استفاده از چسبهای بافتی برای بستن برشهای جراحی
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT with 3 months follow‐up. 72 participants randomised, but only 60 had 3‐month outcome data collected and reported | |
| Participants | 72 participants receiving minimally invasive thyroidectomy using either video‐assisted thyroidectomy or minimal incision thyroidectomy Surgery performed in 1 centre in Ireland. All cases were performed by the senior surgeon Exclusion criteria: autoimmune thyroiditis, diabetes mellitus and/or poor general health Trial conducted in Dublin, Ireland | |
| Interventions | Group 1 (n = 38; results reported for 33): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 34; results reported for 27): staples | |
| Outcomes | Cosmetic appearance by participant and by surgeon (at 3 months) using the Manchester scar scale Patient satisfaction (self assessed at 3 months): collected using a 10 cm VAS line where 0 was poor and 10 was excellent. Data for overall patient satisfaction score (n = 60) was calculated by the review authors using summary data presented in the study report. The patient satisfaction assessment form also measured: cosmesis; ability to shower same day (as operation); need to visit GP for wound; pain on removing clips; pain/tightness of wound; overall wound comfort and allergic reactions. Only ability to shower data was reported in addition to overall satisfaction score ‐ ability to shower data are not presented here | |
| Notes | Cosmetic appearance outcome data not clearly presented for participants or surgeons. Authors contacted Study also reports pain at 1 and 10 days after surgery ‐ not reported here Cost of tissue adhesive reported as EUR22 ‐ no corresponding data for staples | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed prior to commencement of the study as follows: Opaque envelopes were numbered sequentially from 1 to 75. A table of random numbers was generated by a computer program and used for group assignment; if the last digit of the random number was from 0 to 4, the assignment was to Group A (tissue adhesive), and if the last digit was from 5 to 9, the assignment was to Group B (staples)." Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignments were then placed into the opaque envelopes and the envelopes sealed. As eligible participants were entered into the trial, these envelopes were opened in sequential order to give each patient his or her random group assignment. The envelopes were opened by the operating surgeon following patient consent and just prior to the surgical procedure." Comment: use of sealed, numbered opaque envelopes considered robust |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Blinding was not feasible at the time of skin closure, nor was it of any benefit at the two‐week postoperative visit, as wound cosmesis at this stage is not considered predictive of the long‐term cosmetic outcome." Comment: understandably difficult to blind operating surgeon and participants to the intervention.Staff would have been aware of allocation on wound closure and during short‐term post‐operative assessment ‐ however none of these outcomes are reported here. Participants were not blinded. Unclear whether this would have led to bias in the study in terms of satisfaction in favour of 1 treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinding was achieved during evaluation of the surgical wound at or after three months postoperatively as surgeons were unaware of the wound closure method used. A three‐month appointment was arranged for each participant for wound evaluation by a surgeon who was not involved in the patient management." Comment: blinded outcome assessment undertaken at 3 months for surgeon cosmetic outcome data although we were unable to extract these data for the review. Participant assessment not blinded (as noted above) |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Sixty out of the 72 patients agreed to come back for the three‐month postoperative evaluation. The remainder did not attend do to the long travelling [sic]/commuting distance to the tertiary service" Comment: data from only 60 of the 72 participants randomised were available at 3 months. Also possible differential attrition (adhesive 13%, staples 21%). Deemed to be at unclear risk of bias |
| Selective reporting (reporting bias) | High risk | Comment: multiple dimensions of patient satisfaction assessed with ability to shower selectively reported ‐ data not presented here. Study protocol not sought |
| Other bias | Low risk | None noted |
| Methods | RCT with 40 participants with 40 days follow‐up | |
| Participants | 40 women undergoing the Pfannenstiel incision Exclusion criteria: known allergy to trial products Conducted in Turkey | |
| Interventions | Group 1 (n = 20): high viscosity 2‐octylcyanoacrylate (High Viscocity Dermabond®; Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) tissue adhesive Group 2 (n = 20): polypropylene sutures All participants had skin cleansed with povidone iodine and 1 g antibiotic prophylaxis | |
| Outcomes | Wound infection (at 2, 7 and 40 days after surgery: 7‐day data used for analyses, as these data were most clear from the translation) Time for skin closure Participant satisfaction at day 40. Participants were asked how satisfied they were with their skin closure they could select from the following responses: very bad, poor, average, good, very good | |
| Notes | Data extraction based on English language abstract and partial translation of report text that was published in Turkish The study reports an outcome ‐ wound disruption ‐ as yet we have been unable to translate whether this can be interpreted as wound dehiscence Cometic appearance not used in this review as assessed at less than 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unable to gauge from translation |
| Allocation concealment (selection bias) | Unclear risk | Comment: unable to gauge from translation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: unable to gauge from translation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unable to gauge from translation |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unable to gauge from translation. We acknowledge risk of selection bias from reporting infection outcome data from the time point where statistical significance was observed |
| Selective reporting (reporting bias) | Unclear risk | Comment: unable to gauge from translation |
| Other bias | Unclear risk | Comment: unable to gauge from translation |
| Methods | RCT with 1‐month follow‐up | |
| Participants | 217 adults requiring any skin incision closure 4 cm or greater in length. Multicentre study conducted at: Department of Plastic Surgery, University Hospital Gent, Gent, Belgium; Intitute Mutualiste Montsouris, Paris, France; Crestwood Hospital, Huntsville, AL, USA; Gynecologic Oncology Research and Development, LLC, Greenville, SC, USA; Naval Medical Centre, San Diego, CA, USA; University Dental Hospital, Manchester, UK Exclusion criteria: a history of peripheral vascular disease; insulin‐dependent diabetes; a blood‐clotting disorder or taking anticoagulants within 7 days of surgery; concurrent use of steroids; or a personal or family history of keloid formation or hypertrophy; impaired wound healing; or a known allergy to cyanoacrylate or formaldehyde | |
| Interventions | Group 1 (n = 106): high viscosity 2‐octylcyanoacrylate (High Viscocity Dermabond®; Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) tissue adhesive Group 2 (n = 103): any other commercially available device such as sutures, staples, or tapes or compared with low viscosity 2‐octylcyanoacrylate (n = 42; Dermabond®, Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) | |
| Outcomes | Wound dehiscence, infection, patient satisfaction and surgeon satisfaction at 10 days | |
| Notes | Cosmetic appearance data not used as measured at less than 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . patients at each study site were randomised by computer generated code in a 1:1 ratio to epidermal incision closure with high viscosity 2‐octylcyanoacrlate or a commercially available device" Comment: use of computer generated code constitutes low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes containing the concealed treatment allocations were opened in the operating room immediately before epidermal closure." Comment: judgement of unclear risk of bias made as not clear whether envelopes were sequentially numbered and opaque. Not clear who was responsible for allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Blinding to type of treatment was not feasible", due to the nature of the personnel‐led intervention Comment: understandably difficult to fully blind participants and personnel to intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "On day 10, wounds were assessed for adequate progress in healing, wound‐related infection, and indicators of an acute inflammatory reaction." Similarly, " . . . on day 10, physicians completed a questionnaire that measured satisfaction with each use of a device.", also, “a counterpart questionnaire [given to patients] for patient satisfaction for cosmesis, overall comfort, ability to shower, dressing changes, tension at the wound, hygienic problem, allergic reaction, and overall satisfaction" Comment: there is no mention that the wound was assessed by a blinded assessor. Such assessment would be difficult for satisfaction scores ‐ as noted above |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The study was not completed by 4 patients assigned to high viscosity group 2 octylcyanoacrylate because of voluntary withdrawal (n=2), or loss to follow up (n=2) and by 6 patients to the control devices because of voluntary withdrawal (n=2), loss to the follow up (n=3), or death (n=1) [attributed to Burkitt's Lymphoma]". Comment: the numbers were fairly small and were fairly evenly distributed between the treatment arms. Therefore we do not believe this represented a significant risk of bias |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias were detected |
| Methods | Parallel RCT with 6 weeks follow‐up. No withdrawals reported | |
| Participants | 134 children undergoing inguinal herniorrhaphy (ages ranged from 1 month to 12 years) All operations performed by 1 of 4 paediatric surgeons No other inclusion or exclusion data reported Undertaken in 1 centre in the USA | |
| Interventions | Group 1 (n = 64): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 70): sutures (5.0 Monocryl) Group numbers calculated from data in paper as not reported directly | |
| Outcomes | Wound dehiscence (6 weeks) Time for skin closure Relative costs of materials required for skin closure | |
| Notes | Surgeon cosmetic appearance data not used as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Just before wound closure, a sealed envelope indicating randomization to skin adhesive or suture closure was revealed." Comment: no information about how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Just before wound closure, a sealed envelope indicating randomization to skin adhesive or suture closure was revealed." Comment: no information about whether envelopes were opaque and sequential and who prepared or opened these. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Masking as to skin adhesive vs suture closure was not possible for the operating surgeon because of the nature of the intervention." Comment: understandably difficult to blind operating surgeon and participants to the intervention. Staff would have been aware of allocation on wound closure |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "However, subsequent interviewers were masked as to group during assessment of cosmetic outcome measures, as well as those related to efficiency, cost, and complications of wound closure." Comment: deemed at low risk of bias for debridement and costs outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No report of any loss to follow‐up Comment: judged as low risk |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias were detected |
| Methods | RCT with 1‐month follow‐up | |
| Participants | 86 healthy male patients under the age of 12 years requiring elective circumcision. Study conducted at Duchess of Kent Hospital, Hong Kong. No specific exclusion criteria were described | |
| Interventions | Group 1 (n =40): butylcyanoacrylate (Histoacryl®) tissue adhesive Group 2 (n = 46): 4.0 catgut sutures | |
| Outcomes | Dehiscence and infection at days 1, 2, 3, 7 and 30. Cosmetic appearance, bleeding and wound inflammation were also assessed at these time points. Bleeding and wound inflammation were not of interest to this review | |
| Notes | Cosmetic appearance data were not used as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . [patients were] randomised into two groups" Comment: no method of random sequence generation was actively discussed; therefore the risk of bias for this domain is unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: Patients were " . . . randomised into two groups" as previously mentioned Comment: no method of allocation concealment was made clear. Therefore, the risk of bias is again unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether the participants or personnel were blinded to the intervention Comment: as the participants were under the age of 12 and under general anaesthesia, it is reasonable to assume that they were blinded to the intervention. However, the personnel were probably not blinded as they carried out the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The wounds of all the patients were then assessed on day 1, day 2 and day 3, 1 week and 1 month after the operation. Wound inflammation, infection, bleeding, cosmetic result and dehiscence were assessed. A questionnaire was completed" Comment: no mention of whether the assessors of the wound and the cosmetic results were blinded to the intervention. It is not clear if the questionnaire was filled out by parents of the children, who may have been blinded to the intervention, or the assessors, who were likely to know which intervention was given. The judgement for this domain is therefore unclear |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotations, but no losses to follow‐up were reported Comment: therefore judged as low risk |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but the results account for the all the assessment methods identified to qualify the success of the intervention Comment: no evidence of reporting bias, therefore judged as low risk |
| Other bias | Unclear risk | Possible unit of analysis issue for dehiscence outcome |
| Methods | RCT with up to 12 months follow‐up, with assessment at 1, 3, 5‐7 and 14 days, then 1, 3, 6 and 12 months | |
| Participants | 40 participants undergoing elective cranial supratentorial surgery Exclusion criteria: undergoing a procedure for infective pathology; head trauma; admission to the intensive care unit; a dermatologic disease; previous cranial radiotherapy; diabetes mellitus; known blood‐clotting disorders; and allergy to cyanoacrylate products Undertaken in 1 centre in Italy | |
| Interventions | Group 1 (n = 20): n‐butyl‐cyanoacrylate (Liquiband®; MedLogic Global Ltd, Plymouth, Devon, UK) tissue adhesive Group 2 (n = 20): sutures (Monosof 2/0, Tyco United States Surgical, Norwalk, CT, USA) or staples (Auto Suture Appose ULC 35, Tyco United States Surgical) All participants received the same antibiotic prophylaxis (1 dose of 3rd‐generation cephalosporin 20 min prior to surgical incision) Wound dressings were not used for those allocated to tissue adhesive because this formed its own waterproof and antimicrobial wound dressing Participants in the tissue adhesive group (Group 1) were permitted to shower from the day after the surgical procedure, while those participants allocated to sutures were instructed to keep their wounds clean and dry until the removal of the sutures | |
| Outcomes | Wound dehiscence (collected to 7th day postoperatively) Wound infection (collected to 7th day postoperatively; infection not defined) Cosmetic appearance (nurse‐blinded) using modified Hollander Wound Score scale (up to 12 months) Skin closure time | |
| Notes | Cosmetic appearance (patient and surgeon) using a VAS of 1 to 10 where a score of 10 reflected optimal cosmetic outcome. It is not clear at what time points these data were collected. Authors were contacted Supplmentary material referenced in the main paper also checked | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"At the moment of skin closure, they [the patients] were randomly allocated into one of two groups (A or B) of 20 patients each." Comment: no reporting on how the patients were randomly allocated or the method used |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization sequence was arranged on the same day as surgery; each patient’s name was randomly assigned to one of 40 envelopes (20 marked as LiquiBand, 10 as TTS and 10 as SC)." Comment: no indication that the allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotations. Did not report that the participants or personnel were blinded to the intervention Comment: understandably difficult to blind the operating surgeon to the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All of the patients were followed up post‐operatively on days 1, 3, 5‐7 by the same ward nurse who initially recorded details regarding their wound aspects" "After discharge, a second nurse (not from the neurosurgical department), using the same scale, continued the follow‐up, initially at 2 weeks, and then at 1, 3, 6 and 12 months post‐operatively”. Noted in abstract that this second nurse was blinded Comment: although the study intimates that the nurse was not from the department and the second nurse was blinded it is not clear that the first nurse was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Only 39 patients (19 in group A and 20 in group B) were available at the 12 month follow up, as one patient in group A developed a post‐operative intracranial haematoma. This required an emergency evacuation procedure and the wound was subsequently closed with stitched" Comment: given that 39/40 participants had 12‐month data available, we made an overall judgment of low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias detected |
| Methods | Parallel group RCT with 3‐month follow‐up | |
| Participants | 168 patients recruited for laparoscopic procedures at Queen's Medical Centre, Nottingham. 154 participants included in the final analysis. Withdrawals were accounted for. Exclusion criteria included insulin dependant diabetes, prolonged corticosteroid use; known keloid scarring; wounds greater than 5cm in length without deep layer sutures; those undergoing emergency surgery; patients unable to give informed consent. | |
| Interventions | Group 1 (n = 76): n‐butyl‐cyanoacrylate (Liquiband®; MedLogic Global Ltd, Plymouth, Devon, UK) tissue adhesive Group 2 (n = 78): interrupted, non‐absorbable suture | |
| Outcomes | Time to closure, dehiscence, satisfaction, cosmesis and infection (we used 24 to 48 h figures to avoid unit of analysis issues for dehiscence and infection) | |
| Notes | Unclear reference to subcutaneous layers being dealt with. Authors contacted for mean and standard deviations of time to closure and cosmesis, but as we received no reply, this information was not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed using consecutively numbered, sealed envelopes by a person not involved with the study" Comment: although allocation was concealed, there was no specific mention of how the sequence was randomly generated |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed using consecutively numbered, sealed envelopes by a person not involved with the study" Comment: allocation was concealed adequately |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Participants in the trial were not informed of the wound closure method they had been allocated to until they had undergone surgery. The investigators were not blinded" Comment: the risk of performance bias was unclear as it was not possible to blind the investigators to the procedure they were carrying out |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were followed up at 24 to 48 hours, 4 to 6 weeks and 3 months postoperatively by C.C.D, A.D.G., or W.J.S. At these times it was documented if wounds showed any of the following characteristics; erythema, oedema, tenderness, inflammation, drainage/discharge and/or malodorous smell." Concurrently, at the 3 month cosmetic evaluation, "a blinded assessment by a qualified nurse or surgical registrar not involved in the study also being made at 3 months" Comment: adequate blinding of assessment at 3 months. Potential for high risk of detection bias for outcomes reported prior to this |
| Incomplete outcome data (attrition bias) | High risk | Flow chart suggests no loss to follow‐up in first 48 h postoperatively. At 4‐6 weeks 20 participants were lost to follow‐up in the suture arm and 15 in the adhesive arm. There was further loss to follow‐up at 3 months: the suture arm suffered a total loss of 14 (with 7 missing both the 4‐6 week and the 3‐month follow‐up), while the adhesive group had 8 missing the 3‐month follow‐up (with 6 missing both follow‐ups) Comment: relative high attrition of data from study for outcomes collected at later time points |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias identified |
| Methods | 4‐arm parallel RCT with 90 participants with 6 weeks of follow‐up (assessment at 24 h, 3 and 6 weeks). Only data on 75 of participants were reported | |
| Participants | Participants undergoing total knee arthroplasty Exclusion criteria: medical conditions or personal circumstances that would prevent participation and completion of physical therapy and follow‐up visits; current participation in another clinical trial; preoperative systemic infections; uncontrolled diabetes; diseases or conditions known to effect the wound healing process; known hypersensitivity to cyanoacrylate, formaldehyde, or the dye D&C Violet #2 (Aesculap, Inc, Center Valley, PA); prior knee hardware fixation devices; prior knee incisions greater than 9 cm, and arthrofibrosis as evidence by limited ROM of 80° or higher Study performed in 1 centre in the USA | |
| Interventions | Group 1 (n = 19): subcutaneous closure method: sutures at 1.5/cm; skin closure method: 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 18): subcutaneous closure method: sutures at 1.5/cm; skin closure method: butylcyanoacrylate tissue adhesive (Histoacryl Blue tissue adhesive B Braun Corp) Group 3 (n = 19): subcutaneous closure method: sutures at 1.0/cm; skin closure method: staples (Visistat 35W Stapler (Teleflex Corp) Group 4 (n = 19): subcutaneous closure method: sutures at 1.0/cm; skin closure method: Monocryl suture (poliglecaprone 25; Ethicon) Data on 15 participants were excluded from analysis data: it was not reported which trial groups these participants were from. We note there were slight difference to the procedures in each group for the method of closure of the sub‐cutaneous layer. Details for (1) sub‐cutaneous closure methods and (2) skin closure method are provided above. | |
| Outcomes | Wound dehiscence (24 h, 3 weeks, and 6 weeks postoperatively) Wound infection (24 h, 3 weeks, and 6 weeks postoperatively; infection not defined. We have taken the total number of infections reported over the 6‐week period, however, it is not clear whether some participants reported more than 1 infection as the number of infections was reported rather than number of people having an infection Relative costs of materials required for skin closure Skin closure time | |
| Notes | Surgeon cosmetic appearance data not used as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . the eligible subjects were randomly categorized (via a pseudo‐random number generator algorithm) into 1 of 4 cohorts" Comment: adequate random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: " . . . the eligible subjects were randomly categorized (via a pseudo‐random number generator algorithm) into 1 of 4 cohorts". The study also states, "The surgeon was blinded to the closure technique before and during the operation until subcutaneous closure" Comment: although sequence generation was adequate and there is evidence to suggest that allocation was concealed, there is not enough evidence to suggest how this was done and how allocation was concealed to the personnel |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The surgeon was blinded to the closure technique and during the operation until subcutaneous closure" Comment: although attempts were made to conceal the allocated treatment, it would be difficult to blind the operating surgeon to the intended intervention, therefore our judgement is unclear. No information was provided about the extent to which participants were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At each visit, a physical examination was conducted; and adverse events were recorded. The physical examinations after TKA included evaluation of peripheral edema, infection, and dehiscence as well as pain level (0‐10 scale) and cosmesis (100‐mm visual analogue scale (VAS)) evaluation by patient. General health and wellness were further evaluated by an SF‐12v2 survey" Comment: the extent to which the participants were blinded to the intervention is unclear; it is also unclear whether those conducting the examinations were aware of the treatment given. The outcome of cosmesis was evaluated before the our specified time‐frame for the purposes of our review. The judgement remains unclear in this case |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote:"Of the 90 subjects recruited, 15 were excluded because of screen failure; 6 were diagnosed with arthrofibrosis after surgery, 4 failed to follow preferred physical therapy, and 5 sustained unrelated co‐morbidities preventing study completion." "Consequently, a total of 75 subjects, 19 per cohort with the exception of 18 for the n‐butyl‐2 adhesive cohort, completed the study; and their data are presented in the following section." Comment: it seems that these participants were excluded post‐randomisation |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Unclear risk | Possible issues with clustering for infection outcome data |
| Methods | Randomised split‐body design study with 1‐month follow‐up. No participants lost to follow‐up | |
| Participants | 20 adults requiring bilateral blepharoplasty for functional or aesthetic indications (40 eyelids were treated). Procedure conducted at: Division of Otolaryngology ‐ Head and Neck Surgery, Plastic and Reconstructive Surgery, Standford University Medical Centre and Palo Alto Veterans Healthcare System, Palo Alto, California, and Department of Otolayngology ‐ Head and Neck Surgery, Cleveland Clinic Florida, Naples, USA. Used a blepharoplasty model with identical skin sites on the same participant and each participant acted as his or her own control. No specific exclusion criteria were described | |
| Interventions | Group 1 (n = 20): left or right upper eye lid incision closed with 2‐octylcyanoacrylate (Dermabond®, Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 20): other eyelid incision closed with 6.0 suture (10 fast‐absorbing gut or 10 polypropylene, Prolene, Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Blepharoplasties were closed on the tissue adhesive side by using Castroviejo forceps to approximate the skin edges in 15 participants and by using 3‐4 sutures as handles to facilitate apposition and eversion of edges in 5 participants | |
