Administración de suplementos de arginina para la prevención de la enterocolitis necrosante en lactantes prematuros
Resumen
Antecedentes
Se ha propuesto la disminución de la concentración de óxido nítrico como uno de los posibles mecanismos celulares de la enterocolitis necrosante (ECN). La arginina puede actuar como sustrato para la producción de óxido nítrico en los tejidos y la administración de suplementos de arginina puede ayudar a prevenir la ECN.
Objetivos
Examinar el efecto de la administración de suplementos de arginina (administrados por cualquier vía) en la incidencia de ECN en neonatos prematuros. Realizar análisis de subgrupos basados en la dosis de arginina y la edad gestacional de los participantes (≤ 32 semanas, > 32 semanas).
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología (Cochrane Neonatal Group) para realizar búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2016, número 4), MEDLINE a través de PubMed (desde 1966 hasta el 12 de mayo de 2016), Embase (desde 1980 hasta el 12 de mayo de 2016) y el Cumulative Index to Nursing and Allied Health Literature (CINAHL; desde 1982 hasta el 12 de mayo de 2016). También se buscaron ensayos controlados aleatorizados y cuasialeatorizados en las bases de datos de ensayos clínicos, en las actas de congresos y en las listas de referencias de los artículos recuperados.
Criterios de selección
Ensayos controlados aleatorios y cuasialeatorios de la administración de suplementos de arginina (administrados por vía oral o parenteral durante al menos siete días, además de lo que un lactante puede estar recibiendo de una fuente enteral o parenteral) en comparación con placebo o ningún tratamiento.
Obtención y análisis de los datos
La calidad metodológica de los ensayos se evaluó a partir de la información obtenida de los informes de los estudios y mediante la comunicación personal con los autores de los mismos. Se extrajeron los datos sobre los resultados pertinentes y el tamaño del efecto se calculó y se informó como riesgo relativo (RR), diferencia de riesgos (DR) o diferencia de medias (DM), según correspondiera. Se utilizó el enfoque GRADE (Recommendations Assessment, Development, and Evaluation) para evaluar la calidad de la evidencia.
Resultados principales
Se identificaron tres estudios elegibles que incluyeron un total de 285 neonatos (140 recibieron arginina) de tres países. La calidad metodológica general de los estudios incluidos se consideró buena. Se observó una reducción estadísticamente significativa en el riesgo de desarrollo de ECN (en cualquier etapa) entre los neonatos prematuros del grupo de arginina en comparación con el grupo placebo (RR 0,38; intervalo de confianza [IC] del 95%: 0,23 a 0,64; I2 = 27%) (DR ‐0,19; IC del 95%: ‐0,28 a ‐0,10; I2 = 0%) y la calidad de la evidencia se consideró moderada. El número necesario a tratar para obtener un resultado beneficioso adicional (NNTB) que se requiere para prevenir el desarrollo de la ECN (cualquier etapa) fue 6 (IC del 95%: 4 a 10). Los resultados del estudio mostraron una reducción estadísticamente significativa en el riesgo de desarrollo de ECN etapa 1 (RR 0,37; IC del 95%: 0,15 a 0,90; I2 = 52%) (DR ‐0,07; IC del 95%: ‐0,14 a ‐0,01; I2 = 0%) y de ECN etapa 3 (RR 0,13; IC del 95%: 0,02 a 1,03; I2 = 0%) (DR ‐0,05; IC del 95%: ‐0,09 a ‐0,01; I2 = 89%) en el grupo de arginina en comparación con el grupo control; la calidad de la evidencia fue moderada.
La administración de suplementos de arginina se asoció con una reducción significativa de la muerte relacionada con la ECN (RR 0,18; IC del 95%: 0,03 a 1,00; I2 = 0%) (DR ‐0,05; IC del 95%: ‐0,09 a ‐0,01; I2 = 87%). Los resultados mostraron heterogeneidad clínica en las tasas de mortalidad. La mortalidad debido a cualquier causa no fue significativamente diferente entre los grupos de arginina y control o ningún tratamiento (RR 0,77; IC del 95%: 0,41 a 1,45; I2 = 42%) (DR ‐0,03; IC del 95%: ‐0,10 a 0,04; I2 = 79%). Los investigadores no observaron efectos secundarios significativos directamente atribuibles a la arginina, incluida la hipotensión o las alteraciones de la homeostasis de la glucosa. Los datos de seguimiento de un ensayo no revelaron diferencias estadísticamente significativas en los resultados adversos (parálisis cerebral, retraso cognitivo, ceguera bilateral o pérdida de audición que requiera audífonos) a los 36 meses. Las limitaciones de los presentes hallazgos incluyen un tamaño de muestra global relativamente pequeño.
Conclusiones de los autores
La administración de arginina a los lactantes prematuros puede impedir el desarrollo de la ECN. Debido a que la información fue proporcionada por tres pequeños ensayos que incluyeron 285 participantes, los datos no son suficientes en la actualidad para apoyar una recomendación para la práctica. Se necesita un estudio multicéntrico controlado y aleatorizado que se centre en la incidencia de la ECN, especialmente en las etapas más graves (2 y 3).
PICO
Resumen en términos sencillos
Agregado de arginina para prevenir la enterocolitis necrosante en lactantes prematuros
