற்சிகள்
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Low risk of bias | |
| Participants | Hip and knee OA recruitment 116 community volunteers with knee OA Mean age 66 years, 55% female ACR criteria | |
| Interventions | Clinic, individual: 1. Manual therapy: 9 sessions × 50 minutes (over 16 weeks) plus home programme (3 × per week) 2. Exercise (aerobic plus strengthening plus neuromuscular control): 9 sessions × 50 minutes plus home programme (3 × per week) 3. Exercise plus manual therapy: 9 sessions × 50 minutes plus home programme (3 × per week) 4. Usual care alone | |
| Outcomes | At 1 year: Pain (WOMAC) Physical function (WOMAC) No quality of life measure | |
| Notes | Compared only allocation 2 with allocation 4 Outcomes measured only at 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomisation service, stratified for hip or knee OA |
| Allocation concealment (selection bias) | Low risk | Varied block size randomisation, randomisation service kept schedule |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded participants/therapists |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participant self‐reported pain and physical function |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up balanced between allocation groups, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Registered trial |
| Methods | Moderate to high risk of bias | |
| Participants | 28 community volunteers, ACR clinical criteria Mean age 65 years, all female, mean BMI 25 | |
| Interventions | Clinic, classes: 1. Baduanjin (type of Qigong, less physically demanding than Tai Chi), low‐level aerobics and strength, 8 weeks, 5 × 30 minutes 2. No intervention | |
| Outcomes | At 8 weeks: 1. Pain (WOMAC) 2. Physical function (WOMAC) No quality of life scale | |
| Notes | Poor comparability at baseline for WOMAC pain and physical function. Post‐treatment scores indicate non‐normal distribution, i.e. mean (SD) not appropriate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nothing other than 'patients were randomised' |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Not disclosed |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Not disclosed |
| Incomplete outcome data (attrition bias) | High risk | HIgh loss to follow‐up: 3 (21%) and 4 (29%). No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Unblinded assessor | |
| Participants | 46 volunteers, knee OA | |
| Interventions | 1. Home muscle strengthening programme (+ 12 visits) | |
| Outcomes | At 16 weeks: No QoL | |
| Notes | Very closely monitored intensive strengthening programme with 12 home visits over 16 weeks (ankle weights, squats, etc) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by independent statistician |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participant |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 34 participants/volunteers, knee OA | |
| Interventions | Individual programme 1. 12 weeks: providing 36 sessions ROM/walking and education classes | |
| Outcomes | At 12 weeks: No QoL | |
| Notes | Allocation groups very incomparable base pain/BMI/x‐ray with active treatment allocation, demonstrating more severe disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 140 community volunteers, knee OA ACR criteria, pain > 3/10 | |
| Interventions | Individual programme: 1. Taping, knee massage, thoracic mobs and hip muscle strengthening; 12 weeks, 8 sessions | |
| Outcomes | At 12 weeks and 24 weeks: QoL | |
| Notes | Novel intervention with little attention to knee strengthening. Taping, knee massage, thoracic mobs and hip strengthening | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded (sham US control) |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 89 community volunteers, mean age 65 years, mean BMI 28 ACR criteria, KL Grade II+, medial tibiofemoral compartment disease 50% female, 33% KL Grade IV | |
| Interventions | Clinic, individual: 1. Muscle strengthening (targeting hip abductors and adductors), 7 sessions of 15‐30 minutes over 2 months plus home exercise programme with cuff weights/Theraband (5× per week) 2. Waiting list | |
| Outcomes | At 12 weeks: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life measure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, permuted block 4‐6 |
| Allocation concealment (selection bias) | Low risk | Independent investigator, sealed opaque envelopes, central location |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participant |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | SBalanced loss to follow‐up (13% vs 16%), ITT analysis |
| Selective reporting (reporting bias) | Low risk | Registered trial |
| Methods | Moderate risk of bias | |
| Participants | 50 community volunteers 65 years of age and over 70% female, mean age 75 years | |
| Interventions | Clinic, classes: 1. Education + exercises, 4 weeks 1 × 45 minutes clinic classes, home‐based exercise programme strengthening and stretches 2. Short‐wave diathermy 6 sessions 20 minutes | |
| Outcomes | At 4 weeks and 8 weeks: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life | |
| Notes | Scores estimated from graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | 24% dropout each allocation, intention‐to‐treat analysis LOCF |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate risk of bias | |
| Participants | 41 community volunteers 50 years of age and older ACR criteria 85% female, mean age 70 years, mean BMI 28 | |
| Interventions | Clinic, classes: 1. Tai Chi (simplified Yang style) 6 weeks 3 × 40 minutes followed by 6‐week home programme (videotape) 2. Education programme, 6 weeks 3 × 40 minutes | |
| Outcomes | At 6 weeks and 12 weeks: 1. Pain (WOMAC 7‐35) 2. Physical function (WOMAC 17‐85) No quality of life measurement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table stratified by age and sex |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 9% intervention, 27% control; apparent intention‐to‐treat (data from all participants who did not drop out in the first week) |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias (efficacy analysis) | |
| Participants | 41 community volunteers 55‐75 years of age KL Grade III+ | |
| Interventions | Individual, home‐based: 1. Resistance training lower limb, 6 weeks 2 × 30 minutes supervised plus 1 × 30 minutes unsupervised 2. Neuromuscular electrical stimulation (not included in meta‐analysis) 3. Standard care | |
| Outcomes | At week 8 and week 14: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) 3. SF‐36 MCS | |
| Notes | Standard care included OA education, weight loss, pharmacological therapy and physical therapy (no reporting of participation in any of these interventions) Assumed available to the intervention group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, stratified for age and gender |
| Allocation concealment (selection bias) | Unclear risk | Investigator with no clinical role in the study |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unlinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | High loss to follow‐up (29% and 54%), no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Registered trial |
| Methods | Moderate to high risk of bias | |
| Participants | 41 women, KL Grade II or III, knee flexion > 90 degrees Mean age 67 years, mean BMI 25 | |
| Interventions | Clinic, individual: 1. General physiotherapy (SWD, hot packs, TENS, IFC, etc) plus muscle strengthening (Theraband), 8 weeks 2 × 60 minutes 2. General physiotherapy alone, 8 weeks 2 × 30 minutes | |
| Outcomes | At 8 weeks: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life | |
| Notes | All participants 'prohibited' from using Chinese medicine/alternative therapies and non‐habitual exercise during the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned but no description of procedure provided |
| Allocation concealment (selection bias) | Unclear risk | No indication |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded/therapist unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Assessed by lead study author, not clear whether lead study author was also a therapist or was blinded |
| Incomplete outcome data (attrition bias) | High risk | High loss to follow‐up, unbalanced (20% and 44% controls), no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 83 military care patients, knee OA | |
| Interventions | Individual, clinic programme: 1. Manual therapy/strengthening exercises/aerobic exercise, 4 weeks 2 × 60 minutes | |
| Outcomes | At 8 weeks (delayed): No QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generator |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Participants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Particpants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Imbalance in missing data between allocation groups, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate risk of bias | |
| Participants | 142 participants with symptomatic knee OA, 50 years of age and older, osteophytes on x‐ray 76% female, mean age 70 years, mean BMI 25 | |
| Interventions | Home‐based (1 visit for instruction, no monitoring): 1. Quadriceps exercises in sitting or supine, 4 sets of 20 reps (knee extension in sitting) daily. Sandbags for weight, but almost all used just body weight. 2. NSAIDs 3× daily until 'no longer required' | |
| Outcomes | At 8 weeks: 1. Pain (VAS 0‐100) 2. Physical function (total WOMAC score) No quality of life. 1 undefined score reported for SF‐36 (PCS? MCS? One of the 8 domains?) | |
| Notes | Both allocations could use analgesic patches | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Performed by off‐site administrative office in Department of Public Health |
| Blinding of participants and personnel (performance bias) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | 13%‐17% loss to follow‐up at 8 weeks, no intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Registered with Japanese Orthopaedic Association |