| Outcomes | Dehiscence, infection, patient satisfaction, and surgeon satisfaction at 1, 2, and 4 weeks. Time for closure at end of procedure | |
| Notes | Cosmetic appearance data could not be used as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient had been randomised to have either the right or left upper eyelid serve as the experimental closure [with adhesive] . . . and the opposite eyelid as the control with sutures" Comment: despite stating that each patient was randomised to treatment group, there was no specific description of how the randomisation process was done or achieved |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each patient had been randomised . . . " Comment: no description of how allocation was undertaken and whether it was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether participants or personnel were blinded to the intervention Comment: understandably difficult to blind surgeon to the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The photographs were shown to 5 observers blinded to the technique of closure" to evaluate wound quality post operatively." Comment: blinding of outcome assessment achieved for cosmetic appearance, which was not included in this review. Not clear whether blinded assessment was undertaken for other outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The 5 blinded observers using the visual analogue scale to rate the 40 treated eyelids did not find any statistically significant difference between the wound quality . . ." Comment: suggested that all participants were accounted for, as the study had 20 participants (40 eyelids). |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias detected |
| Methods | RCT in which participants (with multiple port wounds) were randomised. Follow‐up was for between 6 and 8 weeks. No withdrawals reported | |
| Participants | Participants undergoing laparoscopic cholecystectomy No other inclusion or exclusion information Undertaken in 1 UK centre | |
| Interventions | Group 1 (12 participants; 48 wounds): 2‐octylcyanoacrylate Group 2 (13 participants; 51 wounds): absorbable sutures Participants in the suture arms had a dressing placed over the wound. Those in the tissue adhesive arm did not require a dressing | |
| Outcomes | Skin closure time (not clear if these data were collected for multiple wounds on the same person) | |
| Notes | Outcome reporting was unclear, so not sure if results were reported for a reference wound for each participant or if outcome data from multiple wounds were collected Cosmetic appearance data could not be used in the review as measured at < 3 months Wound complications reported as an outcome ‐ but nature of the complications was not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by asking the patient to select an envelope out of a hat" Comment: although it seems efforts were in place to randomise patients, it is not wholly clear that a truly randomised sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Randomization was performed by asking the patient to select an envelope out of a hat" Comment: there was no mention of whether allocation was concealed to the investigators |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotations about whether the participants or personnel were blinded in this study. It would be difficult to blind personnel in a surgical procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | No direct quotations about whether the participants or personnel were blinded in this study. It would be difficult to blind personnel in a surgical procedure. The only outcome reported here relevant to the review was time to skin closure. It is not clear if this was assessed by a blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients were followed up. In the suture group one patient refused to have a photograph taken" Comment: adequate outcome data gathered |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Unclear risk | Based on information collected it is not clear whether data were presented for an incorrectly analysed cluster trial |
| Methods | RCT with 7‐month follow‐up. 3 participants lost to follow‐up: 1 from the suture group and 2 from the tissue adhesive group | |
| Participants | 43 people requiring groin incisions (for: inguinal hernia, femoral hernia, sapheno ligations, testicular operations and lymph node biopsies). Skin incisions were closed with either butylcyanoacrylate (Histoacryl) tissue adhesive or Dexon subcuticular suture. In bilateral operations the left side was closed with Histoacryl and the left with Dexon. Conducted at Burton General Hospital, Burton‐on‐Trent, UK | |
| Interventions | Group 1: butylcyanoacrylate (Histoacryl) tissue adhesive Group 2: Dexon suture on straight needle using anchoring knot both ends or opposing the wound with forceps | |
| Outcomes | Infection, inflammation, cosmetic appearance, wound closing time, wound comfort and haematoma | |
| Notes | Exclusion criteria were not stated. Cosmetic appearance data not used in the review, as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"The patients were randomised just prior to skin closure into two groups. Even numbers were closed with Dexon subcuticular suture (Dexon group) and odd numbers were closed with Histoacryl‐Blue tissue adhesive (Histoacryl)" Comment: whilst use of odd and even numbers is detailed in terms of how the randomisation was implemented there was no detail about how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Even numbers were closed with Dexon subcuticular suture (Dexon group) and odd numbers were closed with Histoacryl‐Blue tissue adhesive (Histoacryl)" Comment: no indication mention that this sequence was concealed from surgeons. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotation. Not reported whether participants or personnel were blinded to the intervention. It is possible that participants may have been aware of 2 different methods of closure. "When the patients had bilateral operations, the left side was closed with Histoacryl and the right side with Dexon". Comment: understandably difficult to blind operating surgeon to intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Cosmesis was assessed by an independent observer (the experienced clinic sister) on a scale of one to five . . . " "The patients and wounds were assessed at one week and one month postoperatively in the surgical outpatients clinic. A simple scheme was used to assess the wound . . ." Comment: blinding of outcome assessment achieved for wound appearance, but this was not used in this review. Blinding not clear with regard to the other outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3/43 participants were lost to follow‐up. Considered low risk of bias |
| Selective reporting (reporting bias) | Low risk | All the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | No other sources of bias detected |
| Methods | Parallel RCT in 433 participants with 3 months follow‐up | |
| Participants | 433 participants undergoing a range of laparoscopic procedures Exclusion criteria: known sensitivity to cyanoacrylates; pregnancy or breastfeeding; or conditions known to interfere with wound healing (no further information provided) 4 UK centres | |
| Interventions | Group 1 (216 participants; 636 treated wounds): high viscosity 2‐octylcyanoacrylate (High Viscocity Dermabond®; Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) tissue adhesive Group 2 (217 participants; 618 treated wounds): n‐butyl‐cyanoacrylate (Liquiband®) tissue adhesive (LiquiBand. MedLogic Global Ltd, Plymouth, Devon, UK) | |
| Outcomes | Wound dehiscence (evaluated at 14 days and 3 months postoperatively) Wound infection (evaluated at 14 days and 3 months postoperatively; infection not defined) Cosmetic appearance by surgeon (at 3 months) using a modified Hollander Wound Evaluation scale Participants' satisfaction with incisional wound closure ‐ options were 'satisfied' or 'dissatisfied' Sugeons' satisfaction with wound (considering expression, application, delivery and ease of use for product): options were 'satisfied' or 'dissatisfied' Skin closure time | |
| Notes | Unit of randomisation was person with some data reported per wound (with multiple port incisions on each participant) Cosmetic appearance by participants and surgeon (at 3 months). Participants and surgeons were asked to assess whether they were 'satisfied' or 'dissatisfied' with the overall appearance of the wound. Whilst this was referred to as satisfaction in the study we deemed it to be an unvalidated measure of cosmetic appearance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised into 1 of 2 treatment groups: LB or DB. Although the adhesive user was not masked, both the study subject and evaluators were blinded to the randomised study treatment assignment" Comment. it is unclear how the randomisation sequence was achieved. Judged as unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Subjects were randomised into 1 of 2 treatment groups: LB or DB. Although the adhesive user was not masked, both the study subject and evaluators were blinded to the randomised study treatment assignment" Comment: no method of allocation concealment described, therefore judged as unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The adhesive user was not masked, both the study subject and evaluators were blinded to the randomised study treatment assignment" Comment: this indicates that personnel were not blinded to the procedure, whilst participants were blinded. Given that this was a study investigating two adhesives, blinding might have been possible. The judgement is unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Wound cosmesis was evaluated by a masked panel at the 3‐month visit. Cosmesis was measured using a modified 6‐point Hollander Wound Evaluation Scale (HWES) score." Comment: adequate blinding of outcome assessment Quote: Satisfaction measures were obtained from different study participants throughout the course of this trial. First, the surgical user was asked to indicate his/her satisfaction with the expression, application, delivery as ease of use of products. The user marked on a sheet that he/she was either "satisfied" or "dissatisfied". At the 3‐month follow up visit, the masked evaluators were requested to state whether they were satisfied with the healing and the overall appearance of the incisions" Comment: satisfaction outcome blinded adequately Not clear if wound dehiscence and infection were blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote:"A total of 76 subjects withdrew from the study, primarily because they were lost to follow up, resulting in a loss to follow up rate of 17.6%. Final data analysis was performed on 373 subjects and a total of 1089 incisions" Comment: high number of missing data at the participant level |
| Selective reporting (reporting bias) | Low risk | All the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Unclear risk | Data presented and analysed as an individual trial, but this is a cluster trial (wounds clustered per person) |
| Methods | 3‐arm parallel trial with 187 participants. No withdrawals reported Follow‐up was classed as 'early', which seems to have been the first 3 days after surgery, and then 'late' which was between 8 and 12 weeks after surgery | |
| Participants | 187 participants undergoing either a total knee arthroplasty or a total hip arthroplasty Exclusion criteria: having a revision or with a previous incision in the operative field; a history of keloid formation; allergy to superglue; regular anticoagulation therapy; or an underlying malignancy The study was performed in 1 centre in Australia | |
| Interventions | Group 1 (n = 60): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 64): continuous 3.0 subarticular absorbable poliglecaprone suture (Monocryl, Johnson and Johnson) Group 3 (n = 63): skin staples | |
| Outcomes | Wound infection: (report states that"where cultures were positive or there was clinical evidence of cellulitis, the patients were treated with a course of antibiotics and recorded as having an ‘infection") data collected at 2 time points ‐ early and late Patient satisfaction with the techniques of skin closure was assessed with a VAS between 0 and 100, where 100 represented maximal satisfaction Skin closure time | |
| Notes | Cosmetic appearance data (surgeon) could not be used in this review as measured at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised using a computer‐generated method . . . " Comment: Adequate random sequence generation method utilised |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomised using a computer‐generated method stored in sealed identical opaque envelopes. Allocation took place in the operating theatre after closure of the deep layers. Enrolment, generation of the allocation sequence and assignment of the patients was performed by the lead author." Comment: not clear if envelopes were numbered or otherwise labelled or stored so that allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients and assessors remained blinded to the treatment allocated until the dressings were changed, prior to discharge. At follow‐up, the assessors were not informed of the technique of closure." Comment: no mention of whether the personnel were blinded to the procedure, although this would be understandably difficult for a surgical procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The patients and assessors remained blinded to the treatment allocated until the dressings were changed, prior to discharge. At follow‐up, the assessors were not informed of the technique of closure." |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotation, but no account of a loss to follow‐up, therefore judged as low risk |
| Selective reporting (reporting bias) | Low risk | No direct quotation, but no outcomes selectively reported, therefore judged as adequate |
| Other bias | Unclear risk | Possible issues with clustering for infection outcome data |
| Methods | Split‐body design RCT with 12 weeks of follow‐up. Data missing for 1 participant | |
| Participants | Adults undergoing upper lid blepharoplasty who had not previously undergone this procedure Participants were recruited from a singe site, Henry Ford Health System, USA 1 cosmetic surgeon performed whole procedure Exclusion criteria: taking salicylates and/or anticoagulants; taking oral retinoids in the last 3 months; an unexplained history of excessive bleeding; a history of acute glaucoma or Sjogren’s syndrome; having resurfacing or laser techniques for the eyelid | |
| Interventions | Group 1 (24 participants; 24 eyes): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (24 participants; 24 eyes): standard monofilament suture (6‐0 fast‐absorbing gut or polypropylene) The study authors randomised each participant to have either (1) one eye treated with adhesive and the other with gut sutures; or (2) one eye treated with adhesive and the other with polypropylene sutures; or (3) one eye treated with gut sutures and the other treated with polypropylene sutures. For this review the adhesive data and the suture data from the two separate 'sub‐studies' involving adhesives have been pooled together and treated as one study | |
| Outcomes | Wound dehiscence: assessed at 1 week Cosmetic appearance (surgeon‐rated): assessed at 1 month and 3 months (we report 3‐month data). Scoring performed using a scale of 1 (excellent wound healing, scar matches surrounding skin) to 5 (poor scar wound healing, does not match surrounding skin). This was a blinded assessment. The data presented here were calculated by the review authors from raw data presented in the study report | |
| Notes | The following outcome was reported, but was not thought to be a validated measure for cosmetic appearance, "Participant cosmetic evaluation: (thickness, width, texture, color change, overall cosmetic outcome) was assessed at 3 months. A composite score was calculated as the sum of the scores for thickness, width, texture, and color change." No clear information was reported on wound dehiscence No comparative baseline data reported Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"They [the patients] were randomised for treatment in three subgroups in which each eyelid was repaired with a different material" Comment: no indication that there was randomised sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "They [the patients] were randomised for treatment in three subgroups in which each eyelid was repaired with a different material" Comment: no indication allocation was concealed to the personnel |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotation, but no indication that there was blinding of the participant or the personnel during the procedure. However, there are obvious difficulties in blinding the operating surgeon to the procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Incidence of wound dehiscence and side effects of itching, bleeding, and pain were assessed at 1 week" Comment: no indication that this wound evaluation was undertaken by a blinded assessor Quote. [At 3 months] "A blinded physician assessed cosmetic outcome of wound closure technique using standardized photographs" Comment: adequate blinding of this outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | No direct information regarding drop out rates, however different numbers used in total participants at 1 month to those at 3 months. Therefore the judgement is unclear |
| Selective reporting (reporting bias) | High risk | Quote: "Incidence of wound dehiscence and side effects of itching, bleeding, and pain were assessed at 1 week" Comment: incidence of wound dehiscence not clearly reported in the results |
| Other bias | Low risk | None reported |
| Methods | A parallel RCT with follow‐up at 7 days and 6 weeks postoperatively. No missing data reported, but the number included in analysis was not clear | |
| Participants | 106 participants undergoing saphenous vein harvesting (for coronary artery bypass grafting). Participants were recruited from a single UK site Excluded patients with high risk of vein harvest failure (defined as those with varicose veins, those with small or thin legs and those who required emergency or repeat procedures) | |
| Interventions | Group 1 (n = 53): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 53): sutures (monofilament synthetic absorbable Biosyn 3–0; Covidien PLC, Dublin, Ireland) In the suture group the wound was dressed with Mepore dressing (Mölnlycke Health Care, Manchester, United Kingdom),and a pressure bandage was applied for 48 h In the adhesive group a pressure bandage and Steri‐Strips (3M, St Paul,MN) were applied to hold the edges together for 24 h | |
| Outcomes | Skin closure time | |
| Notes | Cosmetic appearance (surgeon‐reported) data not used in this review as measured at < 3 months An outcome that study authors referred to as a 'patient satisfaction score' was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation; as it was collected at 6 weeks after surgery it is not reported here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized randomization system was used to place patients into two groups of 53 each." Comment: judged as adequate evidence of sequence randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A computerized randomization system was used to place patients into two groups of 53 each." Comment: no indication that allocation was concealed from personnel |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotation or indication that either participants or personnel were blinded from the study. However it is understandably difficult for personnel to be blinded in this case as it is a surgical procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote:"Two surgeons independent of the study who were blinded to the type of skin closure rated photographs at the same time points using a visual analogue scale evaluating cosmetic appearance and the previously validated Hollander wound evaluation scale." Comment: although this would correlate to a perceived low risk of bias, the cosmesis outcome should be excluded at less than 3 months as per the systematic review protocol. No direct quotation available regarding blinding related to the time to skin closure study outcome |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote "We identified and excluded as additional 12 patients . . . " Comment: this quote is presented in the results. It is not clear if these were post‐randomisation exclusions |
| Selective reporting (reporting bias) | Low risk | All the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | None detected |
| Methods | RCT with a 3‐month follow‐up period. In total 13 participants did not contribute data at 3 months | |
| Participants | 90 people having a total hip replacement Exclusion criteria: revision total hip replacement; a previous incision in the operative field, local skin conditions such as psoriasis, eczema or dermatitis; history of keloid formation; underlying malignancy; peripheral vascular disease; insulin‐dependant diabetes; allergy to skin adhesive or metal staples Undertaken in 1 UK centre | |
| Interventions | Group 1 (n = 45): butylcyanoacrylate (LiquiBand®. MedLogic Global Ltd, Plymouth, Devon, UK) Group 2 (n = 45): staples (appose UCL 35W, Tyco, Norfolk, Connecticut) Data at 3 months only available for 38 in Group 1 and 39 in Group 2 thanks to missing data | |
| Outcomes | Wound infection: defined as participant requiring antibiotics specifically for suspected wound infection Cosmetic appearance (participant‐rated) at 3 months: used a 100 mm VAS where 0 = worst outcome and 100 = best outcome. Median rather than mean data presented for this outcome Cosemtic appearance (surgeon‐rated) at 3 months: used a 100 mm VAS where 0 = worst outcome and 100 = best outcome | |
| Notes | Data on participant satisfaction with scar and appearance of the wound in relation to expected appearance were also collected using a VAS scale (as above). Reviewers considered this to be a variation of cosmetic appearance and so it is not reported in the review Surgeons also reported cosmetic appearance using modified version of the Hollander wound evaluation score and modified Vancover scar score. The modified versions were deemed to be not validated (they only included 3 items) Time to wound closure data and ease of wound closure were collected on a sub‐set of 10 participants in each trial arm. There was no information about how these sub‐sets were selected, so these data could not be considered as data from an RCT per se and have not been presented here Follow‐up appointments took place a mean of 14.4 weeks after surgery (no evidence of significant difference between groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomisation was performed, using the sealed envelope method, which involved identical sealed envelopes containing a card stating either 'skin adhesive' or 'staple' being opened by an independent researcher on the day of surgery" Comment: no indication that adequate randomised sequence generation was performed |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed, using the sealed envelope method, which involved identical sealed envelopes containing a card stating either 'skin adhesive' or 'staple' being opened by an independent researcher on the day of surgery. The operating surgeon was blinded to the skin closure method until the patient was in theatre" Comment: although it was not indicated that the envelopes were numbered sequentially, there is reasonable evidence here to describe the allocation concealment as adequate, and as it was undertaken by an independent researcher it has been judged accordingly |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "All patients had the same post‐operative care pathways and were blinded to the method of skin closure until the dressings were changed post‐operatively" Comment: adequate blinding of participants, however study design unable to blind personnel effectively, as a surgical intervention required |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "A researcher collected information on the presence of oozing and wound infection, which was defined as the patient requiring antibiotics specifically for suspected wound infection. Patients whose wound was closed with staples had these removed between ten and 14 days post‐operatively" Comment: indication that those evaluating the wounds were not blinded to the procedure, however this is not explicit, therefore the judgment remains unclear Quote: "An orthopaedic surgeon (AWB) also evaluated the scars using the VAS for cosmetic appearance. Both the plastic and orthopaedic surgeon were blinded to which method of skin closure had been used to each patient, and to each others' scores" Comment: adequate blinding of cosmesis outcome |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "In all, 12 patients (of 90) were lost to follow‐up because of non‐attendance or cancellation of the three‐month outpatient appointment. These patients have been seen subsequently with no reported adverse occurrences, but were not included in the final analysis as the time point for the last follow‐up in the study was three months" Comment: impact of these missing data unclear |
| Selective reporting (reporting bias) | High risk | Quote: "Evaluation of the cosmetic appearance of the scars was completed by a plastic surgeon (CME) using a modified version of the Hollander wound evaluation score and the Vancouver scar score, a Likert Scale and a VAS". . Comment: The results section reports only the Vancouver scar score, the Likert Scale and the VAS . The Hollander wound evaluation score has been omitted the judgement here is of high risk of bias. |
| Other bias | Low risk | None reported |
| Methods | Randomised parallel group study with 16‐month follow‐up. There were no withdrawals, however 7 patients treated with paper tape and 3 with tissue adhesive were converted to the suture group | |
| Participants | 140 adults requiring elective laparoscopic surgery. Patients were excluded if they had undergone previous laparotomy or were pregnant. The study was undertaken at 2 centres, Department of Surgery, Academic Medical Centre, Amsterdam; and The Netherlands and Department of Surgery, Isala Clinics, Zwolle, the Netherlands | |
| Interventions | Group 1 (n = 48): octylcyanoacrylate (Dermabond®, Johnson & Johnson, Amersfoot, the Netherlands) tissue adhesive Group 2 (n = 42): 76 mm x 6 mm adhesive paper tape (SteriStrip® Bioplasty/Uroplasty, Geleen, the Netherlands) Group 3 (n = 50): intracutaneous poliglecaprone (Monocryl®) 4/0, Johnson & Johnson) interrupted sutures | |
| Outcomes | Infection, cosmetic appearance, and surgeon satisfaction at 2 weeks and 3 months, and costs. | |
| Notes | We wrote to the authors who confirmed that there were no withdrawals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to one of the three groups using a computer randomization" Comment: random sequence generation undertaken by computer |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were allocated to one of the three groups using a computer randomization" Comment: not reported whether this allocation was concealed from the operating surgeon |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether participants or personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "At follow up [at 10‐14 days and 3 months], the incidence of wound infection and cosmesis were scored. Patients were also asked to score their own cosmetic results. Surgical residents scored wound infection and cosmetic results, they were blinded to the method used for wound closure" Comment: this infers a low risk of detection bias at both intervals for outcomes assessed by surgeons |
