Эфавиренз или невирапин в составе трехкомпонентной комбинированной терапии с двумя нуклеозидными ингибиторами обратной транскриптазы для стартовой терапии ВИЧ‐инфекции у пациентов, ранее не получавших антиретровирусную терапию
Appendices
Appendix 1. MEDLINE search strategies
| Search | Most recent queries | Result |
| Search #8 AND #9 Limits: Publication Date from 1996 to 2009 | ||
| Search #8 AND #9 | ||
| Search NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING) | ||
| Search #3 AND #4 AND #7 | ||
| Search #5 OR #6 | ||
| Search NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE | ||
| Search EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ | ||
| Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | ||
| Search #1 OR #2 | ||
| Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | ||
| Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | ||
| Search | Query | Items found |
| Search ((#3 AND #4 AND #7)) AND ("2009/05/01"[Date ‐ Publication] : "2014/02/07"[Date ‐ Publication]) | ||
| Search (#3 AND #4 AND #7) | ||
| Search (#5 AND #6) | ||
| Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | ||
| Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | ||
| Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | ||
| Search (#1 AND #2) | ||
| Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab]))) | ||
| Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) |
| Search | Query | Items found |
| Search (((#3 AND #4 AND #7))) AND ("2014/02/07"[Date ‐ Publication] : "2015/03/13"[Date ‐ Publication]) | ||
| Search (#3 AND #4 AND #7) | ||
| Search (#5 AND #6) | ||
| Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | ||
| Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | ||
| Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | ||
| Search (#1 AND #2) | ||
| Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) | ||
| Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) |
| Search | Query | Items found |
| Search (((#3 AND #4 AND #7))) AND ("2015/03/13"[Date ‐ Publication] : "2016/08/12"[Date ‐ Publication]) | ||
| Search (#3 AND #4 AND #7) | ||
| Search (#5 AND #6) | ||
| Search (nevirapine[mh] OR nevirapine[tiab] OR viramune[tiab] OR nevimune[tiab] OR NVP[tiab]) | ||
| Search (efavirenz[tiab] OR sustiva[tiab] OR stocrin[tiab] OR EFV[tiab]) | ||
| Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | ||
| Search (#1 AND #2) | ||
| Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) | ||
| Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) |
Appendix 2. Embase search strategies
| No. | Query | Results | Date |
| #1 | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) | 292,932 | 22 May 2009 |
| #2 | ('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine') OR ('anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab) OR ('anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab) OR ('anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab) OR ('anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab) OR ('anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab) OR ('anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab) OR ('anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab) OR ('anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab) OR ('anti hiv':ti OR 'anti hiv':ab) OR (antiretrovir*:ti OR antiretrovir*:ab) OR ('anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab) OR (haart:ti OR haart:ab) OR ('aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab) OR (('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent')) OR (('antiretrovirus agent'/de OR 'antiretrovirus agent')) OR (('antivirus agent'/de OR 'antivirus agent')) OR (('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy')) | 92,810 | 22 May 2009 |
| #3 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/de OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/de OR 'randomised controlled trial'))))) | 865,259 | 22 May 2009 |
| #4 | #1 OR #2 | 321,839 | 22 May 2009 |
| #5 | ('efavirenz'/de OR 'efavirenz') OR ('sustiva'/de OR 'sustiva') OR ('stocrin'/de OR 'stocrin') OR efv OR efz | 7,680 | 22 May 2009 |
| #6 | ('nevirapine'/de OR 'nevirapine') OR nvp OR ('viramune'/de OR 'viramune') OR nevimune | 9,696 | 22 May 2009 |
| #7 | #5 OR #6 | 12,972 | 22 May 2009 |
| #8 | #3 AND #4 AND #7 | 1,088 | 22 May 2009 |