| Methods | Low risk of bias | |
| Participants | 293 volunteers, knee OA | |
| Interventions | Class‐based programme Ettinger a: aerobic walking, 12 weeks 3 × 1 hour Ettinger b: strengthening upper and lower limbs, 12 weeks 3 × 1 hour | |
| Outcomes | Mean score at 3, 9, 18 months: No quality of life | |
| Notes | Large classes (10‐15 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Web‐based, central |
| Blinding of participants and personnel (performance bias) | High risk | Particpants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis, missing data balanced between allocation groups |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | *Hip and knee OA | |
| Interventions | Class‐based programme (6 weeks); 18 sessions of muscle strengthening, range of motion | |
| Outcomes | At 6 weeks: SF‐12 MCS | |
| Notes | Separate analysis per knee OA only or hip OA, gym‐based group versus controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) | High risk | Participants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Small numbers lost to follow‐up, balanced between allocation groups, intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 54 women 40 years of age and older, OA confirmed by MRI Mean age 66 years, mean BMI 32 | |
| Interventions | Clinic, classes: 1. Progressive resistance training lower limb muscles using pneumatic Keiser machines, progressive to 80% 1RM (15‐18 Borg scale), 3 sets of 8 reps, 24 weeks 3 × 60 minutes 2, Sham exercise: as above, but minimal resistance and no progression, only 2 sets of 8 reps, no hip abduction/adduction, 24 weeks 3 × 60 minutes | |
| Outcomes | At 6 months: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life assessment | |
| Notes | Exercise and sham exercise group trained together at same location | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation stratified for glucosamine/chondroitin use and WOMAC physical function subscale score |
| Allocation concealment (selection bias) | Low risk | Conducted by co‐investigator not involved in testing |
| Blinding of participants and personnel (performance bias) | Unclear risk | Most participants blinded/exercise physiologist supervising treatment unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up: exercise (23%), sham exercise (11%), no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Registered study |
| Methods | Low risk of bias | |
| Participants | 126 participants, knee OA, ACR criteria | |
| Interventions | Individual or class‐based allocation (8 weeks), 16 sessions with muscle strengthening and aerobic components | |
| Outcomes | At 8 weeks: SF‐36 MCS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, sequentially numbered |
| Blinding of participants and personnel (performance bias) | High risk | Participants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | *Hip and knee OA, ACR criteria | |
| Interventions | Class‐based programme: 1. Tai Chi classes, modified style for OA: 12 weeks 2 × 60 minutes | |
| Outcomes | At 12 weeks: 3. SF‐12 MCS | |
| Notes | Disaggregated analysis (hip or knee OA) according to identified signal (most painful) joint | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Central allocation by administrator |
| Blinding of participants and personnel (performance bias) | High risk | Participants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Trial registered NCT00123994 |
| Methods | Moderate to high risk of bias | |
| Participants | 23 volunteers, knee OA | |
| Interventions | Individual programme (8 weeks), 24 sessions of strengthening extensors/flexors (Cybex) | |
| Outcomes | At 8 weeks: | |
| Notes | No medications allowed. Young sample | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Participants/personnel unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Participants unblinded |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Assessor unblinded |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate risk of bias | |
| Participants | 217 participants referred from general practice presenting with persistent knee pain and 55 years of age and older | |
| Interventions | Exercise advice and access to 3‐6 sessions with physiotherapist over a 10‐week period Control: advice/education leaflets with 1 follow‐up telephone call | |
| Outcomes | At 3 months and 6 months: 1. WOMAC pain 2. WOMAC physical function 3. Hospital Anxiety and Depression Scale | |
| Notes | Proportion with knee OA unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generator |
| Allocation concealment (selection bias) | Unclear risk | Small blocks of 6 per practice |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up minimal and balanced, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Registered trial |
| Methods | Moderate to high risk of bias | |
| Participants | *Hip and knee | |
| Interventions | Class, clinic: 1. Education + exercise, 6 weeks 1 × 60 minutes 2. Waiting list control | |
| Outcomes | At 6 weeks: No quality of life measure | |
| Notes | Only 6 treatment occasions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 132 participants, bilateral knee OA | |
| Interventions | Individual clinic: 1. Muscle strengthening (KinCom) extensor/flexor + hotpack/ROM, 8 weeks 3 × 60 minutes | |
| Outcomes | At 8 weeks, 1 year: No quality of life measure | |
| Notes | Combined the 3 muscle strengthening groups for meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential numbers I‐IV (representing treatment allocation) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Uncertain |
| Incomplete outcome data (attrition bias) | Unclear risk | 8% loss to follow‐up, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 70 participants, bilateral moderate knee OA | |
| Interventions | Individual, clinic: 1. Muscle strengthening (KinCom) + hotpack/ROM, 8 weeks 3 × 60 minutes | |
| Outcomes | At 8 weeks, 1 year: No quality of life measure | |
| Notes | Analysed group 1 (exercise only) vs group 4 (control), allocation groups 2 and 3 received US and IA hyaluronan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential numbers I‐IV (representing treatment allocation) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | 9% loss to follow‐up, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | *Hip and knee (combined) | |
| Interventions | Class, clinic: 1. Muscle strengthening plus aerobic walking (1 hour) plus education/discussion (30 minutes), 8 weeks 3 × 1.5 hours 2. Control: arthritis help book and list of available community exercise programmes | |
| Outcomes | 8 weeks, 6 months No quality of life measure | |
| Notes | Large loss to follow‐up at 2 months in controls (40%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessors |
| Incomplete outcome data (attrition bias) | High risk | Imbalance in missing data, efficacy analysis, 40% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate risk of bias | |
| Participants | 418 participants > 50 years of age who consulted a primary care physician for knee pain of > 6 months' duration 70% female, mean age 67 years, mean BMI 30 | |
| Interventions | Clinic, individual or classes (results combined): 1. Strengthening, balance, aerobic and motor control exercises, 6 weeks 2 × 45 minutes 2. Usual primary care (most given analgesics, very few participants referred for other interventions) | |
| Outcomes | At 6 weeks and 6 months: 1. Pain (WOMAC 0‐20): only 6 months 2. Physical function (WOMAC 0‐68) 3. Quality of life (EQ5D 0‐1): only 6 months | |
| Notes | Combined results of 2 exercise‐based interventions: individual and class‐based for all meta‐analyses apart from sensitivity analysis according to delivery mode (individual, class, home) for immediate post‐treatment physical function WOMAC physical function was declared main outcome, with results provided for 6 weeks and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomisation: 54 primary care practices were randomly assigned, not participants. Randomisation list was generated by a study co‐author at an external location |
| Allocation concealment (selection bias) | Low risk | Randomisation list was generated by a study co‐author not involved in execution of the study |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | 18% lost to follow‐up, balanced between allocation groups, no intention‐to‐treat but effect of withdrawal was assessed |
| Selective reporting (reporting bias) | Low risk | Registered study |
| Methods | Moderate to high risk of bias | |
| Participants | 102 participants, bilateral knee pain > 6 months, ACR criteria, KL Grade < IV 80% female, mean age 62 years, mean weight 62 kg | |
| Interventions | Clinic, individual: 1. High resistance training (knee extensors and flexors), 60% 1RM, 3 × 8 reps, 8 weeks 3 × 30 minutes; 10 minutes cycling warmup, 10 minutes cold pack knee post session 2. Low resistance training (knee extensors and flexors), 10% 1RM, 10 × 15 reps, 8 weeks 3 × 50 minutes; 10 minutes cycling warmup, 10 minutes cold pack knee post session 3. Health education | |
| Outcomes | At 8 weeks: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life assessment | |
| Notes | Participants did not take NSAIDs during study Results for high resistance training and low resistance training identical, so combined in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random integer generator used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Unbalanced loss to follow‐up: 4 (13%) in control group, 0 in exercise group, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 106 participants 50 years of age and older (not stated whether clinic or community‐based recruitment) ACR criteria, KL Grade < IV (most KL II) Bilateral knee pain > 6 months 70% female, mean age 62 years, mean weight 63 kg (BMI around 25 calculated) | |