| Incomplete outcome data (attrition bias) | Low risk | No direct relevant quotations, although some patients who underwent closure with adhesive tape or adhesive liquid had to be converted intra‐operatively to suture closure. These seem to be analysed in the groups to which they were randomised (intention‐to‐treat analysis) Comment: judgement of low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct relevant quotations, but all variables listed in the methods section are listed in the tables in the Results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were detected |
| Methods | Parallel RCT in 43 participants. Follow‐up was for 3 months (assessment at 2 weeks and 3 months after surgery). No withdrawals noted | |
| Participants | 43 participants undergoing a skin incision to remove skin cancer Exclusion criteria: known sensitivity to cyanoacrylates; wounds under high tension forces; wounds < 2 cm or > 5 cm in length; or participant pregnant or breastfeeding Study undertaken in USA, number of centres involved was unclear | |
| Interventions | Group 1 (n = 23): high viscosity 2‐octylcyanoacrylate (High Viscocity Dermabond®; Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) tissue adhesive Group 2 (n = 20): n‐butyl‐cyanoacrylate (Liquiband®) tissue adhesive (LiquiBand. MedLogic Global Ltd, Plymouth, Devon, UK) | |
| Outcomes | Wound dehiscence (2 weeks and 3 months after surgery ‐ no events reported at 3 months and 1 event only at 2 weeks, no unit of analysis issue) Wound infection (not defined) Cosmetic appearance at 3 months (blinded evaluators ‐ panel of 6 doctors) using 100 mm VAS scale where 0 = worst scar and 10 = best scar Cosmetic appearance at 3 months (participant‐reported) using VAS scale on worst scar (0) to best scar (10) Surgeon satisfaction (at time of wound closure) assessed using 100 mm VAS scales for: ease of use (0 = impossible; 10 = very easy to use) and satisfaction with device and the closure achieved (0 = completely dissatisfied; 10 = completely satisfied) | |
| Notes | Wound infection listed as outcome, but not clearly reported Cosmetic appearance assessed by blinded evaluator was also measured using Modifed Hollander Wound Evaluation Scale (modified to measure 5 not 6 items). This was not deemed to be validated, and so reviewers did not present the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to receive either LB or DB for wound closure. Randomization occurred immediately following placement of the subcuticular sutures" Comment: unclear how the randomised sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization occurred immediately following placement of the subcuticular sutures. Due to the fact that the devices are packaged differently, it was not possible for the surgeon to be masked from the knowledge of the randomised treatment assignments" Comment: no evidence of adequate concealment of allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Due to the fact that devices are packaged differently, it was not possible for the surgeon to be masked from the knowledge of the randomised treatment assignments, however, the study subjects and the follow up assessments were masked to the device being used" Comment: adequate evidence that participants were blinded to the intervention, but personnel were not blinded. This is understandable due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: " . . . subjects were asked to return at two weeks and three months post surgery at which time any applicable wound complications (erythema, pain, infection, and dehiscence) were captured along with any reported adverse events" Comment: no indication that the 2 week assessment was undertaken by a blinded assessor Quote " . . . the photos were independently evaluated by a masked panel for cosmesis using the same 100mm VAS as well as a modified Hollander Wound Evaluation Scale (HWES)." "The masked panel consisted of 6 physicians: 2 general dermatologists, 1 dermatologic surgeon, 1 oculoplastic surgeon, 1 facial plastic surgeon, and 1 plastic surgeon." Comment: adequate blinding of assessment outcome of cosmetic appearance at 3 months |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 43 subjects participated in this trial completing all study visits including 2‐week and 3‐month follow up" Comment: adequate evidence that no loss to follow‐up occurred |
| Selective reporting (reporting bias) | Low risk | All the variables outlined in the methodology were accounted for in the results Comment: judged as low risk. Study protocol not sought |
| Other bias | Low risk | None detected |
| Methods | RCT with 60 participants and 14 days of follow‐up | |
| Participants | 60 people undergoing a skin biopsy with chronically inflamed skin Exclusion criteria: infection at location where biopsy was planned and known hypersensitivity to cyanoacrylate or other tissue adhesive Surgery was undertaken in 1 centre in Mexico | |
| Interventions | Group 1 (n = 30): 2‐octylcyanoacrylate tissue adhesive (no further details) Group 2 (n = 30): monofilament suture (no further details) | |
| Outcomes | Wound dehiscence (on days 5, 7, 10 and 14 after surgery) Skin closure time | |
| Notes | Data extraction based on English language abstract and partial translation of report text which was published in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study described as randomised. No further detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no detail reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detail reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no detail reported |
| Selective reporting (reporting bias) | Low risk | Comment: wound dehiscence was the only outcome listed in the methods and reported |
| Other bias | Unclear risk | Comment: not noted from the translation available |
| Methods | Parallel RCT with 100 women and 30‐day follow‐up Data on the primary outcome was not available for 2 participants in the adhesive arm and 1 participant in the suture arm | |
| Participants | 100 women undergoing mediolateral episiotomy after a vaginal delivery in the absence of any other perianal or vaginal lesions Exclusion criteria: existing local infections or lesions; body mass index 35 (kg/m2); severe pulmonary disease; collagen disease; known immunodeficiency; diabetes mellitus; or currently receiving immunosuppressive treatment The study was conducted in 1 hospital centre in Portugal | |
| Interventions | Group 1 (n = 53): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 47): continuous subcuticular suture (rapidly absorbable polyglactin 910) | |
| Outcomes | Wound dehiscence (42 h‐68 h post‐partum) | |
| Notes | The main outcome measure for the paper was self‐assessed perineal pain during the first 30 days after delivery. This was not deemed to be an outcome relevant to the review given the outcomes listed Skin closure time not reported ‐ only time for whole procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was decided by opening a previously prepared, opaque, sealed envelope containing a 1:1 computer‐generated random number, assigning the patient to one of the two arms" Comment: use of computer‐generated randomisation adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was decided by opening a previously prepared, opaque, sealed envelope containing a 1:1 computer‐generated random number, assigning the patient to one of the two arms" Comment: use of opaque, sealed envelopes but not noted whether numbered in such a way as to ensure maintenance of robust allocation concealment. Not clear if person who opened the envelopes was independent |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Women were not informed of the technique that was used to close the perineal skin throughout the study period." "As both groups required stitching of other layers, it is believed that they were not able to see or feel the difference between the two types of perineal skin closure, at the time of repair or thereafter." Comment: participants were blinded. Understandably difficult to blind the operating surgeon to the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Other outcomes evaluated were . . . duration of surgical repair and perineal wound complications observed at hospital discharge, 42‐68 hours post ‐partum. This observation was carried out in all cases by one of two authors (RM, FC)" Comment: as the authors were aware of the intervention undertaken, the judgement is that this potentially carries a high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Intra‐operative data was available for all participants. Data from 42‐68 hours post‐op was missing for 2 participants in the adhesive arm and one in the suture arm." Quote: "Eighty‐six women returned the questionnaires, 49 (92%) from the skin adhesive arm and 37 (79%) from the subcuticular suture arm. This difference was very close to achieving statistical difference p= 0.05 . . .". The results also indicated that the reason for loss of follow up was the participants "did not return questionnaire", rather than "discontinued intervention" Comment: unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | Quote: No direct quotations, but all variables listed in methods section were listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | None detected |
| Methods | RCT with 3‐month follow‐up. 50 withdrawals at 3‐month follow‐up | |
| Participants | 59 patients requiring unilateral or bi‐lateral herniotomies at KK Womens' and Childrens' Hospital, Singapore | |
| Interventions | Group 1 (n = 26): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 33): subcuticular polyglecaprone (Monocryl®) suture | |
| Outcomes | Infection, dehiscence, parent satisfaction and time for closure | |
| Notes | Cosmesis scores at 3 weeks not usable as too early and those at 3‐months not usable due to large loss to follow‐up. Viewed as a high risk of bias with 50 participants dropping out | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All enrolled patients were allocated to glue or suture by opening serial sealed envelopes prepared with computerised randomisation" Comment: computerised randomisation probably represents randomised sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "All enrolled patients were allocated to glue or suture by opening serial sealed envelopes prepared with computerised randomisation" Comment: the use of sequential sealed envelopes would reduce risk of selection bias. We have taken serial to mean that envelopes were kept in a pre‐arranged and transparent fixed order and therefore judged this study to be at low risk of bias for this domain |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether the patients or personnel were blinded to the intervention Comment: understandably difficult to blind the operating surgeon to the procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: " . . . assessment was done by an independent, blinded observer (staff nurse) using a previously validated score [The Hollander score] . . . Parent satisfaction with wound cosmesis was recorded at the same time on a 100mm visual analogue scale (VAS)" Comment: reasonable to deduce that this represents a low risk of detection bias for nurse‐assessed cosmetic outcome, but not necessarily participant‐assessed outcomes. Not clear if other outcomes such as wound infection or dehiscence were collected via blinded assessment |
| Incomplete outcome data (attrition bias) | High risk | Quote: "We were unable to do a 3‐month wound assessment on most of the patients, as only 9 returned for late follow up" Comment: it is difficult to draw any conclusions from the results of the long‐term outcomes due to high rates of losses to follow‐up. This leads to a high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in the methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were detected |
| Methods | RCT with 3‐month follow‐up. There were no withdrawals | |
| Participants | 101 people requiring rhinoplasty or septorhinoplasty entered the study Exclusion criteria: a history of peripheral vascular disease; diabetes mellitus; a clotting disorder; keloid or hypertrophic scarring; or an allergy to cyanoacrylate or formaldehyde Conducted at Inonu University Hospital, Turkey | |
| Interventions | Group 1 (n = 34): butylcyanoacrylate (LiquiBand®. MedLogic Global Ltd, Plymouth, Devon, UK) tissue adhesive Group 2 (n = 67): 6.0 polypropylene sutures for columellar skin closure after the majority of the tension had been taken up using 5.0 chromic catgut | |
| Outcomes | Dehiscence and infection at 1 week Cosmesis at 3 months by blinded assessment of photographs using VAS and Hollander scale Time required for skin closure | |
| Notes | We wrote to the authors to clarify the numbers in each group randomised by coin toss and received confirmation that the numbers were correct. We also received clarification that the standard deviations were presented after the means in the results section of the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated by coin toss" Comment: random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated by coin toss" Comment: no mention of allocation concealment from personnel |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel Comment: understandably difficult to blind the operating surgeon to the procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All patients were examined once every week in the first post‐operative month, and once a month for the second and third months. Columellar wounds were examined for infection, inflammation, dehiscence and scarring in addition to cosmetic and functional nasal evaluations". "The three month basal view photograph of each subject were given to two otolaryngologic surgeons who were blinded to the method of repair of the columellar incision" Comment: 3‐month cosmetic assessment blinded. Unclear whether assessments at prior time points were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "One hundred and one patients . . . were eligible for inclusion"; this figure corresponds with the 101 participants accounted for in the results table Comment: no evidence of loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | RCT with follow up at 7 days, 15 days, 1 month, 3 months, 6 months and 12 months. There were no withdrawals | |
| Participants | 70 people who underwent thyroid surgery from November 2005 to May 2007 No inclusion/exclusion criteria were stated In all cases, participants underwent thyroidectomy following a cervical incision The study took place in Italy, although no further information is given regarding the exact location of the trial | |
| Interventions | Group 1 (n = 32): 2‐octylcyanoacrylate (Dermabond, Ethicon Inc) Group 2 (n = 38): skin staples (Proximate, Ethicon Inc) Prophylactic antibiotics in the form of ceftriaxone were administered to both groups | |
| Outcomes | Wound dehiscence (7days) Wound infection (7 days; not defined) Cosmetic appearance (assessments by participants at 3, 6 and 12 months relevant here). Data were recorded using the Stony Brook scar evaluation scale composed of 5 dichotomous, evenly weighted categories. Scars are assigned 0–1 point for the presence or absence of a width greater than 2 mm at any point of the scar, a raised (or depressed) scar, a darker coloration than surrounding skin, any hatch or staples marks, an overall poor appearance, the total score ranging from 0 (worst) to 5 (best) Patient's satisfaction with wound management (7 days). Participants were asked to rate their level of satisfaction with the early postoperative management of the wound (regarding the requirement of a return visit for medications, the possibility of washing oneself, the suture removal) using a numerical scale ranging from 0–10 | |
| Notes | Study records how many closures were considered rapid (between 30 and 60 seconds). These data were not extracted as it was felt that they reported skin closure time in a way that could not be summarised meaningfully Participants were also asked to provide a score using a verbal rating response regarding their scar (at 7 days, 15 days and 3, 6, 9 and 12 months after surgery). Scores could range from 0 to 10: 0–4, poor; 5–6, mild; 7–8, good; and 9–10, excellent. These data were only presented categorically and are not extracted ‐ instead the scar evaluation scale data are presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Only when the surgical procedure was completed, after the closure of the platysma, each patient was randomly assigned to the two treatment groups" Comment: no indication that there was evidence of randomised sequence allocation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Only when the surgical procedure was completed, after the closure of the platysma, each patient was randomly assigned to the two treatment groups" Comment: no indication of allocation concealment from personnel |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotations, but no indication that the participants or the personnel were blinded to treatment. It is understandably difficult to blind personnel to the intervention arm given during an operation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In the follow‐up over 7 and 15 days, 1‐month, 3, 6 and 12‐months periods wound‐healing process was monitored by clinical assessment and documented by digital photographs." Comment: there was no indication that the clinical assessments were made by people who were blinded to the intervention. Therefore the risk of bias here is unclear |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotations, but no losses to follow‐up encountered |
| Selective reporting (reporting bias) | Low risk | No direct quotations, although all methods of evaluating the interventions were accounted for in the results Comment: adequate outcome reporting |
| Other bias | Low risk | None detected |
| Methods | RCT with 6‐week follow‐up. 1 withdrawal but no explanation | |
| Participants | 30 participants recruited for cervicotomy for thyroid and para thyroid surgery at Scunthorpe General Hospital, North Lincs, UK. 29 successfully randomised. No reference to inclusion/exclusion criteria | |
| Interventions | Group 1 (n = 14): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 15): staples (Vivistat®) | |
| Outcomes | Time to closure, neck mobility, cosmesis and adverse events | |
| Notes | Cosmesis results could not be included as they were measured too early to be usable. No explanation of what characterised an adverse event or the occurrence of such outcomes was provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation to glue or stapled closure was performed following induction of general anaesthesia by random envelope allocation" Comment: method of random sequence allocation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation to glue or stapled closure was performed following induction of general anaesthesia by random envelope allocation" Comment: It is not clear whether the envelope was sealed or ordered and marked to be sequential, therefore without further information, the judgement is of an unclear risk of selection bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | No statement made about whether the personnel were blinded to the procedure Comment: understandably difficult to blind the operating surgeon to the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Scar cosmesis assessment by both patient, surgeon and independent blinded assessor at 6 weeks." Comment: this assessment was not included in the review (as conducted at less than 3 months) no other information about blinded assessment of other outcomes provided |
| Incomplete outcome data (attrition bias) | Unclear risk | No direct quotations, although no losses to follow‐up were reported, and all participants accounted for in results Comment: judged as low risk |
| Selective reporting (reporting bias) | Low risk | No direct quotations although all methods of evaluating the interventions were accounted for in the results Comment: adequate outcome reporting |
| Other bias | Low risk | None detected |
| Methods | RCT with 90 days of follow‐up (assessment at 10 and 90 days postoperatively). Assumed all participants' wounds assessed at day 10. There were 6 withdrawals from 90 day follow‐up (2 from adhesives and 4 from strips) | |
| Participants | 49 children undergoing laparoscopic appendectomy Exclusion criteria: presence of concomitant chronic diseases; immunosuppression; malignancies; and conversion to laparotomy or intraoperative enlargement of incisions for intact specimen extractions Conducted in 1 centre in Germany | |
| Interventions | Group 1 (n = 23): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Dermabond, Ethicon, Sommerville, NJ) Group 2 (n = 24): standard adhesive strips ( strips applied in star‐shaped manner) Postoperative antibiotic therapy with cefotaxime and metronidazole was administered intravenously | |
| Outcomes | Wound dehiscence (assessment at 10 and 90 days after surgery) Wound infection (defined as abscess or redness > 3 mm perpendicular to incision) Cosmetic appearance (surgeon‐rated; at day 10 and day 90 ‐ day 90 data reported here) using a 100 mm VAS where 0 = best scar and 100 = worse scar and assessed by 2 blinded surgeons using macrophotos of each scar (3 photos per participant). Mean value of the 2 assessments has been taken Cosmetic appearance (participant‐rated; at day 10 and day 90 ‐ day 90 data reported here) assessed using a dichotomous question (wording of question not clear but results were reported as % expressing dissatisfaction with cosmetic result) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomization, a computer‐generated randomization pattern was implemented and patients were intraoperatively assigned to a procedure by means of a blind envelope system" Comment: adequate random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "For randomization, a computer‐generated randomization pattern was implemented and patients were intraoperatively assigned to a procedure by means of a blind envelope system" Comment: although attempts made to conceal allocation of the intervention, it was not stated whether the envelopes were sealed sequentially, whether they were sealed, or whether they were opaque, therefore the judgement remains unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotations, however it is understandably difficult to blind the operating surgeon from the intervention, therefore the judgement is unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Follow up examinations were carried out on the 10th and 90th postoperative day . . . Follow‐up included clinical investigation and a questionnaire to assess satisfaction with the cosmetic results, postoperative pain at port sites, wound infections, wound dehiscence, and any other adverse events associated with the wound" Comment: no indication that the follow‐up examination on the 10th postoperative day was undertaken by a blinded assessor Quote: [At 90 days] "This assessment was completed by 2 pediatric surgeons blinded to the method of wound repair" Comment: adequate blinding of cosmesis outcome at 3 months |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "A total of 49 patients (24 DermabondTM, 25 Steri‐StripTM) were enrolled between August 2007 and August 2008". "Photographs were taken from 21 patients in the Steri‐StripTM Group and from 22 patients in the DermabondTM Group" Comment: there is a discrepancy concerning 6 participants that were unaccounted for the in the final outcome assessment without clear reporting of why they were not included in the results. Therefore there is a risk of attrition bias, leaving the judgement unclear |
| Selective reporting (reporting bias) | Low risk | No direct quotations, although all methods of evaluating the interventions were accounted for in the results Comment: adequate outcome reporting |
| Other bias | Low risk | None detected |
| Methods | RCT with 2‐week follow‐up. No participants were lost to follow‐up | |
| Participants | 59 participants were enrolled, in whom 228 trocar sites were closed following undergoing laparoscopic surgery performed by one surgeon in the department of Urology, Wilford Hall Medical Center, Texas, USA No inclusion/exclusion criteria were stated | |
| Interventions | Group 1 (30 participants; 118 incisions): 2‐octylcyanoacrylate (Dermabond, Ethicon, Sommerville, NJ) Group 2 (29 participants; 110 incisions): subcuticular suture (4‐0 absorbable sutures, either Vicryl or Monocryl) Data presented at participant, not wound, level The fascia of all sites > 1 cm were closed with absorbable suture. Wounds in both groups that did not closely approximate to 1 cm received interrupted, subcutaneous sutures Those who received subcuticular sutures had their wounds dressed with steri‐strips, a 2 x 2xm gauze pad, and tape or a Tegaderm dressing No dressings were applied to the octylcyanoacrylate‐closed wounds | |
| Outcomes | Wound dehiscence (2 weeks after surgery) Wound infection (2 weeks after surgery; not defined) Mean closure time Cost per patient | |
| Notes | Cosmetic appearance measured at < 3 months so not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients undergoing laparoscopic surgery by one surgeon (JTB) were randomised to receive skin closure with either subcuticular suture of octylcyanoacrylate" Comment: no further information given regarding how the randomised sequence was generated, and therefore the judgement is unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients undergoing laparoscopic surgery by one surgeon (JTB) were randomised to receive skin closure with either subcuticular suture of octylcyanoacrylate" Comment: no further information given regarding how the allocation was concealed to the operating surgeon (if at all), therefore the judgement remains unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | No direct quotations, but no information given regarding blinding of participants or personnel in this study. It is understandably difficult to blind the operating surgeon to the intervention. Therefore the judgement here is unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were evaluated 2 weeks postoperatively for evidence of infection, dehiscence, seroma, and general cosmetic appearance." Comment: unclear whether this evaluation was undertaken by a blinded assessor |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotations, but no reported loss to follow‐up, therefore judgment of low risk |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Postoperative wound complications were similar for both groups. Five patients in the octylcyanoacrylate group had wound complications in 9 incisions. Two patients experienced skin separation in 5 incisions. One patient had a minor wound infection at one incision site treated with oral antibiotics. Two patients experienced small seromas at 2 incision sites, requiring incision opening and healing by secondary intention. We noted small seromas in 2 patients receiving suture closure. These healed by secondary intention after skin opening and drainage. Table 4 presents a summary of the results." Comment: however the table shown does not compare rates of wound complication and the reporting of the outcomes is unclear, it is this author's judgement therefore that the risk of bias here is unclear |
| Other bias | Low risk | None detected |
| Methods | RCT with 9‐month follow‐up. 2 participants were lost to follow‐up from the suture group due to failure to attend and could not be traced by mail or phone | |
