| | Adverse events | Withdrawals |
| Study ID | Treatment | Any | Serious | Adverse event | Other |
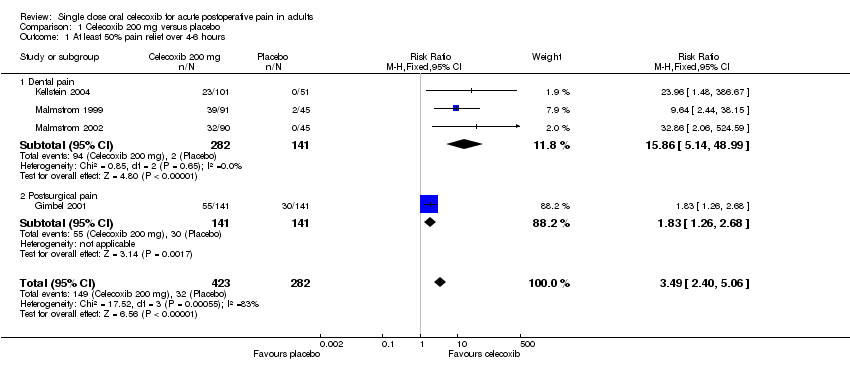
| Malmstrom 1999 | (1) cele 200 mg, n=91 (2) rofe 50 mg, n=90 (3) ibu 400 mg, n=46 (4) placebo, n=45 | no useable data Mostly nausea, vomiting, headache | none reported | (1) 0/91 (4) 1/45 (excessive bleeding) | none |
| Gimbel 2001 | (1) cele 200 mg, n=141 (2) hydrocod/paracet 10/1000 mg, n=136 (3) placebo, n=141 | to 8 hrs: (1) 16/141 (3) 20/141 Mostly nausea, vomiting, somnolence, headache | none reported | (1) 1/141 (3) 0/141 | none |
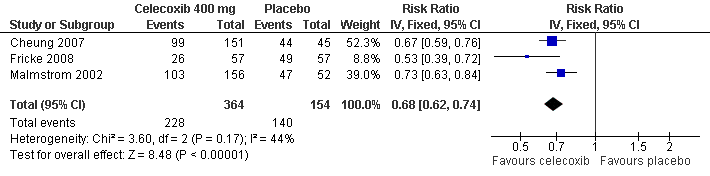
| Malmstrom 2002 | (1) cele 400 mg, n=151 (2) cele 200 mg, n=90 (3) rofe 50 mg, n=150 (4) ibu 400 mg, n=45 (5) placebo, n=45 | to 24 hrs: (1) 38/151 (2) 22/90 (5) 12/45 Mostly nausea and vomiting | (1) 0/151 (2) 1/90 (at post study visit, not related to medication) (5) 0/45 | none reported | none |
| Kellstein 2004 | (1) cele 200 mg, n=101 (2) lumira 400 mg, n=101 (3) rofe 50 mg, n=102 (4) placebo, n=51 | to 24 hrs: (1) 20/101 (4) 9/51 | none | none | none |
| Moberly 2007 | (1) cele 400 mg, n=51 (2) placebo, n=52 Also tested CS‐706 at 10, 50, 100, 200 mg | to 24 hrs: (1) 23/51 (2) 27/52 drug related:(1) 5/51; (2) 13/52 | none | none | I placebo pt had protocol violation (rescue medication early) ‐ excl from ITT analysis |
| Doyle 2002 | (1) cele 200 mg, n=74 (2) ibu liquigel 400 mg, n=74 (3) placebo, n=26 | to 12 hrs: (1) 5/74 (3) 3/26 Most mild to moderate, nausea, vomiting, headache | none | (1) 0/74 (3) 0/26 | 5 pts (2 cele) excl from analysis due to protocol violation, admin reason, withdrew consent |
| Cheung 2007 | (1) cele 400 mg, n=57 (2) ibu 400 mg, n=57 (3) placebo, n=57 | to 24 hrs: (1) 29/57 (3) 39/57 | (1) 1/57 (rhabdomyolysis) (3) 0/57 | (1) 1/57 (3) 3/57 | 1 placebo pt withdrew consent |
| Fricke 2008 | (1) cele 400 mg, n=156 (2) lumira 400 mg, n=156 (3) placebo, n=52 | to 24 hrs: (1) 17/156 (3) 9/52 | none | none | none |