¿Cuál es el problema? La enterocolitis necrosante (ECN) es una afección en la que la inflamación daña el tracto gastrointestinal del lactante. La tasa de ECN varía entre el 4% y el 22% en los lactantes de muy bajo peso al nacer. La enterocolitis necrosante puede ser causada por la inmadurez del lactante, la falta de irrigación sanguínea en el tracto gastrointestinal y la destrucción de la superficie (mucosa) como resultado de una infección o de la alimentación con fórmula. Para proteger el tracto gastrointestinal, el cuerpo produce una sustancia natural (óxido nítrico) a partir del aminoácido arginina. Se ha informado de que las concentraciones de arginina plasmática son bajas en los lactantes de muy bajo peso al nacer y en los lactantes prematuros que desarrollan ECN. Añadir más arginina a la solución alimenticia puede prevenir la ECN.
¿Por qué esto es importante? La ECN puede provocar daños permanentes en el intestino, la necesidad de múltiples cirugías, la prolongación de la estancia hospitalaria, la muerte y el aumento del coste del sistema sanitario.
¿Qué evidencia se encontró? Los autores de la revisión buscaron en la literatura estudios controlados que evaluaran la eficacia y la seguridad de la administración de suplementos de arginina. El agregado adicional de arginina a la alimentación del lactante prematuro redujo el riesgo de ECN en tres estudios de buena calidad que incluyeron a 285 lactantes nacidos con menos de 34 semanas de gestación. Tuvieron que ser tratados seis lactantes, para que uno de ellos se beneficiara del tratamiento. Los investigadores no informaron de efectos secundarios significativos directamente atribuibles al exceso de arginina en los primeros 28 días, y un estudio no informó de un retraso en el desarrollo a largo plazo (36 meses). Los posibles efectos de la administración de suplementos de arginina incluyen la disminución de la presión arterial y cambios en el control de la glucosa en la sangre.
¿Qué quiere decir esto? La administración de suplementos de arginina puede reducir la incidencia y la gravedad de la ECN en los lactantes prematuros. Los resultados son limitados, ya que los estudios incluyeron sólo unos pocos pacientes. Se necesita un gran estudio que incluya a lactantes de múltiples centros para verificar estos hallazgos.
Authors' conclusions
Summary of findings
| Arginine compared with placebo for prevention of necrotising enterocolitis | ||||||
| Patient or population: prevention of necrotising enterocolitis in preterm infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Arginine | |||||
| NEC any stage | 303 per 1000 | 115 per 1000 | RR 0.38 (0.23 to 0.64) | 285 | ⊕⊕⊕⊝ | |
| Stage 1 NEC | 110 per 1000 | 41 per 1000 | RR 0.37 | 285 | ⊕⊕⊕⊝ | |
| Stage 2 NEC | 138 per 1000 | 70 per 1000 | RR 0.51 | 285 | ⊕⊕⊕⊝ | |
| Stage 3 NEC | 55 per 1000 | 7 per 1000 | RR 0.13 | 285 | ⊕⊕⊕⊝ | |
| Mortality due to any cause | 124 per 1000 | 94 per 1000 | RR 0.77 (0.41 to 1.45) | 285 | ⊕⊕⊝⊝ | |
| Side effects ‐ hypotension | 104 per 1000 | 107 per 1000 | RR 1.03 (0.41 to 2.59) | 192 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effects of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMinor sources of indirectness included (1) age and weight of included neonates (< 32 weeks and < 1250 grams vs < 34 weeks and < 1500 grams); (2) placebo composition (saline vs 5% glucose vs no treatment). These were not deemed by review authors to warrant downgrading of recommendations. | ||||||
Background
Description of the condition
Bell 1978 reported that necrotising enterocolitis (NEC) occurs in three different stages; this report was later modified (Walsh 1986). Clinical features of NEC vary from constitutional symptoms such as feeding intolerance to severe systemic symptoms including cardiorespiratory decompensation, coagulopathy, peritonitis and ascites with or without pneumoperitoneum. As the result of marked variability in clinical manifestations, the reported incidence of NEC varies from 4% to 22% among very low birth weight infants at various centres (Stoll 1994).
The exact cause of NEC is unknown. Suspected pathophysiological mechanisms include immaturity, ischaemia, disruption of mucosal integrity, formula feeding, hyperosmolar load to intestine, infection and bacterial translocation (Caplan 2001). Animal experiments have shown that subsequent to ischaemia, intestinal permeability is increased (Langer 1993), and this may lead to passage of bacteria and endotoxins into the intestinal wall, affecting mucosal integrity. Intestinal dysmotility and disruption in mucosal integrity play important roles in the pathogenesis of NEC (Di Lorenzo 1995).
Nitric oxide is important for normal gastrointestinal (GI) function from several perspectives. First, nitric oxide is an important regulator of vasomotor function (Stark 1992). An inadequate concentration of nitric oxide leads to vasoconstriction of the intestinal vessels, which might result in ischaemia and a predisposition to NEC. Second, nitric oxide acts as a neurotransmitter for enteric non‐adrenergic non‐cholinergic neurons that regulate peristalsis (Boeckxstaens 1991). Lack or inadequacy of nitric oxide can alter intestinal motility. Third, nitric oxide inhibits leucocyte adherence and modulates inflammatory responses to various insults within the intestine (Akisu 2002). Therefore, normal concentrations of nitric oxide must be maintained within the GI tract. Owing to its volatile nature, nitric oxide cannot be delivered to the GI tract in its original form. An indirect method of achieving adequate concentration involves supplementing substrates such as arginine to promote production.