| Interventions | Clinic, individual: 1. Progressive weight bearing quadriceps strengthening (sitting, using EN‐Dynamic resistance device), 4 × 6 reps commencing at 50% RM, increasing to 70% RM, 8 weeks 3 × 30 minutes 2. Progressive non‐weight bearing quadriceps strengthening (sitting, using EN‐Tree resistance device), 4 × 6reps commencing at 50% RM, increasing to 70% RM, 8 weeks 3 × 30 minutes 3. No intervention control | |
| Outcomes | At 8 weeks: 1. No pain assessment 2. Physical function (WOMAC 0‐68) No quality of life assessment | |
| Notes | Mean of physical function score taken for the 2 quadriceps strengthening allocations in the meta‐analysis, as no significant difference in physical function at 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up (5 discontinued treatment in the 2 exercise allocations) |
| Selective reporting (reporting bias) | Unclear risk | NCT 9100002377 not found |
| Methods | Low risk of bias | |
| Participants | 389 participants from 5 GP practices in Nottingham, 45 years of age and older BMI > 28, knee pain on most days past month 66% female, mean age 61 years, mean BMI 34, 47% KL Grade II+ | |
| Interventions | Most at home, unmonitored (exercise/control) 1. Diet and exercise 2. Diet 3. Exercise: unsupervised home programme, predominantly strengthening with functional exercises introduced after 2 months and aerobic exercises (walking/stepping up) introduced after 6 months. 2 exercises/d, reps 5 (up to 20) daily for 24 months. Visited every 4 months by a dietitician and received a support telephone call between visits, but the calls were NOT used to reinforce the exercise programme 4. Control: education leaflet (but no information about weight loss or exercise) | |
| Outcomes | At 24 months (delayed): 1. Pain (WOMAC 0‐20) 2, Physical function (WOMAC 0‐68), not estimable as no data for control group No quality of life measure | |
| Notes | Meta‐analysis included data from only 2 allocation groups: Exercise and Control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generator, 2 × 2 factorial design, blocks of 10 stratified by sex, age and BMI |
| Allocation concealment (selection bias) | Low risk | Prepared by trial researcher, kept in locked drawer, opened by co‐ordinator in sequential order |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor (mailed questionnaires) |
| Incomplete outcome data (attrition bias) | Low risk | High loss to follow‐up at 24 months (26% exercise, 14% control), but intention‐to‐treat analysis using multiple imputation methods |
| Selective reporting (reporting bias) | Unclear risk | Study registered, but outcome measures not provided at time of registration. No justification for why only selected domains of SF‐36 (physical function, bodily pain)? |
| Methods | Moderate to high risk of bias | |
| Participants | 259 community volunteers 50 years of age and older, morning stiffness < 30 minutes or crepitus, osteophytes on x‐ray 75% female (81% intervention group, 71% control group), mean age 68 years | |
| Interventions | Clinic, classes: 1. Classes 10‐15 participants, education/discussion plus exercise. Stretching and strengthening 'whole body muscles, especially lower limbs,' 4 weeks 1 × 20 minutes 2. Control, no intervention | |
| Outcomes | At 4 and 8 weeks: 1. No pain (only SF‐36 bodily pain) 2. Physical function (T‐WOMAC 0‐170) 3. Quality of life: SF‐36 MCS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster randomisation of 4 districts: 2 to intervention, 2 to control |
| Allocation concealment (selection bias) | Unclear risk | District allocation would have been known at time of participant screening/recruitment? |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Unbalanced loss to follow‐up: 15% intervention, 27% controls. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Study not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 34 volunteers and participants. Married | |
| Interventions | Class, clinic: 1. 36 aerobic sessions, 24 strengthening sessions, 12 weeks 3 × 1 hour 2. Standard care | |
| Outcomes | At 12 weeks: QoL: AIMS psychological | |
| Notes | Analysed group 3 (exercise only) vs standard care (no spouse intervention groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only 'randomly allocated' |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded particpants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 6% loss to follow‐up, balanced between allocations. Efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 103 participants, knee OA, 84% female, mean age 69 years | |
| Interventions | Class‐based, clinic: 1. Fitness walking/stretch/education, 8 weeks 3 × 60 minutes 2. Control: weekly telephone call regarding ADL function | |
| Outcomes | At 8 weeks: No quality of life measure | |
| Notes | Large classes (20‐30 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data balanced between allocation groups, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | Participants and community volunteers, KL Grade II+, assessed at least 6 months before study entry, 50‐80 years of age 93% female, mean age 69 years, mean BMI 26, most KL Grade II‐III | |
| Interventions | Clinic, classes: 1. Tai Chi Qigong (18 movements). Movements of mixed nature (motor control, ROM). Movements involved gentle body stretches, 8 weeks 2 × 45 minutes 2. No intervention control | |
| Outcomes | At 8 weeks: 1. Pain (WOMAC 0‐35) 2. Physical function (WOMAC 0‐85) 3. Quality of life (SF‐36 MCS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated balanced block randomisation (2:1) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with identification number. Opened in order |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblilnded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up low (n = 3), included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study not registered |
| Methods | Low risk of bias | |
| Participants | 107 community volunteers, tibiofemoral knee OA, ACR criteria, medial knee pain, medial compartment osteophytes and medial joint space narrowing > lateral joint space narrowing. < 5 degrees valgus malalignment on x‐ray 55% female, mean age 66 years, mean BMI 29 | |
| Interventions | Most in home programme: 1. Quadriceps strengthening in varus knee alignment group, 2 × 10 reps (weeks 1‐2), 3 × 10 reps (weeks 3‐12), 5 days a week. Exercise loads progressed frequently, monitored by 7 home visits by physiotherapist 2. Quadriceps strengthening exercise in neutral knee alignment group, as above 3. No intervention control varus knee alignment group 4. No intervention control neutral knee alignment group | |
| Outcomes | At week 13: 1. Pain (WOMAC 0‐100) 2. Physical function (WOMAC 0‐100) No quality of life measure | |
| Notes | Average results for exercise allocations (1,2) vs average results for control allocations (3,4) used in meta‐analysis. Study did demonstrate clearly that effects of quadriceps strengthening greater in neutral alignment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used, stratified by alignment in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Independent researcher randomly assigned participants |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 4%‐18%, but intention‐to‐treat analysis using last observation carried forward |
| Selective reporting (reporting bias) | Low risk | Registered study |
| Methods | Low risk of bias | |
| Participants | 108 participants 50 years of age and older, KL Grade < IV, history of knee pain > 6 months 70% female, mean age 62 years, mean weight 62 kg | |
| Interventions | Clinic, individual: 1. Proprioception exercises, stepping in multiple directions at various speeds, ROM exercises, 8 weeks 3 × 50 minutes 2. Quadriceps strengthening, 50% 1RM 4 × 6 reps. 1RM tested every 2 weeks and a 5% increase in 1RM implemented to training weight, 8 weeks 3 × 50 minutes 3. No intervention control | |
| Outcomes | At 8 weeks: 1. Pain (WOMAC 0‐20) 2. Physical function (WOMAC 0‐68) No quality of life measure | |
| Notes | Only allocations 2 and 3 included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | 6%‐8% loss to follow‐up, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Study not registered |
| Methods | Low risk of bias | |
| Participants | 79 community volunteers, ACR criteria 75% female, mean age 69 years, mean weight 68‐77 kg | |
| Interventions | Clinic, classes: 1. Aquatic exercise 2. Land‐based exercise, mixed strengthening, endurance, balance, stretching, 8 weeks 2 × 50 minutes 3. No intervention control | |
| Outcomes | At 8 weeks and 20 weeks: 1. Pain (KOOS, 100‐0) 2. Physical function (KOOS ADL, 100‐0) 3. Quality of life (KOOS QoL, 100‐0) | |
| Notes | Needed to reverse score KOOS pain and physical function outcomes (KOOS lower score is worse score) and to calculate SD from provided SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope method with blocks of 18 |
| Allocation concealment (selection bias) | Low risk | Envelope method, so screener unaware which will be chosen |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | 7%‐20% loss to follow‐up at 20 weeks; however intention‐to‐treat analysis using LOCF |
| Selective reporting (reporting bias) | Unclear risk | Study not registered |
| Methods | Moderate risk of bias | |
| Participants | 113 participants, knee OA, ACR criteria | |
| Interventions | Individual, clinic: 1. Unilateral quadriceps strengthening only, 8 weeks 3 × 30 minutes 2. 4 education classes | |
| Outcomes | At 8 weeks: No quality of life measure | |
| Notes | Only unilateral exercise but many(?) with bilateral symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data balanced between groups, not study‐related, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 158 obese community volunteers | |
| Interventions | Class, clinic (4 months + optional additional 2 months clinic or home) Four allocations: 1. Exercise 2. Exercise + diet 3. Diet 4. Control Exercise: strengthening and aerobic walking (4 months), then telephone monitored home programme (with weights) | |
