Betamiméticos para la inhibición del trabajo de parto prematuro
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centred randomised controlled trial, 2 arms with individual randomisation. | |
| Participants | Location: Australia, University hospital. | |
| Interventions | 1) Isoxsuprine. | |
| Outcomes | Primary outcomes: perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was described as a double‐blind trial but there was no information on randomisation methods. |
| Allocation concealment (selection bias) | Unclear risk | This was a placebo‐controlled double‐blind trial. It was not clear whether study medications were identical. Methods of allocation at the point of randomisation were not described. |
| Blinding of participants and personnel (performance bias) | Low risk | This was a described as a double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear whether or not those collecting outcome data were aware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not entirely clear how many women were randomised to each group. One table suggests that there were 27 in the intervention group and 24 in the control group, later it said that 48 women were included in the analysis. Women who had not given birth at the end of the trial period were excluded from the analysis (2 in each group). |
| Selective reporting (reporting bias) | Unclear risk | It appeared that some analysis may have been carried out retrospectively as outcomes were not set out in the methods. The main outcome (prolongation of pregnancy) was reported as a mean without SD. |
| Other bias | Unclear risk | There was very little information on study methods. There were 5 multiple pregnancies in the intervention group and 2 in the control group. |
| Methods | Randomised controlled trial (part of multicentre study described in Merkatz 1980). | |
| Participants | Location: USA. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Risk of bias was assessed by the previous review team from a personal communication from the trials authors. This was not available to the current review team and so we have used the previous assessments. |
| Other bias | Unclear risk | Not able to assess. |
| Methods | Single‐centre randomised trial, 2 arms with individual randomisation. | |
| Participants | Location: USA. | |
| Interventions | 1) Terbutaline (in physiologic saline with concentration of terbutaline 20 mcg/mL). | |
| Outcomes | Primary outcomes: RDS. | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | This was described as a double‐blind trial. Study drugs were prepared in pharmacy and the same volume and type of diluent (saline) was used so would have looked similar. |
| Blinding of participants and personnel (performance bias) | Low risk | This was described as a double‐blind trial. It was stated that investigators and staff were not aware of which treatment arm women were in. |
| Blinding of outcome assessment (detection bias) | Low risk | “all outcomes were ascertained by personnel blinded to the women’s treatment assignment”. |
| Incomplete outcome data (attrition bias) | Low risk | “All the enrolled patients were included in the analysis”. 5 women (0.7% did not receive the assigned treatment, but were included in an intention‐to‐treat analysis. Unclear for length of prolongation. 8/100 women randomised were withdrawn from the treatment due to side effects, and the length of prolongation was up to the time of treatment withdrawal rather than the birth (so this outcome may be more susceptible to bias – similar numbers of women in the two arms were withdrawn for side effects). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Not apparent. Groups were reported to be similar at baseline. |
| Methods | Single‐centre randomised controlled trial, individual randomisation. | |
| Participants | Location: USA, University Hospital. | |
| Interventions | 1) Terbutaline. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS. | |
| Notes | Prenatal corticosteroid use: 14 participants, distributed evenly among three groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were “randomized” method not described. |
| Allocation concealment (selection bias) | Unclear risk | Women were “randomized” method not described. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not mentioned. Staff would have been aware of study treatment allocations as iv regimens were different in the different arms of the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned but staff aware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | 57 women randomised, 54 analysed, 1 women from each group required more than one tocolysis treatment and both were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Little information on study methods. It was stated that 14 women received steroids for fetal lung maturation. It was not clear which groups these were in (reported as evenly distributed among the groups) or whether this related to fetal survival. assessment from published study report. |
| Other bias | Unclear risk | Very little information on methods. Groups appeared similar at baseline. |
| Methods | Type of study: multicentre randomised controlled trial (6 University hospitals). | |
| Participants | Location: Canada. | |
| Interventions | 1) Ritodrine (in physiologic saline with concentration of ritodrine 400 mcg/mL). | |
| Outcomes | Primary outcomes: birth within 48 hrs; perinatal death; abnormal long‐term neurodevelopmental delay; RDS. | |
| Notes | Prenatal corticosteroid use: 34.6% in ritodrine group (vs) 36.0% in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation lists in each participating centre (randomisation stratified by gestational age). |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial with external randomisation by pharmacy at each centre. |
| Blinding of participants and personnel (performance bias) | Low risk | Women, and staff were reported to be blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment was reported to have been carried out by staff who were blind to women’s treatment group, |
| Incomplete outcome data (attrition bias) | Low risk | A small number of women did not receive the therapy assigned due to rapid labour progression (0.7%). All women randomised were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the trial registration were reported in the paper. There was no evidence of outcome reporting bias. |
| Other bias | Unclear risk | More women in the placebo group (19.2%) had cervical dilatation >=4 at randomisation compared with women in the treatment group (12.6%). Other characteristics at baseline appeared similar. |
| Methods | Described as a double‐blind, double‐placebo study, in 2 centres. RCT, 2 arms with individual randomisation | |
| Participants | Setting: 2 hospitals in France. Inclusion criteria: 60 women recruited with threatened preterm labour (confirmed by observation and external tocography). Women were between 24‐37 weeks’ gestation. Exclusion criteria: women for whom betamimetic therapy was not suitable, or already receiving tocolytic treatment. | |
| Interventions | 60 women were randomised, 8 were excluded after randomisation. It was not clear how many were randomised to each group Experimental intervention: (28 analysed) Slow release salbutamol (iv over 48 hrs, oral after 24 hrs: slow release (2/days + 2 placebo) for further 4 days). 16 mg per day in 2 oral capsules. Comparison intervention: (24 analysed). Normal release salbutamol (iv over 48 hrs, oral after 24 hrs: with normal release (4/days) for further 4 days). 16 mg/day in 4 oral capsules | |
| Outcomes | Birth within 4 days, birth before 36 completed weeks, CS, Apgar scores, side effects, pregnancy continuation (mean but no SDs reported). | |
| Notes | Before the oral salbutamol was administered women received an average of 31.4mg (slow release) vs 38.9 mg (normal release). So total dose greater in the normal release group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Original report in French. Sequence generation not described. This was described as a randomised study, but it was stated that women were allocated to groups after being matched (in the abstract and in translated notes). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double blind‐double placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear (translated notes). |
| Incomplete outcome data (attrition bias) | High risk | 60 women were randomised, 8 were excluded after randomisation. It was not clear how many were randomised to each group. 52 included in the analysis. Others were not “ready to come off the intravenous drip within the specified timeframe”, possibly indicating that women who continued in preterm labour were systematically excluded. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report and translated notes. |
| Other bias | Unclear risk | Little information on methods and assessment of risk of bias from translated notes. |
| Methods | Single‐centre randomised trial, 2 arms with individual randomisation. | |
| Participants | Location: The Netherlands, University Hospital. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcome: RDS. | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Staff would have been aware of different treatments. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned. 18/114 women crossed over onto the other treatment so staff and outcome assessors must have been aware of treatment allocation. |
| Incomplete outcome data (attrition bias) | High risk | 18/114 women (9 in each group) crossed over to the other treatment because of “intolerance”. These women were not included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were some baseline differences between groups (previous number of miscarriages and maternal age). |
| Methods | Single‐centre randomised trial, 2 arms with individual randomisation. | |
| Participants | Location: Helsinki. | |
| Interventions | 1) Hexoprenaline. | |
| Outcomes | Secondary outcomes: adverse effects. | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Assessment was from translated notes. |
| Other bias | Unclear risk | Little information on study methods. Not clear if there were any baseline differences between groups. |
| Methods | Randomised controlled trial (part of multicentre study described by Merkatz 1980). | |
| Participants | Location: USA. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Risk of bias was assessed by the previous review team from a personal communication from the trials authors. This was not available to the current review team and so we have used the previous assessments. |
| Other bias | Unclear risk | Not able to assess. |
| Methods | Multicentre RCT in 5 hospitals. 2 arms with individual randomisation. | |
| Participants | Location: The Netherlands, 5 teaching hospitals. | |
| Interventions | 1) Loading dose ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; periventricular haemorrhage grade 3‐4. | |
| Notes | Prenatal corticosteroid: yes (do not state exact number). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method for generating the randomisation sequence was not stated. |
| Allocation concealment (selection bias) | Low risk | Allocations were in numbered, opaque, sealed envelopes. All envelopes were accounted for. |
| Blinding of participants and personnel (performance bias) | High risk | It was not clear if women were aware of treatment group. Staff would have been aware of treatment as dosing regimens were different in the two groups. Clinical decisions appear to have been affected by treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | It was stated that data entry sheets were assessed by blind outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Nine women were excluded post randomisation as they did not meet inclusion criteria. Of the remaining 203 women 2 women (1 in each group) were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent (protocol not available). |
| Other bias | High risk | There was considerable deviation from protocol in the control group. It was stated that the strict interval for dose augmentation was only observed for 30% of the control group “This was because the participating clinicians were used to being more cautious with the conventional infusion scheme, often applying it in a more gradual way than officially prescribed.” |
| Methods | Single‐centre randomised controlled trial, 2 arms with individual randomisation. | |
| Participants | Location: The Netherlands, University Hospital. | |
| Interventions | 1) Terbutaline (in 5% glucose solution). | |
| Outcomes | Primary outcomes: birth within 48 hrs. | |
| Notes | Prenatal corticosteroid: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Thirty sets of coded ampules and tablets of identical appearance, 15 containing terbutaline and 15 placebo, were given a number from 1 to 30 in a randomized way. The sets were taken in numerical order for the patients as they entered the trial”. |
| Allocation concealment (selection bias) | Low risk | “The code key was not available to the investigator”. |
| Blinding of participants and personnel (performance bias) | Low risk | “The two groups were treated in a double‐blind manner”. Fluid infusion was set up to avoid confounding: “This ensured that the patients in the placebo group did not get more fluid intravenously than the patients in the terbutaline group." |
| Blinding of outcome assessment (detection bias) | Low risk | “The code key was not available to the investigator”. |
| Incomplete outcome data (attrition bias) | Low risk | All women appeared to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. Main outcome was birth after 37 weeks. Mean prolongation was not reported as this was described as not meaningful. Time to birth was reported as a category variable. |
| Other bias | Low risk | Groups appeared similar at baseline. |
| Methods | (Brief abstract) randomised trial. | |
| Participants | 61 women in premature labour. No other details provided. | |
| Interventions | 32 women were given clenbuterol tablets. 29 women were given fenoterol perlongettes. | |
| Outcomes | Electrocadiographic changes. | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by computer". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Different regimens. Staff would be aware of treatment group |
| Blinding of outcome assessment (detection bias) | High risk | Different regimens. Staff would be aware of treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published abstract. |
| Other bias | Unclear risk | Very little information on methods. |
| Methods | Randomised trial; 3 arms with individual randomisation. | |
| Participants | Study in hospital setting in Sweden. 13 women with established premature contractions, 27‐35 weeks' gestation with intact membranes. | |
| Interventions | 1. Terbutaline impregnated vaginal polymer ring (5 g) (5 women). 2. Terbutaline vaginal gel (4 women). 3. Control, placebo vaginal polymer ring (5 women). | |
| Outcomes | Percentage reduction in uterine contractions over a 2‐ hr period; maternal pulse and blood pressure over a 2‐hr period, and peripheral blood mean terbutaline concentration over 2 hrs of treatment. | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | "randomized". Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Different regimens. Staff would be aware of treatment group |
| Blinding of outcome assessment (detection bias) | High risk | Different regimens. Staff would be aware of treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Not apparent. Small sample size. |
| Methods | Multicentre randomised controlled trial, 4‐arm trial, (3 different doses/routes of ritodrine and 1 placebo group) | |
| Participants | Location: Denmark. | |
| Interventions | 1) Long ritodrine infusion group (ritodrine plus 5.5% glucose). | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal deaths. | |
| Notes | Prenatal corticosteroid: betamethasone 12 + 12 mg, number of participants treated by steroids not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | “allocated randomly”, no other description. |
| Blinding of participants and personnel (performance bias) | High risk | It was stated that women in the control group were given an iv 5% glucose infusion to “blind” them As the other arms had different regimens and routes (one group had im) it was not clear whether blinding was effective. Staff would have been aware of allocation due to different treatment regimens. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned. Staff would have been aware of the treatment regimens. |
| Incomplete outcome data (attrition bias) | Unclear risk | 176/199 women were included in the analysis. 23 women were excluded after randomisation. There were some missing data for some variables. |
| Selective reporting (reporting bias) | Unclear risk | Assessed from published reports. |
| Other bias | Low risk | Not apparent. Groups appeared comparable at baseline. |
| Methods | Single‐centre randomised controlled trial, 2 arms with individual randomisation. | |
| Participants | Location: USA, University Hospital. Time frame: 1982‐1985. | |
| Interventions | 1. Ritodrine (150 mg in 500 physiologic saline). | |
| Outcomes | Primary outcomes: birth within 48 hrs; perinatal death (at 7 days). | |
| Notes | Prenatal corticosteroid use: not used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “pregnancies were randomly assigned to treatment or control groups by means of a random number table”. |
| Allocation concealment (selection bias) | Low risk | “with group allocation predetermined and placed in sealed envelopes”. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned, but same dosage of ritodrine and placebo given which suggests some blinding. Women may have been unaware, but it is likely that staff were aware of the treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Treatment allocation would have been apparent. Impact on outcomes of lack of blinding not clear. |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised appeared to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups reported to be similar at baseline. Other bias not apparent. |
| Methods | Multicentre randomised trials, 2 arms with individual randomisation. | |
| Participants | Location: USA. | |
| Interventions | 1) Hexoprenaline. | |
| Outcomes | Secondary outcomes: adverse effects. | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomized” no description of method. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomized” no description of allocation concealment method. |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned (likely to be high risk of bias). |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned (likely to be high risk of bias). |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was not described. |
| Selective reporting (reporting bias) | Unclear risk | Assessed from published study report. |