| Participants | 79 adults requiring varicose vein surgery on the leg. Trial conducted at Ludwick Boltzmann Institure, Linz, Austria Exclusion criteria: a history of chronic venous insufficiency with dermatosclerosis; previous phlebectomies; or allergy to plaster or octylcyanoacrylate | |
| Interventions | Mullerian phlebectomy performed creating an average wound length of 5 mm. Used 5 min wound compression followed by skin closure with: Group 1 (n = 26): octylcyanoacrylate tissue adhesive Group 2 (n = 28): 5.0 monofilament suture Group 3 (n = 25): tape A small plaster was placed over each wound | |
| Outcomes | Wound dehiscence, infection at 10 days, and patient and surgeon satisfaction, and cosmetic appearance at 1 year and costs. Time required for incision closure was also recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomisation was done by a computer [during] the morning of the operation day" Comment: this was judged to be an adequate method of generating the randomisation sequence i.e. ‘by computer’ |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Randomisation was done by a computer [during] the morning of the operation day". Comment: there is no mention of the method used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel Comment: understandably difficult to blind the operating surgeon to the procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The follow up by the operating surgeon was performed [at] 10 days and 6 weeks after the operation" and, "Eight to 14 months after the operation all patients had been investigated by one senior dermatologist who was blinded to the method of skin closure, the scars were examined for color, width, and cosmetic appearance . . ." Comment: blinded outcome assessment achieved for outcomes collected after 8 months. Not clear if blinding was implemented for earlier follow‐up times when wound dehiscence and infection were assessed |
| Incomplete outcome data (attrition bias) | Low risk | Quote:"Only 2 of 79 patients were lost to follow‐up, resulting in 77 patients with postoperative control" Comment: no reasons reported for those lost to follow‐up, but as they only represented a small percentage of the total participants, this was judged overall to be at low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | RCT with 6‐month follow‐up. 6 participants were lost to follow‐up: 5 in tissue adhesive and 1 in suture group | |
| Participants | 50 adults requiring hand or wrist surgery (carpal tunnel syndrome, trigger finger, De Quervain's tenosynovitis, ganglions of wrist and hand, and cysts of fingers) Exclusion criteria: requiring surgery for Dupuytren's contracture; repeat surgery; a history of skin allergy or keloid formation; diabetes; or corticosteroid use Trial conducted at Monklands Hospital, Airdre, UK | |
| Interventions | Skin approximated with skin hooks then: Group 1 (n = 20): application of butylcyanoacrylate adhesive (Indermil), or Group 2 (n = 24): suturing with 4.0 monofilament All cases had local anaesthetic infiltration with or without general anaesthesia | |
| Outcomes | Dehiscence, infection, cosmetic appearance at 10 days, 2 weeks and 6 weeks | |
| Notes | Cosmetic appearance data not used as assessed at < 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to wound adhesive or suture on the basis of 50 previously prepared and sealed envelopes (25 of each)" Comment: whilst the trial was reported as randomised there was no information about how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised to wound adhesive or suture on the basis of 50 previously prepared and sealed envelopes (25 of each)" Comment: judgement of unclear risk of bias, as not clear if envelopes were sequentially numbered and opaque. Not clear who was responsible for allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided about blinding of participants or surgeons Comment: it would be difficult to blind the operating surgeon |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were subsequently assessed at 2 and 6 weeks post‐surgery by a designated tissue viability nurse who was blinded to the method of closure" Comment: outcome assessment blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of the study 50 participants, six were lost to follow up (five in the adhesive group and one in the suture group)" Comment: the sample size of the study is small, 6 participants lost represents over 20% of the total sample. No information reported on reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | RCT with 3‐month follow‐up. No reference to withdrawals | |
| Participants | 14 participants recruited for removal of basal cell carcinoma or squamous cell carcinoma of the head and neck using the Mohs technique at the Department of Dermatology at University Hospital Iowa | |
| Interventions | Split wound design, so both methods of closure were used for part of each wound Intervention 1: 2‐octylcyanoacrylate (Dermabond®; Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Intervention 2: polypropylene cuticular suture | |
| Outcomes | Dehiscence, infection and cosmesis | |
| Notes | As a split wound design the data were paired. The authors were contacted for the mean difference and the standard deviation of the cosmetic scores on a participant basis. No reply was received, therefore this information was not included in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Half of the surgical wound was randomly selected (by coin toss) for epidermal approximation . . ." Comment: use of coin toss judged to constitute adequate random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Half of the surgical wound was randomly selected (by coin toss) for epidermal approximation . . ." Comment: no information given about whether the personnel were concealed to the allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No reporting of either participants or personnel being blinded to the intervention Comment: as the nature of the intervention was that the two methods of closure were placed together to close the same incision, there is potential that participants may have understood the method of closure. Similarly it is understandably difficult to blind the operating surgeon to the intervention. The judgement is therefore unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients returned in 7 days for suture removal and were evaluated for early complications such as wound separation, or dehiscence, inflammation or infection. High resolution photographs were obtained immediately post‐operatively and 3 months postoperatively" and, "The primary outcome measure was scar cosmesis, evaluated by comparing both halves of the scar from the 3 month photographs by five blinded dermatologists" Comment: unclear whether assessment at day 7 was blinded. 3‐month assessment of cosmesis was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 14 patients completed all study end points" Comment: judged as low risk. |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | RCT with 4‐week follow‐up. 2 withdrawals accounted for | |
| Participants | 45 participants recruited for elective repair of inguinal hernias at the Eisenhower Army Medical Centre, Fort Gordon, Georgia, USA Inclusion/exclusion criteria not reported | |
| Interventions | Group 1 (n = 24): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Group 2 (n = 22): subcuticular polyglecaprone (Monocryl®) suture | |
| Outcomes | Dehiscence, infection, cosmesis and time taken to closure | |
| Notes | The cosmetic data were not included as the data were taken at < 3 months. The authors were contacted for the mean and standard deviations for the time to closure, but since no response has been received these data were not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . patients were then randomised with the use of a computerized random number generator to receive either 2‐octylcyanoacrylate tissue adhesive or subcuticular running 4‐0 poliglecaprone 25 (Monocryl, Ethicon, Inc) skin closure" Comment: judgement of a low risk of selection bias due to use of computer to generate random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: " . . . patients were then randomised with the use of a computerized random number generator to receive either 2‐octylcyanoacrylate tissue adhesive or subcuticular running 4‐0 poliglecaprone 25 (Monocryl, Ethicon, Inc) skin closure" Comment: No specific mention of how sequence was allocated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported whether participants or personnel were blinded to the intervention Comment: understandably difficult to blind operating surgeon to intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were monitored postoperatively for complications of their closures, and all patients were scheduled for post‐operative visits at 2 weeks and at 4 weeks" Comment: it is not reported whether those monitoring for post‐operative complications were blinded to the intervention. Cosmesis assessment was blinded, but these data were not extracted here as taken at < 3 months after surgery |
| Incomplete outcome data (attrition bias) | Low risk | Not directly stated, but no participants were reported to have been lost to follow‐up and all were accounted for in results Comment: judged as low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | None detected |
| Methods | Split body design RCT with 3 months follow‐up | |
| Participants | 8 participants undergoing surgery for non‐melanomas skin cancer Exclusion criteria: on immuno‐suppressive medication and/or recipients of organ transplants Undertaken in 1 centre in the USA | |
| Interventions | 8 participants, each having half of incisional wound randomised to each intervention Intervention 1 (8 half wounds): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) Intervention 2 (8 half wounds): rapid absorbing gut suture | |
| Outcomes | Wound dehiscence (1 week postoperatively) Wound infection (1 week postoperatively ‐ no definition provided) | |
| Notes | Cosmetic appearance (surgeon) at 3 months and cosmetic appearance (participant) at 3 months were collected but the data are not clear and not presented here. The report suggests that outcome data were reported on a 1 to 4 point scale but some data are > 4 so this is unclear. Also unclear what the scale measured Patient reference data not extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised for epidermal closure with one half of the wounds (chest (n=6) and upper extremities n=2)) with rapid absorbing gut structure and on the other half with 2‐octylethylcyanoacrylate tissue adhesive" Comment: no information given about the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised for epidermal closure with one half of the wounds (chest (n=6) and upper extremities n=2)) with rapid absorbing gut structure and on the other half with 2‐octylethylcyanoacrylate tissue adhesive" Comment: no information given about whether allocation was concealed from the participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "All wounds were closed by a single surgeon (DJK) using a linear, bilayered closure method . . ." Comment: no information given about whether the participants or the personnel were blinded to the intervention. However, it would be difficult to blind personnel to a surgical intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Patients were seen for evaluation at postoperative visits at both 1 week and 3 months after the procedure. Incidence of wound dehiscence and side effects of itching, bleeding, and pain were assessed at 1 week" Comment: unclear whether those evaluating the wounds at 1 week were blinded to the intervention |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotations, although there was no loss to follow‐up reported within the study |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | None detected |
| Methods | RCT with 1‐year follow‐up. 11 participants were lost to follow‐up, but the groups from which they came were not specified | |
| Participants | People over 1 year of age requiring elective surgery for benign skin lesions predominantly in face and neck Exclusion criteria: a history of significant trauma; peripheral vascular disease; diabetes mellitus; blood clotting disorder; keloid or hypertrophy scarring; known allergy to cyanoacrylate or formaldehyde Trial conducted at University of Illinois, Chicago, USA | |
| Interventions | Incisions with and without subcutaneous sutures were randomised for closure with: Group 1: 2‐octylcyanoacrylate, or Group 2: 5.0 or 6.0 nylon suture | |
| Outcomes | Dehiscence, infection, cosmesis and closure time | |
| Notes | Participants lost to follow up were not reported by group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using clinical indications as evaluated by the surgeon, patients were assigned to one of the two treatment groups". Later trial report mentions "Patients were randomised . . ." Comment: no method of randomised sequence generation stated. The initial quotation suggests clinicians had some autonomy in deciding which participants were to receive which treatment, which in turn represents a high risk of bias, but this is unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Using clinical indications as evaluated by the surgeon, patients were assigned to one of the two treatment groups" Comment: no mention of allocation concealment and possible evidence that surgeon was aware of allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel Comment: due to the nature of the surgical procedure, it would be difficult to blind either the operating surgeon or participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At each postoperative visit, wounds were examined for infection, inflammation, wound dehiscence or separation, and scarring." and, "At the 90 day follow up visit, the wounds were graded for cosmesis using the modified Hollander wound evaluation scale”, and, at one year, the patients were assessed by way of a standardised method of photography of the wound, "The photographs were then given to two facial plastic surgeons that were unfamiliar with the study design, the purpose or the site of the surgical incision, or the type of treatment received" Comment: no indication of blinded outcome assessment for outcomes assessed prior to one year. Blinded outcome assessment of wound appearance at 12 month follow‐up |
| Incomplete outcome data (attrition bias) | Unclear risk | No direct quotations given, but results show that of the 100/111 (90%) patients enrolled in the study for their long term 1‐year follow‐up wound evaluation Comment: 10% loss of follow‐up data at 12 months |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | 2‐arm RCT with 6 week follow‐up | |
| Participants | 100 children undergoing correction of hernia inguinalis No inclusion or exclusion criteria stated | |
| Interventions | Group 1 (n = 50): butylcyanoacrylate (Indermil) tissue adhesive Group 2 (n = 50): polyglactin 5‐0 (Vicryl) sutures | |
| Outcomes | Wound dehiscence (10 days after surgery) Wound infection (not defined; 10 days after surgery) Mean closure time | |
| Notes | It is noted that 100 participants were randomised and that some had surgery on both sides of the abdomen. It was not clear from the study results whether outcomes were presented at the participant level. In one instance where % data are presented the denominator used was 50 for each group suggesting that there is no unit of analysis issue but this is not clear Wound cosmesis assessed at 6 weeks ‐ not presented here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were selected randomly to receive wound adhesive or suture on the basis of 100 previously prepared and sealed envelopes containing slips for either suture closure or the use of Indermil (50 of each)" Comment: use of opaque, sealed envelopes, but not noted whether numbered in such a way as to ensure robust allocation concealment maintained. Not clear if person who opened envelopes was independent |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel Comment: due to the nature of the surgical procedure, it would be difficult to blind either the operating surgeon |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinded outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | No mention of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No direct quotations, but all variables listed in methods are listed in the tables in the results section Comment: a low risk of reporting bias is inferred |
| Other bias | Unclear risk | Comment: it is noted that 100 participants were randomised and that some had surgery on both sides of the abdomen. It is not clear from the study results if outcomes are presented at the participant level. Where % data is presented in one case the denominator used was 50 for each group suggesting that there is no unit of analysis issue but this is not clear |
Abbreviations
h = hour(s)
min = minute(s)
RCT = randomised controlled trial
ROM = range of motion
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not relevant wound type (not surgical wound) | |
| No data given in the paper. We have contacted authors but did not receive a reply | |
| Does not include any outcome relevant to this review | |
| Does not include any outcome relevant to this review | |
| Not an RCT | |
| After full translation, found not to be an RCT | |
| All participants in the intervention group had a precautionary subcuticular suture placed. The reviewers viewed this as an inherent bias to the outcome of this group and thus the study was excluded | |
| All participants had a subcuticular suture placed and described the intervention as a method of "superficial" closure. The reviewers viewed this as unnecessary and counter‐intuitive as the aim of the study is to assess the adhesive as an alternative to other methods of closure of incisions, not as an adjunct | |
| Not an RCT ‐ see quotation below ‐ considered alternation Quote: "Randomization was performed weekly. Each odd numbered week of the study (first week, third week, etc. was randomized to either OCA or suturing by a biostatistician, and then the subsequent even numbered week (second week, fourth week, etc) received the opposite treatment." | |
| Not considered an RCT | |
| Skin closure method was not the only systematic difference between groups | |
| There was no reference to randomisation. The authors have been contacted but we have received no reply | |
| Not relevant wound type (not surgical wound) | |
| Not an RCT | |
| Not an RCT | |
| Data could not be used because combined with data for lacerations. We wrote to the authors requesting data by group but did not receive a reply | |
| Not an RCT | |
| Not an RCT | |
| No relevant outcomes (based on translation) | |
| Not relevant wound type (not surgical wounds) |
Abbreviation
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Citation retrieved through handsearching awaiting full text retrieval |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Citation retrieved through handsearching awaiting full text retrieval |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Conference abstract with limited data ‐ contacting authors to find out if there is a pending publication or if further information available |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Citation retrieved through handsearching awaiting full text retrieval |
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence: all studies Show forest plot | 17 | 1225 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [1.53, 7.33] |
| Analysis 1.1  Comparison 1 Adhesive versus suture, Outcome 1 Dehiscence: all studies. | ||||
| 2 Dehiscence: sensitivity analysis Show forest plot | 13 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.95, 7.68] |
| Analysis 1.2  Comparison 1 Adhesive versus suture, Outcome 2 Dehiscence: sensitivity analysis. | ||||
| 3 Infection: all studies Show forest plot | 18 | 1239 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.94, 3.16] |
| Analysis 1.3  Comparison 1 Adhesive versus suture, Outcome 3 Infection: all studies. | ||||
| 4 Infection: sensitivity analysis Show forest plot | 15 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.80, 5.12] |
| Analysis 1.4  Comparison 1 Adhesive versus suture, Outcome 4 Infection: sensitivity analysis. | ||||
| 5 Cosmetic appearance rated by patient Show forest plot | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐7.20, 2.95] |
| Analysis 1.5 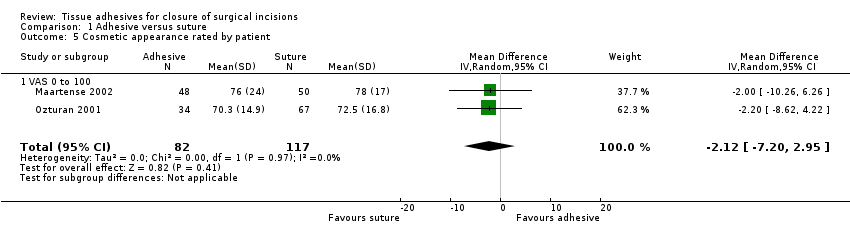 Comparison 1 Adhesive versus suture, Outcome 5 Cosmetic appearance rated by patient. | ||||
| 5.1 VAS 0 to 100 | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐7.20, 2.95] |
| 6 Cosmetic appearance rated by surgeon Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Adhesive versus suture, Outcome 6 Cosmetic appearance rated by surgeon. | ||||
| 6.1 VAS scale 0 to 100 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Scar assessment (0 to 5 scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Patient/parent satisfaction (% satisfied) Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| Analysis 1.7 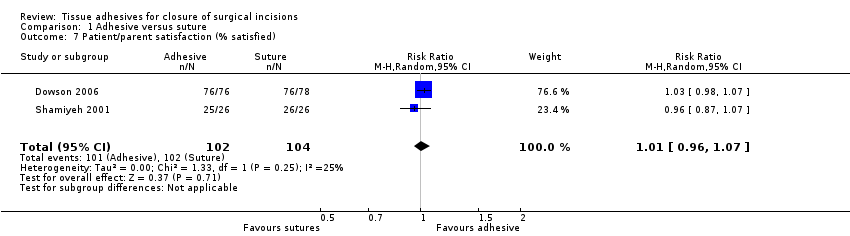 Comparison 1 Adhesive versus suture, Outcome 7 Patient/parent satisfaction (% satisfied). | ||||
| 8 Patient/parent satisfaction (VAS Scale 0 to 100) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Adhesive versus suture, Outcome 8 Patient/parent satisfaction (VAS Scale 0 to 100). | ||||
| 9 Surgeon satisfaction (% satisfied) Show forest plot | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.58, 2.19] |
| Analysis 1.9  Comparison 1 Adhesive versus suture, Outcome 9 Surgeon satisfaction (% satisfied). | ||||
| 10 Time taken for wound closure Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Adhesive versus suture, Outcome 10 Time taken for wound closure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Adhesive versus adhesive tape, Outcome 1 Dehiscence. | ||||
| 2 Infection Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.39, 4.81] |
| Analysis 2.2 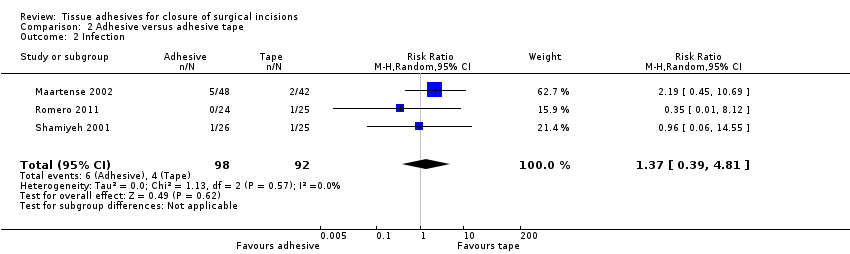 Comparison 2 Adhesive versus adhesive tape, Outcome 2 Infection. | ||||
| 3 Cosmetic appearance rated by patient (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 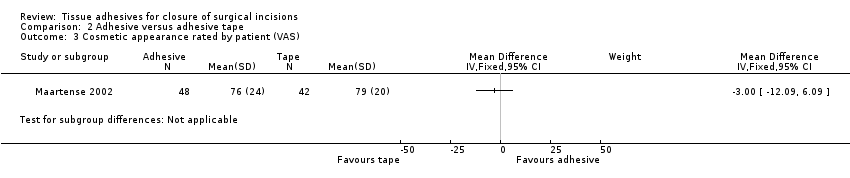 Comparison 2 Adhesive versus adhesive tape, Outcome 3 Cosmetic appearance rated by patient (VAS). | ||||
| 4 Cosmetic appearance rated by patient (% satisfied) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 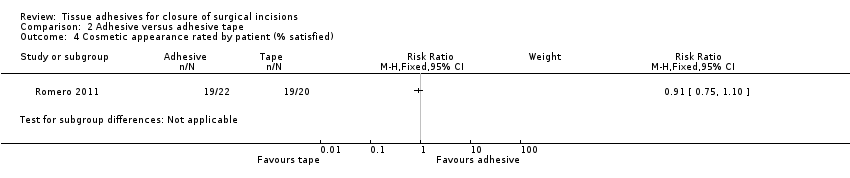 Comparison 2 Adhesive versus adhesive tape, Outcome 4 Cosmetic appearance rated by patient (% satisfied). | ||||
| 5 Cosmetic appearance rated by surgeon (VAS) Show forest plot | 2 | 139 | Mean Difference (IV, Random, 95% CI) | 9.56 [4.74, 14.37] |
| Analysis 2.5  Comparison 2 Adhesive versus adhesive tape, Outcome 5 Cosmetic appearance rated by surgeon (VAS). | ||||
| 6 Patient satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Adhesive versus adhesive tape, Outcome 6 Patient satisfaction. | ||||
| 7 Surgeon satisfaction Show forest plot | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| Analysis 2.7  Comparison 2 Adhesive versus adhesive tape, Outcome 7 Surgeon satisfaction. | ||||
| 8 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8 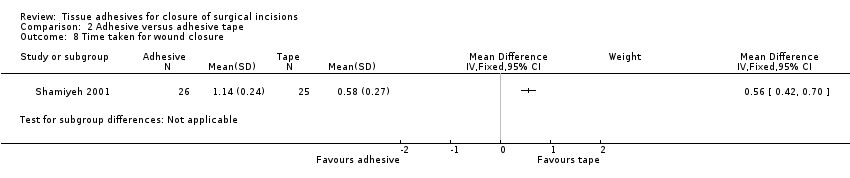 Comparison 2 Adhesive versus adhesive tape, Outcome 8 Time taken for wound closure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.33] |
| Analysis 3.1  Comparison 3 Adhesive versus staples, Outcome 1 Dehiscence. | ||||
| 2 Infection Show forest plot | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.30, 6.54] |
| Analysis 3.2  Comparison 3 Adhesive versus staples, Outcome 2 Infection. | ||||
| 3 Cosmetic appearance rated by patient (scar scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3 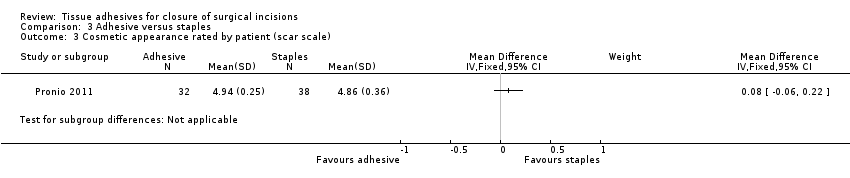 Comparison 3 Adhesive versus staples, Outcome 3 Cosmetic appearance rated by patient (scar scale). | ||||
| 4 Cosmetic appearance by plastic surgeons (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 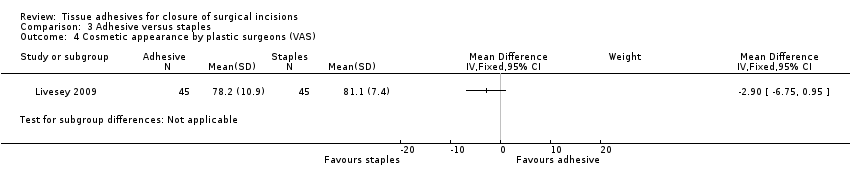 Comparison 3 Adhesive versus staples, Outcome 4 Cosmetic appearance by plastic surgeons (VAS). | ||||
| 5 Patient satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Adhesive versus staples, Outcome 5 Patient satisfaction. | ||||
| 6 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Adhesive versus staples, Outcome 6 Time taken for wound closure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.38] |
| Analysis 4.1  Comparison 4 Adhesive versus other method, Outcome 1 Dehiscence. | ||||
| 2 Infection Show forest plot | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.60] |
| Analysis 4.2  Comparison 4 Adhesive versus other method, Outcome 2 Infection. | ||||
| 3 Patient satisfaction Show forest plot | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.10, 0.70] |
| Analysis 4.3 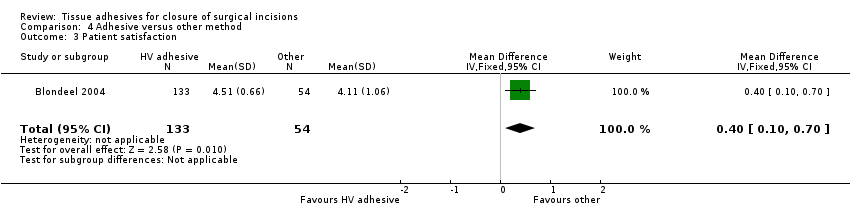 Comparison 4 Adhesive versus other method, Outcome 3 Patient satisfaction. | ||||
| 4 Clinician satisfaction Show forest plot | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.29, 0.77] |
| Analysis 4.4  Comparison 4 Adhesive versus other method, Outcome 4 Clinician satisfaction. | ||||
| 5 Time taken for wound closure Show forest plot | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.79, ‐0.31] |
| Analysis 4.5  Comparison 4 Adhesive versus other method, Outcome 5 Time taken for wound closure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 1 Dehiscence. | ||||
| 2 Infection Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 2 Infection. | ||||
| 3 Patient satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 3 Patient satisfaction. | ||||
| 4 Clinician satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 4 Clinician satisfaction. | ||||
| 5 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5 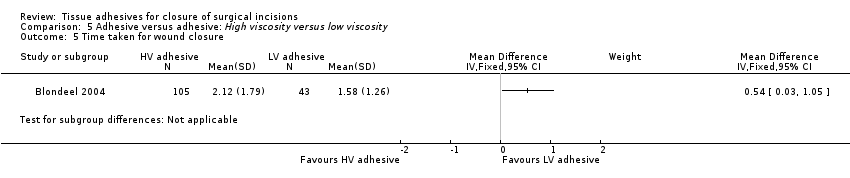 Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 5 Time taken for wound closure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.19, 11.30] |
| Analysis 6.1 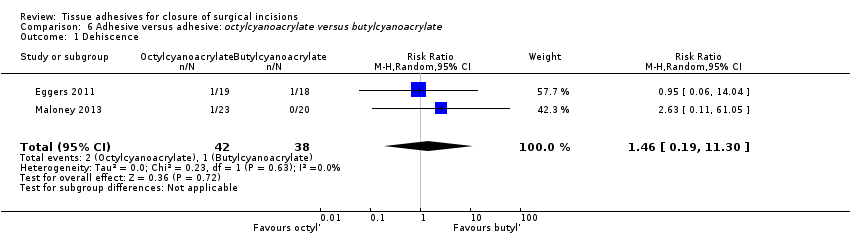 Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 1 Dehiscence. | ||||
| 2 Infection Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2 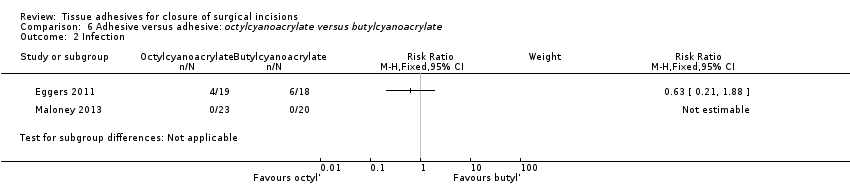 Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 2 Infection. | ||||
| 3 Cosmetic assessment rated by patient (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.3 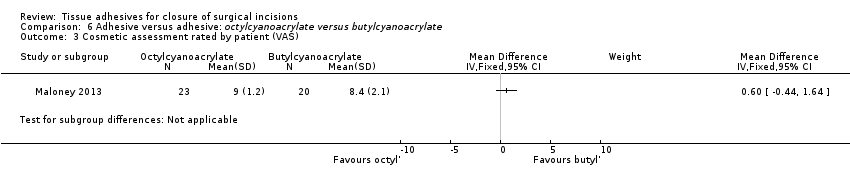 Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 3 Cosmetic assessment rated by patient (VAS). | ||||
| 4 Cosmetic assessment rated by surgeon (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 4 Cosmetic assessment rated by surgeon (VAS). | ||||
| 5 Surgeon satisfaction (with device) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.5  Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 5 Surgeon satisfaction (with device). | ||||
| 6 Surgeon satisfaction (with closure) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.6 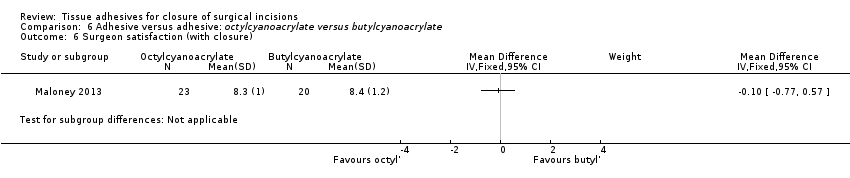 Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 6 Surgeon satisfaction (with closure). | ||||
| 7 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.7  Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 7 Time taken for wound closure. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
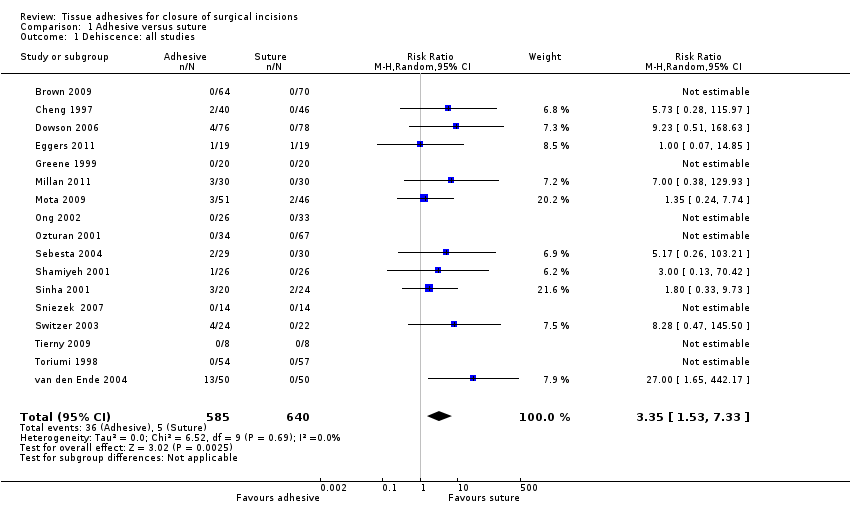
Comparison 1 Adhesive versus suture, Outcome 1 Dehiscence: all studies.
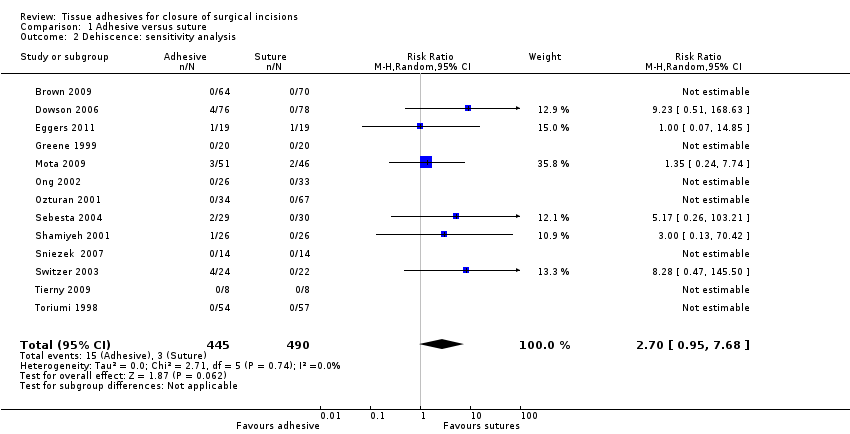
Comparison 1 Adhesive versus suture, Outcome 2 Dehiscence: sensitivity analysis.
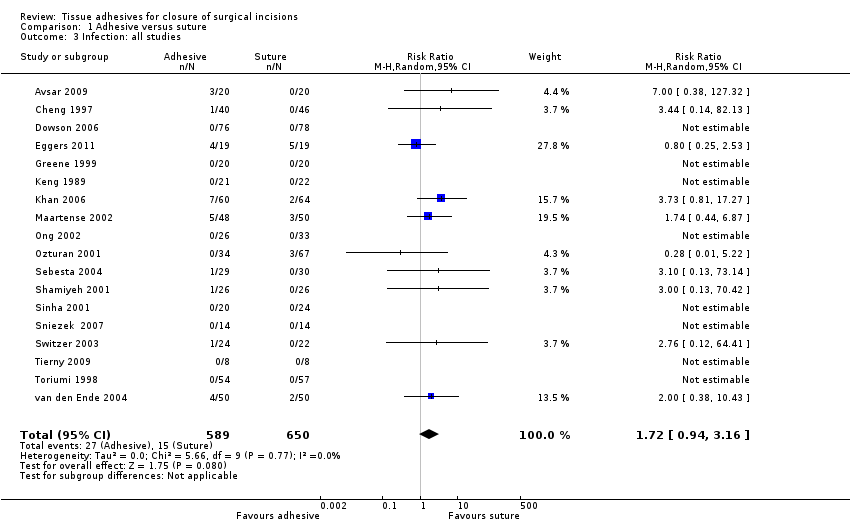
Comparison 1 Adhesive versus suture, Outcome 3 Infection: all studies.
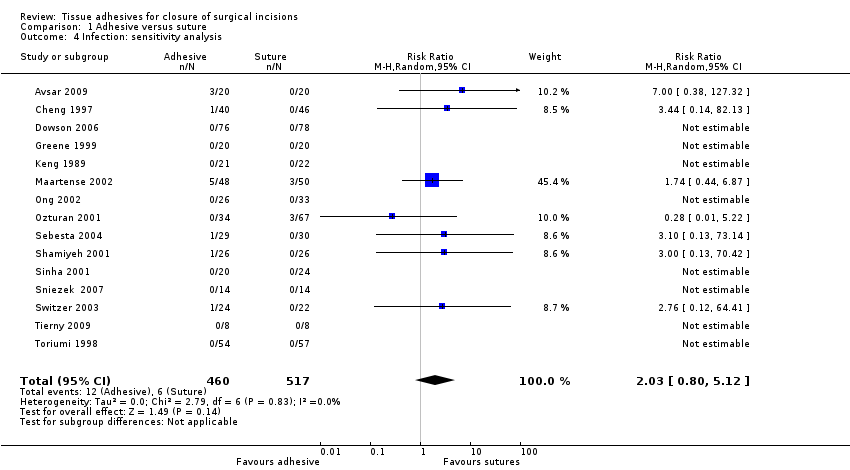
Comparison 1 Adhesive versus suture, Outcome 4 Infection: sensitivity analysis.