| Outcomes | At 6 and 18 months (delayed): No quality of life measure | |
| Notes | Analysis of exercise only vs healthy lifestyle control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Web‐based |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 37 community volunteers in OA/pain strata | |
| Interventions | Clinic (0‐12 months), then home programme thereafter (12‐30 months): 2. ROM control | |
| Outcomes | 30 months (delayed): 3. SF‐36 MCS | |
| Notes | Analysis only of participants with knee OA/pain. Twice‐weekly clinic‐based classes in the first 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information apart from 'randomized' |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | 21% loss to follow‐up at 30 months but intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 80 participants/volunteers, knee OA, 80% female, mean age 64 years | |
| Interventions | Class, clinic: 1. Aerobic walking, 12 weeks 3 × 1 hour | |
| Outcomes | At 12 weeks and 1 year: QoL (AIMS depression) | |
| Notes | Large classes (max 12 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Uncertain blinding assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Efficacy analysis, 7% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 180 volunteers, knee OA, 66% female, mean age 62 years | |
| Interventions | Home programme: 1. Lower limb strengthening (4 home visits to monitor) + lifestyle advice 2. Lifestyle advice only | |
| Outcomes | At 6 months (delayed): 3. Quality of life (HADS depression) | |
| Notes | Community sample, most with mild radiographic/symptomatic disease (only 41% > KL Grade I) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported whether sealed envelopes were opaque with sequential numbers for audit trail |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded partcipants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessors |
| Incomplete outcome data (attrition bias) | Low risk | 6% loss to follow‐up, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 137 volunteers, knee OA, 70% female, mean age 66 years | |
| Interventions | Class‐based, clinic: 1. Aerobic and strengthening/stretching exercise, 12 weeks 3 × 1 hour | |
| Outcomes | At 12 weeks: No quality of life measure | |
| Notes | Excluded people with severe disease: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Imbalance in missing data between allocation groups, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 87 community volunteers with patellofemoral pain | |
| Interventions | 1. 9 physiotherapy sessions over 10 weeks 2. Standard care | |
| Outcomes | At 5 and 12 months: No quality of life measure | |
| Notes | Zelen randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, sequentially numbered for audit trail |
| Blinding of participants and personnel (performance bias) | Low risk | Unblinded to intervention, but Zelen randomisation resulted in blinded control group |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | Low risk | Blinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 25 participants, knee OA ACR criteria and +x‐ray (> KL Grade II) | |
| Interventions | Class‐based programme: 1. Complex mix of exercises, 12 weeks 2 × 1 hour | |
| Outcomes | At 12 weeks and 1 year: No quality of life measure | |
| Notes | Moderate to severe disease. Only median (IQR) provided. Baseline differences in pain scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data minimal and balanced between allocation groups, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 37 participants/community volunteers, KL Grades I‐III > 90 degrees knee flexion and exclude patellofemoral pain precluding stationary cycling 77%‐60% female, mean age 53‐61 years, mean BMI 22‐27, experimental/control | |
| Interventions | Clinic, classes: 1. Aerobic cycling (modified 'spinning,' 70% maximum heart rate) 12 weeks 2 × 60 minutes 2. Wait list control | |
| Outcomes | At 12 weeks: Pain (WOMAC): reverse score Physical function (WOMAC): reverse score KOOS QoL | |
| Notes | Baseline incomparability between groups for BMI and age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Preset randomisation scheme with computer assignment |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | High risk | 17% (control)‐32%(intervention) lost to follow‐up and not included in analysis |
| Selective reporting (reporting bias) | Low risk | Registered |
| Methods | Moderate to high risk of bias | |
| Participants | 75 community volunteers 45‐65 years of age, leading sedentary life ACR criteria, KL Grade I or II, mean age 57 years, 83% female, mean BMI 32 | |
| Interventions | Clinic, individual: 1. Concentric‐eccentric exercise programme (8 weeks 3 × 60 minutes individual, used isokinetic dynamometer) + PRN paracetamol to max 2 grams per day 2. Isometric exercise programme (8 weeks 3 × 60 minutes individual, used isokinetic dynamometer) + PRN paracetamol to max 2 grams per day 3. Control (PRN paracetamol to max 2 grams per day) | |
| Outcomes | At week 8 and week 20: Pain (VAS motion) Physical function (WOMAC) No quality of life | |
| Notes | Early radiographic disease, SF‐36 MCS scores appear extremely high (70.1) Participants assigned to concentric‐eccentric experienced a short period of difficulty in adaptation, but no later adverse effects were observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided, major differences between allocation groups in physical function/SF‐36 MCS at baseline |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Only 4/75 lost fo follow‐up (5%), balanced between allocation groups |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 20 participants, knee OA, 85% female, mean age 66 years | |
| Interventions | Individual, clinic: 1. Strengthening bilateral knee extensors and flexors, 8 weeks 3 × 1 hour 2. No intervention control | |
| Outcomes | At 8 weeks: No quality of life measure | |
| Notes | All training on Cybex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Uncertain |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate risk of bias | |
| Participants | 35 participants, ACR criteria, KL Grade II+ 90% female, mean age 70 years, mean BMI 27‐30 | |
| Interventions | Clinic classes: 1. Squat exercises on a vibratory platform 2. Cycle (70% maximum heart rate) and squatting exercises (progressive 20 × 6 reps), 12 weeks 3 × 30 minutes 3. Telephone calls to confirm adherence to routine activities, i.e. not starting exercise programme (control) | |
| Outcomes | At 12 weeks (median, IQR provided): 1. Pain (WOMAC 0‐500) 2. Physical function (WOMAC 0‐1700) No QoL | |
| Notes | The 2 allocation groups were incomparable at baseline for BMI (27 vs 30) WOMAC pain and function Large proportion same KL 4 (27%‐40%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No procedure described |
| Allocation concealment (selection bias) | Low risk | Serial numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant in each allocation lost to follow‐up at 12 weeks |
| Selective reporting (reporting bias) | Low risk | Registered |
| Methods | Moderate risk of bias | |
| Participants | 72 sedentary female participants, knee OA (confirmed by email), clinical and radiographic criteria | |
| Interventions | Class‐based programme, clinic: 1. Tai Chi classes, 16 1‐hour sessions 2. Control: weekly telephone call | |
| Outcomes | At 12 weeks: No quality of life measure | |
| Notes | About 40% dropout | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | 43% missing data, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 34 participants, knee OA, ACR criteria | |
| Interventions | Home programme: 1. 12 ASMP classes plus home‐based pedometer walking programme | |
| Outcomes | At 12 week and 24 weeks: No physical function measure No quality of life measure | |
| Notes | Evaluating the addition of a home‐based pedometer monitored walking programme to the Arthritis Self‐Management Programme (ASMP) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | High risk | Unblinded assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Minimal missing data, balanced between allocation groups, efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 786 participants, knee pain | |
| Interventions | Home programme: 1. Daily muscle strength training, bilateral, with Theraband plus 4 home visits during first 2 months, then 1 visit per 6 months (8, 14, 20 months?) | |
| Outcomes | At 24 months (delayed): No quality of life measure | |
| Notes | Participants with knee pain, all may not be OA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central administration, sequential list audit trail |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 65 participants (identified by radiologists/orthopaedic surgeons) with radiographic knee OA (KL Grade III or higher) and long‐standing knee pain Between 35 and 65 years of age | |
| Interventions | Clinic‐based classes: 1. Intensive muscle strengthening programme, 6 weeks 2 × 1 hour 2. Control: waiting list for 6 months | |
| Outcomes | At 6 weeks and 6 months: 1. KOOS pain 2. KOOS ADL 3. SF‐36 MCS | |
| Notes | Younger sample and more severe radiographic disease than most RCTs evaluating exercise for OA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No sequence generation, sealed envelopes produced before randomisation |
| Allocation concealment (selection bias) | Low risk | Participants selected sealed envelope |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Uncertain |
| Incomplete outcome data (attrition bias) | Low risk | Only 7%‐10% loss to follow‐up, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 102 volunteers, ACR clinical criteria | |
| Interventions | Class‐based, clinic: 1. Muscle strengthening (dynamic or isometric) with Theraband, 15 weeks 1 × 1 hour (clinic), home 16 weeks 2 × 1 hour 2. Control: no intervention | |
| Outcomes | At 16 weeks: No quality of life measure | |
