Sistemas de financiamiento de la atención sanitaria para el aumento del uso del tratamiento del tabaquismo
Appendices
Appendix 1. Specialised Register Search Strategy
Search strategy used for Specialised Register (using Cochrane Register of Studies (CRS) software)
#1 MeSH DESCRIPTOR Insurance Explode All
#2 MeSH DESCRIPTOR Insurance Coverage Explode All
#3 MeSH DESCRIPTOR Insurance, Health Explode All
#4 MeSH DESCRIPTOR Reimbursement Mechanisms Explode All
#5 MeSH DESCRIPTOR Insurance, Health, Reimbursement Explode All
#6 MeSH DESCRIPTOR social control policies Explode All
#7 MeSH DESCRIPTOR health care costs Explode All
#8 MeSH DESCRIPTOR Quality of Health Care Explode All
#9 MeSH DESCRIPTOR Fee‐for‐Service Plans Explode All
#10 MeSH DESCRIPTOR Physician Incentive Plans Explode All
#11 MeSH DESCRIPTOR Costs and Cost Analysis Explode All
#12 MeSH DESCRIPTOR Cost‐Benefit Analysis Explode All
#13 health care costs
#14 health insurance
#15 coverage*:AB,TI
#16 reimburse*
#17 payment*
#18 remunerat*
#19 incentive*
#20 salary or salaries
#21 fee or fees
#22 deductible*
#23 co?insurance
#24 co?payment
#25 capita*
#26 fund?hold*
#27 prepay or prepaid
#28 financ* NEAR incentive*:AB,TI
#29 cost? NEAR (shar* or free or no):TI,AB
#30 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 or #28
Appendix 2. MEDLINE search strategy
The following topic related terms were combined with MeSH and free text terms concerning smoking and tobacco use, and with terms to identify trials and other evaluations of healthcare effects used by Cochrane Tobacco Addiction for regular searches of MEDLINE. (See Specialized Register section of Tobacco Addiction Group Module). The free text term 'time series' was included in the trials identification set.
exp Insurance, Health, Reimbursement/ or exp Insurance/ or exp Insurance Coverage/ or exp Insurance, Health/ or exp Reimbursement Mechanisms/ or exp Insurance, Health, Reimbursement/ or exp social control policies/ or exp health care costs/ or "Quality of Health Care"/ec or exp Fee‐for‐Service Plans/ or exp Managed Care Programs/ or exp Physician Incentive Plans/ or exp Employee Incentive Plans/ or (coverage or reimburs$ or target$ or payment$ or remunerat$ or incentive$ or financ$ or salar$ or fee or fees or deductible$ or coinsurance or copayment or capita$ or cost$ or payment$ or fundhold$ or prepay$ or prepaid).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
Appendix 3. Glossary of terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, may be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation'. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' |
| Continuous abstinence | Also called 'sustained abstinence' |
| 'Cold turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support |
| Craving | A very intense urge or desire [to smoke]. |