| #9 | ('nnrti'/de OR 'nnrti') OR 'non‐nucleoside reverse transcriptase inhibitor' OR 'non nucleoside reverse transcriptase inhibitor' OR ('nonnucleoside reverse transcriptase inhibitor'/de OR 'nonnucleoside reverse transcriptase inhibitor') OR 'non‐nucleoside reverse transcriptase inhibitors' OR 'non nucleoside reverse transcriptase inhibitors' OR 'nonnucleoside reverse transcriptase inhibitors' OR 'pi sparing' OR 'protease inhibitor sparing' OR 'protease‐inhibitor sparing' OR 'non‐protease inhibitor containing' OR 'non‐pi containing' OR 'non protease inhibitor containing' | 13,324 | 22 May 2009 |
| #10 | #8 AND #9 AND [1996‐2009]/py | 523 | 22 May 2009 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [embase]/lim AND [1‐5‐2009]/sd NOT [7‐2‐2014]/sd | 554 |
| #13 | #3 AND #9 AND #12 | 1115 |
| #12 | #10 AND #11 | 7336 |
| #11 | 'nevirapine'/de OR nevirapine OR 'viramune'/de OR viramune OR 'nevimune'/de OR nevimune OR nvp | 16047 |
| #10 | 'efavirenz'/de OR efavirenz OR 'sustiva'/de OR sustiva OR 'stocrin'/de OR stocrin OR efv | 13719 |
| #9 | #4 NOT #8 | 1551893 |
| #8 | #5 NOT #7 | 4886059 |
| #7 | #5 AND #6 | 1247730 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 14535586 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6133789 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1782714 |
| #3 | #1 AND #2 | 116833 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy':ab,ti | 165003 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 384563 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [7‐2‐2014]/sd NOT [13‐3‐2015]/sd | 85 |
| #13 | #3 AND #9 AND #12 | 796 |
| #12 | #10 AND #11 | 7647 |
| #11 | 'nevirapine'/de OR nevirapine:ab,ti OR 'viramune'/de OR viramune:ab,ti OR 'nevimune'/de OR nevimune:ab,ti OR nvp:ab,ti | 16066 |
| #10 | 'efavirenz'/de OR efavirenz:ab,ti OR 'sustiva'/de OR sustiva:ab,ti OR 'stocrin'/de OR stocrin:ab,ti OR efv:ab,ti | 14587 |
| #9 | #4 NOT #8 | 1438290 |
| #8 | #5 NOT #7 | 5079643 |
| #7 | #5 AND #6 | 1341085 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 15656505 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6420728 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1617084 |
| #3 | #1 AND #2 | 126453 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine' OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent' OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent' OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy' OR 'highly active antiretroviral therapy':ab,ti | 178964 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 410883 |
| No. | Query | Results |
| #14 | #3 AND #9 AND #12 AND [13‐3‐2015]/sd NOT [12‐8‐2016]/sd | 113 |
| #13 | #3 AND #9 AND #12 | 907 |
| #12 | #10 AND #11 | 8277 |
| #11 | 'nevirapine'/de OR nevirapine:ab,ti OR 'viramune'/de OR viramune:ab,ti OR 'nevimune'/de OR nevimune:ab,ti OR nvp:ab,ti | 17485 |
| #10 | 'efavirenz'/de OR efavirenz:ab,ti OR 'sustiva'/de OR sustiva:ab,ti OR 'stocrin'/de OR stocrin:ab,ti OR efv:ab,ti | 16217 |
| #9 | #4 NOT #8 | 1648816 |
| #8 | #5 NOT #7 | 5394456 |
| #7 | #5 AND #6 | 1491807 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 17407367 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6886263 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1847056 |
| #3 | #1 AND #2 | 140484 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy':ab,ti | 198729 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti | 448264 |
Appendix 3. CENTRAL search strategies
| ID | Search | Hits |
| #1 | 8223 | |
| #2 | 3577 | |
| #3 | 8432 | |
| #4 | 315 | |
| #5 | 227 | |
| #6 | 487 | |
| #7 | 493 | |
| #8 | 146 | |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 7543 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2495 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 12832 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 21 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 20 |
| #6 | #1 or #2 or #3 or #4 or #5 | 12913 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 961 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 2570 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3288 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 312 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 5678 |
| #12 | #7 or #8 or #9 or #10 or #11 | 8955 |
| #13 | #6 and #12 from 1980 to 2014, in Trials (Word variations have been searched) | 4943 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 562 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 523 |
| #16 | #14 and #15 | 121 |
| #17 | #13 and #16 from2009 to 2014, in Trials | 61 |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 8172 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2638 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 14285 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 21 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 23 |
