Leki przeciwdepresyjne w zapobieganiu depresji poporodowej
Appendices
Appendix 1. CCDANCTR update search ‐ Feb 2018
#1. antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*
#2. Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233
#3. Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*
#4. Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone
#5. (#1 or #2 or #3 or #4 or #5)
#6. (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptoms") AND (postpartum or "post partum" or post‐partum or postnatal or "post natal" or post‐natal or puerper*)
#7. (#5 and #6)
Appendix 2. Earlier searches conducted to June 2007
Earlier searches, conducted for the first version of this review used the following search strategies (to 11‐June‐2007):
1. TheCCDANCTR‐Studies Register was searched using the following controlled search terms:
Diagnosis = Depression, Postpartum
AND
Intervention = (Antidepress* or "Monoamine Oxidase Inhibitors" or "Selective Serotonin Reuptake Inhibitors" or "Tricyclic Drugs" or Acetylcarnitine or Alaproclate or Amersergide or Amiflamine or Amineptine or Amitriptyline or Amoxapine or Befloxatone or Benactyzine or Brofaromine or Bupropion or Butriptyline or Caroxazone or Chlorpoxiten or Cilosamine or Cimoxatone or Citalopram or Clomipramine or Clorgyline or Clorimipramine or Clovoxamine or Deanol or Demexiptiline or Deprenyl or Desipramine or Dibenzipin or Diclofensine or Dothiepin or Doxepin or Duloxetine or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Fluvoxamine or Idazoxan or Imipramine or Iprindole or Iproniazid or isocarboxazid or Litoxetine or Lofepramine or Maprotiline or Medifoxamine or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nomifensine or Nortriptyline or Noxiptiline or Opipramol or Oxaflozane or Oxaprotiline or Pargyline or Paroxetine or Phenelzine or Piribedil or Pirlindole or Pivagabine or Prosulpride or Protriptyline or Quinupramine or Reboxetine or Rolipram or Sertraline or Setiptiline or SSRI* or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptine or Toloxatone or Tomoxetine or Tranylcypromine or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Viqualine or Zimeldine)
2. The CCDANCTR‐References Register was searched using a more sensitive set of free‐text terms to identify additional untagged/uncoded reports of RCTs:
Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder*" or "Affective Symptoms"
AND
Free‐text = Postpartum or "post partum" or post‐partum or Postnatal or "post natal" or post‐natal
AND
Free‐text = Antidepress* or "Monoamine Oxidase Inhibitors" or "Selective Serotonin Reuptake Inhibitors" or SSRI* or "Tricyclic Drugs" or Acetylcarnitin* or Alaproclat* or Amersergid* or Amiflamin* or Amineptin* or Amitriptylin* or Amoxapin* or Befloxaton* or Benactyzin* or Brofaromin* or Bupropion or Butriptylin* or Caroxazon* or Chlorpoxiten or Cilosamin* or Cimoxaton* or Citalopram or Clomipramin* or Clorgylin* or Clorimipramin* or Clovoxamin* or Deanol or Demexiptilin* or Deprenyl or Desipramin* or Dibenzipin or Diclofensin* or Dothiepin or Doxepin or Duloxetin* or Escitalopram or Etoperidon* or Femoxetin* or Fluotracen or Fluoxetin* or Fluparoxan or Fluvoxamin* or Idazoxan or Imipramin* or Iprindol* or Iproniazid or isocarboxazid or Litoxetin* or Lofepramin* or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemid* or Nefazodon* or Nialamid* or Nomifensin* or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozan* or Oxaprotilin* or Pargylin* or Paroxetin* or Phenelzin* or Piribedil or Pirlindol* or Pivagabin* or Prosulprid* or Protriptylin* or Quinupramin* or Reboxetin* or Rolipram or Sertralin* or Setiptilin* or Teniloxin* or Tetrindol* or Thiazesim or Thozalinon* or Tianeptin* or Toloxaton* or Tomoxetin* or Tranylcypromin* or Trazodon* or Trimipramin* or Venlafaxin* or Viloxazin* or Viqualin* or Zimeldin*
3. The Cochrane Central Register of Controlled Trials (CENTRAL) and the specialised register of the Cochrane Pregnancy and Childbirth Review Group were searched with the following terms: Postnatal or postpartum or puerper*
4. Searches for grey literature included:
-
The HSRProj in the National Library of Medicine, Washington D.C was checked for unpublished trials.