Comparison 1 Adhesive versus suture, Outcome 5 Cosmetic appearance rated by patient.
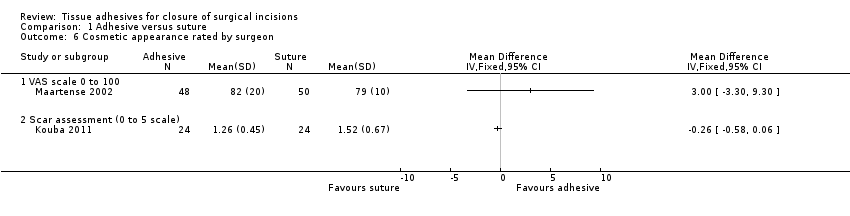
Comparison 1 Adhesive versus suture, Outcome 6 Cosmetic appearance rated by surgeon.

Comparison 1 Adhesive versus suture, Outcome 7 Patient/parent satisfaction (% satisfied).
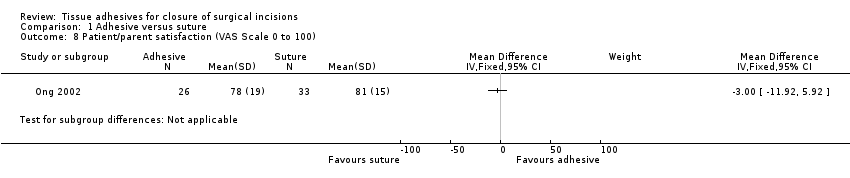
Comparison 1 Adhesive versus suture, Outcome 8 Patient/parent satisfaction (VAS Scale 0 to 100).