| Notes | Clinic‐based classes 1× per week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | Uncertain |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 113 participants, knee OA, ACR criteria | |
| Interventions | Individual, clinic: 1. Physiotherapy + GP education, 12 weeks, 17 sessions total 2. GP education | |
| Outcomes | At 12 weeks: No quality of life measure | |
| Notes | Recruited participants with hip and knee OA. Separate results provided for knee OA. Most with early disease, as approximately 50% of sample had symptom duration < 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, sequential numbering for audit trail |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Low risk of bias | |
| Participants | 84 community volunteers, 55 years of age and older Physician diagnosis of OA Not currently exercising > 60 minutes per week, past 2 months | |
| Interventions | Clinic, classes: 1. Land‐based exercise, PACE programme (flexibility and aerobic), 12 weeks 3 × 60 minutes 2. Aquatic exercise programme 3. Control (no intervention) | |
| Outcomes | At 12 weeks: 1. KOOS pain (0‐100), reverse scored 2. KOOS ADL (0‐100), reverse scored 3. KOOS quality of life (0‐100) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | External, researcher not recruiting participants |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Only 7% loss to follow‐up, 2/28 in each allocation, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Methods | Moderate to high risk of bias | |
| Participants | 182 participants 50 years of age and older, ACR clinical criteria Mean age 65 years, 84% female | |
| Interventions | Clinic, classes: 1. ASMP + stretching/walking/Tai Chi (8 movements), 6 weeks 1 × 120 minutes (15 minutes for exercise) 2. No intervention | |
| Outcomes | At week 7 and week 23: 1. Current pain (0‐100) No physical function (HAQ score inappropriate: most upper limb function; scores inaccurate: outside 0‐3 range) No quality of life | |
| Notes | Large loss to follow‐up due to SARS (Hong Kong): discouraged from attending hospital clinics Health Assessment Questionnaire for rheumatoid arthritis developed (not specific to lower limb disability) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) ‐ subjective self‐reported outcomes (pain, function, quality of life) | High risk | Unblinded participants |
| Blinding of outcome assessment (detection bias) ‐ other outcomes | Unclear risk | No indication that outcomes assessors blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss to follow‐up post treatment due to SARS: 25% control, 10% intervention; 16 weeks: 44% control, 24% intervention. ITT analysis conducted but method not clarified |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
1RM: One‐repetition maximum.
ACR: American College of Rheumatology.
ADL: Activity of daily living.
AFI: Arthritis Function Index.
AIMS: Arthritis Impact Measurement Scales.
ASMP: Arthritis Self‐Management Programme.
BMI: Body mass index.
EQ5D: Standardised measure of health outcome.
FAST: Fitness Arthritis and Seniors Trial.
GP: General practitioner.
HADS: Hospital Anxiety Depression Scale.
IFC: International Functional Classification.
IQR: Interquartile range.
IRGL: Influence of Rheumatic Disease on Health and Lifestyle scale.
ITT: Intention‐to‐treat.
KL: Kellgren and Lawrence.
KOOS: Knee Osteoarthritis Outcome Scale.
LOCF: Last observation carried forward.
MCS: Mental Component Summary.
MRI: Magnetic resonance imaging.
NSAIDs: Non‐steroidal anti‐inflammatory drugs.
OA: Osteoarthritis.
OASI: Osteoarthritis Screening Index.
PACE: Patient‐centered Assessment and Counseling for Exercise.
PCS: Physical Component Summary.
QoL: Quality of life.
RCT: Randomised controlled trial.
ROM: Range of motion.
SARS: Severe acute respiratory syndrome.
SD: Standard deviation.
SF: Short Form.
SWD: Short Wave Diathermy.
TENS: Transcutaneous electrical nerve stimulation.
US: Ultrasound.
VAS: Visual analogue scale.
WOMAC: Western Ontario and McMasters Universities Osteoarthritis Index.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No non‐exercise control, not randomised | |
| Large baseline differences between 2 small comparator groups in pain and physical function scores | |
| Secondary analysis (Aglamis 2009) | |
| No non‐exercise control | |
| No non‐exercise control | |
| No non‐exercise control | |
| Prehabilitation (home stretching programme) | |
| Physical therapy did not include an exercise programme (IR, short‐wave diathermy, interferential) | |
| No non‐exercise control | |
| No non‐exercise control, not randomised, no self‐report measures | |
| Patients scheduled for joint replacement surgery | |
| No pain/physical function/quality of life measures | |
| All non‐arthroplasty patients had RA | |
| Secondary analysis (Bulthuis 2007) | |
| Unable to ascertain effect size, as only provided with median % improvements without baseline scores and with extremely wide confidence intervals because of small sample size | |
| No non‐exercise control | |
| No non‐exercise control | |
| No appropriate control. Assessed benefit of SWD added to exercise | |
| No control group. Control group used extremely effective sham TENS | |
| Secondary analysis of Cheing 2002. Only gait and muscle strength evaluated | |
| No non‐exercise control, not randomised | |
| Secondary analysis (Veenhof 2007) | |
| Prehabilitation | |
| No non‐exercise control | |
| Unable to extract change (SD) or post‐treatment (SD) scores from published manuscript. Unusually, published manuscript provided only median/test statistic/degrees of freedom data | |
| No non‐exercise control | |
| All study patients taking fixed‐dose NSAIDs (meloxicam 15 mg daily) | |
| No non‐exercise control | |
| No non‐exercise control | |
| No non‐exercise control | |
| No non‐exercise control | |
| Not a randomised trial. Patients were 'separated' into 3 groups | |
| Prehabilitation | |
| No non‐exercise control | |
| No self‐reported pain/physical function/quality of life | |
| No non‐exercise control | |
| No non‐exercise control | |
| Aquatic exercise | |
| Secondary analysis (Foroughi 2011) | |
| No non‐exercise control | |
| Aquatic exercise | |
| No randomly assigned allocation | |
| No non‐exercise control | |
| No appropriate control. Assessed benefit of hydrotherapy added to home exercise | |
| Inappropriate control group (biomagnetic therapy) | |
| Advice and exercise given in control group. Evaluated treatment was acupuncture | |
| No self‐reported pain/function outcomes | |
| No randomly assigned allocation | |
| Aquatic exercise | |
| No non‐exercise control | |
| No non‐exercise control. Manual therapy vs exercise | |
| Earlier version of Huang 2005 (1) | |
| Secondary analysis | |
| Not even quasi‐randomised | |
| Secondary analysis | |
| 18‐ and 30‐month outcomes for a 6‐week intervention (Hurley 2007). Already submitted 6‐month outcomes for sustainability evaluation | |
| Not even quasi‐randomised | |
| Preliminary analysis for Lin 2009 | |
| No non‐exercise control | |
| Aquatic exercise | |
| No non‐exercise control | |
| Inappropriate control: weekly intra‐articular hyaluronate injections | |
| No randomly assigned allocation | |
| No randomly assigned allocation | |
| No pain/function/patient global outcome assessment. Only outcome is muscle strength | |
| Cluster random sampling | |
| No control group in analysis of results. No pain/function/patient global outcome assessment | |
| No non‐exercise control | |
| No non‐exercise control | |
| Water exercise programme | |
| Preliminary analysis (Lin 2009) | |
| No non‐exercise control | |
| No appropriate control group. Both allocations on stationary cycling, high vs low intensity | |
| Exercise only a small component of the experimental allocation (pharmacist‐led education programme) | |
| No non‐exercise control | |
| No non‐exercise control | |
| No appropriate control group (comprehensive and well‐monitored self‐management programme, including exercise component) | |
| No randomly assigned allocation | |
| Secondary analysis (Ettinger 1997). Gait assessment | |
| No appropriate control group. Assessed benefit of dietary therapy added to an exercise programme | |
| Secondary analysis (Ettinger 1997a/b). Balance assessment | |
| No non‐exercise control | |
| No pain, physical function, quality of life outcomes | |
| Secondary analysis (ADAPT study) | |
| No exercise group; patients passive for mobilisation | |
| No non‐exercise control | |
| No non‐exercise control | |
| No non‐exercise control | |
| Secondary analysis (Messier 2004). Outcomes limited to markers of chronic inflammation | |
| No non‐exercise control | |
| Secondary analysis (Ettinger 1997a/b) | |
| Secondary analysis (Ettinger 1997a/b) | |
| No non‐exercise allocation | |
| No non‐exercise allocation, no pain/physical function measures | |
| No non‐exercise allocation | |
| Secondary analysis (Kovar 1992). Gait assessment | |
| All study patients taking fixed‐dose NSAIDs (oxaprozin 1200 mg daily) | |
| No non‐exercise allocation | |
| No non‐exercise allocation, no pain/function/quality of life outcomes | |
| No non‐exercise allocation | |
| Secondary analyses | |
| Secondary analysis | |
| No non‐exercise allocation | |
| No appropriate control group. Assessed benefit of interferential therapy or SWD added to exercise | |
| No non‐exercise allocation | |
| Cluster‐randomised trial | |
| No non‐exercise allocation | |
| No self‐report pain/physical function/quality of life outcomes | |
| Secondary analysis (Ettinger 1997a/b) | |
| No non‐exercise control | |
| Not randomised | |
| Not randomised | |
| No non‐exercise allocation | |
| No non‐exercise control | |
| Secondary analysis ADAPT study | |
| No non‐exercise allocation | |
| Not randomised, no specific pain/function/quality of life outcomes | |
| Not randomised | |
| No non‐exercise allocation | |
| Not randomised | |