| #6 | #1 or #2 or #3 or #4 or #5 | 14369 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 1056 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 2753 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3434 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 336 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 6380 |
| #12 | #7 or #8 or #9 or #10 or #11 | 9803 |
| #13 | #6 and #12 in Trials (Word variations have been searched) | 5538 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 633 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 634 |
| #16 | #14 and #15 | 148 |
| #17 | #13 and #16 Publication Year from 2014 to 2015, in Trials | 16 |
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 8983 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2834 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 16377 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 23 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 25 |
| #6 | #1 or #2 or #3 or #4 or #5 | 16462 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 1164 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 3041 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3784 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 375 |
| #11 | (anti hiv) or antiretroviral* or (anti near retroviral*) or (aids near vaccin*) (Word variations have been searched) | 7445 |
| #12 | #7 or #8 or #9 or #10 or #11 | 11216 |
| #13 | #6 and #12 in Trials (Word variations have been searched) | 6006 |
| #14 | nevirapine:ti,ab,kw or viramune:ti,ab,kw or nevimune:ti,ab,kw or NVP:ti,ab,kw | 767 |
| #15 | efavirenz:ti,ab,kw or sustiva:ti,ab,kw or efv:ti,ab,kw or stocrin:ti,ab,kw | 859 |
| #16 | #14 and #15 | 226 |
| #17 | #13 and #16 Publication Year from 2015 to 2016, in Trials | 0 |
Appendix 4. NLM Gateway search strategy
| Search | Search | Items |
| #8 | Search: ((((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND ((EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE))) AND ((NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS)) OR ((NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING))) Limit: 1996:2009 | 260 |
| #7 | Search: (NNRTI OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR) OR (NON‐NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (NON NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS)) OR ((NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) OR (PI SPARING) OR (PROTEASE INHIBITOR SPARING) OR (PROTEASE‐INHIBITOR SPARING) OR (NON‐PROTEASE INHIBITOR CONTAINING) OR (NON‐PI CONTAINING) OR (NON PROTEASE INHIBITOR CONTAINING)) | 16334 |
| #6 | Search: (((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND ((EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE)) | 756 |
| #5 | Search: (EFAVIRENZ OR SUSTIVA OR STOCRIN OR EFV OR EFZ) OR (NEVIRAPINE OR NVP OR VIRAMUNE OR NEVIMUNE) | 6894 |
| #4 | Search: (((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) | 5030795 |
| #3 | Search: ((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw]))))) | 390683 |
| #2 | Search: (("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) | 122073 |
| #1 | Search: (((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL))) | 366953 |
Appendix 5. Cochrane 'Risk of bias' assessment tool
| Domain | Support for judgement | Review authors’ judgement |
| Selection bias. | ||
| Random sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Performance bias. | ||
| Blinding of participants and personnelAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Detection bias. | ||
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Attrition bias. | ||
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to amount, nature or handling of incomplete outcome data. |
| Reporting bias. | ||
| Selective reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other bias. | ||
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. | Bias due to problems not covered elsewhere in the table. |

Flow diagram of study screening and selection

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

Funnel plot of comparison: 1 Efavirenz 600 mg versus Nevirapine all doses, outcome: 1.1 Virological success.

Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 1 Virological success.

Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 2 Mortality.

Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 3 Progression to AIDS.
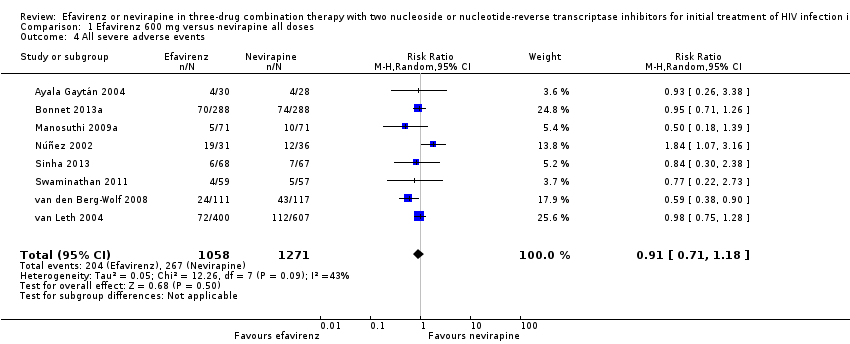
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 4 All severe adverse events.

Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 5 Discontinuation rate.
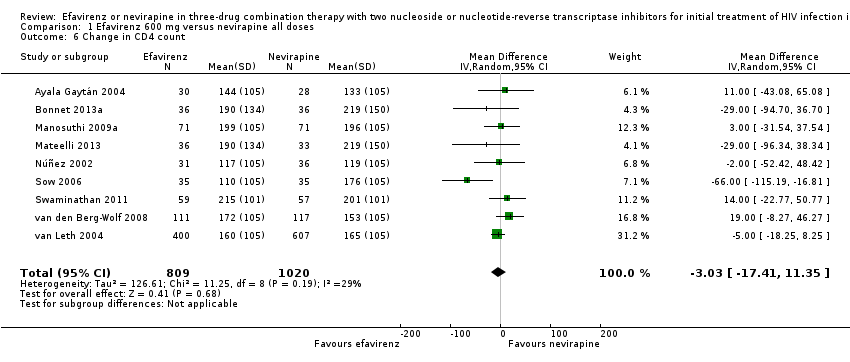
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 6 Change in CD4 count.
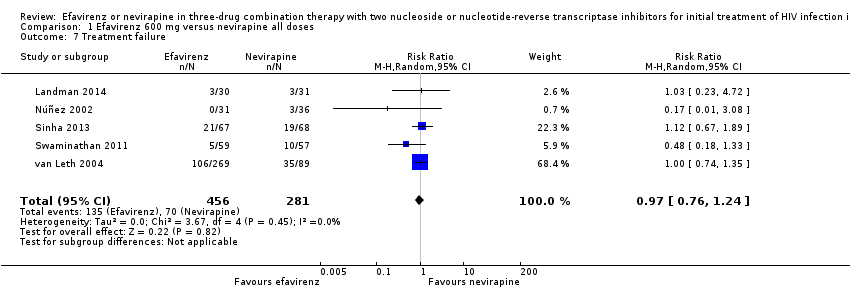
Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 7 Treatment failure.

Comparison 1 Efavirenz 600 mg versus nevirapine all doses, Outcome 8 Development of drug resistance.
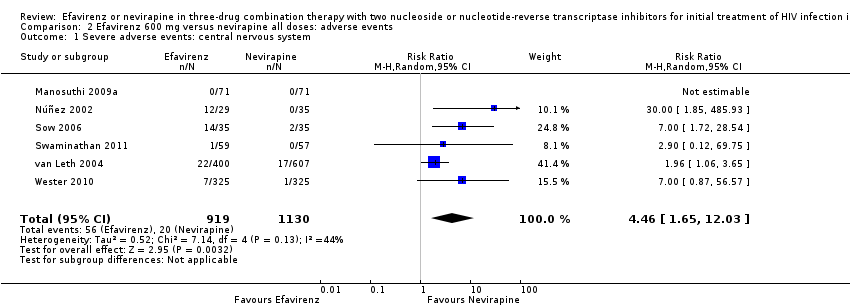
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 1 Severe adverse events: central nervous system.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 2 Severe adverse events: gastrointestinal tract.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 3 Severe adverse events: pyrexia.
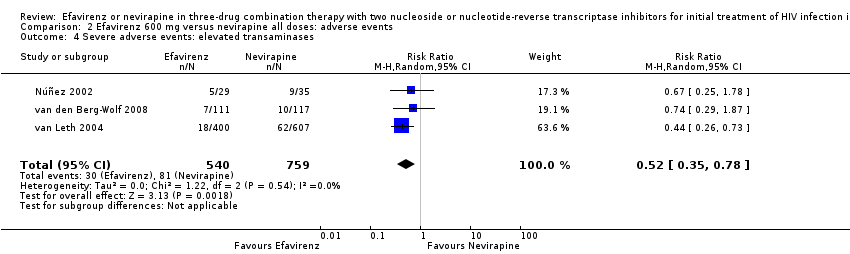
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 4 Severe adverse events: elevated transaminases.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 5 Severe adverse events: elevated alkaline phosphatase.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 6 Severe adverse events: elevated amylase.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 7 Severe adverse events: elevated triglycerides.