Comparison 1 Adhesive versus suture, Outcome 9 Surgeon satisfaction (% satisfied).
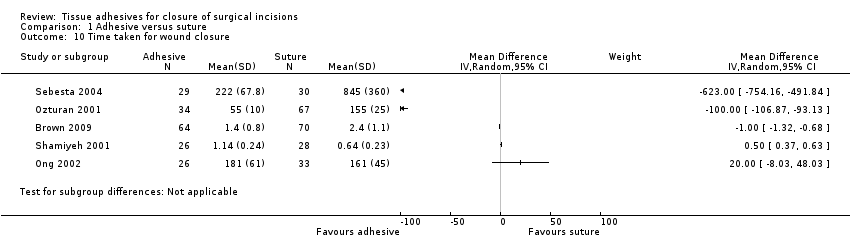
Comparison 1 Adhesive versus suture, Outcome 10 Time taken for wound closure.
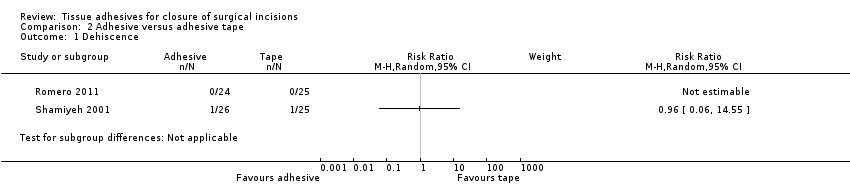
Comparison 2 Adhesive versus adhesive tape, Outcome 1 Dehiscence.
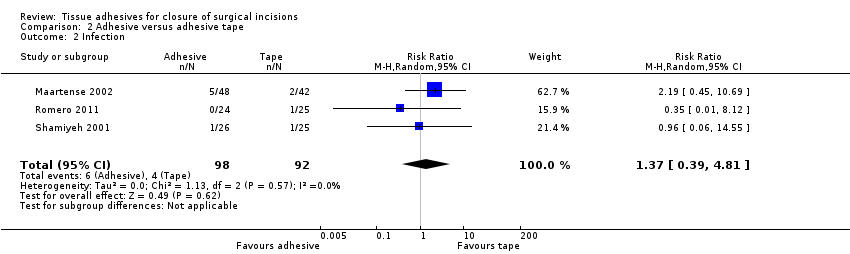
Comparison 2 Adhesive versus adhesive tape, Outcome 2 Infection.
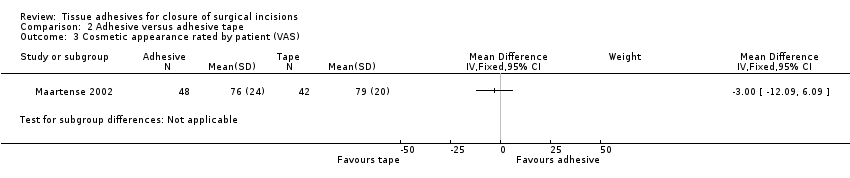
Comparison 2 Adhesive versus adhesive tape, Outcome 3 Cosmetic appearance rated by patient (VAS).