| No pain/function/quality of life outcomes | |
| No non‐exercise allocation | |
| Not randomised or quasi‐randomised—sequentially assigned. In addition, all patients received hyaluronan (5 or 3 weekly injections) | |
| Not randomised | |
| Secondary analysis (1‐year follow‐up) (Kovar 1992) | |
| Secondary analysis (Topp) | |
| No appropriate control. Hydrotherapy compared with exercise plus SWD (N = 14) | |
| Secondary analysis (Fitzgerald 2011), no non‐exercise control | |
| No non‐exercise allocation | |
| Not randomised | |
| No non‐exercise allocation | |
| Prehabilitation | |
| No non‐exercise allocation | |
| No non‐exercise allocation | |
| No non‐exercise control | |
| Secondary analysis (van Baar 1998) (follow‐up study) | |
| Secondary analysis ADAPT study | |
| No non‐exercise allocation | |
| Prehabilitation | |
| No land‐based exercise group | |
| Aquatic exercise only | |
| No pain/function/quality of life outcomes | |
| No non‐exercise control | |
| No non‐exercise control | |
| No non‐exercise allocation | |
| Patients awaiting knee replacement surgery | |
| Prehabilitation | |
| No non‐exercise control | |
| No non‐exercise allocation | |
| Secondary analysis | |
| Secondary analysis |
ADAPT: Arthritis Diet and Activity Promotion Trial
IR: Infra‐Red
NSAIDs: Non‐steroidal anti‐inflammatory drugsav
RA: Rheumatoid Arthritis
SD: standard deviation
SWD: Short Wave Diathermy
TENS: Transcutaneous electrical nerve stimulation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3537 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.59, ‐0.39] |
| Analysis 1.1  Comparison 1 Post treatment, Outcome 1 Pain. | ||||
| 1.1 Change scores | 28 | 2136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.62, ‐0.38] |
| 1.2 End of treatment scores | 16 | 1401 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.65, ‐0.29] |
| 2 Physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| Analysis 1.2 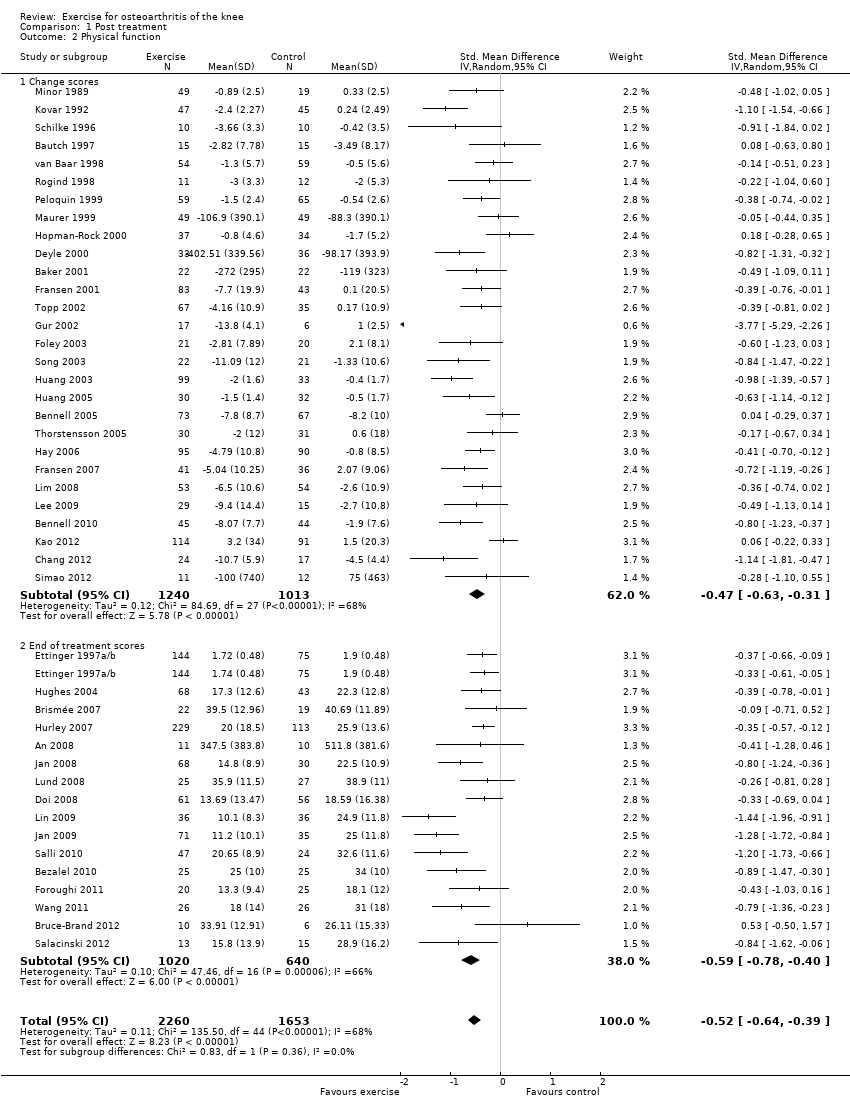 Comparison 1 Post treatment, Outcome 2 Physical function. | ||||
| 2.1 Change scores | 28 | 2253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 2.2 End of treatment scores | 16 | 1660 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.78, ‐0.40] |
| 3 Quality of Life Show forest plot | 13 | 1073 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.15, 0.40] |
| Analysis 1.3  Comparison 1 Post treatment, Outcome 3 Quality of Life. | ||||
| 3.1 Change scores | 8 | 848 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.13, 0.42] |
| 3.2 End of treatment scores | 5 | 225 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.04, 0.57] |
| 4 Study withdrawals Show forest plot | 45 | 4607 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| Analysis 1.4 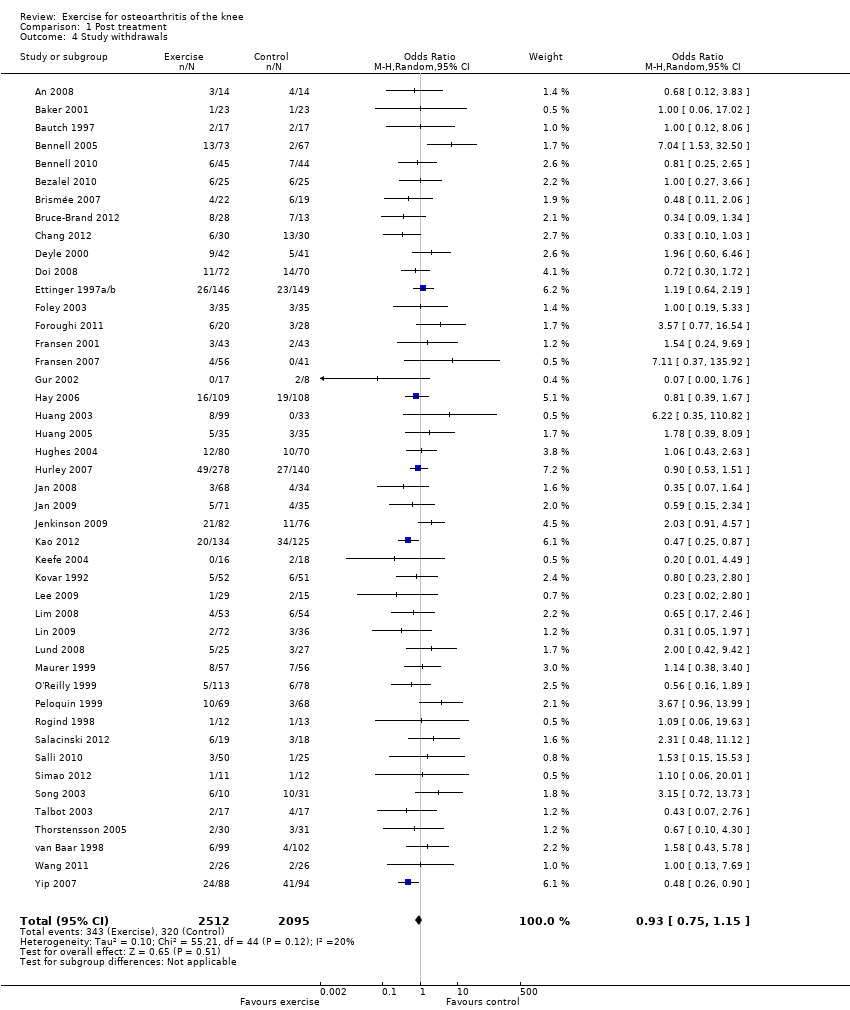 Comparison 1 Post treatment, Outcome 4 Study withdrawals. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 12 | 1468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.35, ‐0.14] |
| Analysis 2.1  Comparison 2 Treatment sustainability 2‐6 months, Outcome 1 Pain. | ||||
| 1.1 Change | 4 | 563 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.36, ‐0.02] |
| 1.2 End of follow‐up | 8 | 905 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.15] |
| 2 Physical function Show forest plot | 10 | 1279 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| Analysis 2.2  Comparison 2 Treatment sustainability 2‐6 months, Outcome 2 Physical function. | ||||
| 2.1 Change scores | 4 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.02] |
| 2.2 End of follow‐up | 6 | 713 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.32, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 8 | 1272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.01, ‐0.03] |
| Analysis 3.1  Comparison 3 Treatment sustainability > 6 months, Outcome 1 Pain. | ||||
| 1.1 Change | 4 | 1024 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.35, 0.26] |
| 1.2 End of follow‐up | 4 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.02, ‐0.04] |
| 2 Physical function Show forest plot | 8 | 1266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.05, ‐0.10] |
| Analysis 3.2 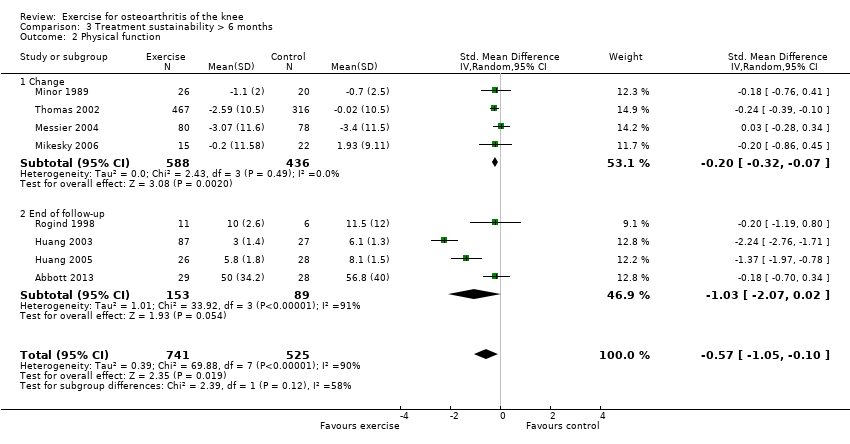 Comparison 3 Treatment sustainability > 6 months, Outcome 2 Physical function. | ||||
| 2.1 Change | 4 | 1024 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.32, ‐0.07] |
| 2.2 End of follow‐up | 4 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.07, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| Analysis 4.1 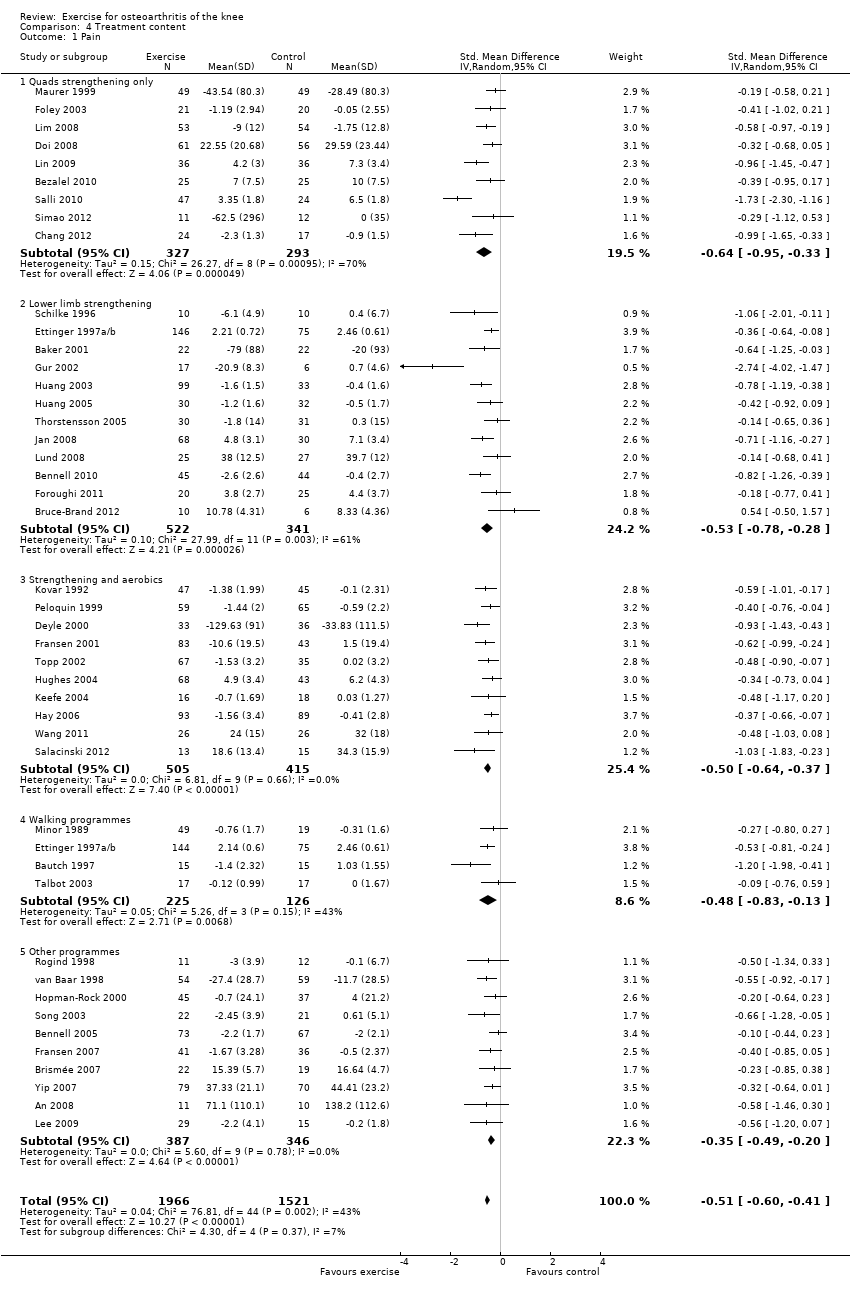 Comparison 4 Treatment content, Outcome 1 Pain. | ||||
| 1.1 Quads strengthening only | 9 | 620 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.95, ‐0.33] |
| 1.2 Lower limb strengthening | 12 | 863 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.28] |
| 1.3 Strengthening and aerobics | 10 | 920 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.64, ‐0.37] |
| 1.4 Walking programmes | 4 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.83, ‐0.13] |
| 1.5 Other programmes | 10 | 733 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.49, ‐0.20] |
| 2 Physical function Show forest plot | 44 | 4255 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.62, ‐0.39] |
| Analysis 4.2 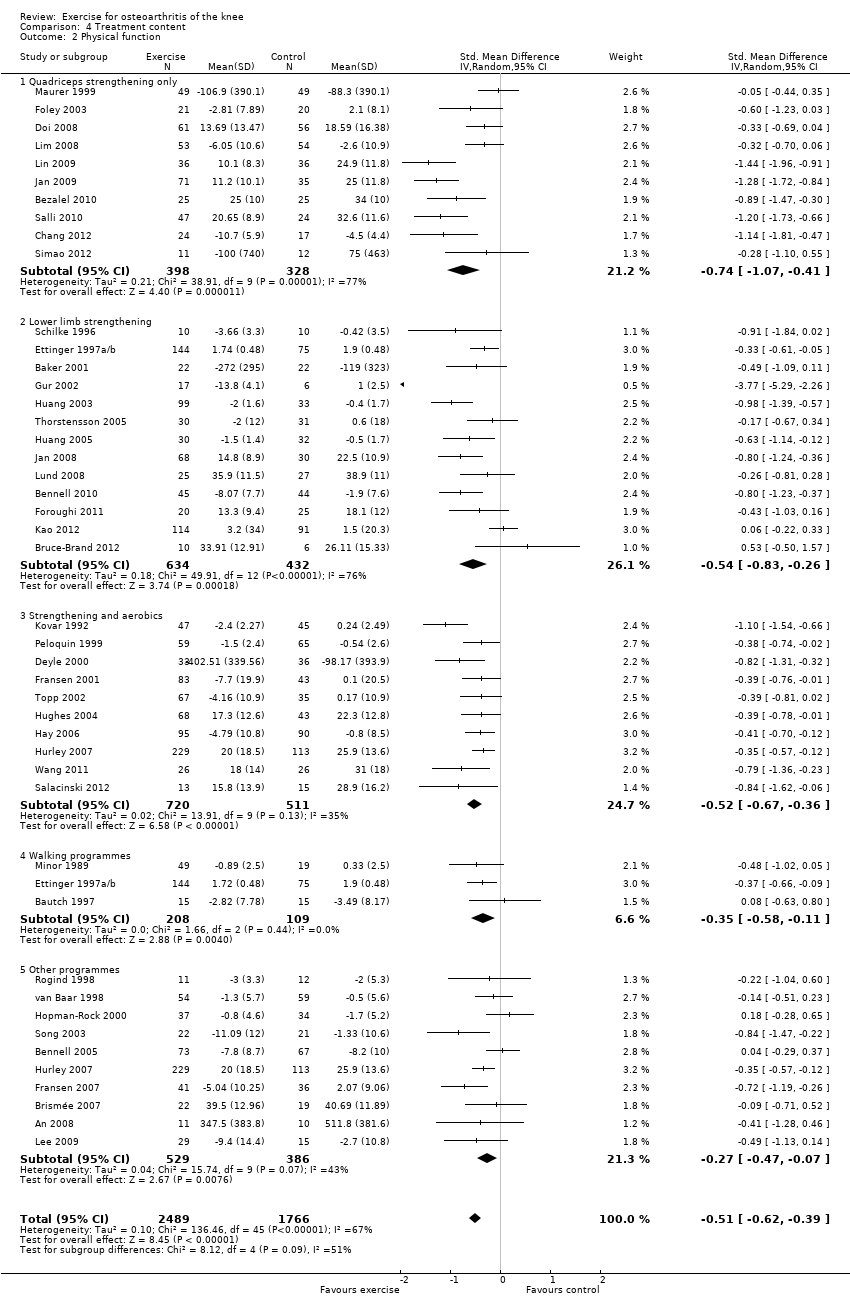 Comparison 4 Treatment content, Outcome 2 Physical function. | ||||
| 2.1 Quadriceps strengthening only | 10 | 726 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.07, ‐0.41] |
| 2.2 Lower limb strengthening | 13 | 1066 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.83, ‐0.26] |
| 2.3 Strengthening and aerobics | 10 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.67, ‐0.36] |
| 2.4 Walking programmes | 3 | 317 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.58, ‐0.11] |
| 2.5 Other programmes | 10 | 915 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.47, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3588 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.60, ‐0.41] |
| Analysis 5.1 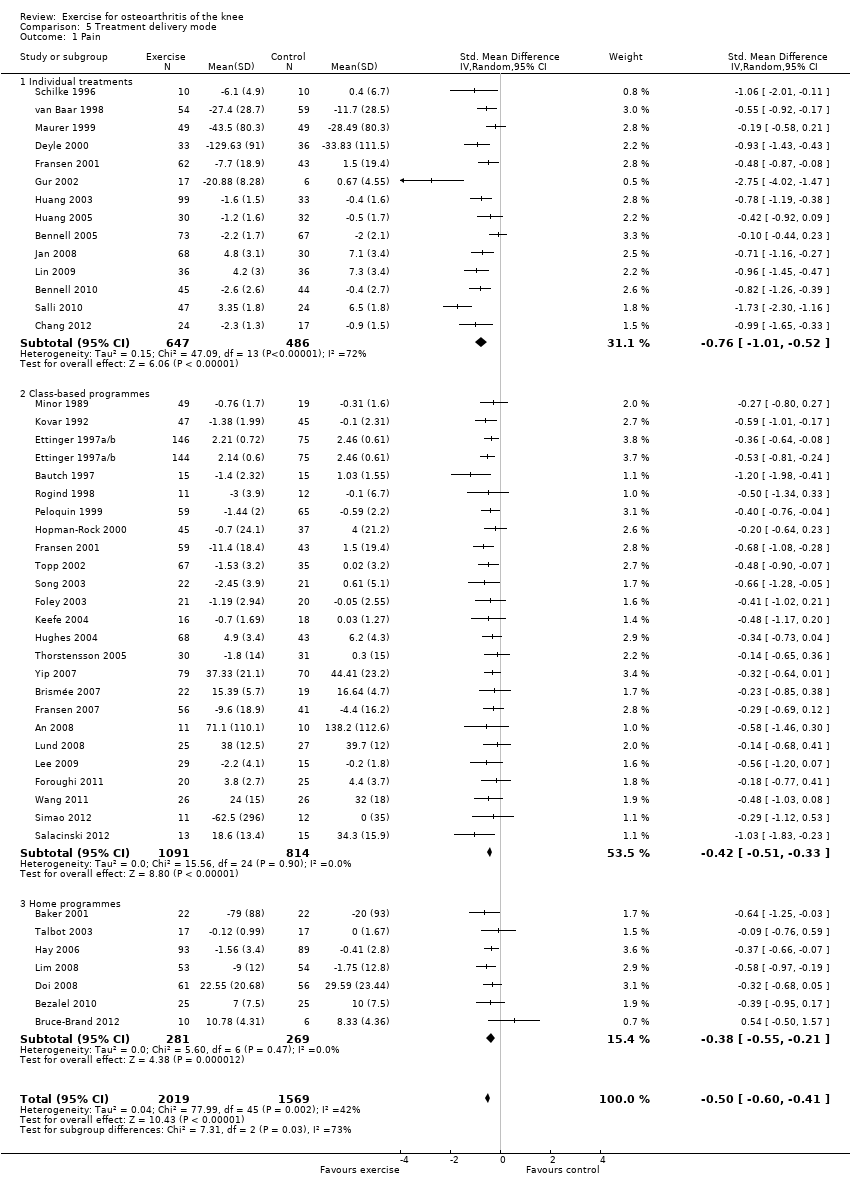 Comparison 5 Treatment delivery mode, Outcome 1 Pain. | ||||
| 1.1 Individual treatments | 14 | 1133 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.01, ‐0.52] |
| 1.2 Class‐based programmes | 24 | 1905 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.51, ‐0.33] |
| 1.3 Home programmes | 7 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.55, ‐0.21] |
| 2 Physical Function Show forest plot | 45 | 4344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.61, ‐0.38] |
| Analysis 5.2 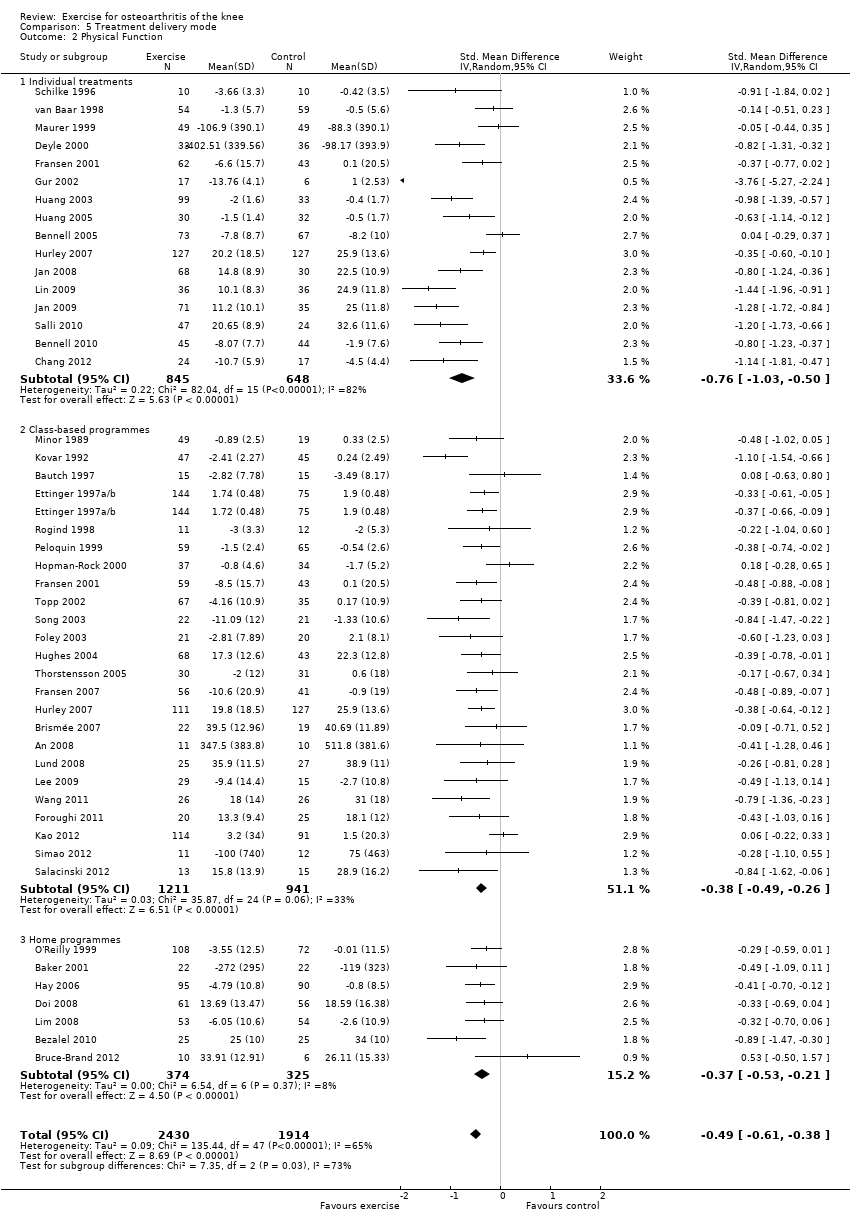 Comparison 5 Treatment delivery mode, Outcome 2 Physical Function. | ||||
| 2.1 Individual treatments | 16 | 1493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.03, ‐0.50] |
| 2.2 Class‐based programmes | 24 | 2152 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.49, ‐0.26] |
| 2.3 Home programmes | 7 | 699 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.53, ‐0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| Analysis 6.1  Comparison 6 Number of contact occasions, Outcome 1 Pain. | ||||
| 1.1 Fewer than 12 occasions | 10 | 1019 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.56, ‐0.24] |
| 1.2 12 or more occasions | 34 | 2468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.66, ‐0.43] |
| 2 Physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.64, ‐0.39] |
| Analysis 6.2 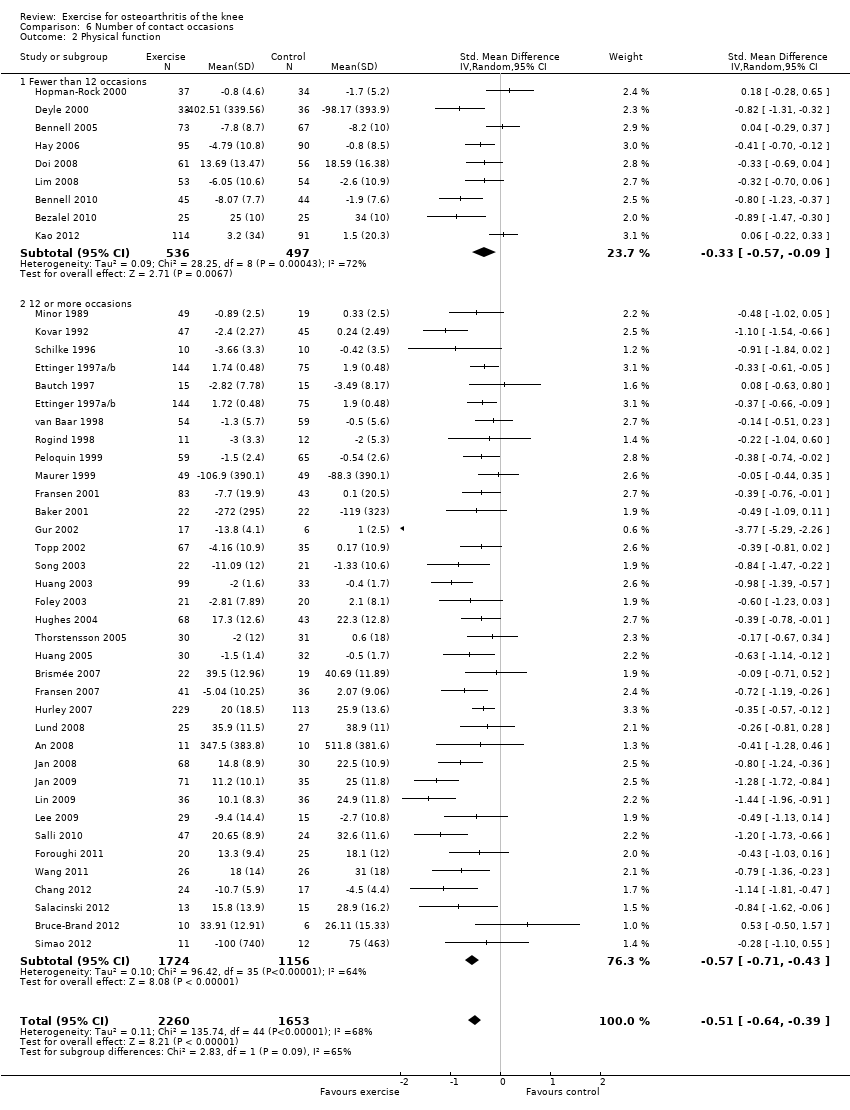 Comparison 6 Number of contact occasions, Outcome 2 Physical function. | ||||
| 2.1 Fewer than 12 occasions | 9 | 1033 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.57, ‐0.09] |
| 2.2 12 or more occasions | 35 | 2880 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.71, ‐0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Selection and attrition bias: pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| Analysis 7.1 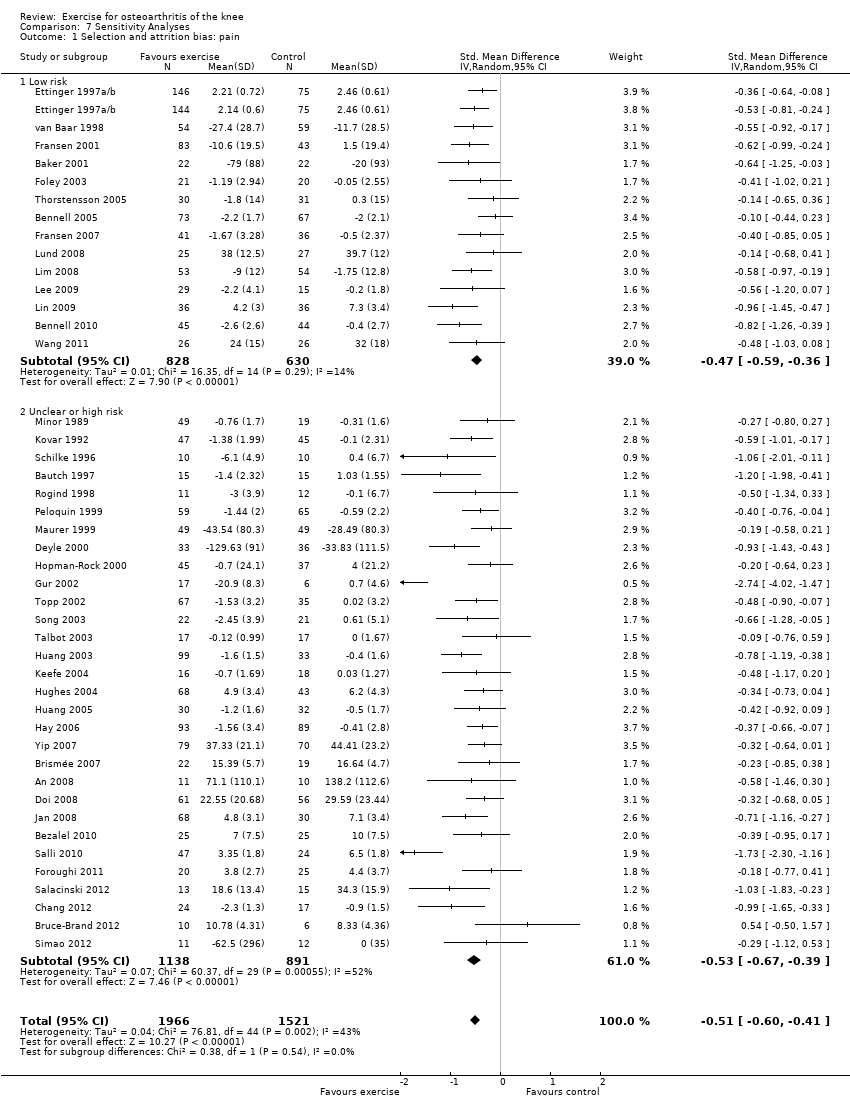 Comparison 7 Sensitivity Analyses, Outcome 1 Selection and attrition bias: pain. | ||||
| 1.1 Low risk | 14 | 1458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.59, ‐0.36] |
| 1.2 Unclear or high risk | 30 | 2029 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.67, ‐0.39] |
| 2 Selection and attrition bias: physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| Analysis 7.2 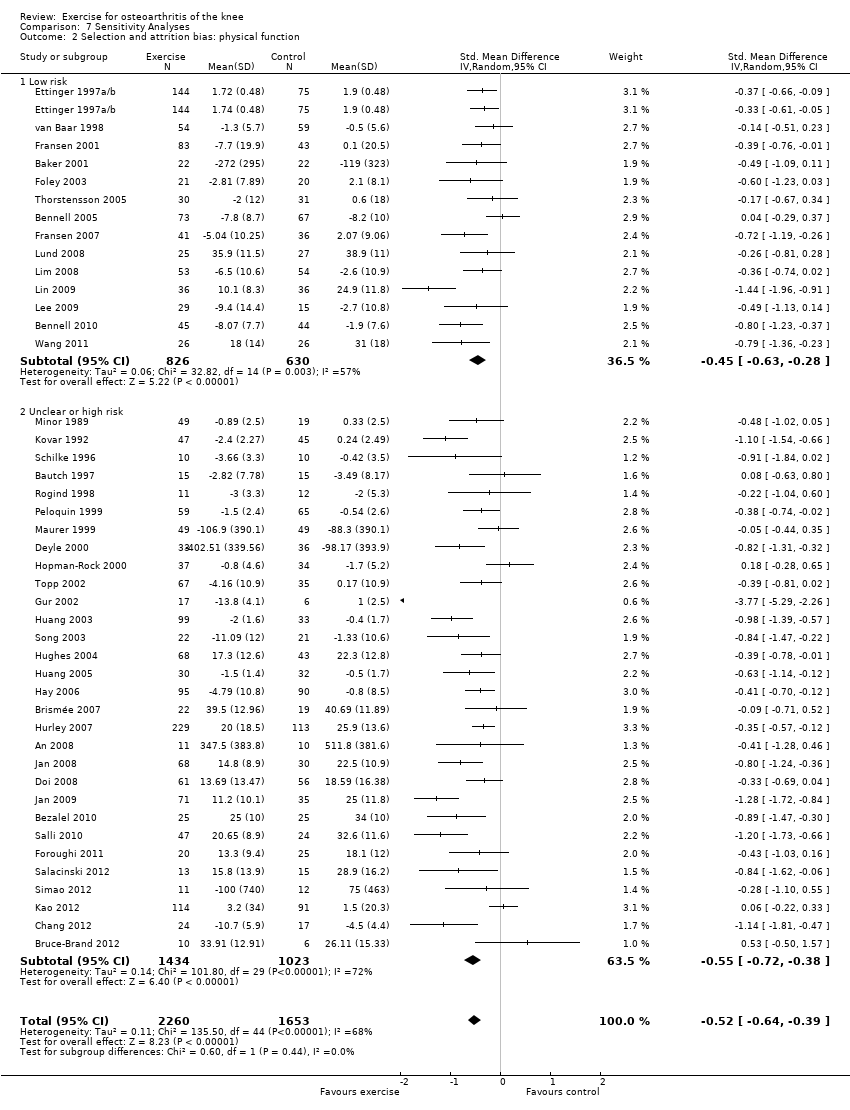 Comparison 7 Sensitivity Analyses, Outcome 2 Selection and attrition bias: physical function. | ||||
| 2.1 Low risk | 14 | 1456 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.63, ‐0.28] |
| 2.2 Unclear or high risk | 30 | 2457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.72, ‐0.38] |
| 3 Detection bias: pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| Analysis 7.3  Comparison 7 Sensitivity Analyses, Outcome 3 Detection bias: pain. | ||||
| 3.1 Low risk | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 3.2 Unclear or high risk | 41 | 3261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.61, ‐0.42] |
| 4 Detection bias: physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| Analysis 7.4 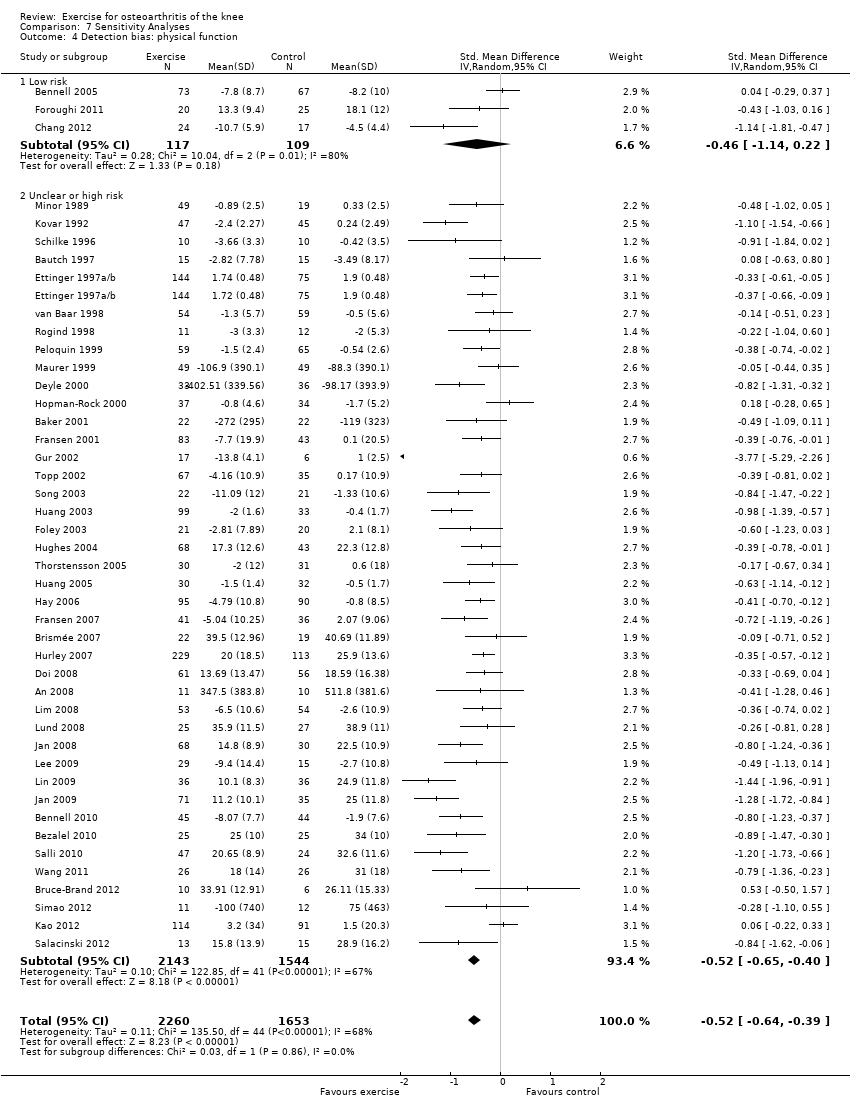 Comparison 7 Sensitivity Analyses, Outcome 4 Detection bias: physical function. | ||||
| 4.1 Low risk | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.14, 0.22] |
| 4.2 Unclear or high risk | 41 | 3687 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.65, ‐0.40] |
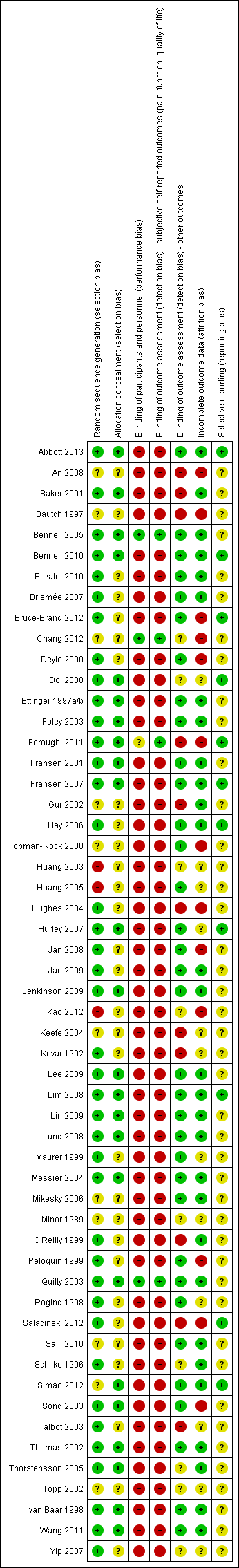
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Post treatment, outcome: 1.1 Pain.

Forest plot of comparison: 1 Post treatment, outcome: 1.2 Physical function.
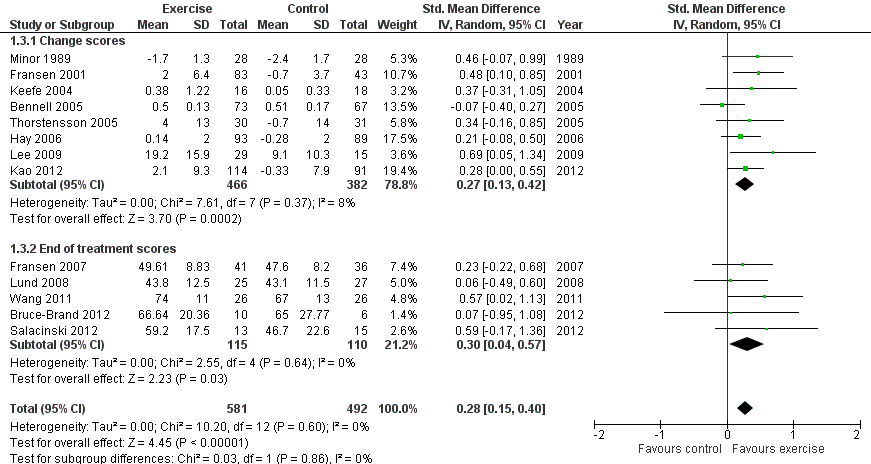
Forest plot of comparison: 1 Post treatment, outcome: 1.3 Quality of life.
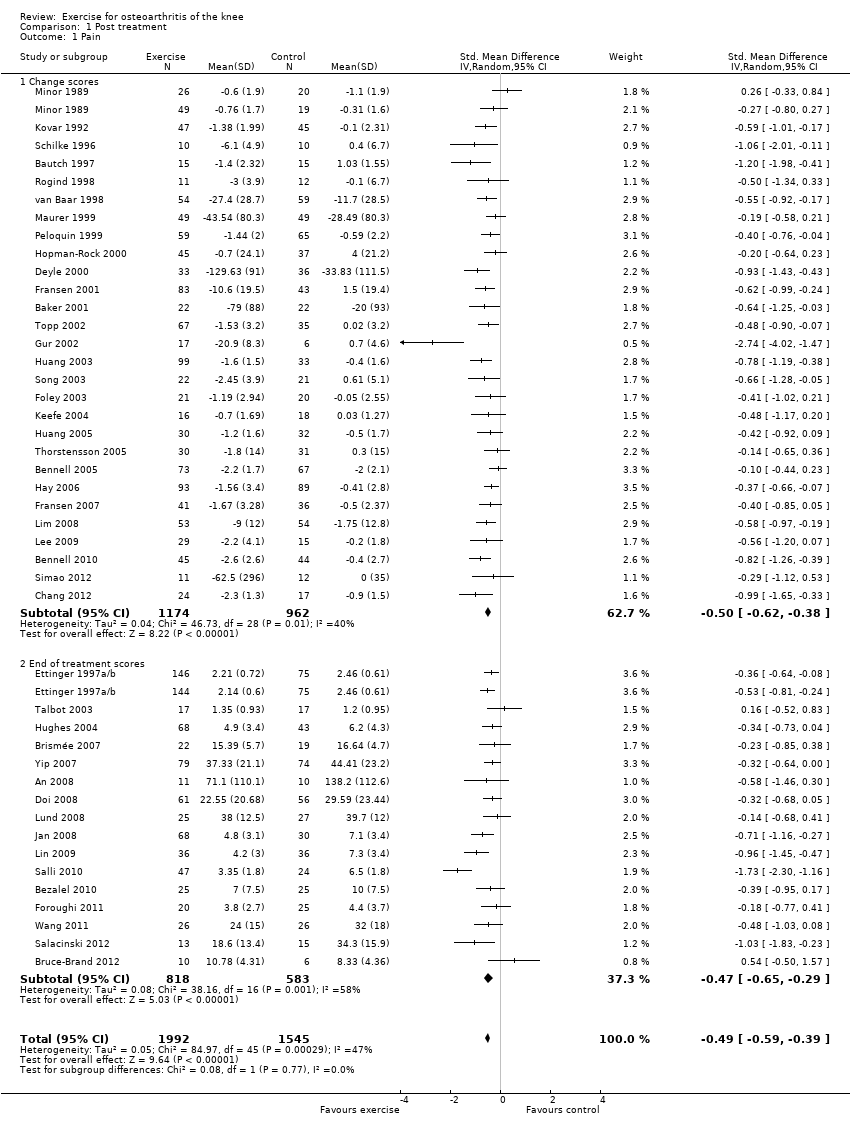
Comparison 1 Post treatment, Outcome 1 Pain.
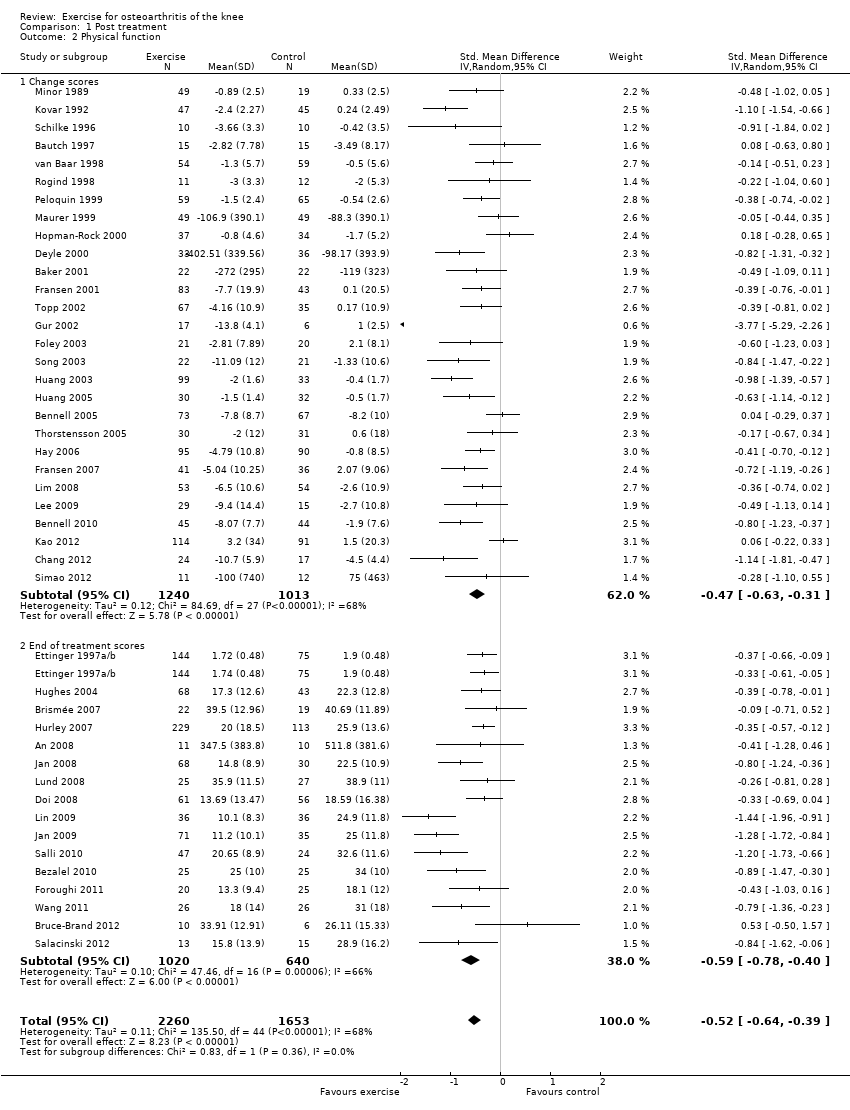
Comparison 1 Post treatment, Outcome 2 Physical function.