| Other bias | Unclear risk | Very little information on study methods. |
| Methods | Randomised controlled trial (part of multicentre study described by Merkatz 1980 ). | |
| Participants | Location: USA. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Risk of bias was assessed by the previous review team from a personal communication from the trials authors. This was not available to the current review team and so we have used the previous assessments. |
| Other bias | Unclear risk | Not able to assess. |
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: hospital (tertiary care) in Iran, recruitment between April‐September 2009 Inclusion criteria: 287 assessed and 200 eligible. Women between 20 and 37 weeks were monitored over 1 hr and were in threatened preterm labour (2 contractions within a 10‐min period) with cervical dilation 0‐3cm for primiparous and 1‐3cm for multiparous women, and less than 50% cervical effacement. Exclusion criteria: Cervix dilated > 5cm, polyhydramnios, oligohydramnios, macrosomia, suspected infection, IUGR, antepartum haemorrhage, ruptured membranes, medical disorder, previous CS, multiple pregnancy, pregnancy hypertension,low BP, fetal distress or abnormality, previous treatment with or contraindication to betamimetics. | |
| Interventions | Group 1: (100 women) subcutaneous terbutaline 250 mcg loading dose, same dose every 45 mins until contractions ceased. If contractions ceased maintenance therapy of 20 mg daily oral terbutaline. Group 2: (100 women) iv salbutamol, bolus 0.1 mg with same boluses every 5 mins, if contractions stopped maintenance therapy of 24 mg/day of oral salbutamol. | |
| Outcomes | Prolongation of pregnancy beyond 48 hrs, mean prolongation of pregnancy, pregnancy outcomes (for those women delivering after 37 weeks only) side effects, adverse events, neonatal weight, Apgar score, umbilical arterial and venous pH values, hyperbilirubinaemia. Neonatal complications such as haemorrhage or infections. | |
| Notes | Women who delivered during the treatment period, whose contractions did not cease within 48 hrs or who developed complications were not included in analysis for pregnancy outcomes or complications (44/200 – 22%).Data for these outcomes have not been included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque numbered envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Different regimens, not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Different regimens, not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Low risk of bias for primary outcome but high risk of bias for all secondary outcomes (> 20% no data for pregnancy outcomes). We have not included outcome data for these secondary outcomes as there was > 20% missing data and the data are likely to be at high risk of bias. |
| Selective reporting (reporting bias) | High risk | Outcomes for babies delivered before 37 weeks were not reported. Results are therefore difficult to interpret. |
| Other bias | Low risk | Other bias not apparent. |
| Methods | RCT in one centre, 2 treatment arms (with stratification by gestational age). | |
| Participants | Setting: Hospital in Milan, Italy. Inclusion criteria: 82 randomised. Inclusion criteria not clear, women for whom “tocolytic treatment was considered necessary” (not clear that all women had threatened preterm labour). All women had intact membranes. (Only two of the three stratified groups are relevant to this review – 16/82 randomised were 14‐20 weeks gestation) Exclusion criteria: Blood loss, hypertension, hyperthyroidism or heart problems. | |
| Interventions | Group 1: Clenbuterol (a beta‐2 mimetic): vials: 100 mcg in 5 mL doses (equal to 20 mcg/mL), tablets: 20 mcg Group 2: Isoxsuprine: vials: 100 mg in 2 mL doses, tablets 10 mg. (In both groups 41 women were randomised, 8 had a gestational age of < 21 wks; 33 women in each group are relevant to this review). “The administration plan was the same for both drugs. The tocolytic treatment was composed of a first, more intense, phase carried out intravenously and maintained until a valid tocolysis was obtained. In second phase, the tocolytic treatment was administered orally until the 37th week of pregnancy.” | |
| Outcomes | Maternal and fetal heart rate during treatment (reported as average) and minimum and maximum blood pressure during iv treatment. There were also figures reported for birth before 37 weeks (in graphs) but denominators are not clear and data includes those women recruited before 21 weeks' gestation. | |
| Notes | We were unable to include any outcome data from this study as separate data were not available for women whose gestational age was greater than 20 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, sample stratified by gestational age. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Not described (different regimens) Staff likely to have been aware of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Not described (different regimens) Staff likely to have been aware of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All women appear to have been accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from translated notes. |
| Other bias | Unclear risk | Little information on study methods. |
| Methods | Double‐blind RCT. | |
| Participants | 33 women in premature labour (women with ruptured membranes, placental abruption or cervical cerclage were excluded). | |
| Interventions | iv terbutaline 15 women) versus placebo (18 women). | |
| Outcomes | "prolongation index". Outcomes were reported in graphs and results were not reported by randomisation group. | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as a double‐blind study. |
| Blinding of outcome assessment (detection bias) | Low risk | Described as a double‐blind study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Results were not reported by randomisation group. It was not clear if there was any loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Results from published report. |
| Other bias | Unclear risk | Little information about methods. |
| Methods | Randomised trial with 2 arms. | |
| Participants | 23 women with premature labour between 27‐35 weeks' gestation. | |
| Interventions | iv ritodrine (12 women) versus iv isotonic glucose (11 women). | |
| Outcomes | Water and salt metabolism and blood chemistry. | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo‐controlled trial. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up apparent. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Not apparent. |
| Methods | Randomised controlled trial (part of multicentre study described by Merkatz 1980 ). | |
| Participants | Location: USA. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Risk of bias was assessed by the previous review team from a personal communication from the trials authors. This was not available to the current review team and so we have used the previous assessments. |
| Other bias | Unclear risk | Not able to assess. |
| Methods | Randomised double‐blind trial with 2 arms. | |
| Participants | 10 women in premature labour (no further details of participants provided). | |
| Interventions | Not clear how many women randomised to the active treatment and placebo groups. Interventions group: Ritodrine infusion over 12 hrs. Control: Placebo iv infusion (saline). | |
| Outcomes | Maternal blood analyses (including glucose, insulin, glucagon) at half‐hourly intervals. | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from brief conference abstract. |
| Other bias | Unclear risk | Little information on study methods. |
| Methods | Randomised trial (2 arms). | |
| Participants | 29 women in premature labour, 24 to 35 weeks' gestation. | |
| Interventions | Ritodrine (15 women) versus placebo (14 women). | |
| Outcomes | Maternal metabolism (glucose, insulin, glucagon, triglycerides, cholesterol, lactogen, chorionic gonadotropin). | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo‐controlled trial. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up apparent (some missing data). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups described as similar at baseline. |
| Methods | Randomised controlled trial (part of multicentre study described by Merkatz 1980 ). | |
| Participants | Location: USA. | |
| Interventions | 1) Ritodrine. | |
| Outcomes | Primary outcomes: birth within 48 hrs; RDS; perinatal death (7 days). | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Risk of bias was assessed by the previous review team from a personal communication from the trials authors. This was not available to the current review team and so we have used the previous assessments. |
| Other bias | Unclear risk | Not able to assess. |
| Methods | Multicentre randomised study. | |
| Participants | No information on inclusion or exclusion criteria. 82 women in premature labour. | |
| Interventions | Two iv doses or ritodrine were compared (fixed versus variable dose). Group 1: (35 women) starting dose of 0.050 mg/min augmented every 5 mins to a maximum of 0.300 mg/min. Group 2: (37 women) starting dose between 0.200 to 0.300 mg/min adapted to clinical effects (variable dose). | |
| Outcomes | Mean average dosage and mean average duration of perfusion, "delay to obtain tocolysis". Maternal tolerance (results not reported). | |
| Notes | Although this study met the inclusion criteria, no relevant outcome data were reported, and this study does not contribute any data to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Different doses. No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from brief abstract. |
| Other bias | Unclear risk | Very little information on study methods. |
| Methods | Multicentre RCT in 10 centres, 2 arms, placebo‐controlled, individual randomisation. | |
| Participants | 10 centres between 1981 to 1982. Inclusion criteria: 47 women between 24‐36 weeks’ gestation with threatened preterm labour (2 or more uterine contractions during 40 mins monitoring period), estimated fetal body weight of less than 2500 g, dilation of the external os of uterus at no more than 5 cm, Exclusion criteria: Ruptured membranes, fetal malformation, infection or non‐viable fetus, fetal distress, maternal pregnancy complication (toxaemia, placental abruption) or pre‐existing illness, maternal infection. | |
| Interventions | Experimental intervention: (22 women) 50 mg of ritodrine hydrochloride in a 5ml ampoule in 500 mL saline iv (initial dose 100 mcg/min) increased to 150 mcg/min after 30 mins if no effect tocolytic effect observed and increased to 200 mcg/min after 30 mins. After one hr if no effect observed other interventions could be introduced at the discretion of the doctor. Control intervention: (25 women) placebo, same regimen. After one hr if no effect observed other interventions could be introduced at the discretion of the doctor. 6 women in the placebo group had treatment stopped after 1 hr as no effect was observed and they received other treatment. These women were included in the analysis. | |
| Outcomes | Monitoring of uterine contractions and women’s assessment of labour pains over 2 hrs of treatment. Side effects. | |
| Notes | We were unable to include most of the data included in this study in the data and analysis in the review. The primary outcome was uterine contractions during treatment period. It was stated that there were no significant differences in pregnancy outcome attributable to the drugs but data were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | External randomisation. |
| Allocation concealment (selection bias) | Low risk | External randomisation by external trial controller. “the allocation table was sealed and stored by the controller during the evaluation period”. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled. Placebo had the same appearance and volume as active drug. Doctor could intervene if no progress. In 6 out of 25 cases in the placebo group there was some discretionary intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | It was stated that randomisation code was not broken during the randomisation period. |
| Incomplete outcome data (attrition bias) | Low risk | All women appeared to be accounted for in the analysis. 6 women in the placebo group were withdrawn and given alternative treatment after 1 hr, but there was an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report. |
| Other bias | Low risk | Not apparent. No significant differences in baseline characteristics between groups. |
| Methods | Single‐centre randomised trial. | |
| Participants | Location: USA. | |
| Interventions | 1) Terbutaline. | |
| Outcomes | Primary outcomes: birth within 48 hrs; perinatal death. | |
| Notes | Prenatal corticosteroid use: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | 83 women. Not clear if any women were lost to follow‐up. Some missing data for some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from brief conference abstract. |
| Other bias | Unclear risk | Assessment from brief conference abstract. Very little information on study methods. |
BP: blood pressure
cm: centimetre
CS: caesarean section
g: gram
hr(s): hour(s)
im: intramuscular
ITT: intention‐to‐treat
IUGR: intrauterine growth restriction
iv: intravenous
mcg: microgram
mg: milligram
mL: millilitre
min(s): minute(s)
NEC: necrotising enterocolitis
RCT: randomised controlled trial
RDS: respiratory distress syndrome
Rh: rhesus
SD: standard deviation
vs: versus
wk(s): week(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The interventions were ritodrine compared with ritodrine plus magnesium gluconate. | |
| Loss to follow‐up was more than 20% (26%). | |
| The interventions were orciprenaline compared with other uterine sedatives (trasentine, hyoscine bromide, benzodiazepine and progesterone). | |
| The interventions were ritodrine compared with indomethacin. | |
| The interventions were fenoterol compared with nitroglycerin patches. | |
| The interventions were ritodrine compared with indomethacin. | |
| The randomisation was inadequate. | |
| The interventions were ritodrine compared with nifedipine. | |
| The participants were not randomised. | |
| Study reported in brief abstract. Not clear that this was a randomised trial. | |
| Method of generation of allocation was inadequate. | |
| The participants were not randomised. | |
| The study was conducted in preterm premature rupture of membranes without preterm labour. | |
| The participants were not randomised. | |
| The participants were not randomised and the interventions were isoxsuprine compared with sedatives, analgesics and antispasmodics. | |
| The interventions compared were dysmalgine with placebo. It was not clear that the study drug was a betamimetic (described as antispasmodic). Results were not reported for both randomised groups. | |
| The study was conducted in preterm premature rupture of membranes without uterine contractions. | |
| The interventions were ritodrine plus magnesium sulfate compared with ritodrine plus placebo. | |
| The interventions were betamimetics (hexoprenaline) compared with hexoprenaline plus magnesium sulfate. | |
| The interventions were ritodrine plus placebo compared with ritodrine plus indomethacin or placebo plus indomethacin. | |
| The interventions were ritodrine plus placebo compared with ritodrine plus indomethacin or placebo plus indomethacin. | |
| Loss to follow‐up more than 20% after randomisation. | |
| The interventions were isoxuprine with micronised progesterone. | |
| Method of generation of allocation was inadequate. | |
| The study was conducted in participants with premature uterine contraction. | |
| Method of generation of allocation was inadequate. | |
| The study was conducted in participants without signs of preterm labour. | |
| The study was conducted in participants without signs of preterm labour. | |
| The interventions were ritodrine compared with ritodrine plus magnesium sulfate. | |
| The interventions were fenoterol plus oral magnesium sulfate compared with other regimens of magnesium sulfate. | |
| Loss to follow‐up 35% after randomisation. | |
| The interventions were magnesium sulfate plus ritodrine compared with control group (unclear). | |
| The interventions were ritodrine compared with a urinary trypsin inhibitor. | |
| The participants were not randomised. | |
| The interventions were betamimetics (ritodrine) compared with ritodrine plus indomethacin. | |
| The participants were not randomised. | |
| Method of generation of allocation was inadequate. | |
| Loss to follow‐up was more than 20%. | |
| The participants had preterm premature rupture of membranes without labour. | |
| The participants were not randomised. | |
| The study was conducted in participants with preterm premature rupture of membranes without evidence of true labour. | |
| The study was conducted in participants with elective inductions of labour. | |
| The interventions were magnesium sulfate compared with nifedipine. | |
| All trials in this multi‐centred study have been reported individually. | |
| Participants were premature uterine contractions without mention of cervical dilatations or inclusion or exclusion criteria. Also, the outcomes did not contribute to the review. | |
| The interventions were ritodrine compared with Atosiban (oxytocin receptor antagonist). | |
| The participants were not randomised. | |
| The interventions were nifedipine compared with isoxsuprine. | |
| The interventions were salbutamol compared with salbutamol plus ethanol. | |
| The interventions were betamimetics (fenoterol) compared with fenoterol plus naproxen. | |
| The participants were not randomised. | |
| The interventions were betamimetics (ritodrine) compared with ritodrine plus calcium antagonist or buphenine plus calcium antagonists. | |
| The interventions were terbutaline plus metoprolol compared with terbutaline plus placebo. | |
| The participants were not randomised. | |
| The study was conducted in participants with normal labour. | |
| The interventions were crossed‐over between betamimetics (iv ritodrine, subcutaneous terbutaline) and magnesium sulfate. | |
| The randomisation was inadequate. | |
| Method of generation of allocation was not truly randomisation. | |
| Method of generation of allocation was inadequate. | |
| The study was conducted in participants before cesarean section without labour. | |
| The interventions were salbutamol compared with nicardipine. | |
| The participants were not randomised. | |
| Recurrent preterm was considered as new case. The outcomes did not contribute to the review. | |
| This paper did not report the findings of a randomised trial, rather there is secondary analysis of data for women who had undergone long‐term tocolysis (it was stated that this was "mainly with Fenoterol"). Women were divided into groups retrospectively according to the interval between the end of tocolysis and delivery. |