Description of the intervention
Arginine is an amino acid that is a precursor for proteins (Wu 1998) and nitric oxide synthase. The availability of plasma arginine is important for formation of nitric oxide (Castillo 1995; Zamora 1998). Plasma arginine concentration was found to be 95 ± 25 μmol/L in term breast‐fed (N = 16) infants (Wu 1986). In extremely low birth weight infants (birth weight < 1000 grams) who were given formula feeds (N = 2), the mean arginine concentration was found to be 37 μmol/L (range 13 to 60 μmol/L), and the mean arginine concentration was 53 μmol/L (range 3 to 116 μmol/L) in breast‐fed (N = 9) infants (Ventura 1987). In a case control study (Becker 2000), researchers found that arginine and glutamine levels were similar at day 3, but were significantly lower at days 7 and 14 in infants who developed NEC. Zamora 1997 reported statistically significantly reduced plasma arginine concentrations in preterm infants who developed NEC compared with control infants, even after adjustments were made for intake of arginine and day of life. Recommended minimum and maximum concentrations of arginine in preterm infant formula are 72 mg/100 kcal and 104 mg/100 kcal, respectively (Klein 2002). These requirements are greater in infants who are stressed owing to higher utilisation in conditions such as NEC or pulmonary hypertension. The concentration of arginine found in formula today is 47 to 51 mg/100 kcal (according to manufacturer specifications). Therefore, preterm infants have low levels of arginine and low intake. Supplementation of arginine may help prevent NEC by promoting nitric oxide synthesis.
How the intervention might work
In an experimental model of hypoxaemia/reoxygenation‐induced NEC in mice, dietary supplementation of arginine had a protective effect, as evidenced by lower injury scores on histopathological examination (Akisu 2002). In a piglet model of NEC, continuous infusion of arginine reduced the severity of intestinal injury (Di Lorenzo 1995). However, in a rat model of ischaemia‐reperfusion injury, investigators found that nitric oxide did not play a protective role in the immature or newborn intestine (Chan 2002). Therefore, conflicting results have been reported when animal experiments were conducted to determine the role of nitric oxide in the pathogenesis of NEC.
Arginine supplementation can cause alterations in glucose homeostasis (both hypoglycaemia and hyperglycaemia) and histaminergic side effects (Vosatka 1994). Researchers found that inhibition of nitric oxide synthesis reduced hypotension in rats with endotoxin‐mediated septic shock; thus promotion of nitric oxide synthesis in infants with sepsis may lead to irreversible septic shock (Thiemermann 1990). This makes it imperative that any human studies of arginine supplementation to prevent NEC should be rigorously evaluated for both benefit and risk.
Why it is important to do this review
The objective of this review was to evaluate the efficacy and safety of arginine supplementation in decreasing the incidence of all stages of NEC among preterm neonates. As the rise in plasma levels of arginine depends on the dose of arginine used, we planned a subgroup analysis based on the dose of arginine administered. In addition, as the baseline incidence of NEC is low among infants after 32 weeks' gestation, we planned a subgroup analysis based on this cut‐off for the purpose of examining the efficacy of arginine in infants of lower gestational age.
Objectives
To examine the effect of arginine supplementation (administered by any route) on the incidence of NEC in preterm neonates. To conduct subgroup analyses based on the dose of arginine and the gestational age of participants (≤ 32 weeks, > 32 weeks).
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials.
Types of participants
Preterm infants before 37 weeks' gestation at birth.
Types of interventions
Arginine supplementation (in addition to what an infant may be receiving from an enteral or parenteral source) versus placebo or no treatment administered orally or parenterally for at least seven days to achieve plasma arginine levels at or above the upper end of the normal range (145 μmol/L in term breast‐fed infants).
Types of outcome measures
Primary outcomes
Incidence of NEC (any stage and specific stage (1, 2 or 3)) based on Bell's criteria (Bell 1978; Walsh 1986) and diagnosed before discharge.
Secondary outcomes
-
Death before discharge
-
Death attributed to NEC at any time
-
Surgery for NEC (including placement of peritoneal drains)
-
Duration of total parenteral nutrition administration (days)
-
Plasma concentrations of arginine and glutamine (μmol/L) measured at least every seven days after the start of supplementation
-
Side effects of arginine supplementation