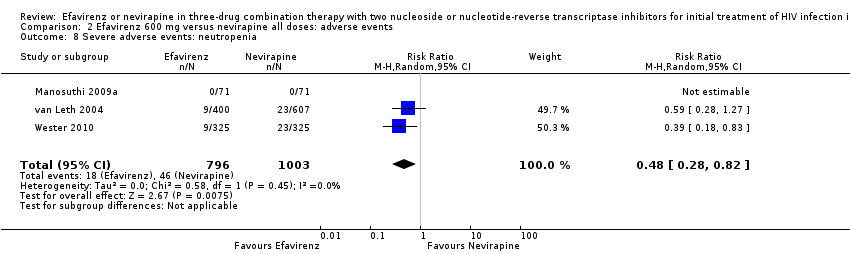
Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 8 Severe adverse events: neutropenia.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 9 Severe adverse events: rash.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 10 Severe adverse events: elevated SGOT.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 11 Severe adverse events: elevated SGPT.

Comparison 2 Efavirenz 600 mg versus nevirapine all doses: adverse events, Outcome 12 Severe adverse events: elevated cholesterol.
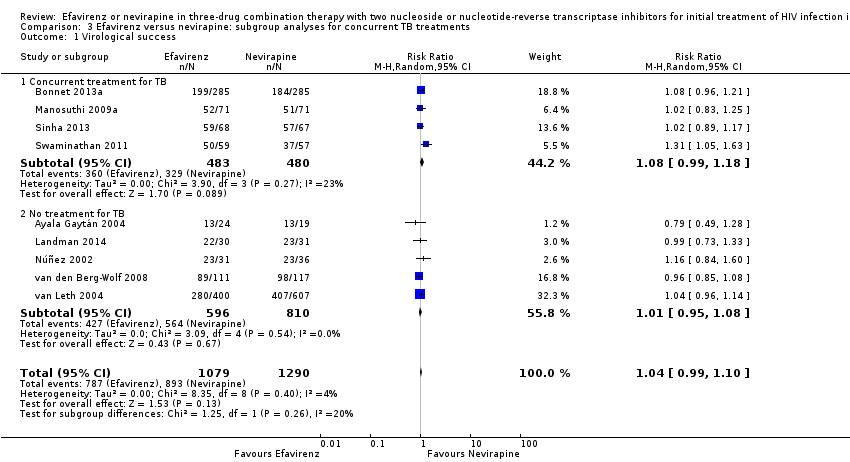
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 1 Virological success.

Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 2 Mortality.
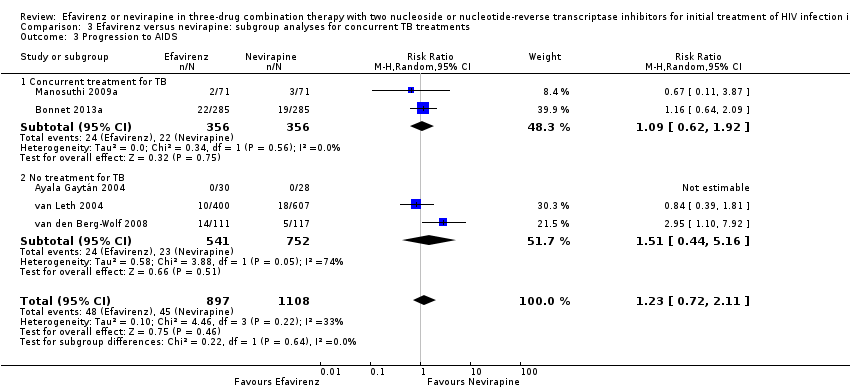
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 3 Progression to AIDS.