iv: intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not clear. |
| Participants | 50 women in preterm labour (28‐36 weeks' gestation). |
| Interventions | 25 women received iv ritodrine, 25 received iv isoxsuprine. |
| Outcomes | Side effects, gestational age at birth, prolongation of pregnancy, mode of birth, neonatal death, Apgar score < 7 at 1 and 5 minutes. |
| Notes | Study methods were not clear. In the methods it was stated that women in the 2 groups were matched, but also referred to randomisation. We have written to the study authors for more information. Mr Vijay Roy [email protected] (contacted on 19th August 2013, awaiting response). |
iv: intravenous
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours of treatment Show forest plot | 10 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.88] |
| Analysis 1.1  Comparison 1 All betamimetics versus placebo, Outcome 1 Birth within 48 hours of treatment. | ||||
| 2 Perinatal death (7 days) Show forest plot | 11 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.55] |
| Analysis 1.2  Comparison 1 All betamimetics versus placebo, Outcome 2 Perinatal death (7 days). | ||||
| 3 Respiratory distress syndrome Show forest plot | 8 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.08] |
| Analysis 1.3  Comparison 1 All betamimetics versus placebo, Outcome 3 Respiratory distress syndrome. | ||||
| 4 Cerebral palsy Show forest plot | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.63] |
| Analysis 1.4  Comparison 1 All betamimetics versus placebo, Outcome 4 Cerebral palsy. | ||||
| 5 Birth within 7 days Show forest plot | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.98] |
| Analysis 1.5  Comparison 1 All betamimetics versus placebo, Outcome 5 Birth within 7 days. | ||||
| 6 Birth less than 37 weeks' gestation Show forest plot | 10 | 1212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| Analysis 1.6  Comparison 1 All betamimetics versus placebo, Outcome 6 Birth less than 37 weeks' gestation. | ||||
| 7 Maternal death Show forest plot | 2 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.7  Comparison 1 All betamimetics versus placebo, Outcome 7 Maternal death. | ||||
| 8 Pulmonary oedema Show forest plot | 3 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.23] |
| Analysis 1.8  Comparison 1 All betamimetics versus placebo, Outcome 8 Pulmonary oedema. | ||||
| 9 Cardiac arrhythmias Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.74, 16.92] |
| Analysis 1.9  Comparison 1 All betamimetics versus placebo, Outcome 9 Cardiac arrhythmias. | ||||
| 10 Myocardial ischemia Show forest plot | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.53 [0.72, 216.91] |
| Analysis 1.10  Comparison 1 All betamimetics versus placebo, Outcome 10 Myocardial ischemia. | ||||
| 11 Hypotension Show forest plot | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.12, 20.86] |
| Analysis 1.11  Comparison 1 All betamimetics versus placebo, Outcome 11 Hypotension. | ||||
| 12 Cessation of treatment due to adverse drug reaction Show forest plot | 5 | 1081 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.38 [5.21, 24.86] |
| Analysis 1.12  Comparison 1 All betamimetics versus placebo, Outcome 12 Cessation of treatment due to adverse drug reaction. | ||||
| 13 Palpitation Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 9.91 [6.46, 15.20] |
| Analysis 1.13  Comparison 1 All betamimetics versus placebo, Outcome 13 Palpitation. | ||||
| 14 Tachycardia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 All betamimetics versus placebo, Outcome 14 Tachycardia. | ||||
| 15 Chest pain Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.29 [3.81, 33.46] |
| Analysis 1.15  Comparison 1 All betamimetics versus placebo, Outcome 15 Chest pain. | ||||
| 16 Dyspnoea Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.21, 6.77] |
| Analysis 1.16  Comparison 1 All betamimetics versus placebo, Outcome 16 Dyspnoea. | ||||
| 17 Tremor Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [6.20, 18.59] |
| Analysis 1.17  Comparison 1 All betamimetics versus placebo, Outcome 17 Tremor. | ||||
| 18 Hyperglycaemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [2.05, 4.09] |
| Analysis 1.18  Comparison 1 All betamimetics versus placebo, Outcome 18 Hyperglycaemia. | ||||
| 19 Hypokalaemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [4.00, 9.20] |
| Analysis 1.19  Comparison 1 All betamimetics versus placebo, Outcome 19 Hypokalaemia. | ||||
| 20 Nausea or vomiting Show forest plot | 3 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.29, 2.42] |
| Analysis 1.20  Comparison 1 All betamimetics versus placebo, Outcome 20 Nausea or vomiting. | ||||
| 21 Nasal stuffiness Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.64, 5.12] |
| Analysis 1.21  Comparison 1 All betamimetics versus placebo, Outcome 21 Nasal stuffiness. | ||||
| 22 Headaches Show forest plot | 3 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.07 [2.60, 6.35] |
| Analysis 1.22  Comparison 1 All betamimetics versus placebo, Outcome 22 Headaches. | ||||
| 23 Neonatal death Show forest plot | 6 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.27, 3.00] |
| Analysis 1.23  Comparison 1 All betamimetics versus placebo, Outcome 23 Neonatal death. | ||||
| 24 Infant death Show forest plot | 1 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.64] |
| Analysis 1.24  Comparison 1 All betamimetics versus placebo, Outcome 24 Infant death. | ||||
| 25 Necrotising enterocolitis Show forest plot | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.06, 2.78] |
| Analysis 1.25  Comparison 1 All betamimetics versus placebo, Outcome 25 Necrotising enterocolitis. | ||||
| 26 Sepsis or infection Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.26  Comparison 1 All betamimetics versus placebo, Outcome 26 Sepsis or infection. | ||||
| 27 Fetal hypoglycaemia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.27  Comparison 1 All betamimetics versus placebo, Outcome 27 Fetal hypoglycaemia. | ||||
| 28 Fetal tachycardia Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.12, 5.13] |
| Analysis 1.28  Comparison 1 All betamimetics versus placebo, Outcome 28 Fetal tachycardia. | ||||
| 29 Infant long‐term neurological development (Bayley score: Psychomotor development) Show forest plot | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐2.74, 5.34] |
| Analysis 1.29  Comparison 1 All betamimetics versus placebo, Outcome 29 Infant long‐term neurological development (Bayley score: Psychomotor development). | ||||
| 30 Infant long‐term neurological development (Bayley score: Mental development) Show forest plot | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [0.56, 9.84] |
| Analysis 1.30  Comparison 1 All betamimetics versus placebo, Outcome 30 Infant long‐term neurological development (Bayley score: Mental development). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.77, 5.48] |
| Analysis 2.1  Comparison 2 Terbutaline versus ritodrine, Outcome 1 Birth within 48 hours. | ||||
| 2 Perinatal death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.2  Comparison 2 Terbutaline versus ritodrine, Outcome 2 Perinatal death. | ||||
| 3 Respiratory distress syndrome Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.93, 4.27] |
| Analysis 2.3  Comparison 2 Terbutaline versus ritodrine, Outcome 3 Respiratory distress syndrome. | ||||
| 4 Birth within 7 days Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.10] |
| Analysis 2.4  Comparison 2 Terbutaline versus ritodrine, Outcome 4 Birth within 7 days. | ||||
| 5 Birth less than 28 weeks' gestation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.55, 7.87] |
| Analysis 2.5  Comparison 2 Terbutaline versus ritodrine, Outcome 5 Birth less than 28 weeks' gestation. | ||||
| 6 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.92] |
| Analysis 2.6  Comparison 2 Terbutaline versus ritodrine, Outcome 6 Cessation of treatment due to adverse drug reactions. | ||||
| 7 Any maternal adverse effects Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| Analysis 2.7  Comparison 2 Terbutaline versus ritodrine, Outcome 7 Any maternal adverse effects. | ||||
| 8 Chest pain Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| Analysis 2.8  Comparison 2 Terbutaline versus ritodrine, Outcome 8 Chest pain. | ||||
| 9 Shortness of breath or dyspnea Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| Analysis 2.9  Comparison 2 Terbutaline versus ritodrine, Outcome 9 Shortness of breath or dyspnea. | ||||
| 10 Hyperglycaemia or abnormal glucose tolerance test Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.05, 3.03] |
| Analysis 2.10  Comparison 2 Terbutaline versus ritodrine, Outcome 10 Hyperglycaemia or abnormal glucose tolerance test. | ||||
| 11 Palpitations Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.78, 1.79] |
| Analysis 2.11  Comparison 2 Terbutaline versus ritodrine, Outcome 11 Palpitations. | ||||
| 12 Tachycardia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.00] |
| Analysis 2.12  Comparison 2 Terbutaline versus ritodrine, Outcome 12 Tachycardia. | ||||
| 13 Arrhythmia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Analysis 2.13  Comparison 2 Terbutaline versus ritodrine, Outcome 13 Arrhythmia. | ||||
| 14 Hypotension Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.49] |
| Analysis 2.14  Comparison 2 Terbutaline versus ritodrine, Outcome 14 Hypotension. | ||||
| 15 Nausea or vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.71, 3.20] |
| Analysis 2.15  Comparison 2 Terbutaline versus ritodrine, Outcome 15 Nausea or vomiting. | ||||
| 16 Headache Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| Analysis 2.16  Comparison 2 Terbutaline versus ritodrine, Outcome 16 Headache. | ||||
| 17 Anxiety Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.75] |
| Analysis 2.17  Comparison 2 Terbutaline versus ritodrine, Outcome 17 Anxiety. | ||||
| 18 Necrotising enterocolitis Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.67] |
| Analysis 2.18  Comparison 2 Terbutaline versus ritodrine, Outcome 18 Necrotising enterocolitis. | ||||
| 19 Neonatal death Show forest plot | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.42, 3.91] |
| Analysis 2.19  Comparison 2 Terbutaline versus ritodrine, Outcome 19 Neonatal death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| Analysis 3.1  Comparison 3 Fenoterol versus ritodrine, Outcome 1 Perinatal death. | ||||
| 2 Respiratory distress syndrome Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.42] |
| Analysis 3.2  Comparison 3 Fenoterol versus ritodrine, Outcome 2 Respiratory distress syndrome. | ||||
| 3 Tachycardia Show forest plot | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.45] |
| Analysis 3.3  Comparison 3 Fenoterol versus ritodrine, Outcome 3 Tachycardia. | ||||
| 4 Hypoglycaemia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| Analysis 3.4  Comparison 3 Fenoterol versus ritodrine, Outcome 4 Hypoglycaemia. | ||||
| 5 Fetal bradycardia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.54] |
| Analysis 3.5  Comparison 3 Fenoterol versus ritodrine, Outcome 5 Fetal bradycardia. | ||||
| 6 Neonatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.96] |
| Analysis 3.6  Comparison 3 Fenoterol versus ritodrine, Outcome 6 Neonatal death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.60, 1.91] |
| Analysis 4.1  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 1 Birth within 48 hours. | ||||
| 2 Respiratory distress syndrome Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.41] |
| Analysis 4.2  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 2 Respiratory distress syndrome. | ||||
| 3 Periventricular haemorrhage grade 3‐4 Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| Analysis 4.3  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 3 Periventricular haemorrhage grade 3‐4. | ||||
| 4 Birth less than 34 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.70, 1.45] |
| Analysis 4.4  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 4 Birth less than 34 weeks. | ||||
| 5 Birth less than 37 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.13] |
| Analysis 4.5  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 5 Birth less than 37 weeks. | ||||
| 6 Any maternal adverse effects Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.43, 1.11] |
| Analysis 4.6  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 6 Any maternal adverse effects. | ||||
| 7 Palpitations Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.13] |
| Analysis 4.7  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 7 Palpitations. | ||||
| 8 Tachycardia Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.35] |
| Analysis 4.8  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 8 Tachycardia. | ||||
| 9 Nausea or vomiting Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.38, 3.84] |
| Analysis 4.9  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 9 Nausea or vomiting. | ||||
| 10 Headache Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| Analysis 4.10  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 10 Headache. | ||||
| 11 Sepsis Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| Analysis 4.11  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 11 Sepsis. | ||||
| 12 Neonatal death Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| Analysis 4.12  Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 12 Neonatal death. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.93] |
| Analysis 5.1  Comparison 5 Hexoprenaline versus ritodrine, Outcome 1 Cessation of treatment due to adverse drug reactions. | ||||
| 2 Any maternal adverse effects Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| Analysis 5.2  Comparison 5 Hexoprenaline versus ritodrine, Outcome 2 Any maternal adverse effects. | ||||
| 3 Palpitations Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| Analysis 5.3  Comparison 5 Hexoprenaline versus ritodrine, Outcome 3 Palpitations. | ||||
| 4 Hypotension Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.96] |
| Analysis 5.4  Comparison 5 Hexoprenaline versus ritodrine, Outcome 4 Hypotension. | ||||
| 5 Nausea or vomiting Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| Analysis 5.5  Comparison 5 Hexoprenaline versus ritodrine, Outcome 5 Nausea or vomiting. | ||||
| 6 Increase in fetal heart rate Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.98] |
| Analysis 5.6  Comparison 5 Hexoprenaline versus ritodrine, Outcome 6 Increase in fetal heart rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Respiratory distress syndrome Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| Analysis 6.1  Comparison 6 Hexoprenaline versus salbutamol, Outcome 1 Respiratory distress syndrome. | ||||
| 2 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.67] |
| Analysis 6.2  Comparison 6 Hexoprenaline versus salbutamol, Outcome 2 Cessation of treatment due to adverse drug reactions. | ||||
| 3 Any maternal adverse effects Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.80] |
| Analysis 6.3  Comparison 6 Hexoprenaline versus salbutamol, Outcome 3 Any maternal adverse effects. | ||||
| 4 Tachycardia Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.85] |
| Analysis 6.4  Comparison 6 Hexoprenaline versus salbutamol, Outcome 4 Tachycardia. | ||||
| 5 Nausea or vomiting Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.95] |
| Analysis 6.5  Comparison 6 Hexoprenaline versus salbutamol, Outcome 5 Nausea or vomiting. | ||||
| 6 Headache Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.13] |
| Analysis 6.6  Comparison 6 Hexoprenaline versus salbutamol, Outcome 6 Headache. | ||||
| 7 Tremor Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| Analysis 6.7  Comparison 6 Hexoprenaline versus salbutamol, Outcome 7 Tremor. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BIrth within 48 hours Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| Analysis 7.1  Comparison 7 Terbutaline versus salbutamol, Outcome 1 BIrth within 48 hours. | ||||
| 2 Side effects (overall) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.70] |
| Analysis 7.2  Comparison 7 Terbutaline versus salbutamol, Outcome 2 Side effects (overall). | ||||
| 3 Tachycardia Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.30, 0.75] |
| Analysis 7.3  Comparison 7 Terbutaline versus salbutamol, Outcome 3 Tachycardia. | ||||
| 4 Dyspnea Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.14] |
| Analysis 7.4  Comparison 7 Terbutaline versus salbutamol, Outcome 4 Dyspnea. | ||||
| 5 Nausea Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.09] |
| Analysis 7.5  Comparison 7 Terbutaline versus salbutamol, Outcome 5 Nausea. | ||||
| 6 Anxiety Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.84] |
| Analysis 7.6  Comparison 7 Terbutaline versus salbutamol, Outcome 6 Anxiety. | ||||
| 7 Chills Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| Analysis 7.7  Comparison 7 Terbutaline versus salbutamol, Outcome 7 Chills. | ||||
| 8 Oedema Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.27] |
| Analysis 7.8  Comparison 7 Terbutaline versus salbutamol, Outcome 8 Oedema. | ||||
| 9 Duration of hospital admission (days) Show forest plot | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.19, ‐0.41] |
| Analysis 7.9  Comparison 7 Terbutaline versus salbutamol, Outcome 9 Duration of hospital admission (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth (before 37 weeks) Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.29, 23.13] |
| Analysis 8.1  Comparison 8 Slow release versus normal release salbutamol, Outcome 1 Preterm birth (before 37 weeks). | ||||
| 2 Caesarean section Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.23, 7.07] |
| Analysis 8.2  Comparison 8 Slow release versus normal release salbutamol, Outcome 2 Caesarean section. | ||||
| 3 Side effects leading to discontinuation of treatment Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 8.3  Comparison 8 Slow release versus normal release salbutamol, Outcome 3 Side effects leading to discontinuation of treatment. | ||||
| 4 Nausea Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.06, 12.98] |
| Analysis 8.4  Comparison 8 Slow release versus normal release salbutamol, Outcome 4 Nausea. | ||||
| 5 Apgar score less than 7 at 5 minutes Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 8.5  Comparison 8 Slow release versus normal release salbutamol, Outcome 5 Apgar score less than 7 at 5 minutes. | ||||
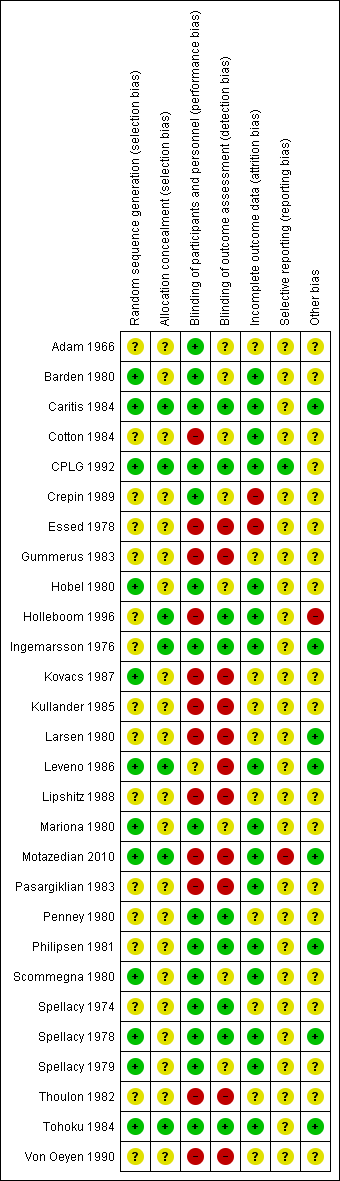
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
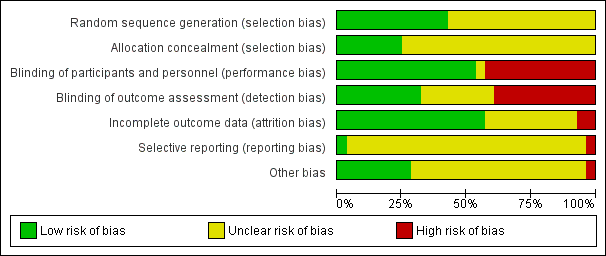
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
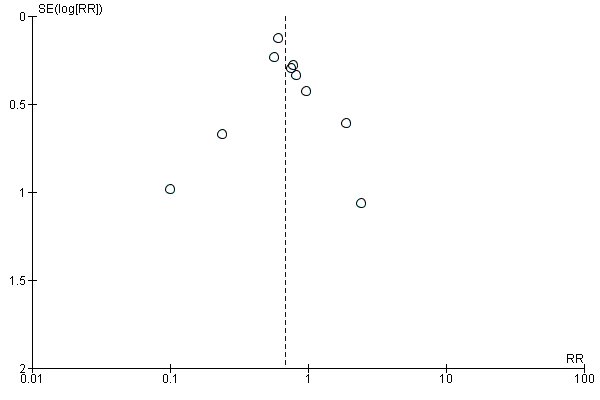
Funnel plot of comparison: 1 All betamimetics versus placebo, outcome: 1.1 Birth within 48 hours of treatment.