-
Systemic hypotension, defined as mean blood pressure below the mean for corrected gestational age during the period of intervention
-
Alteration in glucose homeostasis (number of infants with blood glucose levels < 2.6 mmol/L or > 8 mmol/L during the period of intervention)
-
Post hoc secondary outcomes
Neurodevelopmental outcomes among survivors (including incidences of cerebral palsy, cognitive delay, blindness and deafness), patent ductus arteriosus (PDA), intraventricular haemorrhage (IVH) grades III and IV, faecal calprotectin levels and median age at NEC diagnosis were included on the basis of outcomes reported in the included studies.
Search methods for identification of studies
For the May 2016 update, we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library; MEDLINE via PubMed (from 1996 to 12 May 2016); Embase (from 1980 to 12 May 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; from 1982 to 12 May 2016) and used the following search terms: (L‐Arginine OR Arginine, L‐Isomer OR Arginine, L Isomer OR L‐Isomer Arginine OR DL‐Arginine Acetate, Monohydrate OR DL Arginine Acetate, Monohydrate OR Monohydrate DL‐Arginine Acetate), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry) for ongoing and recently completed trials.
For previous publications of this review, review authors searched MEDLINE (from 1966 to May 2016) using the following medical subject heading (MeSH) terms: enterocolitis necrotizing; enterocolitis; enteritis; colitis; enterocolitis, pseudomembranous; enterocolitis, acute; infant, premature; infant, newborn; infant, premature, disease; clinical trials; randomised controlled trials; random allocation; prospective studies; and arginine.
Review authors searched other databases as well, including Embase (from 1980 to May 2016); CINAHL (from 1982 to May 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 4) in the Cochrane Library; the reference lists of identified trials; and abstracts from annual meetings of the Society for Pediatric Research, the American Pediatric Society and Pediatric Academic Societies, which were published in Pediatric Research (1991 to 2016). Review authors applied no language restrictions.
Review authors excluded the following types of articles: letters, editorials/commentaries, reviews and lectures.
We updated the first search in August 2010, and again in May 2016. See Appendix 2.
We searched clinical trials registries (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp) for ongoing and recently completed trials.
Data collection and analysis
We employed standard methods and followed Guidelines of the Cochrane Neonatal Review Group in creating this update.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. Two review authors (LK, PS) reviewed search results and separately selected studies for inclusion. Review authors resolved disagreements by discussion or with involvement of the third review author (VS).
Data extraction and management
We assessed retrieved articles and independently abstracted data. We resolved discrepancies between review authors by reaching consensus.
Assessment of risk of bias in included studies
Two review authors used the Cochrane ‘Risk of bias’ tool (Higgins 2011) to independently assess risk of bias (low, high or unclear) of all included trials according to the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessors (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a detailed description of risk of bias for each domain.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Measures of treatment effect
We performed all statistical analyses using Review Manager software. We analysed categorical data according to risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB). We analysed continuous data using weighted mean difference (WMD) and reported the 95% confidence interval (CI) for all estimates.
Assessment of heterogeneity
We planned to estimate treatment effects in individual trials and to examine heterogeneity between trials by inspecting forest plots and quantified the impact of heterogeneity by using the I2 statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by performing post hoc subgroup analyses.
Data synthesis
If appropriate, we planned to perform meta‐analysis by using Review Manager software (RevMan 5) supplied by Cochrane. For estimates of typical risk ratio and risk difference, we planned to use the Mantel‐Haenszel method. For measured quantities, we planned to use the inverse variance method. We planned to perform meta‐analyses by using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We performed unplanned subgroup analyses based on stage of NEC (stage 1, 2 or 3) because of the subjective nature of the diagnosis of stage 1 NEC. We planned to perform subgroup analysis to investigate arginine concentrations based on gestational age. We have reported heterogeneity scores (I2 values) for all analyses.
Results
Description of studies
We identified three eligible studies ‐ Amin 2002, Polycarpou 2013a and El‐Shimi 2015 (Figure 1). We have provided clinical details of participants, interventions and outcomes in the Characteristics of included studies table.