| Dopamine | A neurotransmitter in the brain that regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size' |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | Neural nicotinic acetylcholine receptors |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine replacement therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free. The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. See prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke (ETS) |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC (Summary of Product Characteristics) | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Tar | The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Varenicline | A pharmaceutical drug prescribed to treat nicotine addiction; trade names Chantix and Champix |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. |
Appendix 4. Quality assessment of economic evaluations
| Item | An 2008 | Curry 1998 | Halpin 2006 | Hughes 1991 | Joyce 2008 | Kaper 2006a | Salize 2009 (Twardella 2007) | Schauffler 2001 |
| 1. Is the study population clearly described? | no | no | yes | yes | yes | yes | yes | no |
| 2. Are competing alternatives clearly described? | yes | yes | yes | yes | yes | yes | yes | yes |
| 3. Is a well‐defined research question posed in answerable form? | yes | yes | yes | yes | yes | yes | yes | yes |
| 4. Is the economic study design appropriate to the stated objective? | yes | yes | yes | yes | yes | yes | yes | yes |
| 5. Is the chosen time horizon appropriate in order to include relevant costs and consequences? | no | yes | yes | yes | no | yes | yes | yes |
| 6. Is the actual perspective chosen appropriate? | no | yes | yes | yes | no | yes | yes | no |
| 7. Are all important and relevant costs for each alternative identified? | no | no | no | no | no | yes | yes | no |
| 8. Are all costs measured appropriately in physical units? | no | no | no | no | no | yes | no | no |
| 9. Are costs valued appropriately? | no | no | no | no | no | yes | no | no |
| 10. Are all important and relevant outcomes for each alternative identified? | no | no | no | no | no | no | no | no |
| 11. Are all outcomes measured appropriately? | no | no | yes | yes | no | yes | yes | no |
| 12. Are outcomes valued appropriately? | yes | yes | yes | yes | no | yes | yes | yes |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | no | no | no | no | yes | yes | yes | no |
| 14. Are all future costs and outcomes discounted appropriately? | yes | yes | yes | yes | no | yes | yes | yes |
| 15. Are all important variables appropriately subjected to sensitivity analysis? | no | no | no | no | no | no | yes | no |
| 16. Do the conclusions follow from the data reported? | yes | yes | yes | no | no | yes | yes | yes |
| 17. Does the study discuss the generalisability of the results to others settings/ patients? | Yes | no | yes | yes | no | no | yes | yes |
| 18. Does the article indicate that there is no potential conflict of interest of researchers and funders? | yes | no | yes | no | yes | yes | yes | no |
| 19. Are ethical and distributional issues discussed appropriately? | no | no | no | no | no | no | no | no |
| Total score | 8 | 8 | 12 | 10 | 6 | 15 | 15 | 8 |
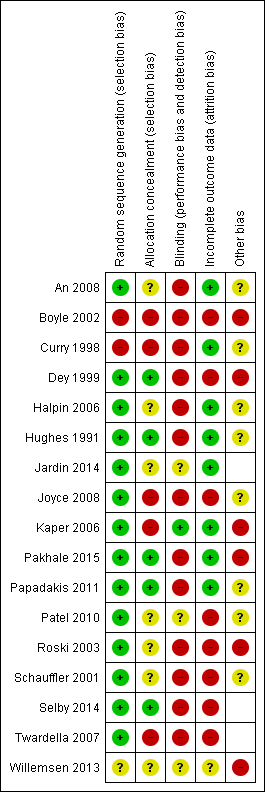
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
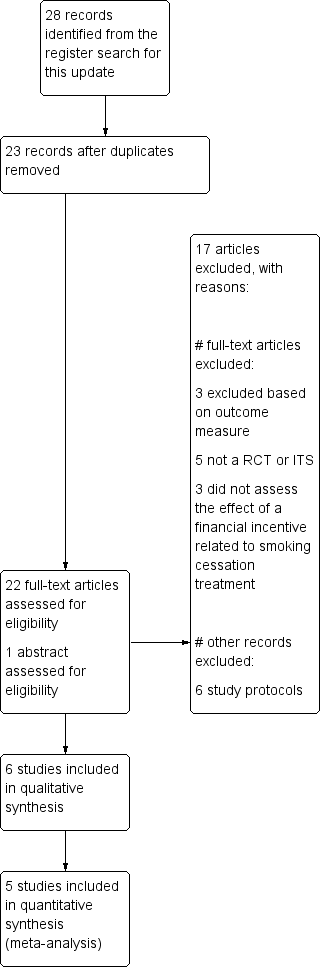
Prisma study flow diagram of studies included in most recent update of this review