Funnel plot of comparison: 1 All betamimetics versus placebo, outcome: 1.2 Perinatal death (7 days).
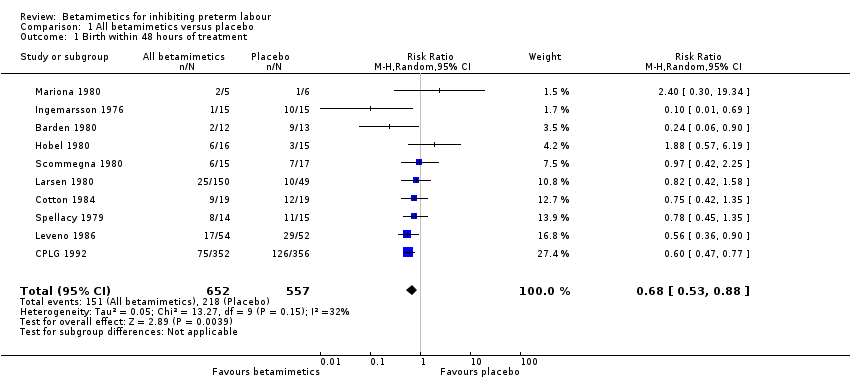
Comparison 1 All betamimetics versus placebo, Outcome 1 Birth within 48 hours of treatment.
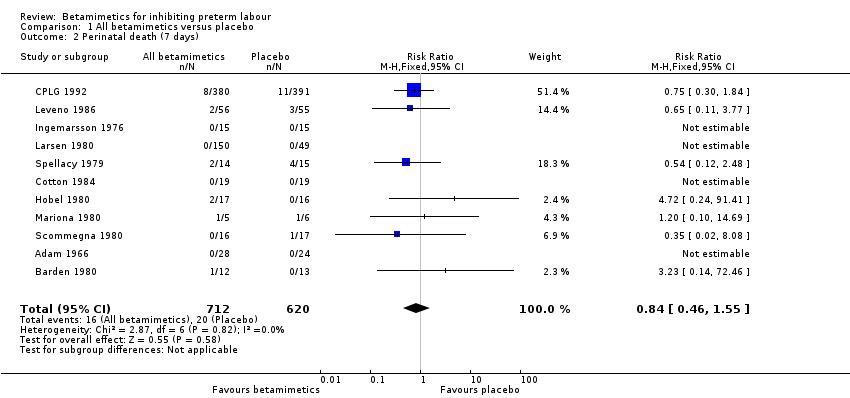
Comparison 1 All betamimetics versus placebo, Outcome 2 Perinatal death (7 days).
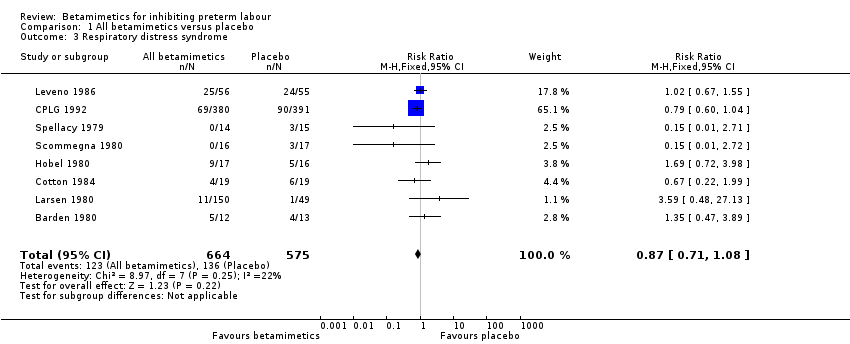
Comparison 1 All betamimetics versus placebo, Outcome 3 Respiratory distress syndrome.
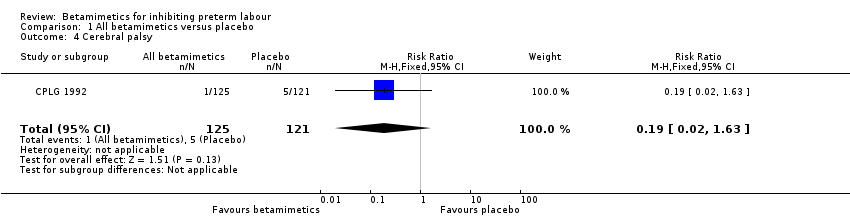
Comparison 1 All betamimetics versus placebo, Outcome 4 Cerebral palsy.