Study flow diagram: review update.
In Amin 2002, investigators randomised preterm neonates at ≤ 32 weeks and at ≤ 1250 grams to receive L‐arginine (1.5 mmol/kg/d) or placebo (normal saline) in equivalent volume. Researchers added L‐arginine to the parenteral nutrition solution until infants were able to tolerate more than 40% of feeds, after which investigators provided L‐arginine or placebo supplementation via the enteral route. Researchers administered study medication at between two and five days of age and continued the medication until 28 days of age. The goal was to increase plasma arginine concentrations from a baseline level of 99 μmol/L to 164 μmol/L (upper limit of normal in healthy full‐term breast‐fed infants was 148 μmol/L). Other clinical management decisions were left to the clinical care team responsible for the participants. Investigators enrolled a total of 152 participants (75 in the L‐arginine group and 77 in the placebo group).
The primary outcome assessed in Amin 2002 was the incidence of NEC (stage 1, 2 and 3, according to Bell 1978). Secondary outcomes assessed included incidences of various stages of NEC, death due to NEC, death due to any cause and concentrations of arginine, glutamine and ammonia at baseline and at 14 and 28 days of age. Both groups recorded incidences of IVH, sepsis, hypotension and PDA and followed up all survivors until completion of the study (28 days of age). They reported long‐term outcome data in abstract format and published a report of the follow‐up study in 2009 (Amin 2002).
In Polycarpou 2013a, researchers randomised preterm neonates at ≤ 34 weeks and at ≤ 1500 grams to receive L‐arginine (1.5 mmol/kg/d) or placebo (5% glucose in equivalent volume). They provided L‐arginine in liquid form in two equal doses, with nasogastric tube enteral feeds from three to 28 days of age. Investigators commenced enteral feeds at 10 to 25 mL/kg/d on the first day of life and increased these feeds on day 4 up to a maximum tolerance of 150 to 180 mL/kg/d. This study enrolled a total of 83 participants (40 in the L‐arginine group and 43 in the placebo group).
The primary outcome in Polycarpou 2013a was the incidence of NEC (all stages according to Bell 1978) in the first three months of life. Secondary outcomes assessed included age at NEC diagnosis and age at death due to NEC. Both groups evaluated the incidences of IVH, respiratory distress and PDA. Researchers monitored the intervention group for arginine‐related side effects (diarrhoea, vomiting, low blood pressure and hypo/hyperglycaemia). A previous report revealed levels of faecal calprotectin in these 83 neonates (Polycarpou 2013b).
In El‐Shimi 2015, researchers randomised 75 neonates born at ≤ 34 weeks to three study arms: arginine (N = 25), glutamine (N = 25) and no treatment (control; N = 25). They administered L‐arginine orally or via nasogastric tube, starting with the introduction of enteral feeds until day 30 of postnatal life. The primary outcome assessed in El‐Shimi 2015 was the incidence of any stage of NEC from the time of enrolment to discharge, death or completion of 30 days of life.
These studies were conducted in three different settings: Canada (Amin 2002), Egypt (El‐Shimi 2015) and Greece (Polycarpou 2013a). The baseline population was ≤ 32 weeks in Amin 2002 and ≤ 34 weeks in the remaining two studies. However, baseline rates of any NEC were 17% (Amin 2002), 18% (El‐Shimi 2015) and 30% (Polycarpou 2013a). Thus, we anticipated a degree of clinical heterogeneity in the results. However, we found little methodological heterogeneity between studies (all of which were relatively well‐conducted RCTs) and lower risk of bias. Thus, we decided to pool results statistically and to explore heterogeneity if identified by using a random‐effects model.
Risk of bias in included studies
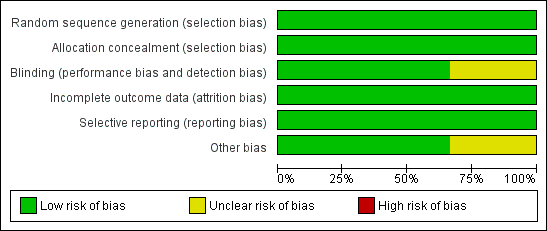
For studies included in the review, we have provided assessments of methodological quality in the Characteristics of included studies table and have summarised findings in Figure 2 and Figure 3. We extracted methodological details of each study by reviewing published information and by contacting the primary author.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In Amin 2002, the pharmacist performed randomisation centrally (information provided by study author ‐ Dr Amin). In this study, allocation was concealed and the intervention was masked, so that investigators, parents and caretakers were unaware of treatment allocation. Cross‐over was not allowed (information provided by study author ‐ Dr Amin) and contamination was not possible because the study drug was not available outside the trial. Outcome assessors (at various stages of NEC before study completion) were blinded and were not aware of treatment allocation. All randomised patients were accounted for in the analysis of outcomes.
Amin 2002 reported outcomes among survivors at 36 months' postmenstrual age. Individuals who assessed outcomes at follow‐up were blinded to the intervention. Among 145 infants eligible for follow‐up, 135 (93%) were followed.
In Polycarpou 2013a, the randomisation sequence was computer generated, as described in Polycarpou 2013b. A pharmacist prepared intervention and control solutions in non‐transparent bottles at equal volumes, meaning that nurses who administered the dilutions were blinded to the allocated intervention. Neonatalogists and radiologists evaluating x‐rays of the abdomen were also blinded to the treatment arm. All participants were accounted for in the analysis of outcomes. Investigators included an additional evaluation of neonates whose parents declined consent.
Researchers in El‐Shimi 2015 used a simple, computer‐generated randomisation approach and concealed allocation (information obtained from study author ‐ Dr Khafagy). Participants, attending physicians, nurses and data analysts were blinded to the group to which babies were assigned (information obtained from study author ‐ Dr Khafagy). Investigators reported all outcomes and accounted for all randomised participants in the analysis of outcomes. It is difficult to understand blinding of nurses and doctors involved in the study to interventions, as the control group received no intervention. We contacted study authors to request clarification but received no response.
Effects of interventions
Arginine supplementation versus placebo
Primary outcomes