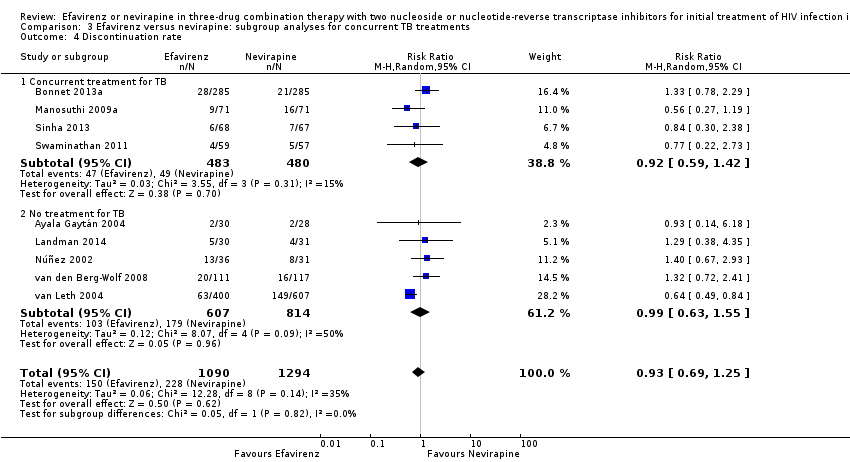
Comparison 3 Efavirenz versus nevirapine: subgroup analyses for concurrent TB treatments, Outcome 4 Discontinuation rate.

Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 1 Virological success.
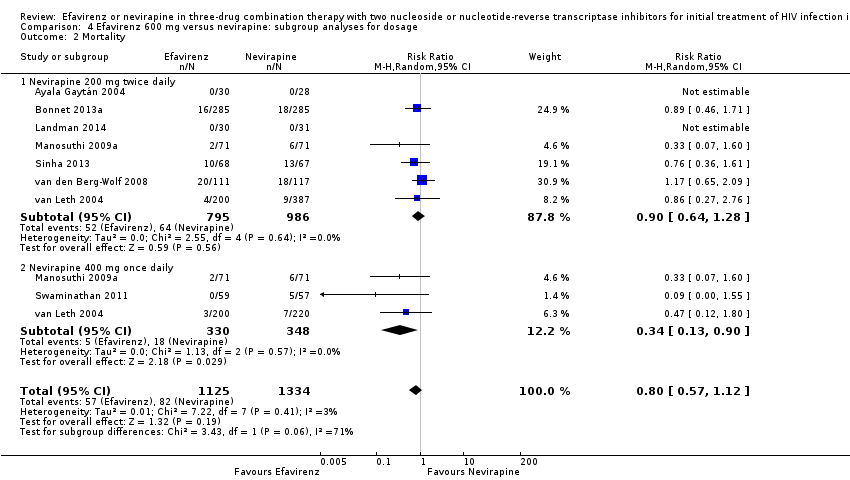
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 2 Mortality.
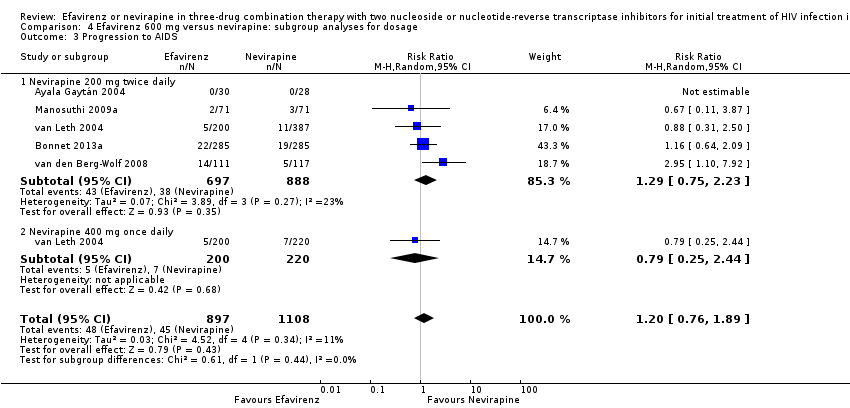
Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 3 Progression to AIDS.