Comparison 1 Interventions directed at individuals: abstinence from smoking, Outcome 1 Full versus no financial coverage.

Comparison 1 Interventions directed at individuals: abstinence from smoking, Outcome 2 Full versus partial financial coverage.

Comparison 1 Interventions directed at individuals: abstinence from smoking, Outcome 3 Partial versus no financial coverage.
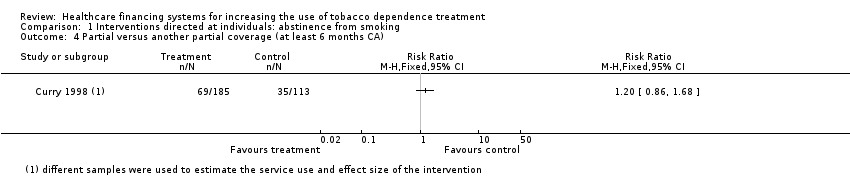
Comparison 1 Interventions directed at individuals: abstinence from smoking, Outcome 4 Partial versus another partial coverage (at least 6 months CA).
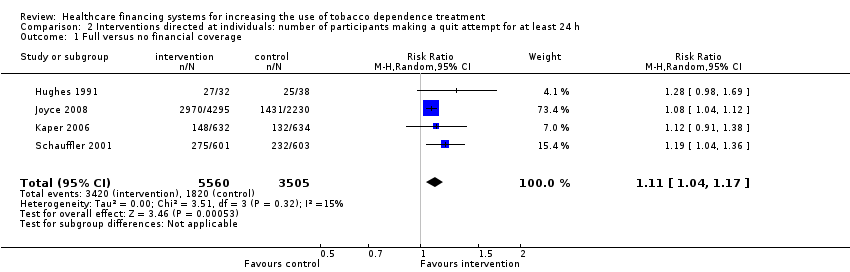
Comparison 2 Interventions directed at individuals: number of participants making a quit attempt for at least 24 h, Outcome 1 Full versus no financial coverage.
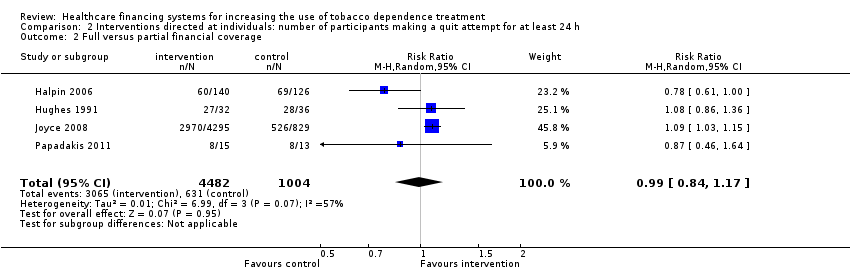
Comparison 2 Interventions directed at individuals: number of participants making a quit attempt for at least 24 h, Outcome 2 Full versus partial financial coverage.

Comparison 2 Interventions directed at individuals: number of participants making a quit attempt for at least 24 h, Outcome 3 Partial versus no financial coverage.

Comparison 3 Interventions directed at individuals: use of smoking cessation treatment, Outcome 1 Full versus no financial coverage.

Comparison 3 Interventions directed at individuals: use of smoking cessation treatment, Outcome 2 Full versus partial financial coverage.

Comparison 3 Interventions directed at individuals: use of smoking cessation treatment, Outcome 3 Partial versus no financial coverage.
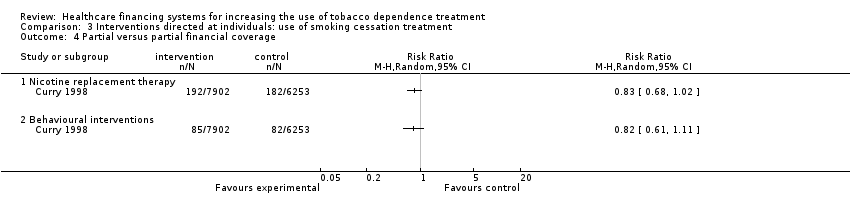
Comparison 3 Interventions directed at individuals: use of smoking cessation treatment, Outcome 4 Partial versus partial financial coverage.
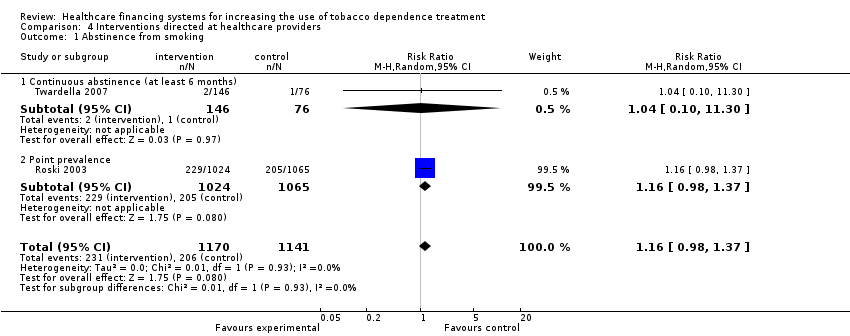
Comparison 4 Interventions directed at healthcare providers, Outcome 1 Abstinence from smoking.