Incidence of NEC (at any stage according to Bell 1978) among all randomised participants (Outcome 1.1)
Results showed a statistically significant reduction in risk of development of NEC (any stage) in the arginine group as compared with the placebo group (RR 0.38, 95% CI 0.23 to 0.64; N = 285; three studies; I2 = 27%) (RD ‐0.19, 95% CI ‐0.28 to ‐0.10; N = 285; three studies; I2 = 0%). The number of participants needed to treat with arginine for an additional beneficial outcome of preventing development of NEC among preterm infants was 6 (95% CI 4 to 10). It should be noted that investigators in Amin 2002 followed neonates for 28 days, and El‐Shimi 2015 for 30 days; Polycarpou 2013a followed neonates for three months (Analysis 1.1).
We have reported below unplanned subgroup analyses according to various stages of NEC.
NEC stage 1 (according to Bell 1978 staging criteria) (Outcome 1.2)
Results showed a statistically significant reduction in risk of developing NEC stage 1 (RR 0.37, 95% CI 0.15 to 0.90; N = 285; three studies; I2 = 52%) (RD ‐0.07, 95% CI ‐0.14 to ‐0.01; N = 285; three studies; I2 = 0%). The number of participants needed to treat with arginine to prevent development of stage 1 NEC among preterm infants was 15 (95% CI 8 to 100) (Analysis 1.2).
NEC stage 2 (according to Bell 1978 staging criteria) (Outcome 1.3)
Results showed no reduction in risk of developing NEC stage 2 (RR 0.51, 95% CI 0.25 to 1.06; N = 285; three studies; I2 = 2%) (RD ‐0.07, 95% CI ‐0.14 to 0.00; N = 285; three studies; I2 = 51%) (Analysis 1.3).
NEC stage 3 (according to Bell 1978 staging criteria) (Outcome 1.4)
Results for stage 3 NEC were different depending upon the type of estimate used for analysis because none of the participants in two studies (Amin 2002 and El‐Shimi 2015) developed NEC stage 3. Thus the RR for developing NEC stage 3 (RR 0.13, 95% CI 0.02 to 1.03; N = 285; three studies; I2 = 0%) was not significantly different but the risk difference was statistically significantly different (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; N = 285; three studies; I2 = 89%). The risk difference revealed that the number of patients needed to treat with arginine to prevent development of stage 3 NEC among preterm infants was 20 (95% CI 12 to 100) (Analysis 1.4).
Secondary outcomes
Mortality (Outcome 1.5)
Death due to any cause
In Amin 2002, three deaths were reported in the arginine group and none in the placebo group. Polycarpou 2013a reported eight deaths in the arginine group and 12 in the placebo group. In El‐Shimi 2015, two deaths occurred in the arginine group and six in the control group. Combined results from all three studies showed no differences in risk of mortality between the two groups (RR 0.77, 95% CI 0.41 to 1.45; N = 285; three studies; I2 = 42%) (RD ‐0.03, 95% CI ‐0.10 to 0.04; N = 285; three studies; I2 = 79%). Clinical heterogeneity was noted in the populations included in these three studies, with Amin 2002 reporting overall mortality of 2%, El‐Shimi 2015 16% and Polycarpou 2013a 25% in the entire cohort (Analysis 1.5).
Death due to NEC
Results showed a statistically significant reduction in risk of death due to NEC (RR 0.18, 95% CI 0.03 to 1.00; N = 285; three studies; I2 = 0%) (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; N = 285; three studies; I2 = 87%). The number of patients needing treatment with arginine to prevent NEC‐related mortality was 20 (95% CI 12 to 100) (Analysis 1.5).
Surgery for NEC
In all three included studies, none of the participants in either group required surgical intervention for NEC.
Duration of total parenteral nutrition administration
Data on this outcome are not available.
Plasma concentrations of arginine and glutamine at baseline and after the start of supplementation (Outcomes 1.6, 1.7)
In Amin 2002, plasma arginine concentrations at baseline before supplementation were not significantly different between the two groups (MD ‐10 μmol/L, 95% CI ‐23.85 to 3.85). However, plasma arginine concentrations were statistically significantly higher in the group supplemented with arginine at 14 days (MD 70.00 μmol/L, 95% CI 50.40 to 89.60) and at 28 days of age (MD 79.00 μmol/L, 95% CI 57.09 to 100.91). Plasma glutamine concentrations at baseline before supplementation (MD ‐42 μmol/L, 95% CI ‐91.88 to 7.88) and at 14 days after supplementation (MD 27.00 μmol/L, 95% CI ‐22.88 to 76.88) were not statistically significantly different between the two groups; however, results showed a statistically significant difference (higher in the arginine‐supplemented group) in plasma glutamine concentrations at 28 days of age (MD 82.00 μmol/L, 95% CI 17.72 to 146.28).
El‐Shimi 2015 evaluated serum arginine concentrations at baseline, at day 14 and at time of NEC diagnosis. Baseline arginine levels were significantly lower in the arginine group (6.1 ng/mL vs 8.4 ng/mL; P = 0.009). In both groups, plasma arginine levels significantly increased over the 14‐day period. Baseline and day 14 arginine levels were reported in El‐Shimi 2015 as median and interquartile range (IQR). Study authors clarified that the single value reported for IQR was the difference between the 25th and 75th centiles, but without values for the IQR, we were unable to pool these data points.
Side effects of arginine supplementation (Outcome 1.8)
Outcome 1.8.1 ‐ Hypotension
In Amin 2002, results showed no statistically significant difference in risk of developing hypotension after 24 hours of age between treatment and placebo groups (RR 1.03, 95% CI 0.41 to 2.59; RD 0.00, 95% CI ‐0.05 to 0.06; N = 285; three studies; I2 = 0%). Polycarpou 2013a and El‐Shimi 2015 reported that hypotension was not observed in neonates receiving arginine (Analysis 1.8).
Outcome 1.8.2 ‐ Alteration in glucose homeostasis
In Amin 2002, Polycarpou 2013a and El‐Shimi 2015, no participants in either group developed hypoglycaemia during the study period (RD 0.00, 95% CI ‐0.02 to 0.02; N = 285; three studies; I2 = 0%) (Analysis 1.8).
Review authors did not undertake planned subgroup analyses based on gestational age and dose of arginine owing to lack of available data.
Post hoc secondary outcomes (Outcomes 1.9 to 1.18)
Cerebral palsy (Outcome 1.9)
At 36‐month follow‐up, results showed no statistically significant differences in risk of cerebral palsy between the two groups (RR 0.88, 95% CI 0.21 to 3.80; N = 135; one study; I2 = 0%).
Cognitive delay (cognitive index < 70) (Outcome 1.10)