Comparison 2 Adhesive versus adhesive tape, Outcome 4 Cosmetic appearance rated by patient (% satisfied).
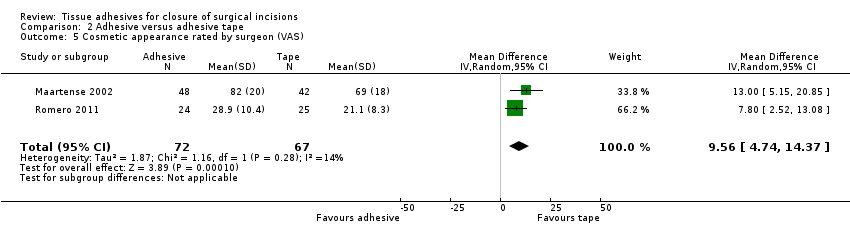
Comparison 2 Adhesive versus adhesive tape, Outcome 5 Cosmetic appearance rated by surgeon (VAS).

Comparison 2 Adhesive versus adhesive tape, Outcome 6 Patient satisfaction.
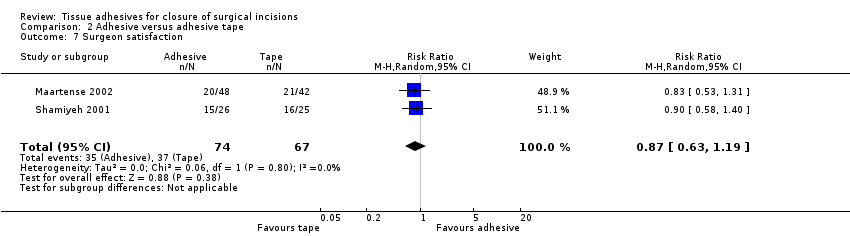
Comparison 2 Adhesive versus adhesive tape, Outcome 7 Surgeon satisfaction.
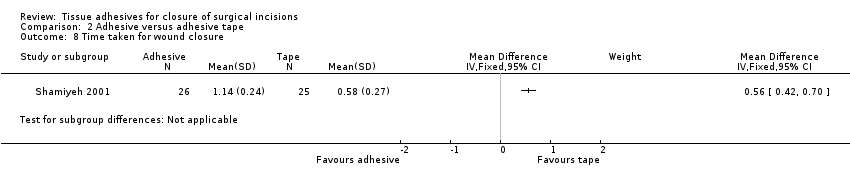
Comparison 2 Adhesive versus adhesive tape, Outcome 8 Time taken for wound closure.