Comparison 1 All betamimetics versus placebo, Outcome 5 Birth within 7 days.

Comparison 1 All betamimetics versus placebo, Outcome 6 Birth less than 37 weeks' gestation.
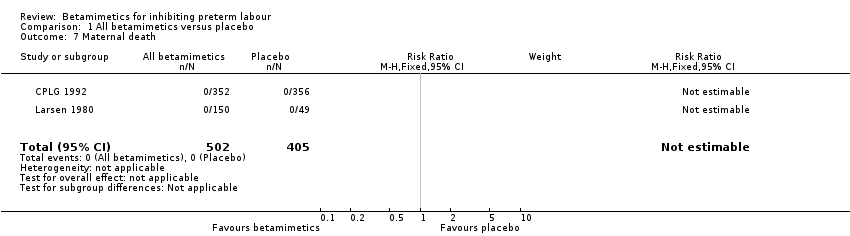
Comparison 1 All betamimetics versus placebo, Outcome 7 Maternal death.

Comparison 1 All betamimetics versus placebo, Outcome 8 Pulmonary oedema.
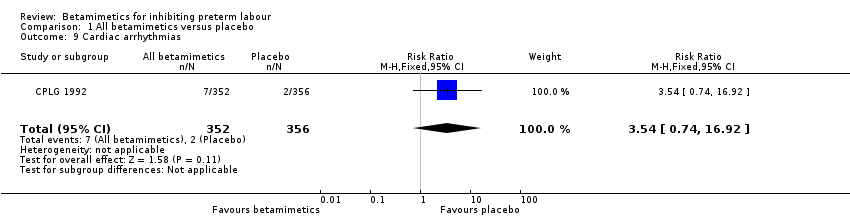
Comparison 1 All betamimetics versus placebo, Outcome 9 Cardiac arrhythmias.

Comparison 1 All betamimetics versus placebo, Outcome 10 Myocardial ischemia.
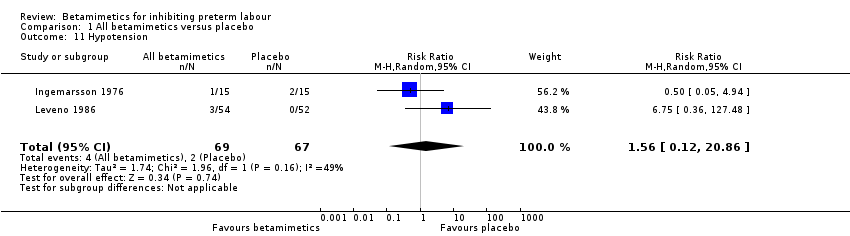
Comparison 1 All betamimetics versus placebo, Outcome 11 Hypotension.

Comparison 1 All betamimetics versus placebo, Outcome 12 Cessation of treatment due to adverse drug reaction.
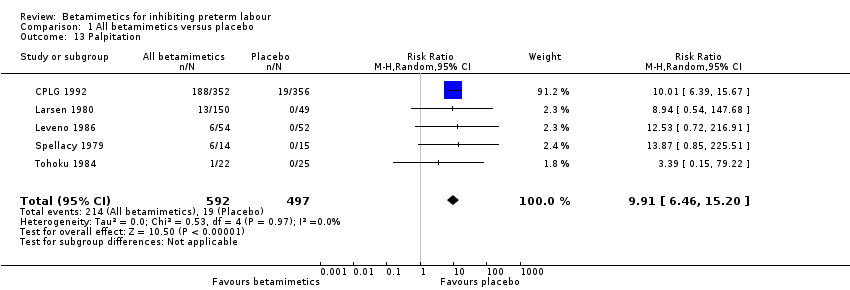
Comparison 1 All betamimetics versus placebo, Outcome 13 Palpitation.
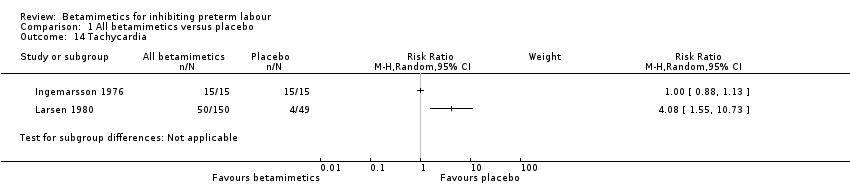
Comparison 1 All betamimetics versus placebo, Outcome 14 Tachycardia.