At 36‐month follow‐up, results showed no statistically significant differences in risk of cerebral palsy between the two groups (RR 0.78, 95% CI 0.14 to 4.55; N = 135; one study; I2 = 0%).
Visual impairment (Outcome 1.11)
None of the infants in either group developed visual impairment.
Deafness (Outcome 1.12)
At 36‐month follow‐up, results showed no statistically significant differences in risk of deafness requiring a hearing aid between the two groups (RR 3.52, 95% CI 0.15 to 84.98; N = 135; one study; I2 = 0%).
Major neurodevelopmental disability (Outcome 1.13)
Results showed no statistically significant differences in risk of major neurodevelopmental disability between the two groups (RR 0.65, 95% CI 0.23 to 1.83; N = 132; one study; I2 = 0%) at 36 months.
Patent ductus arteriosis (Outcome 1.14)
Results showed no statistically significant differences in the incidence of PDA (RR 0.96, 95% CI 0.75 to 1.24; N = 285; three studies; I2 = 0%).
Intraventricular haemorrhage (Outcome 1.16)
Results showed no statistically significant differences in risk of grade II or III IVH (RR 0.85, 95% CI 0.43 to 1.68; N = 235; two studies; I2 = 0%).
Faecal calprotectin (Outcome 1.17)
Polycarpou 2013a measured effects of arginine on intestinal inflammation, as estimated by faecal calprotectin levels and reported no statistically significant differences between groups in calprotectin levels at day 3 (MD 32.10, 95% CI ‐114.31 to 178.51), at day 14 (MD 55.80, 95% CI ‐46.13 to 157.73) and at day 28 (MD ‐13.40, 95% CI ‐105.05 to 78.25).
Age at NEC diagnosis (Outcome 1.18)
Only median values for age at NEC diagnosis were reported. Amin 2002 reported a borderline significantly higher median age at diagnosis of 20 days in the arginine group (vs 10 days in the control group; P = 0.049). El‐Shimi 2015 reported a mean age at diagnosis of 10.7 ± 2.5 days in the arginine group (9.8 ± 5.2 days in the control group), which was not significantly different. Polycarpou 2013a reported a median age at diagnosis of 17 days in the arginine group and 15 days in the control group, which was not significantly different. Without variance measures for two of the studies (Amin 2002; Polycarpou 2013a), we were unable to pool these outcomes.
Discussion
We conducted an extensive literature search to identify trials that might qualify for inclusion in this review. However, we identified only three trials (Amin 2002; El‐Shimi 2015; Polycarpou 2013a). The number of neonates included in this analysis was 285. It appears from these studies that arginine may be effective in preventing necrotising enterocolitis (NEC).
The overall quality of the included studies was good, suggesting that extracted data were valid. However, review authors noted some issues of concern. In Amin 2002, the incidence of NEC in the control group was 27.3% ‐ a rate well above that (5% to 10%) reported by studies including similar populations. Study authors included stage 1 NEC as an outcome, but diagnosis of this disorder is highly subjective and non‐specific. Use of stage 1 NEC as an outcome in a prophylactic study is questionable owing to the inherent possibility of over‐diagnosis (i.e. labelling cases as NEC when patients do not have NEC). Because of masking in this study, it is unlikely that any such over‐diagnosis was biased. Factors responsible for the high incidence of NEC at this centre are unknown. When only participants with stage 2 NEC were analysed, a trend towards reduction that was not statistically significant was noted. Lack of significance of these post hoc analyses may be due to reduced sample size at each stage. Follow‐up reports reveal no increase in risk of neurodevelopmental impairment among survivors.
In El‐Shimi 2015, neonates who developed NEC were of significantly lower gestational age and weight. Owing to the small sample size, study authors were unable to apply regression modelling, which presented a limitation in interpretation, as the effects of arginine cannot be isolated. Results of this study showed a significant baseline imbalance in the control group, which included smaller neonates (based on birth weight) with significantly higher baseline arginine levels. A larger sample size may have resulted in groups that were more comparable.
Based on data provided by the three included studies, we have downgraded recommendations for the use of arginine to prevent NEC to moderate. The included studies are of good methodological design, are consistent in their findings regarding the overall incidence of NEC and appear free of publication bias. We noted minor sources of indirectness related to populations examined in the included studies (< 32 weeks vs < 34 weeks) and to composition of the comparator (placebo‐glucose vs placebo‐saline vs no treatment); however, we believe this would not affect the overall strength of the recommendation. We downgraded evidence as the result of imprecision resulting from the relatively small sample size. We graded evidence on the use of arginine supplementation to reduce mortality to low owing to clinical heterogeneity in baseline mortality rates, likely reflecting variation in population demographics and perinatal health services across regions.
Arginine supplementation for prevention of NEC could be an important avenue for further research. However, the sample size required for prevention of stage 2 or 3 NEC will be significantly larger than the sample previously studied (for an absolute risk reduction of 50% from a baseline incidence of 6%, with an alpha of 0.05 and power of 0.8, each arm would have to include 750 participants), requiring a multi‐centre design. The fact that researchers in Amin 2002 were able to demonstrate reduced concentrations of arginine among patients who develop NEC compared with those who do not develop NEC points towards the possibility of increased utilisation or reduced absorption of the substrate, thereby supporting the rationale for undertaking such a study. Conversely, El‐Shimi 2015 reported similar arginine levels at the time of enrolment among neonates who developed NEC compared with those who did not. The findings of El‐Shimi 2015 may be confounded by baseline imbalances and significantly higher arginine concentrations in the control group at the time of enrolment.
Results showed no significant side effects with supplementation of arginine at a dose of 1.5 mmol/kg, despite arginine levels that were marginally higher than those of normal breast‐fed term newborn infants. This finding further supports the rationale for future research.