Comparison 4 Interventions directed at healthcare providers, Outcome 2 Use of nicotine replacement therapy and/or bupropion.
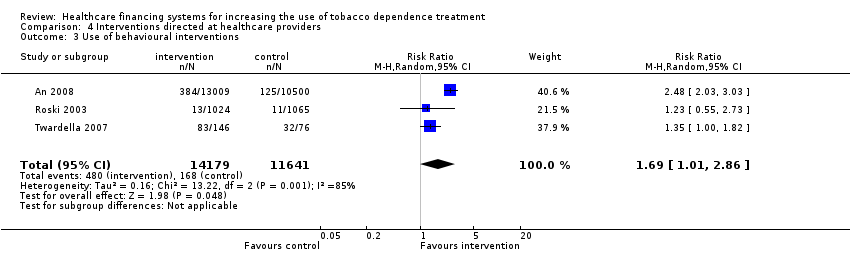
Comparison 4 Interventions directed at healthcare providers, Outcome 3 Use of behavioural interventions.
| Interventions directed at individuals: full financial coverage compared to no financial coverage for increasing abstinence from smoking | ||||||
| Patient or population: smokers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no coverage | Risk with full financial coverage | |||||
| Abstinence from smoking | Study population | RR 1.77 | 9333 | ⊕⊕⊕⊝ | ||
| 84 per 1000 | 149 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias: all studies except for Hughes 1991 had a serious risk of bias. | ||||||
| Interventions directed at healthcare providers compared to no interventions for increasing the use of smoking cessation treatment | ||||||
| Patient or population: physicians and clinics from a multispecialty group practice | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no interventions | Risk with interventions directed at healthcare providers | |||||
| Abstinence from smoking | Study population | RR 1.16 | 2311 | ⊕⊕⊕⊝ | ||
| 181 per 1000 | 209 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because both studies were judged to be at serious risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full versus no financial coverage Show forest plot | 6 | 9333 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.37, 2.28] |
| 1.1 Continuous abstinence (at least 6 months) | 2 | 1486 | Risk Ratio (M‐H, Random, 95% CI) | 4.38 [1.94, 9.87] |
| 1.2 Point prevalence abstinence | 4 | 7847 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.45, 1.86] |
| 2 Full versus partial financial coverage Show forest plot | 5 | 5914 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.48] |
| 2.1 Continuous abstinence (at least 6 months) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.50, 9.35] |
| 2.2 Point prevalence abstinence | 4 | 5886 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.44] |
| 3 Partial versus no financial coverage Show forest plot | 5 | 7108 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.02, 1.59] |
| 3.1 Continuous abstinence (at least 6 months) | 2 | 3707 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.45] |
| 3.2 Point prevalence | 3 | 3401 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.98, 2.24] |
| 4 Partial versus another partial coverage (at least 6 months CA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full versus no financial coverage Show forest plot | 4 | 9065 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.04, 1.17] |
| 2 Full versus partial financial coverage Show forest plot | 4 | 5486 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.17] |
| 3 Partial versus no financial coverage Show forest plot | 5 | 6944 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.98, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full versus no financial coverage Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Nicotine replacement therapy | 7 | 9455 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.54, 2.09] |
| 1.2 Bupropion | 3 | 6321 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.41, 7.34] |
| 1.3 Behavioural interventions | 4 | 9215 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.19, 2.65] |
| 1.4 Pharmacotherapy, not specified | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.68] |
| 2 Full versus partial financial coverage Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Nicotine replacement therapy | 4 | 22380 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.27, 2.43] |
| 2.2 Bupropion | 2 | 3700 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.84, 2.41] |
| 2.3 Behavioural interventions | 1 | 16922 | Risk Ratio (M‐H, Random, 95% CI) | 3.95 [3.15, 4.95] |
| 2.4 Pharmacotherapy, not specified | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.02] |
| 3 Partial versus no financial coverage Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Nicotine replacement therapy | 5 | 6944 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.91] |
| 3.2 Bupropion | 3 | 6765 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.03, 1.29] |
| 3.3 Varenicline | 1 | 1380 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.68, 2.03] |
| 3.4 Behavioural interventions | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.22, 2.71] |
| 4 Partial versus partial financial coverage Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Nicotine replacement therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Behavioural interventions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence from smoking Show forest plot | 2 | 2311 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.98, 1.37] |
| 1.1 Continuous abstinence (at least 6 months) | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.10, 11.30] |
| 1.2 Point prevalence | 1 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.98, 1.37] |
| 2 Use of nicotine replacement therapy and/or bupropion Show forest plot | 2 | 2311 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.18] |
| 3 Use of behavioural interventions Show forest plot | 3 | 25820 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.01, 2.86] |