Comparison 1 All betamimetics versus placebo, Outcome 15 Chest pain.

Comparison 1 All betamimetics versus placebo, Outcome 16 Dyspnoea.
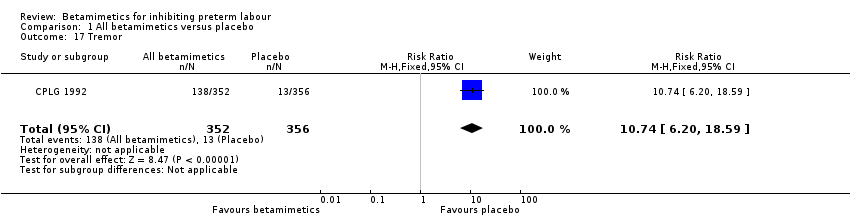
Comparison 1 All betamimetics versus placebo, Outcome 17 Tremor.
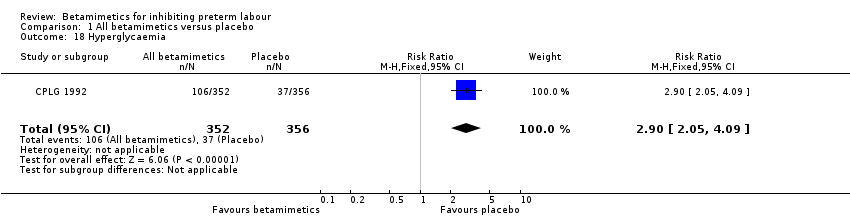
Comparison 1 All betamimetics versus placebo, Outcome 18 Hyperglycaemia.

Comparison 1 All betamimetics versus placebo, Outcome 19 Hypokalaemia.

Comparison 1 All betamimetics versus placebo, Outcome 20 Nausea or vomiting.
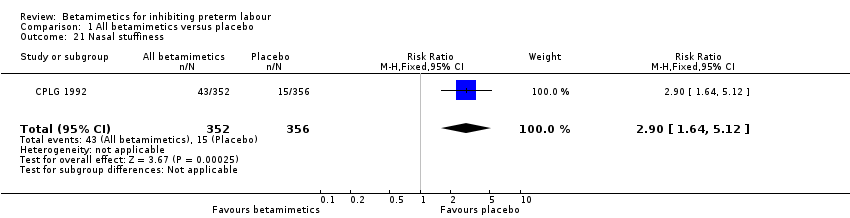
Comparison 1 All betamimetics versus placebo, Outcome 21 Nasal stuffiness.

Comparison 1 All betamimetics versus placebo, Outcome 22 Headaches.

Comparison 1 All betamimetics versus placebo, Outcome 23 Neonatal death.

Comparison 1 All betamimetics versus placebo, Outcome 24 Infant death.

Comparison 1 All betamimetics versus placebo, Outcome 25 Necrotising enterocolitis.

Comparison 1 All betamimetics versus placebo, Outcome 26 Sepsis or infection.
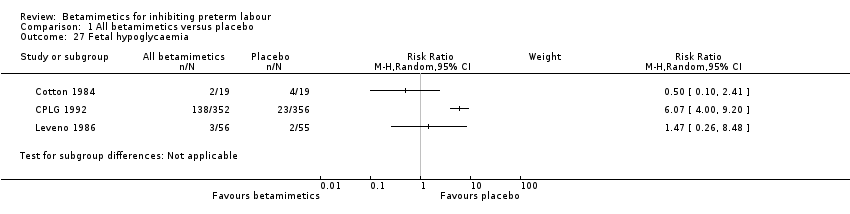
Comparison 1 All betamimetics versus placebo, Outcome 27 Fetal hypoglycaemia.

Comparison 1 All betamimetics versus placebo, Outcome 28 Fetal tachycardia.

Comparison 1 All betamimetics versus placebo, Outcome 29 Infant long‐term neurological development (Bayley score: Psychomotor development).
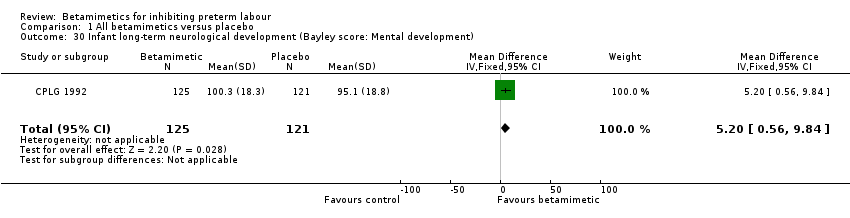
Comparison 1 All betamimetics versus placebo, Outcome 30 Infant long‐term neurological development (Bayley score: Mental development).

Comparison 2 Terbutaline versus ritodrine, Outcome 1 Birth within 48 hours.
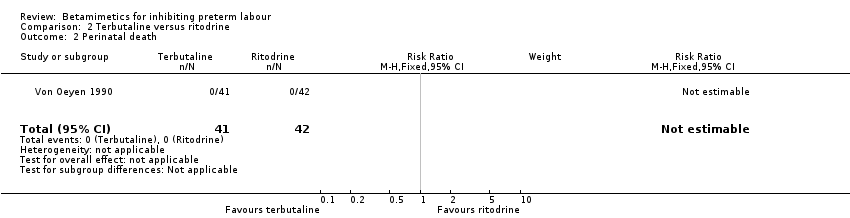
Comparison 2 Terbutaline versus ritodrine, Outcome 2 Perinatal death.
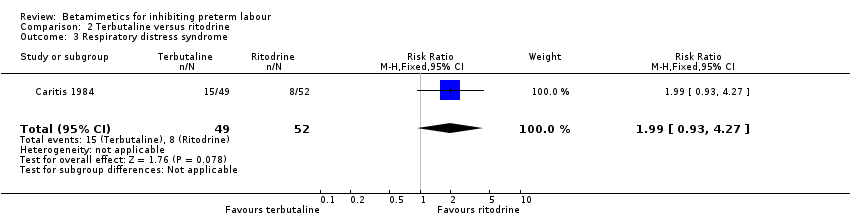
Comparison 2 Terbutaline versus ritodrine, Outcome 3 Respiratory distress syndrome.

Comparison 2 Terbutaline versus ritodrine, Outcome 4 Birth within 7 days.
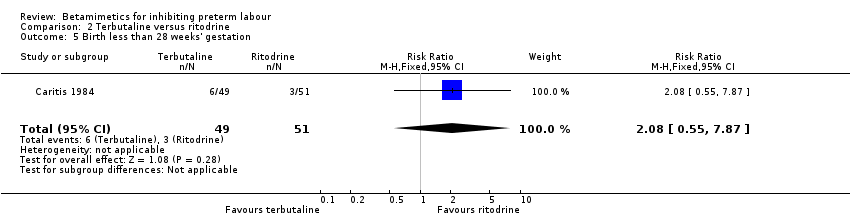
Comparison 2 Terbutaline versus ritodrine, Outcome 5 Birth less than 28 weeks' gestation.

Comparison 2 Terbutaline versus ritodrine, Outcome 6 Cessation of treatment due to adverse drug reactions.
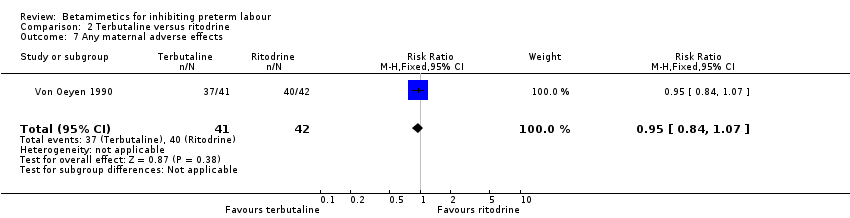
Comparison 2 Terbutaline versus ritodrine, Outcome 7 Any maternal adverse effects.
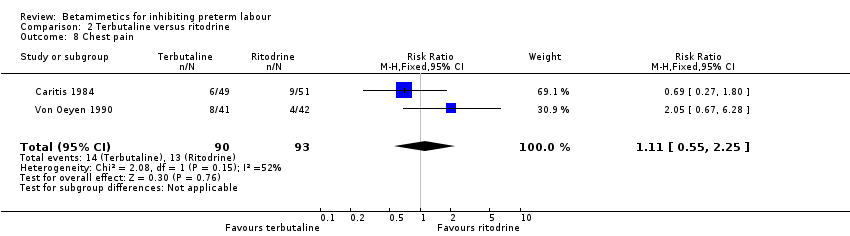
Comparison 2 Terbutaline versus ritodrine, Outcome 8 Chest pain.

Comparison 2 Terbutaline versus ritodrine, Outcome 9 Shortness of breath or dyspnea.

Comparison 2 Terbutaline versus ritodrine, Outcome 10 Hyperglycaemia or abnormal glucose tolerance test.

Comparison 2 Terbutaline versus ritodrine, Outcome 11 Palpitations.
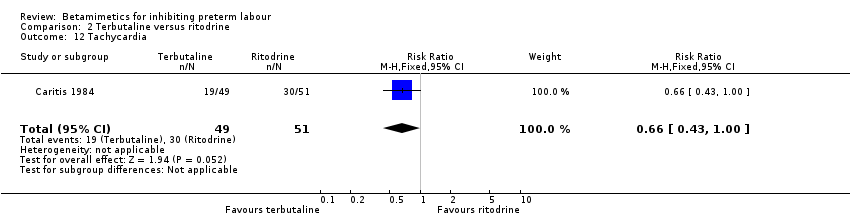
Comparison 2 Terbutaline versus ritodrine, Outcome 12 Tachycardia.

Comparison 2 Terbutaline versus ritodrine, Outcome 13 Arrhythmia.
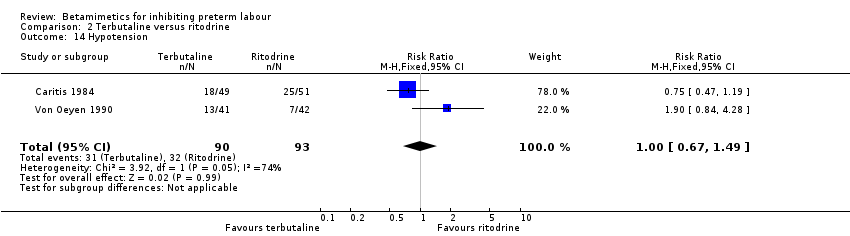
Comparison 2 Terbutaline versus ritodrine, Outcome 14 Hypotension.
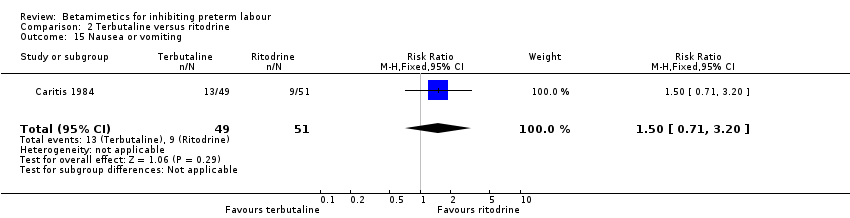
Comparison 2 Terbutaline versus ritodrine, Outcome 15 Nausea or vomiting.
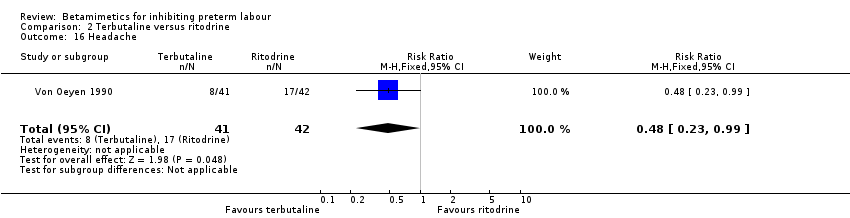
Comparison 2 Terbutaline versus ritodrine, Outcome 16 Headache.
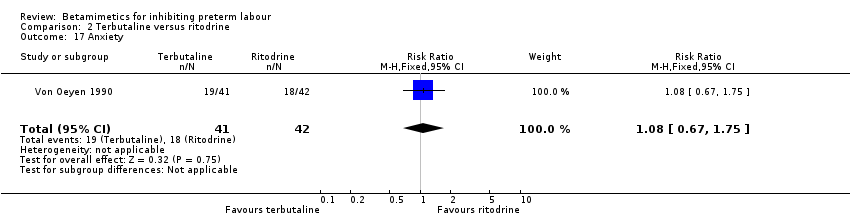
Comparison 2 Terbutaline versus ritodrine, Outcome 17 Anxiety.