-
References from MIDIRS Midwifery Database was searched.
-
Current Controlled Trials web site: http://www.controlled trials.com/terms2.cfm was examined for ongoing studies.
-
The Science Citation Index was checked for references to all included studies.
-
Conference proceedings were examined where possible (details available from authors on request).
5. Reference lists
The reference lists of all included studies were checked to identify additional studies missed from the original electronic searches (for example unpublished or in‐press citations). Relevant book chapters and their bibliographies were searched (details available from authors on request).
6. Correspondence
Pharmaceutical companies were contacted directly for any relevant unpublished data.
Contact was made with authors of identified trials and with experts in the field including a search for non‐English material (Professor L Appleby, Professor P Boyce, Dr T Brugha, Dr L Cohen, Dr N Glangeaud, Dr S Glasser, Dr M Marks, Dr A Wieck, Professor K Wisner).
Contact with the Marcé Society through the newsletter was made.
Self help groups were contacted (the National Childbirth Trust (NCT), The Association for Postnatal Illness, Postnatal Distress Association of Ireland, Postpartum Support International (PSI) and PaNDA).
Appendix 3. Update searches conducted to February 2018
Database: CENTRAL
Host: Wiley
Data Parameters: Issue 1 of 12, January 2018
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 18
Search strategy:
ID Search Hits
#1 (postpartum or post partum or postnatal or post natal or puerper*) 9779
#2 MeSH descriptor: [Antidepressive Agents] explode all trees 5750
#3 (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*) .mp. 1080
#4 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) 22191
#5 #2 or #3 or #4 24723
#6 #1 and #5 252
Notes: CENTRAL only = 18 records in date range 2016‐current
File: GS51 CENTRAL18.txt
Databases: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: OVID
Data Parameters: 1946 to Present
Date Searched: Tuesday, February 13th 2018
Searched by: Chris
Hits: 28
Search strategy:
Search Strategy:
| # | Searches | Results |
| 1 | exp Antidepressive Agents/ | 134025 |
| 2 | exp Neurotransmitter Uptake Inhibitors/ | 133352 |
| 3 | exp Monoamine Oxidase Inhibitors/ | 20964 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 228950 |
| 5 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab,kw. | 76654 |
| 6 | 1 or 2 or 3 or 4 or 5 | 374868 |
| 7 | Depression, Postpartum/ | 4532 |
| 8 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab,kw. | 5566 |
| 9 | 7 or 8 | 6988 |
| 10 | randomized controlled trial.pt. | 453387 |
| 11 | controlled clinical trial.pt. | 92150 |
| 12 | (randomized or randomised).ti,ab. | 517074 |
| 13 | (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. | 433913 |
| 14 | placebo.ab. | 186351 |
| 15 | ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy)).ti,ab,kw. | 156034 |
| 16 | (control* adj3 (trial or study or group*)).ti,ab,kw. | 692411 |
| 17 | (group* adj3 (allocat* or assign* or control* or divide* or division or distribut* or treat*)).ti,ab,kw. | 730860 |
| 18 | trial.ti,ab. | 491130 |
| 19 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 | 1726074 |
| 20 | 6 and 9 and 19 | 133 |
| 21 | (2016* or 2017* or 2018*).yr,dt,ed,ep. | 3328698 |
| 22 | 20 and 21 | 28 |
Notes: N/A
File: GS51MEDLINE28.txt
Database: Embase
Host: OVID
Data Parameters: 1974 to 2018 February 12