Comparison 4 Efavirenz 600 mg versus nevirapine: subgroup analyses for dosage, Outcome 4 Discontinuation rate.
| Efavirenz (600 mg) versus nevirapine (all doses) for three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïveindividuals | |||||
| Patient or population: antiretroviral‐naïve individuals | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with nevirapine all doses | Risk with efavirenz 600 mg | ||||
| Virological success | 688 per 1000 | 715 per 1000 | RR 1.04 | 2438 | ⊕⊕⊕⊕ |
| Mortality | 64 per 1000 | 54 per 1000 | RR 0.84 | 2317 | ⊕⊕⊕⊝ |
| Progression to AIDS | 41 per 1000 | 50 per 1000 | RR 1.23 | 2005 | ⊕⊕⊕⊝ |
| All severe adverse events | 192 per 1000 | 216 per 1000 | RR 0.91 | 2329 | ⊕⊝⊝⊝ |
| Discontinuation rate | 176 per 1000 | 164 per 1000 | RR 0.93 | 2384 | ⊕⊕⊕⊝ |
| Change in CD4 count | The mean change in CD4 count was 0 | MD 3.03 lower | — | 1829 | ⊕⊕⊕⊝ |
| Treatment failure | 249 per 1000 | 242 per 1000 | RR 0.97 | 737 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Three trials used a cut‐off point of 400 copies/mL (Ayala Gaytán 2004; Swaminathan 2011; Sinha 2013), but we did not downgrade for this. | |||||
| Efavirenz (600 mg) versus nevirapine (all doses): adverse events for three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïveindividuals | |||||
| Patient or population: antiretroviral‐naïve individuals | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with nevirapine all doses: adverse events | Risk with efavirenz 600 mg | ||||
| Severe adverse events: central nervous system | 2 per 1000 | 7 per 1000 | RR 4.46 | 2049 | ⊕⊕⊕⊝ |
| Severe adverse events: gastrointestinal | 18 per 1000 | 14 per 1000 | RR 0.76 | 2049 | ⊕⊕⊝⊝ |
| Severe adverse events: pyrexia | 0 per 1000 | 0 per 1000 | RR 0.65 | 1799 | ⊕⊕⊝⊝ |
| Severe adverse events: raised transaminases | 257 per 1000 | 134 per 1000 | RR 0.52 | 1299 | ⊕⊕⊕⊕ |
| Severe adverse events: raised alkaline phosphatase | 12 per 1000 | 7 per 1000 | RR 0.65 | 1007 | ⊕⊕⊝⊝ |
| Severe adverse events: raised amylase | 14 per 1000 | 20 per 1000 | RR 1.40 | 1071 | ⊕⊕⊝⊝ |
| Severe adverse events: raised triglycerides | 7 per 1000 | 7 per 1000 | RR 1.10 | 1071 | ⊕⊕⊝⊝ |
| Severe adverse events: neutropenia | 38 per 1000 | 18 per 1000 | RR 0.48 | 1799 | ⊕⊕⊕⊕ |
| Severe adverse events: rash | 229 per 1000 | 133 per 1000 | RR 0.58 | 2277 | ⊕⊕⊕⊝ |
| Severe adverse events: serum glutamic oxaloacetic transaminase (SGOT) | The mean severe adverse events: SGOT was 0 | MD 3.3 higher | — | 135 | ⊕⊕⊕⊝ |
| Severe adverse events: serum glutamic‐ pyruvic transaminase (SGPT) | The mean severe adverse events: SGPT was 0 | MD 5.7 higher | — | 135 | ⊕⊕⊕⊝ |
| Raised cholesterol | 29 per 1000 | 172 per 1000 | RR 6.03 | 64 | ⊕⊕⊕⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Five trials (Manosuthi 2009a; Núñez 2002; Swaminathan 2011; van Leth 2004; Wester 2010) were open‐label. One trial did not report blinding (Sow 2006). We downgraded by 1 for this. | |||||
| Trial ID | Location | NVP dosage | NRTI combination drugs | Co‐infection with tuberculosis | Virological success cut‐off point |
| Mexico | 200 mg twice daily | AZT 300 mg and 3TC 150 mg | No | < 400 copies/mL | |
| Mozambique | 200 mg twice daily | 3TC and d4T/AZT1 | No | < 50 copies/mL | |
| Cameroon and Senegal | 200 mg twice daily | TDF 300 mg and FTC 200 mg | No | < 50 copies/mL | |
| Thailand | 400 mg once daily | 3TC 150 mg and D4T 30 or 40 mg | Yes | < 50 copies/mL | |
| Burkina Faso | 200 mg twice daily | D4T and 3TC1 | Yes | Not reported | |
| Spain | 400 mg once daily | D4T 40 mg and DDI 400 mg | No | < 50 copies/mL | |
| India | 200 mg twice daily | AZT, d4T, 3TC1 | Yes | < 400 copies/mL | |
| Senegal | 200 mg twice daily | AZT 300 mg and 3TC 150 mg | No | Not reported | |
| India | 400 mg once daily | DDI 250 mg or 400 mg and 3TC 300 mg | Yes | Not reported | |
| USA | 200 mg twice daily | ABC/3TC, DDI/d4T, AZT/3TC, d4T/3TC1 | No | < 50 copies/mL | |
| North/South America, Australia, Europe, South Africa, and Thailand | 200 mg twice daily and 400 mg twice daily | D4T 40 mg and 3TC 150 mg | No | < 50 copies/mL | |
| Botswana | Not reported | AZT or d4T/3TC or DDI1 | No | Not reported | |
| 1Dosage not specified. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 10 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 2 Mortality Show forest plot | 8 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 3 Progression to AIDS Show forest plot | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.72, 2.11] |