Comparison 2 Terbutaline versus ritodrine, Outcome 18 Necrotising enterocolitis.
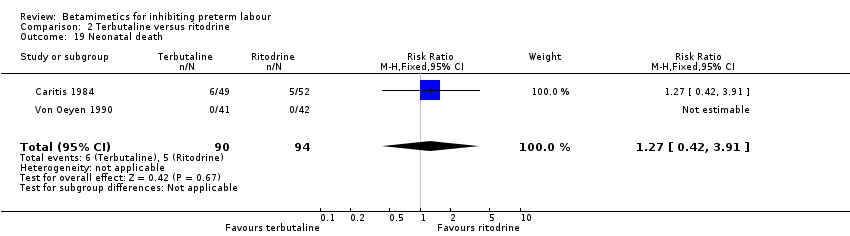
Comparison 2 Terbutaline versus ritodrine, Outcome 19 Neonatal death.
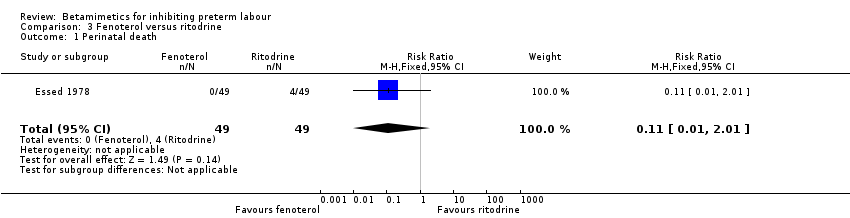
Comparison 3 Fenoterol versus ritodrine, Outcome 1 Perinatal death.

Comparison 3 Fenoterol versus ritodrine, Outcome 2 Respiratory distress syndrome.

Comparison 3 Fenoterol versus ritodrine, Outcome 3 Tachycardia.
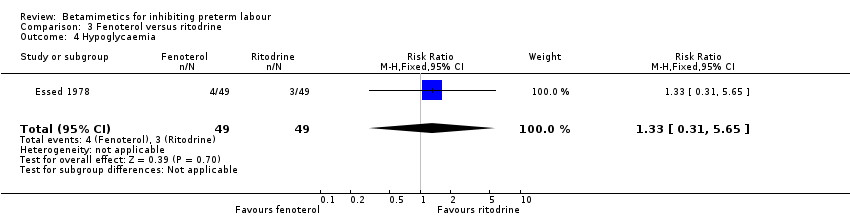
Comparison 3 Fenoterol versus ritodrine, Outcome 4 Hypoglycaemia.
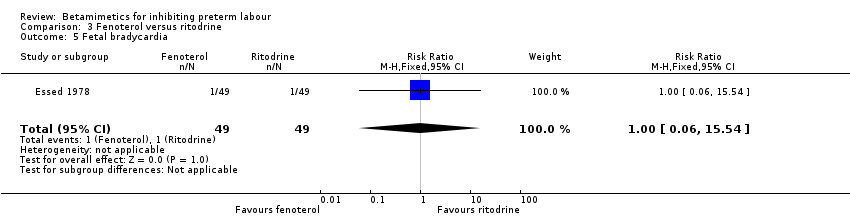
Comparison 3 Fenoterol versus ritodrine, Outcome 5 Fetal bradycardia.

Comparison 3 Fenoterol versus ritodrine, Outcome 6 Neonatal death.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 1 Birth within 48 hours.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 2 Respiratory distress syndrome.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 3 Periventricular haemorrhage grade 3‐4.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 4 Birth less than 34 weeks.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 5 Birth less than 37 weeks.
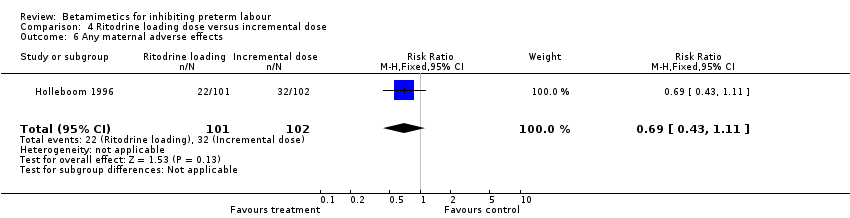
Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 6 Any maternal adverse effects.
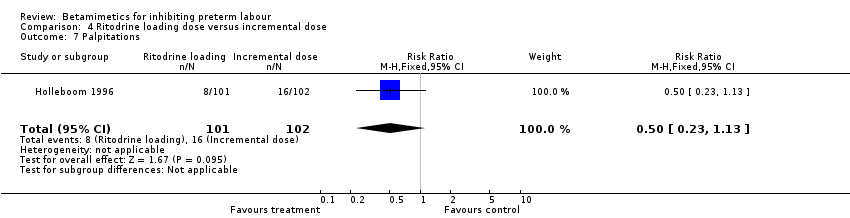
Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 7 Palpitations.
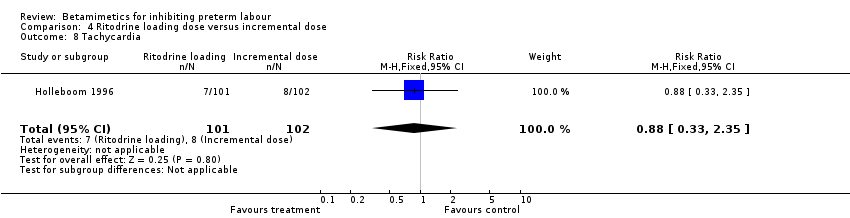
Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 8 Tachycardia.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 9 Nausea or vomiting.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 10 Headache.

Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 11 Sepsis.
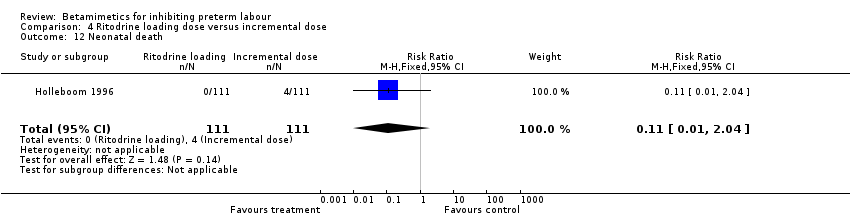
Comparison 4 Ritodrine loading dose versus incremental dose, Outcome 12 Neonatal death.
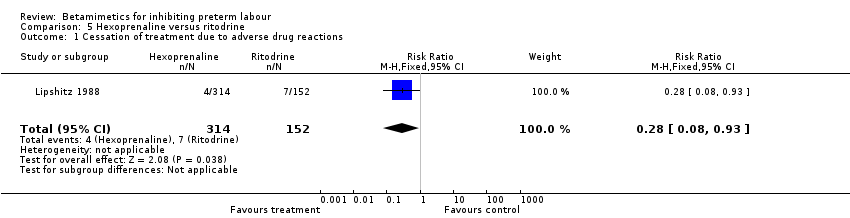
Comparison 5 Hexoprenaline versus ritodrine, Outcome 1 Cessation of treatment due to adverse drug reactions.
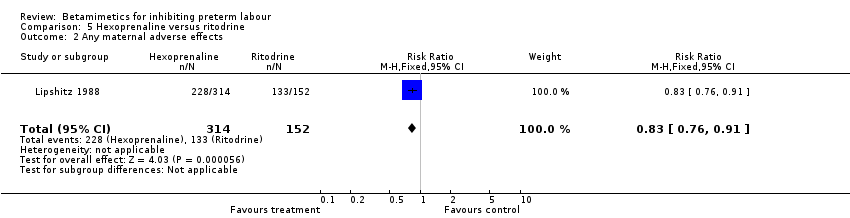
Comparison 5 Hexoprenaline versus ritodrine, Outcome 2 Any maternal adverse effects.

Comparison 5 Hexoprenaline versus ritodrine, Outcome 3 Palpitations.