Comparison 1 Post treatment, Outcome 3 Quality of Life.

Comparison 1 Post treatment, Outcome 4 Study withdrawals.
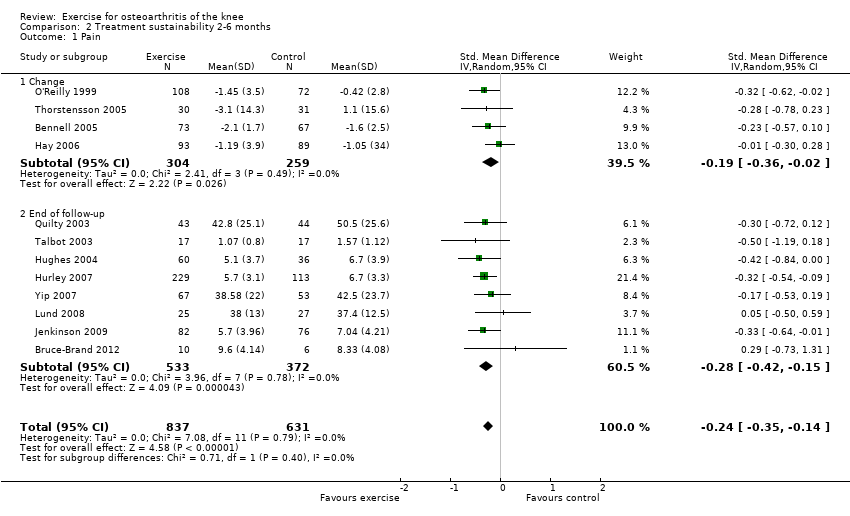
Comparison 2 Treatment sustainability 2‐6 months, Outcome 1 Pain.
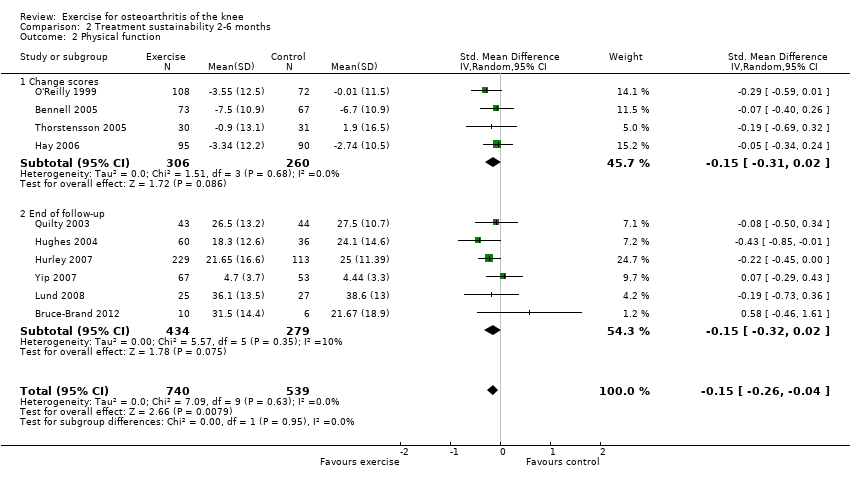
Comparison 2 Treatment sustainability 2‐6 months, Outcome 2 Physical function.
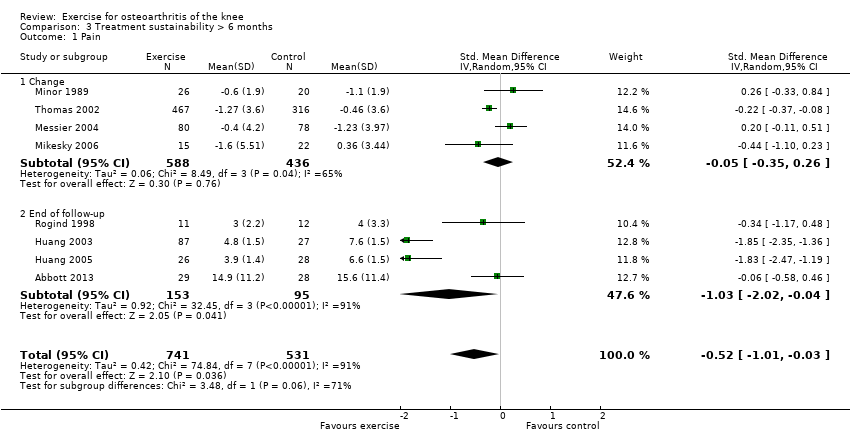
Comparison 3 Treatment sustainability > 6 months, Outcome 1 Pain.
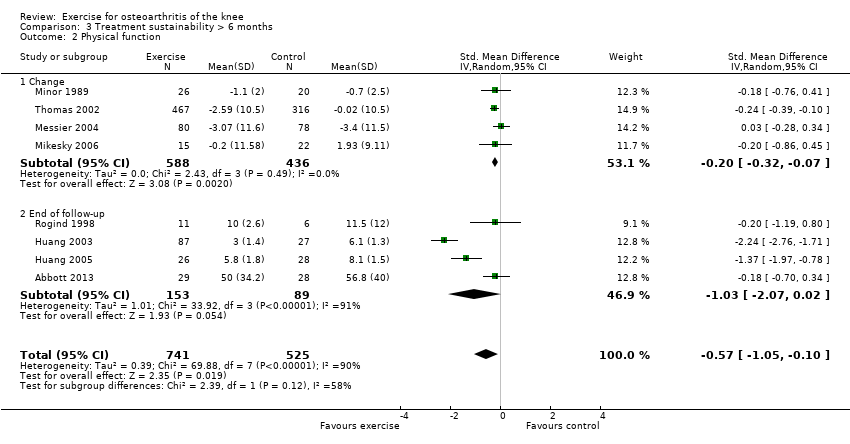
Comparison 3 Treatment sustainability > 6 months, Outcome 2 Physical function.

Comparison 4 Treatment content, Outcome 1 Pain.
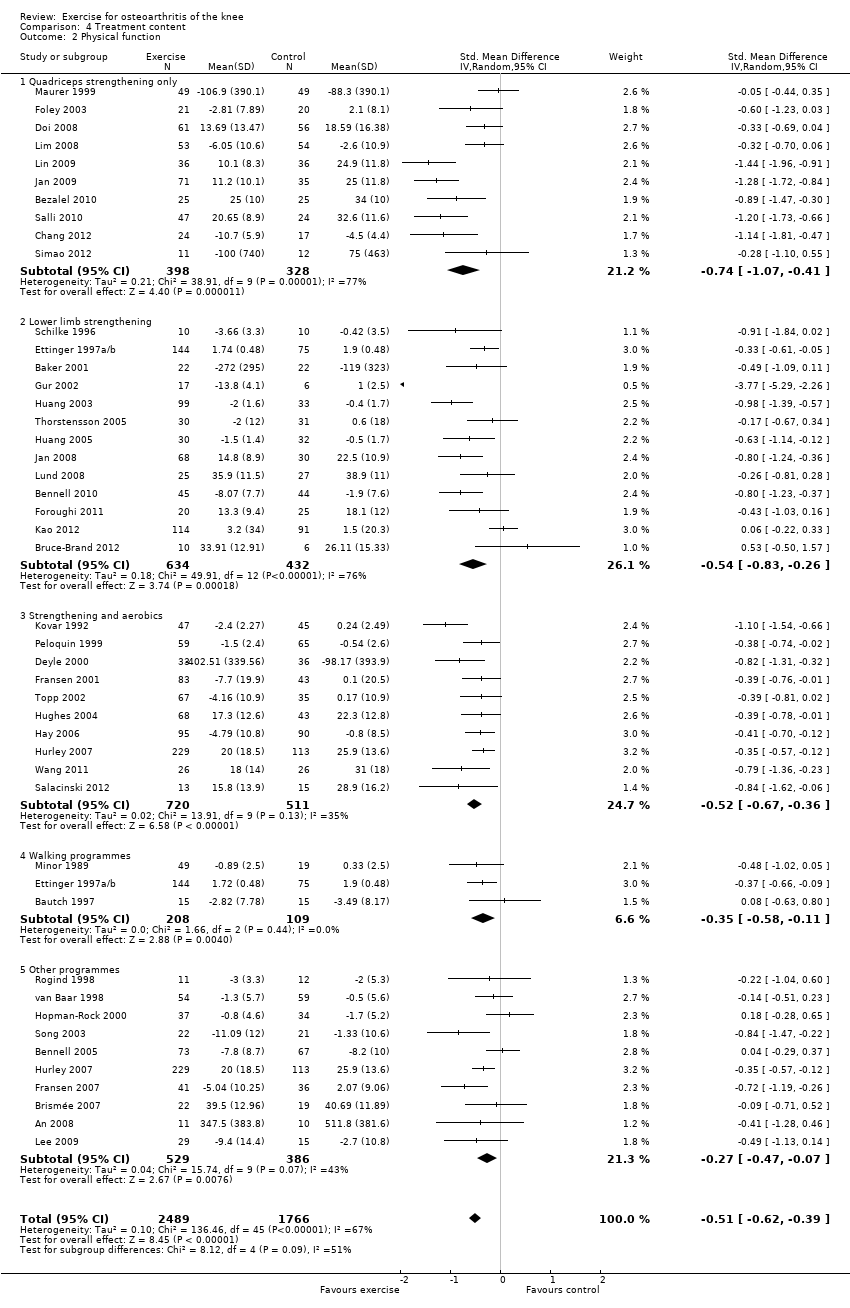
Comparison 4 Treatment content, Outcome 2 Physical function.
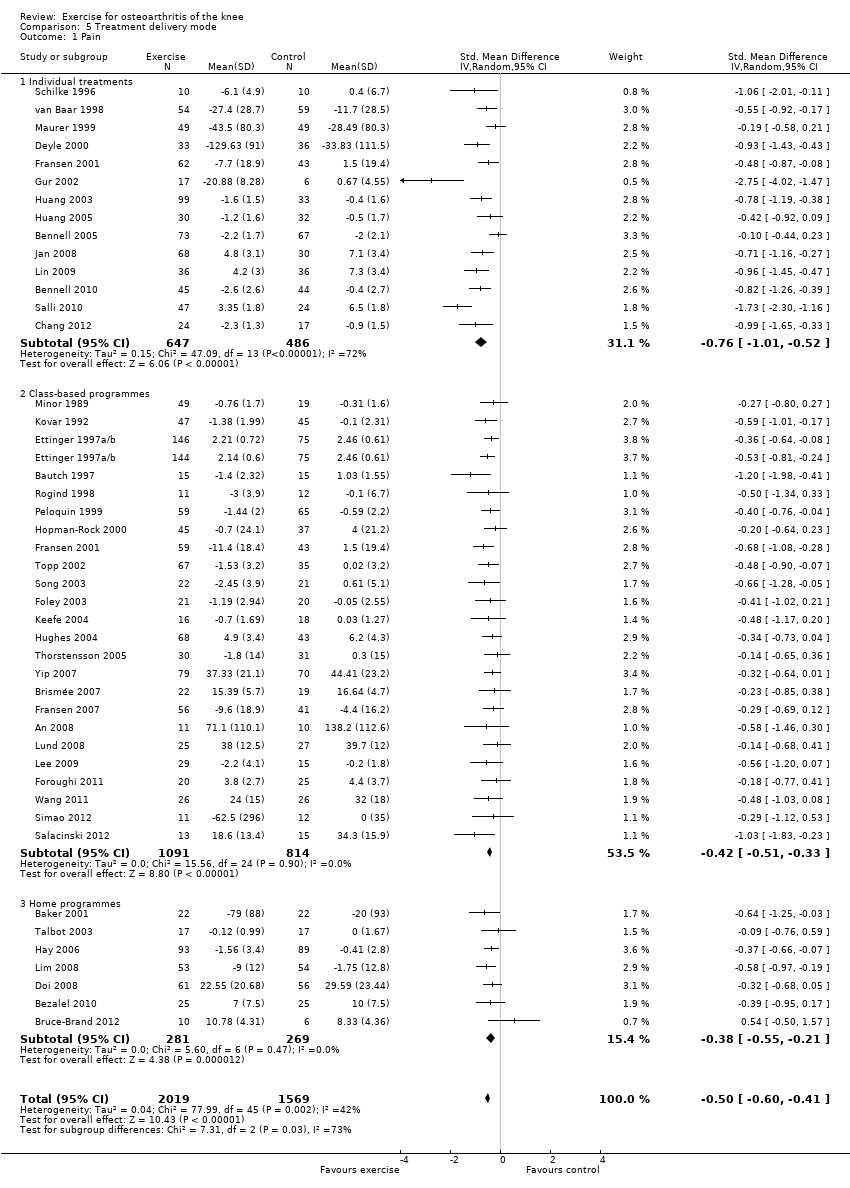
Comparison 5 Treatment delivery mode, Outcome 1 Pain.

Comparison 5 Treatment delivery mode, Outcome 2 Physical Function.

Comparison 6 Number of contact occasions, Outcome 1 Pain.