| 4 All severe adverse events Show forest plot | 8 | 2329 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 5 Discontinuation rate Show forest plot | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.25] |
| 6 Change in CD4 count Show forest plot | 9 | 1829 | Mean Difference (IV, Random, 95% CI) | ‐3.03 [‐17.41, 11.35] |
| 7 Treatment failure Show forest plot | 5 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 8 Development of drug resistance Show forest plot | 4 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe adverse events: central nervous system Show forest plot | 6 | 2049 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.65, 12.03] |
| 2 Severe adverse events: gastrointestinal tract Show forest plot | 6 | 2049 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.48, 1.21] |
| 3 Severe adverse events: pyrexia Show forest plot | 3 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.73] |
| 4 Severe adverse events: elevated transaminases Show forest plot | 3 | 1299 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.78] |
| 5 Severe adverse events: elevated alkaline phosphatase Show forest plot | 1 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.17, 2.50] |
| 6 Severe adverse events: elevated amylase Show forest plot | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.72, 2.73] |
| 7 Severe adverse events: elevated triglycerides Show forest plot | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.39, 3.13] |
| 8 Severe adverse events: neutropenia Show forest plot | 3 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.82] |
| 9 Severe adverse events: rash Show forest plot | 7 | 2277 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 10 Severe adverse events: elevated SGOT Show forest plot | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 3.30 [‐2.06, 8.66] |
| 11 Severe adverse events: elevated SGPT Show forest plot | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 5.70 [‐4.23, 15.63] |
| 12 Severe adverse events: elevated cholesterol Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [0.75, 48.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 9 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.10] |
| 1.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.99, 1.18] |
| 1.2 No treatment for TB | 5 | 1406 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 2 Mortality Show forest plot | 8 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 2.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.19] |
| 2.2 No treatment for TB | 4 | 1354 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.62, 1.64] |
| 3 Progression to AIDS Show forest plot | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.72, 2.11] |
| 3.1 Concurrent treatment for TB | 2 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.62, 1.92] |
| 3.2 No treatment for TB | 3 | 1293 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.44, 5.16] |
| 4 Discontinuation rate Show forest plot | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.25] |
| 4.1 Concurrent treatment for TB | 4 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.42] |
| 4.2 No treatment for TB | 5 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.63, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 9 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 1.1 Nevirapine 200 mg twice daily | 7 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.09] |
| 1.2 Nevirapine 400 mg once daily | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.35] |
| 2 Mortality Show forest plot | 8 | 2459 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 2.1 Nevirapine 200 mg twice daily | 7 | 1781 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.28] |
| 2.2 Nevirapine 400 mg once daily | 3 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.90] |
| 3 Progression to AIDS Show forest plot | 5 | 2005 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.76, 1.89] |
| 3.1 Nevirapine 200 mg twice daily | 5 | 1585 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.75, 2.23] |
| 3.2 Nevirapine 400 mg once daily | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.25, 2.44] |
| 4 Discontinuation rate Show forest plot | 9 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.14] |
| 4.1 Nevirapine 200 mg twice daily | 7 | 1781 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.21] |
| 4.2 Nevirapine 400 mg once daily | 3 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.57] |