Comparison 5 Hexoprenaline versus ritodrine, Outcome 4 Hypotension.
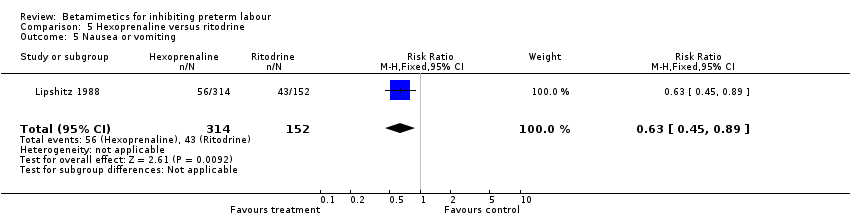
Comparison 5 Hexoprenaline versus ritodrine, Outcome 5 Nausea or vomiting.
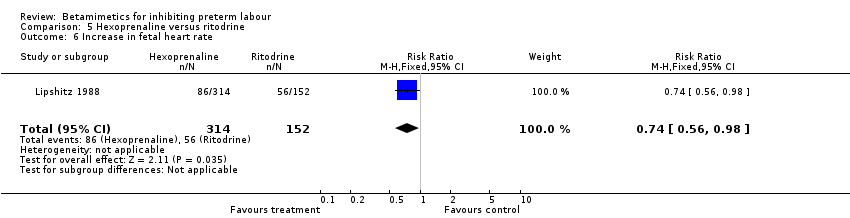
Comparison 5 Hexoprenaline versus ritodrine, Outcome 6 Increase in fetal heart rate.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 1 Respiratory distress syndrome.
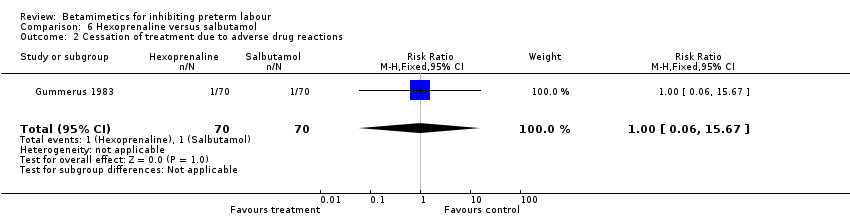
Comparison 6 Hexoprenaline versus salbutamol, Outcome 2 Cessation of treatment due to adverse drug reactions.
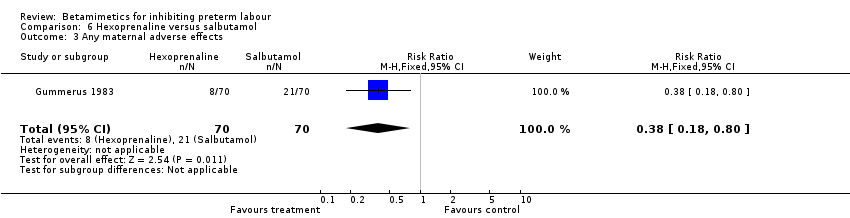
Comparison 6 Hexoprenaline versus salbutamol, Outcome 3 Any maternal adverse effects.
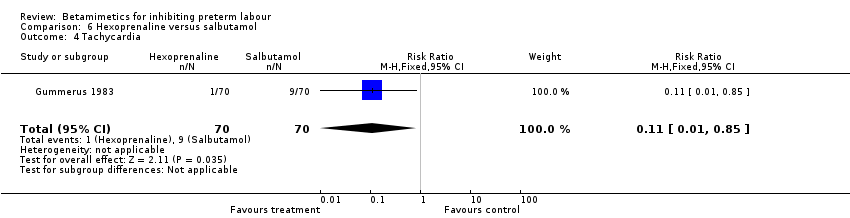
Comparison 6 Hexoprenaline versus salbutamol, Outcome 4 Tachycardia.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 5 Nausea or vomiting.
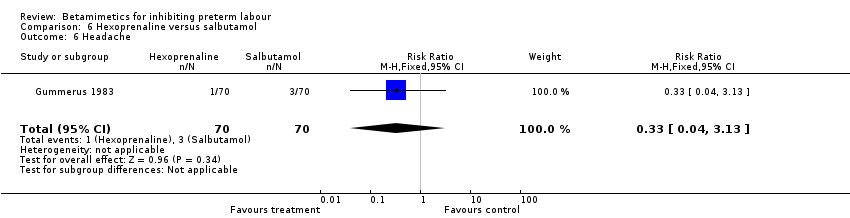
Comparison 6 Hexoprenaline versus salbutamol, Outcome 6 Headache.
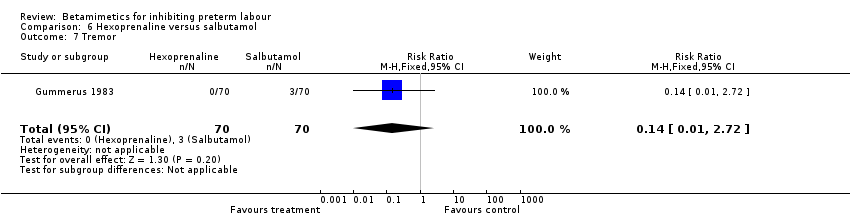
Comparison 6 Hexoprenaline versus salbutamol, Outcome 7 Tremor.

Comparison 7 Terbutaline versus salbutamol, Outcome 1 BIrth within 48 hours.
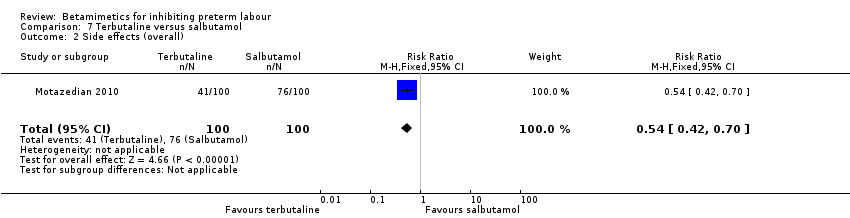
Comparison 7 Terbutaline versus salbutamol, Outcome 2 Side effects (overall).

Comparison 7 Terbutaline versus salbutamol, Outcome 3 Tachycardia.

Comparison 7 Terbutaline versus salbutamol, Outcome 4 Dyspnea.

Comparison 7 Terbutaline versus salbutamol, Outcome 5 Nausea.
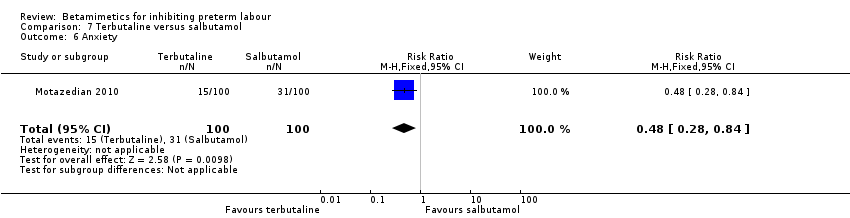
Comparison 7 Terbutaline versus salbutamol, Outcome 6 Anxiety.

Comparison 7 Terbutaline versus salbutamol, Outcome 7 Chills.

Comparison 7 Terbutaline versus salbutamol, Outcome 8 Oedema.
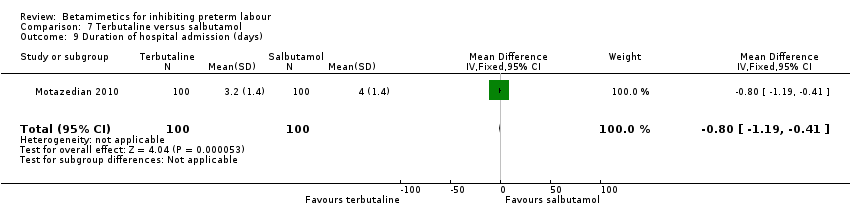
Comparison 7 Terbutaline versus salbutamol, Outcome 9 Duration of hospital admission (days).
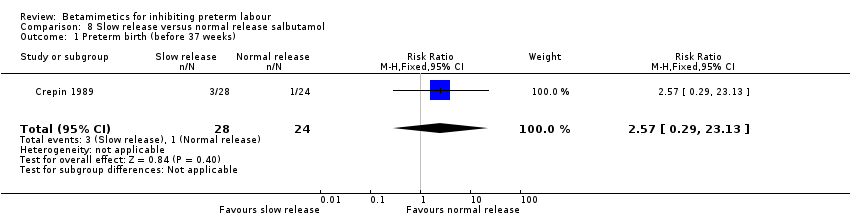
Comparison 8 Slow release versus normal release salbutamol, Outcome 1 Preterm birth (before 37 weeks).

Comparison 8 Slow release versus normal release salbutamol, Outcome 2 Caesarean section.

Comparison 8 Slow release versus normal release salbutamol, Outcome 3 Side effects leading to discontinuation of treatment.

Comparison 8 Slow release versus normal release salbutamol, Outcome 4 Nausea.

Comparison 8 Slow release versus normal release salbutamol, Outcome 5 Apgar score less than 7 at 5 minutes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours of treatment Show forest plot | 10 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.88] |
| 2 Perinatal death (7 days) Show forest plot | 11 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.55] |
| 3 Respiratory distress syndrome Show forest plot | 8 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.08] |
| 4 Cerebral palsy Show forest plot | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.63] |
| 5 Birth within 7 days Show forest plot | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.98] |
| 6 Birth less than 37 weeks' gestation Show forest plot | 10 | 1212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 7 Maternal death Show forest plot | 2 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Pulmonary oedema Show forest plot | 3 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.23] |
| 9 Cardiac arrhythmias Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.74, 16.92] |
| 10 Myocardial ischemia Show forest plot | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.53 [0.72, 216.91] |
| 11 Hypotension Show forest plot | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.12, 20.86] |
| 12 Cessation of treatment due to adverse drug reaction Show forest plot | 5 | 1081 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.38 [5.21, 24.86] |
| 13 Palpitation Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 9.91 [6.46, 15.20] |
| 14 Tachycardia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Chest pain Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.29 [3.81, 33.46] |
| 16 Dyspnoea Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.21, 6.77] |
| 17 Tremor Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [6.20, 18.59] |
| 18 Hyperglycaemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [2.05, 4.09] |
| 19 Hypokalaemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [4.00, 9.20] |
| 20 Nausea or vomiting Show forest plot | 3 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.29, 2.42] |
| 21 Nasal stuffiness Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.64, 5.12] |
| 22 Headaches Show forest plot | 3 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.07 [2.60, 6.35] |
| 23 Neonatal death Show forest plot | 6 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.27, 3.00] |
| 24 Infant death Show forest plot | 1 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.64] |
| 25 Necrotising enterocolitis Show forest plot | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.06, 2.78] |
| 26 Sepsis or infection Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27 Fetal hypoglycaemia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 28 Fetal tachycardia Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.12, 5.13] |
| 29 Infant long‐term neurological development (Bayley score: Psychomotor development) Show forest plot | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐2.74, 5.34] |
| 30 Infant long‐term neurological development (Bayley score: Mental development) Show forest plot | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [0.56, 9.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.77, 5.48] |
| 2 Perinatal death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Respiratory distress syndrome Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.93, 4.27] |
| 4 Birth within 7 days Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.10] |
| 5 Birth less than 28 weeks' gestation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.55, 7.87] |
| 6 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.92] |
| 7 Any maternal adverse effects Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Chest pain Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| 9 Shortness of breath or dyspnea Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 10 Hyperglycaemia or abnormal glucose tolerance test Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.05, 3.03] |
| 11 Palpitations Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.78, 1.79] |
| 12 Tachycardia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.00] |
| 13 Arrhythmia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| 14 Hypotension Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.49] |
| 15 Nausea or vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.71, 3.20] |
| 16 Headache Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 17 Anxiety Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.75] |
| 18 Necrotising enterocolitis Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.67] |
| 19 Neonatal death Show forest plot | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.42, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| 2 Respiratory distress syndrome Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.42] |
| 3 Tachycardia Show forest plot | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.45] |
| 4 Hypoglycaemia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| 5 Fetal bradycardia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.54] |
| 6 Neonatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.60, 1.91] |
| 2 Respiratory distress syndrome Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.41] |
| 3 Periventricular haemorrhage grade 3‐4 Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| 4 Birth less than 34 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.70, 1.45] |
| 5 Birth less than 37 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.13] |
| 6 Any maternal adverse effects Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.43, 1.11] |
| 7 Palpitations Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.13] |
| 8 Tachycardia Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.35] |
| 9 Nausea or vomiting Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.38, 3.84] |
| 10 Headache Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 11 Sepsis Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 12 Neonatal death Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.93] |
| 2 Any maternal adverse effects Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 3 Palpitations Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4 Hypotension Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.96] |
| 5 Nausea or vomiting Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 6 Increase in fetal heart rate Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Respiratory distress syndrome Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| 2 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.67] |
| 3 Any maternal adverse effects Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.80] |
| 4 Tachycardia Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.85] |
| 5 Nausea or vomiting Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.95] |
| 6 Headache Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.13] |
| 7 Tremor Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BIrth within 48 hours Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| 2 Side effects (overall) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.70] |
| 3 Tachycardia Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.30, 0.75] |
| 4 Dyspnea Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.14] |
| 5 Nausea Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.09] |
| 6 Anxiety Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.84] |
| 7 Chills Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| 8 Oedema Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.27] |
| 9 Duration of hospital admission (days) Show forest plot | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.19, ‐0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth (before 37 weeks) Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.29, 23.13] |
| 2 Caesarean section Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.23, 7.07] |
| 3 Side effects leading to discontinuation of treatment Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Nausea Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.06, 12.98] |
| 5 Apgar score less than 7 at 5 minutes Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