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 50
Search strategy:
Search Strategy:
| # | Searches | Results |
| 1 | exp Antidepressant Agent/ | 401718 |
| 2 | Neurotransmitter Uptake Inhibitors/ | 155 |
| 3 | exp Monoamine Oxidase Inhibitor/ | 45668 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 319952 |
| 5 | Psychopharmacology/ | 26480 |
| 6 | Psychotropic Agent/ | 27346 |
| 7 | Serotonin Receptor Affecting Agent/ or Serotonin Uptake Inhibitor/ or Serotonin Noradrenalin Reuptake Inhibitor/ or Triple Reuptake inhibitor/ | 46515 |
| 8 | Dopamine Receptor Affecting Agent/ or Dopamine Uptake Inhibitor/ | 1482 |
| 9 | Adrenergic Receptor Affecting Agent/ or Noradrenalin Uptake Inhibitor/ | 3576 |
| 10 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab,kw. | 100164 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 572354 |
| 12 | postnatal depression/ | 644 |
| 13 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab,kw. | 7804 |
| 14 | 12 or 13 | 8082 |
| 15 | randomized controlled trial.de. | 486416 |
| 16 | randomization.de. | 76792 |
| 17 | placebo.de. | 318178 |
| 18 | placebo?.ti,ab. | 266278 |
| 19 | (randomized or randomised).ti,ab. | 725053 |
| 20 | double blind procedure/ | 146068 |
| 21 | (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. | 586635 |
| 22 | trial.ti. | 241798 |
| 23 | ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy)).ti,ab. | 211307 |
| 24 | (control* adj3 (trial or study or group*)).ti,ab. | 954393 |
| 25 | (group* adj3 (allocat* or assign* or control* or divide* or division or distribut* or treat*)).ti,ab. | 1039132 |
| 26 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 2251280 |
| 27 | 11 and 14 and 26 | 260 |
| 28 | (2016* or 2017* or 2018*).yr,dc. | 3762342 |
| 29 | 27 and 28 | 50 |
Notes: N/A
File: GS51 Embase50.txt
Database: PscyINFO
Host: OVID
Data Parameters: 1806 to February Week 1 2018
Date searched: Tuesday, February 13th 2018
Searched by: Chris Cooper
Hits: 44
Search strategy:
| # | Searches | Results |
| 1 | exp antidepressant drugs/ | 36266 |
| 2 | exp Neurotransmitter Uptake Inhibitors/ | 13404 |
| 3 | monoamine oxidase inhibitors/ | 1391 |
| 4 | (antidepress* or anti depress* or MAOI* or RIMA* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or "re‐uptake")) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. | 65272 |
| 5 | (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Amfebutamone or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine or Clorgyline or Clovoxamine or CX157 or Tyrima or Demexiptiline or Deprenyl or Desipramine* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or Hyperforin or Hypericum or "St John*" or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or "LY2216684" or Edivoxetine or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).ti,ab. | 32880 |
| 6 | 1 or 2 or 3 or 4 or 5 | 83585 |
| 7 | exp Postpartum Depression/ | 4151 |
| 8 | ((postpartum or post partum or postnatal or post natal or puerper*) adj3 (depress* or dysthymi* or "adjustment disorder*" or "mood disorder*" or "affective disorder*" or "affective symptom*")).ti,ab. | 4586 |
| 9 | 7 or 8 | 5597 |
| 10 | 6 and 9 | 370 |
| 11 | (2016* or 2017* or 2018*).yr,dc,mo. | 377668 |
| 12 | 10 and 11 | 44 |
Notes: N/A
File: GS51 PsycINFO44.txt

Study flow diagram.
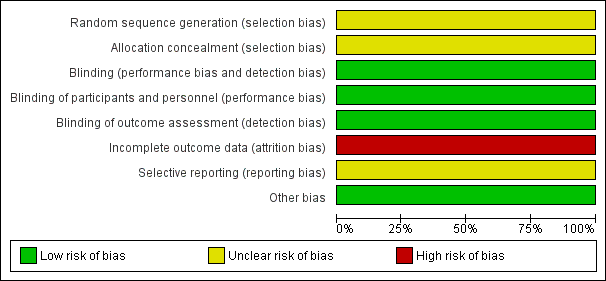
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Nortriptyline versus placebo, outcome: 1.1 Recurrence of postpartum major depressive disorder.