Comparison 3 Adhesive versus staples, Outcome 1 Dehiscence.

Comparison 3 Adhesive versus staples, Outcome 2 Infection.

Comparison 3 Adhesive versus staples, Outcome 3 Cosmetic appearance rated by patient (scar scale).

Comparison 3 Adhesive versus staples, Outcome 4 Cosmetic appearance by plastic surgeons (VAS).
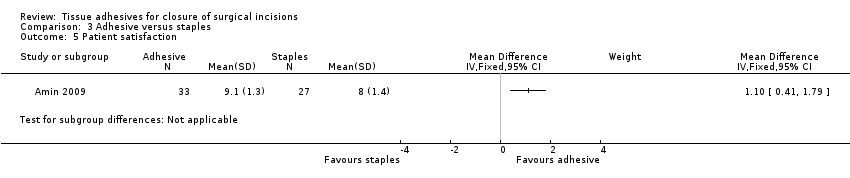
Comparison 3 Adhesive versus staples, Outcome 5 Patient satisfaction.
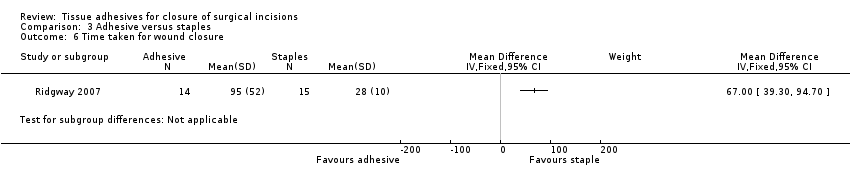
Comparison 3 Adhesive versus staples, Outcome 6 Time taken for wound closure.
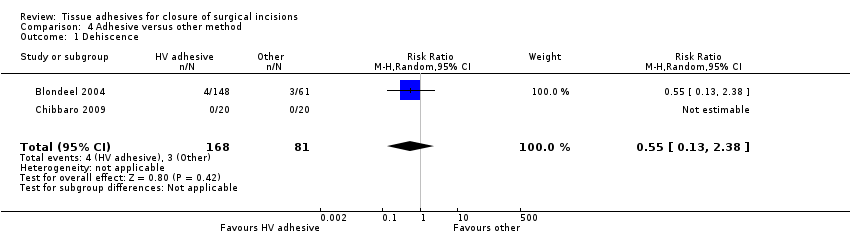
Comparison 4 Adhesive versus other method, Outcome 1 Dehiscence.

Comparison 4 Adhesive versus other method, Outcome 2 Infection.

Comparison 4 Adhesive versus other method, Outcome 3 Patient satisfaction.
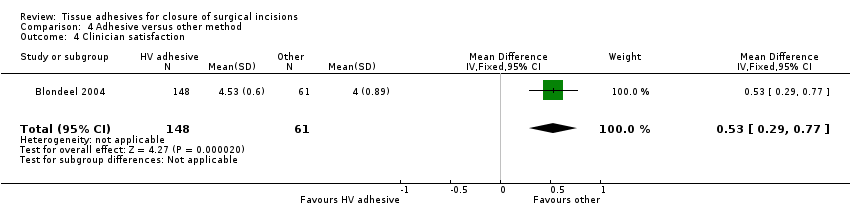
Comparison 4 Adhesive versus other method, Outcome 4 Clinician satisfaction.
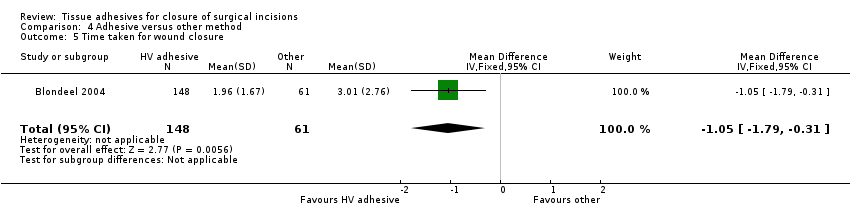
Comparison 4 Adhesive versus other method, Outcome 5 Time taken for wound closure.

Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 1 Dehiscence.
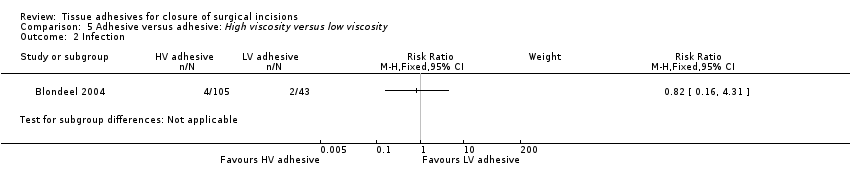
Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 2 Infection.

Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 3 Patient satisfaction.
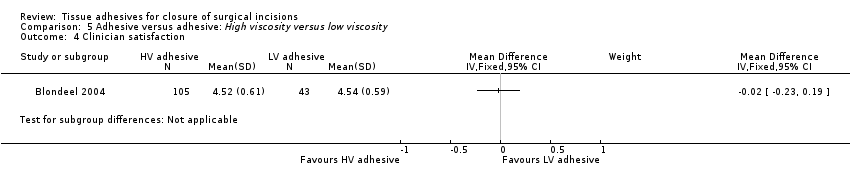
Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 4 Clinician satisfaction.
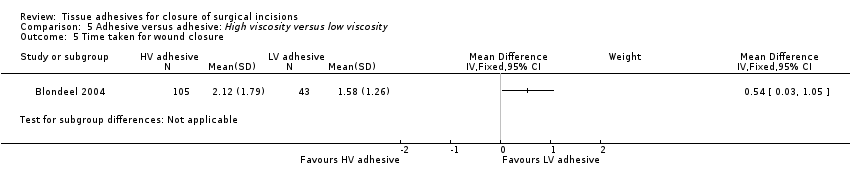
Comparison 5 Adhesive versus adhesive: High viscosity versus low viscosity, Outcome 5 Time taken for wound closure.
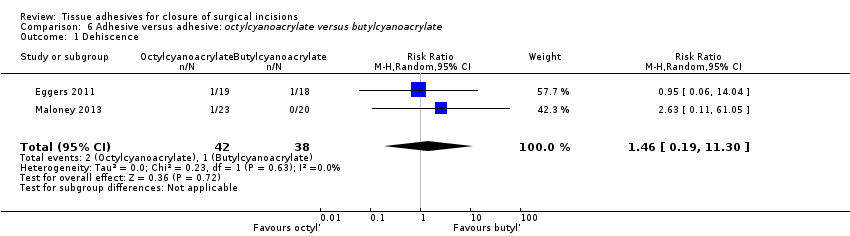
Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 1 Dehiscence.
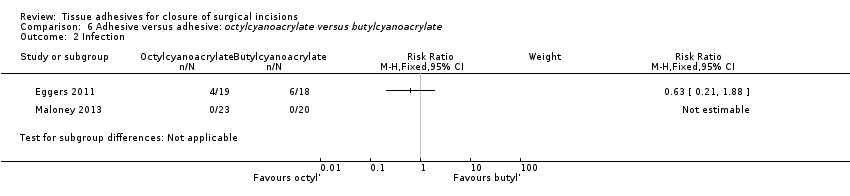
Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 2 Infection.
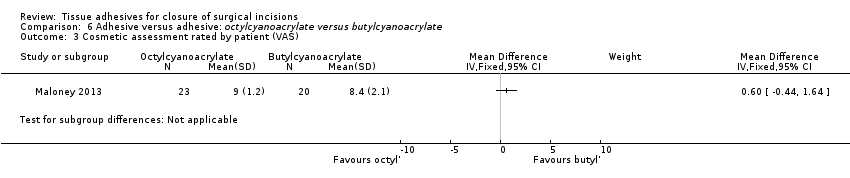
Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 3 Cosmetic assessment rated by patient (VAS).

Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 4 Cosmetic assessment rated by surgeon (VAS).
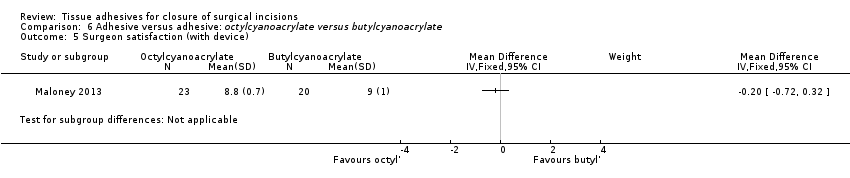
Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 5 Surgeon satisfaction (with device).

Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 6 Surgeon satisfaction (with closure).

Comparison 6 Adhesive versus adhesive: octylcyanoacrylate versus butylcyanoacrylate, Outcome 7 Time taken for wound closure.
| Tissue adhesive compared to sutures for surgical incisions | ||||||
| Patient or population: People with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks*4 (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sutures | Tissue adhesive | |||||
| Wound dehiscence | Study population | RR 3.35 | 736 | ⊕⊕⊝⊝ | ||
| 13 per 1000 | 45 per 1000 | |||||
| Moderate | ||||||
| Wound infection | Study population | RR 1.72 | 744 | ⊕⊝⊝⊝ | ||
| 38 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Possible unit of analyses issues. A sensitivity analysis changes a statistically significant difference to a non‐statistically significant difference | ||||||
| Tissue adhesive compared to adhesive tape for surgical incisions | ||||||
| Patient or population: people with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks*3 (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Adhesive tape | Tissue adhesive | |||||
| Wound dehiscence | Study population | RR 0.96 | 50 | ⊕⊕⊝⊝ | ||
| 42 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| Wound infection | Study population | RR 1.37 | 190 | ⊕⊕⊝⊝ | ||
| 43 per 1000 | 60 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study 95% CIs are very wide | ||||||
| Tissue adhesive compared to staples for surgical incisions | ||||||
| Patient or population: people with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks*3 (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Staples | Tissue adhesive | |||||
| Wound dehiscence | Study population | RR 0.53 | 37 | ⊕⊕⊝⊝ | ||
| 105 per 1000 | 56 per 1000 | |||||
| Moderate | ||||||
| Wound infection | Study population | RR 1.39 | 250 | ⊕⊝⊝⊝ | ||
| 71 per 1000 | 99 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study 95% CIs are very wide. | ||||||
| Tissue adhesive compared to other methods for surgical incisions | ||||||
| Patient or population: people with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other methods | Tissue adhesive | |||||
| Wound dehiscence | Study population | RR 0.55 | 209 | ⊕⊕⊝⊝ | ||
| 49 per 1000 | 27 per 1000 | |||||
| Moderate | ||||||
| Wound infection | Study population | RR 0.41 | 209 | ⊕⊕⊝⊝ | ||
| 66 per 1000 | 27 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study 95% CIs are very wide | ||||||
| High viscosity tissue adhesive compared to low viscosity tissue adhesive for surgical incisions | ||||||
| Patient or population: people with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low viscosity tissue adhesive | High viscosity tissue adhesive | |||||
| Wound dehiscence | Study population | RR 3.74 | 148 | ⊕⊝⊝⊝ | ||
| Could not be calculated | Could not be calculated | |||||
| Wound infection | Study population | RR 0.82 | 148 | ⊕⊝⊝⊝ | ||
| 47 per 1000 | 38 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study 95% CIs are very wide | ||||||
| Octylcyanoacrylate compared to butylcyanoacrylate for surgical incisions | ||||||
| Patient or population: people with surgical incisions | ||||||
| Outcomes | Illustrative comparative risks*2 (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Butylcyanoacrylate | Octylcyanoacrylate | |||||
| Wound dehiscence | 26 per 1000 | 38 per 1000 (5 to 297) | RR 1.46 (0.19 to 11) | 80 | ⊕⊕⊝⊝ | |
| Wound infection | Study population | RR 0.63 | 37 | ⊕⊕⊝⊝ | ||
| 333 per 1000 | 210 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The 95% CI estimate around the RR of 1.46 is very wide | ||||||
| Tissue adhesive vs tissue adhesive (Comp 5) | Mixed control (Comp 4) | Butyl‐2‐ cyanoacrylate vs staples (Comp 3) | 2‐octyl cyanoacrylate vs staples (Comp 3) | 2‐octyl cyanoacrylate vs tape (Comp 2) | Butyl‐2‐ cyanoacrylate vs sutures (Comp 1) | 2‐octyl cyanoacrylate vs sutures (Comp1) | Trial ID | 2‐octyl cyanoacrylate | Butyl‐2‐ cyanoacrylate | Sutures | Staples | Adhesive tape/strips | Mixed sutures and staples | All non‐tissue adhesive closure methods | Other viscosity 2‐octyl cyanoacrylate |
| 3 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 5 | 4 | ✓ | ✓ | ✓ | |||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 4 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 5 | 3 | 3 | 1 | 1 | ✓ | ✓ | ✓ | ✓ | |||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 5 | ✓ | ✓ | |||||||||||||
| 3 | 1 | ✓ | ✓ | ✓ | |||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 3 | ✓ | ✓ | |||||||||||||
| 2 | 1 | ✓ | ✓ | ✓ | |||||||||||
| 5 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 3 | ✓ | ✓ | |||||||||||||
| 3 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 2 | 1 | ✓ | ✓ | ✓ | |||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| 2 | ✓ | ✓ | |||||||||||||
| 1 | ✓ | ✓ | |||||||||||||
| Abbreviation Comp = comparison | |||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence: all studies Show forest plot | 17 | 1225 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [1.53, 7.33] |
| 2 Dehiscence: sensitivity analysis Show forest plot | 13 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.95, 7.68] |
| 3 Infection: all studies Show forest plot | 18 | 1239 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.94, 3.16] |
| 4 Infection: sensitivity analysis Show forest plot | 15 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.80, 5.12] |
| 5 Cosmetic appearance rated by patient Show forest plot | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐7.20, 2.95] |
| 5.1 VAS 0 to 100 | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐7.20, 2.95] |
| 6 Cosmetic appearance rated by surgeon Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 VAS scale 0 to 100 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Scar assessment (0 to 5 scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Patient/parent satisfaction (% satisfied) Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 8 Patient/parent satisfaction (VAS Scale 0 to 100) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Surgeon satisfaction (% satisfied) Show forest plot | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.58, 2.19] |
| 10 Time taken for wound closure Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Infection Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.39, 4.81] |
| 3 Cosmetic appearance rated by patient (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Cosmetic appearance rated by patient (% satisfied) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Cosmetic appearance rated by surgeon (VAS) Show forest plot | 2 | 139 | Mean Difference (IV, Random, 95% CI) | 9.56 [4.74, 14.37] |
| 6 Patient satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Surgeon satisfaction Show forest plot | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| 8 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.33] |
| 2 Infection Show forest plot | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.30, 6.54] |
| 3 Cosmetic appearance rated by patient (scar scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Cosmetic appearance by plastic surgeons (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Patient satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.38] |
| 2 Infection Show forest plot | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.60] |
| 3 Patient satisfaction Show forest plot | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.10, 0.70] |
| 4 Clinician satisfaction Show forest plot | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.29, 0.77] |
| 5 Time taken for wound closure Show forest plot | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.79, ‐0.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Infection Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Patient satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Clinician satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dehiscence Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.19, 11.30] |
| 2 Infection Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Cosmetic assessment rated by patient (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Cosmetic assessment rated by surgeon (VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Surgeon satisfaction (with device) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Surgeon satisfaction (with closure) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Time taken for wound closure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