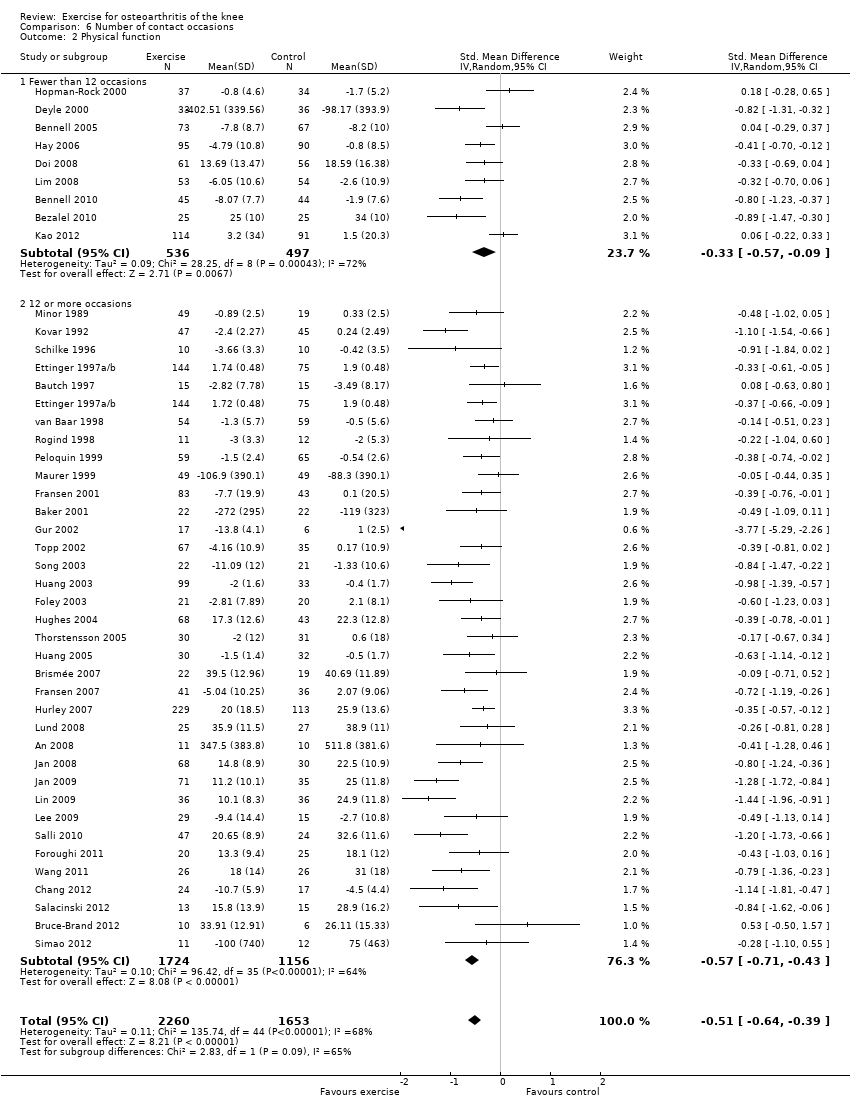
Comparison 6 Number of contact occasions, Outcome 2 Physical function.

Comparison 7 Sensitivity Analyses, Outcome 1 Selection and attrition bias: pain.
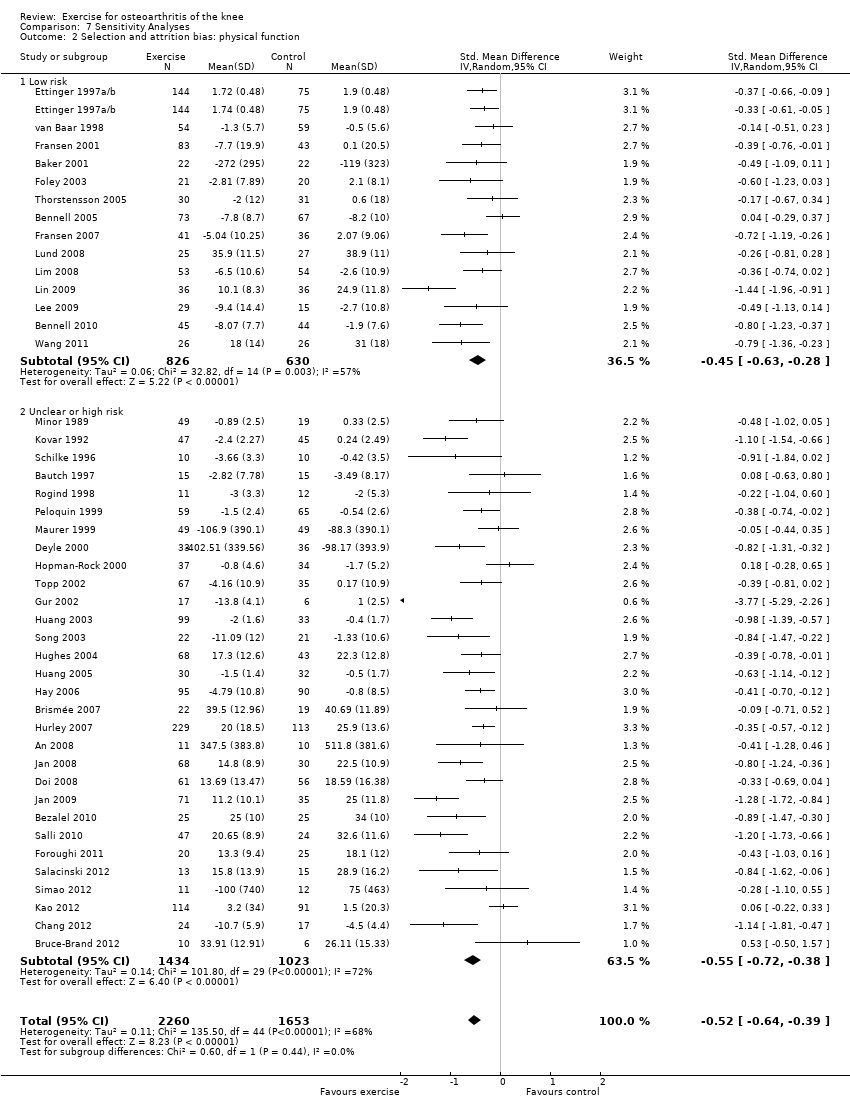
Comparison 7 Sensitivity Analyses, Outcome 2 Selection and attrition bias: physical function.

Comparison 7 Sensitivity Analyses, Outcome 3 Detection bias: pain.
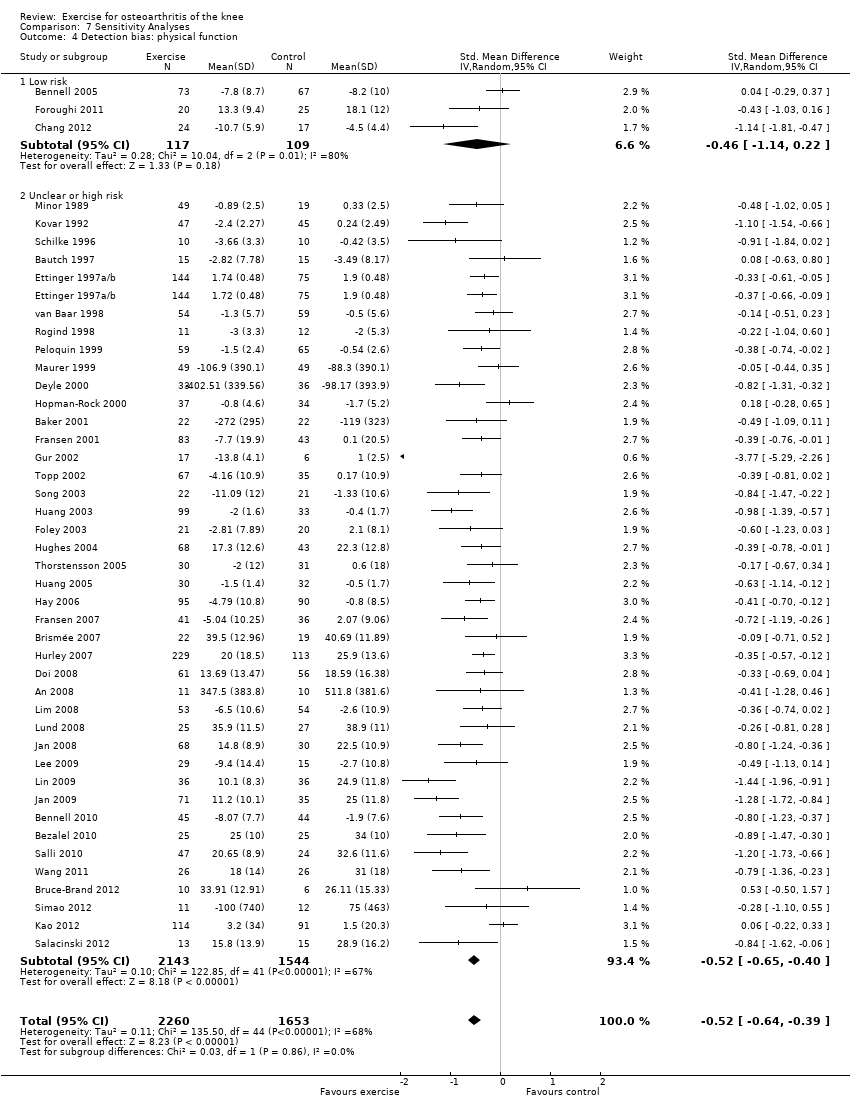
Comparison 7 Sensitivity Analyses, Outcome 4 Detection bias: physical function.
| Immediate post‐treatment effects of exercise for osteoarthritis of the knee | ||||||
| Patient or population: patients with knee OA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No exercise | Land‐based exercise | |||||
| Pain | Mean pain in the control groups was | Mean pain in intervention groups was This translates to an absolute mean reduction of 12 (10‐15) points compared with control group on a 0‐100 scalea | 3537 | ⊕⊕⊕⊕ | SMD ‐0.49 (‐0.39 to ‐0.59) Absolute reduction in pain 12% (10%‐15%); relative change 27% (21%‐32%)a NNTB 4 (3‐5)b | |
| Physical function | Mean physical function in control groups was | Mean physical function in intervention groups was This translates to an absolute mean improvement of 10 (8‐13) points on a 0‐100 scalec | 3913 | ⊕⊕⊕⊝ | SMD ‐0.52 (‐0.39 to ‐0.64) Absolute improvement 10% (8%‐13%); relative improvement 26% (20%‐32%)c NNTB 4 (3‐5)b | |
| Quality of life | Mean quality of life in control groups was | Mean quality of life in intervention groups was This translates to an absolute improvement of 4 (2‐5) points on a 0‐100 scalee | 1073 | ⊕⊕⊕⊕ | SMD 0.28 (0.15‐0.40) Absolute improvement 4% (2%‐5%); relative improvement 9% (5%‐13%)e NNTB 8 (5‐14)b | |
| Study withdrawals or dropouts | 153 per 1000 | 137 per 1000 | 4607 (44 studies) | ⊕⊕⊕⊕ | OR 0.93 (0.75‐1.15) Absolute risk reduction: 1% fewer events with exercise (2% fewer‐2% more); relative risk reduction 6% fewer events with exercise (21% fewer‐12% more) NNTH n/ab | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aCalculations based on the control group baseline mean (SD) pain: 44.3 (24.4) points on 0‐100 scale (from Yip 2007). bNumber needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH) not applicable (n/a) when result was not statistically significant. Number needed to treat (NNT) for continuous outcomes calculated using the Wells calculator (from the CMSG Editorial office; http://musculoskeletal.cochrane.org/), and for dichotomous outcomes using the Cates NNT calculator (www.nntonline.net/visualrx/). cCalculations based on the control group baseline mean (SD) function: 40.0 (20.0) points on 0‐100 scale (from Hurley 2007). dPhysical function downgraded for inconsistency (heterogeneity, I2 = 68%). eCalculated on the basis of the control group baseline mean (SD): 39.2 (13.1) points on 0‐100 KOOS subscale (from Lund 2008). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3537 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.59, ‐0.39] |
| 1.1 Change scores | 28 | 2136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.62, ‐0.38] |
| 1.2 End of treatment scores | 16 | 1401 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.65, ‐0.29] |
| 2 Physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| 2.1 Change scores | 28 | 2253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 2.2 End of treatment scores | 16 | 1660 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.78, ‐0.40] |
| 3 Quality of Life Show forest plot | 13 | 1073 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.15, 0.40] |
| 3.1 Change scores | 8 | 848 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.13, 0.42] |
| 3.2 End of treatment scores | 5 | 225 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.04, 0.57] |
| 4 Study withdrawals Show forest plot | 45 | 4607 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 12 | 1468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.35, ‐0.14] |
| 1.1 Change | 4 | 563 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.36, ‐0.02] |
| 1.2 End of follow‐up | 8 | 905 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.15] |
| 2 Physical function Show forest plot | 10 | 1279 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| 2.1 Change scores | 4 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.02] |
| 2.2 End of follow‐up | 6 | 713 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.32, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 8 | 1272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.01, ‐0.03] |
| 1.1 Change | 4 | 1024 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.35, 0.26] |
| 1.2 End of follow‐up | 4 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.02, ‐0.04] |
| 2 Physical function Show forest plot | 8 | 1266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.05, ‐0.10] |
| 2.1 Change | 4 | 1024 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.32, ‐0.07] |
| 2.2 End of follow‐up | 4 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.07, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| 1.1 Quads strengthening only | 9 | 620 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.95, ‐0.33] |
| 1.2 Lower limb strengthening | 12 | 863 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.28] |
| 1.3 Strengthening and aerobics | 10 | 920 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.64, ‐0.37] |
| 1.4 Walking programmes | 4 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.83, ‐0.13] |
| 1.5 Other programmes | 10 | 733 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.49, ‐0.20] |
| 2 Physical function Show forest plot | 44 | 4255 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.62, ‐0.39] |
| 2.1 Quadriceps strengthening only | 10 | 726 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.07, ‐0.41] |
| 2.2 Lower limb strengthening | 13 | 1066 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.83, ‐0.26] |
| 2.3 Strengthening and aerobics | 10 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.67, ‐0.36] |
| 2.4 Walking programmes | 3 | 317 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.58, ‐0.11] |
| 2.5 Other programmes | 10 | 915 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.47, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3588 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.60, ‐0.41] |
| 1.1 Individual treatments | 14 | 1133 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.01, ‐0.52] |
| 1.2 Class‐based programmes | 24 | 1905 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.51, ‐0.33] |
| 1.3 Home programmes | 7 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.55, ‐0.21] |
| 2 Physical Function Show forest plot | 45 | 4344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.61, ‐0.38] |
| 2.1 Individual treatments | 16 | 1493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.03, ‐0.50] |
| 2.2 Class‐based programmes | 24 | 2152 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.49, ‐0.26] |
| 2.3 Home programmes | 7 | 699 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.53, ‐0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| 1.1 Fewer than 12 occasions | 10 | 1019 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.56, ‐0.24] |
| 1.2 12 or more occasions | 34 | 2468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.66, ‐0.43] |
| 2 Physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.64, ‐0.39] |
| 2.1 Fewer than 12 occasions | 9 | 1033 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.57, ‐0.09] |
| 2.2 12 or more occasions | 35 | 2880 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.71, ‐0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Selection and attrition bias: pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| 1.1 Low risk | 14 | 1458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.59, ‐0.36] |
| 1.2 Unclear or high risk | 30 | 2029 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.67, ‐0.39] |
| 2 Selection and attrition bias: physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| 2.1 Low risk | 14 | 1456 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.63, ‐0.28] |
| 2.2 Unclear or high risk | 30 | 2457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.72, ‐0.38] |
| 3 Detection bias: pain Show forest plot | 44 | 3487 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| 3.1 Low risk | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 3.2 Unclear or high risk | 41 | 3261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.61, ‐0.42] |
| 4 Detection bias: physical function Show forest plot | 44 | 3913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.64, ‐0.39] |
| 4.1 Low risk | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.14, 0.22] |
| 4.2 Unclear or high risk | 41 | 3687 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.65, ‐0.40] |