Forest plot of comparison: 2 Sertraline versus placebo, outcome: 2.1 Recurrence of postpartum major depressive disorde.

Comparison 1 Nortriptyline versus placebo, Outcome 1 Recurrence of postpartum major depressive disorder.
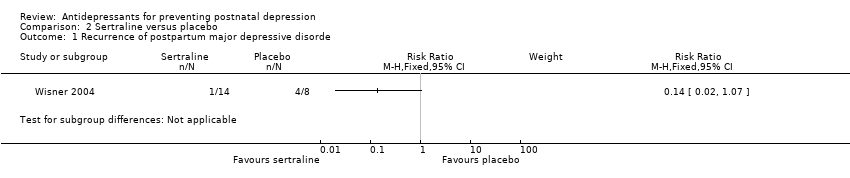
Comparison 2 Sertraline versus placebo, Outcome 1 Recurrence of postpartum major depressive disorde.
| Nortripyline for the prevention of postnatal depression | ||||||
| Patient or population: women with a history of postnatal depression Control: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tricyclic antidepressants | |||||
| Postnatal depression (17 weeks) | 240 per 10001 | 230 per 1000 | RR 0.96 | 51 | ⊕⊝⊝⊝ | |
| Adverse effects experienced by mother and/or foetus or nursing baby | 51 | ⊕⊝⊝⊝ | 1 woman assigned to nortriptyline developed mania within the first week. Constipation was reported more frequently in the nortriptyline than placebo group (78% among women taking nortriptyline and 22% among women taking placebo; Fischer's exact test P < 0.001). This was the only side effect that was more common among women taking nortriptyline than placebo, but the other side effects assessed and the proportion of women experiencing these side effects was not reported. | |||
| Acceptability of treatment (17 weeks) | 51 | ⊕⊝⊝⊝ | Acceptability of treatment was not assessed directly but 1 woman was lost to follow‐up from the nortriptyline arm (and 1 women in the nortriptyline arm withdrew after developing mania in the first postpartum week) and 3 withdrew from the placebo arm (owing to side effects, personal reasons and pregnancy). 4 participants declined to take the study drug after randomisation. | |||
| Overall maternal satisfaction (17 weeks) | No data available | |||||
| Improvement in the maternal relationship with the baby (17 weeks) | No data available | |||||
| Establishment or continuation of breastfeeding (17 weeks) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk calculated as the proportion of women on placebo with the outcome (postnatal depression) multiplied by 1000 2Downgraded due to high risk of bias in 1 domain (incomplete outcome data) | ||||||
| Sertraline for the prevention of postnatal depression | ||||||
| Patient or population: women with a history of postnatal depression Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Selective serotonin reuptake inhibitors | |||||
| Postnatal depression (17 weeks) | 500 per 1000 | 70 per 1000 | RR 0.14 | 22 | ⊕⊝⊝⊝ | |
| Adverse effects experienced by mother and/or fetus or nursing baby | 22 | ⊕⊝⊝⊝ | 1 woman taking sertraline had a hypomanic episode. 2 side effects (dizziness and drowsiness) were more common among women taking sertraline than women taking placebo. | |||
| Acceptability of treatment (17 weeks) | 22 | ⊕⊝⊝⊝ | Acceptability of treatment was not assessed directly but no difference was found between the antidepressant and placebo groups in the number of women withdrawing from the study (P = 0.35, Fisher’s exact test). | |||
| Overall maternal satisfaction (17 weeks) | No data available | |||||
| Improvement in the maternal relationship with the baby (17 weeks) | No data available | |||||
| Establishment or continuation of breastfeeding (17 weeks) | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk calculated as the proportion of women on placebo with the outcome (postnatal depression) multiplied by 1000 2Downgraded due to high risk of bias in 1 domain (incomplete outcome data) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of postpartum major depressive disorder Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of postpartum major depressive disorde Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