Study flow diagram: review update.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Arginine versus placebo, Outcome 1 NEC any stage.

Comparison 1 Arginine versus placebo, Outcome 2 Stage 1 NEC.

Comparison 1 Arginine versus placebo, Outcome 3 Stage 2 NEC.

Comparison 1 Arginine versus placebo, Outcome 4 Stage 3 NEC.

Comparison 1 Arginine versus placebo, Outcome 5 Mortality.

Comparison 1 Arginine versus placebo, Outcome 6 Plasma arginine concentrations (μmol/L).

Comparison 1 Arginine versus placebo, Outcome 7 Plasma glutamine concentrations (μmol/L).

Comparison 1 Arginine versus placebo, Outcome 8 Side effects.

Comparison 1 Arginine versus placebo, Outcome 9 Cerebral palsy.

Comparison 1 Arginine versus placebo, Outcome 10 Cognitive delay (cognitive index < 70).

Comparison 1 Arginine versus placebo, Outcome 11 Visual impairment.

Comparison 1 Arginine versus placebo, Outcome 12 Deafness.

Comparison 1 Arginine versus placebo, Outcome 13 Major neurodevelopmental disability.

Comparison 1 Arginine versus placebo, Outcome 14 PDA (%).

Comparison 1 Arginine versus placebo, Outcome 15 IVH grade III or IV.

Comparison 1 Arginine versus placebo, Outcome 16 Faecal calprotectin (μg/g).
| Study | Age of NEC diagnosis, median (SD if available) (Arginine group) | Age of NEC diagnosis, median (SD if available) (Control group) | Reported p‐value |
| Amin 2002 | 20 days | 10 days | 0.049 |
| El‐Shimi 2015 | 10.67 (2.5) days | 9.83 (5.2) days | NS |
| Polycarpou 2013a | 17 days | 15 days | |
Comparison 1 Arginine versus placebo, Outcome 17 Median age at NEC diagnosis.
| Arginine compared with placebo for prevention of necrotising enterocolitis | ||||||
| Patient or population: prevention of necrotising enterocolitis in preterm infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Arginine | |||||
| NEC any stage | 303 per 1000 | 115 per 1000 | RR 0.38 (0.23 to 0.64) | 285 | ⊕⊕⊕⊝ | |
| Stage 1 NEC | 110 per 1000 | 41 per 1000 | RR 0.37 | 285 | ⊕⊕⊕⊝ | |
| Stage 2 NEC | 138 per 1000 | 70 per 1000 | RR 0.51 | 285 | ⊕⊕⊕⊝ | |
| Stage 3 NEC | 55 per 1000 | 7 per 1000 | RR 0.13 | 285 | ⊕⊕⊕⊝ | |
| Mortality due to any cause | 124 per 1000 | 94 per 1000 | RR 0.77 (0.41 to 1.45) | 285 | ⊕⊕⊝⊝ | |
| Side effects ‐ hypotension | 104 per 1000 | 107 per 1000 | RR 1.03 (0.41 to 2.59) | 192 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effects of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMinor sources of indirectness included (1) age and weight of included neonates (< 32 weeks and < 1250 grams vs < 34 weeks and < 1500 grams); (2) placebo composition (saline vs 5% glucose vs no treatment). These were not deemed by review authors to warrant downgrading of recommendations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NEC any stage Show forest plot | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.64] |
| 2 Stage 1 NEC Show forest plot | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| 3 Stage 2 NEC Show forest plot | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.06] |
| 4 Stage 3 NEC Show forest plot | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.03] |
| 5 Mortality Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mortality due to any cause | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 5.2 Mortality due to NEC | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.03, 1.00] |
| 6 Plasma arginine concentrations (μmol/L) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Baseline | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐23.85, 3.85] |
| 6.2 At day 14 | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | 70.0 [50.40, 89.60] |
| 6.3 At day 28 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 79.0 [57.09, 100.91] |
| 7 Plasma glutamine concentrations (μmol/L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Baseline | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐40.00 [‐91.88, 7.88] |
| 7.2 At day 14 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 27.0 [‐22.88, 76.88] |
| 7.3 At day 28 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [17.72, 146.28] |
| 8 Side effects Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Hypotension | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.41, 2.59] |
| 8.2 Alteration in glucose homeostasis | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Cerebral palsy Show forest plot | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.21, 3.80] |
| 10 Cognitive delay (cognitive index < 70) Show forest plot | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.14, 4.55] |
| 11 Visual impairment Show forest plot | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Deafness Show forest plot | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [0.15, 84.98] |
| 13 Major neurodevelopmental disability Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.83] |
| 14 PDA (%) Show forest plot | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.24] |
| 15 IVH grade III or IV Show forest plot | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.43, 1.68] |
| 16 Faecal calprotectin (μg/g) Show forest plot | 1 | 249 | Mean Difference (IV, Random, 95% CI) | 20.13 [‐41.66, 81.92] |
| 16.1 At day 3 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 32.10 [‐114.31, 178.51] |
| 16.2 At day 14 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 55.80 [‐46.13, 157.73] |
| 16.3 At day 28 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐13.40 [‐105.05, 78.25] |
| 17 Median age at NEC diagnosis Show forest plot | Other data | No numeric data | ||

